Abstract
Vagus nerve stimulation has recently been reported to improve symptoms of migraine. Cortical spreading depression is the electrophysiological event underlying migraine aura, and a trigger for headache. We tested whether vagus nerve stimulation inhibits cortical spreading depression to explain its anti-migraine effect. Vagus nerve stimulation was delivered either non-invasively through the skin or directly by electrodes placed around the vagus nerve unilaterally. Systemic physiology was monitored throughout the study. Both non-invasive transcutaneous and invasive direct vagus nerve stimulation significantly suppressed spreading depression susceptibility in the occipital cortex in rats. The electrical stimulation threshold to evoke a spreading depression was elevated by more than two-fold, the frequency of spreading depressions during continuous topical 1M KCl was reduced by ~40%, and propagation speed of spreading depression was reduced by ~15%. This effect developed within 30 minutes after vagus nerve stimulation. Non-invasive transcutaneous vagus nerve stimulation was as efficacious as direct invasive vagus nerve stimulation, and the efficacy did not differ between the ipsilateral and contralateral hemispheres. Our findings provide a potential mechanism by which vagus nerve stimulation may be efficacious in migraine, and suggest that susceptibility to spreading depression is a suitable platform to optimize its efficacy.
Keywords: Migraine, Cortical spreading depression, Vagus nerve stimulation
1. Introduction
Migraine is a highly prevalent and disabling disease with tremendous socioeconomic impact. Despite intense research, therapeutic options are limited. Even the most effective acute abortive drugs, triptans, can only achieve a 30% two-hour pain-free response rate at best [28]. Considering the complex pathophysiology and genetics of migraine [2; 52; 54], new therapeutic modalities are needed for patients who do not respond to currently available treatments.
Vagus nerve stimulation (VNS) is an FDA-approved treatment for seizures and depression, currently under investigation as a non-pharmacological therapy in migraine. Patients with refractory epilepsy or major depression have reported remarkable improvement in their migraines after VNS [11; 17; 34; 43; 59], and a small case series suggested efficacy in chronic refractory migraine [48]. In recent open-label, single-arm, multiple-attack studies, VNS afforded a 22% two-hour pain-free rate for moderate or severe attacks [10; 30]. Despite these promising early results, the mechanism of action of VNS in migraine is unknown, hampering the efforts to optimize its efficacy and minimize potential side effects.
Cortical spreading depression (CSD) is a slowly propagating wave of neuronal and glial depolarization that underlies migraine aura. Once triggered, CSD activates downstream inflammatory and nociceptive pathways leading to headache that can last many hours and sometimes days [6; 15; 37; 64; 65]. Animal models of CSD have been widely used as a validated platform for screening pharmacological therapies for migraine [5; 7]. Indeed, migraine prophylactic drugs belonging to different pharmacological classes all inhibit CSD as a common mode of action [7; 23], suggesting that CSD is a headache trigger and a viable therapeutic target in migraine.
Previous work in animal models has shown that VNS activates nucleus tractus solitarius (NTS), locus ceruleus (LC) and dorsal raphe nuclei (DRN) [4; 21; 22; 32], all of which can suppress CSD susceptibility by enhancing serotonergic or β-adrenergic activity [29; 42; 46; 56]. VNS has been effective for the treatment of seizures and depression which show a strong clinical association with migraine [27], and many migraine prophylactic drugs are antiepileptics or antidepressants, and suppress CSD [5]. Moreover, VNS has reproducibly shown efficacy in animal models of ischemic [3; 33; 35] or traumatic brain injury[61], where CSD plays a major pathophysiologic role [40]. We, therefore, hypothesized that VNS suppresses CSD susceptibility as one mechanism of action in migraine, and tested this electrophysiologically in previously validated animal models under full systemic physiological monitoring.
2. Methods
2.1 Ethics statement
Experiments were carried out in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, 1996), and approved by the institutional review board (Massachusetts General Hospital Subcommittee on Research Animal Care).
2.2 General surgical preparation
A total of 66 adult male Sprague-Dawley rats (230–405g, Harlan Laboratories, Indianapolis, IN; Charles River Laboratories, Wilmington, MA) were anesthetized with isoflurane, intubated via a tracheostomy, and mechanically ventilated (SAR-830; CWE, Ardmore, PA). The femoral artery was catheterized for continuous blood pressure and heart rate monitoring (ADInstruments, Ardmore, PA), and intermittent arterial blood gas and pH measurements (Rapidlab 248 blood gas/pH analyzer, Siemens HealthCare, Eschborn, Germany). Rectal temperature was maintained at 37 °C. All systemic physiological parameters were within normal ranges for anesthetized rats and did not differ among experimental groups (Table 1).
Table 1.
