Abstract
Background. In May 2013, a wild poliovirus type 1 (WPV1) outbreak reported in Somalia provided an opportunity to examine the contribution of testing contacts to WPV detection.
Methods. We reviewed acute flaccid paralysis (AFP) case-patients and linked contacts reported in the Somalia Surveillance Database from May 9 to December 31, 2013. We restricted our analysis to AFP case-patients that had ≥3 contacts and calculated the contribution of each contact to case detection.
Results. Among 546 AFP cases identified, 328 AFP cases had ≥3 contacts. Among the 328 AFP cases with ≥3 contacts, 93 WPV1 cases were detected: 58 cases (62%; 95% confidence interval [CI], 52%–72%) were detected through testing stool specimens from AFP case-patients; and 35 cases (38%; 95% CI, 28%–48%) were detected through testing stool specimens from contacts, including 19 cases (20%; 95% CI, 14%–30%) from the first contact, 11 cases (12%; 95% CI, 7%–20%) from the second contact, and 5 cases (5%; 95% CI, 2%–12%) from the third contact. Among the 103 AFP cases with ≥4 contacts, 3 (6%; 95% CI, 2%–16%) of 52 WPV1 cases were detected by testing the fourth contact. No additional WPV1 cases were detected by testing >4 contacts.
Conclusions. Stool specimens from 3 to 4 contacts of persons with AFP during polio outbreaks are needed to maximize detection of WPV cases.
Keywords: acute flaccid paralysis, contact stool specimens, eradication, polio, Somalia
In 1988, the World Health Assembly (WHA) of the World Health Organization (WHO) resolved to interrupt wild poliovirus (WPV) transmission worldwide and launched the Global Polio Eradication Initiative (GPEI) [1]. In 2012, the WHA declared the completion of polio eradication a programmatic emergency for public health [2], and it reported that annual WPV cases increased from 223 in 2012 to 416 in 2013 [3], an increase partly caused by several large outbreaks in previously polio-free countries in the Horn of Africa, Middle East, and Central Africa [3–8]. On May 9, 2013, a WPV type 1 (WPV1) case, genetically linked to WPV in Nigeria, was confirmed in a 32-month-old girl from Mogadishu, which is located in the Banadir Region of Somalia; the ensuing outbreak ultimately spread into 2 additional countries in the Horn of Africa [9, 10]. Before this outbreak, Somalia had been polio-free since March 2007 [5, 11]. We reviewed acute flaccid paralysis (AFP) surveillance data from the 2013 polio outbreak in Somalia to examine the added contribution of WPV detection from stool specimens collected from contacts of persons with AFP.
METHODS
Acute Flaccid Paralysis Surveillance System
During 2013, AFP cases in Somalia were detected by passive weekly reporting from approximately 500 designated reporting sites across the country. In addition, active surveillance is conducted by an extensive network of trained locally recruited polio officers and volunteers supervised by international technical staff [12] who visit health facilities and communities to conduct active searches for AFP cases. An AFP case was defined as (1) any occurrence of acute onset flaccid paralysis in a child <15 years of age or (2) any case of paralytic illness in a person of any age in whom poliomyelitis is suspected. An AFP contact was defined as a child <15 years of age residing in the same household or neighboring households, and who has been in direct contact with the AFP case-patient at any time from 1 week before the onset of paralysis up to 2 weeks after onset of paralysis [13]. Two stool specimens of adequate volume (8–10 grams) were collected from each AFP case-patient within 14 days of paralysis onset and ≥24 hours apart. Attempts were made to collect stool specimens from ≥3 AFP case contacts. All stool specimens were transported to the Kenya Medical Research Institute polio laboratory for virologic diagnosis as per GPEI recommendation [14, 15]. An AFP case was confirmed to be polio if WPV1 was isolated from stool specimens of either the AFP case-patient or any of their contacts.
Statistical Methods
We reviewed epidemiological and laboratory information for AFP cases and linked contacts reported during the polio outbreak in Somalia from May 9 to December 31, 2013. Data from case investigation forms and laboratory results of all AFP cases and contacts were obtained from an EpiData software surveillance database maintained by the WHO Somalia office in Nairobi [16]. We combined all WPV cases (identified by either AFP case or linked contact) and linked contacts (WPV positive and/or negative) into a dataset for analysis. We estimated the added contribution of each contact specimen using a method similar to that described by Gary et al [17], which derives maximum-likelihood estimates for specimen sensitivities of each stool specimen. This method has previously been used to assess sensitivities of the first and second stool specimens collected from AFP case-patients, by assuming each stool specimen to be independent of each other; therefore, each stool specimen functions as the gold standard estimate for the other specimen [18, 19].
