Abstract
Background. The efficacy of live, attenuated live attenuated influenza vaccine(LAIV) and inactivated influenza vaccine(IIV) is poorly explained by either single or composite immune responses to vaccination. Protective biomarkers were therefore studied in response to LAIV or IIV followed by LAIV challenge in children.
Methods. Serum and mucosal responses to LAIV or IIV were analyzed using immunologic assays to assess both quantitative and functional responses. Cytokines and chemokines were measured in nasal washes collected before vaccination, on days 2, 4, and 7 after initial LAIV, and again after LAIV challenge using a 63-multiplex Luminex panel.
Results. Patterns of immunity induced by LAIV and IIV were significantly different. Serum responses induced by IIV, including hemagglutination inhibition, did not correlate with detection or quantitation of LAIV on subsequent challenge. Modalities that induced sterilizing immunity seen after LAIV challenge could not be defined by any measurements of mucosal or serum antibodies induced by the initial LAIV immunization. No single cytokine or chemokine was predictive of protection.
Conclusions. The mechanism of protective immunity observed after LAIV could not be defined, and traditional measurements of immunity to IIV did not correlate with protection against an LAIV challenge.
Keywords: humoral immunity, influenza vaccine, mucosal immunity, vaccine
There are 2 distinct approaches to the prevention of influenzal illness. Live, attenuated influenza vaccines (LAIVs) are reassortants between internal protein genes from attenuated master strains and contemporary wild-type hemagglutinin (HA) and neuraminidase (NA) genes representing circulating influenza viruses. Live, attenuated influenza vaccines are delivered by large-particle aerosol spray into the upper respiratory tract where they replicate and mimic many aspects of the pathogenesis of wild-type influenza infection. Inactivated influenza vaccines (IIVs) are given intramuscularly and contain purified, inactivated, and structurally disrupted virus particles enriched for HA and NA and standardized to HA content.
The use of LAIV as an experimental challenge to predict protection against subsequent infection afforded by different approaches to vaccination has been reported for both polio and influenza [1–3]. An earlier paper by our group demonstrated decreased shedding in children immunized with seasonal LAIV, compared with IIV, following challenge with LAIV 1 month after the initial vaccination [4]. In children challenged with LAIV after IIV, 10 of 15 shed 1 or more of the influenza virus strains in the trivalent vaccine with 21 of 45 possible strains recovered. In contrast, when LAIV recipients were challenged with LAIV, 1 of 11 shed virus—although that child shed all 3 strains after both vaccinations.
The current study included quantitative, strain-specific virus shedding and multiple measurements of mucosal and systemic immunity at the time of, and after, each vaccine dose. Data from this study allowed us to examine the following: (1) correlations between measurements of systemic and mucosal immunity, (2) patterns of immunity induced by LAIV and IIV, and (3) correlates of protection upon LAIV challenge. With the limitations of a relatively small sample size and the short duration between vaccination and challenge, we demonstrated different patterns of immunity induced by the 2 vaccines. However, none of the standard measures of either systemic or mucosal immunity predicted the comparative efficacy of the 2 vaccines in limiting virus shedding on subsequent LAIV challenge.
METHODS
Study samples were generated as part of a clinical trial conducted as a collaboration between the Vaccine Research Unit at the University of Rochester Medical Center and the Geisel School of Medicine at Dartmouth with support from the Laboratory of Infectious Diseases at the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Informed assent or consent was obtained from all participants and their parents using protocols and consent forms approved by each institutional investigational review board. In brief, the trial used a sequence of LAIV or IIV followed by an LAIV challenge 1 month later in children 2 to 9 years of age (ClinicalTrials.gov identifier NCT01246999). Detailed results were previously published [4] and are summarized in Table 1.
Table 1.
Virus Recovery by Group From the Parent Pediatric Study [4]
| Vaccine Dose 1 | Volunteers per Group | Influenza Strains Recovereda | Volunteers Shedding Influenza | Vaccine Dose 2 | Volunteers per Group | Influenza Strains Recovereda | Volunteers Shedding Influenza |
|---|---|---|---|---|---|---|---|
| LAIV | 13 | 27 | 10 | LAIV | 11 | 3 | 1 |
| IIV | 18 | N/A | N/A | LAIV | 15 | 21 | 10 |
Abbreviations: IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine; N/A; not applicable; PCR, polymerase chain reaction.
a As reflected in growth of H1, H3, and B influenza virus in culture or identification by PCR on days 2, 4, and/or 7 after challenge.