Systemic physiology
| Body weight (g) | Hemisphere | BP (mmHg) | pH | pCO2 (mmHg) | pO2 (mmHg) | ||
|---|---|---|---|---|---|---|---|
| nVNS | Sham | 369±29 | 1st | 104±2 | 7.44±0.01 | 37±1 | 118±6 |
| 2nd | 96±4 | 7.44±0.01 | 38±1 | 114±2 | |||
| Stimulated | 353±29 | 1st | 96±3 | 7.43±0.01 | 39±1 | 112±5 | |
| 2nd | 100±3 | 7.43±0.01 | 39±1 | 111±3 | |||
| iVNS | Naïve | 349±16 | 1st | 112±3 | 7.43±0.01 | 39±1 | 115±7 |
| 2nd | 110±5 | 7.42±0.01 | 37±1 | 104±7 | |||
| Sham | 300±20 | 1st | 120±5 | 7.41±0.02 | 39±2 | 124±9 | |
| 2nd | 117±4 | 7.41±0.01 | 37±1 | 113±5 | |||
| Stimulated | 336±22 | 1st | 110±6 | 7.42±0.01 | 38±1 | 103±4 | |
| 2nd | 111±6 | 7.42±0.01 | 36±1 | 116±9 |
2.3 VNS paradigms
VNS was delivered either non-invasively through the skin or directly by electrodes placed around the vagus nerve (Figure 1A). For non-invasive transcutaneous VNS (nVNS), two disc electrodes (6 mm diameter, 5 mm electrode separation) were connected to a customized stimulator modified from the current gammaCore nVNS device (electroCore LLC, Basking Ridge, NJ), and placed on the intact skin directly over the cervical portion of the right vagus nerve (approximately 5–8 mm lateral from midline at the level of larynx), after shaving and application of a conductive gel to ensure proper electrode contact. To control for the potential effects of cervical skin and muscle stimulation that occur during nVNS, in a separate cohort we tested non-invasive transcutaneous stimulation of femoral nerve branches (nFNS) in the anterior thigh area overlying the quadriceps femoris muscle using the same protocol, electrode apparatus and device as in nVNS. This area was chosen for the paucity of autonomic nerve fibers to minimize inadvertent stimulation of visceral afferents. For invasive direct VNS (iVNS), self-constructed bipolar helical electrodes [3] were surgically placed around the mid-cervical portion of the right vagus nerve (at the level of larynx; electrode separation 1.5 mm). Sham controls had identical electrode placements but without stimulation. Sham-operated rats in the iVNS group were separately compared with naïve rats that did not undergo surgical exploration of the vagus nerve to control for the effects of electrode placement.
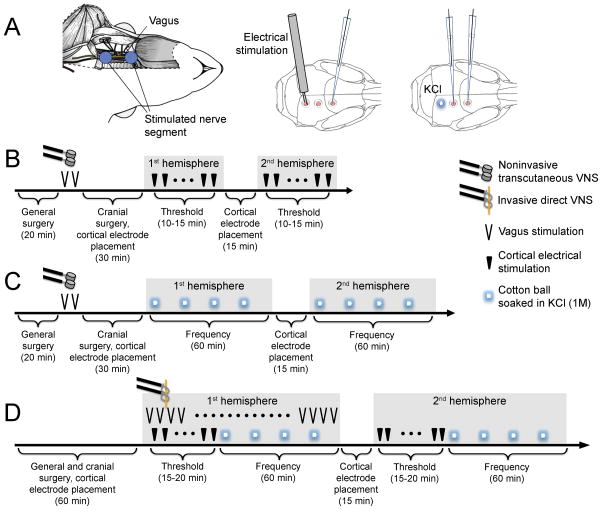
Fig. 1. Experimental setup and VNS paradigms.
A) Left: Surgical exposure of the vagus nerve for iVNS and the vagus nerve segment stimulated using the helical electrode are shown. For nVNS, transcutaneous disc electrodes were placed where indicated by blue circles. Middle and right: Craniotomies were prepared on occipital cortex for electrical or KCl stimulation, and on parietal and frontal cortices for electrophysiological recordings. B) Experimental timeline to test nVNS on electrical CSD threshold. C) Experimental timeline to test nVNS on KCl-induced CSD frequency. D) Experimental timeline to test iVNS on electrical CSD threshold and KCl-induced CSD frequency. All symbols are defined on the right. Details of stimulation parameters are provided in the Methods.
In the nVNS paradigm (Figures 1B and C), stimulation was delivered while the rat was in a supine position after general surgical preparation but prior to placement on the stereotaxic frame. Two 2-min stimulus trains (1ms pulse of 5kHz sine waves at 25 Hz) were delivered with an interval of 5 minutes. Systemic physiology was monitored during the stimulation. Rats were then transferred to the stereotaxic frame for cranial surgical preparation. CSD susceptibility testing always started in the ipsilateral hemisphere approximately 30 minutes after nVNS. The CSD frequency during topical KCl application and electrical CSD threshold were determined in separate cohorts.
In the iVNS paradigm (Figure 1D), electrodes were placed around the right vagus nerve as part of general surgical preparation, rats were transferred to the stereotaxic frame, and cranial surgery completed. We delivered 30 sec trains of 0.5 ms, 0.5 mA square pulses at 20 Hz (S48 stimulator, and stimulus isolation and constant current unit, Grass Instruments, West Warwick, RI) every 5 minutes, as reported previously [3; 61]. The first iVNS started 2.5 minutes before the first cortical electrical stimulus, and repeated iVNS trains were interleaved with incremental steps of cortical stimulation to determine the CSD threshold. iVNS was continued using the same parameters during the subsequent CSD frequency determination during topical KCl application. Therefore, iVNS was delivered during the entire CSD susceptibility testing in the first hemisphere, and then stopped during the testing in the second hemisphere. In iVNS, CSD testing started either in the right or in the left hemisphere in alternating order in each successive rat. Therefore, in half of the rats the hemisphere contralateral to iVNS was studied first.
2.4 Cranial surgery
Animals were placed on a stereotactic frame (Stoelting, Wood Dale, IL, USA), and craniotomies were drilled under saline cooling for occipital stimulation (mm from bregma: 4.5 posterior, 2 lateral; diameter 2 mm), and parietal and frontal recordings (1.5 posterior, 2 lateral, and 1.5 anterior, 2 lateral; diameter 1 mm) (Figure 1A). Dura was carefully removed. Following surgical preparation and cortical electrode insertion, the cortex was allowed to rest for 15 minutes under saline irrigation. The electrocorticogram and direct current (DC) potential were recorded with glass capillary microelectrodes and amplified with a DC pre-amplifier (EX1 differential amplifiers, Dagan Corporation, Minneapolis, MN, USA) and continuously digitized (PowerLab, ADInstruments, Colorado Springs, CO, USA).
