We used an improved ADCC assay to investigate HA-ADCC antibody responses in human sera following either seasonal or avian influenza vaccination. Our results suggest that detection of both neutralizing and non-neutralizing antibodies may better reflect protective capacity of HA-specific antibodies induced by avian influenza vaccines.
Keywords: antibody, antibody-dependent cell-mediated cytotoxicity, hemagglutinin, influenza A virus, vaccines
Abstract
Background. Detection of neutralizing antibodies (nAbs) to influenza A virus hemagglutinin (HA) antigens by conventional serological assays is currently the main immune correlate of protection for influenza vaccines However, current prepandemic avian influenza vaccines are poorly immunogenic in inducing nAbs despite considerable protection conferred. Recent studies show that Ab-dependent cell-mediated cytotoxicity (ADCC) to HA antigens are readily detectable in the sera of healthy individuals and patients with influenza infection.
Methods. Virus neutralization and ADCC activities of serum samples from individuals who received either seasonal or a stock-piled H5N1 avian influenza vaccine were evaluated by hemagglutination inhibition assay, microneutralization assay, and an improved ADCC natural killer (NK) cell activation assay.
Results. Immunization with inactivated seasonal influenza vaccine led to strong expansion of both nAbs and ADCC-mediating antibodies (adccAbs) to H3 antigen of the vaccine virus in 24 postvaccination human sera. In sharp contrast, 18 individuals vaccinated with the adjuvanted H5N1 avian influenza vaccine mounted H5-specific antibodies with strong ADCC activities despite moderate virus neutralization capacity. Strength of HA-specific ADCC activities is largely associated with the titers of HA-binding antibodies and not with the fine antigenic specificity of anti-HA nAbs.
Conclusions. Detection of both nAbs and adccAbs may better reflect protective capacity of HA-specific antibodies induced by avian influenza vaccines.
Hemagglutinin (HA) is the major glycoprotein expressed on the surface of influenza A viruses. A total of 18 antigenically different HA subtypes have been identified so far, with each sharing 40%–60% amino acid sequence identity [1]. Seasonal H1 and H3 subtypes of influenza A viruses have been circulating among human populations for decades and cause annual influenza epidemics. Recently, emerging avian influenza A viruses, including H5, H7, and H9 subtypes, have caused serious infections in humans and pose a new threat for public health.
Vaccination is an efficient approach to prevent human influenza illness. The current seasonal trivalent inactivated influenza vaccines (TIVs) are highly immunogenic in inducing neutralizing antibodies (nAbs) to the HA antigens [2]. Two types of HA-specific nAbs have been identified so far: conventional nAbs (cnAbs) and broadly nAbs (bnAbs). Conventional nAbs primarily recognize antigenic sites located within the HA globular head [3]. Whereas the globular head undergoes constant antigenic drift, cnAbs are often strain-specific [4]. Nevertheless, detection of cnAbs by conventional serological assays such as hemagglutination inhibition (HI) assay and/or microneutralization (MN) assay is currently the main way of assessing protection after influenza virus infection or vaccination. In addition to strain-specific cnAbs, bnAbs to HA antigens have been identified [5]. These bnAbs primarily target amino acid sequences within the membrane proximal HA stem region that are conserved among diverse HA subtypes [5]. Levels of bnAbs are extremely low or undetectable in human sera after seasonal influenza vaccination [6].
In contrast to seasonal TIV, prepandemic avian influenza vaccines containing H5, H7, or H9 subtypes are poorly immunogenic in inducing nAbs [7, 8]. Multiple dosing or coadministration with an adjuvant is generally required to elicit detectable levels of nAbs after immunization [9]. However, it has been shown in animal models that avian influenza vaccines can provide considerable protection despite no or low levels of nAbs induced [10]. This raises an important question of whether detection of cnAbs alone can fully reflect the protective capacity of antiavian HA Abs after vaccination with current avian influenza vaccines.
In a recent study, Jegaskanda et al [11] reported that pre-existing HA-specific Abs in normal human sera (NHS) possessed cross-reactive Ab-dependent cell-mediated cytotoxicity (ADCC) against a wide range of HA subtypes, including both seasonal and avian HA subtypes. Notably, they found that ADCC activities toward H1 antigen of the 2009 pandemic H1N1 virus was inversely correlated with the age of the patients [12], a finding that is in line with the epidemiological data [13]. Further study in a rhesus macaque model suggested that influenza virus-specific ADCC may be associated with control of pandemic H1N1 virus infection [14]. Taken together, current evidence clearly suggests potential involvement of HA-specific ADCC in protection against influenza illness. Despite recent progress, several important questions remain unanswered, partially due to the lack of a robust ADCC assay. First, what is the relation between HA-associated virus neutralization (VN) and ADCC activities after seasonal versus avian influenza vaccination? Second, which factors primarily determine the strength of HA-specific ADCC activities in human sera? Third, what potential values could in vitro measurement of HA-specific ADCC add to immune correlates of protection after influenza vaccination?