We hypothesized that each strain of influenza in the vaccine (A/California/07/09 [H1N1], A/Perth/16/09 [H3N2], and B/Brisbane/60/08) would be recovered independently and that immune responses would be strain-specific as has been previously shown for other simultaneously delivered LAIV A and B strains [5]. In this study, the numbers shedding virus are consistent with our assumption that (1) each strain would be recovered with approximately equal frequency and (2) the likelihood of shedding multiple strains would be proportional to the frequency with which the individual strains were shed. The observed distributions provided justification for aggregating virus recovery data regardless of strain when considering correlates of immunity.
Immunologic assays were performed as previously described [4, 6]. Serum antibodies to HA and NA, as well as immunoglobulin G (IgG) and IgA class-specific antibodies to whole inactivated virus, neutralizing antibodies, and antibody-dependent cellular cytotoxicity (ADCC) were each measured on days 0, 28, and 56. Strain-specific mucosal IgA and IgG antibodies in respiratory secretions were measured by kinetic enzyme-linked immunosorbent assay (kELISA) and Luminex on days 0, 28, and 56. Cellular immunity was measured using CD4 and CD8 influenza A virus-specific IFN-γ+ and TNF-α+ cells. As previously reported (Supplemental data in [4]), influenza-specific cellular immunity was not detected in any volunteers 1 month after primary immunization, thus these data did not discriminate and were not included in the current analyses. Levels of 63 individual cytokines or chemokines were quantified by Luminex in nasal swab samples obtained on days 0, 2, 4, and 7 after initial LAIV and on the same days after challenge with LAIV 1 month later [7].
Preimmunization samples were used to assess whether the assays correlated with each other or whether they measured discordant facets of immunity. The effect of distantly induced immunity on virus shedding was derived from evaluating the correlation of serologic values and virus shedding on day 0 after the first LAIV dose.
A full history of past influenza exposure through natural infection or different vaccine regimens was not available. However, none of the subjects had received a prior influenza vaccine during the calendar year in which they participated in the study, which was carried out from August to October. Data on the effect of recent immunity induced by LAIV and IIV were derived by determining the difference or fold-change in immune parameter values measured between days 0 and 28 in relation to virus shedding after LAIV challenge.
Spearman correlations were calculated between pairs of immunologic responses as well as between immunologic responses and virus shedding. Results from immunologic assays were examined in 2 ways: (1) activity on day 0 and (2) change (difference or fold-change) in response between days 0 and 28. Virus shedding was evaluated by determining the peak amount of LAIV shed either after the initial dose of LAIV or after challenge with LAIV. Correlations presented as matrices represent pairs within the immunologic assays performed or associations between immunologic assay results and virus shedding. P values were adjusted using a Bonferroni correction. This correction accounts for multiple comparisons with the number of comparisons equal to the number of correlations presented in a given matrix.
Principal component analysis (PCA) was performed to examine the association between multivariate immunologic assay responses and the type of vaccine (IIV vs LAIV) administered as dose 1. Principal components were computed based on the change (difference or fold-change) in immunologic assay response between days 0 and 28 for each subject and for each of the 3 virus subtypes. Before analysis, all variables were scaled to have unit variance. The association between changes in immunologic response and vaccine type given as dose 1 (either LAIV or IIV) was measured by fitting a logistic regression model with vaccine type as the dependent variable and the first 2 principal component analyses as the independent variables.