2.5 Electrophysiology and CSD susceptibility
Susceptibility to CSD was evaluated by measuring the electrical threshold for CSD, followed by analysis of CSD frequency and propagation speed during continuous topical application of KCl, as previously described in detail [7; 8]. The electrical threshold to induce CSD was determined by direct cortical stimulation using a stimulus isolator (WPI, Sarasota, FL) and a bipolar stimulation electrode (400 μm tip diameter, 1 mm tip separation; Frederick Haer Company, Bowdoin, ME, USA) placed on the pial surface. Single-square pulses of increasing duration and intensity (50–4,000 μC) were applied at 5-minute intervals until a CSD was observed. Migraine-susceptible brains may develop repetitive/reverberating CSDs [24; 26], and analysis of CSD frequency therefore provides another tool to assess CSD susceptibility and the efficacy of migraine treatments. For analysis of CSD frequency and propagation speed, a cotton ball (1.5–2 mm diameter) soaked with KCl (1M) was placed on the occipital cortex and changed every 15 minutes for 1 hour. CSDs were continuously recorded, and only counted if their amplitudes were 5 mV or higher. Other attributes of CSD, such as propagation speed, amplitude, and duration at half-maximal amplitude, were also measured.
2.6 Cerebral blood flow (CBF)
To examine the acute effects of nVNS on CBF, we placed one laser Doppler flowmetry probe (Perimed, Sweden) over each temporal bone, and continuously measured CBF during nVNS and for 30 minutes thereafter under isoflurane anesthesia. Arterial blood pressure and blood gases were measured as described in the general surgical preparation section above. Data were expressed as % of baseline.
2.7 Study design and statistics
None of the animals died or experienced severe complications during electrode implantation or recordings. Animals were randomly assigned to VNS or sham control groups. Effective blinding was not possible during the experiment due to the transient hypotensive effect of VNS. However, measurements were confirmed post-hoc by an independent, blinded investigator. Sample sizes were estimated to detect an effect size of 35% based on a standard deviation of 25% of the mean (α=0.05; 80% power). In anticipation of potential surgical failures, two more rats were added a priori in the iVNS group. Data are expressed as whisker-box plots (whisker, full range; box interquartile range; line, median, cross, mean), mean±SEM or median [interquartile range]. A priori exclusion criteria were surgical failure and poor systemic physiology. In the nVNS paradigm only, one rat was excluded because of femoral artery catheter dislocation. Continuous variables were analyzed with paired or unpaired Student’s t-tests, Pearson correlation, or two-way analysis of variance (ANOVA) for repeated measures with post-hoc test as appropriate (GraphPad Prism). A p-value of <0.05 was considered statistically significant.
3. Results
3.1 Effect of nVNS on CSD susceptibility
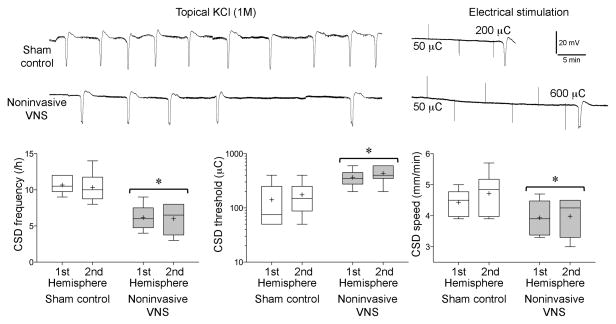
Noninvasive transcutaneous VNS (nVNS) 30 minutes prior to testing significantly suppressed CSD susceptibility (Figure 2). The frequency of repetitive CSDs triggered by continuous topical KCl application was reduced by 40% (n=6 each, nVNS and sham), and the cortical electrical stimulation intensity required to initiate a CSD was elevated by ~2.5 fold (n=6 each, nVNS and sham). Propagation speed of CSD was also significantly reduced. Importantly, these effects were nearly identical in both hemispheres, studied sequentially but independently in the same animal. Based on the experimental timeline (Figures 1B and C), CSD suppression was fully established within 30 minutes after nVNS, and lasted at least 3 hours in this paradigm. Non-invasive stimulation of femoral nerve (nFNS) to control for potential nonspecific effects of cutaneous and muscle stimulation did not alter CSD attributes compared to sham controls in a separate cohort (Supplemental Figure).
Fig. 2. Effect of nVNS on KCl-induced SD frequency, electrical SD threshold, and SD propagation speed.
Representative intracortical microelectrode recordings are provided from the second hemispheres of control and nVNS animals. Graphs show the data from the first and second hemispheres of sham control and nVNS groups (n=6 each), expressed as whisker-box plots (whisker, full range; box interquartile range; line, median, cross, mean). Note that the vertical axis of CSD threshold is in log scale. *p<0.05 vs. sham controls; two-way ANOVA for repeated measures.
