Preemptive antiviral therapy for active CMV infection in allogeneic stem cell transplant recipients guided by immunological and virological parameters minimizes the risk of recurrent viremia in a subset of patients.
Keywords: allogeneic stem cell transplantation, cytomegalovirus, IFN-γ CD8+ T cells, immunological monitoring, preemptive antiviral therapy
Abstract
Background. Preemptive antiviral therapy for active cytomegalovirus (CMV) infection in allogeneic stem cell transplant recipients (Allo-SCT) results in overtreatment and a high rate of recurrences. Monitoring of CMV-specific T-cell immunity may help to individualize treatments and minimize these problems.
Methods. We conducted a prospective, multicenter, matched comparison-group study to evaluate the efficacy and safety of a novel strategy that consisted of interrupting anti-CMV therapy upon CMV DNAemia clearance and concurrent detection of phosphoprotein 65/immediate-early-1-specific interferon-γ-producing CD8+ T cells at levels of >1 cell/µL (within 30 days after the initiation of therapy). Immunological monitoring was performed on days +7, +14, +21, and +28 after treatment initiation. The primary endpoint was the cumulative incidence of recurrent DNAemia within 2 months after treatment cessation. Secondary endpoints were the length of antiviral treatment courses and the incidence of hematological toxicity.
Results. Sixty-one patients were enrolled in the study group. Fifty-six patients were included in the matched-control group. Eleven patients (18%) fulfilled the criteria for antiviral treatment interruption. The cumulative incidence of recurrent CMV DNAemia was significantly lower (P = .02) in these patients than in patients in the comparative groups. Likewise, the length of antiviral treatment courses was significantly shorter in these patients than that in patients in the matched-control group (P = .003). No significant differences in the incidence of hematological toxicity was observed between the comparative groups.
Conclusions. Our data support the clinical utility of combining immunological and virological monitoring for the management of CMV infection in a subset of Allo-SCT recipients.
Preemptive antiviral therapy is currently the mainstay strategy for the prevention of cytomegalovirus (CMV) disease in allogeneic stem cell transplant recipients (Allo-SCT) [1–3]. Although this strategy is highly effective in minimizing the risk of CMV end-organ disease developing within the first 3 months after transplant (early disease) [1–5], it results in overtreatment leading to drug-related toxicity, an exceedingly high rate of recurrence, and, to a lesser extent, late CMV end-organ disease [6, 7]. Control of CMV viremia in Allo-SCT patients is largely dependent upon a prompt and adequate expansion of functional CMV-specific T cells [8–12], even in patients treated with antivirals [13, 14]. In this context, sequential immunological monitoring of CMV-specific T-cell immunity during episodes of active CMV infection may help to tailor individually the length of antiviral therapy courses and minimize these problems [4, 15]. Nevertheless, to date, there are scarce data supporting this assumption [16, 17]. Previous work by our group showed that patients treated preemptively with antivirals and expanding CMV-specific phosphoprotein (pp) 65 and immediate-early (IE)-1 interferon (IFN)-γ CD8+ T cells at levels >1 cell/µL at the time of CMV DNAemia clearance had a reduced risk of developing recurrent episodes of viremia [14]. In light of this finding, an intervention strategy based on combined virological and immunological monitoring was designed and tested in a small group of patients [17]. Our data indicated that this strategy was indeed highly effective in minimizing the incidence of recurrent viremia in a subset of patients, even using shorter courses of antiviral therapy. To further assess the clinical efficacy of this intervention approach, we conducted a multicenter, closely matched comparison-group study.
METHODS
Study Design
This prospective, multicenter, open-label study (No. EudraCT: 2011-001449-34) compared a novel preemptive antiviral therapy strategy guided by plasma CMV deoxyribonucleic acid (DNA) load and peripheral blood counts of pp65/IE-1-IFN-γ-producing CD8+ T cells (study group) with a classic strategy guided solely by plasma CMV DNA load (control group). The study was conducted between June 2011 and November 2014, and it was carried out in 7 centers in Spain in accordance with national and local regulations. The control group was initially planned to consist of an historical cohort including patients enrolled in a multicenter, Phase III, randomized study comparing oral valganciclovir versus intravenous ganciclovir (GCV) for the prevention of CMV disease in patients with active CMV infection (Convince study, EudraCT No. 2009-015965-29). This trial was suspended prematurely due to a low rate of enrollment. As a result, the current study was redesigned to compare the study group with a closely matched control group, as detailed below. At the conclusion of the study, an unblinded review of clinical, virological, and safety data was performed by an independent committee. The study was designed by 3 of the authors (C. S., D. N., and R. d. l. C.) and was reviewed by all of the authors. All patients gave written informed consent for inclusion, and the study was approved by local ethics committees.