The analysis was among AFP cases with at least 3 contacts. To determine the incremental contribution of each contact, we further restricted our analysis to the contribution of the first, second, third, and fourth contact. We created 2 subsets: (1) AFP cases that had ≥3 contacts and (2) AFP cases that had ≥4 contacts. All stool specimens within each subset were mutually exclusive. The total number of WPV-positive stools (ie, including those identified from testing AFP case-patients or any of their case-contacts) was the denominator for each subset. We calculated the number and percentage of WPV cases identified by testing (1) AFP case-patients and (2) case-contacts for the specified contact number (first, second, third, and fourth). We were unable to do statistical tests of significance comparing the contributions of third and fourth contact stool because the analysis of those with ≥4 contacts is not independent of the analysis of those with ≥3 contacts (ie, stools of AFP case-patients with ≥4 contacts are a subset of those with ≥3 contacts).
To demonstrate any potential increase in case detection after testing >4 contacts, we determined the proportion of WPV-positive stool specimens provided by AFP case-patients or case-contacts for the first through the sixth contact. However, denominators for each calculation are not constant, and the proportions are not independent; therefore, they do not add up to the overall total.
The added contribution of each contact specimen is presented with Wilson score 95% confidence intervals (CIs). Data were analyzed using SAS version 9.3 (SAS Institute Inc.) and R version 2.12.1 (R Foundation, Vienna, Austria). Indicators of stool adequacy, timeliness of specimen collection, condition of arrival at laboratory, and median time from collection of first and second AFP stool specimen were also calculated.
RESULTS
Of the 546 reported AFP case-patients during the 2013 polio outbreak in Somalia, 479 (88%) were children aged <5 years; the median age was 24 months (range, 1 month–40 years), and 324 (59%) were male. Two adequate specimens were collected for 545 (99.8%) AFP cases, 540 (99.1%) had an interval of 24–48 hours from collection of specimen 1 to collection of specimen 2, and 543 (99.6%) had 2 stool specimens arrive in the laboratory in good condition. The first specimen was collected within a median of 6 days after paralysis onset (range, 0–138 days), and the second stool specimen was collected within a median of 7 days (range, 1–139 days) after paralysis onset. The interval between the 2 specimens ranged from 1 to 4 days. The median interval from onset of paralysis to arrival of the second stool specimen to the laboratory was 13 days (range, 2–144 days).
Among the 546 AFP cases, 41 (7.5%) had a single contact tested, 49 (9.0%) had 2 contacts, 225 (41.2%) had 3 contacts, 31 (5.7%) had 4 contacts, 56 (10.3%) had 5 contacts, 16 (2.9%) had 6 or more contacts, and 128 (23.4%) had no contact stool specimens. In total, 1738 AFP contact stool specimens were collected; 1581 (90.9%) of contact stool specimens were collected within 7 days of onset of paralysis in linked AFP cases. The majority, 1165 (67%), of AFP case contacts were children <5 years old; the median age was 36 months (range, 1 month–83 years), and 1172 (67.5%) were male.
Wild poliovirus type 1 cases were detected during April–October 2013, and they peaked in June when collection of stool specimen from contacts also peaked (Figure 1). Of the 194 WPV1 cases identified during the 2013 polio outbreak in Somalia, 145 (74.7%) were detected through testing stool specimens collected from AFP case-patients, and 49 (25.3%) were detected only through testing stool specimens collected from AFP case contacts. For the 145 WPV1 cases identified by testing stool specimens collected from AFP case-patients only, the median interval from onset of paralysis to arrival of the second stool specimen to the laboratory was 13 days (range, 2–49 days). The median interval from onset of paralysis to arrival of the second AFP stool specimen in the laboratory for WPV1 cases identified by testing contact stool specimens only was 16 days (range, 5–48 days).
Figure 1.
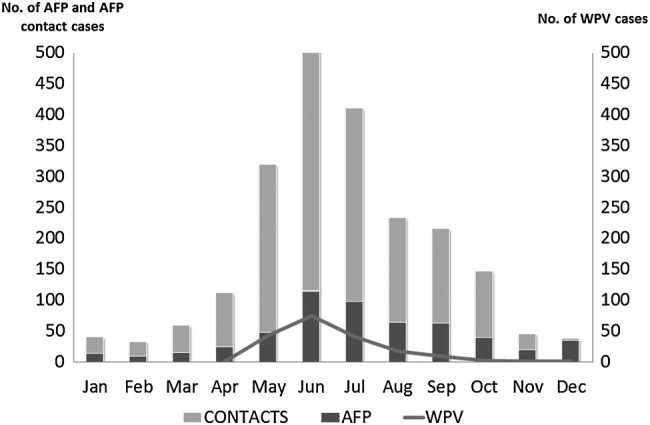
Acute flaccid paralysis (AFP) cases, AFP case-contacts, and confirmed wild poliovirus (WPV) cases reported, by month—Somalia, 2013.