RESULTS
Correlations Between Measurements of Immunity Before Vaccination
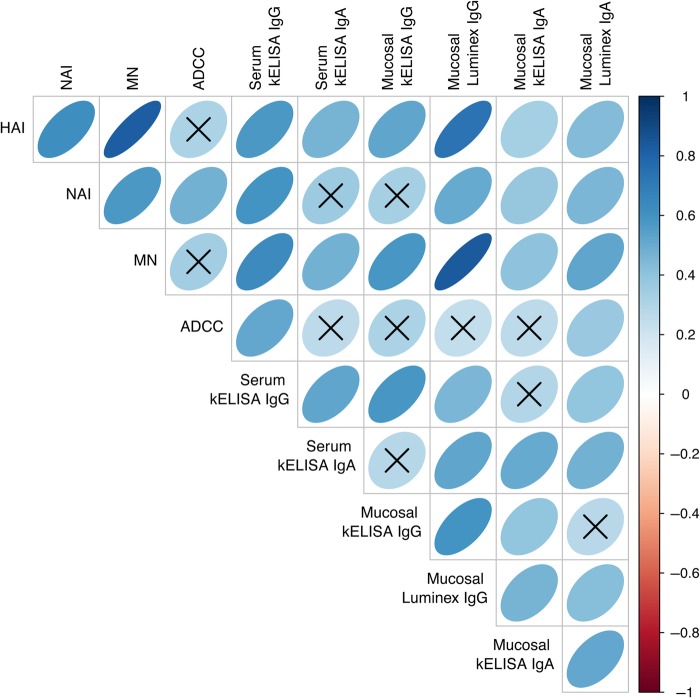
The strong correlation of serum HA inhibition (HAI) titer and microneutralization (MN) titer (r = 0.83; P < .001) reinforces the finding that HAI antibody reflects serum's ability to neutralize influenza (Figure 1). A pairwise correlation matrix demonstrates visually the relative strengths of correlations between individual measurements of serum and mucosal antibodies on day 0 (Figure 2). Relationships between all pairs of variables are positive, as indicated by blue, right-leaning ellipses. Correlations for a majority of the assay comparisons are statistically significant. Nonsignificant comparisons have an X through the corresponding ellipse. On day 0 the strongest correlations (deepest blue and narrowest elliptical forms) exist between (1) serum HAI and serum MN titers (also shown in Figure 1) and (2) mucosal IgG as measured by Luminex. As previously shown, serum ADCC did not correlate well with HAI or MN titer and appears to measure a different facet of immunity [6]. On day 0, upper respiratory tract IgA antibodies collected by nasal wick and measured by Luminex or kELISA showed weak correlations with most serum IgG and IgA measurements, as depicted visually by plumper, paler blue ovals. Immunoglobulin A antibodies in serum and nasal wicks correlated with each other. When looked at by individual influenza virus strain, the same patterns are seen for H3, H1, and B strains although the smaller number of comparisons leads to fewer associations that remain significant (data not shown).
Figure 1.
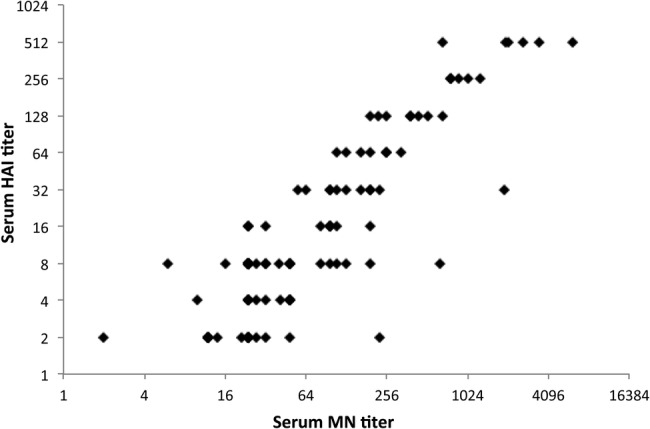
Correlation of serum microneutralization (MN) titer and hemagglutination inhibition (HAI) titer on day 0 before live, attenuated influenza vaccine or inactivated influenza vaccine. Results represent an aggregate of data from all 3 vaccine strains. The Spearman correlation is 0.83 with a P value of <.001.
Figure 2.
Heat map representation of correlations between measurements of immune parameters. The intensity of blue and narrowness of shape indicate the strength of the correlation between measured parameters from 31 participants (93 strain-specific assays) on day 0. The vertical bar indicates the color associated with the strength of a direct correlation (blue ellipse leaning to the right) or an inverse correlation (red ellipse leaning to the left). An X through the ellipse indicates that the correlation was not significant. Data represent aggregate immune responses to all 3 influenza virus strains in the vaccine. Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; HAI, hemagglutination inhibition; Ig, immunoglobulin; kELISA, kinetic enzyme-linked immunosorbent assay; MN, microneutralization; NAI, neuraminidase inhibition.
