Abstract
Childhood functional gastrointestinal disorders (FGIDs) affect a large number of children throughout the world. Carbohydrates (which provide the majority of calories consumed in the Western diet) have been implicated both as culprits for the etiology of symptoms and as potential therapeutic agents (e.g., fiber) in childhood FGIDs. In this review, we detail how carbohydrate malabsorption may cause gastrointestinal symptoms (e.g., bloating) via the physiologic effects of both increased osmotic activity and increased gas production from bacterial fermentation. Several factors may play a role, including: (1) the amount of carbohydrate ingested; (2) whether ingestion is accompanied by a meal or other food; (3) the rate of gastric emptying (how quickly the meal enters the small intestine); (4) small intestinal transit time (the time it takes for a meal to enter the large intestine after first entering the small intestine); (5) whether the meal contains bacteria with enzymes capable of breaking down the carbohydrate; (6) colonic bacterial adaptation to one’s diet, and (7) host factors such as the presence or absence of visceral hypersensitivity. By detailing controlled and uncontrolled trials, we describe how there is a general lack of strong evidence supporting restriction of individual carbohydrates (e.g., lactose, fructose) for childhood FGIDs. We review emerging evidence suggesting that a more comprehensive restriction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) may be effective. Finally, we review how soluble fiber (a complex carbohydrate) supplementation via randomized controlled intervention trials in childhood functional gastrointestinal disorders has demonstrated efficacy.
Keywords: Lactose, Fructose, FODMAP, Irritable bowel syndrome, Fiber, Recurrent abdominal pain
Introduction
Childhood abdominal pain-related functional gastrointestinal disorders (AP-FGIDs) affect up to 20% of children worldwide and account for at least 5% of all pediatric office visits in the United States [1, 2] . These disorders do not have an identifiable organic etiology based on conventional diagnostic testing; nevertheless, children with AP-FGIDs have both decreased quality of life and increased school absences as compared to their peers [3]. The gastrointestinal (GI) symptoms (e.g., abdominal pain) associated with these disorders may persist for years and into adulthood [4]. Successful interventions that ameliorate these symptoms in childhood AP-FGIDs may have an impact into adulthood [4]. Unfortunately, despite growing interest and research, current conventional clinical interventions for these disorders are often ineffective.
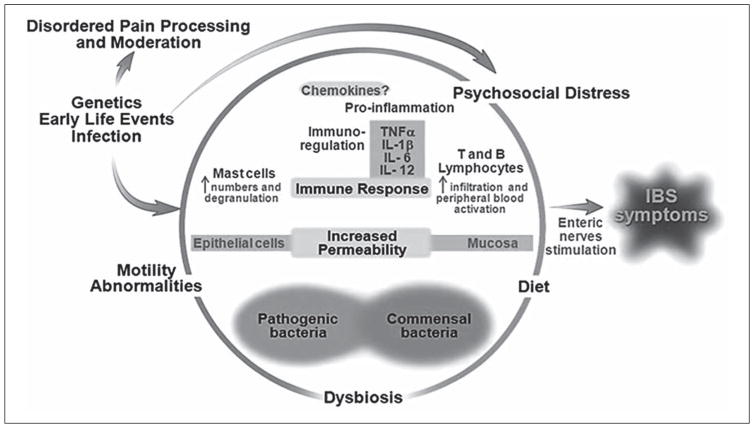
Though previously defined under the broad category of recurrent abdominal pain (RAP), using Rome III criteria, AP-FGIDs are now classified into: irritable bowel syndrome (IBS), functional dyspepsia, functional abdominal pain, and abdominal migraine [5]. The etiology of AP-FGIDs is believed to be multifactorial and is best approached using the biopsychosocial model (fig. 1). Potential factors include: psychosocial distress (e.g., somatization, anxiety); alterations in the composition and function of the gut microbiome; low-grade gut inflammation; increased gut permeability; visceral hypersensitivity; altered GI motor function, and diet. Diet in particular has generated interest as a culprit given that children with AP-FGIDs often associate symptoms with intake of particular foods [6].
Fig. 1.
Biopsychosocial model of FGIDs. Modified from Rodriguez-Fandino et al. [45] with permission from the Journal of Neurogastroenterology and Motility.
Carbohydrates provide the majority of calories consumed in the Western diet [7]. For many decades, individual carbohydrates (e.g., lactose) have been implicated both as culprits for the etiology of symptoms and as potential therapeutic agents (e.g., fiber) in children with AP-FGIDs. This review will examine the proposed patho-physiology of carbohydrate intolerance and the current evidence implicating individual carbohydrates and groupings of carbohydrates in causing or improving symptoms in childhood AP-FGIDs. It will also briefly examine the proposed digestive physiology of fiber and the current evidence pointing toward the use of fiber in children with AP-FGIDs.