In the present study, we demonstrate that a combination of both nAbs and ADCC-mediating Abs (adccAbs) may provide a better correlate of protection than nAbs alone in assessment of protective efficacy of avian influenza vaccines.
MATERIALS AND METHODS
Human Serum Samples
One panel of single NHS, sampled from 72 healthy adults (median age, 40 years; range, 20–65) between 1999 and 2006, and 2 panels of paired human sera were tested in the present study. The first panel of paired sera consists of 24 paired sera sampled from 24 healthy adults (median age, 32.5 years; range, 21–48) pre- (day 0) and postvaccination (day 20–21) with 1 dose of 2011–2012 seasonal TIV. The sera were acquired through a contract and received as anonymous samples. Thus, a review by the Centers for Disease Control and Prevention (CDC) institutional review board was exempted. The second panel of paired sera were collected from 18 healthy adult volunteers (median age, 41.3 years; range, 30–62) who participated in a clinical trial of an avian H5N1 vaccine under informed consent. The paired sera were sampled pre- (day 0) and postvaccination (day 21–60) after 2 doses of 3.75 µg per dose of AS03-adjuvanted inactivated avian H5N1 vaccine, derived from A/Indonesia/05/2005 virus. Use of the sera in the present study was approved by the CDC National Center for Immunization and Respiratory Diseases human subjects review.
Chimeric Monoclonal Antibodies
Details of 6 HA-specific chimeric mAbs were described previously [15].
Hemagglutination Inhibition Assay
The HI assay was performed according to the standard procedure using 0.5% turkey red blood cells as described previously [16].
Microneutralization Assay
Virus neutralization titers of human sera were determined by a standard MN assay as described previously [16].
Antibody-Dependent Cell-Mediated Cytotoxicity Natural Killer Cell Activation Assay
Antibody-dependent cell-mediated cytotoxicity natural killer (NK) cell activation assay was improved from a flow cytometry-based ADCC method described previously [17]. Ninty-six-well nickel-coated plates (Thermo Scientific) were coated with 200 ng/well of full-length, trimeric, recombinant HA antigens with Histidine Tag (Influenza Reagent Resource) at 4°C overnight. The plates were then washed 5 times with 200 µL/well of sterile 10 mM phosphate-buffered saline (PBS) (pH 7.2). Human serum samples were serially diluted with PBS and added into each well at 100 µL/well. The start dilution was 1:40. The plates were incubated for 1 hour at 37°C and then washed 5 times. Human NK cell lines expressing either high-affinity (158 V/V) or low-affinity (158 F/F) FcγRIIIa receptor and the parental NK-92 control cells were used as effector cells as described previously [18]. Natural killer cells were mixed with appropriately diluted (usually 1:25) phycoerythrin-conjugated mouse antihuman CD107a (BD Pharmingen) in the presence of 1:1500 diluted protein transport inhibitor containing monensin (BD Bioscience). Natural killer cells (5 × 105) in 100 µL of the above mixture were then added into each 96 well of the plates and incubated for 4 hours at 37°C. The cells were washed twice and fixed with 250 µL/well 4% paraformaldehyde (Sigma-Aldrich). Data acquisition was performed on an LSR II flow cytometer (Becton Dickenson). The results were expressed as end-point titers, eg, the highest serum dilution that achieved the 3% of the arbitrary threshold. Each serum sample was tested in duplicate. The final titer was the geometric mean titer (GMT) of the duplicate titers. Evaluation of human NK cell lines as effector cells and the arbitrary threshold of the assay are described in detail in the Supplementary Material.
Enzyme-Linked Immunosorbent Assay
Total influenza HA-specific immunoglobulin (Ig)G Abs in human sera were determined by an enzyme-linked immunosorbent assay (ELISA) method described previously using the same recombinant HA antigens as described above as coating antigens [19].
RESULTS
Assessment of Both Neutralizing Antibodies (Abs) and Ab-Dependent Cell-Mediated Cytotoxicity Abs to Hemagglutinin Antigens of Influenza A Viruses in Human Sera
We developed an improved ADCC NK cell activation assay utilizing human NK cell lines as effector cells (Supplementary Figures 1 and 2). To examine the relation between HA-specific nAbs and adccAbs in human sera, we first measured VN and ADCC activities to HA antigen of a then representative seasonal A/New Caledonia/20/1999 H1N1 virus at the time frame when a panel of 72 NHS were collected between 1999 and 2006. As expected, sera with a “protective” level of pre-existing nAbs to the seasonal H1N1 virus were common (33.33%) among the 72 sera tested (Table 1). Unexpectedly, a substantially higher proportion of the NHS panel (69.44%) had the H1-specific ADCC titers above the arbitrary 1:160 positive threshold of HA-specific ADCC. Note that the ADCC titers of the 24 sera with pre-existing HI Abs to the H1N1 virus were 4.2-fold higher than the rest of the 48 sera without detectable levels of HI Abs (GMT: 1031 vs 245).