Study Participants
Adults (>18 years of age) undergoing any modality of Allo-SCT were eligible for inclusion in the study if they were CMV seropositive or CMV seronegative but received an allograft from a CMV-seropositive donor, and they developed a first episode of CMV DNAemia within the first 100 days after transplant that required preemptive antiviral therapy according to local guidelines (this being administered up to 72 hours after CMV DNA load reached the established threshold for treatment initiation). Females of reproductive age were required to have a negative pregnancy test at the time of enrollment. The exclusion criteria were the following: (1) enrollment in another clinical trial using investigational drugs; (2) receipt of an autologous or syngenic transplant; (3) known allergy or hypersensitivity to GCV or foscarnet; (4) proven or suspected CMV end-organ disease at the time of enrollment; (5) prior episode of CMV DNAemia; (6) prior exposure to GCV (intravenous [i.v.] or oral), foscarnet, or any other drugs with intrinsic activity against CMV; (7) relapse of the baseline disease; (8) severe kidney impairment (creatinine clearance <10 mL/minute or dialysis); (9) severe liver insufficiency (bilirubinemia ≥10 mg/dL); (10) neutrophil count <0.5 × 109 cells/L at the time of enrollment; (11) platelet count <25 × 109/L at the time of study entry; and (12) weight <50 kg. At the conclusion of the study, a well matched comparison-group study was retrospectively selected that included patients who had developed the first episodes of CMV DNAemia within the same time frame as those in the study group and had been managed following the guidelines in use at the participating centers. Prioritized parameters for matching were the following: age (±10 years), source of stem cells, type of Allo-SCT, human leukocyte antigen (HLA)-matching, in vivo T-cell depletion, recipient and donor CMV serology, presence and severity of graft-versus-host disease (GvHD), treatment with corticosteroids at doses ≥0.5 mg/kg, and the underlying disease. The control group included patients who underwent Allo-SCT at the participating centers between 2011 and 2015. None of these patients had been given the option to participate in the intervention and declined. These patients had been previously enrolled in other clinical trials using drugs with no activity against CMV.
Study Procedures
Preemptive antiviral therapy was initiated upon detection of CMV DNA in plasma by quantitative real-time polymerase chain reaction (PCR) at a predefined threshold according to local guidelines. Valganciclovir (900 mg twice daily) was prescribed to outpatients. Hospitalized patients received i.v. GCV (at doses of 5 mg/kg every 12 hours). Foscarnet was primarily given (at a dose of 60 mg/kg every 12 hours) to patients with neutropenia. In accordance with the study design, anti-CMV therapy was scheduled to be withdrawn upon CMV DNAemia clearance (first undetectable PCR result) and concurrent detection of pp65/IE-1-specific IFN-γ-producing CD8+ T cells at levels >1 cell/µL (at any time within 1 month after therapy inception). Virological monitoring was performed once or twice a week until the completion of the study (2 months after treatment cessation), and immunological monitoring was performed on days +7, +14, +21, and +28 after initiation of antiviral therapy. Patients who met the criteria for treatment interruption were immunologically monitored at days 30 and 60 after discontinuation of antiviral therapy. Immunological analyses were centralized at Hospital Clínico Universitario (HCU) of Valencia and were performed within 24–36 hours after collection. Specimens obtained at the participating centers were kept at room temperature until shipment to HCU. Cytomegalovirus-specific IFN-γ-producing CD8+ T-cell counts were provided to the attending physician within 48 hours of receipt of the specimen. After day +28, the patients left the study and antiviral therapy was interrupted exclusively on the basis of locally established criteria. Preemptive antiviral therapy in patients in the matched-control group was also initiated according to locally validated criteria and discontinued upon CMV DNAemia clearance (first undetectable CMV DNA PCR result). Anti-CMV therapy at maintenance doses was allowed for patients (excluding those who met the criteria for treatment interruption) with severe acute GvHD who had cleared the episode of CMV DNAemia. Matched-control patients were not immunologically monitored.
Efficacy and Safety Analyses
The primary efficacy endpoint of this study was the incidence of recurrent CMV DNAemia, as defined by the reappearance of CMV DNA in plasma (at any level) after at least 15 days after the resolution of the initial episode of CMV DNAemia, within 2 months after discontinuation of antiviral therapy. A secondary efficacy endpoint was the length of antiviral treatment courses (duration in days). Safety endpoints were focused on hematological toxicity, namely, the incidence of grade III (<1.0 × 109/L) or grade IV (<0.5 × 109/L) neutropenia and thrombocytopenia (<0.5 × 109/L) after engraftment. The incidence of CMV end-organ disease, as previously defined [18], was assessed in the comparison groups.
Virological Monitoring
Plasma CMV DNA load was quantitated by means of commercially available real-time PCR methods (affigene CMV trender from Cepheid, Sunnyvale, CA, at 3 centers; Q-CMV Real-Time from Nanogen, San Diego, CA, at 2 centers; CMV PCR Kit from Abbott, Lake Forest, Illinois, at 1 center; and the LightCycler CMV Quant Kit from Roche, Indianapolis Indiana, at 1 center). Cytomegalovirus DNA load thresholds prompting the initiation of preemptive antiviral therapy ranged from 150 to 1000 copies/mL after locally validated criteria [5].