Immunity Induced by Live, Attenuated Influenza Vaccine and Inactivated Influenza Vaccine
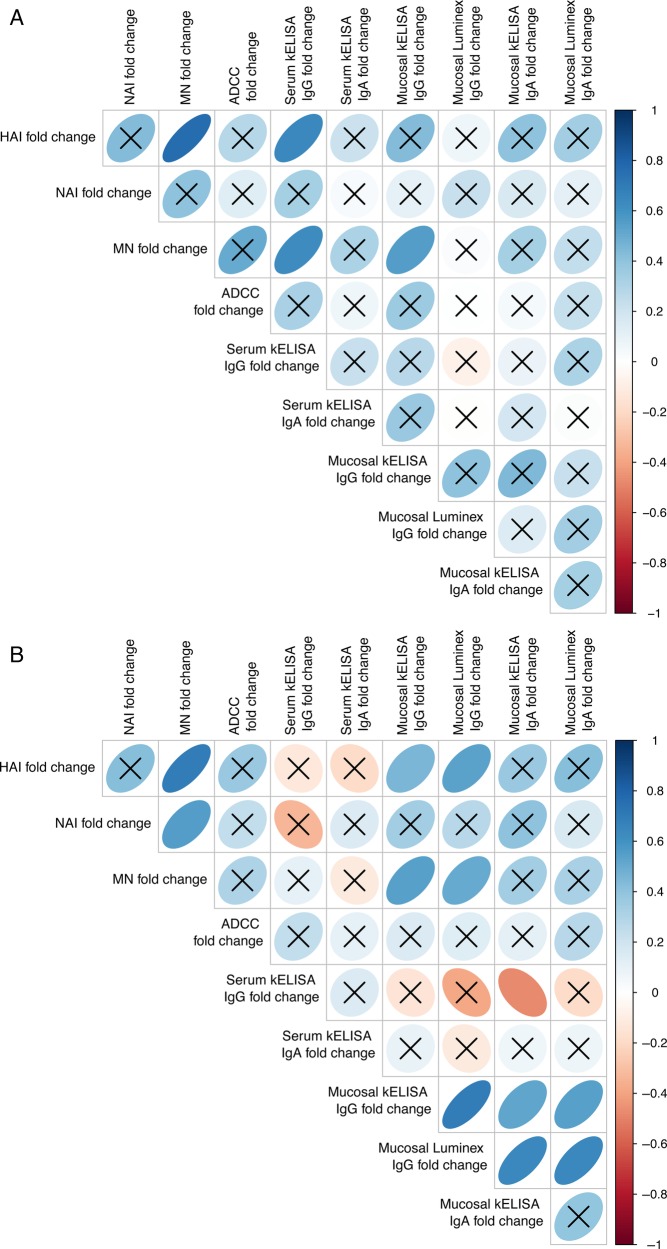
Induction of immunity by LAIV or IIV was measured as the difference or fold-change in the immunologic response between days 0 and 28. After initial LAIV, there was no significant correlation between most of the induced immunologic markers (Figure 3A). Serum antibody responses (HAI, MN titer, and serum ELISA) showed the greatest correlation with each other after LAIV administration. Comparison of immunity induced by IIV again showed that most correlations were not significant (Figure 3B). The highest correlations were found between HAI, MN titer, and mucosal IgG responses. There was an inverse correlation between mucosal IgG and mucosal IgA (Figure 3B).
Figure 3.
(A) Heat map representation of fold-changes in immune parameters from days 0 to 28 in samples from 13 participants (39 strain-specific assays) receiving live, attenuated influenza vaccine on day 0. (B) Heat map representation of fold-changes in immune parameters from days 0 to 28 in samples from 18 participants (54 strain-specific assays) receiving inactivated influenza vaccine on day 0. The vertical bar indicates the color associated with the strength of a direct correlation (blue ellipse leaning to the right) or an inverse correlation (red ellipse leaning to the left). An X through the ellipse indicates that the correlation was not significant. Data represent aggregate immune responses to all 3 influenza virus strains in the vaccine. Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; HAI, hemagglutination inhibition; Ig, immunoglobulin; kELISA, kinetic enzyme-linked immunosorbent assay; MN, microneutralization; NAI, neuraminidase inhibition.
Principal component analysis revealed that immune response profiles across all 10 immunologic assays were significantly different depending on the vaccine given (P < .001; Figure 4). Examination of PCA variable weights for the first 2 principal components, which together explained approximately 50% of the variance in the data, indicated that the immune responses driving much of this association were MN titer, NA inhibition (NAI), and serum IgG as measured by kELISA. Antibody-dependent cellular cytotoxicity rises were preferentially induced after IIV, whereas a minimal increase in ADCC occurred after LAIV. One generalization is that IIV induced stronger serum antibody responses and LAIV induced better mucosal responses, although this was not uniformly observed.
Figure 4.