Table 1.
Neutralizing and ADCC-Mediating Antibodies to HA Antigen of a Common Seasonal H1N1 Influenza A Viruses in Normal Human Seraa
| Serum Group | HI |
ADCC |
||
|---|---|---|---|---|
| GMT (95% CI) | No. Titers ≥40 (%) | GMT (95% CI) | No. Titers ≥160 (%) | |
| Total | 15 (11–21) | 24/72 (33.33) | 427 (311–586) | 50/72 (69.44) |
| HI ≥ 40 | 82 (56–121) | 24/24 (100) | 1031 (688–1545) | 24/24 (100) |
| HI < 40 | 7 (7–8) | 0/48 (0.00) | 245 (155–387) | 26/48 (54.17) |
Abbreviations: ADCC, antibody-dependent cell-mediated cytotoxicity; CI, confidence interval; GMT, geometric mean titer; HA, hemagglutinin; HI, hemagglutination inhibition; NK, natural killer.
a Normal human sera were collected from a total of 72 healthy individuals and tested with HI and ADCC NK cell activation assay against seasonal A/New Caledonia/20/1999 (H1N1) virus and recombinant HA antigen derived from the H1N1 virus, respectively.
We then measured VN and ADCC activities to the H3 antigen of 2011–2012 seasonal TIV H3N2 vaccine virus in 24 paired sera collected from a cohort of the TIV-immunized healthy adults. As expected from the prescreening of this serum panel, relatively low levels of both nAbs and adccAbs (GMT: 36 and 94, respectively) were detected, with approximately 45% of the 24 prevaccination sera having titers greater than 40 by MN (Table 2). Vaccination with the seasonal TIV led to considerable expansion of both nAbs and adccAbs to the H3 antigen (GMT: 370 and 446, respectively) in the 24 postvaccination sera, and a high percentage of the 24 postvaccination sera had levels of H3-specific VN and ADCC activities above their respective thresholds of 40 and 160 (100% and 83.83%, respectively).
Table 2.
Neutralizing and ADCC-Mediating Antibodies Induced After Seasonal vs Avian Influenza Vaccination
| HA Specificity | Serum | VN |
ADCC |
||
|---|---|---|---|---|---|
| GMT (95% CI) | No. Titers ≥40 (%) | GMT (95% CI) | No. Titers ≥160 (%) | ||
| A/Perth/16/2009a | Pre- | 36 (21–62) | 11/24 (45.83) | 94 (66–133) | 10/24 (41.8) |
| (H3N2) | Post- | 370 (249–549) | 24/24 (100) | 446 (248–803) | 20/24 (83.83) |
| A/Indonesia/05/2005b | Pre- | 5 (5–5) | 0 (0.00) | 436 (260–729) | 17 (94.44) |
| (H5N1) | Post- | 62 (32–119) | 13 (72.22) | 2765 (1953–3914) | 18 (100) |
Abbreviations: ADCC, antibody-dependent cell-mediated cytotoxicity; CI, confidence interval; GMT, geometric mean titer; HA, hemagglutinin; MN, microneutralization; NK, natural killer; VN, virus neutralization.
a Paired serum samples were collected from 24 adult volunteers on pre- (day 0) and post-vaccination (day 20–21) with 1 dose of 2011–2012 inactivated trivalent influenza vaccine containing A/Perth/16/2009 (H3N2) vaccine component, respectively. The sera were tested with MN assay to determine titers of VN antibodies against A/Perth/16/2009 (H3N2) virus and with ADCC NK cell activation assay to determine the H3-specific ADCC activity, respectively.
b Paired serum samples were collected from 18 healthy adult volunteers on pre- (day 0) and post-vaccination (day 21–60) with 2 doses of AS03-adjuvanted H5N1 vaccine (A/Indonesia/05/2005), respectively. The sera were tested with MN assay for VN antibodies against A/Indonesia/05/2005 (H5N1) virus and with ADCC NK cell activation assay for the H5 HA-specific ADCC activity, respectively.
Finally, we assessed nAbs and adccAbs in 18 paired sera collected from healthy adults who volunteered to receive an AS03-adjuvanted prepandemic H5N1 avian influenza vaccine containing A/Indonesia/05/2005 H5N1 virus antigens. As shown in Table 2, none of the 18 prevaccination sera contained detectable levels of nAbs to the H5N1 vaccine virus. Unexpectedly, almost all of the 18 prevaccination sera (94.44%) had detectable levels of adccAbs to the H5 antigen (GMT: 436). At present, the reasons for a high baseline ADCC titers to the H5 antigen in this serum panel are not known. High ADCC Abs titers to H5N1 and H7N9 avian influenza A viruses were also observed in healthy adults and children in a recent independent study [20].
