Abstract
The examination of antibody responses in human immunodeficiency virus (HIV)-1-infected individuals in the setting of antiretroviral treatment (ART) interruption can provide insight into the evolution of antibody responses during viral rebound. In this study, we assessed antibody responses in 20 subjects in AIDS Clinical Trials Group A5187, wherein subjects were treated with antiretroviral therapy during acute/early HIV-1 infection, underwent analytic treatment interruption, and subsequently demonstrated viral rebound. Our data suggest that early initiation of ART arrests the maturation of HIV-1-specific antibody responses, preventing epitope diversification of antibody binding and the development of functional neutralizing capacity. Antibody responses do not appear permanently blunted, however, because viral rebound triggered the resumption of antibody maturation in our study. We also found that antibody responses measured by these assays did not predict imminent viral rebound. These data have important implications for the HIV-1 vaccine and eradication fields.
Keywords: antibody, HIV eradication, HIV vaccine, microarray, treatment interruption
A priority in the field of human immunodeficiency virus (HIV)-1 research is to develop novel therapeutic interventions that may lead to long-term suppression of HIV-1 replication in the absence of antiretroviral therapy (ART) [1]. Such novel interventions include broadly neutralizing antibodies, latency reversal agents, and therapeutic vaccination. Analytic treatment interruption (ATI) is an essential tool to assess the efficacy of such therapeutic interventions, but it carries the risk of HIV-1 replication and acute retroviral syndrome. As a result, there is interest in exploring early antibody recall responses after ATI as a potential predictor of imminent viral rebound. In addition, early antibody recall responses after ATI may also provide important lessons about the evolution of antibody specificity and function in the setting of expanding viral replication.
Due to the importance of understanding early antibody recall responses after ATI, we performed a comprehensive assessment of antibody responses after viral rebound in a cohort of 20 subjects enrolled in AIDS Clinical Trials Group (ACTG) A5187, a clinical trial testing the safety and immunogenicity of therapeutic deoxyribonucleic acid (DNA) vaccination in individuals treated with ART during early HIV-1 infection [2].
METHODS
Study Population
Plasma samples were obtained from 20 HIV-1-infected subjects from the United States, aged 26–47 years old, who had been enrolled in ACTG A5187, a phase I/II, randomized, placebo-controlled, double-blinded trial to evaluate the safety and immunogenicity of an HIV-1 DNA vaccine (VRC-HVDNA009-00-VP) in subjects treated with ART during acute/early HIV-1 infection (NCT00125099) [2]. All subjects gave written informed consent, and the study was approved by the ACTG, the National Institutes of Health Division of AIDS, and the human protection committees of each participating institution. All subjects had started ART during early HIV-1 infection, defined as positive HIV ribonucleic acid, a negative or indeterminate Western blot, or a nonreactive detuned enzyme-linked immunosorbent assay (ELISA). All subjects had been on ART with suppressed HIV-1 viral load (<50 copies/mL) for at least 6 months when they were randomized to receive either DNA HIV-1 vaccine or placebo at weeks 0, 4, 8, and 24 postrandomization. At week 30 postrandomization, all subjects underwent ATI, and set-point HIV-1 viral loads and CD4 T-cell counts were determined 17–23 weeks after treatment discontinuation. No differences in immunogenicity were detected in subjects receiving vaccine versus placebo, and there were no significant differences in set-point HIV-1 viral loads or CD4 T-cell counts after ATI. For the current study, plasma samples were available from 20 subjects at baseline, 17 subjects 4 weeks post-ATI, and 15 subjects 20 weeks post-ATI. Plasma samples from 4 HIV-negative subjects were also used as assay controls. A5187 subjects were divided into 2 groups based on whether or not their plasma HIV viral load was greater or less than 50 copies/mL at 4 weeks post-ATI. Group 1 represented 7 subjects that had undetectable viral load at week 4 post-ATI, all of whom would have viral rebound in the coming weeks (6 of 7 within just 2 weeks). This group represented subjects in an eclipse period of viral replication. Group 2 represented 10 subjects that already had detectable viral load by week 4 post-ATI.
Enzyme-Linked Immunosorbent Assay
Human immunodeficiency virus-1-specific humoral immune responses were assessed by envelope (Env) ELISAs [3, 4] using antigens produced in stably transfected 293 T cells or commercially purchased (Polymun). These antigens were trimeric HIV-1 glycoprotein (gp)140 (Env) proteins, and were from 6 major clades/circulating recombinant forms (CRFs) (A, B, C, D, F, 01_AE, and mosaic): PV0.4, C97ZA, CN54, UG37, UG92, BR29, UG21, A244, and Mos1. This panel allowed the quantitation of antibody titers to intact soluble trimeric gp140 Envs that included conformational epitopes.