Carbohydrate Intolerance
Proposed Common Pathophysiologic Mechanisms
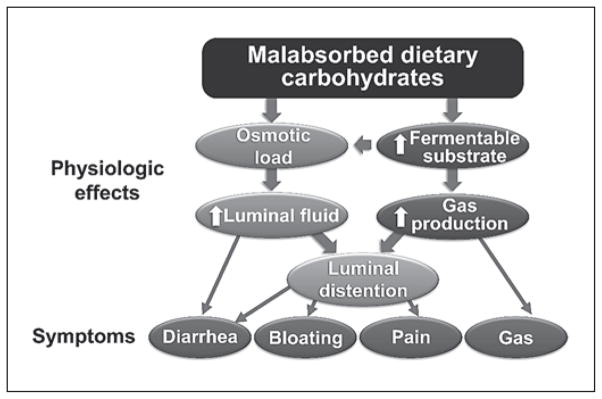
In children with AP-FGIDs, several individual carbohydrates (including lactose and fructose) have been implicated as exacerbating GI symptoms [8, 9]. As a group, these individual carbohydrates in combination with fructans, galactans, and polyols are termed fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) carbohydrates (table 1). FODMAP carbohydrates are rapidly fermented by colonic bacteria and are osmotically active (fig. 2). These physiologic effects can cause colonic distention from influx of water into the lumen and may lead to symptoms of abdominal pain, flatus, bloating, and loose and/or more frequent stools. These nonimmunologic adverse reactions to carbohydrates are termed carbohydrate intolerance.
Table 1.
Listing of carbohydrates and examples of foods which contain them within the FODMAP group
| Carbohydrate | Structure | Common foods |
|---|---|---|
| Fructose | Monosaccharide | Certain fruits: apples, pears; honey; juices |
| Lactose | Disaccharide | Dairy products: cow’s milk, cheese |
| Fructans | Fructose polymers | Wheat, onions, rye |
| Galactans | Galactose polymers | Beans, legumes, asparagus |
| Polyols | Sugar alcohols | Certain fruits and vegetables: apricots, cherries, pears |
Fig. 2.
Proposed pathophysiologic mechanism of malabsorbed carbohydrates in FGIDs. Modified from Barrett et al. [46] with permission from Practical Gastroenterology.
Though grouped together as FODMAP carbohydrates, each individual malabsorbed carbohydrate may have a different physiologic effect. Magnetic resonance imaging evaluation in healthy adults demonstrates that fructose significantly increases the small bowel water content; however, fructans increase the small bowel water content to a much smaller degree [10]. In comparison, in healthy adults, fructans significantly distend the colonic lumen; however, fructose increases colonic distention to a much smaller degree [10]. The individual effects of lactose, galactans, and polyols on GI physiologic function as measured by magnetic resonance imaging remain to be elucidated. In addition, whether these same physiologic changes occur following FODMAP carbohydrate ingestion in children (or adults) with AP-FGIDs is unknown.
Additional factors which may play a role in the generation of symptoms in subjects with lactose intolerance include low-grade gut inflammation and visceral hypersensitivity [11]. This was demonstrated by Yang et al. [11] in adults with IBS following lactose challenge and breath hydrogen testing to identify lactose malabsorbers (increased hydrogen production following the lactose challenge). Those with IBS who had lactose malabsorption were categorized into one of two groups: lactose malabsorption with lactose intolerance (concomitant increase in GI symptoms with the lactose challenge) and lactose malabsorption without lactose intolerance (no increase in GI symptoms). Yang et al. [11] found that adults with IBS with lactose malabsorption and intolerance (in comparison to those with malabsorption alone) had both increased mast cells in ileocolonic biopsies and increased visceral hypersensitivity. Whether these factors also play a role in carbohydrate intolerance in childhood AP-FGIDs is currently unknown.
Lactose
Hippocrates first described lactose intolerance around 400 years BC, but the clinical symptoms have only become recognized in the past 50 years [12] . Lactose is a disaccharide (two conjoined sugars) that is unique in that it is only present in mammalian milk with estimates of 5.5–8.0 g/100 ml in human breast milk and 4.5–5.0 g/100 ml in cow’s milk [13]. In order to be utilized, lactose needs to be broken down (hydrolyzed) by the enzyme lactase. Lactase is found on the tips of the villi of the small intestine and breaks down lactose into two monosaccharides – galactose and glucose. These monosaccharides are then absorbed by the small intestine and metabolized.
At the time of birth in humans, lactase activity is at its peak. However, lactase activity begins to decrease in early childhood in approximately 70% of humans; by adulthood, lactase activity is very low or undetectable [14, 15]. Approximately 30% of the population has lactase persistence whereby lactase activity remains beyond weaning and into adulthood [14, 15]. Lactase persistence occurs primarily in people of northern European descent.
The amount of lactose needed to induce symptoms in someone who is lactose intolerant varies depending on numerous factors. These include: (1) the amount of lactose ingested; (2) whether ingestion is accompanied by a meal or other food; (3) the rate of gastric emptying (how quickly the meal enters the small intestine); (4) small intestinal transit time (the time it takes for a meal to enter the large intestine after first entering the small intestine); (5) whether the meal contains bacteria with enzymes (beta-galactosidase) capable of breaking down lactase, and (6) colonic bacterial adaptation to one’s previous diet [16, 17]. For example, in one study, single lactose loads of ≥ 15 g produced symptoms in the majority of lactase-deficient adults; however, lactose loads of up to 12 g (particularly if spread throughout the day) resulted in minimal or no symptoms [18]. Similarly, feeding subjects dairy-based yogurts with viable microbial cultures that contain bacteria with beta-galactosidase activity resulted in fewer signs of lactose malabsorption than feeding them pasteurized yogurts with little beta-galactosidase activity [17] . The amount of lactose needed to induce symptoms in a child with lactose malabsorption (either healthy or with an AP-FGID) is currently unknown.