After vaccination with 2 low-dose AS03-adjuvanted H5N1 vaccine, only relatively moderate increases of H5-specific nAbs were detected (GMT: 62). Of the 18 sera, 72.22% had a protective level of MN titer to the H5N1 virus. In sharp contrast, the GMT of H5-specific adccAbs reached 1:2765 in the 18 postvaccination sera, a 6.3-fold increase in titers relative to the baseline. All of the 18 sera had a ≥160 ADCC titer to the H5 antigen.
Antibody (Ab)-Dependent Cell-Mediated Cytotoxicity Activities of Neutralizing Abs Versus Nonneutralizing Abs to Hemagglutinin Antigens
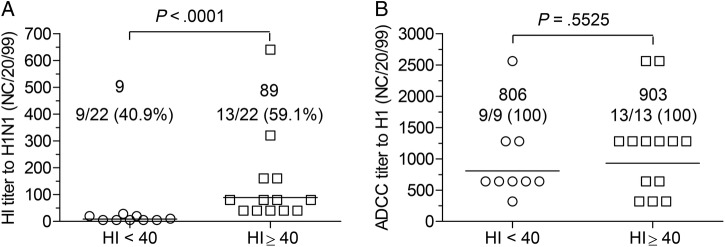
The finding that NHS with HI Abs to the seasonal H1N1 virus manifested considerably higher ADCC activities than those without HI Abs (Table 1) suggests that nAbs and non-neutralizing antibodies might be different quantitatively and/or qualitatively in their ability to trigger ADCC activities in vitro. To test this possibility, a group of 22 sera with equivalent amounts of HA-binding Abs to the seasonal H1 antigen (ELISA titer: 102 400) were selected from the 72 NHS tested and divided into 2 subgroups according to the titers of HI Abs for further analysis.
As shown in Figure 1A, 59.1% of the selected 22 sera had a HI titer ≥40 to the seasonal H1N1 virus. Irrespective of the HI titers, the 13 sera with HI Abs and the 9 sera without HI Abs showed a similar level of ADCC activity to the H1 antigen (903 vs 806) (Figure 1B). The difference was statistically not significant (P = .5525).
Figure 1.
Antibody-dependent cell-mediated cytotoxicity (ADCC) activities of neutralizing vs nonneutralizing anti-hemagglutinin (HA) antibodies in human sera with equivalent titers of HA-binding antibodies. Twenty-two normal human sera with an enzyme-linked immunosorbent assay binding titer of 1:102 400 to recombinant H1 antigen derived from A/New Caledonia/20/1999 H1N1 virus were selected from the panel of 72 normal human sera (described under Materials and Methods) for further analysis. Statistical differences between the groups were analyzed by Mann-Whitney U test using commercial Prism 5 software. (A) Geometric mean titer and frequency of H1-specific hemagglutination inhibition (HI) titers of the 22 sera with or without detectable HI titers to the H1N1 virus. (B) Geometric mean titer and frequency of H1-specific ADCC activities of the 22 sera with or without detectable HI antibodies to the H1N1 virus.
Antibody (Ab)-Dependent Cell-Mediated Cytotoxicity-Mediating Capability of Chimeric Monoclonal Abs With a Wide Range of Virus Neutralization Capability
The data thus far reveal that, at the polyclonal level, HI Abs and HA-specific nnAbs were indistinguishable in their capability to induce ADCC in vitro, provided that equivalent amounts of HA-binding Abs were present in the sera. However, this does not rule out the possibility that VN and ADCC are mediated by 2 separate subsets of HA-specific Abs in the polyclonal human sera. Therefore, we used a panel of 6 HA-specific chimeric mAbs to dissect this possibility. All of the 6 mAbs have identical Fc fragments derived from human IgG1 and differ in the Fab portions that recognizes diverse antigenic sites on the globular head of the H1 antigen [15]. As shown in Table 3, 3 of the 6 mAbs (069-A09, 145-D11, and 146-C07) possessed high VN capability (MN titers: 12–24 ng/mL). Two of the mAbs (065-D01 and 065-C05) showed intermediate (16–32-fold lower) VN capability (MN titers: 195–391 ng/mL). Monoclonal Ab 145-C09 had the lowest VN capability among the 6 mAbs tested (MN titer: 3125, 130- to 260-fold lower). However, independent of the differences in the fine antigenic specificity and VN capability, all of the 6 mAbs showed similar strength of ADCC activity (ADCC titers: 12–50 ng/mL).
Table 3.