Immunological Monitoring
Cytomegalovirus pp65 and IE-1-specific IFN-γ CD8+ T cells were enumerated by flow cytometry for intracellular cytokine staining (BD Fastimmune, BD Becton Dickinson and Company, and BD Biosciences, San Jose, CA) as previously reported [19, 20]. Whole blood (0.5 mL) was simultaneously stimulated for 6 hours with 2 sets of 15-mer overlapping peptides encompassing the entire sequence of pp65 and IE-1 CMV proteins (1 μg/mL per peptide) obtained from JPT Peptide Technologies GmbH (Berlin, Germany), in the presence of l μg/mL of costimulatory monoclonal antibodies to CD28 and CD49d. Samples mock stimulated with phosphate-buffered saline (PBS)/dimethyl sulfoxide (without peptides) and costimulatory antibodies or stimulated with 1 mg/mL phytohemagglutinin (Sigma-Aldrich, St. Louis, MO) were run in parallel. Brefeldin A (10 lg/mL) was added for the last 4 hours of incubation. Stimulated blood was washed, permeabilized, and stained with a combination of labeled monoclonal antibodies (anti-IFN-γ-FITC, anti-CD69-PE, anti-CD8-PerCP-Cy5.5, and anti-CD3-APC) for 30 minutes at room temperature. Appropriate isotype controls were used (BD FastImmune γ2a/γ1/CD8/CD3). Cells were then washed, resuspended in 200 μL of 1% paraformaldehyde in PBS, and analyzed within 2 hours on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences Immunocytometry Systems). Cells were first gated for lymphocytes (SSC-A vs FSC-A). The lymphocyte population was analyzed for their surface expression of CD3 and CD8. CD3/CD8− expressing cells were further gated for their expression of the activation marker CD69 and the intracellular cytokine IFN-γ. Data files usually contained at least 1000 positive events for CD8+ within the lymphocyte gate. The total number of CMV-specific CD8+ T cells was calculated by multiplying the percentages of CMV-specific T cells producing IFN-γ on stimulation (after background subtraction) by the absolute CD8+ T-cell counts. A positive (detectable) response was that >0.1% (2 standard deviations higher than median responses of 10 CMV-seronegative subjects).
Statistical Analysis
On the basis of published data and our own experience, we assumed the following: (1) the rate of plasma CMV DNAemia clearance on day +28 after the start of therapy with oral valganciclovir or i.v. ganciclovir varies between 60% and 80% [4, 6, and unpublished observations]; (2) the overall incidence of recurrent CMV DNAemia within the first 3 months after transplant ranges between 35% and 70%, depending upon the patient's risk factor constellation ([6] and unpublished observations). The maximum difference acceptable to confirm equivalence was set at 20%. At a 1-sided significance level of 0.05 and an early dropout rate of <20%, we calculated that we would need to enroll 58 patients in each group for the study to have 80% power to detect this difference. To compare the characteristics of cases with those of controls, percentages for categorical variables, as well as medians and ranges for ages at transplantation, were calculated. Comparisons from 2 × 2 tables were made by means of a χ2 test (for nonmatched groups) or the McNemar χ2 test (for matched groups). Cumulative incidence curves up were produced for recurrent CMV DNAemia episodes taking baseline disease relapse and death as a competing events. Conditional logistic regression models adjusting for baseline characteristics of the patients were used for univariate and multivariate analyses of risk factors for recurrent CMV infection. Variables for the multivariate models were selected with backward stepwise elimination with significance exceeding 0.1 as the criterion for removal from the models. Median cell counts (neutrophils, platelets, CMV-specific T cells) were compared using the Mann-Whitney U test (nonmatched groups) or the Wilcoxon paired test (matched groups). Tests of significance were 2-sided, with a significance level of α = 0.05. All statistical analyses were performed using SPSS version 21.0 (SPSS, Chicago, IL), with the exception of the cumulative incidence analyses, which were carried out using R version 2.12.2 (The CRAN project) with packages, survival v2.36-10, Design 2.3-0, prodlim v1.2.1, and cmprsk v2.2-2.
RESULTS
Patient Characteristics and Study Developments
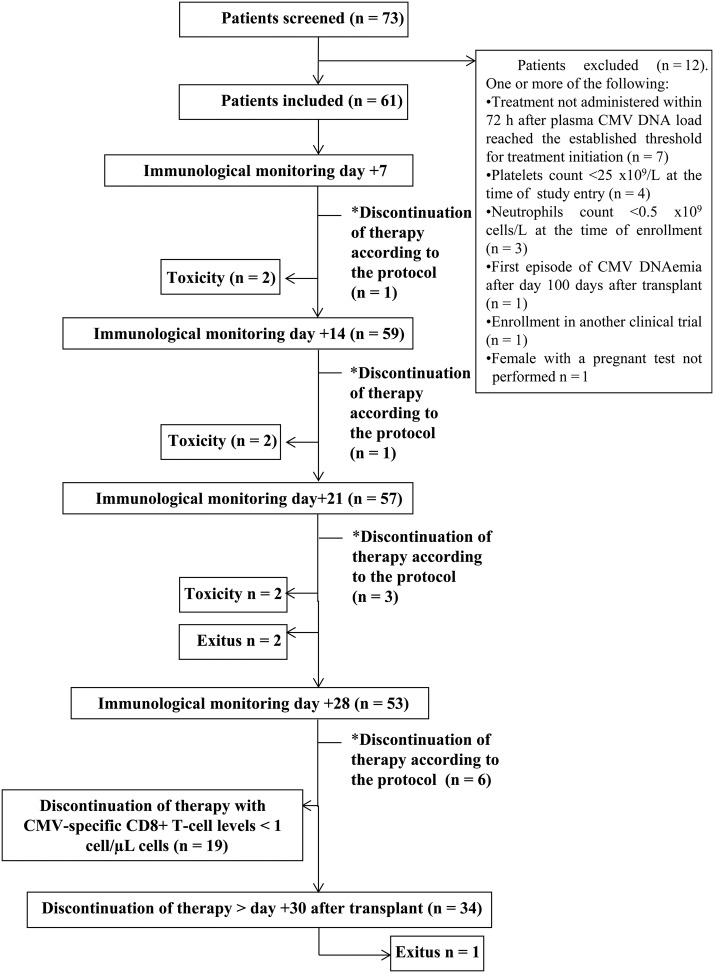
As shown in Figure 1, of 73 screened patients, 12 were excluded and 61 met the inclusion criteria and were enrolled (Study group or A). In turn, 56 patients were included in the matched-control group (B). Both groups were comparable in terms of demographics, clinical characteristics, the time at which antiviral treatment was initiated (Table 1), and the first-line drug used (data not shown). Eleven patients in group A (18%) fulfilled the criteria for antiviral treatment interruption according to the novel strategy (subgroup A1). In these patients, antiviral therapy was discontinued after virological and immunological monitoring performed on day +7 (n = 1), +14 (n = 1), day +21 (n = 3), or day +28 (n = 6) (Figure 1). Median CMV-specific IFN-γ CD8+ T-cell counts at the time of treatment interruption was 3.1 cells/µL (range, 1.03–77.0 cells/µL). The remaining 50 patients did not meet these criteria (subgroup A2). Antiviral treatment was interrupted within 72 hours after virological and immunological monitoring performed on day +28 in 19 patients and after day +31 in 23 patients.