Two other studies support a dose-response gradient of lactose exposure and symptoms. One study challenged 13 healthy adults with lactose maldigestion with varying amounts of lactose (0, 2, 6, 12, and 20 g) [16]. This study found that doses of up to 6 g (representing 120 ml of milk) were well tolerated, but symptoms began to emerge at 12 g of exposure [16]. Similar findings were noted in adults challenged with increasing quantities of lactose in a lactose-hydrolyzed milk preparation; the severity of symptoms was primarily dependent on the amount of lactose present [19].
Lactose-Related Studies in Children with AP-FGIDs
In children with AP-FGIDs, three randomized controlled trials have been completed, though none evaluated a restricted lactose diet in a controlled fashion (table 2). There have been a greater number of observational or uncontrolled trials (table 3). Lebenthal et al. [20] used both a randomized controlled challenge and an uncontrolled treatment component. The majority of the trials employed either a lactose challenge to identify those who were lactose intolerant based on the development of GI symptoms following the challenge or a lactose hydrogen breath test to identify children who were malabsorbing lactose by demonstrating an excessive amount of hydrogen production within a specified time after lactose ingestion.
Table 2.
Prospective randomized controlled trials of lactose in children with AP-FGIDs
| Authors [Ref.], country (year) | Design | Results |
|---|---|---|
| Lebenthal et al. [20], USA (1981) | Children with RAP (n = 38: 21 LHBT positive/ 17 LHBT negative) 6-week baseline period followed by double-blind crossover challenges × 6 weeks (chocolate cow’s milk vs. chocolate soy milk) |
10/21 with a positive LHBT versus 4/17 (p = 0.13) with a negative LHBT had worsening of pain with cow’s milk versus regular diet 7/21 with a positive LHBT versus 4/17 (p = 0.51) with a negative LHBT had worsening of pain with soy milk versus regular diet |
| Dearlove et al. [22], UK (1983) | Children with RAP (n = 21: 8 lactose intolerant and 13 lactose tolerant) 2-week baseline followed by 2-week lactose-free diet followed by double-blind crossover challenge × 2 weeks (tonic with vs. without lactose) |
1/8 lactose-intolerant versus 4/13 (p = 0.34) lactose-tolerant improved with lactose-free diet 1/8 lactose-intolerant versus 2/13 (p = 1.0) lactose-tolerant worsened with the lactose tonic |
| Gremse et al. [21] USA (2003) | Children with RAP (n = 30) all with lactose malabsorption by LHBT 14-day double-blind crossover challenge (lactose-containing vs. lactose-hydrolyzed milk, 12 g/240 ml); subjects had been instructed to otherwise maintain a lactose-free diet throughout |
Abdominal pain scores were significantly lower on the lactose hydrolyzed milk (p = 0.02); bloating, diarrhea, flatulence scores were lower but not statistically significantly different on the lactose-hydrolyzed milk |
LHBT = Lactose hydrogen breath test.
Table 3.
Observational or uncontrolled lactose-related studies in children with AP-FGIDs
| Authors [Ref.], country (year) | Methods | Findings |
|---|---|---|
| Liebman et al. [47], USA, (1979) | RAP (n = 38) versus controls (n = 29) completed lactose dietary challenges 17 RAP (n = 11 malabsorbers and n = 6 absorbers) underwent lactose-free diet |
11/38 RAP versus 1/29 controls with positive lactose challenge (malabsorbers) (p < 0.01) 10/11 malabsorbers versus 0/6 absorbers had total relief of symptoms on diet (p < 0.001) |
| Barr et al. [8], USA (1979) | RAP (n = 80) completed LHBT 28 with positive LHBT placed on 6-week non-blinded dietary trial (2 weeks lactose free, 2 weeks lactose, 2 weeks lactose free) |
32/80 (40%) LHBT positive 20/28 (71%) exacerbation of pain on lactose versus lactose-free diet (p < 0.01) |
| Christensen [41], Denmark (1986) | RAP (n = 50) versus controls (n = 40) completed lactose tolerance testing (2 g/kg); determined positive if abdominal pain and/or diarrhea developed | 1/50 RAP children found to be lactose intolerant versus 0/40 controls (p = 1.0) |
| Lebenthal et al. [20], USA (1981) | RAP (n = 69 underwent LHBT) 12-month milk elimination diet in positive LHBT (n = 15) versus negative LHBT (n = 13) |
21/69 RAP LHBT positive 6/15 positive LHBT versus 5/13 negative LHBT had elimination of pain (p = 0.93) |
| Blumenthal et al. [48], UK (1981) | RAP (n = 26) given LHBT (2 g/kg up to 50g, 20% solution) | 3/26 (12%) LHBT positive 1 of 2 children with positive LHBT improved on lactose-free diet |
| Wald et al. [49], USA (1982) | RAP (n = 40) completed LHBT (2 g/kg, up to 50 g) Dietary intervention (total 6 weeks): 2 weeks lactose elimination, 2 weeks lactose-containing, 2 weeks lactose elimination |
12/40 (30%) LHBT positive at 2 g/kg 3/12 (25%) positive LHBT versus 5/28 (18%) negative LHBT (p = 0.68) with significant improvement on lactose-free diet |
| Bhan et al. [50], India (1982) | RAP (n = 70) versus controls (n = 50) lactose tolerance testing All RAP children started on 4-week lactose-free diet |
