Abstract
We examined the effect of adding species-appropriate environmental enrichment items to breeding cages of BALB/cAnNCrl and 129S2/SvPasCrl mice. The 3 enrichment conditions were: 1) cotton nesting material; 2) nesting material plus a paper shelter and rolled paper bedding; and 3) an igloo dome with an exercise wheel in addition to the shelter-group enrichments. We measured litter size, litter survival to weaning age, average pup weight at 21 d, and the interlitter interval to evaluate reproductive performance. A random subset of the first- or second-litter offspring from each enrichment condition and strain was assessed in multiple behavioral tests. Enrichment significantly affected anxiety-like behavior and sociability, with the direction of change dependent on strain and sex. Litter parity had greater effects on some reproductive parameters than did the enrichment condition, and this effect was not solely due to a difference between the first compared with subsequent litters. The significant effects of litter parity on the number of pups born and weaned, female pup weight, and interlitter interval were dependent on the enrichment condition in BALB/c but not 129/Sv mice. Offspring from the first or second litter were included in a generational component to investigate whether enrichment effects on reproduction persist in adult offspring after transfer to a different facility for breeding. Natal cage enrichment had no effect on any reproductive parameter in the transferred mice. Overall, additional enrichment beyond nesting material had a beneficial effect on the interlitter interval in BALB/c mice and on the number of pups weaned in 129/Sv mice.
Over the past few years, agencies, institutions, and investigators have shared an increased focus on the benefits of species-appropriate environmental enrichment for laboratory animals. In addition, the 8th edition of the Guide for the Care and Use of Laboratory Animals 11 emphasizes the use of enrichment where possible. In the case of laboratory mice, the available options to enhance caging conditions are generally limited to either changing the size of the cage or adding items to the cage. One study examining the effects of cage size and environmental enrichment on behavior in adult female mice found that cage complexity was more important than the quantity of floor space.4 Scientific studies using environmental enrichment tend to focus on adding multiple items to a cage in formats that can be elaborate or rotating items in ways that can be difficult to implement as a practical enrichment paradigm throughout a research program. Adding items to a cage may benefit mice by reducing aggression,4,10,32 decreasing corticosterone levels,9 and decreasing anxiety-like behavior,20 although effects can be highly dependent on the strain and sex of the mice and on the item added.12,19,28,31 Some studies have shown increased variability in experimental measures with enrichment,10,31 whereas other studies have not,38 but because the effects of variation between laboratories is so great, this may remain unresolved.1,24,25 Several reviews of the effects of a wide variety of environmental enrichment systems on rodents are available,20,30 and many studies have concluded that nesting material is the most beneficial enrichment in mice,20,32 although not all reports agree.12,30 However, little work has been done investigating enrichment in breeding cages.27
There are many ways to evaluate the effects of enrichment, including physiologic values, behavior, or performance measures. Reproductive performance can serve as an indicator of a mouse's overall well-being, because reproduction is physiologically costly and requires favorable energy balances.15 Stressors such as physical disturbance21 or a negative metabolic energy balance15 can negatively affect reproductive performance. Estrus cycling, ovulation, and implantation can be affected by stress.23 Other studies have shown enrichment effects on reproductive parameters. For example, the addition of nesting material increased the number of pups weaned per dam per week compared with bedding-only cages for C57BL/6NCrl, BALB/cAnNCrl, and Crl:CD1(Icr) breeding pairs and BALB/c nude (CAnN.Cg-Foxn1nu /Crl) and Crl:NU-Foxn1nu mice.5,6
In a previous study on the effects of cage size, we demonstrated that simply increasing amount of cage space had no effects on reproductive parameters measured in C57BL/6 mice.37 In addition, cage size during the prenatal and postnatal period did not have overt or consistent effects on the performance of offspring in elevated plus maze or acoustic startle tests or on measures of activity in a novel environment.37 In a subsequent study, we examined whether environmental enrichment, together with cage size, had effects on reproductive performance and behavior in C57BL/6 mice.36 We showed significant effects of enrichment and cage size conditions during the prenatal and postnatal periods on offspring behavior, dependent on the specific behavioral measure and sex of the mice.37 Specifically, we found that enrichment had a significant effect on reproductive parameters. Litters from the nonenriched cages weighed less (by 9.3% to 14.4%, or 0.9 to 1.3 g) and weaned fewer pups (23.3% to 30.7% fewer) than did those from enriched cages.37
Because enrichment can significantly improve the survival and weight of mouse pups, it would be beneficial to know which enrichment items improve breeding efficiency. In addition, some strains have behavioral complications that might be alleviated by enrichment in the natal cage and that of young adults. The present study examined the effects of cage enrichment in 2 inbred strains: 129S2/SvPasCrl and BALB/cAnNCrl. Previous work has shown that the 129 and BALB/c substrains are characterized by anxiety-like behavior and low sociability.18,26,34 Although litter sizes are comparable between 129/Sv and BALB/c mice (average number of pups per litter, 5.1 for 129/Sv and 5.6 for BALB/c), 129/Sv mice have a lower survival rate at weaning than do BALB/c mice.29 In the current study, we investigated whether enrichment strategies would improve the reproductive efficiency of either strain or lead to different behavioral profiles in the postweaning period.