VN and ADCC Capability of Chimeric Monoclonal Antibodies Specific for Diverse Antigenic Sites on the HA Head of A/California/07/2009 (H1N1) Virus
| cmAb | Antigenic Sites Recognizeda | MNb (ng/mL) | ADCCb (ng/mL) |
|---|---|---|---|
| 069A09 | Sa | 12.21 | 36.63 |
| 145D11 | Ca2 | 24.41 | 48.82 |
| 146C07 | Ca2 | 24.41 | 24.41 |
| 065D01 | Sa | 195.31 | 36.63 |
| 065C05 | Cb | 390.63 | 48.82 |
| 145C09 | Sb/Ca2 | 3125.00 | 12.21 |
Abbreviations: ADCC, antibody-dependent cell-mediated cytotoxicity; cmAb, chimeric monoclonal antibodies; MN, microneutralization; NK, natural killer.
a Antigenic sites recognized by the chimeric monoclonal antibodies were mapped previously (Wilson et al [15]).
b Minimal concentrations of the chimeric monoclonal antibodies required to neutralize 100 TCID50 (50% tissue culture infective dose) of A/California/07/2009 (H1N1) virus (MN) or produce ≥3% of CD107a-positive NK cells (ADCC), respectively.
Correlation Between Antibody (Ab)-Dependent Cell-Mediated Cytotoxicity-Mediating, Virus Neutralization and Hemagglutinin Antigens-Binding Abs
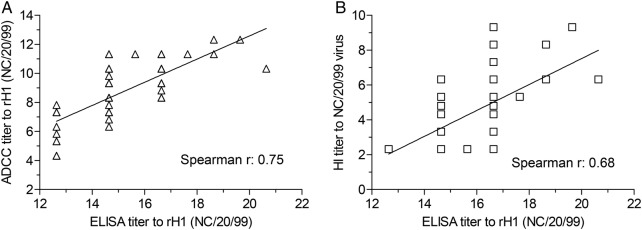
Finally, we analyzed the potential correlation between adccAb, nAb and HA-binding Abs to the seasonal H1N1 virus from the 72 NHS tested above. As shown in Figure 2A, overall, H1-specific ADCC titers are positively correlated with the amount of HA-binding Abs (Spearman coefficient: 0.75). Similar levels of correlation were observed when HA ELISA titers and HI titers from the same serum panel were analyzed (Spearman coefficient: 0.68) (Figure 2B). Note that the titers of HA-specific adccAbs were unable to reach high enough levels among the total HA-binding Abs to achieve a stronger correlation in the serum groups analyzed (Supplementary Table 1).
Figure 2.
Correlation between neutralizing, antibody-dependent cell-mediated cytotoxicity (ADCC)-mediating and anti-hemagglutinin (HA)-binding antibodies. A total of 72 normal human sera were tested for titers of neutralizing, ADCC mediating, and HA binding to the H1 specificity of A/New Caledonia/20/1999 H1N1 virus. Correlation between neutralizing, ADCC-mediating, and HA-binding antibodies was analyzed by Spearman correlation analysis using commercial Prism 5 software. All of the titers were expressed in log2 scale.
DISCUSSION
In the present study, we improved the robustness of the influenza-associated ADCC assays by incorporating human NK cell lines as effector cells (Supplementary Figure 1). This circumvents a major limitation associated with the usage of fresh human peripheral blood mononuclear cells effectors in this type of analysis [17, 20]: eg, (1) limited sources of fresh human blood donors for routine analysis; and (2) potential interassay variations associated with different blood donor sources with heterogeneous NK cell activation status and uncharacterized phenotypes of FcγRIIIa receptors. In addition, a target-free surrogate assay for the conventional ADCC assays using influenza virus-infected targets, as proposed originally by Jegaskanda et al [17], eliminates the potentially inevitable batch-to-batch variations of virus infectivity to target cells.
We then used the improved ADCC NK cell activation assay to evaluate the potential of measuring HA-specific ADCC activities as a possible new addition to the current immune correlates of protection after influenza vaccination. Our results suggest that in vitro measurement of HA-specific ADCC activities may complement conventional serological methods in assessment of the full spectrum of in vitro functionality of anti-HA Abs induced by avian influenza vaccines.
Thus far, the data from us and others have provided sufficient evidence that nnAbs to influenza HA antigens possess the capability to induce ADCC in vitro [12, 21] and in the present work. The question remains whether this subset of anti-HA Abs is biologically relevant in protection against human influenza illness. Several lines of evidence obtained in animal models support this possibility. First, vaccination with either seasonal or avian influenza vaccines often conferred a certain degree of cross-protection against lethal challenges with heterologous viruses in the absence of nAbs [8, 22, 23]. Second, titers of HA-specific nnAbs were correlated with protection observed under such circumstances [24, 25]. Third, passive transfer of HA-specific mAbs or polyclonal, HA-monospecific immune sera without detectable levels of HA-related VN capability led to complete resolution of influenza infection and/or improved viral clearance in the lung of the recipient mice [26–28]. It has become clear that although nnAbs cannot prevent influenza infection, they may reduce severity of clinical influenza illness considerably [22, 29]. Mitigation of laboratory-confirmed human influenza by current seasonal influenza vaccines, including aversion of influenza-associated hospitalization or death, has been well documented in the literature [30, 31]. The exact immune mechanism(s) that correlate with this protective effect are not fully understood at present. It is conceivable that HA-specific nnAbs, alone or together with other humoral immune components such as neuraminidase-inhibition Abs and anti-M2 Abs, may contribute to the observed protection via FcR-dependent mechanisms such as ADCC. Prospective clinical trials may help establish the biological relevance of HA-specific ADCC activities in protection against human influenza illness.