33/70 RAP (47%) versus 9/50 (18%) controls were lactose malabsorbers (p < 0.01) 11/33 (33%) lactose malabsorbers improved versus 6/37 (16%) lactose absorbers on diet (p < 0.01) |
| Webster et al. [51], USA (1995) | RAP (n = 137) given LHBT (1 g/kg, 10% solution) Those with positive LHBT recommended to have lactose elimination diets; telephone follow-up in 115/137 (84%) |
LHBT positive in 33/137 (24%) In follow-up, 20/27 (74%) of lactose malabsorbers had less frequent abdominal pain versus 28/88 (32%) lactose absorbers (p < 0.001) |
| Ceriani et al. [52], Italy (1988) | RAP (n = 32) given LHBT (2 g/kg up to 50 g) 18 LHBT positive placed on lactose-free diet |
24/32 (75%) LHBT positive 14/18 (78%) LHBT positive improved on the diet |
| Gremse et al. [53], USA (1999) | RAP (n = 146) given LHBT (1 g/kg, 10% solution) | LHBT positive in 50/146 (34%) |
| Boey [54], Malaysia (2001) | RAP (n = 24) given LHBT (2 g/kg) All participants started on lactose-free diet |
17/24 (71%) LHBT positive None responded to lactose-free diet |
| Gijsbers et al. [55], The Netherlands (2012) | RAP (n = 220) given LHBT (2 g/kg) In those with positive LHBT: initial lactose elimination, provocation with lactose if symptoms resolved, followed by double-blind placebo controlled challenge undertaken for ‘definite proof’ of causal relationship |
57/210 (27%) LHBT positive 24/38 (63%) with positive LHBT improved on lactose elimination diet 7/23 (30%) of those who improved had worsening symptoms with provocation None of the 6 subsequently investigated via double-blind challenge had specific lactose-related symptoms |
| Ockeloen et al. [56], The Netherlands (2012) | Retrospective review of LHBT testing in children with chronic abdominal pain (n = 91); subsequent follow-up following lactose-free diet in those with positive LHBT | 22/91 (24%) LHBT positive 21/22 (95%) improved on lactose free-diet at 5 months, and 14/22 (64%) continued with improvement at 15 months’ follow-up |
| Dabritz et al. [27], Germany (2014) | AP-FGID children, of whom 161 completed LHBT | 35/161 (22%) LHBT positive Unclear how many specifically improved on a lactose free-diet |
LHBT = Lactose hydrogen breath test.
The randomized controlled trials provide mixed results regarding the ability of lactose to worsen symptoms in children with AP-FGIDs. One trial identified worsening abdominal pain (but not other symptoms) in children with AP-FGIDs with lactose malabsorption when using nonhydrolyzed lactose milk versus hydrolyzed lactose milk [21] . However, the other two trials did not find that lactose significantly worsened symptoms in children with AP-FGIDs and lactose malabsorption [20, 22]. Similarly, the uncontrolled studies have had mixed results with respect to both the proportion of children with AP-FGIDs who have lactose malabsorption and the efficacy of a low-lactose diet in these children. Further prospective randomized controlled studies are needed, particularly with respect to evaluation of a lactose-restricted diet in children with AP-FGIDs who are lactose malabsorbers. Additionally, the potential role of low-grade gut inflammation in producing symptoms in lactose malabsorbers needs to be investigated in children.
Fructose
Fructose is a monosaccharide of which American children consume a mean of 54.7 g/day, representing approximately 10% of their daily caloric intake [23]. Fructose is dependent on the glucose transporter 5 (GLUT5) and glucose transporter 2 (GLUT2) for passive absorption.
Fructose Studies in Children with AP-FGIDs
One prospective randomized controlled trial using a fructose-restricted diet has been conducted in children with RAP. Wirth et al. [24] randomized 103 children with RAP to either a fructose-restricted diet (n = 51) or a no dietary intervention group (n = 52) for 2 weeks. Those on the fructose-restricted diet (irrespective of their fructose hydrogen breath test result) had less pain intensity; however, they did not have a decrease in pain frequency [24]. In a prospective observational study, Wintermeyer et al. [25] placed 75 children with RAP, all of whom had a positive fructose breath test, on a restricted fructose diet. Overall pain frequency and pain severity decreased while on the diet [25].
Three retrospective studies have been completed. In 32 children with an AP-FGID, Gomara et al. [9] completed fructose hydrogen breath testing using various doses of fructose, including 1, 15, and 45 g. They found that 11 (34%) of the 32 studied children had fructose malabsorption at the 15 or 45 g doses. Of these 11, 9 (82%) had a significant improvement on a 2-week dietitian-recommended fructose-restricted diet [9] . Escobar et al. [26] completed fructose breath testing using 1 g/kg (up to 25 g) in 222 children with AP-FGIDs. Of these, 121 (55%) had fructose malabsorption, of whom 93 (77%) had improvement on a dietitian-recommended low-fructose diet. Dabritz et al. [27] included fructose hydrogen breath testing in their review and found that 55/142 (39%) children had fructose malabsorption. As several in the Dabritz cohort had multiple positive carbohydrate tests, the number of children who specifically responded to a fructose-restricted diet is unclear.