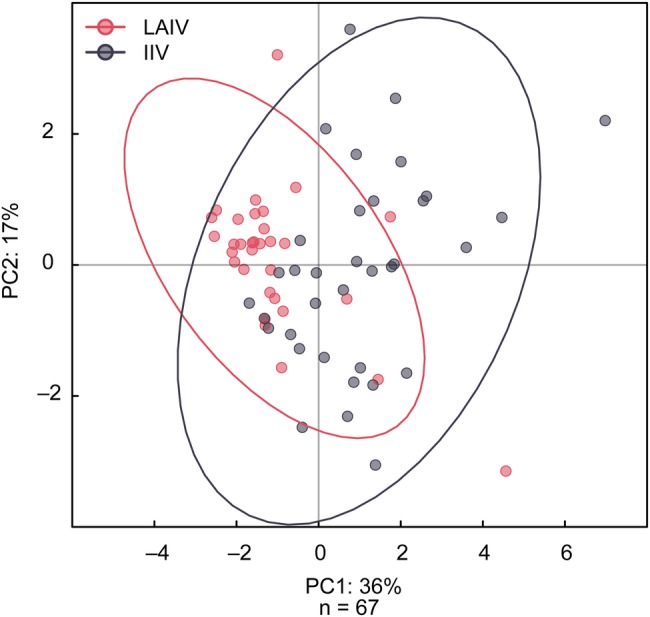
Principal components plot of immune responses to live, attenuated influenza vaccine (LAIV) and inactivated influenza vaccine (IIV) demonstrating differences in the immune response to each vaccine. The first 2 principal components together explained approximately 50% of the variance in the data. The immune responses driving much of this variation were microneutralization titer, neuraminidase inhibition, and serum kinetic enzyme-linked immunosorbent assay immunoglobulin G.
Correlation of Immune Parameters With Virus Shedding
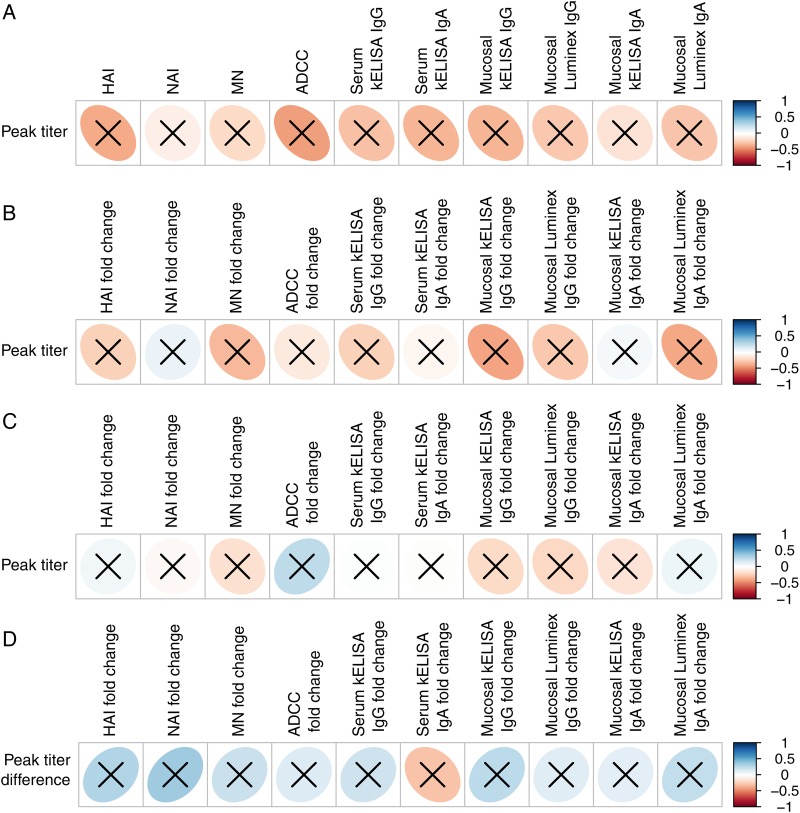
Our results did not reveal any measure of immunity resulting from prior exposure to LAIV that correlated significantly with suppression of virus shedding, although all immune responses were in the predicted inverse direction (Figure 5A). As a measure of the induction of immunity, values generated on day 0 were subtracted from those generated on day 28 for children receiving an initial dose of either LAIV (Figure 5B) or IIV (Figure 5C). Again, we found no significant correlations between the amount of virus shed and increases in immunity generated by prior LAIV or IIV. The generation of HAI by IIV, a traditional measure of protection against influenza, did not correlate with virus shedding (a white circle in Figure 5C). Finally, we examined the decrease in peak virus titer between the first and second dose of LAIV and found that this did not correlate with an increase in the immune parameters measured in response to prior LAIV (Figure 5D).
