Abstract
Background. Viral loads (VLs) detectable at low levels are not uncommon in patients on combination antiretroviral therapy (cART). We investigated whether a single quantifiable VL predicted virological failure (VF).
Methods. We analyzed patients receiving standard regimens with at least 1 VL measurement below the limit of quantification (BLQ) in their treatment history. The first VL measurement after 6 months of unmodified cART served as baseline VL for the subsequent analyses of the time to reach single VL levels of ≥200, ≥400, and ≥1000 copies/mL. Roche TaqMan 2.0 was used to quantify human immunodeficiency virus-1 ribonucleic acid. Factors associated with VF were determined by Cox proportional hazards models.
Results. Of 1614 patients included in the study, 68, 44, and 34 experienced VF ≥200, ≥400, and ≥1000 copies/mL, respectively. In multivariable analyses, compared with patients who were BLQ, a detectable VL ≤ 50 and VL 51–199 copies/mL predicted VF ≥ 200 copies/mL (hazards ratio [HR] = 2.19, 95% confidence interval [CI] = 1.06–4.55 and HR = 4.21, 95% CI = 2.15–8.22, respectively). In those with VL 51–199 copies/mL, a trend for an increased risk of VF ≥400 and VF ≥1000 copies/mL could be found (HR = 2.13, 95% CI = 0.84–5.39 and HR = 2.52, 95% CI = 0.96–6.60, respectively).
Conclusions. These findings support closer monitoring and adherence counseling for patients with a single measurement of quantifiable VL <200 copies/mL.
Keywords: cART, HIV, low-level viremia, viral load, virological failure
The goal of combination antiretroviral therapy (cART) is to obtain and maintain viral suppression. Although guidelines have recommended viral loads (VLs) to be below the limit of quantification (BLQ) of clinically accessible assays [1–4], it remains unclear whether VLs detectable at low levels, typically below 200 copies/mL, are clinically important with regard to subsequent treatment failures. At the time VL reveals 51 copies/mL or more, it is uncertain how it will subsequently develop. It may possibly turn out to be only a viral blip, generally indicating episodes of transiently detectable VL above 50 copies/mL [3, 5] followed by a subsequent undetectable VL measurement, or persistent detectable VL [4] or even virological failure (VF). Definitions of VF vary between guidelines and, accordingly, some guidelines seem to be more conservative, recommending lower levels of VL (>50 copies/mL) to be reviewed due to a potential risk of rebound [1]. Others defined VF as having a VL of >200 copies/mL [2–4], and one guideline sets higher thresholds for defining VF, namely VL of >1000 copies/mL [5]. Despite several investigations that focused on consequences of persistent low-level viremia (LLV), which was determined by 2 or more VL measurements [6–12], studies considering 1 single VL measurement to be predictive of VF are very rare. Only 1 study observed patients with a single quantifiable VL below 50 copies/mL to be at higher risk of rebound of >50 and >400 copies/mL [13]. Different commercial techniques are able to detect VLs at low levels. It seems that low-level, positive VL results appear to be more common with some VL assays including the Roche Cobas AmpliPrep/Cobas TaqMan 2.0 [14–16]. However, this result needs to be confirmed.
In this study, we present data from a cohort of well defined human immunodeficiency virus (HIV)-infected patients on unmodified cART with standard regimens over a recent period of more than 6 months in Austria. The aim of the study was to evaluate the impact of LLV, measured by TaqMan 2.0 assay, and other factors that could increase risk of VF. Virological failure was defined as HIV ribonucleic acid (RNA) levels of ≥200, ≥400, and ≥1000 copies/mL.
PATIENTS AND METHODS
The Austrian HIV Cohort Study
The Austrian HIV Cohort Study (AHIVCOS) is an open, multicenter, prospective, observational cohort study of HIV-infected individuals observed at 7 HIV treatment centers in Austria. The study was initiated in 2001 as an incorporated association by representatives of 5 Austrian HIV treatments centers (AKH Vienna, Otto-Wagner-Hospital Vienna, AKH Linz, LKH Innsbruck, and LKH Graz West). In 2008, 2 additional HIV treatment centers (LKH Salzburg and LKH Klagenfurt) joined AHIVCOS, thus patients are currently enrolled actively and prospectively at 7 public hospital-based HIV treatment centers, which covers approximately 80% of all treated HIV-infected patients in Austria.
By July 1, 2014, AHIVCOS had included information on 8097 individuals. Demographic, clinical, laboratory, and treatment data have been collected prospectively starting at the time of inclusion into the cohort. Type of and changes in cART, including reasons for interruptions, are recorded, as well as the entire medication history including all drugs, duration of intake, and doses patients receive. Acquired immune deficiency syndrome events as well as coinfections, such as hepatitis B and C, syphilis, etc, were collected. Laboratory parameters were recorded continuously and measured in each single center.
Study Design
A retrospective analysis was conducted of individuals on cART who enrolled in AHIVCOS between July 1, 2012 and July 1, 2013. Approval for this study was obtained from the local ethical committees of the following participating centers: Vienna Medical University (No. 898/2010), City of Vienna (No. 12-216-VK/2013), Salzburg Federal Government (No. 1159/ 2010), Graz Medical University (No. 21-431/2010), Innsbruck Medical University (No. 283/4.4/2009), Upper Austria Federal Government (No. C-3-10/2010), and Carinthian Federal State (No. A-13-11/2011). The patients gave written informed consent to store their information in the hospital database and to use the data for research.