Sorbitol
No prospective studies regarding sorbitol restriction have been completed in children with AP-FGIDs. Hyams [28] published a case report describing a 15-year-old girl with chronic abdominal pain attributed to sorbitol ingestion from sugar-free gum which improved with elimination of the sorbitol source. In their retrospective study of hydrogen breath testing in children with RAP, Dabritz et al. [27] found that 109/146 (75%) children had sorbitol malabsorption; 27/31 (87%) improved on a sorbitol-restricted diet.
FODMAP Carbohydrates (table 1)
FODMAP carbohydrates include fructose and lactose, fructans, galactans, and polyols (such as sorbitol). Fructans are consumed primarily from wheat in the Western diet, with an average consumption ranging from approximately 4.4 to 6.7 g/day in children in the United States [29]. In the Western diet, galactans are consumed primarily from legumes and beans. Polyols are sugar alcohols such as sorbitol and xylitol. Fructans, galactans, and polyols have unique bonds which are not able to be hydrolyzed by human enzymes. As such, following ingestion, the vast majority of these sugars enter the human colon essentially intact [30]. Within the colon, they may be metabolized by the gut bacteria, which contain enzymes with the ability to metabolize complex carbohydrates [31].
FODMAP Evidence in Childhood AP-FGIDs
Two prospective studies – one an open-label pilot study and the second one a randomized controlled trial – have evaluated a low-FODMAP diet in children with IBS. In the open-label study, 8 children with IBS were instructed by a dietitian to follow a low-FODMAP diet for 1 week [32]. The group as a whole had a decrease in abdominal pain frequency, with 4 children having a ≥50% decrease in abdominal pain frequency as compared to baseline [32]. In the randomized double-blind crossover trial, children with IBS (n = 32) received a low- FODMAP or typical American childhood diet for 48 h [33]. The group as a whole had fewer abdominal pain episodes during the low-FODMAP diet. Those who had significant improvement (>50% decrease in abdominal pain frequency) on the low-FODMAP diet, compared to those who did not, had a gut microbiome composition that was enriched in bacteria with high saccharolytic potential (e.g., Faecalibacterium prausnitzii) [33].
Sucrose and Starch
Sucrose and starch are normally easily hydrolyzed and absorbed in the small intestine through the activity of sucrase-isomaltase (which hydrolyzes both sucrose and starch) and maltase-glucoamylase (which hydrolyzes starch) [34]. The activities of these enzymes may be evaluated using the Dahlqvist method from duodenal biopsy tissue obtained during upper endoscopy [35].
Carbohydrate enzyme deficiencies related to sucrose and starch digestion have been investigated in children with AP-FGIDs. Children with AP-FGID (n = 44) undergoing upper GI endoscopy for the evaluation of abdominal pain, vomiting, or gastroesophageal reflux were evaluated for lactase, sucrase, and glucoamylase activities [36] . Low enzyme activities were found in 32, 34, and 28% of children, respectively, with some children having a combination of enzyme deficiencies [36]. These findings are supported by those of El-Chammas et al. [37] who evaluated disaccharidase activity in 203 children with AP-FGIDs. Low enzyme levels for lactase, sucrose, glucoamylase, and palatinase (a measure of isomaltase) were found in 37, 21, 25, and 8%, respectively [37]. However, neither study found a correlation between enzyme activity levels and GI symptoms. In addition, the studies did not have a dietary intervention component. Future studies are needed to further investigate the significance of disaccharidase deficiencies and potential dietary or other interventions in children with AP-FGIDs.
Fiber Therapy in Childhood AP-FGIDs
Proposed Mechanisms
The rationale for fiber therapy for AP-FGIDs primarily relates to using soluble fiber, which is able to absorb water and maintain the hydration of stool, and/or using insoluble fiber, the particles of which have the potential ability to mechanically stimulate/irritate the gut mucosa and induce a laxative effect through secretion of mucous and water, resulting in more rapid transit through the colon [38]. Fiber that is not easily fermented and is able to maintain its gel-like property, such as psyllium, provides increased water-holding capacity and regulation of stool form [38]. In contrast to soluble fiber, insoluble fiber, such as bran, has been shown to potentially exacerbate GI symptoms in adults with IBS [39].
Evidence in Childhood AP-FGIDs
Soluble fiber has demonstrated efficacy in randomized controlled trials in children with AP-FGIDs. In a double-blind randomized controlled trial with 52 children with RAP, Feldman et al. [40] compared 10 g of corn fiber (a type of soluble fiber) supplementation for 2 weeks to placebo. With the primary endpoint of a 50% reduction in abdominal pain frequency, 13/26 (50%) of those with fiber supplementation achieved the endpoint compared to 7/26 (27%) of those receiving placebo (p < 0.04) [40]. In a double-blind randomized controlled trial with 32 children with RAP who were hospitalized for their abdominal pain, Christensen [41] reported no benefit using 7 weeks of ispaghula husk (psyllium – containing greater soluble than insoluble fiber properties) [38] . However, as this report is in the form of a letter to the editor, important information is missing (e.g., p values, standard deviations), making interpretation of the results difficult [41]. A prospective randomized controlled trial recently published as an abstract suggests that psyllium may be beneficial in children with IBS, though full details are still not published as of the time of this review [42] . Retrospective studies suggest a benefit of increased fiber intake in reducing the risk of abdominal pain in children [43, 44].