Global Human Immunodeficiency Virus-1 Peptide Microarray
The magnitude of immunoglobulin (Ig)G binding to HIV epitopes was measured with global HIV-1 peptide microarrays (JPT Peptide Tech) as we have described [5]. In brief, microarrays consist of 3 identical subarrays containing 6564 overlapping 15 amino acid peptides, covering 46%–72% of HIV-1 Env, Gag, Pol, Nef, Rev, Vif, and Tat sequences contained in the Los Alamos National Laboratory sequence database. Plasma was incubated with the microarray and Alexa Fluor 647-conjugated Anti-Human IgG; readout and image processing was performed with Genepix 4300A scanner/software. Signal intensity equaled the median of triplicate peptides. Signal intensity was corrected by subtracting values from matched peptides on control microarrays incubated with secondary antibody alone [6]. The threshold for positivity was >5× noise distribution of the sample slide.
TZM-bl-Neutralizing Antibody Assay
Standard TZM-bl virus neutralization assays were performed [7–9] using a panel that included 3 tier 1A Env pseudotyped strains from HIV-1 clades B, C, and CRF01_AE, and 4 tier 1B strains from HIV-1 clades A, B, C, and CRF 02_AG, as well as a negative control virus (murine leukemia virus [MuLV]). Of note, this assay could not be performed on baseline samples because of the antiretroviral drugs present in plasma at this time point.
Statistical Analysis
Antibody responses in Group 1 were compared with Group 2, and between 2 time points, using unpaired Student t test and 2-tailed P values, with a threshold for significance of P ≤ .05. Antibody responses within the same group but between 2 time points were compared using paired t tests. Associations were calculated using non-parametric Spearman correlation and 2-tailed P values, with a threshold for significance of P ≤ .05. All calculations were performed using GraphPad Prism 6 (V6.0f).
RESULTS
In this study, we report the results of a comprehensive assessment of antibody responses in a cohort of 20 subjects enrolled in ACTG A5187, a randomized, double-blind, placebo-controlled trial of an HIV-1 DNA vaccine (VRC-HVDNA009-00-VP) in subjects treated with ART during acute/early HIV-1 infection (NCT00125099) [2]. In ACTG A5187, all subjects underwent ATI at week 30 after immunization; no differences in immunogenicity were detected in subjects receiving vaccine versus placebo, and there were no significant differences in set-point HIV-1 viral loads or CD4+ T-cell counts after ATI. We performed our analysis on 3 time points: at baseline (beginning of study) before ATI, at week 4 post-ATI, and at week 20 post-ATI.
Cross-Clade Envelope-Specific Immunoglobulin G Binding Titers Increased After Analytic Treatment Interruption When Plasma Human Immunodeficiency Virus Viral Loads Were Greater Than 50 Copies per mL
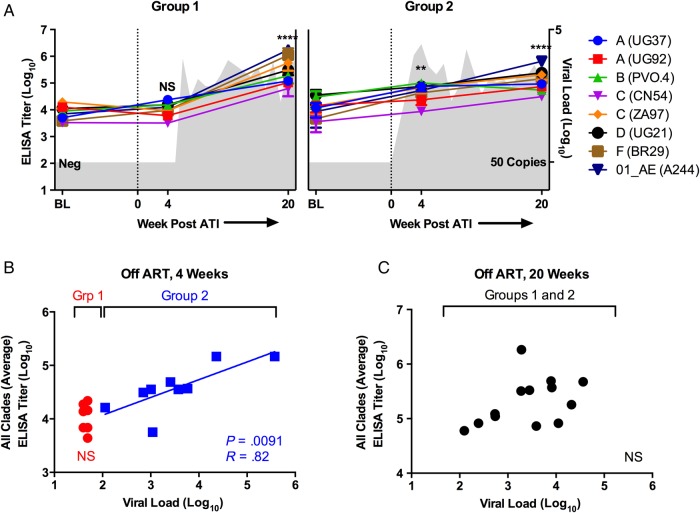
We began by quantifying IgG binding at baseline to a panel of trimeric gp140 HIV-1 Env proteins by ELISA [3, 4]. We found that the average all-clade IgG titer—defined as the average IgG titer across the 8 tested proteins—ranged from 3.2 to 5.5 log10 in HIV-1-infected subjects who received early-initiated ART at baseline before ATI (Figure 1A), and that titers against the different clades/CRFs were largely equivalent. The all-clade IgG titer was significantly greater in the ART-suppressed, HIV-1-infected subjects than in HIV-1-negative controls (3.9 vs 2.0 log10 IgG titer, P < .0001; data not shown), and there was no difference in average IgG titers between subjects in Group 1 and Group 2 at baseline (3.9 vs 3.8 mean log10 titer, P = not significant [NS]). These data suggest that the initiation of ART during the first 6 months of HIV-1 infection did not prevent the development of gp140 binding titers early in infection or impair the ability to maintain IgG binding while on suppressive ART.