Inclusion Criteria
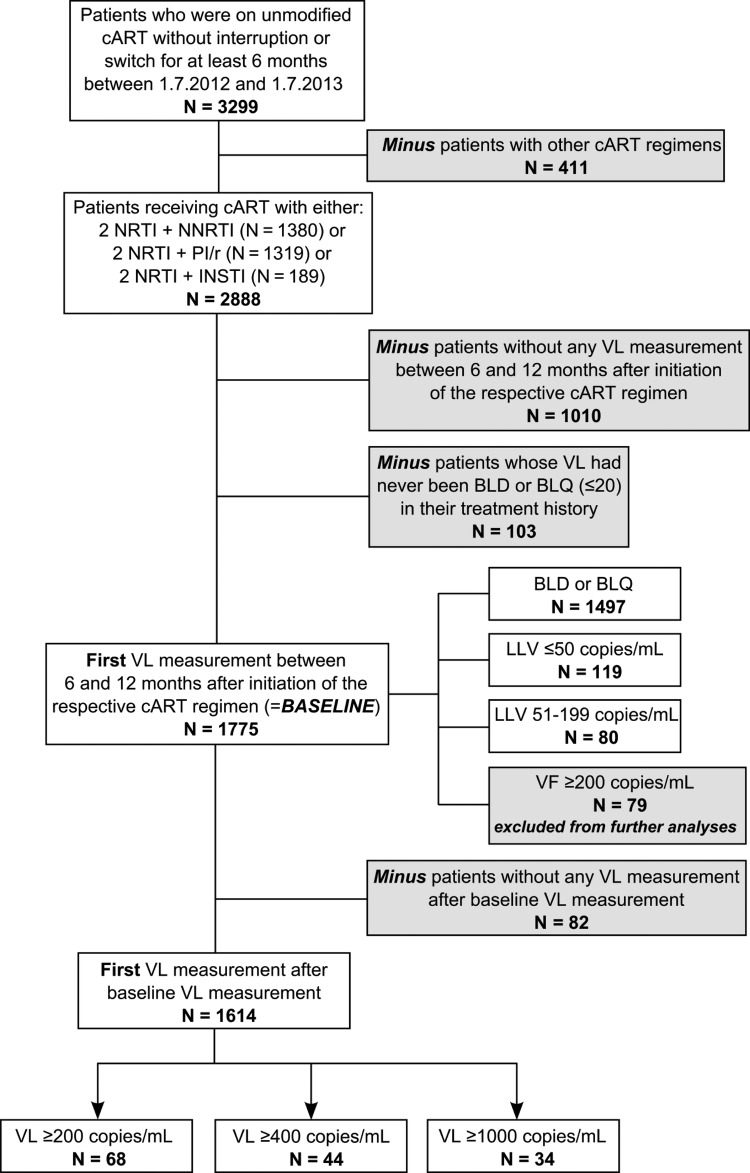
Patients with at least 1 VL measurement BLQ after initiating cART had to receive unmodified cART for more than 6 months between July 1, 2012 and July 1, 2013 (N = 3299), irrespective of therapies the patients might have taken before this period. Patients who were not on stable cART due to either interruptions or switches were excluded. During these 6 months, patients had to receive cART with 2 nucleoside reverse-transcriptase inhibitors (NRTIs) and either a non-NRTI (NNRTI) or a boosted protease inhibitor (PI/r) or an integrase inhibitor (INSTI). Individuals with other cART regimens than those mentioned above were excluded (N = 411). A total of 1010 patients did not have any VL measurement between 6 and 12 months after initiation of the respective cART regimen and were also excluded. We further excluded 103 individuals who never had VL BLQ. In the end, 1775 patients fulfilled the inclusion criteria, and their first VL measurement between 6 and 12 months after initiation of the respective cART regimen served as baseline for the analyses of the time to single HIV RNA levels of ≥200, ≥400, and ≥1000 copies/mL, respectively (Figure 1).
Figure 1.
Flowchart of inclusion criteria in patient selection. Abbreviations: BLD, below the limit of detection; BLQ, below the limit of quantification; cART, combination antiretroviral therapy; INSTI, integrase inhibitor; LLV, low-level viremia; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI/r, boosted protease inhibitor; VF, virological failure; VL, viral load.
In order to have a consistent lower limit of quantification of 20 copies/mL, 5 of 7 HIV treatment centers that measured VL by the Roche Cobas AmpliPrep/Cobas TaqMan 2.0 (Taqman, Roche Diagnostics, Mannheim, Germany) were included in the analysis. The remaining 2 centers used the Abbott RealTime HIV-1 assay (Abbott Diagnostics, Wiesbaden, Germany) and were excluded. The observation period ended in March 2014.
Definition of Low-Level Viremia and Virological Failure
All single quantifiable measurements of <200 copies/mL were classified as LLV. For further analyses, LLV was divided into 2 categories: HIV RNA levels of 51–199 copies/mL and HIV RNA levels of ≤50 copies/mL. Three definitions for VF were used: the “broad” definition reflects a single HIV RNA level of ≥200 copies/mL, the “restricted” definition includes a single HIV RNA level of ≥400 copies/mL, and the “stringent” definition uses a single HIV RNA level of ≥1000 copies/mL.
Statistical Methods
Baseline data are presented as number (%) or median (interquartile range [IQR]). Characteristics of patients who developed VF (defined as HIV RNA levels ≥200, ≥400, and ≥1000 copies/mL) and individuals who did not experience VF in each case were compared using χ² tests or Fisher's exact tests where appropriate for categorical data and non-parametric Wilcoxon rank-sum tests for continuous data. To assess the predictive value of LLV as well as the influence of various demographic and clinical parameters on the occurrence of VF, univariable and multivariable Cox proportional hazards regression models were applied. For each definition of VF (HIV RNA levels of ≥200, ≥400, and ≥1000 copies/mL) models were run separately. Multivariable models were adjusted for age and VL at baseline, CD4 count at baseline, HIV transmission category, nationality, and prior cART interruptions. Adjusted time-to-event curves were performed, holding all adjusted covariables fixed at their mean level. The proportional hazards assumptions were assessed by testing for zero slopes of the scaled Schoenfeld residuals.