Figure 1.
Flow chart showing the screening, the enrollment status, and the developments in the study group (patients guided by the plasma cytomegalovirus [CMV] deoxyribonucleic acid [DNA] load and peripheral blood counts of pp65/IE1-interferon [IFN]-γ-producing CD8+ T cells). Anti-CMV therapy was scheduled to be withdrawn upon CMV DNAemia clearance and concurrent detection of pp65/IE-1-specific IFN-γ-producing CD8+ T cells at levels >1 cell/µL at any time within 1 month after therapy inception (*). Patients who left the study because of drug-related toxicity: 2 between days 7 and 14, 2 between days 14 and 21, and 2 between days 21 and 28. Two patients died between days 21 and 28. Antiviral treatment was interrupted within the study period (on day 28) with CMV-specific T-cell counts <1 cell/µL. Antiviral therapy was suspended in 23 patients after day 30 on the basis of virological criteria (exclusively).
Table 1.
Demographic and Clinical Characteristics of Patients Included in the Study Group and in the Matched-Control Group
| Parameter | Patients |
P Valuea | |
|---|---|---|---|
| Study Group (n = 61) No. (%) | Matched-Control Group (n = 56) No. (%) | ||
| Age, median (range) | 54 (26–71) | 52 (19–66) | .17 |
| Sex (male/female) | 31/30 | 39/17 | .08 |
| Underlying disease | .47 | ||
| Aplastic anemia | 3 (4.9) | 2 (3.6) | |
| Acute lymphocytic leukemia | 3 (4.9) | 5 (8.9) | |
| Acute myeloid leukemia | 26 (42.6) | 26 (46.4) | |
| Chronic lymphocytic leukemia | 5 (8.2) | 4 (7.1) | |
| Hodgkin's lymphoma | 5 (8.2) | 3 (5.4) | |
| Myelodysplastic syndrome | 12 (19.7) | 7 (12.5) | |
| Myelofibrosis | 2 (3.3) | 1 (1.8) | |
| Non-Hodgkin's lymphoma | 5 (8.2) | 8 (14.3) | |
| Stem cell source | .62 | ||
| Peripheral blood | 54 (88.5) | 52 (92.9) | |
| Bone marrow | 3 (4.9) | 1 (1.8) | |
| Umbilical cord blood | 4 (6.6) | 3 (5.4) | |
| HLA-matching | .50 | ||
| Mismatched | 26 (42.6) | 23 (41.1) | |
| Matched | 35 (57.3) | 33 (58.9) | |
| Donor type | .81 | ||
| Related | 34 (55.7) | 30 (53.6) | |
| Unrelated | 27 (44.3) | 26 (46.4) | |
| Conditioning regimen | .79 | ||
| Myeloablative | 35 (58.3) | 34 (60.7) | |
| Nonmyeloablative | 25 (41.7) | 22 (39.3) | |
| Treatment with ATG | .76 | ||
| Yes | 19 (31.1) | 16 (28.6) | |
| No | 42 (68.9) | 40 (71.4) | |
| Graft-vs-host disease prophylaxis | .38 | ||
| Cyclosporin A/methotrexate | 28 (45.9) | 28 (54.9) | |
| Cyclosporin A/mycophenolate mofetil | 16 (26.2) | 9 (17.6) | |
| Cyclosporin A/prednisone | 3 (4.9) | 2 (3.9) | |
| Tacrolimus/methotrexate | 4 (6.6) | 4 (7.8) | |
| Tacrolimus/mycophenolate | 0 (0.0) | 1 (2.0) | |
| Tacrolimus/sirolimus | 6 (9.8) | 7 (13.7) | |
| Any combination plus ATG | 4 (6.6) | 5 (4.3) | |
| CMV serostatus | .22 | ||
| D+/R+ | 31 (50.8) | 37 (66.1) | |
| D−/R+ | 28 (45.9) | 17 (30.4) | |
| D+/R− | 2 (3.3) | 2 (3.6) | |
| Acute graft-vs-host disease | .66 | ||
| No | 34 (55.7) | 35 (62.5) | |
| Grade I | 9 (14.8) | 5 (8.9) | |
| Grade II | 9 (14.8) | 10 (17.8) | |
| Grades III and IV | 9 (14.8) | 6 (10.7) | |
| Receipt of corticosteroids ≥0.5 mg/kg | .67 | ||
| Yes | 23 (37.7) | 19 (33.9) | |
| No | 38 (62.3) | 37 (66.1) | |
| Median time to initiation of antiviral therapy after transplant (range) | 31 (7–57) | 32 (5–77) | .83 |
Abbreviations: ATG, antithymocyte globulin; CMV, cytomegalovirus; D, donor; HLA, human leukocyte antigen; R, recipient.