Of the 546 AFP case-patients, 328 (60%) had ≥3 contacts. Testing stool specimens from these AFP case-patients and their contacts identified a total of 93 WPV1 cases. Of these, 58 (62%; 95% CI, 52%–72%) WPV1 cases were detected by testing stool specimens from AFP case-patients, 19 (20%; 95% CI, 14%–30%) cases were detected by testing the first contact, 11 (12%; 95% CI, 7%–20%) cases were detected by testing the second contact, and 5 (5%; 95% CI, 2%–12%) cases were detected by testing the third contact. Cumulatively, testing 3 case-contacts detected 35 (38%; 95% CI, 28%–48%) additional WPV1 cases (Table 1).
Table 1.
Proportion of Positive Stools Detected From Testing Stool Specimens From AFP Case-Patients With at Least Three Case-Contacts, Somalia 2013 (n = 93)
| Stool Specimen Source | Number WPV Detected | %WPV Detected | 95% CI |
|---|---|---|---|
| Positive AFP case | 58 | 62.4 | 52–72 |
| 1 | 19 | 20.4 | 14–30 |
| 2 | 11 | 11.8 | 7–20 |
| 3 | 5 | 5.4 | 2–12 |
| Total positive | 93 | 100 |
Abbreviations: AFP, acute flaccid paralysis; CI, confidence interval; WPV, wild poliovirus.
Of the 546 AFP cases, 103 (19%) had ≥4 contacts. Testing stool specimens from these AFP case-patients and their contacts identified a total of 52 WPV1-positive stools. Of these, 29 (56%; 95% CI, 42%–68%) WPV1 cases were detected only by testing AFP stool specimens, 9 (17%; 95% CI, 9%–30%) cases were detected by testing the first contact, 7 (13%; 95% CI, 7%–25%) cases were detected by testing the second contact, 4 (8%; 95% CI, 3%–18%) cases were detected by testing the third contact, and 3 (6%; 95% CI, 2%–16%) cases were detected by testing the forth contact. Cumulatively, testing 4 case-contacts detected 23 (44%; 95% CI, 32%–58%) additional WPV1 cases (Table 2).
Table 2.
Proportion of Positive Stools Detected From Testing Stool Specimens From AFP Case-Patients With at Least 4 Case-Contacts, Somalia 2013 (n = 52)
| Stool Specimen Source | Number WPV Detected | % WPV Detected | 95% CI |
|---|---|---|---|
| Positive AFP case | 29 | 55.8 | 42–68 |
| 1 | 9 | 17.3 | 9–30 |
| 2 | 7 | 13.5 | 7–25 |
| 3 | 4 | 7.7 | 3–18 |
| 4 | 3 | 5.8 | 2–16 |
| Total positive | 52 | 100 |
Abbreviations: AFP, acute flaccid paralysis; CI, confidence intervals; WPV, wild poliovirus.
Among the 49 WPV cases detected only by testing contact stool specimens, 25 (18%; 95% CI, 13%–26%) were detected by testing the first contact, 13 (11%; 95% CI, 7%–18%) were detected by the second contact, 5 (5%; 95% CI, 2%–12%) were detected by the third contact, 3 (6%; 95% CI, 2%–16%) were detected by the fourth contact, and none were detected by the fifth and sixth contacts (Table 3). Three WPV cases were detected by testing >6 AFP case-contacts and were excluded from analysis.
Table 3.
Proportion of Cases Detected From Testing Stool Specimens From AFP Case-Patients and Contacts, Somalia 2013 (N = 418)
| Number of Contacts (K) | Number Positive Stools Detected by AFP Case-Patient or Kth Contact | Number WPV Detected by Kth Contact Onlya | %WPV Detected by Kth Contact Only | 95% CI |
|---|---|---|---|---|
| 1 | 137 | 25 | 18.2 | 12.7–25.6 |
| 2 | 114 | 13 | 11.4 | 6.8–18.5 |
| 3 | 93 | 5 | 5.4 | 2.3–12.0 |
| 4 | 52 | 3 | 5.8 | 2.0–15.6 |
| 5 | 36 | 0 | 0.0 | .0–9.6 |
| 6 | 10 | 0 | 0.0 | .0–27.8 |
Abbreviations: AFP, acute flaccid paralysis; CI, confidence interval; K, number of specified contacts; WPV, wild poliovirus.
a Three WPV cases were detected by testing >6 AFP case-contacts and were excluded from analysis.