Additional sensitivity analyses were run for different criteria in patient selection: one analysis was performed without considering a VL measurement below the limit of detection (BLD) or BLQ in treatment history in order to exclude patients with very short cART durations; one analysis was performed excluding patients with cART interruptions or a cART stop after baseline VL measurement; one analysis was conducted regarding a shorter recruitment period of 6 months instead of 12 months; one analysis included patients receiving unmodified cART for >9 months instead of >6 months; and finally, one analysis was performed with a shorter recruitment period of 6 months, an unmodified cART for >3 months, and without cART interruptions or a cART stop after baseline VL measurement. All analyses were conducted using Stata software, version 13.1 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics Stratified by Virological Failure
A total of 1775 patients fulfilled the inclusion criteria: 79 of them with a VF of ≥200 copies/mL and another 82 of them without any further VL measurement after baseline were excluded. Therefore, 1614 patients were eligible for analyses (Figure 1) in this study. Differences in demographic and laboratory characteristics between the patients who developed VF and individuals who did not experience VF are described in Table 1. Stratification was made according to the 3 definitions of VF; therefore, 68 (4.2%), 44 (2.7%), and 34 (2.1%) of 1614 incorporated patients progressed to a VF defined as an HIV RNA level of ≥200, ≥400, and ≥1000 copies/mL, respectively. Median follow-up time for all patients regarding VF of ≥200 copies/mL as endpoint was 9.2 months (IQR, 7.8–11.5). Each VF group differed significantly from the non-VF group in VL at baseline, age at baseline, HIV transmission category, CD4 count at baseline, prior cART interruptions, as well as cART regimen. Compared with individuals who experienced VF, patients who did not develop a VF were older and had a higher CD4 cell count at baseline. The proportion of injecting drug users (IDUs) was higher, and there were fewer men who have sex with men (MSM) in the VF group. Patients who had a VF ≥ 400 and ≥1000 copies/mL originated more frequently from high-prevalence countries. Patients with VF had more prior cART interruptions, were more often on 2 NRTIs with a PI/r instead of a NNRTI or an INSTI, and VL at baseline was higher compared with individuals who did not experience VF. More importantly, in patients who had a VF defined as HIV RNA levels of ≥200 copies/mL, VL before initiating cART was higher compared with the non-VF group, but not for patients with VF defined as HIV RNA level of ≥400 and ≥1000 copies/mL. No difference between the groups could be found in ever having had a VF before VL at baseline, in prior cART duration and in first-line cART of the respective regimen.
Table 1.
Characteristics of Patients Stratified by Virological Failure Defined as HIV RNA Levels ≥200, ≥400, and ≥1000 copies/mL vs Nonvirological Failure in Each Casea
| All Patients (N = 1614) |
All Patients (N = 1614) |
All Patients (N = 1614) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Broad Definition |
Restricted Definition |
Stringent Definition |
|||||||
| VL < 200 |
VF ≥ 200 |
VL < 400 |
VF ≥ 400 |
VL < 1000 |
VF ≥ 1000 |
||||
| N = 1546 |
N = 68 |
N = 1570 |
N = 44 |
N = 1580 |
N = 34 |
||||
| No. of Patients | N (%) | N (%) | P Value | N (%) | N (%) | P Value | N (%) | N (%) | P Value |
| VL at baseline | <.001 | .012 | .008 | ||||||
| 51–199 copies/mL | 62 (4.0) | 13 (19.1) | 69 (4.4) | 6 (13.6) | 69 (4.4) | 6 (17.7) | |||
| ≤50 copies/mL | 103 (6.7) | 9 (13.2) | 108 (6.9) | 4 (9.1) | 110 (7.0) | 2 (5.9) | |||
| BLD + BLQ | 1381 (89.3) | 46 (67.7) | 1393 (88.7) | 34 (77.3) | 1401 (88.7) | 26 (76.5) | |||
| Age at baseline | .028 | .045 | .019 | ||||||
| <30 yr | 141 (9.1) | 11 (16.2) | 145 (9.3) | 7 (15.9) | 146 (9.3) | 6 (17.7) | |||
| 30–50 yr | 996 (64.4) | 47 (69.1) | 1011 (64.4) | 32 (72.7) | 1018 (64.4) | 25 (73.5) | |||
| >50 yr | 409 (26.5) | 10 (14.7) | 414 (26.4) | 5 (11.4) | 416 (26.3) | 3 (8.8) | |||
| HIV transmission category | <.001 | <.001 | .001 | ||||||
| Male injecting drug user | 133 (8.6) | 14 (20.6) | 135 (8.6) | 12 (27.3) | 137 (8.7) | 10 (29.4) | |||
| Female injecting drug user | 46 (3.0) | 4 (5.9) | 47 (3.0) | 3 (6.8) | 48 (3.0) | 2 (5.9) | |||
| Male heterosexual | 340 (22.0) | 8 (11.8) | 343 (21.9) | 5 (11.4) | 343 (21.7) | 5 (14.7) | |||
| Female heterosexual | 339 (21.9) | 21 (30.9) | 348 (22.2) | 12 (27.3) | 351 (22.2) | 9 (26.5) | |||
| Other | 65 (4.2) | 6 (8.8) | 68 (4.3) | 3 (6.8) | 69 (4.4) | 2 (5.9) | |||
| Men who have sex with men | 623 (40.3) | 15 (22.1) | 629 (40.1) | 9 (20.5) | 632 (40.0) | 6 (17.7) | |||
| Nationality | .133 | .012 | .005 | ||||||
| High prevalence country | 143 (9.3) | 10 (14.7) | 144 (9.2) | 9 (20.5) | 145 (9.2) | 8 (23.5) | |||
| Low prevalence country | 1403 (90.8) | 58 (85.3) | 1426 (90.8) | 35 (79.6) | 1435 (90.8) | 26 (76.5) | |||
| CD4 count at baseline | .001 | .015 | .015 | ||||||
| Missing | 41 (2.7) | 7 (10.3) | 44 (2.8) | 4 (9.1) | 45 (2.9) | 3 (8.8) | |||
| <200 cells/µL | 48 (3.1) | 5 (7.4) | 49 (3.1) | 4 (9.1) | 49 (3.1) | 4 (11.8) | |||
| 200–349 cells/µL | 174 (11.3) | 7 (10.3) | 176 (11.2) | 5 (11.4) | 176 (11.1) | 5 (14.7) | |||
| 350–499 cells/µL | 264 (17.1) | 12 (17.7) | 267 (17.0) | 9 (20.5) | 271 (17.2) | 5 (14.7) | |||
| ≥500 cells/µL | 1019 (65.9) | 37 (54.4) | 1034 (65.9) | 22 (50.0) | 1039 (65.8) | 17 (50.0) | |||
| Prior cART interruptionsb | <.001 | <.001 | <.001 | ||||||
| ≥1 | 339 (21.9) | 30 (44.1) | 348 (22.2) | 21 (47.7) | 352 (22.3) | 17 (50.0) | |||
| None | 1207 (78.1) | 38 (55.9) | 1222 (77.8) | 23 (52.3) | 1228 (77.7) | 17 (50.0) | |||
| cART regimen | .001 | .024 | .026 | ||||||
| 2 NRTI + PI/r | 611 (39.5) | 40 (58.8) | 626 (39.9) | 25 (56.8) | 631 (39.9) | 20 (58.8) | |||