a McNemar χ2 test for discrete variables and Wilcoxon test for continuous variables.
Incidence of Recurrent Cytomegalovirus DNAemia
As shown in Figure 2A, overall, the cumulative incidence of recurrent CMV DNAemia was similar in groups A and B (P = .90). Nevertheless, the incidence was significantly lower (P = .02) in subgroup A1 than in both subgroup A2 and group B (9%, 44%, and 37.5%, respectively) (Figure 2B). In fact, only 1 patient of 11 in subgroup A1 had recurrent DNAemia, this occurring on day +30 after treatment interruption in the setting of a grade III acute GvHD (aGvHD) treated with high-dose corticosteroids. At this time point, the patient displayed undetectable levels of CMV-specific IFN-γ CD8+ T cells. All but 2 patients in the A1 subgroup maintained CMV-specific IFN-γ-producing CD8+ T cells >1 cell/µL after antiviral treatment interruption (data not shown). One of these patients eventually developed CMV end-organ disease (gastrointestinal). The demographic and clinical characteristics of the patients in subgroups A1 and A2 did not differ significantly (Table 2). Conditional logistic regression analyses failed to identify any significant risk factors for the occurrence of recurrent CMV DNAemia (Table 3).
Figure 2.
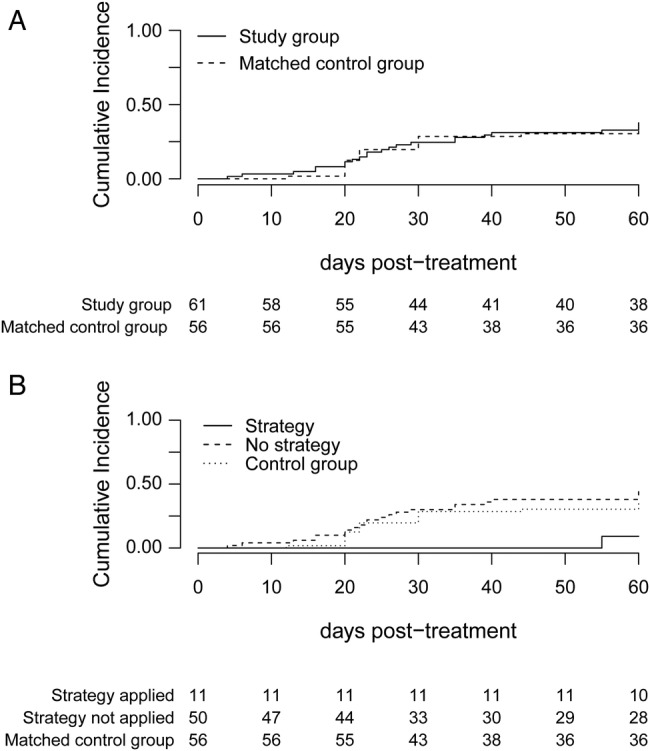
Cumulative incidence of recurrent cytomegalovirus (CMV) DNAemia within 2 months after interruption of antiviral therapy in patients guided by combined virological and immunological monitoring (study group) vs matched-control patients (A) and in patients who met the criteria for antiviral therapy interruption; that is, upon CMV DNAemia clearance and concurrent detection of pp65/IE-1-specific interferon-γ-producing CD8+ T cells at levels >1 cell/µL at any time within 1 month after therapy inception (strategy applied) vs patients who did not meet them (strategy not applied) and matched-control patients (B).
Table 2.