Figure 5.
(A) Correlation of immune response measured on day 0 to peak amount of live, attenuated influenza vaccine (LAIV) shed (N = 13, aggregate of 39 strain comparisons). (B) Correlation of the fold-change in LAIV immune responses measured between days 0 and 28 to the amount of virus shed on LAIV challenge (N = 11 prior recipients of LAIV, aggregate of 33 strain comparisons). (C) Correlation of the fold-change in inactivated influenza vaccine immune responses measured between days 0 and 28 to the amount of virus shed on LAIV challenge (N = 15 prior recipients of IIV, aggregate of 45 strain comparisons). (D) Correlation of the fold-change in immune response measured between days 0 and 28 to the decrease in peak virus titer between the first and second doses of LAIV (N = 11 participants, aggregate of 33 strain comparisons). The vertical bar indicates the color associated with the strength of a direct correlation (blue ellipse leaning to the right) or an inverse correlation (red ellipse leaning to the left) correlations. An X through the ellipse indicates that the correlation was not significant. Data represent aggregate immune responses to all 3 influenza virus strains in the vaccine. Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; HAI, hemagglutination inhibition; kELISA, kinetic enzyme-linked immunosorbent assay; MN, microneutralization; NAI, neuraminidase inhibition.
Correlation of Mucosal Cytokines and Chemokines With Virus Shedding
Cytokine and chemokine levels were quantified in samples obtained from anterior nares swabs using a 63-multiplex Luminex panel. Although cytokine and chemokine levels were undoubtedly influenced by variation in sample collection, we were able to detect measureable levels for all but 7 factors in the panel. For analysis of the resulting data, subjects receiving LAIV followed by LAIV challenge (N = 10) were divided into 3 groups; individuals who did not shed virus with either dose of LAIV (N = 3), those who shed virus with the first dose but not the second (N = 6), and individuals who shed all 3 strains of virus after each dose (N = 1). Those cytokines and chemokines with a ≥4-fold rise from baseline were considered significant and were examined for each of the 3 groups.
Our results revealed no distinctive patterns of proinflammatory or anti-inflammatory cytokine or chemokine responses in those shedding virus after the initial or subsequent dose of LAIV nor did the level of any individual cytokine or chemokine at the time of LAIV administration provide an explanation for protection against subsequent virus shedding (Supplemental Data, Figure 1). One striking observation was that the 1 child who consistently shed virus after both LAIV doses was unique in having no detectable transforming growth factor-beta (TGFβ) at baseline or in response to vaccination. This child was 53 months old, compared with the mean age of other recipients of LAIV (69 months) and IIV (64 months).
DISCUSSION
This study examined whether correlate(s) of immunity could be defined with current immunologic assays that might explain the greater ability of prior LAIV to restrict virus replication in the upper respiratory tract on LAIV challenge compared with IIV. In addition, this provided an opportunity to explore more broadly our understanding of protective immunity to influenza virus within the context of the 3 arms of the study.
In the first arm, 13 LAIV-dose 1 recipients, whose prior influenza virus exposure could have included LAIV, IIV, and/or wild-type infection, were partially protected against influenza infection. However, vaccine strains were still recovered 72% of the time; H1N1 (N = 9), H3N2 (N = 9), and B (N = 10). This level of protection is representative of the effectiveness of distantly induced immunity to influenza virus, because at least 6 months had passed since possible influenza exposure during the previous winter.
In the second arm, a dose of LAIV was given 1 month after initial LAIV. In this group, only 1 of 10 children shed influenza virus. That single child shed all 3 vaccine strains after both the first and second dose of LAIV. Inferences from observations in a single subject are limited, but this child uniquely had no detectable mucosal TGFβ, which has been shown in animal models to be a key regulator of influenza virus replication [8–10].
In the third arm, an LAIV challenge was given 1 month after IIV to 15 children. An influenza strain was recovered 56% of the time from children in this group; H1N1 (N = 6), H3N2 (N = 5), and B (N = 10). Thus, IIV provides limited protection to LAIV challenge when compared with the response generated by initial LAIV after distant influenza virus exposure (P = .027 by Fisher's exact test).