| 2 NRTI + NNRTI/INSTI | 935 (60.5) | 28 (41.2) | 944 (60.1) | 19 (43.2) | 949 (60.1) | 14 (41.2) | |||
| Ever VF before VL at baseline | .140 | .340 | .373 | ||||||
| <200 copies/mL | 83 (5.4) | 2 (2.9) | 83 (5.3) | 2 (4.6) | 84 (5.3) | 1 (2.9) | |||
| 200–399 copies/mL | 416 (26.9) | 26 (38.2) | 428 (27.3) | 14 (31.8) | 430 (27.2) | 12 (35.3) | |||
| 400–999 copies/mL | 303 (19.6) | 8 (11.8) | 307 (19.6) | 4 (9.1) | 308 (19.5) | 3 (8.8) | |||
| ≥1000 copies/mL | 744 (48.1) | 32 (47.1) | 752 (47.9) | 24 (54.6) | 758 (48.0) | 18 (52.9) | |||
| VL before initiating cART | .045 | .399 | .330 | ||||||
| Missing | 272 (17.6) | 13 (19.1) | 277 (17.6) | 8 (18.2) | 280 (17.7) | 5 (14.7) | |||
| >99.999 copies/mL | 559 (36.2) | 34 (50.0) | 573 (36.5) | 20 (45.5) | 577 (36.5) | 16 (47.1) | |||
| 10.000–99.999 copies/mL | 527 (34.1) | 18 (26.5) | 531 (33.8) | 14 (31.8) | 533 (33.7) | 12 (35.3) | |||
| ≤9.999 copies/mL | 188 (12.2) | 3 (4.4) | 189 (12.0) | 2 (4.6) | 190 (12.0) | 1 (2.9) | |||
| Prior cART durationc | 1.000 | .873 | .710 | ||||||
| <9 mo | 61 (4.0) | 2 (2.9) | 61 (3.9) | 2 (4.6) | 61 (3.9) | 2 (5.9) | |||
| 9–18 mo | 139 (9.0) | 6 (8.8) | 141 (9.0) | 4 (9.1) | 142 (9.0) | 3 (8.8) | |||
| >18 mo | 1346 (87.1) | 60 (88.2) | 1368 (87.1) | 38 (86.4) | 1377 (87.2) | 29 (85.3) | |||
| Antiretroviral therapy-naived | .146 | .218 | .181 | ||||||
| Yes | 290 (18.8) | 8 (11.8) | 293 (18.7) | 5 (11.4) | 295 (18.7) | 3 (8.8) | |||
| No | 1256 (81.2) | 60 (88.2) | 1277 (81.3) | 39 (88.6) | 1285 (81.3) | 31 (91.2) | |||
| Follow-up time in months | 9.3 (8.0–11.5) | 6.4 (3.3–8.2) | <.001 | 9.4 (8.0–11.5) | 7.0 (5.0–8.4) | <.001 | 9.4 (8.0–11.6) | 7.1 (5.8–8.7) | <.001 |
Abbreviations: BLD, below the limit of detection; BLQ, below the limit of quantification; cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; INSTI, integrase inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI/r, boosted protease inhibitor; RNA, ribonucleic acid; VF, virological failure; VL, viral load.
a The first VL measurement after baseline VL measurement (baseline: the first detectable VL measurement within 6 months after receiving unmodified cART for >6 months with standard regimens between July 2012 and 2013, excluding patients without any VL measurement BLQ in their treatment history as well as patients without any baseline VL measurement). [Number (%) or median (interquartile range)].
b Interruptions prior to 6 months of unmodified cART of the respective cART regimen.
c cART duration until 6 months of unmodified cART of the respective cART regimen.
d Whether the respective cART regimen is a first-line cART or not.
Predictors of Virological Failure
Table 2 provides results from univariable and multivariable Cox proportional hazards models for the occurrence of VF according to the broad definition of VF (defined as HIV RNA level of ≥200 copies/mL). Hazard ratios (HRs) for the development of VF regarding the restricted and stringent definition of VF (defined as HIV RNA level of ≥400 and ≥1000 copies/mL, respectively) are shown in the Supplemental Table 1. A total of 1614 patients were observed until the development of VF or last VL measurement or death, whichever came first.
Table 2.
Univariable and Multivariable Cox Regression Results: Association Between Different Factors and Virological Failure Defined as HIV RNA Levels ≥200 Copies/mL (Broad Definition)
| Outcome | VF ≥ 200 |
|
|---|---|---|
| No. of Patients Included | N = 1614 |
|
| No. of Failures | N = 68 |
|
| Univariable |
Multivariable |
|
| HR (95% CI) | HR (95% CI) | |
| VL at baseline | ||
| 51–199 copies/mL | 5.84 (3.15–10.82) | 4.21 (2.15–8.22) |
| ≤50 copies/mL | 2.47 (1.21–5.05) | 2.19 (1.06–4.55) |
| BLD + BLQ | 1.00 (Reference) | 1.00 (Reference) |
| Age at baseline | ||
| <30 y | 3.31 (1.41–7.81) | 2.98 (1.24–7.21) |
| 30–50 yr | 1.94 (.98–3.84) | 1.91 (.95–3.84) |
| >50 yr | 1.00 (Reference) | 1.00 (Reference) |
| HIV transmission category | ||
| Male injecting drug user | 4.53 (2.19–9.40) | 3.21 (1.50–6.88) |
| Female injecting drug user | 3.83 (1.27–11.54) | 3.83 (1.25–11.74) |
| Male heterosexual | 1.00 (.42–2.35) | 1.07 (.44–2.59) |
| Female heterosexual | 2.64 (1.36–5.13) | 2.25 (1.08–4.66) |
| Other | 3.72 (1.44–9.59) | 3.66 (1.40–9.56) |
| Men who have sex with men | 1.00 (Reference) | 1.00 (Reference) |
| Nationality | ||
| High prevalence country | 1.62 (.83–3.16) | 1.07 (.48–2.36) |
| Low prevalence country | 1.00 (Reference) | 1.00 (Reference) |
| CD4 count at baseline | ||
| Missing | 4.60 (2.05–10.32) | 3.31 (1.40–7.81) |
| <200 cells/µL | 2.91 (1.14–7.41) | 1.66 (.62–4.43) |
| 200–349 cells/µL | 1.11 (.50–2.49) | 0.95 (.42–2.16) |
| 350–499 cells/µL | 1.25 (.65–2.39) | 1.48 (.76–2.87) |
| ≥500 cells/µL | 1.00 (Reference) | 1.00 (Reference) |
| Prior cART interruptionsa | ||
| ≥1 | 2.80 (1.73–4.52) | 2.28 (1.37–3.77) |
| None | 1.00 (Reference) | 1.00 (Reference) |
| cART regimen | ||
| 2 NRTI + PI/r | 2.13 (1.31–3.45) | |
| 2 NRTI + NNRTI/INSTI | 1.00 (Reference) | |
| Ever VF before VL at baseline | ||
| <200 copies/mL | 0.58 (.14–2.42) | |
| 200–399 copies/mL | 1.40 (.84–2.36) | |
| 400–999 copies/mL | 0.59 (.27–1.29) | |
| ≥1000 copies/mL | 1.00 (Reference) | |
| VL before initiating cART | ||
| Missing | 2.91 (.83–10.21) | |
| >99.999 copies/mL | 3.49 (1.07–11.37) | |
| 10.000–99.999 copies/mL | 2.10 (.62–7.14) | |
| ≤9.999 copies/mL | 1.00 (Reference) | |
| Prior cART durationb | ||
| <9 mo | 0.85 (.21–3.47) | |
| 9–18 mo | 0.96 (.42–2.22) | |
| >18 mo | 1.00 (Reference) | |
| Antiretroviral therapy-naivec | ||
| Yes | 0.55 (.26–1.15) | |
| No | 1.00 (Reference) | |
Abbreviations: BLD, below the limit of detection; BLQ, below the limit of quantification; cART, combination antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; INSTI, integrase inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI/r, boosted protease inhibitor; RNA, ribonucleic acid; VF, virological failure; VL, viral load.