3.2 Effect of iVNS on CSD susceptibility
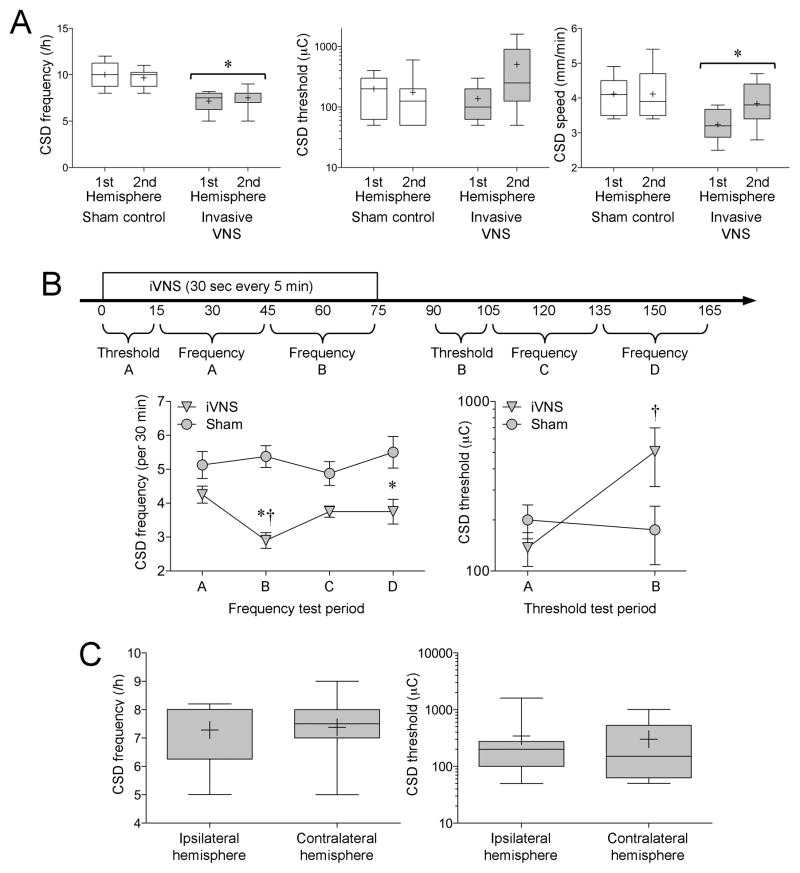
Invasive direct VNS (iVNS) also suppressed CSD susceptibility (n=8 each, iVNS and sham; Figure 3A). In this paradigm, iVNS was started simultaneously with CSD testing (Figure 1D). Consequently, CSD suppression differed between the initial and subsequent stages of testing. To better define the time-dependence of iVNS efficacy, we analyzed the data separately in each successive experimental stage (Figure 3B). The first CSD threshold (Threshold A) tested during the first 15 minutes of iVNS did not differ from sham controls, whereas the threshold tested more than 90 minutes after iVNS onset (Threshold B) was significantly higher. Similarly, KCl-induced CSD frequency between 15 and 45 minutes after iVNS onset (Frequency A) was only mildly reduced (~17%; p>0.05), whereas the frequency between 45 and 75 minutes (Frequency B) significantly decreased by 46% compared to sham controls, and remained lower throughout the rest of the experiment (Frequency C and D). Because in the iVNS paradigm we placed the electrode around the right vagus nerve, and started CSD testing first in the right or left hemisphere in alternating order in each successive rat, we were able to compare whether the efficacy of VNS differed between the ipsilateral and contralateral hemispheres independent of the effect of time. This comparison once again showed that iVNS-induced CSD suppression was nearly identical in the two hemispheres (Figure 3C). Importantly, CSD susceptibility in naïve controls that did not undergo any surgery (CSD frequency 10.4±0.8 and 9.5±0.4 CSDs/h, CSD threshold 200 [200–350] and 350 [125–600] μC, CSD propagation speed 4.2±0.5 and 4.2±0.2 mm/min, in the first and second hemispheres, respectively, in naïve rats; n=8) did not differ from sham operated rats.
Fig. 3. Effect of iVNS on KCl-induced SD frequency, electrical SD threshold, and SD propagation speed.
A) Graphs show the data from the first and second hemispheres of sham control and iVNS groups (n=8 each), expressed as whisker-box plots (whisker, full range; box interquartile range; line, median, cross, mean). *p<0.05 vs. sham controls; two-way ANOVA for repeated measures. B) Because CSD susceptibility testing was started simultaneously with the iVNS, time was a significant factor. Therefore, we analyzed the data for each time period separately as indicated on the timeline, and compared each time period in iVNS group to the corresponding one in sham controls as well as to the other time periods in the same group. Graphs show the data from each time period in sham control and iVNS groups, expressed as mean±SEM. Note that the vertical axis of CSD threshold is in log scale. *p<0.05 vs. sham controls; †p<0.05 vs. iVNS test period A; two-way ANOVA for repeated measures. C) We found no difference between the ipsilateral and contralateral hemisphere to the iVNS side independent of time (see Methods and Results), suggesting that iVNS efficacy was bilateral (t-test).
3.3 Effect of VNS on blood pressure, heart rate, and cerebral blood flow
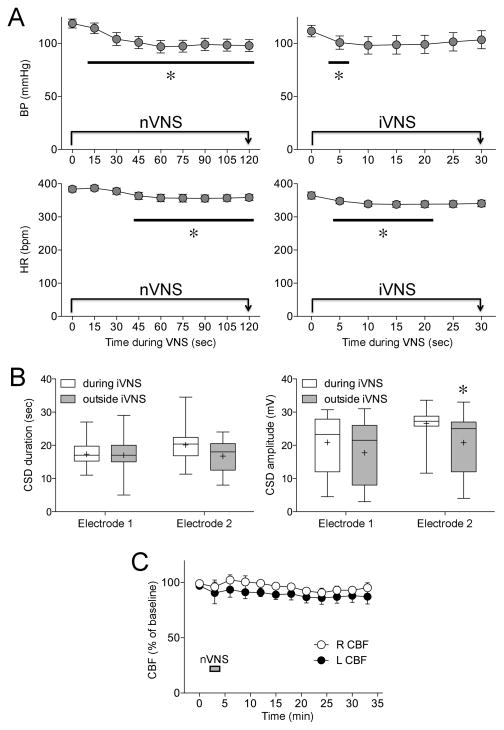
Neither nVNS nor iVNS had any effect on CSD amplitude or duration (Table 2). However, in both paradigms, we observed transient and mild blood pressure and heart rate drops during the stimulation (Figure 4A), which recovered promptly upon stimulus cessation, as reported previously [3]. The magnitude of blood pressure drop during nVNS did not correlate with CSD frequency, threshold, or propagation speed in individual rats (R2=0.18–0.33, p=0.23–0.47 for nVNS, and R2=0.00–0.20, p=0.31–0.99 for iVNS). Since iVNS was delivered during CSD susceptibility testing in the first hemisphere (Figure 1D), some KCl-induced CSDs coincided with the 30-second iVNS periods. The duration of CSDs that coincided with iVNS did not significantly differ from CSDs that did not coincide with iVNS, but their amplitude was slightly larger (Figure 4B). Lastly, in order to test whether changes in CSD susceptibility might be caused by changes in cerebral perfusion upon VNS, we studied CBF in both ipsilateral and contralateral hemispheres in a separate group of rats (n=4), and found no significant change in CBF during or for 30 minutes after nVNS (Figure 4C).