Conclusions/Future Opportunities
Carbohydrate malabsorption can lead to GI symptoms through direct physiologic effects within the GI tract. However, current evidence does not strongly support restriction of single carbohydrates in children with AP-FGIDs. Rather, as in adults with IBS, FODMAP restriction is emerging as a better clinical strategy. In addition, soluble fiber supplementation appears to be an effective therapy in childhood AP-FGID.
Future research opportunities related to carbohydrates in childhood AP-FGIDs include: further elucidating the mechanisms of action of both FODMAP carbohydrates to induce symptoms and soluble fiber to ameliorate symptoms; determining the role of the gut microbiome in carbohydrate metabolism and associated symptoms; understanding the long-term effects of dietary carbohydrate-related interventions on the gut microbiome composition and function, and identifying those children with AP-FGIDs who would benefit most from dietary carbohydrate-related interventions.
Key Messages.
Carbohydrate malabsorption may cause gastrointestinal symptoms (e.g., bloating) via the physiologic effects of both increased osmotic activity and increased gas production from bacterial fermentation.
There is a general lack of strong evidence supporting a restriction of individual carbohydrates (e.g., lactose) for childhood functional gastrointestinal disorders; however, emerging evidence suggests that a restriction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) may be effective.
Soluble fiber (a complex carbohydrate) supplementation via randomized controlled intervention trials in childhood functional gastrointestinal disorders has demonstrated efficacy.
Footnotes
Disclosure Statement
B.P.C. has received research support from the National Institutes of Health and QOL Medical, Inc., and was a consultant for Mead Johnson Nutrition. R.J.S. has received research support from the National Institutes of Health and is a consultant for Mead Johnson Nutrition.
Financial and/or intellection support during the completion of this review was provided by NIH K23 DK101688 (B.P.C.) and NIH R01 NR013497 as well as the Daffy’s Foundation (R.J.S.), the USDA/ARS under Cooperative Agreement No. 6250-51000-043 (R.J.S.), and P30 DK56338, which funds the Texas Medical Center Digestive Disease Center (B.P.C., R.J.S.).
The writing of this article was supported by Nestlé Nutrition Institute
References
- 1.Chitkara DK, Rawat DJ, Talley NJ. The epidemiology of childhood recurrent abdominal pain in Western countries: a systematic review. Am J Gastroenterol. 2005;100:1868–1875. doi: 10.1111/j.1572-0241.2005.41893.x. [DOI] [PubMed] [Google Scholar]
- 2.Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr. 1996;129:220–226. doi: 10.1016/s0022-3476(96)70246-9. [DOI] [PubMed] [Google Scholar]
- 3.Youssef NN, Murphy TG, Langseder AL, Rosh JR. Quality of life for children with functional abdominal pain: a comparison study of patients’ and parents’ perceptions. Pediatrics. 2006;117:54–59. doi: 10.1542/peds.2005-0114. [DOI] [PubMed] [Google Scholar]
- 4.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasquin A, DiLorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson MJ, Moore CE, Tsai CM, Shulman RJ, Chumpitazi BP. Child and parent perceived food-induced gastrointestinal symptoms and quality of life in children with functional gastrointestinal disorders. J Acad Nutr Diet. 2014;114:403–413. doi: 10.1016/j.jand.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am J Clin Nutr. 2011;93:836–843. doi: 10.3945/ajcn.110.000141. [DOI] [PubMed] [Google Scholar]
- 8.Barr RG, Levine MD, Watkins JB. Recurrent abdominal pain of childhood due to lactose intolerance. N Engl J Med. 1979;300:1449–1452. doi: 10.1056/NEJM197906283002602. [DOI] [PubMed] [Google Scholar]
- 9.Gomara RE, Halata MS, Newman LJ, Bost-wick HE, Berezin SH, Cukaj L, et al. Fructose intolerance in children presenting with abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47:303–308. doi: 10.1097/MPG.0b013e318166cbe4. [DOI] [PubMed] [Google Scholar]
- 10.Murray K, Wilkinson-Smith V, Hoad C, Costigan C, Cox E, Lam C, et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109:110–119. doi: 10.1038/ajg.2013.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Deng Y, Chu H, Cong Y, Zhao J, Pohl D, et al. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:262–268. doi: 10.1016/j.cgh.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Matthews SB, Waud JP, Roberts AG, Campbell AK. Systemic lactose intolerance: a new perspective on an old problem. Postgrad Med J. 2005;81:167–173. doi: 10.1136/pgmj.2004.025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusch G, Choi A, Rochow N, Fusch C. Quantification of lactose content in human and cow’s milk using UPLC-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3759–3762. doi: 10.1016/j.jchromb.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 14.Deng Y, Misselwitz B, Dai N, Fox M. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients. 2015;7:8020–8035. doi: 10.3390/nu7095380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misselwitz B, Pohl D, Fruhauf H, Fried M, Vavricka SR, Fox M. Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterol J. 2013;1:151–159. doi: 10.1177/2050640613484463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am J Clin Nutr. 1996;64:232–236. doi: 10.1093/ajcn/64.2.232. [DOI] [PubMed] [Google Scholar]