a Interruptions prior to 6 months of unmodified cART of the respective cART regimen.
b cART duration until 6 months of unmodified cART of the respective cART regimen.
c Whether the respective cART regimen is a first-line cART or not.
In univariable models, several significant factors turned out to be associated with the development of VF. These factors were (1) a higher VL at baseline (compared with patients who were BLD or BLQ), (2) a lower CD4 cell count at baseline (compared with patients with ≥500 cells/µL), (3) younger age (<30 years compared with above 50 years), (4) IDU, (5) women who acquired HIV through heterosexual contact (compared with MSM), (6) patient origin from high-prevalence countries (compared with low-prevalence countries), (7) prior cART interruptions (compared with uninterrupted cART), (8) 2 NRTI with a PI/r as cART regimen (compared with 2 NRTI with an NNRTI or INSTI), and finally (9) a higher VL before initiating cART (compared with a VL ≤9.999 copies/mL).
Multivariable Cox proportional hazards models for each definition of VF were calculated and controlled for VL at baseline, CD4 count at baseline, age at baseline, HIV transmission category, nationality, and prior cART interruptions. The resulting adjusted cumulative hazard curves are shown in Figure 2. Compared with patients who were BLD or BLQ, patients with a quantifiable VL of ≤50 copies/mL at baseline had a 2.19 times higher risk of VF of ≥200 copies/mL; however, this did not encompass the restricted and stringent definition of VF. Viral load between 51 and 199 copies/mL at baseline also increased the risk of VF ≥200 copies/mL, compared with patients who were BLD or BLQ at baseline (HR = 4.21, 95% CI = 2.15–8.22). In addition, a trend for a higher risk of VF ≥400 and ≥1000 copies/mL could be found (HR = 2.13, 95% CI = 0.84–5.39 and HR = 2.52, 95% CI = 0.96–6.60, respectively). The association between lower CD4 count at baseline and the development of VF found in univariable analyses vanished after adjustment. The hazard ratio for VF was higher in patients under 30 years compared with individuals older than 50 years: HIV RNA levels of ≥200 copies/mL, HR = 2.98 and 95% CI = 1.24–7.21; HIV RNA levels of ≥400 copies/mL, HR = 3.67 and 95% CI = 1.13–11.96; HIV RNA levels of ≥1000 copies/mL, HR = 5.02 and 95% CI = .20–21.04, respectively. Injecting drug use was associated with an increased risk of VF in males: HIV RNA levels of ≥200 copies/mL, HR = 3.21 and 95% CI = 1.50–6.88; HIV RNA levels of ≥400 copies/mL, HR = 4.57 and 95% CI = 1.85–11.33; HIV RNA levels of ≥1000 copies/mL, HR = 5.25 and 95% CI = 1.81–15.22, respectively, compared with MSM. Injecting drug use was also associated with an increased risk of VF in females: HIV RNA levels of ≥200 copies/mL, HR = 3.83 and 95% CI = 1.25–11.74; HIV RNA levels of ≥400 copies/mL, HR = 4.52 and 95% CI = 1.20–17.03, respectively, compared with MSM. Female heterosexuals showed a higher risk of VF of ≥200 copies/mL (HR = 2.25, 95% CI = 1.08–4.66), but not for VF defined by higher VL levels. Patients originating from high-prevalence countries were at higher risk to develop VF ≥1000 copies/mL compared with individuals from low-prevalence countries (HR = 2.94, 95% CI = 1.03–8.43). The hazard ratio of VF for all 3 definitions was higher in patients who have had prior cART interruptions: HIV RNA levels of ≥200 copies/mL, HR = 2.28 and 95% CI = 1.37–3.77; HIV RNA levels ≥400 copies/mL, HR = 2.77 and 95% CI = 1.48–5.19; HIV RNA levels ≥1000 copies/mL, HR = 2.88 and 95% CI = 1.41–5.87. However, all additional analyses did not reveal any substantial differences in HRs compared with our primary analysis (data not shown).
Figure 2.