Table 2.
SD amplitude and duration (data are from the first SD detected at the posterior electrode)
| Hemisphere | Experimental group | Duration (sec) | Amplitude (mV) | |
|---|---|---|---|---|
| nVNS | 1st | Sham | 23±1 | 27±1 |
| Stimulated | 23±1 | 26±1 | ||
| 2nd | Sham | 20±2 | 24±2 | |
| Stimulated | 22±2 | 22±2 | ||
| iVNS | 1st | Sham | 19±1 | 29±1 |
| Stimulated | 21±1 | 28±0 | ||
| 2nd | Sham | 19±1 | 27±2 | |
| Stimulated | 19±1 | 27±1 |
Fig. 4. Systemic physiological, electrophysiological and cerebral blood flow effects.
A) The time course of arterial blood pressure (BP) and heart rate (HR) during nVNS (n=12 stimulations) or iVNS (n=98 stimulations). Note that some error bars are too small to be visible. *p<0.05 vs. baseline (time 0); two-way ANOVA for repeated measures. B) Because CSD susceptibility testing was started simultaneously with the iVNS, some KCl-induced CSDs coincided with the 30 sec iVNS delivery. The duration of CSDs that occurred during iVNS (n=20) did not differ from those that occurred when iVNS was not on (n=32), although their amplitude was slightly larger. The significance of this finding, however, is unclear. *p<0.05 vs. during iVNS; two-way ANOVA for repeated measures. C) Cerebral blood flow (CBF) was studied in a separate cohort of rats (n=4), and did not significantly change in either the right or left hemisphere during nVNS delivered on the right side.
Discussion
In this study, we show for the first time that VNS acutely suppresses CSD susceptibility as a potential mechanism for its therapeutic efficacy in migraine. The degree of CSD suppression by VNS was comparable to suppression by migraine prophylactic drugs previously demonstrated in the same experimental paradigm [8; 13; 25]. We demonstrate the efficacy using both non-invasive transcutaneous and invasive VNS approaches currently approved for clinical use, employing experimental techniques accepted as the gold standard to assess CSD susceptibility, and under full systemic physiological monitoring to eliminate potential confounders [7].
Several case series and one open-label trial suggest that VNS can be an effective treatment against migraine [11; 17; 30; 34; 43; 48; 59]. In retrospective case series (n=24), headache frequency was reduced in 75% of patients using iVNS as a preventive treatment [11; 17; 34; 43; 48; 59]. The VNS paradigms employed in these cases (20–30 Hz, 30 sec trains of 0.25–0.5 ms/0.25–2.25 mA square pulses, every 5 min), were nearly identical to our iVNS paradigm (20 Hz, 30 sec trains of 0.5 ms/0.5 mA square pulses, every 5 min). In a prospective open-label trial of nVNS in 30 patients, the 2-hour pain-free rate was 22% for moderate to severe attacks [30], comparable to triptans [28] and non-steroidal anti-inflammatory drugs [41]. The clinical nVNS paradigm (two 90 sec sinusoidal nVNS of the right cervical vagus nerve separated by 15 minutes) was once again nearly identical to our study in rats (two 120 sec sinusoidal nVNS of right cervical vagus nerve separated by 5 minutes). None of the clinical studies investigated the effect of VNS on aura.
These data are also congruent with the efficacy of iVNS in epilepsy and ischemic stroke, two conditions that show a strong clinical association with migraine [57]. For example, iVNS has been safe and effective in refractory partial or generalized epilepsy [20; 50], with more than 50% seizure reduction in more than half of the patients [20; 50]. Efficacious iVNS paradigms in epilepsy typically consisted of 30 Hz, 30 sec trains of 0.25–0.5 ms/1.0–1.5 mA, every 5 minutes [39], nearly identical to our study. In experimental models, similar iVNS paradigms reduced spiking frequency and increased seizure threshold [1]. Experimental data also suggest that iVNS (20 Hz, 30 sec trains of 0.3–0.5 ms/0.5 mA square pulses, every 5 minutes for 1–3h) reduces infarct size and improves functional outcome in animal models of focal cerebral ischemia [3; 33]. Taken together, our data implicate SD inhibition as one mechanism by which VNS may improve stroke outcomes as well [25; 60].
CSD is associated with activation of voltage-gated and other large conductance cation channels causing massive transmembrane K+, Na+, Ca2+ and water fluxes that last up to a minute [9]. As a result, extracellular K+ and glutamate concentrations increase during CSD by an order of magnitude or more. It is generally accepted that the K+ and glutamate released from the depolarized tissue diffuse into adjacent tissues and trigger the same depolarization cycle, allowing CSD to slowly propagate by way of contiguity. Although glutamatergic receptors, particularly of NMDA subtype, are critical for CSD, numerous other neurotransmitter, neuromodulator and ion channel systems, including noradrenaline, serotonin and cytokines, have also been shown to modulate CSD susceptibility, one or more of which are likely to be responsible for VNS efficacy [5; 7; 23].