- 17.Martini MC, Smith DE, Savaiano DA. Lactose digestion from flavored and frozen yogurts, ice milk, and ice cream by lactase-deficient persons. Am J Clin Nutr. 1987;46:636–640. doi: 10.1093/ajcn/46.4.636. [DOI] [PubMed] [Google Scholar]
- 18.Savaiano DA, Levitt MD. Milk intolerance and microbe-containing dairy foods. J Dairy Sci. 1987;70:397–406. doi: 10.3168/jds.S0022-0302(87)80023-1. [DOI] [PubMed] [Google Scholar]
- 19.Gudmand-Hoyer E, Simony K. Individual sensitivity to lactose in lactose malabsorption. Am J Dig Dis. 1977;22:177–181. doi: 10.1007/BF01072273. [DOI] [PubMed] [Google Scholar]
- 20.Lebenthal E, Rossi TM, Nord SK, Branski D. Recurrent abdominal pain and lactose absorption in children. Pediatrics. 1981;67:828–832. [PubMed] [Google Scholar]
- 21.Gremse DA, Greer AS, Vacik J, DiPalma JA. Abdominal pain associated with lactose ingestion in children with lactose intolerance. Clin Pediatr (Phila) 2003;42:341–345. doi: 10.1177/000992280304200406. [DOI] [PubMed] [Google Scholar]
- 22.Dearlove J, Dearlove B, Pearl K, Primavesi R. Dietary lactose and the child with abdominal pain. Br Med J. 1983;286:1936–1936. doi: 10.1136/bmj.286.6382.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 24.Wirth S, Klodt C, Wintermeyer P, Berrang J, Hensel K, Langer T, et al. Positive or negative fructose breath test results do not predict response to fructose restricted diet in children with recurrent abdominal pain: results from a prospective randomized trial. Klin Padiatr. 2014;226:268–273. doi: 10.1055/s-0034-1383653. [DOI] [PubMed] [Google Scholar]
- 25.Wintermeyer P, Baur M, Pilic D, Schmidt-Choudhury A, Zilbauer M, Wirth S. Fructose malabsorption in children with recurrent abdominal pain: positive effects of dietary treatment. Klin Padiatr. 2012;224:17–21. doi: 10.1055/s-0031-1279747. [DOI] [PubMed] [Google Scholar]
- 26.Escobar MA, Jr, Lustig D, Pflugeisen BM, Amoroso PJ, Sherif D, Saeed R, et al. Fructose intolerance/malabsorption and recurrent abdominal pain in children. J Pediatr Gastroenterol Nutr. 2014;58:498–501. doi: 10.1097/MPG.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 27.Dabritz J, Muhlbauer M, Domagk D, Voos N, Hennebohl G, Siemer ML, et al. Significance of hydrogen breath tests in children with suspected carbohydrate malabsorption. BMC Pediatr. 2014;14:59. doi: 10.1186/1471-2431-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyams JS. Chronic abdominal pain caused by sorbitol malabsorption. J Pediatr. 1982;100:772–773. doi: 10.1016/s0022-3476(82)80586-6. [DOI] [PubMed] [Google Scholar]
- 29.Moshfegh AJ, Friday JE, Goldman JP, Ahuja JK. Presence of inulin and oligofructose in the diets of Americans. J Nutr. 1999;129:1407S–1411S. doi: 10.1093/jn/129.7.1407S. [DOI] [PubMed] [Google Scholar]
- 30.Barrett JS, Gibson PR. Fermentable oligosac-charides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol. 2012;5:261–268. doi: 10.1177/1756283X11436241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chumpitazi BP, Hollister EB, Oezguen N, Tsai CM, McMeans AR, Luna RA, et al. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes. 2014;5:165–75. doi: 10.4161/gmic.27923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chumpitazi BP, Cope JL, Hollister EB, Tsai CM, McMeans AR, Luna RA, et al. Randomised clinical trial: gut microbiome bio-markers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther. 2015;42:418–427. doi: 10.1111/apt.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robayo-Torres CC, Quezada-Calvillo R, Nichols BL. Disaccharide digestion: clinical and molecular aspects. Clin Gastroenterol Hepatol. 2006;4:276–287. doi: 10.1016/j.cgh.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Dahlqvist A, Hammond JB, Crane RK, Dunphy JV, Littman A. Assay of disaccharidase activities in peroral biopsies of the small-intestinal mucosa. Acta Gastroenterol Belg. 1964;27:543–555. [PubMed] [Google Scholar]