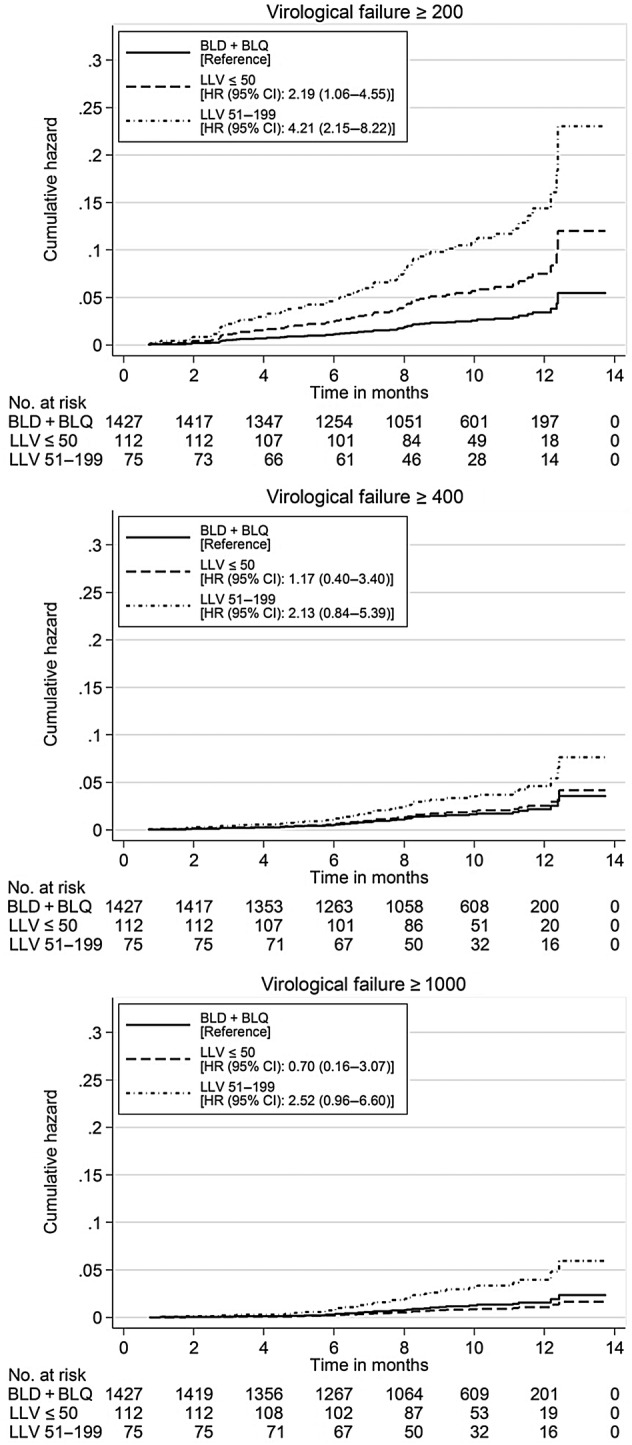
Adjusted cumulative hazard curves for the development of virological failure based on a single viral load at baseline. Abbreviations: BLD, below the limit of detection; BLQ, below the limit of quantification; CI, confidence interval; HR, hazards ratio; LLV, low-level viremia.
DISCUSSION
In this study, we specifically investigated the impact of LLV on VF, defined as HIV RNA level of ≥200, ≥400, and ≥1000 copies/mL, among a cohort of HIV-positive individuals in Austria on unmodified cART with standard regimens over a recent period of more than 6 months. Other potential risk factors were also studied.
We observed that 1 single measurement of quantifiable VL below 200 copies/mL under unmodified cART predicted the occurrence of VF, according to the broad definition of VF (≥200 copies/mL). The risk to develop such VF was 2.19 times higher for individuals whose VL was ≤50 copies/mL and 4.21 times higher for those with VL of 51–199 copies/mL compared with patients who were BLD or BLQ at baseline. This finding was independent of other covariates including CD4 count at baseline, age at baseline, HIV transmission category, nationality, and prior cART interruptions, which may influence the outcome. Regarding the restricted and the stringent definition of VF, a trend for an increased risk of VF could be found in patients having had a VL of 51–199 copies/mL at baseline.
Our results extend and refine data from several recent studies showing the predictive value of LLV. Although these studies differ in study designs and methods, they all conclude that persistent LLV (determined by repeated measurements) elevates the risk of VF [6–11].
In clinical routine healthcare, providers encounter 1 single measurement of VL. Only 1 study has hitherto addressed this important issue; however, a different commercial HIV RNA assay was used in this study, namely the Abbott RealTime assay. It pointed out the strong predictive value for VF in patients with 1 single VL measurement below 50 copies/mL [13]. Our study, based on the Roche Cobas AmpliPrep/Cobas TaqMan 2.0 assay, essentially confirms these data, but we provide more detail and use this in conjunction with other parameters. In our study, the risk of the development of VF decreased with increasing age and escalated with stricter threshold definitions of VF. However, our finding that younger age was an independent factor of viral rebound in patients with a single measurement of VL <50 copies/mL was also confirmed by other studies [17, 18]; however, these were studies on persistent LLV. In addition, we demonstrated a higher risk of VF in male and female IDUs compared with MSM. The risk was higher in men and increased with higher VF thresholds. In our study, patients from high-prevalence countries had an almost 3-fold higher risk of experiencing VF ≥1000 copies/mL after a single measurement of LLV, similar to the correlation to persistent LLV [17].
Because adherence to therapy is difficult to measure accurately, cART interruptions before 6 months under stable cART of the respective cART regimen were considered as a proxy for adherence. Treatment interruptions, defined as cART discontinuation for at least 8 days after having started cART, were observed more frequently in patients who developed VF (approximately 44% vs 22%). In adjusted analyses, the risk of VF of ≥200 was 2.3-fold higher in patients who had prior cART interruptions compared with those who never interrupted treatment. As a consequence, we demonstrated reduced adherence as a predictor of VF. Doyle et al [13] found major resistance-associated mutations in less than half of the patients with rebound of >400 copies/mL, and they concluded that poor adherence was as a cause of rebound. A decline in VF was shown for patients who have had virologic suppression for longer time periods than individuals suppressed for shorter periods [19, 20]. However, in our study, prior cART duration did not predict VF. We recently found that duration of cART is significantly associated with LLV [21], which is in line with other reports [22, 23] but not all [24].