It has been long recognized that HA-specific nAbs, classically measured by HI assay, were positively correlated with the probability of protection among naturally infected or vaccinated individuals [32]. An HI titer of 1:40 is generally considered as an immune correlate corresponding to a 50% reduction in the risk of contracting seasonal influenza in adults. According to this threshold, approximately one third of the 72 NHS tested had pre-existing protective levels of nAbs to the then circulating seasonal H1N1 virus (Table 1). Vaccination with a seasonal TIV boosted nAbs to the H3N2 vaccine component considerably in the 24 healthy adults. Protective levels of H3-specific nAbs were detected in all of the 24 postvaccination sera (Table 2). At present, it is not clear whether both VN and ADCC are required in order for HA-specific nAbs to clear influenza A viruses efficiently in vivo. Earlier studies have shown that immune protection mediated by HA-specific nAbs was independent of Fc fragment-associated functional activities [33, 34].
We noted that 24 of the 72 NHS showed strong activities in both VN and ADCC to the seasonal H1 antigen examined (Table 1). Moreover, the titers of adccAbs in the sera were 4.2-fold higher than those 48 sera without nAbs. At first glance, this seemed to indicate that the subset of nAbs might possess stronger capability to trigger ADCC activities than the nnAb subset. Detailed analyses revealed no evidence to support this assumption. First, 2 subgroups of sera with equivalent titers of H1-binding Abs showed similar strength of ADCC activities to the H1 antigen, independent of nAbs (Figure 1). Second, a panel of mAbs with substantially different VN capability, yet identical affinity to FCγRIIIa receptor on NK cells, displayed similar strength of ADCC activities (Table 3). These observations demonstrate that both nAbs and nnAbs can trigger equally strong ADCC activities in vitro. In contexts where the main Abs induced are neutralizing, as with the anti-H3 response after seasonal TIV vaccination (Table 2), the titers of adccAbs will mirror the MN titers.
We observed that relatively low levels of nAbs to the H5N1 vaccine virus were detected in the 18 postvaccination sera after vaccination with 2 low doses of H5N1 avian vaccine (Table 2). This result is not surprising, in light of well documented observations in the literature that inactivated avian H5N1 vaccines were generally poorly immunogenic in inducing nAbs [7, 35]. However, we noted that the titers of anti-H5 adccAbs expanded substantially despite weak induction of nAbs after the H5N1 vaccination. This implies that a large proportion of H5-specific Abs induced were nonneutralizing yet capable of triggering ADCC activities in vitro. Although not tested in this study, this may hold true for other HA subtypes of avian influenza vaccines as well. In fact, it was observed recently in the mouse model that after vaccination with either inactivated or recombinant H7 vaccines, the titers of H7-binding Abs were approximately equivalent to those specific for seasonal H1 or H3 antigen tested in parallel, although the levels of the H7-specific nAbs in the sera were substantially lower [36]. Similar results were also obtained in humans who were vaccinated with H7 influenza vaccines [37]. In addition to HA, other influenza antigens, such as NA and M2, are also capable of triggering ADCC activity that may contribute to cross-protection against influenza. In a recent study, Terajima et al [20] reported a high level of ADCC titers to avian viruses in healthy adults and older children. In this study, whole influenza virus-infected cells were used as targets of which ADCC activity to all surface antigens, including HA, NA, and M2, were detected. Nonetheless, it is worth noting that high ADCC Ab titers to avian influenza A viruses were detected in human sera by both ADCC assay formats ([20] and Table 2).
We found that multiple factors may affect strength of in vitro ADCC activities. The strength of HA-specific ADCC activity is largely associated with the titers of HA-binding Abs in the human sera tested (Figure 2 and Supplementary Table 1) and, to lesser extent, the affinity of FcγRIIIa receptor on NK cells (Supplementary Figure 1B) and IgG subclasses of the HA-specific Abs (Supplementary Table 2). Diverse antigenic sites on the HA globular head did not appear to have a major impact on the strength of HA-associated ADCC activities, because a panel of 6 mAbs with identical Fc fragments derived from a human IgG1 showed similar strengths of ADCC activities, independent of the obvious differences in their VN capability (Table 3). This observation is different from the result of a recent study showing that whereas 5 anti-HA stalk human mAbs induced superior ADCC activities, 3 anti-HA head mAbs tested in parallel failed to do so [38]. At present, we do not know the reasons for this discrepancy, because similar ADCC NK cell activation protocols were used by both research groups. One remote possibility is that the anti-HA head mAbs tested by both groups may happen to belong to 2 different classes of anti-HA nAbs [38]. Whereas our anti-HA head chimeric mAbs might be selected from those that require FcγR effector mechanism to induce ADCC, the 3 anti-HA head mAbs tested by Dillilo et al did not [38]. Nevertheless, it appears that both anti-HA head and anti-HA stalk Abs are able to induce ADCC activities in vitro.