Demographic and Clinical Characteristics of Patients to Whom the Novel Preemptive Antiviral Strategy Could or Could Not Be Applied
| Parameter | Patients |
P Valueb | |
|---|---|---|---|
| Strategy Applied (n = 10)a No. (%) | Strategy Not Applied (n = 50) No. (%) | ||
| Age, median (range) | 60 (34–71) | 53.50 (26–71) | .22 |
| Sex | .17 | ||
| Male | 3 (30.0) | 27 (54.0) | |
| Female | 7 (70.0) | 23 (46.0) | |
| Underlying disease | .82 | ||
| Aplastic anemia | 0 (0.0) | 3 (6.0) | |
| Acute lymphocytic leukemia | 0 (0.0) | 3 (6.0) | |
| Acute myeloid leukemia | 6 (6.0) | 19 (38.0) | |
| Chronic lymphocytic leukemia | 1 (10.0) | 4 (8.0) | |
| Hodgkin's lymphoma | 1 (10.0) | 4 (8.0) | |
| Myelodysplastic syndrome | 2 (20.0) | 10 (20.0) | |
| Myelofibrosis | 0 (0.0) | 2 (4.0) | |
| Non-Hodgkin's lymphoma | 0 (0.0) | 5 (10.0) | |
| Stem cell source | .45 | ||
| Peripheral blood | 10 (100.0) | 43 (86.0) | |
| Bone marrow | 0 (0.0) | 3 (6.0) | |
| Umbilical cord blood | 0 (0.0) | 4 (8.0) | |
| HLA-matching | .14 | ||
| Mismatched | 2 (20.0) | 26 (52.0) | |
| Matched | 8 (80.0) | 24 (48.0) | |
| Donor type | .73 | ||
| Related | 6 (60.0) | 27 (54.0) | |
| Unrelated | 4 (40.0) | 23 (46.0) | |
| Conditioning regimen | .64 | ||
| Myeloablative | 5 (50.0) | 29 (58.0) | |
| Nonmyeloablative | 5 (50.0) | 21 (42.0) | |
| Treatment with ATG | .90 | ||
| Yes | 3 (30.0) | 16 (32.0) | |
| No | 7 (70.0) | 34 (68.0) | |
| CMV serostatus | .28 | ||
| D+/R+ | 6 (60.0) | 24 (48.0) | |
| D−/R+ | 3 (30.0) | 25 (50.0) | |
| D+/R− | 1 (10.0) | 1 (2.0) | |
| Acute graft-vs-host disease | .83 | ||
| No | 7 (70.0) | 27 (54.0) | |
| Grade I | 1 (10.0) | 8 (16.0) | |
| Grade II | 1 (10.0) | 8 (16.0) | |
| Grades III and IV | 1 (10.0) | 7 (14.0) | |
| Receipt of corticosteroids ≥0.5 mg/kg | .23 | ||
| Yes | 2 (20.0) | 20 (40.0) | |
| No | 8 (80.0) | 30 (60.0) | |
Abbreviations: ATG, antithymocyte globulin; CMV, cytomegalovirus; D, donor; HLA, human leukocyte antigen; R, recipient.
a The patient who developed recurrent CMV DNAemia was excluded from this analysis.
b χ2 test for discrete variables and Mann-Whitney U test for continuous variables.
Table 3.
Risk Factors for Recurrent CMV DNAemiaa
| Factor | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Sex (male vs female) | 0.76 (.40–1.44) | .76 | ||
| Age (>60 y) | 1.64 (.71–3.79) | .25 | ||
| HLA-matching (mismatched vs matched) | 1.50 (.74–3.03) | .26 | ||
| Stem cell source | ||||
| Umbilical cord blood vs peripheral blood | 8.51 (1.06–68.18) | .04 | 3.61 (.37–35.37) | .27 |
| Bone marrow vs peripheral blood | 3.29 (.82–13.15) | .09 | 2.15 (.54–8.63) | .28 |
| Acute graft-vs-host disease (grades II to IV) | 1.33 (.73–2.44) | .36 | ||
| CMV-specific T CD8+ cells >1 cell/µLb | 0.31 (.09–1.06) | .06 | 0.34 (.1–1.18) | .09 |
| Combined virological and immunological strategy (study group) vs virological monitoring (control group) | 0.61 (.08–4.87) | .64 | ||
Abbreviations: ATG, antithymocyte globulin; CI, confidence interval; CMV, cytomegalovirus; HLA, human leukocyte antigen; OR, odds ratio.
a Conditional logistic regression analysis.
b At day +28 after initiation of therapy irrespective of the presence or absence of detectable plasma CMV DNA. Conditional logistic regression models adjusting for baseline characteristics: type of donor (related/unrelated), donor and recipients CMV serostatus, type on conditioning and treatment with ATG.
Incidence of Cytomegalovirus End-Organ Disease
Three cases of CMV end-organ disease (gastrointestinal) were diagnosed, 1 in each comparison (sub)group-A1, A2, and B (P = .35).
Duration of Preemptive Antiviral Treatment
The length of antiviral treatment courses did not differ significantly between groups A (median, 28 days; range, 12–77 days) and B (median, 31 days; range, 11–97) (P = .17); nevertheless, it was significantly (P = .003) shorter in subgroup A1 patients (median, 22 days; range, 12–31 days) than in group B (median, 31 days; range, 11–97 days).
Hematological Toxicity
The incidence of moderate (<1.0 × 109/L) and severe (<0.5 × 109/L) neutropenia and thrombocytopenia (<0.5 × 109/L) after engraftment was evaluated on day +28 after antiviral treatment inception, because the average time between the introduction of GCV and the occurrence of neutropenia is approximately 30 days [21, 22]. At this time point, 10%, 25%, and 12.2% of patients in subgroups A1 and A2 and group B, respectively, had moderate neutropenia (P = .12), whereas 0%, 10.6%, and 2.0%, respectively, had severe neutropenia (P = .80). In turn, severe thrombocytopenia was seen in 11.1%, 31.9%, and 36% of patients in the A1, A2, and B groups, respectively (P = .34). As shown in Table 4, neither neutrophil nor platelets cell counts differed significantly between comparison (sub)groups.
Table 4.