Figure 1.
Immunoglobulin G (IgG) binding titers to a cross-clade panel of human immunodeficiency virus (HIV)-1 envelope proteins by enzyme-linked immunosorbent assay (ELISA) in HIV-infected subjects treated with antiretroviral therapy (ART) at baseline (BL), 4 weeks post-analytic treatment interruption (ATI) and 20 weeks post-ATI. (A) Immunoglobulin G binding titers are plotted over time for subjects that had undetectable (Group 1, left) and detectable (Group 2, right) viral load at week 4 post-ATI. Mean viral load for each group is plotted over time in gray in the background. Average all-clade IgG titers at week 4 and 20 were compared with BL in both groups. (B) Immunoglobulin G titers are plotted by viral load for both groups at 4 weeks post-ATI. (C) Immunoglobulin G titers are plotted by viral load for both groups at 20 weeks post-ATI. **P < .01; ****P < .0001. Abbreviation: NS, not significant.
We then examined whether gp140 antibody binding titers increased after ATI and whether a rise in titer might predict imminent viral rebound. For the analysis presented here, A5187 subjects were divided into 2 groups: Group 1 represented 7 subjects that had undetectable viral load at week 4 post-ATI. Group 2 represented 10 subjects that already had detectable viral load by week 4 post-ATI. We found that 4 weeks after ATI, subjects who still had an undetectable viral load (Group 1) had no increase in IgG binding titers against all clades compared with baseline (3.9 vs 4.0 log10 titer, P = NS) (Figure 1A), despite the fact that 6 of 7 subjects became viremic within the next 2 weeks. In contrast, subjects who had a detectable viral load at 4 weeks after ATI (Group 2) had a significant boost in all-clade IgG binding titers at 4 weeks compared with baseline (3.8 vs 4.6 log10 titer, P = .0044) (Figure 1A). In addition, there was a significant difference between Groups 1 and 2 at week 4 post-ATI (4.0 vs 4.6 log10 titer, P = .0122) (Figure 1B), as well as a significant and strong positive association between viral load and all-clade IgG titer within Group 2 (P = .0091, R = 0.82) (Figure 1B). These data show that gp140 IgG antibody binding titers are not a predictor of imminent viral rebound. On the contrary, it appears that increases in viral load likely drive subsequent increases in antibody titers.
By 20 weeks after ATI, Env-specific IgG titers boosted in both Groups 1 and 2 compared with baseline and at 4 weeks (Figure 1A), because all subjects had experienced viral rebound and reached viral setpoint by this time point. In Group 1, all-clade antibody titers increased from 3.9 to. 5.5 log10 (P < .0001); in Group 2, titers increased 3.8 vs 5.2 log10 (P < .0001). For all subjects, all-clade IgG titers significantly increased between 4 and 20 weeks (4.3 vs 5.3 log10, P < .0001; data not shown). By week 20, gp140-specific IgG titers were essentially equal among all subjects and no longer correlated with viral load (Figure 1C). Of note, there was no significant difference in all-clade IgG titers at 4 weeks or 20 weeks after ATI between subjects who received vaccine versus placebo (unpaired t test, P = NS; data not shown).