This is supported by data on the mechanisms of action of VNS in other related disease models such as epilepsy and stroke. Eighty percent of the vagus nerve is comprised of sensory afferents, which terminate in the NTS bilaterally, and then project to the LC, DRN, nucleus basalis and cerebellar fastigial nucleus among other structures [18; 19]. VNS upregulates c-fos expression in the NTS and the LC [4; 21], and increases neuronal firing in the LC [22; 32] and the DRN [22]. There is a large body of data strongly suggesting that the efficacy of VNS in epilepsy and stroke depends on activation of these afferent fibers and their projections to subcortical nuclei [4; 21; 22; 29; 32; 38; 45; 51; 58; 62]. These subcortical nuclei likely mediate CSD suppression by VNS as well. For example, electrical stimulation of the fastigial nucleus elevated the CSD threshold by almost 3 fold, and reduces the propagation speed by a third [31]. VNS induces norepinephrine release from LC [29], and norepinephrine inhibits CSD [56]. VNS also increases serotonin in dorsal raphe [46], and serotonin depletion has been shown to facilitate CSD [42]. Furthermore, VNS inhibits the release of pro-inflammatory cytokines [16; 36] that are known to facilitate CSD [55]. VNS increases GABA in the cerebrospinal fluid and cortical GABA(A) receptor density in patients with epilepsy [12; 47], although the relevance of these in CSD suppression is not clear [5]. Lastly, VNS inhibits glutamate release [12; 49], the neurotransmitter critical for CSD initiation and spread, and reduces the excitability of pyramidal cells in cortical layer V [63]. More work is needed to dissect the contribution of each one of these mechanisms to CSD suppression after VNS. Although vagus nerve also carries the principal parasympathetic input to thoracoabdominal organs, these autonomic efferents are unlikely to suppress CSD in occipital cortex. Of course, mechanisms independent of CSD may also contribute to the efficacy of VNS in migraine. For example, VNS has been shown to reduce chemically-induced trigeminal pain and allodynia, as well as neuronal firing, c-fos expression and glutamate release in trigeminal nucleus caudalis [14; 44; 53].
4.1 Conclusion
In summary, we show for the first time that VNS acutely suppresses CSD susceptibility, reminiscent of its efficacy in epilepsy and stroke, two comorbid conditions with migraine. Therefore, our data provide a mechanism by which VNS may be efficacious in migraine. Because of the brief but nevertheless clinically relevant latency between VNS onset and SD suppression in this experimental model (~30 min), we believe VNS may find clinical utility as an acute preemptive intervention. It remains to be determined whether chronic daily VNS affords prophylactic efficacy that is comparable to or stronger than the acute preemptive efficacy tested in this study.
Supplementary Material
Acknowledgments
This work was funded in part by the American Heart Association (10SDG2610275, KEH; 10SDG2600218, IA), the Massachusetts General Hospital (Claflin Distinguished Award, KEH), and an unrestricted research gift from Electrocore LLC.
Footnotes
Author contributions:
Dr. Chen - Study concept and design, experiments, acquisition of data, analysis and interpretation, manuscript writing
Dr. Ay - Study concept and design, experiments, acquisition of data, critical revision of the manuscript for important intellectual content
Dr. Lopes de Morais - experiments, acquisition of data and analysis.
Dr. Qin - acquisition of data, analysis and interpretation
Dr. Zheng - critical revision of the manuscript for important intellectual content
Dr. Sadhegian - acquisition of data, analysis and interpretation
Dr. Oka- acquisition of data, analysis and interpretation
Dr. Simon - Study concept and design, critical revision of the manuscript for important intellectual content
Dr. Eikermann-Haerter - Study concept and design, study supervision, and manuscript writing
Dr. Ayata - Study concept and design, study supervision, and manuscript writing
Conflict of Interest:
Dr. Bruce Simon is an employee of ElectroCore LLC, and joined the project after completion of the experiments investigating the effect of invasive vagus nerve stimulation. The other authors declare no competing financial interests.
References
- 1.Aalbers M, Vles J, Klinkenberg S, Hoogland G, Majoie M, Rijkers K. Animal models for vagus nerve stimulation in epilepsy. Exp Neurol. 2011;230(2):167–175. doi: 10.1016/j.expneurol.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Anttila V, Winsvold BS, Gormley P, Kurth T, Bettella F, McMahon G, Kallela M, Malik R, de Vries B, Terwindt G, Medland SE, Todt U, McArdle WL, Quaye L, Koiranen M, Ikram MA, Lehtimaki T, Stam AH, Ligthart L, Wedenoja J, Dunham I, Neale BM, Palta P, Hamalainen E, Schurks M, Rose LM, Buring JE, Ridker PM, Steinberg S, Stefansson H, Jakobsson F, Lawlor DA, Evans DM, Ring SM, Farkkila M, Artto V, Kaunisto MA, Freilinger T, Schoenen J, Frants RR, Pelzer N, Weller CM, Zielman R, Heath AC, Madden PA, Montgomery GW, Martin NG, Borck G, Gobel H, Heinze A, Heinze-Kuhn K, Williams FM, Hartikainen AL, Pouta A, van den Ende J, Uitterlinden AG, Hofman A, Amin N, Hottenga JJ, Vink JM, Heikkila K, Alexander M, Muller-Myhsok B, Schreiber S, Meitinger T, Wichmann HE, Aromaa A, Eriksson JG, Traynor BJ, Trabzuni D, Rossin E, Lage K, Jacobs SB, Gibbs JR, Birney E, Kaprio J, Penninx BW, Boomsma DI, van Duijn C, Raitakari O, Jarvelin MR, Zwart JA, Cherkas L, Strachan DP, Kubisch C, Ferrari MD, van den Maagdenberg AM, Dichgans M, Wessman M, Smith GD, Stefansson K, Daly MJ, Nyholt DR, Chasman DI, Palotie A North American Brain Expression C, Consortium UKBE, International Headache Genetics C. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45(8):912–917. doi: 10.1038/ng.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ay I, Lu J, Ay H, Gregory Sorensen A. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett. 2009;459(3):147–151. doi: 10.1016/j.neulet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Ay I, Napadow V, Ay H. Electrical stimulation of the vagus nerve dermatome in the external ear is protective in rat cerebral ischemia. Brain Stimul. 2015;8(1):7–12. doi: 10.1016/j.brs.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayata C. Spreading depression: from serendipity to targeted therapy in migraine prophylaxis. Cephalalgia. 2009;29(10):1095–1114. doi: 10.1111/j.1468-2982.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 6.Ayata C. Cortical spreading depression triggers migraine attack: pro. Headache. 2010;50(4):725–730. doi: 10.1111/j.1526-4610.2010.01647.x. [DOI] [PubMed] [Google Scholar]