An additional concern regarding mouse reproductive performance has been the possible disruptive effect of transferring mice from commercial facilities or between academic settings, with subsequent housing under cage conditions markedly different from those at the original institution. Therefore, the present study included a generational component to investigate whether any enrichment effect on reproduction persists in adult offspring after their transfer to a different facility and placement into a different housing environment. Offspring from the 129/Sv and BALB/c strains were raised in the defined enrichment conditions of the study until the mice were 6 wk of age, when they were transferred from the University of North Carolina to a commercial facility (Charles River Laboratories, Wilmington, MA) and placed in breeding pairs under that facility's standard housing condition. Reproductive parameters, including litter weight and pup survival to weaning, were recorded to determine whether enrichment in the natal cage led to persistent effects on adult breeding success after the mice were transferred to a different housing facility and caging system.
Materials and Methods
Parental reproductive study.
Mice.
Mice were housed and tested in an AAALAC-accredited facility. All procedures were approved by the University of North Carolina IACUC and conducted under the guidelines in theGuide for the Care and Use of Laboratory Animals .11 At the start of this study, 6-wk-old 129S2/SvPasCrl (129/Sv) male (n = 90) and female (n = 90) mice and BALB/cAnNCrl (BALB/c) male (n = 90) and female (n = 90) mice from Charles River Laboratories (Wilmington, MA) were arbitrarily set up as breeder pairs. Mice had unrestricted access to food (Purina 5058 [protein, 20%; fat, 9% or greater; fiber, 4% or less; ash, 6.5% or less], LabDiet, St Louis, MO) and water (from bottles filled with reverse-osmosis–treated water). Mice were checked daily as part of routine animal care. Temperature in the housing room was 72 °F (range, 70 to 75 °F [22 °C, 21 to 24 °C]), relative humidity was controlled to between 30% and 45%, and the photoperiod was 12:12-h light:dark. Mouse colonies were monitored every 4 mo by using a dirty-bedding sentinel program, and the mice in this study were free of tested viruses (ectromelia virus, lymphocytic choriomeningitis virus, Theiler mouse encephalomyelitis virus, minute virus of mice, mouse parvovirus, mouse cytomegalovirus, mouse adenovirus 1 and 2, mouse hepatitis virus, pneumonia virus of mice, polyoma virus, murine rotavirus [epizootic diarrhea of infant mice], reovirus 3, Sendai virus, and mouse norovirus), the bacterial pathogens Mycoplasma pulmonis and CAR bacillus, and external and internal parasites.
Cages and enrichment.