Neutralizing Antibody Titers Were Low Through 4 Weeks After Analytic Treatment Interruption but Slowly Developed Against Some Isolates After Viral Rebound
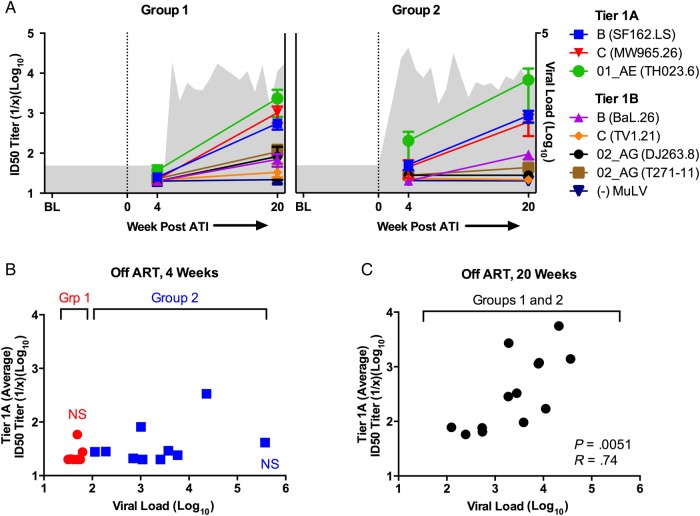
We next assessed neutralizing antibody (NAb) responses against a panel of tier 1 (easiest to neutralize) HIV isolates in all subjects at 4 and 20 weeks after ATI by TZM-bl luciferase-based virus neutralization assay [7, 8]. Of note, this assay could not be performed on baseline samples because of the antiretroviral drugs present in plasma at this time point, nor did we test neutralization against autologous viruses. We found that NAb titers against tier 1A or 1B viruses 4 weeks post-ATI were not significantly higher than NAb titers against the MuLV control virus (Figure 2A), although NAb titers against the clade B virus SF162.LS trended towards being significant within Group 2 (P = .0179 by paired t test, NS when corrected for 7 comparisons). There was also no difference in average tier 1A or 1B NAb titers between Groups 1 and 2 four weeks post-ATI (Figure 2B), nor was there an association between neutralization and viral load within Group 2.
Figure 2.
Neutralizing antibody (NAb) responses in human immunodeficiency virus (HIV)-infected subjects treated with antiretroviral therapy (ART) at baseline (BL), 4 weeks post-analytic treatment interruption (ATI) and 20 weeks post-ATI. (A) Neutralizing antibody titers against a panel of tier 1 HIV isolates are plotted over time for subjects that had undetectable (Group 1, left) and detectable (Group 2, right) viral load at week 4 post-ATI. Mean viral load for each group is plotted over time in gray in the background. (B) Average tier 1A NAb titers are plotted by viral load for both groups at 4 weeks post-ATI. (C) Average tier 1A NAb titers are plotted by viral load for both groups at 20 weeks post-ATI. Abbreviation: NS, not significant.
By 20 weeks after ATI, mean NAb titers among all subjects (Groups 1 and 2 combined) were detectable against all tier 1A viruses, as well as the tier 1B viruses BaL.26 (clade B) and T271-11 (CRF 02_AG), when compared with control NAb titers (1.7–2.7 vs 1.3 log10 NAb titer, Ps < .001 by paired t test) (Figure 2A). There was a significant increase in mean NAb titers against tier 1A viruses among all subjects between 4 weeks and 20 weeks after ATI (1.5 vs 2.6 log10 NAb titer, P < .0001). Mean NAb titers against tier 1A viruses (Figure 2C) were also significantly positively associated with viral load at 20 weeks post-ATI (P = .0051). There was no difference in NAb titers against tier 1A viruses between subjects who received vaccine versus placebo at either week 4 or 20 after ATI (unpaired t test, P = NS; data not shown).
The above data suggest that the early initiation of ART during the first 6 months of HIV-1 infection essentially arrests the development of NAb responses against heterologous viruses maintaining the phenotype of early infection [10], with minimal NAb titers against even highly neutralization-sensitive tier 1A isolates 4 weeks after discontinuation of suppressive ART. However, it appears that after subjects become viremic, NAb responses begin to develop slowly and sequentially: titers against an exquisitely sensitive heterologous clade B virus rise first (ie, SF162.LS), followed by cross-clade titers against other tier 1 isolates. Taken together, these data (Figures 1 and 2) suggest that viral replication post-ATI drives the rapid development of binding antibodies and the slower development of NAbs.