- 7.Ayata C. Pearls and pitfalls in experimental models of spreading depression. Cephalalgia. 2013;33(8):604–613. doi: 10.1177/0333102412470216. [DOI] [PubMed] [Google Scholar]
- 8.Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59(4):652–661. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- 9.Ayata C, Lauritzen M. Spreading Depression, Spreading Depolarizations, and the Cerebral Vasculature. Physiol Rev. 2015;95(3):953–993. doi: 10.1152/physrev.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbanti P, Grazzi L, Egeo G, Padovan AM, Liebler E, Bussone G. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain. 2015;16(1):542. doi: 10.1186/s10194-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basic S, Sporis D, Chudy D, Grahovac G, Nevajda B. The effect of vagus nerve stimulation on migraine in patient with intractable epilepsy: case report. Neurol Sci. 2013;34(5):797–798. doi: 10.1007/s10072-012-1135-5. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, Treig T, Stefan H, Ramsay RE, Wernicke JF, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221–227. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 13.Bogdanov VB, Multon S, Chauvel V, Bogdanova OV, Prodanov D, Makarchuk MY, Schoenen J. Migraine preventive drugs differentially affect cortical spreading depression in rat. Neurobiol Dis. 2011;41(2):430–435. doi: 10.1016/j.nbd.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Bohotin C, Scholsem M, Multon S, Martin D, Bohotin V, Schoenen J. Vagus nerve stimulation in awake rats reduces formalin-induced nociceptive behaviour and fos-immunoreactivity in trigeminal nucleus caudalis. Pain. 2003;101(1–2):3–12. doi: 10.1016/s0304-3959(02)00301-9. [DOI] [PubMed] [Google Scholar]
- 15.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8(2):136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 16.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 17.Cecchini AP, Mea E, Tullo V, Curone M, Franzini A, Broggi G, Savino M, Bussone G, Leone M. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci. 2009;30(Suppl 1):S101–104. doi: 10.1007/s10072-009-0073-3. [DOI] [PubMed] [Google Scholar]
- 18.Chae JH, Nahas Z, Lomarev M, Denslow S, Lorberbaum JP, Bohning DE, George MS. A review of functional neuroimaging studies of vagus nerve stimulation (VNS) J Psychiatr Res. 2003;37(6):443–455. doi: 10.1016/s0022-3956(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 19.Cheyuo C, Jacob A, Wu R, Zhou M, Coppa GF, Wang P. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab. 2011;31(5):1187–1195. doi: 10.1038/jcbfm.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor DE, Jr, Nixon M, Nanda A, Guthikonda B. Vagal nerve stimulation for the treatment of medically refractory epilepsy: a review of the current literature. Neurosurg Focus. 2012;32(3):E12. doi: 10.3171/2011.12.FOCUS11328. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by Vagal nerve stimulation. Neuropsychopharmacology. 2008;33(8):1884–1895. doi: 10.1038/sj.npp.1301570. [DOI] [PubMed] [Google Scholar]
- 22.Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318(2):890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- 23.Eikermann-Haerter K, Can A, Ayata C. Pharmacological targeting of spreading depression in migraine. Expert Rev Neurother. 2012;12(3):297–306. doi: 10.1586/ern.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eikermann-Haerter K, Dilekoz E, Kudo C, Savitz SI, Waeber C, Baum MJ, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest. 2009;119(1):99–109. doi: 10.1172/JCI36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eikermann-Haerter K, Lee JH, Yalcin N, Yu ES, Daneshmand A, Wei Y, Zheng Y, Can A, Sengul B, Ferrari MD, van den Maagdenberg AM, Ayata C. Migraine prophylaxis, ischemic depolarizations, and stroke outcomes in mice. Stroke; a journal of cerebral circulation. 2015;46(1):229–236. doi: 10.1161/STROKEAHA.114.006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eikermann-Haerter K, Yuzawa I, Qin T, Wang Y, Baek K, Kim YR, Hoffmann U, Dilekoz E, Waeber C, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C. Enhanced subcortical spreading depression in familial hemiplegic migraine type 1 mutant mice. J Neurosci. 2011;31(15):5755–5763. doi: 10.1523/JNEUROSCI.5346-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrari MD, Klever RR, Terwindt GM, Ayata C, van den Maagdenberg AM. Migraine pathophysiology: lessons from mouse models and human genetics. The Lancet Neurology. 2015;14(1):65–80. doi: 10.1016/S1474-4422(14)70220-0. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358(9294):1668–1675. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]
- 29.Fornai F, Ruffoli R, Giorgi FS, Paparelli A. The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. Eur J Neurosci. 2011;33(12):2169–2178. doi: 10.1111/j.1460-9568.2011.07707.x. [DOI] [PubMed] [Google Scholar]
- 30.Goadsby P, Grosberg B, Mauskop A, Cady R, Simmons K. Effect of noninvasive vagus nerve stimulation on acute migraine: An open-label pilot study. Cephalalgia. 2014 doi: 10.1177/0333102414524494. [DOI] [PubMed] [Google Scholar]
- 31.Golanov EV, Reis DJ. Neuroprotective electrical stimulation of cerebellar fastigial nucleus attenuates expression of periinfarction depolarizing waves (PIDs) and inhibits cortical spreading depression. Brain Res. 1999;818(2):304–315. doi: 10.1016/s0006-8993(98)01169-x. [DOI] [PubMed] [Google Scholar]