In the latter 2 settings, the LAIV challenge was administered after a short interval, raising questions as to the possibility of innate immunity that is not influenza-specific. Such heterotypic protection against virus shedding has been reported with sequential infections with different rhinoviruses [11]. Cytokine or chemokine levels in nasal secretions collected before and 2, 4, and 7 days after both LAIV doses did not reveal a distinctive pattern, although it has been shown that selected cytokine levels are elevated and predict severity of disease with wild-type influenza infection [12].
Early in the development of LAIV, Clements et al [13] challenged seronegative young adults with wild-type virus 1 to 2 months after receipt of monovalent H1N1 or H3N2. Fifty-two percent of LAIV vaccinees shed virus on challenge, compared with 66% of IIV vaccinees and 79% of those without prior immunization. It was reported that serum HAI and NAI induced by IIV inhibited virus replication and that serum NAI and mucosal IgA antibodies induced by LAIV were protective [14]. Notably, when a challenge was done 7 months postvaccination, protection against virus shedding was not seen with either vaccine [13]. However, in a separate pediatric study of children who received LAIV 1 year after a prior dose of either LAIV or IIV, it was found that prior LAIV but not IIV continued to afford enhanced protection [1]. In that study, LAIV induced more persistent serum HAI, IgG, and IgA antibody responses than IIV, and nasal IgA antibody was persistent over the 1-year period [1].
Several additional studies have looked at protection afforded by LAIV vs IIV over an influenza season and have shown that protection was higher with LAIV than IIV [15–17]. This led to the recommendation of LAIV in 2014 as the preferred vaccine in children ages 2 to 8 without a history of asthma [18, 19]. However, subsequent observational trials have shown very mixed comparative effectiveness of both vaccines [20], leading the Centers for Disease Control and Prevention to recently recommend each vaccine without a preference [21].
The limitations and limited generalizability of this study are important to emphasize. Live, attenuated influenza vaccine challenge measures only recent immunity conveyed by the 2 vaccines. This protection against influenza infection should theoretically include protection against illness. However, illness, which is the defining event in virtually all observational studies of vaccine protection, might be prevented by vaccination approaches that still allow infection.
In addition, a relatively broad age range (2–9 years) was represented in this study. However, with the exception of increasing influenza B HAI seropositivity with age, we found no differences with respect to age in an earlier study of influenza vaccines in children [4]. That study used 1 dose of vaccine, rather than the recommended 2 doses, in potentially influenza naive subjects. However, all subjects had evidence of prior influenza exposures. There were no symptoms associated with the attenuated vaccine in the trial associated with this study, despite evidence of robust viral replication [4].
Another consideration is that the behavior of LAIV in adults is clearly different than in children. Limited vaccine virus recovery is seen in adults even when the infecting strains are those to which the adult has no prior exposure [21–23]. An immunologic explanation for this resistance to LAIV in adults is not yet apparent.
In spite of the relatively large number of assays performed, it remains possible that the most discriminating assays were not done, eg, neutralizing antibody in mucosal secretions, which we have previously shown to be a distinctive marker of protection afforded by live, oral polio vaccine [3]. In addition, others have implicated cellular immunity in protection induced by LAIV [24]; however, this was not detected in the current study. It is worth noting that the mucosal assays, in particular Luminex assays, are sensitive detectors of mucosal IgA and IgG responses, but all mucosal assays suffer from variability in collection and processing.
In considering the methods used for data analysis, the Bonferroni correction for multiple comparisons is recognized as conservative, and in a few instances we found significance without it. However, for most comparisons the correlation coefficients were weak, and it was unclear whether important correlations would emerge with inclusion of additional subjects. This underscores the small size of the current study and the subsequent need to focus our primary analyses on aggregate responses to all 3 viruses in the vaccine recipients.
Finally, there is an assumption that the events surrounding an attenuated vaccine virus infection mimic wild-type infection and that immunologic measurements from the upper respiratory tract and serum reflect lower respiratory tract protection. Neither may be true. As an example, protection against upper respiratory tract influenza infection in mice is mediated by IgA, whereas lower respiratory tract protection is mediated by IgG [25].
CONCLUSIONS
These limitations do not negate the fact that profound differences are seen in the immunity induced, and the protection afforded, by the 2 different influenza vaccine approaches. We could not explain the differences in protection by the comprehensive collection of currently available immunologic assays performed. In particular, HAI is generally accepted as a measure of vaccine-induced protection against illness, but we found no correlation of HAI with protection against virus shedding on LAIV challenge. These observations highlight the need to understand more fully the pathogenesis and correlates of protection against influenza infection at the mucosal level in humans.