During 2012, the nonpolio AFP (NPAFP) rate was 2.9 in 2012, the year before the outbreak began, and there were 2 polio-compatible cases reported that year. The NPAFP rate was 6.4 and 7.4 in 2013 and 2014, respectively. There were 15 and 4 polio-compatible cases reported during 2013 and 2014, respectively. There is no environmental surveillance in Somalia.
DISCUSSION
This analysis demonstrates that testing of stool specimens from contacts of AFP case-patients increased WPV case detection during the polio outbreak in Somalia from May 2013 to December 2013. In addition to an overall increase in sensitivity by contact sampling, the first confirmed WPV cases in Central and South regions of Somalia were detected by contact sampling alone, which enabled initiation of targeted outbreak response vaccination. Collecting the first and second contact stool specimens had the greatest impact on the proportion of WPV cases detected with diminishing contribution detected by the third and subsequent contact stool specimens. This is because as more specimens are collected, it is more likely that 1 of the preceding stools was positive, diminishing the contribution of the contact in question. Our findings support the existing policy in the Eastern Mediterranean Region of WHO to collect 1 stool specimen from ≥3 contacts of every AFP case-patient to improve WPV detection [20, 21]. Contact stools should be collected within 2 months from the date of onset of paralysis in the AFP case-patient.
The proportion of WPV1 cases identified by testing contacts during this outbreak is higher than previously reported from the Americas region, where testing 5 contact stool specimens for every APF case resulted in an increased WPV case detection of 10% in the period immediately before WPV circulation was interrupted [22, 23]. However, testing any contacts was later discontinued because the number of polio cases declined in the region, and the increased sensitivity provided by contact sampling did not justify the required resources. Routine testing of stool specimen from all AFP cases has proven to be an effective strategy to detect polio cases in South Sudan and Somalia, which have hard-to-reach areas [20].
Collecting contact stool specimens increases case detection, but the contribution of testing additional contacts should be balanced with the associated increased testing burden on the surveillance system. In addition, increased laboratory workload caused by testing many contact stool specimens might potentially reduce laboratory efficiency and result in a delay in processing other programmatically important specimens. In this outbreak, testing of 3 contacts identified 94% of WPV1 cases, and testing of 4 contacts identified 100% of WPV1 cases. However, the sample sizes for AFP cases with 5 or 6 contact stool specimens were too small to draw meaningful conclusions. Some laboratories often test pools of specimens from contacts of a single AFP case-patient to reduce laboratory work and increase timeliness of reporting poliovirus testing results [24]. However, this was not done during this outbreak.
A limitation of our analysis is that no statistical test comparing the contribution of each contact with the one preceding it could be applied in our calculations because contact stool specimens used in each successive calculation were a subset of the previous contacts and overlapped. For example, case-patients with 4 contacts were also included in the analysis for 3 contacts. Therefore, our evaluation measured the proportion of WPV cases detected by each contact stool specimen in the order recorded by the polio staff. Moreover, AFP cases had different numbers of case contacts and the contribution could not be compared.
CONCLUSIONS
Multiple outbreaks caused by importation of poliovirus in polio-free countries have reinforced the need to maintain effective AFP surveillance to detect and guide an effective public health response to poliovirus importations [5, 7]. In Somalia, the high proportion of stool specimens arriving in the laboratory in good condition within 24–48 hours after collection demonstrates a strong commitment to high-quality AFP surveillance by public and private health practitioners, polio volunteers, and traditional healers, despite the challenges of conflict and insecurity. Such commitments to maximize the quality and sensitivity of AFP surveillance systems in the remaining polio-endemic countries and countries at high risk for reinfection are important to limit poliovirus spread from importations.
Acknowledgments
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Aylward RB, Alwan A. Polio in Syria. Lancet 2014; 383:489–91. [DOI] [PubMed] [Google Scholar]
- 2.Arie S. Polio virus spreads from Syria to Iraq. BMJ 2014; 348:g2481. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). Notes from the field: outbreak of poliomyelitis--Somalia and Kenya, May 2013. MMWR Morb Mortal Wkly Rep 2013; 62:484. [PMC free article] [PubMed] [Google Scholar]
- 4.Mahamud A, Kamadjeu R, Webeck J et al. . Effectiveness of oral polio vaccination against paralytic poliomyelitis: a matched case-control study in Somalia. J Infect Dis 2014; 210(suppl 1):S187–93. [DOI] [PubMed] [Google Scholar]
- 5.Moturi EK, Porter KA, Wassilak SG et al. . Progress toward polio eradication -- worldwide, 2013–2014. MMWR Morb Mortal Wkly Rep 2014; 63:468–72. [PMC free article] [PubMed] [Google Scholar]
- 6.Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol 2010; 172:1213–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polio: Global Eradication Initiative . Data and monitoring. Available at: http://www.polioeradication.org/dataandmonitoring.aspx. Accessed 16 November 2015.