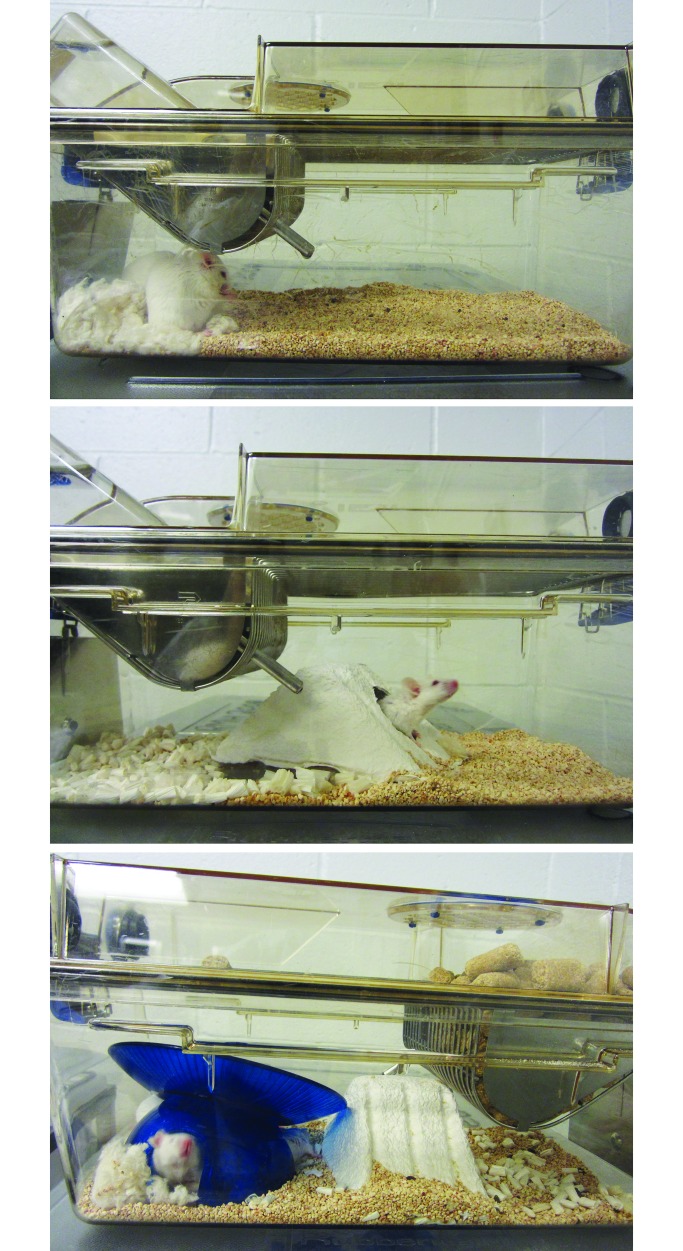
Mice were housed in IVC (365 mm × 207 mm × 140 mm; model 1284L, Seal-Safe, Tecniplast, Buguggiate, Italy) containing irradiated 1/4-in. bedding (Bed-o'Cobs, The Andersons, Maumee, OH). The ventilation condition was an air-exchange rate of 70 air changes hourly. Three in-cage enrichment conditions were created for each of the 2 strains, so that each condition comprised 30 cages per strain. The enrichment conditions were: 1) nest only, in which a cotton square (Ancare, Bellmore, NY) was provided for nesting material; 2) nest and shelter, comprising a cotton square, a Shepherd Shack (Shepherd Specialty Papers, Watertown, TN) to add shelter-type enrichment, and 10 to 11 g Enrich-n'Nest material (The Andersons, Maumee, OH) for foraging activity and nesting material; and 3) nest, shelter, and wheel, which represented enrichment consisting of a cotton square, a Shepherd Shack, 10 to 11 g Enrich-n'Nest, and an InnoWheel (Bio-Serv, Frenchtown, NJ) consisting of an igloo dome with an exercise wheel on top for additional shelter and for activity (Figure 1). The cages were changed every 2 wk. New nesting squares, shelters, Enrich-n'Nest, and wheels were placed in the clean cage every time cages were changed.
Figure 1.
Enrichment conditions for BALB/c mice. Top, nest only; middle, shelter and nest (shelter group); bottom, wheel with shelter and nest (wheel group).
Reproductive parameters.