Antibody Responses Against Linear Peptides Were Primarily Against Peptides From the Third Hypervariable Loop of Glycoprotein (gp)120 and the CC Loop of gp41 After Analytic Treatment Interruption
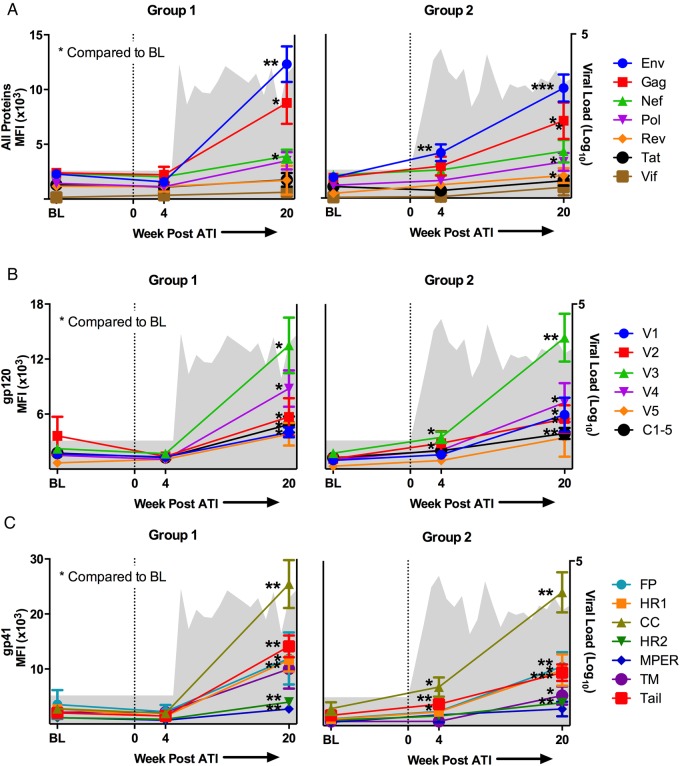
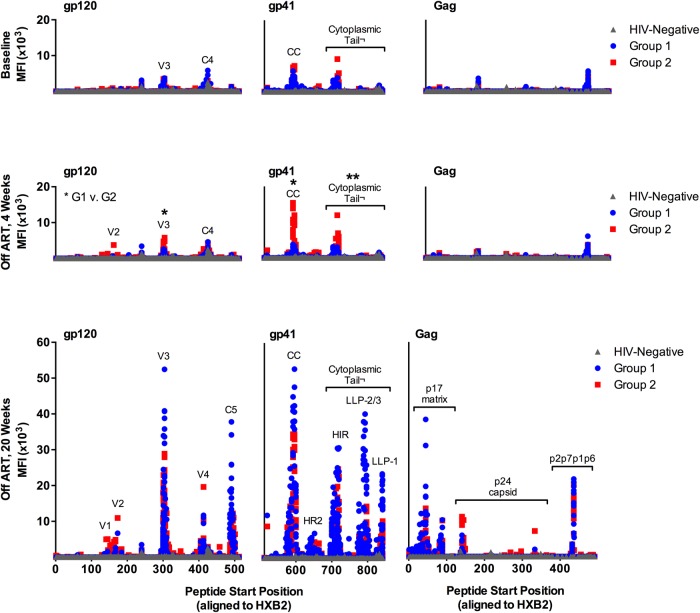
We next evaluated the epitope specificity of Env-specific IgG responses in these individuals using global HIV-1 peptide microarrays that contained 6564 overlapping linear HIV-1 peptides covering common HIV-1 variants in the HIV-1 sequence database at Los Alamos National Laboratory (LANL) [5]. At baseline, subjects in both Groups 1 and 2 had low binding to Env, Gag, Nef, Pol, Rev, and Tat peptides (Figure 3A). The Env binding patterns in both groups were focused on peptides from the third hypervariable loop (V3) and the fourth constant region (C4) of gp120, as well as the CC loop of gp41 and the cytoplasmic tail (Figure 4). These patterns are consistent with previously reported patterns of antibody binding in early HIV-1 infection [10, 11].
Figure 3.
Immunoglobulin G (IgG) responses to linear human immunodeficiency virus (HIV)-1 peptides in HIV-infected subjects treated with antiretroviral therapy (ART) at baseline (BL), 4 weeks post-analytic treatment interruption (ATI) and 20 weeks post-ATI. Mean fluorescent intensity (MFI) of IgG binding is plotted over time in (A–C) for subjects that had undetectable (Group 1, left) and detectable (Group 2, right) viral load at week 4 post-ATI. Mean viral load for each group is plotted over time in gray in the background. The average MFI per protein or protein subregion is depicted for complete HIV proteins (A), envelope (Env) gp120 subregions (B), and glycoprotein (gp)41 regions (C). For all figures, average group MFI at week 4 and 20 was compared with BL: *P < .05; **P < .01; ***P < .001.
Figure 4.
Detailed immunoglobulin G (IgG) responses to linear human immunodeficiency virus (HIV)-1 peptides in HIV-infected subjects treated with antiretroviral therapy (ART) at baseline (BL), 4 weeks post-analytic treatment interruption (ATI), and 20 weeks post-ATI. Mean fluorescent intensity (MFI) of IgG binding to glycoprotein (gp)120, gp41, and Gag peptides is plotted by peptide start position (ie, peptide location on HXB2 reference strain). At 4 weeks post-ATI, Group 1 (G1) and Group 2 (G2) responses are compared to each other; *P < .05; **P < .01.