Neutrophil and Platelet Cell Counts on Day +28 After Initiation of Antiviral Therapy
| Groups Compared | Parameter; Median (Range) |
P Valuea | |
|---|---|---|---|
| 1 | 2 | ||
| Study (1) vs matched controls (2) | |||
| Neutrophils count (109/µL) | 1.86 (0.00–14.50) | 1.90 (0.40–18.80) | .46 |
| Platelet count (109/µL) | 80.50 (12–433) | 72 (10–336) | .55 |
| Strategy applied (1) vs matched controls (2) | |||
| Neutrophils count (109/µL) | 1.27 (0.63–6.10) | 1.90 (0.40–18.80) | .12 |
| Platelet count (109/µL) | 114 (12–234) | 72 (10–336) | .27 |
a Wilcoxon test for matched groups and Mann-Whitney U test for unpaired groups.
DISCUSSION
We report here on the results of a multicenter, matched-control study to evaluate a novel preemptive antiviral therapy strategy for active CMV infection in Allo-SCT recipients guided by a combined virological and immunological monitoring approach. The incidence of recurrent CMV DNAemia within 2 months after antiviral treatment cessation was chosen as the primary endpoint of the study. It must be stressed in this context that the overall incidence of recurrent CMV DNAemia within the first 3 months after transplant is high, varying approximately between 35% and 70%, depending upon the patient's risk factor constellation ([6] and unpublished observations). In addition, in our experience, most episodes of recurrent viremia develop within the next 2 months after clearance of the initial episode (unpublished observation). The data indicate that this novel strategy had a major impact both on the incidence of recurrent CMV DNAemia episodes and on the length of antiviral treatment course (secondary endpoint) in a subset of patients. In effect, the cumulative incidence of recurrent CMV DNAemia was 9% in patients who met the criteria for treatment interruption (A1), whereas it was approximately 40% in the comparative (sub)groups. Recurrent viremia was rarely observed in patients in subgroup A1 (1 of 11), even in those (n = 2) who were unable to maintain CMV-specific CD8+ T-cell levels >1 cell/µL over time, suggesting that the magnitude of the expansion of these functional T cells upon viral replication within the first episodes of viremia modulates the risk of recurrent viremia. Regrettably, a low number of patients (18%) could benefit from this novel therapeutic scheme. This was not entirely unexpected, considering previous observations made by ours and other groups [6, 12–14, 23–25]. Patients displaying 1 or more of the following conditions were overrepresented in the A1 subgroup: (1) CMV-seropositive donor, (2) receipt of an HLA-matched allograft, (3) no treatment with corticosteroids at doses ≥0.5 mg/kg. These patients are particularly prone to reconstitution of CMV-specific T-cell immunity relatively early after engraftment [6, 12, 14, 23–25]. In turn, the negative impact of corticosteroids on the reconstitution of T-cell immunity in Allo-SCT recipients has been conclusively proven [14, 23–25]. Conditional logistic regression analyses revealed a trend towards an association between the presence of CMV-specific IFN-γ-producing CD8+ T-cell counts >1 cell/µL at day +28, irrespective of the plasma CMV DNA load, and a reduced risk of recurrent CMV DNAemia. The incidence of CMV end-organ disease was low in all comparative groups. In fact, only 1 case was diagnosed in each group. For the case registered in the A1 subgroup, it is noteworthy to mention that it developed in the setting of a severe aGvHD treated with corticosteroids at high doses (occurring 3 weeks after antiviral treatment interruption), and that at the time of diagnosis, no traces of functional CMV-specific CD8+ T cells were detected. In our opinion, the occurrence of CMV disease in this patient cannot be causally linked to the implementation of the novel strategy.
Patients in the A1 subgroup received shorter courses of antiviral therapy in comparison with those in the matched-control group (a median of 1 week shorter). Understandably, this shortening was likely insufficient to result in tangible benefits in terms of hematological toxicity, which we found to develop at comparable frequencies in patients in the comparison groups.
The current study has several limitations that deserve comment. A major one is inherent in case-matched control studies, namely, the enormous difficulty in recruiting exact matches. Moreover, the retrospective selection of matched controls may introduce bias in either direction. In this context, we felt that conducting a randomized clinical trial was premature in light of the data reported in our proof-of-concept study [17]. Other limitations are the following: (1) real-time PCR assays with different limits of detection (sensitivity) were used, and some episodes of low-level CMV DNAemia that resolved spontaneously could have been missed at some centers; (2) no homogeneous criteria existed across centers for both antiviral treatment inception (variable CMV DNA loads triggering the initiation of therapy) and interruption. To some extent, this undermines the robustness of our analyses addressing the duration of antiviral therapy courses in patients in the comparison groups.
CONCLUSIONS
In summary, the data presented herein support the feasibility and potential clinical utility of combining immunological and virological monitoring for the management of CMV infection in a subset of Allo-SCT recipients (low-intermediate risk). Nevertheless, conducting a randomized controlled clinical trial seems to be the next logical step in validating the results reported herein.
Acknowledgments
We are indebted to the Residents from the Microbiology Service of Hospital Clínico Universitario and the staff from the Hematology Units of the participating centers for their cooperation. We thank Prof. José María Aguado and Dr. Mario Fernández-Ruiz for helpful comments. Paula Amat was a research fellow of the Asociación Española contra el Cáncer.