To evaluate reproductive performance, we recorded the litter size, litter survival to weaning age, average pup weight at 21 d, days to first litter, and the interlitter interval. Cages were checked daily for litters, pups from new litters were counted, and the date of birth was recorded. On day 21, pups were separated into a male group and a female group, and the average weight of each sex was recorded. Pups were weaned on day 21. The number of pups in a litter that survived from birth to weaning was recorded also. The number of days between consecutive litters was recorded for each dam, and each cage of breeders was kept in the study until either the female mouse had 4 litters or for 6 mo total. The breeding pair remained together in the same cage throughout the study.
Weaning for behavioral testing.
Mice were weaned into cages containing the same enrichment items as their natal cage. We arbitrarily selected one male and one female mouse from either litter 1 or litter 2 for each of the cage conditions and each strain until a total of 9 to 14 mice per sex per condition was achieved. Offspring were housed at 2 to 4 mice per cage for testing by using the Mouse Behavioral Phenotyping Core (described later), with a total of 9 to 14 mice per sex per condition for each strain.
Weaning for generational study.
The remaining offspring were weaned, separated according to sex, and housed at 2 to 5 mice per cage in cages that contained the same enrichment as their natal cage. Offspring were then held for 3 wk under the designated enrichment condition until mice were 6 wk old. At that time, mice were shipped to a collaborator at a different institution (Charles River Laboratories, Wilmington, MA) for breeding and tracking of reproductive parameters (that is, the first-generation reproductive study described later). The total numbers of offspring transferred to the collaborator were 29 to 30 mice per enrichment condition for each sex and strain.
Behavioral testing of offspring.
Behavioral testing was conducted by the Mouse Behavioral Phenotyping Laboratory, a core facility at the University of North Carolina. In addition, a set of C57BL/6NCrl mice (male, 10; female, 10; age, 3 wk on arrival; Charles River Laboratories) was tested to confirm typical wild-type behavioral phenotypes under standard housing conditions.
Testing was initiated when mice were 6 wk of age, during the 4th week of postweaning exposure to the different housing conditions. All behavioral testing was conducted between 0800 and 1600, during the light phase (Table 1).
Table 1.
Numbers of mice from each enrichment condition that underwent behavioral testing
| Enrichment condition |
||||
| Strain | Sex | Nest | Nest and shelter | Nest, shelter, and wheel |
| BALB/c | Male | 10 | 11 | 9 |
| Female | 10 | 12 | 10 | |
| 129/Sv | Male | 11 | 11 | 12 |
| Female | 10 | 14 | 11 | |
| B6 | Male | 10 | Not tested | Not tested |
| Female | 10 | Not tested | Not tested | |
Elevated plus maze test of anxiety-like behavior.
This procedure is based on a natural tendency of mice to actively explore a new environment, compared with a fear of being in an open area. In the present study, mice (age, 6 wk) underwent a single 5-min trial on the plus maze, which had 2 walled arms (the closed arms; height, 20 cm) and 2 open arms. The maze was elevated 50 cm from the floor, and each arm was 30 cm long. Mice were placed on the center section (8 cm × 8 cm) and allowed to freely explore the maze. The times spent in the open and closed arms of the maze and the total number of arm entries (an index of activity during the test) were recorded.
Open field test.
At 2 to 4 d after the elevated plus maze test, mice (age, 6 wk) were evaluated for exploratory activity in a novel environment. The 1-h trial was conducted in an open field chamber (41 cm × 41 cm × 30 cm) crossed by a grid of photobeams (VersaMax System, AccuScan Instruments, Columbus, OH). Locomotor activity (total distance traveled) and time spent in the center region of the open field (an index of anxiety-like behavior during the test) were measured every 5 min throughout the session.
Social approach.
At 7 wk of age, mice were tested for sociability in a 3-chamber choice task. The test was designed to assess preference or avoidance of a social stimulus (an unfamiliar stranger mouse). The procedure comprised 2 phases (10 min each): a habituation period and a test for sociability. For the sociability assay, mice were given a choice between being in the proximity of an unfamiliar conspecific and being alone. The test was conducted in a rectangular, 3-chambered box. Dividing walls had doorways allowing access into each chamber. Photocells were embedded in each doorway to allow automatic quantification of entries and duration in each side of the social test box.