Supplementary Material
Acknowledgments
We acknowledge the contributions of Andrew J. Giustini, Molly Housman, and Alexey Khalenkov to the conduct of the initial study. Vaccine antigens used in kinetic enzyme-linked immunosorbent assay and Luminex assays were a kind gift of Novartis Vaccines and Diagnostics (Cambridge, MA).
Financial support. This work was funded by a contract with the University of Rochester from the National Institutes of Health (NIH) Respiratory Pathogens Research Center (contract HHSN272201200005C) and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
References
- 1.Johnson PR, Feldman S, Thompson JM et al. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J Infect Dis 1986; 154:121–7. [DOI] [PubMed] [Google Scholar]
- 2.Mohammed AJ, AlAwaidy S, Bawikar S et al. Fractional doses of inactivated poliovirus vaccine in Oman. N Engl J Med 2010; 362:2351–9. [DOI] [PubMed] [Google Scholar]
- 3.Wright PF, Wieland-Alter W, Ilyushina NA et al. Intestinal immunity is a determinant of clearance of poliovirus after oral vaccination. J Infect Dis 2014; 209:1628–34. [DOI] [PubMed] [Google Scholar]
- 4.Ilyushina NA, Haynes BC, Hoen AG et al. Live attenuated and inactivated influenza vaccines in children. J Infect Dis 2015; 211:352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright PF, Bhargava M, Johnson PR et al. Simultaneous administration of live, attenuated influenza A vaccines representing different serotypes. Vaccine 1985; 3:305–8. [DOI] [PubMed] [Google Scholar]
- 6.Jegaskanda S, Job ER, Kramski M et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 2013; 190:1837–48. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg-Hasson Y, Hansmann L, Liedtke M et al. Effects of serum and plasma matrices on multiplex immunoassays. Immunol Res 2014; 58:224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall HD, Ray JP, Laidlaw BJ et al. The transforming growth factor beta signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa. Elife 2015; 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, Hao R, Li P et al. MicroRNA expression profile of mouse lung infected with 2009 pandemic H1N1 influenza virus. PLoS One 2013; 8:e74190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu CI, Becker C, Wang Y et al. CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-β. Immunity 2013; 38:818–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleet WF, Couch RB, Cate TR, Knight V. Homologous and heterologous resistance to rhinovirus common cold. Am J Epidemiol 1965; 82:185–96. [DOI] [PubMed] [Google Scholar]
- 12.Oshansky CM, Gartland AJ, Wong SS et al. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am J Respir Crit Care Med 2014; 89:449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol 1986; 24:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements ML, Betts RF, Tierney EL, Murphy BR. Resistance of adults to challenge with influenza A wild-type virus after receiving live or inactivated virus vaccine. J Clin Microbiol 1986; 23:73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson PR Jr, Feldman S, Thompson JM et al. Comparison of long-term systemic and secretory antibody responses in children given live, attenuated, or inactivated influenza A vaccine. J Med Virol 1985; 17:325–35. [DOI] [PubMed] [Google Scholar]
- 16.Belshe RB, Mendelman PM, Treanor J et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med 1998; 338:1405–12. [DOI] [PubMed] [Google Scholar]
- 17.Belshe RB, Edwards KM, Vesikari T et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007; 356:685–96. [DOI] [PubMed] [Google Scholar]
- 18.Committee on Infectious Diseases; American Academy Pediatrics. Recommendations for prevention and control of influenza in children, 2014-2015. Pediatrics 2014; 134:e1503–19. [DOI] [PubMed] [Google Scholar]
- 19.Grohskopf LA, Olsen SJ, Sokolow LZ et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Chung JR, Flannery B, Thompson MG et al. Seasonal effectiveness if live attenuated and inactivated influenza vaccine. Pediatrics 2016; 137:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grohskopf LA, Sokolow LZ, Olsen SJ et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 influenza season. MMWR Morb Mortal Wkly Rep 2015; 64:818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karron RA, Talaat K, Luke C et al. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine 2009; 27:4953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babu TM, Levine M, Fitzgerald T et al. Live attenuated H7N7 influenza vaccine primes for a vigorous antibody response to inactivated H7N7 influenza vaccine. Vaccine 2014; 32:6798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoft DF, Babusis E, Worku S et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 2011; 204:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renegar KB, Small PA Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 2004; 173:1978–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.