Furthermore, we found that the type of cART regimen was associated with low-level rebound [8] and that NNRTI-based regimens were able to exert a stronger inhibitory effect on viral replication than PI-based regimens [13, 25, 26]. In a previous study, we showed that patients BLD or BLQ were more likely to be treated with 2 NRTIs and an NNRTI or INSTI compared with those with LLV, most of whom tended to be on 2 NRTIs and a PI/r. In contrast, we found a 1.5-fold increased risk of LLV for patients receiving cART with a PI/r-based regimen compared with those on a NNRTI- or INSTI-based regimen [21]. However, this effect could be confounded by a selection bias because PI/r-based cART regimens may preferentially be prescribed to individuals with concerns regarding adherence. A recent retrospective study in patients on cART with viremia between 50 and 100 copies/mL found that plasma drug levels at the first LLV episode were associated with subsequent VF, which was defined as HIV RNA ≥1000 copies/mL. In addition, it was shown that together with resistance data, a higher proportion of treatment failures can be explained than either measure alone [27]. However, at this time, HIV cannot be eradicated by cART; therefore, despite being on stable cART, it remains a challenge for some patients to achieve HIV RNA levels BLD or BLQ [28]. Low-level viremia might arise from the following: (1) ongoing cycles of viral replication in a sanctuary site where drug levels are suboptimal; (2) long-lived infected cells that produce virus; or (3) activation of virus expression from latently infected CD4 T-cell reservoirs [29]. Studies on the effect of cART intensification in patients with LLV did not find evidence of ongoing replication [30], whereas others concluded that active replication persists in some individuals on cART [31]. In our study, only a small proportion of the patients with VL BLD or BLQ at baseline experienced a VF of ≥200 copies/mL (3%).
A major strength of this study is the open, observational design with complete follow up. Our study provides data of HIV-positive individuals in Austria and is representative of an entire country; therefore, selection bias was minimized. This study has several limitations. There was a small number of VFs, especially with regard to the stringent definition, which limits the number of variables for which the analysis could be adjusted. We were not able to adjust for several parameters that might influence cART pharmacokinetics such as body mass index and renal function. However, no association could be found between these factors and LLV [7]. Furthermore, we were not able to adjust for socioeconomic and lifestyle factors such as smoking, which may also influence the results, because of missing or incomplete data. Moreover, we did not analyze the association between VF and the development of resistance mutations. Previous studies documented the risk of acquiring resistance mutations during LLV [32, 33] and in patients who experienced rebound [13].
CONCLUSIONS
This study of well defined patients on unmodified cART over a period of more than 6 months gives insights into different predictors for the development of VF. Our main finding of an increased risk of VF in patients with 1 single quantifiable VL measurement below 200 copies/mL supports the need to evaluate strategies for managing LLV in cART-treated patients. These results provide implications for patient management by emphasizing closer monitoring and adherence counseling. Furthermore, our findings could form the basis for future methods and strategies to improve the outcome in single quantifiable VL studies.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We are grateful to all clinicians, data managers, and research nurses in participating human immunodeficiency virus (HIV) treatments centers listed in the Appendix. Furthermore, a special thank goes to DI Heinz Appoyer (now called network vita) who developed the HIV Patient Management System.
Disclaimer. Companies involved in marketing HIV drugs (AbbVie, Boehringer-Ingelheim, Bristol-Myers-Squibb, GILEAD, GSK Austria on behalf of Viif, Janssen, MSD) provided equal contributions, irrespective of their market shares.
Financial support. This work was funded by the Austrian Agency for Health and Food Safety (AGES), participating hospitals running HIV treatment centers, and partners in the pharmaceutical industry.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
APPENDIX
The Austrian HIV Cohort Study ([AHIVCOS] Steering Committee): Alexander Egle, Maria Geit, Bernhard Haas, Manfred Kanatschnig, Armin Rieger, Andrea Steuer, Robert Zangerle (Chair).
HIV Treatment Centers: LKH Innsbruck (coordinating center): Martin Gisinger, Maria Kitchen, Elisabeth Rieser, Brigitte Rühr, Mario Sarcletti, Robert Zangerle. LKH Salzburg: Alexander Egle, Richard Greil, Michaela Schachner, Ninon Taylor. AKH Linz: Jörg Berg, Maria Geit, Angela Öllinger. AKH Vienna: Regina Aichwalder, Katharina Grabmeier-Pfistershammer, Armin Rieger, Veronique Touzeau. Otto-Wagner Hospital Vienna: Piotr Cichon, Manfred Gartner, Brigitte Schmied, Andrea Steuer. LKH Graz-West: Bernhard Haas, Andreas Kapper, Elmar Wallner. LKH Klagenfurt: Silvana Achatz, Manfred Kanatschnig, Georg Schober.
Virology: Elisabeth Puchhammer-Stöckl (Vienna).
Data Management Group: Heinz Appoyer (IT-related), Gisela Leierer (AHIVCOS), Michaela Rappold (AHIVCOS), Stefanie Strickner (AHIVCOS).
Data Safety and Protection: Klaus Schindelwig (Innsbruck).
Scientific Advisory Board: Bruno Ledergerber (Zurich), Gerd Fätkenheuer (Cologne).