- 36.Karnsakul W, Luginbuehl U, Hahn D, Sterchi E, Avery S, Sen P, et al. Disaccharidase activities in dyspeptic children: biochemical and molecular investigations of maltase-glucoamylase activity. J Pediatr Gastroenterol Nutr. 2002;35:551–556. doi: 10.1097/00005176-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 37.El-Chammas K, Williams SE, Miranda A. Disaccharidase deficiencies in children with chronic abdominal pain. JPEN J Parenter Enteral Nutr. 2015 doi: 10.1177/0148607115594675. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.McRorie JW., Jr Evidence-based approach to fiber supplements and clinically meaningful health benefits. Part 2. What to look for and how to recommend an effective fiber therapy. Nutr Today. 2015;50:90–97. doi: 10.1097/NT.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hebden JM, Blackshaw E, D’Amato M, Perkins AC, Spiller RC. Abnormalities of GI transit in bloated irritable bowel syndrome: effect of bran on transit and symptoms. Am J Gastroenterol. 2002;97:2315–2320. doi: 10.1111/j.1572-0241.2002.05985.x. [DOI] [PubMed] [Google Scholar]
- 40.Feldman W, McGrath P, Hodgson C, Ritter H, Shipman RT. The use of dietary fiber in the management of simple, childhood, idiopathic, recurrent abdominal pain. Results in a prospective, double-blind, randomized, controlled trial. Am J Dis Child. 1985;139:1216–1218. doi: 10.1001/archpedi.1985.02140140050025. [DOI] [PubMed] [Google Scholar]
- 41.Christensen MF. Recurrent abdominal pain and dietary fiber. Am J Dis Child. 1986;140:738–739. doi: 10.1001/archpedi.1986.02140220020009. [DOI] [PubMed] [Google Scholar]
- 42.Shulman RJ, Cain K, Czyzewski D, Self M, Weidler E, Devaraj S, et al. Randomized, double blind trial of psyllium fiber in children with irritable bowel syndrome (IBS) Gastroenterology. 2015;148:S120. doi: 10.1016/j.cgh.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang RC, Palmer LJ, Forbes DA. Prevalence and pattern of childhood abdominal pain in an Australian general practice. J Paediatr Child Health. 2000;36:349–353. doi: 10.1046/j.1440-1754.2000.00513.x. [DOI] [PubMed] [Google Scholar]
- 44.Paulo AZ, Amancio OM, de Morais MB, Tabacow KM. Low-dietary fiber intake as a risk factor for recurrent abdominal pain in children. Eur J Clin Nutr. 2006;60:823–827. doi: 10.1038/sj.ejcn.1602386. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Fandino O, Hernandez-Ruiz J, Schmulson M. From cytokines to toll-like receptors and beyond – current knowledge and future research needs in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:363–373. doi: 10.5056/jnm.2010.16.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrett JSG, Gibson PR. Clinical ramifications of malabsorption of fructose and other short-chain carbohydrates. Pract Gastroenterol. 2007;53:51–65. [Google Scholar]
- 47.Liebman WM. Recurrent abdominal pain in children: lactose and sucrose intolerance, a prospective study. Pediatrics. 1979;64:43–45. [PubMed] [Google Scholar]
- 48.Blumenthal I, Kelleher J, Littlewood JM. Recurrent abdominal pain and lactose intolerance in childhood. Br Med J (Clin Res Ed) 1981;282:2013–2014. doi: 10.1136/bmj.282.6281.2013-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wald A, Chandra R, Fisher SE, Gartner JC, Zitelli B. Lactose malabsorption in recurrent abdominal pain of childhood. J Pediatr. 1982;100:65–68. doi: 10.1016/s0022-3476(82)80236-9. [DOI] [PubMed] [Google Scholar]
- 50.Bhan MK, Arora NK, Ghai OP, Dhamija NK, Nayyar S, Fotedar A. Lactose and milk intolerance in recurrent abdominal pain of childhood. Indian J Pediatr. 1982;49:199–202. doi: 10.1007/BF02830749. [DOI] [PubMed] [Google Scholar]
- 51.Webster RB, Dipalma JA, Gremse DA. Lactose maldigestion and recurrent abdominal pain in children. Dig Dis Sci. 1995;40:1506–1510. doi: 10.1007/BF02285199. [DOI] [PubMed] [Google Scholar]
- 52.Ceriani R, Zuccato E, Fontana M, Zuin G, Ferrari L, Principi N, Paccagnini S, Mussini E. Lactose malabsorption and recurrent abdominal pain in Italian children. J Pediatr Gastroenterol Nutr. 1988;7:852–857. doi: 10.1097/00005176-198811000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Gremse DA, Nguyenduc GH, Sacks AI, Dipalma JA. Irritable bowel syndrome and lactose maldigestion in recurrent abdominal pain in childhood. South Med J. 1999;92:778–781. doi: 10.1097/00007611-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Boey CC. Lactase deficiency among Malaysian children with recurrent abdominal pain. J Paediatr Child Health. 2001;37:157–160. doi: 10.1046/j.1440-1754.2001.00622.x. [DOI] [PubMed] [Google Scholar]
- 55.Gijsbers CF, Kneepkens CM, Buller HA. Lactose and fructose malabsorption in children with recurrent abdominal pain: results of double-blinded testing. Acta Paediatr. 2012;101:e411–e415. doi: 10.1111/j.1651-2227.2012.02721.x. [DOI] [PubMed] [Google Scholar]
- 56.Ockeloen LE, Deckers-Kocken JM. Short- and long-term effects of a lactose-restricted diet and probiotics in children with chronic abdominal pain: a retrospective study. Complement Ther Clin Pract. 2012;18:81–84. doi: 10.1016/j.ctcp.2011.11.002. [DOI] [PubMed] [Google Scholar]