We then examined whether the antibody binding patterns evolved after ATI and whether a change in the pattern prior to detectable viremia might predict viral rebound. We found that 4 weeks after ATI, subjects who still had an undetectable viral load (Group 1) had no increase in any HIV-1 protein-specific binding (Figure 3A) nor any increase in binding to V3, C4, CC loop, or cytoplasmic tail peptides (Figure 3B and 3C). Subjects who had a detectable viral load at 4 weeks after ATI (Group 2) had a significant increase in Env-specific binding (1905 vs 4143 mean fluorescent intensity (MFI), P = .0078 by paired t test) (Figure 3A). As shown in Figure 3B and 3C, this increase was driven by significant increases in binding to V3 (1738 vs 3463 MFI, P = .0251), CC loop (3101 vs 7047 MFI, P = .0182), and cytoplasmic tail peptides (1888 vs 3917 MFI, P = .0057) (all comparisons by paired t tests). In addition, there was a significant difference between Groups 1 and 2 at week 4 post-ATI for Env-specific binding (1574 vs 4143 MFI, P = .0160) (Figure 4), as well as for binding to V3 (1687 vs 3464, P = .0369), CC loop (1944 vs 7047, P = .0323), and cytoplasmic tail peptides (1471 vs 3917 MFI, P = .0094) (all comparisons by unpaired t test). These data show that HIV-1 epitope-specific IgG responses measured by peptide microarray were not a predictor of imminent viral rebound using the assay parameters that were tested. Instead, these titers increased in magnitude only after there was an increase in viral load. The antibody binding patterns also reveal that the earliest response to viral replication was an increase in binding to linear peptides from V3, the CC loop, and the cytoplasmic tail of Env, at least among subjects who initiated ART early in infection.
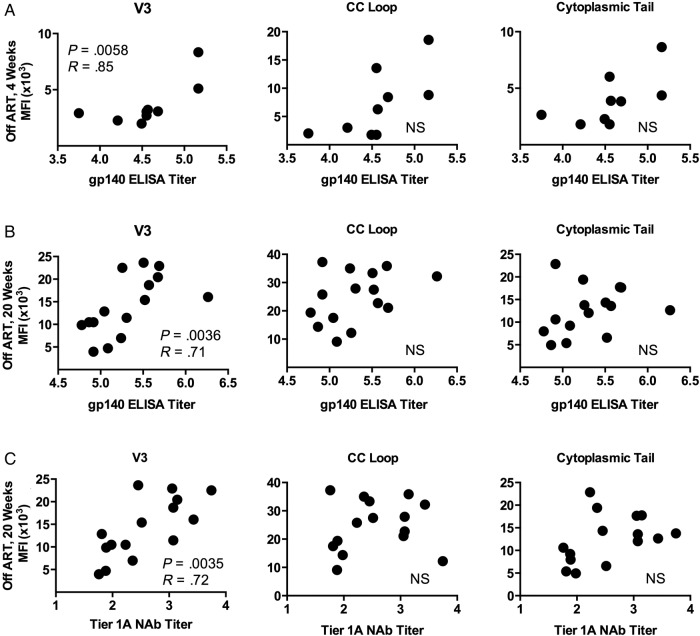
Given that our ELISA data showed a significant increase in mean gp140 titers in Group 2 at 4 weeks post-ATI, we then asked whether 1 of the targeted peptide regions—V3, CC loop, or cytoplasmic tail—might be associated with binding to the intact Env trimer. We found that V3-specific peptide binding correlated strongly with gp140 ELISA titers (P = .0058 and R = 0.85, Spearman rank correlation test) (Figure 5A), whereas binding to CC loop peptides did not (Figure 5A). Not surprisingly, binding to cytoplasmic tail peptides (sequences that are not included in the gp140) also did not correlate with the magnitude of trimer binding.
Figure 5.
The association between immunoglobulin G (IgG) epitope specificity and glycoprotein (gp)140 enzyme-linked immunosorbent assay (ELISA) titer and/or tier 1A neutralizing antibody (NAb) responses in human immunodeficiency virus (HIV)-infected subjects treated with antiretroviral therapy (ART) at 4 weeks post-analytic treatment interruption (ATI) and 20 weeks post-ATI. Average mean fluorescent intensity (MFI) of IgG binding to third hypervariable loop (V3), CC loop, and cytoplasmic tail peptides is plotted against (A) gp140 ELISA titer at 4 weeks post-ATI, (B) gp140 ELISA titer at 20 weeks post-ATI, and (C) tier 1A NAb titer at 20 weeks post-ATI. Abbreviation: NS, not significant.