- 8.Nathanson N, Martin JR. The epidemiology of poliomyelitis: enigmas surrounding its appearance, epidemicity, and disappearance. Am J Epidemiol 1979; 110:672–92. [DOI] [PubMed] [Google Scholar]
- 9.Burki TK. Somalia: a gathering storm? Lancet 2013; 382:1237–8. [DOI] [PubMed] [Google Scholar]
- 10.Kamadjeu R, Assegid K, Naouri B et al. . Measles control and elimination in Somalia: The good, the bad, and the ugly. J Infect Dis 2011; 204(suppl 1):S312–7. [DOI] [PubMed] [Google Scholar]
- 11.Grünewald F. Aid in a city at war: the case of Mogadishu, Somalia. Disasters 2012; 36(suppl 1):S105–25. [DOI] [PubMed] [Google Scholar]
- 12.WHO Somalia. AFP Surveillance Handbook, Somalia. 2012. [Google Scholar]
- 13.World Health Organization: Regional Office for the Eastern Mediterranean. Intercountry meeting of the Chairpersons of National Committees for Certification of Poliomyelitis Eradication, 2010. Available at: http://applications.emro.who.int/docs/who_em_pol_387_e_en.pdf?ua=1 Accessed 9 June 2016.
- 14.Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. Chapter 8: Meningococcal Disease. 2012. Available at: http://www.cdc.gov/vaccines/pubs/surv-manual/chpt08-mening.html. Accessed 9 June 2016. [Google Scholar]
- 15.World Health Organization. Polio laboratory manual. Available at: http://apps.who.int/iris/bitstream/10665/68762/1/WHO_IVB_04.10.pdf. Accessed 9 June 2016.
- 16.Birmingham ME, Linkins RW, Hull BP, Hull HF. Poliomyelitis surveillance: the compass for eradication. J Infect Dis 1997; 175(suppl 1):S146–50. [DOI] [PubMed] [Google Scholar]
- 17.Gary HE, Sanders R, Pallansch MA. A theoretical framework for evaluating the sensitivity of surveillance for detecting wild poliovirus: II. Factors affecting detection sensitivity in a population with circulating wild poliovirus. J Infect Dis 1997; 175(suppl):S141–5. [DOI] [PubMed] [Google Scholar]
- 18.Cardemil CV, Rathee M, Gary H et al. . Surveillance during an era of rapidly changing poliovirus epidemiology in India: the role of one vs. two stool specimens in poliovirus detection, 2000–2010. Epidemiol Infect 2014; 142:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohler KA, Deshpande JM, Gary HE Jr et al. . Contribution of second stool specimen to increased sensitivity of poliovirus detection in India, 1998–2000. Epidemiol Infect 131:711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Technical Advisory Group on Polio Eradication for the Horn of Africa Countries. 9th Meeting Report. Available at: http://www.polioeradication.org/Portals/0/Document/Aboutus/Governance/IMB/9IMBMeeting/11.2_9IMB.pdf. Accessed 9 June 2016. [Google Scholar]
- 21.World Health Organization: Regional Office for the Eastern Mediterranean. Regional Committee for the Eastern Mediterranean . Progress on Polio Eradication. Available at: http://applications.emro.who.int/docs/EM_RC57_inf_doc_1_en.pdf?ua=1. Accessed June 9, 2016.
- 22.de Quadros CA, Hersh BS, Olive J-M, Andrus JK, da Silveira CM, Carrasco PA. Eradication of wild poliovirus from the Americas: acute flaccid paralysis surveillance, 1988–1995. J Infect Dis 1997; 175(suppl 1):S37–42. [DOI] [PubMed] [Google Scholar]
- 23.Debanne SM, Rowland DY. Statistical certification of eradication of poliomyelitis in the America. Math Biosci 1998; 150:83–103. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Polio Laboratory Network Quarterly Updates, 2008; Available at: http://archives.who.int/vaccines/en/poliolab/1996/21.pdf. [Google Scholar]