Financial support. This research was funded by a grant (EC10-317) from the Program “Fomento de la Investigación Clínica Independiente” from Ministerio de Sanidad, Política Social e Igualdad, Spain).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 2009; 113:5711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungman P, de la Camara R, Cordonnier C et al. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant 2008; 42:227–40. [DOI] [PubMed] [Google Scholar]
- 3.Tomblyn M, Chiller T, Einsele H et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez Romero P, Blanco P, Giménez E et al. An update on the management and prevention of cytomegalovirus infection following allogeneic hematopoietic stem cell transplantation. Future Virol 2015; 10:113–34. [Google Scholar]
- 5.Solano C, de la Cámara R, Vázquez L et al. Cytomegalovirus infection management in allogeneic stem cell transplant recipients: a National Survey in Spain. J Clin Microbiol 2015; 53:2741–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solano C, Muñoz-Cobo B, Giménez E et al. Pre-emptive antiviral therapy for active CMV infection in adult allo-SCT patients guided by plasma CMV DNAemia quantitation using a real-time PCR assay: Clinical experience at a single center. Bone Marrow Transplant 2013; 48:1010–2. [DOI] [PubMed] [Google Scholar]
- 7.Boeckh M, Nichols WG, Chemaly RF et al. Valganciclovir for the prevention of complications of late cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a randomized trial. Ann Intern Med 2015; 162:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinnan GV, Kirmani N, Rook AH et al. Cytotoxic T cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med 1982; 307:7–13. [DOI] [PubMed] [Google Scholar]
- 9.Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 1991; 78:1373–80. [PubMed] [Google Scholar]
- 10.Riddell SR, Watanabe KS, Goodrich JM et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 1992; 257:238–41. [DOI] [PubMed] [Google Scholar]
- 11.Li CR, Greenberg PD, Gilbert MJ et al. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood 1994; 83:1971–9. [PubMed] [Google Scholar]
- 12.Einsele H, Roosnek E, Rufer N et al. Infusion of cytomegalovirus (CMV)-specific T-cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002; 99:3916–22. [DOI] [PubMed] [Google Scholar]
- 13.Tormo N, Solano C, Benet I et al. Lack of prompt expansion of cytomegalovirus pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells is associated with rising levels of pp65 antigenemia and DNAemia during pre-emptive therapy in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2010; 45:543–9. [DOI] [PubMed] [Google Scholar]
- 14.Tormo N, Solano C, Benet I et al. Kinetics of cytomegalovirus (CMV) pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells during episodes of viral DNAemia in allogeneic stem cell transplant recipients: potential implications for the management of active CMV infection. J Med Virol 2010; 82:1208–15. [DOI] [PubMed] [Google Scholar]
- 15.Ljungman P. Would monitoring CMV immune responses allow improved control of CMV in stem cell transplant patients. J Clin Virol 2006; 35:493–5. [DOI] [PubMed] [Google Scholar]
- 16.Avetisyan G, Aschan J, Hägglund H et al. Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT. Bone Marrow Transplant 2007; 40:865–9. [DOI] [PubMed] [Google Scholar]
- 17.Solano C, Benet I, Remigia MJ et al. Immunological monitoring for guidance of preemptive antiviral therapy for active cytomegalovirus infection in allogeneic stem-cell transplant recipients: a pilot experience. Transplantation 2011; 92:e17–20. [DOI] [PubMed] [Google Scholar]
- 18.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34:1094–7. [DOI] [PubMed] [Google Scholar]
- 19.Solano C, Benet I, Clari MA et al. Enumeration of cytomegalovirus-specific interferongamma CD8+ and CD4+ T cells early after allogeneic stem cell transplantation may identify patients at risk of active cytomegalovirus infection. Haematologica 2008; 93:1434–6. [DOI] [PubMed] [Google Scholar]
- 20.San-Juan R, Navarro D, García-Reyne A et al. Effect of long-term prophylaxis in the development of cytomegalovirus-specific T-cell immunity in D+/R− solid organ transplant recipients. Transpl Infect Dis 2015; 17:637–46. [DOI] [PubMed] [Google Scholar]
- 21.Salzberger B, Bowden RA, Hackman RC et al. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood 1997; 90:2502–8. [PubMed] [Google Scholar]
- 22.Venton G, Crocchiolo R, Fürst S et al. Risk factors of ganciclovir-related neutropenia after allogeneic stem cell transplantation: a retrospective monocentre study on 547 patients. Clin Microbiol Infect 2014; 20:160–6. [DOI] [PubMed] [Google Scholar]
- 23.Hakki M, Riddell SR, Storek J et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood 2003; 102:3060–7. [DOI] [PubMed] [Google Scholar]
- 24.Lilleri D, Fornara C, Chiesa A et al. Human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in adult allogeneic hematopoietic stem cell transplant recipients and immune control of viral infection. Haematologica 2008; 93:248–53. [DOI] [PubMed] [Google Scholar]
- 25.Tormo N, Solano C, Benet I et al. Reconstitution of CMV pp65 and IE-1-specific IFN-γ CD8(+) and CD4(+) T-cell responses affording protection from CMV DNAemia following allogeneic hematopoietic SCT. Bone Marrow Transplant 2011; 46:1437–43. [DOI] [PubMed] [Google Scholar]