By 20 weeks after ATI, IgG binding to Env peptides increased in both Groups 1 and 2 compared with baseline (Figure 3A), similar to what was observed for gp140 trimer antibody binding titers. In Group 1, mean Env peptide binding increased from 2286 to 12 313 MFI (P < .0001 by unpaired t tests); in Group 2, Env binding increased from 1905 to 10 052 MFI (P < .0001 by unpaired t tests). Immunoglobulin G binding to Gag, Nef, and Pol peptides also increased significantly in both groups (Figure 3A); Group 2 had significantly increased binding to Rev peptides as well. At this time point, both groups also had an expansion of the antibody binding pattern to Env peptides (Figure 4). In particular, new binding peaks were observed at C5 and within the cytoplasmic tail; smaller peaks were also observed at V1, V2, and V4. Nevertheless, the highest levels of binding were still observed to V3 and CC loop peptides. Of note, there was no significant difference in the levels of binding to V3 or CC loop peptides between subjects who received vaccine versus placebo at 4 weeks or 20 weeks post-ATI (unpaired t test, P = NS; data not shown).
We again asked whether binding to the intact gp140 trimer was associated with binding to any particular Env region, and we found that V3 peptide binding was strongly associated with the all-clade gp140 ELISA antibody titer (P = .0036 and R = 0.71, Spearman rank correlation test) (Figure 5B), whereas binding to CC loop and cytoplasmic tail peptides were not. Because neutralization of tier 1A isolates was significantly higher at week 20 compared with baseline, we also asked whether neutralizing function was associated with binding to any particular Env region. We found that V3 peptide binding was again associated with tier 1A NAb titers (P = .0035 and R = 0.72, Spearman rank correlation test) (Figure 5C), whereas binding to CC loop and cytoplasmic tail peptides were not. These data are consistent with the reported exquisite sensitivity of SF162.LS and MW965.26 to the monoclonal antibody 447-52D (<0.02 ID50 titer [µg/L]) [8], a well described V3-loop antibody [12, 13], as well as the sensitivity of TH023.6 to V3-specific neutralizing antibodies isolated from subjects in the RV144 vaccine trial [14].
DISCUSSION
In this study, we examined antibody responses in a cohort of HIV-1-infected subjects who initiated ART during acute/early HIV-1 infection and who discontinued ART as part of ACTG A5187, a therapeutic vaccine trial of a DNA vaccine that did not impact viral rebound [2]. We found that cross-clade Env-specific IgG binding titers were detectable in these individuals prior to ATI. After ATI, IgG binding titers did not increase until plasma HIV-1 viral loads were greater than 50 copies per mL. Neutralizing antibodies, even against neutralization-sensitive HIV-1 isolates, were largely undetectable up to 4 weeks after ATI, and they slowly developed against some isolates by 20 weeks. After ATI, binding antibodies against linear sequences were predominantly raised against peptides from the V3 loop of gp120 and the CC loop of gp41 and increased in magnitude after plasma viral loads were greater than 50 copies per mL. The V3 peptide binding, but not CC loop peptide binding, correlated with ELISA titers and NAb titers to neutralization-sensitive isolates.
Our data suggest that the initiation of ART early in the course of HIV-1 infection arrests the maturation of HIV-1-specific antibody responses at the pattern typical of acute/early HIV-1 infection, and that viral rebound then stimulates first binding antibodies, primarily against linear V3 and CC epitopes, followed by NAbs against heterologous viruses. One limitation of our analysis is that acute/early HIV-1 infection was defined broadly in A5187, and thus subjects were heterogeneous in the exact timing of ART initiation. In addition, the sample size of our cohort is small. Nevertheless, our data are consistent with prior studies that showed that in 21 subjects with acute/early HIV-1 infection, IgG responses were focused on the HR1/CC loop of gp41 and the V3 loop of gp120, and they had no neutralizing activity until at least 8 weeks after infection, when only V3-mediated neutralization of tier 1A viruses was first observed [11]. Our data demonstrate a similar pattern of linear binding antibody responses among subjects treated with ART during acute/early HIV-1 infection after ATI and viral rebound.