- 32.Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett. 2005;379(3):174–179. doi: 10.1016/j.neulet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 33.Hiraki T, Baker W, Greenberg JH. Effect of vagus nerve stimulation during transient focal cerebral ischemia on chronic outcome in rats. Journal of neuroscience research. 2012;90(4):887–894. doi: 10.1002/jnr.22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagus nerve stimulation on migraines. J Pain. 2003;4(9):530–534. doi: 10.1016/j.jpain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y, Li L, Liu B, Zhang Y, Chen Q, Li C. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS One. 2014;9(7):e102342. doi: 10.1371/journal.pone.0102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston GR, Webster NR. Cytokines and the immunomodulatory function of the vagus nerve. Br J Anaesth. 2009;102(4):453–462. doi: 10.1093/bja/aep037. [DOI] [PubMed] [Google Scholar]
- 37.Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, Dalkara T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339(6123):1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 38.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 39.Labiner DM, Ahern GL. Vagus nerve stimulation therapy in depression and epilepsy: therapeutic parameter settings. Acta Neurol Scand. 2007;115(1):23–33. doi: 10.1111/j.1600-0404.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 40.Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31(1):17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Law S, Derry S, Moore RA. Naproxen with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;10:CD009455. doi: 10.1002/14651858.CD009455.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.le Grand SM, Supornsilpchai W, Saengjaroentham C, Srikiatkhachorn A. Serotonin depletion leads to cortical hyperexcitability and trigeminal nociceptive facilitation via the nitric oxide pathway. Headache. 2011;51(7):1152–1160. doi: 10.1111/j.1526-4610.2011.01931.x. [DOI] [PubMed] [Google Scholar]
- 43.Lenaerts ME, Oommen KJ, Couch JR, Skaggs V. Can vagus nerve stimulation help migraine? Cephalalgia. 2008;28(4):392–395. doi: 10.1111/j.1468-2982.2008.01538.x. [DOI] [PubMed] [Google Scholar]
- 44.Lyubashina OA, Sokolov AY, Panteleev SS. Vagal afferent modulation of spinal trigeminal neuronal responses to dural electrical stimulation in rats. Neuroscience. 2012;222:29–37. doi: 10.1016/j.neuroscience.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Magdaleno-Madrigal VM, Martínez-Vargas D, Valdés-Cruz A, Almazán-Alvarado S, Fernández-Mas R. Preemptive effect of nucleus of the solitary tract stimulation on amygdaloid kindling in freely moving cats. Epilepsia. 2010;51(3):438–444. doi: 10.1111/j.1528-1167.2009.02337.x. [DOI] [PubMed] [Google Scholar]
- 46.Manta S, El Mansari M, Debonnel G, Blier P. Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int J Neuropsychopharmacol. 2013;16(2):459–470. doi: 10.1017/S1461145712000387. [DOI] [PubMed] [Google Scholar]
- 47.Marrosu F, Serra A, Maleci A, Puligheddu M, Biggio G, Piga M. Correlation between GABA(A) receptor density and vagus nerve stimulation in individuals with drug-resistant partial epilepsy. Epilepsy Res. 2003;55(1–2):59–70. doi: 10.1016/s0920-1211(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 48.Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25(2):82–86. doi: 10.1111/j.1468-2982.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 49.Miyamoto O, Pang J, Sumitani K, Negi T, Hayashida Y, Itano T. Mechanisms of the anti-ischemic effect of vagus nerve stimulation in the gerbil hippocampus. Neuroreport. 2003;14(15):1971–1974. doi: 10.1097/00001756-200310270-00018. [DOI] [PubMed] [Google Scholar]
- 50.Morris GL, 3rd, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(16):1453–1459. doi: 10.1212/WNL.0b013e3182a393d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011;189:207–214. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 52.Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154(Suppl 1):S44–53. doi: 10.1016/j.pain.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 53.Oshinsky ML, Murphy AL, Hekierski H, Jr, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155(5):1037–1042. doi: 10.1016/j.pain.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- 55.Pusic KM, Pusic AD, Kemme J, Kraig RP. Spreading depression requires microglia and is decreased by their M2a polarization from environmental enrichment. Glia. 2014;62(7):1176–1194. doi: 10.1002/glia.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richter F, Mikulik O, Ebersberger A, Schaible HG. Noradrenergic agonists and antagonists influence migration of cortical spreading depression in rat-a possible mechanism of migraine prophylaxis and prevention of postischemic neuronal damage. J Cereb Blood Flow Metab. 2005;25(9):1225–1235. doi: 10.1038/sj.jcbfm.9600120. [DOI] [PubMed] [Google Scholar]
- 57.Rogawski MA. Migraine and Epilepsy-Shared Mechanisms within the Family of Episodic Disorders. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 58.Rutecki P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia. 1990;31(Suppl 2):S1–6. doi: 10.1111/j.1528-1157.1990.tb05843.x. [DOI] [PubMed] [Google Scholar]
- 59.Sadler RM, Purdy RA, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia. 2002;22(6):482–484. doi: 10.1046/j.1468-2982.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 60.Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26(8):1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- 61.Smith DC, Modglin AA, Roosevelt RW, Neese SL, Jensen RA, Browning RA, Clough RW. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. Journal of neurotrauma. 2005;22(12):1485–1502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker BR, Easton A, Gale K. Regulation of limbic motor seizures by GABA and glutamate transmission in nucleus tractus solitarius. Epilepsia. 1999;40(8):1051–1057. doi: 10.1111/j.1528-1157.1999.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 63.Zagon A, Kemeny AA. Slow hyperpolarization in cortical neurons: a possible mechanism behind vagus nerve simulation therapy for refractory epilepsy? Epilepsia. 2000;41(11):1382–1389. doi: 10.1111/j.1528-1157.2000.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011;69(5):855–865. doi: 10.1002/ana.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010;30(26):8807–8814. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.