CONCLUSIONS
We wish to point out that HA-specific ADCC activities measured in vitro may not reflect the ADCC activities in vivo after natural influenza infection, which is most likely a well balanced process modulated by multiple factors under normal circumstances. However, immunopathology associated with high titers of influenza antigen-specific, low-avidity, nnAbs may occur under certain circumstances in nature [39, 40]. It is conceivable that FcR-dependent mechanisms such as ADCC may be involved in the detrimental process. In this regard, monitoring ADCC activities associated with nonneutralizing anti-HA Abs may provide new insights into the immune mechanisms of Ab-enhanced influenza virus respiratory diseases.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank the Centers for Disease Control and Prevention (CDC) Influenza Serology Team for assistance with determination of virus neutralization titers of human sera by microneutralization assay. We also thank the following team members who participated in the serum testing: Eric Gillis, F. Liaini Gross, Stacie N. Jefferson, Bonnie Dighero-Kemp, Heather R. Tatum, Leilani Thomas, and David Wang; Jin Kim for flow cytometer-based sorting of H7-293FT transfectants; and NantKwest Inc. and Dr. Kerry Campbell (Fox Chase Cancer Center) for kindly providing human NK cell lines for the ADCC NK cell activation assay.
Disclaimer. The views expressed in this study solely represent those of the authors and do not reflect the official policy of the CDC.
Financial support. This work was funded by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Yoon SW, Webby RJ, Webster RG. Evolution and ecology of influenza A viruses. Curr Top Microbiol Immunol 2014; 385:359–75. [DOI] [PubMed] [Google Scholar]
- 2.Zakay-Rones Z. Human influenza vaccines and assessment of immunogenicity. Expert Rev Vaccines 2010; 9:1423–39. [DOI] [PubMed] [Google Scholar]
- 3.Gerhard W, Yewdell J, Frankel ME et al. . Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature 1981; 290:713–7. [DOI] [PubMed] [Google Scholar]
- 4.Wilson IA, Cox NJ. Structural basis of immune recognition of influenza virus hemagglutinin. Annu Rev Immunol 1990; 8:737–71. [DOI] [PubMed] [Google Scholar]
- 5.Sui J, Hwang WC, Perez S et al. . Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 2009; 16:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sui J, Sheehan J, Hwang WC et al. . Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies. Clin Infect Dis 2011; 52:1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treanor JJ, Campbell JD, Zangwill KM et al. . Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354:1343–51. [DOI] [PubMed] [Google Scholar]
- 8.Cox RJ, Madhun AS, Hauge S et al. . A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine 2009; 27:1889–97. [DOI] [PubMed] [Google Scholar]
- 9.Mulligan MJ, Bernstein DI, Winokur P et al. . Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA 2014; 312:1409–19. [DOI] [PubMed] [Google Scholar]
- 10.Hessel A, Schwendinger M, Holzer GW et al. . Vectors based on modified vaccinia Ankara expressing influenza H5N1 hemagglutinin induce substantial cross-clade protective immunity. PLoS One 2011; 6:e16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jegaskanda S, Vandenberg K, Laurie KL et al. . Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity in intravenous immunoglobulin as a potential therapeutic against emerging influenza viruses. J Infect Dis 2014; 210:1811–22. [DOI] [PubMed] [Google Scholar]
- 12.Jegaskanda S, Laurie KL, Amarasena TH et al. . Age-associated cross-reactive antibody-dependent cellular cytotoxicity toward 2009 pandemic influenza A virus subtype H1N1. J Infect Dis 2013; 208:1051–61. [DOI] [PubMed] [Google Scholar]
- 13.Jhung MA, Swerdlow D, Olsen SJ et al. . Epidemiology of 2009 pandemic influenza A (H1N1) in the United States. Clin Infect Dis 2011; 52(Suppl 1):S13–26. [DOI] [PubMed] [Google Scholar]
- 14.Jegaskanda S, Weinfurter JT, Friedrich TC et al. . Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol 2013; 87:5512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson JR, Guo Z, Tzeng WP et al. . Diverse antigenic site targeting of influenza hemagglutinin in the murine antibody recall response to A(H1N1)pdm09 virus. Virology 2015; 485:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Global Influenza Surveillance Network. Serological diagnosis of influenza by microneutralization assay. In: Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza . Geneva, Switzerland, World Health Organization; 2011: pp 59–77. [Google Scholar]
- 17.Jegaskanda S, Job ER, Kramski M et al. . Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 2013; 190:1837–48. [DOI] [PubMed] [Google Scholar]