The test mouse was first placed in the middle chamber and allowed to explore for 10 min with the doorways into the 2 side chambers open. After the habituation period, the test mouse was enclosed in the center compartment of the social test box, and an unfamiliar C57BL/6J mouse (the stranger), sex-matched with the test mouse, was placed in one of the side chambers. The unfamiliar mouse (the ‘stranger’) was enclosed in a small wire cage, which allowed nose contact between the bars. An identical empty wire cage was placed at the opposite side of the chamber. After the placement of the stranger and the empty wire cage, the doors were reopened, and the subject was allowed to freely explore the entire social test box for 10 min. The amount of time spent in each chamber and number of entries into each chamber were recorded by the automated testing system. Each stranger mouse was used in only one test each day.
Marble burying.
This assay was used to measure defensive burying behavior in a novel environment, a measure of neophobia, anxiety, and repetitive behavior,13 a response which is typically robust in wild-type mice. Mice (age, 8 wk) were tested in an acrylic cage (20 cm × 35.5 cm × 12.7 cm) located in a sound-attenuating chamber with a ceiling light and fan. The cage contained corncob bedding (depth, 5 cm) with 20 black glass marbles (diameter, 14 mm) arranged in an equidistant 5 × 4 grid on top of the bedding. Mice were given access to the marbles for 30 min. The number of marbles buried (2/3 of the marble covered by the bedding) was recorded.
Acoustic startle.
At 2 to 4 d after the marble-burying test, mice (age, 8 wk) were evaluated for auditory function and sensorimotor gating in an acoustic startle test. The test was based on the reflexive whole-body flinch, or startle response, that follows exposure to a sudden noise. Startle magnitude and prepulse inhibition, which occurs when a weak prestimulus leads to a reduced startle in response to a subsequent louder noise, were measured. For this study, mice were tested by using an SR-Lab system (San Diego Instruments, San Diego, CA). Briefly, mice were placed in a small acrylic cylinder (length, 12.7 cm; diameter, 3.8 cm) within a larger, sound-attenuating chamber. The cylinder was seated on a piezoelectric transducer, which allowed vibrations to be quantified and displayed on a computer. The chamber included a ceiling light, fan, and speaker for the acoustic stimuli. Background sound levels (70 dB) and calibration of the acoustic stimuli were confirmed by using a digital sound-level meter (San Diego Instruments).
Each session consisted of 42 trials, which began with a 5-min habituation period. There were 7 different types of trials: the no-stimulus trials, trials with the acoustic startle stimulus (40 ms, 120 dB) alone, and trials in which a prepulse stimulus (20 ms; 74, 78, 82, 86, or 90 dB) occurred 100 ms before the onset of the startle stimulus. For each trial, the startle amplitude was measured across a 65-msec sampling window, and an overall analysis was performed for each subject's data for levels of prepulse inhibition at each prepulse sound level (calculated as 100 – [(response amplitude for prepulse stimulus and startle stimulus together / response amplitude for startle stimulus alone) × 100].
First-generation reproduction study.
All mice used in this portion of the study were housed at the AAALAC-accredited facilities of Charles River Laboratories (Wilmington, MA), and the experimental protocol was approved by the Charles River IACUC. Animals were housed in 6-foot flexible film isolators. The temperatures within isolators were maintained at 21° + 1 °C (70° ± 2 °F) with 60% ± 10% relative humidity. Mice were kept on a 12:12 light: dark cycle and provided unlimited access to feed (Lab Diet 5L79 [protein, 18%; fat, 5% or greater; fiber, 5% or less; ash, 8% or less]; Purina Mills, Richmond, IN) and hyperchlorinated, ultrafiltered water (delivered by water bottle). Mice were housed in solid-bottomed cages containing heat-treated hardwood shavings (NEPCO, Warrensburg, NY). Enrichment was provided in the form of 8 to 10 g of Enviro-Dri (Shepherd Specialty Papers, Watertown, TN) once weekly at cage change. Further health monitoring was not performed.