Our data are also consistent with previous clinical studies that demonstrated that ART initiated during acute/early HIV-1 infection might blunt HIV-1-specific antibody responses and in rare cases lead to seroreversion [15–17]. For example, it has been demonstrated that early initiation of ART decreases the pool of B cells that secrete HIV-1-specific antibodies [15], and that early initiation of ART leads to profound reductions in the concentration of all anti-HIV IgG subclasses, without changing the ratio between these different subclasses or affecting anti-HIV-1 IgG avidity [16]. It has also been reported that affinity maturation of HIV-1-specific antibodies was blocked in 8 subjects who started ART during early HIV-1 infection [17]. Our data extend these prior studies by showing that early initiation of ART also blunts antibody binding diversity and functional neutralizing capacity. In addition, our data suggest that the development of antibody responses are not permanently blunted by early initiation of ART and can resume once the antigen stimulus returns.
A primary focus of the HIV-1 eradication field is the search for biomarkers that may predict viral rebound. In our study, neither ELISA antibody titers, NAb titers, nor epitope-specific linear binding antibodies before viral rebound could predict subsequent viral rebound. Instead, the expansion of first binding antibodies and then NAbs appeared to follow viremia. Nevertheless, it remains possible that more frequent sampling or different assay conditions may be able to pick up small changes in antibody responses at earlier time points.
CONCLUSIONS
In summary, antibody responses in HIV-1-infected individuals in the setting of ART treatment discontinuation can provide insight into the evolution of antibody responses during viral rebound. In this study, we analyzed cross-clade Env-specific IgG binding antibodies, epitope-specific linear peptide binding antibodies, and neutralizing antibodies in a cohort of 20 HIV-1-infected subjects who were treated with ART during acute/early HIV-1 infection and who underwent ATI and demonstrated viral rebound. Our data suggest that the initiation of ART early in the course of HIV-1 infection arrests the development of HIV-1-specific antibody responses with a pattern characteristic of acute/early infection, and that viral rebound triggers the further development of these antibody responses. We also found that antibody responses as measured by these assays did not predict imminent viral rebound. These data have important implications for the HIV-1 vaccine and eradication fields.
Acknowledgments
Financial support. This research was funded by the National Institutes of Health (AI060354 [to K. E. S.]; AI078526, AI084794, AI095985, and AI096040 [to D. H. B.]) and the Ragon Institute of MGH, MIT, and Harvard (to K. E. S. and D. H. B.). Plasma samples from human subjects were obtained from the AIDS Clinical Trials Group.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science 2014; 345:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg ES, Graham BS, Chan ES et al. Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/early HIV-1 infection. PLoS One 2010; 5:e10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch DH, O'Brien KL, Simmons NL et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med 2010; 16:319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nkolola JP, Peng H, Settembre EC et al. Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs. J Virol 2010; 84:3270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephenson KE, Neubauer GH, Reimer U et al. Quantification of the epitope diversity of HIV-1-specific binding antibodies by peptide microarrays for global HIV-1 vaccine development. J Immunol Methods 2015; 416:105–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahtman T, Jernberg A, Mahdavifar S et al. Validation of peptide epitope microarray experiments and extraction of quality data. J Immunol Methods 2007; 328:1–13. [DOI] [PubMed] [Google Scholar]
- 7.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol 2005; Chapter 12:Unit 12, 11. [DOI] [PubMed] [Google Scholar]
- 8.Seaman MS, Janes H, Hawkins N et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 2010; 84:1439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarzotti-Kelsoe M, Bailer RT, Turk E et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods 2014; 409:131–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomaras GD, Haynes BF. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr Opin HIV AIDS 2009; 4:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomaras GD, Yates NL, Liu P et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 2008; 82:12449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conley AJ, Gorny MK, Kessler JA et al. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J Virol 1994; 68:6994–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta M, Liu J, Roux KH, Taylor KA. Visualization of retroviral envelope spikes in complex with the V3 loop antibody 447-52D on intact viruses by cryo-electron tomography. J Virol 2014; 88:12265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montefiori DC, Karnasuta C, Huang Y et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 2012; 206:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris L, Binley JM, Clas BA et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med 1998; 188:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adalid-Peralta L, Grangeot-Keros L, Rudent A et al. Impact of highly active antiretroviral therapy on the maturation of anti-HIV-1 antibodies during primary HIV-1 infection. HIV Med 2006; 7:514–9. [DOI] [PubMed] [Google Scholar]
- 17.Selleri M, Orchi N, Zaniratti MS et al. Effective highly active antiretroviral therapy in patients with primary HIV-1 infection prevents the evolution of the avidity of HIV-1-specific antibodies. J Acquir Immune Defic Syndr 2007; 46:145–50. [DOI] [PubMed] [Google Scholar]