- 18.Binyamin L, Alpaugh RK, Hughes TL et al. . Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol 2008; 180:6392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li ZN, Carney PJ, Lin SC et al. . Improved specificity and reduced subtype cross-reactivity for antibody detection by ELISA using globular head domain recombinant hemagglutinin. J Virol Methods 2014; 209:121–5. [DOI] [PubMed] [Google Scholar]
- 20.Terajima M, Co MD, Cruz J et al. . High antibody-dependent cellular cytotoxicity antibody titers to H5N1 and H7N9 Avian influenza A viruses in healthy US adults and older children. J Infect Dis 2015; 212:1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Co MD, Terajima M, Thomas SJ et al. . Relationship of preexisting influenza hemagglutination inhibition, complement-dependent lytic, and antibody-dependent cellular cytotoxicity antibodies to the development of clinical illness in a prospective study of A(H1N1)pdm09 Influenza in children. Viral Immunol 2014; 27:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipatov AS, Hoffmann E, Salomon R et al. . Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J Infect Dis 2006; 194:1040–3. [DOI] [PubMed] [Google Scholar]
- 23.Ellebedy AH, Ducatez MF, Duan S et al. . Impact of prior seasonal influenza vaccination and infection on pandemic A (H1N1) influenza virus replication in ferrets. Vaccine 2011; 29:3335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donnell CD, Wright A, Vogel LN et al. . Effect of priming with H1N1 influenza viruses of variable antigenic distances on challenge with 2009 pandemic H1N1 virus. J Virol 2012; 86:8625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roos A, Roozendaal R, Theeuwsen J et al. . Protection against H5N1 by multiple immunizations with seasonal influenza vaccine in mice is correlated with H5 cross-reactive antibodies. Vaccine 2015; 33:1739–47. [DOI] [PubMed] [Google Scholar]
- 26.Mozdzanowska K, Furchner M, Washko G et al. . A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J Virol 1997; 71:4347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Impagliazzo A, Milder F, Kuipers H et al. . A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015; 349:1301–6. [DOI] [PubMed] [Google Scholar]
- 28.Yassine HM, Boyington JC, McTamney PM et al. . Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med 2015; 21:1065–70. [DOI] [PubMed] [Google Scholar]
- 29.Endo A, Itamura S, Iinuma H et al. . Homotypic and heterotypic protection against influenza virus infection in mice by recombinant vaccinia virus expressing the haemagglutinin or nucleoprotein of influenza virus. J Gen Virol 1991; 72(Pt 3):699–703. [DOI] [PubMed] [Google Scholar]
- 30.Deiss RG, Arnold JC, Chen WJ et al. . Vaccine-associated reduction in symptom severity among patients with influenza A/H3N2 disease. Vaccine 2015; 33:7160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferdinands JM, Olsho LE, Agan AA et al. . Effectiveness of influenza vaccine against life-threatening RT-PCR-confirmed influenza illness in US children, 2010–2012. J Infect Dis 2014; 210:674–83. [DOI] [PubMed] [Google Scholar]
- 32.Hobson D, Curry RL, Beare AS et al. . The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mozdzanowska K, Feng J, Gerhard W. Virus-neutralizing activity mediated by the Fab fragment of a hemagglutinin-specific antibody is sufficient for the resolution of influenza virus infection in SCID mice. J Virol 2003; 77:8322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramisse F, Deramoudt FX, Szatanik M et al. . Effective prophylaxis of influenza A virus pneumonia in mice by topical passive immunotherapy with polyvalent human immunoglobulins or F(ab’)2 fragments. Clin Exp Immunol 1998; 111:583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bresson JL, Perronne C, Launay O et al. . Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 2006; 367:1657–64. [DOI] [PubMed] [Google Scholar]
- 36.Blanchfield K, Kamal RP, Tzeng WP et al. . Recombinant influenza H7 hemagglutinins induce lower neutralizing antibody titers in mice than do seasonal hemagglutinins. Influenza Other Respir Viruses 2014; 8:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson LA, Campbell JD, Frey SE et al. . Effect of varying doses of a monovalent H7N9 influenza vaccine with and without AS03 and MF59 adjuvants on immune response: a randomized clinical trial. JAMA 2015; 314:237–46. [DOI] [PubMed] [Google Scholar]
- 38.DiLillo DJ, Tan GS, Palese P et al. . Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 2014; 20:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monsalvo AC, Batalle JP, Lopez MF et al. . Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med 2011; 17:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khurana S, Loving CL, Manischewitz J et al. . Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med 2013; 5:200ra114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.