Contributor Information
Collaborators: for the Austrian HIV Cohort Study Group, Alexander Egle, Maria Geit, Bernhard Haas, Manfred Kanatschnig, Armin Rieger, Andrea Steuer, Robert Zangerle, Martin Gisinger, Maria Kitchen, Elisabeth Rieser, Brigitte Rühr, Mario Sarcletti, Robert Zangerle, Richard Greil, Michaela Schachner, Ninon Taylor, Jörg Berg, Angela Öllinger, Regina Aichwalder, Katharina Grabmeier-Pfistershammer, Veronique Touzeau, Piotr Cichon, Manfred Gartner, Brigitte Schmied, Andrea Steuer, Bernhard Haas, Andreas Kapper, Elmar Wallner, Elisabeth Puchhammer-Stöckl, Heinz Appoyer, Gisela Leierer, Michaela Rappold, Stefanie Strickner, Klaus Schindelwig, Bruno Ledergerber, and Gerd Fätkenheuer
References
- 1.European Guidelines for treatment of HIV-infected adults in Europe, Version 8.0, October 2015. European AIDS Clinical Society. Brussels, Belgium, 2015. Available at: http://www.eacsociety.org/files/2015_eacsguidelines_8.0-english_revised-20151104.pdf Accessed 25 March 2016. [Google Scholar]
- 2.British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015. British HIV Association, 2015. Available at: http://www.bhiva.org/documents/Guidelines/Treatment/2015/2015-treatment-guidelines.pdf. Accessed 25 March 2016. [DOI] [PubMed]
- 3.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Rockville, MD, 2016. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 25 March 2016. [Google Scholar]
- 4.Gunthard HF, Aberg JA, Eron JJ et al. . Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA 2014; 312:410–25. [DOI] [PubMed] [Google Scholar]
- 5.Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: What's New. World; Health Organization; Geneva; 2015. Available at: http://apps.who.int/iris/bitstream/10665/198064/1/9789241509893_eng.pdf?ua=1 Accessed 25 March 2016. [Google Scholar]
- 6.Antiretroviral Therapy Cohort Collaboration (ART-CC), Vandenhende MA, Ingle S et al. . Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS 2015; 29:373–83. [DOI] [PubMed] [Google Scholar]
- 7.Boillat-Blanco N, Darling KE, Schoni-Affolter F et al. . Virological outcome and management of persistent low-level viraemia in HIV-1-infected patients: 11 years of the Swiss HIV Cohort Study. Antivir Ther 2015; 20:165–75. [DOI] [PubMed] [Google Scholar]
- 8.Geretti AM, Smith C, Haberl A et al. . Determinants of virological failure after successful viral load suppression in first-line highly active antiretroviral therapy. Antivir Ther 2008; 13:927–36. [PubMed] [Google Scholar]
- 9.Laprise C, de Pokomandy A, Baril JG et al. . Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis 2013; 57:1489–96. [DOI] [PubMed] [Google Scholar]
- 10.Pernas B, Grandal M, Pertega S et al. . Any impact of blips and low-level viraemia episodes among HIV-infected patients with sustained virological suppression on ART? J Antimicrob Chemother 2016; 71:1051–5. [DOI] [PubMed] [Google Scholar]
- 11.Sungkanuparph S, Groger RK, Overton ET et al. . Persistent low-level viraemia and virological failure in HIV-1-infected patients treated with highly active antiretroviral therapy. HIV Med 2006; 7:437–41. [DOI] [PubMed] [Google Scholar]
- 12.Vandenhende MA, Perrier A, Bonnet F et al. . Risk of virological failure in HIV-1-infected patients experiencing low-level viraemia under active antiretroviral therapy (ANRS C03 cohort study). Antivir Ther 2015; 20:655–60. [DOI] [PubMed] [Google Scholar]
- 13.Doyle T, Smith C, Vitiello P et al. . Plasma HIV-1 RNA detection below 50 copies/ml and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis 2012; 54:724–32. [DOI] [PubMed] [Google Scholar]
- 14.Sire JM, Vray M, Merzouk M et al. . Comparative RNA quantification of HIV-1 group M and non-M with the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 v2.0 and Abbott Real-Time HIV-1 PCR assays. J Acquir Immune Defic Syndr 2011; 56:239–43. [DOI] [PubMed] [Google Scholar]
- 15.Taylor N, Grabmeier-Pfistershammer K, Egle A et al. . Cobas ampliprep/cobas TaqMan HIV-1 v2.0 assay: consequences at the cohort level. PLoS One 2013; 8:e74024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swenson LC, Cobb B, Geretti AM et al. . Comparative performances of HIV-1 RNA load assays at low viral load levels: results of an international collaboration. J Clin Microbiol 2014; 52:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansi LK, Benzie AA, Phillips AN et al. . Are previous treatment interruptions associated with higher viral rebound rates in patients with viral suppression? AIDS 2008; 22:349–56. [DOI] [PubMed] [Google Scholar]
- 18.Henrich TJ, Wood BR, Kuritzkes DR. Increased risk of virologic rebound in patients on antiviral therapy with a detectable HIV load <48 copies/mL. PLoS One 2012; 7:e50065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima VD, Bangsberg DR, Harrigan PR et al. . Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr 2010; 55:460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenblum M, Deeks SG, van der Laan M, Bangsberg DR. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One 2009; 4:e7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leierer G, Grabmeier-Pfistershammer K, Steuer A et al. . Factors associated with low-level viraemia and virological failure: results from the Austrian HIV cohort study. PLoS One 2015; 10:e0142923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascual-Pareja JF, Martinez-Prats L, Luczkowiak J et al. . Detection of HIV-1 at between 20 and 49 copies per milliliter by the Cobas TaqMan HIV-1 v2.0 assay is associated with higher pretherapy viral load and less time on antiretroviral therapy. J Clin Microbiol 2010; 48:1911–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vancoillie L, Demecheleer E, Callens S et al. . Markers associated with persisting low-level viraemia under antiretroviral therapy in HIV-1 infection. J Antimicrob Chemother 2014; 69:1098–103. [DOI] [PubMed] [Google Scholar]
- 24.Charpentier C, Landman R, Laouenan C et al. . Persistent low-level HIV-1 RNA between 20 and 50 copies/mL in antiretroviral-treated patients: associated factors and virological outcome. J Antimicrob Chemother 2012; 67:2231–5. [DOI] [PubMed] [Google Scholar]
- 25.Bonora S, Nicastri E, Calcagno A et al. . Ultrasensitive assessment of residual HIV viraemia in HAART-treated patients with persistently undetectable plasma HIV-RNA: a cross-sectional evaluation. J Med Virol 2009; 81:400–5. [DOI] [PubMed] [Google Scholar]
- 26.Widdrington J, Payne B, Medhi M et al. . The significance of very low-level viraemia detected by sensitive viral load assays in HIV infected patients on HAART. J Infect 2011; 62:87–92. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Serna A. Untimed drug levels and resistance in patients experiencing low-level viremia. In: Conference on Retroviruses and Opportunistic Infections Seattle, Washington February 23–26, 2015 (Abstract 117). [Google Scholar]
- 28.Palmer S, Maldarelli F, Wiegand A et al. . Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2008; 105:3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer S, Josefsson L, Coffin JM. HIV reservoirs and the possibility of a cure for HIV infection. J Intern Med 2011; 270:550–60. [DOI] [PubMed] [Google Scholar]
- 30.McMahon D, Jones J, Wiegand A et al. . Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis 2010; 50:912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buzon MJ, Massanella M, Llibre JM et al. . HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med 2010; 16:460–5. [DOI] [PubMed] [Google Scholar]
- 32.Nettles RE, Kieffer TL, Simmons RP et al. . Genotypic resistance in HIV-1-infected patients with persistently detectable low-level viremia while receiving highly active antiretroviral therapy. Clin Infect Dis 2004; 39:1030–7. [DOI] [PubMed] [Google Scholar]
- 33.Taiwo B, Gallien S, Aga E et al. . Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis 2011; 204:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.