Six-week old mice were housed in the isolator on the day of arrival. Mice from the same strain and same environmental enrichment group were paired randomly as breeders by using a random integer generator from random.org. When possible, an equal number of pairs from each treatment were allocated to each isolator. Each isolator housed 29 or 30 pairs of mice on 3 rows of racking. Pairs were allocated to the rows in a balanced fashion by treatment and comprised 30 pairs of BALB/c mice raised under nest-only conditions, 29 pairs from the nest + shelter environment, and 29 pairs from the nest + shelter + wheel enrichment; 129/Sv pairs included 30 pairs from each of the 3 enrichment conditions.
Mice were checked daily as part of routine animal care. Births of litters were recorded daily. Pups from the first litter were sorted by sex, and groups were weighed at weaning for an average weight. After a second litter was born, or when 45 d had elapsed without the birth of a second litter (secondary nonproductive), the mice were euthanized. In cages in which one animal had died, the mice were euthanized. Cages with no pups weaned 60 d after setup were categorized as primary nonproductive, and the mice were euthanized. Pups were weaned at 17 to 23 d after birth, and statistical analyses controlled for the variation in weaning age.
Statistical analysis.
Reproductive parameters.
Analyses were conducted for the mean value per female of each reproductive parameter and for litter parity (litters 1 through 4). A mixed linear models analysis with unstructured covariance was conducted on reproductive parameters for each female mouse of each strain (across all litters, 1 to 4). Mixed linear models were applied per strain to evaluate the effects of enrichment, litter parity, and the interaction of enrichment and litter parity. Similar analyses were performed for the first-generation study, but across fewer litter parities (litter 1, with number born for litter 2) and adjusted for weaning day. The least-squares mean of each reproductive characteristic for each enrichment condition was calculated, as well as estimated mean differences, and a P value for the hypothesis that the difference between group means equals 0. ANOVA was conducted on summary total reproductive variables (total weaned and total number born) to analyze differences between cage enrichment conditions. Analyses were performed by using SAS (SAS Institute, Cary, NC). The significance level in all tests was α = 0.05.
Behavioral analysis. Data were analyzed by using Statview (SAS Institute). For the BALB/c and 129/Sv strains, data were first analyzed with 3-way or repeated-measures ANOVA, with factors of strain, housing condition, and sex. Separate within-strain comparisons were then conducted to determine effects of housing condition and sex. Significant effects of housing condition were further explored in male and female groups by using separate ANOVA, to determine whether effects were sex-dependent. Fisher protected least-significant difference tests were used for comparing group means only when a significant F value was determined in the overall ANOVA. Data from the B6 mice were analyzed separately, by using 1-way or repeated-measures ANOVA, with sex as the factor. Within-group ANOVA was used to determine sociability. For all comparisons, significance was set at a P value of less than 0.05.
Results
Parental reproductive parameters.
From each strain (BALB/c and 129/Sv), one breeding pair in the nest+shelter condition failed to produce any pups. All other breeding pairs in both strains in all enrichment conditions produced at least one litter. Most of the breeding female mice of both strains produced 4 litters (165 of 180 female mice overall: 86 of 90 BALB/c, 79 of 90 129/Sv). Number of litters (mean ± SEM) was 3.92 ± 0.04 for BALB/c dams and 3.76 ± 0.07 for 129/Sv dams across all enrichment conditions.
Enrichment condition did not significantly affect the number of pups born (across all 4 litters) in either the BALB/c or 129/Sv strain or the number of pups weaned in the BALB/c strain. However, significantly more 129/Sv pups were weaned in the nest+shelter (P = 0.026) and nest+shelter+wheel (P = 0.006) enrichment conditions than in the nest-only condition (Table 2). Neither male nor female pup weight at weaning differed significantly by enrichment condition (across all 4 litters) in either the BALB/c or 129/Sv strains, and enrichment condition did not affect the interlitter interval or time to first litter across all 4 litters in 129/Sv strain. In contrast, for the BALB/c strain, the interlitter interval or time to first litter was longer for the nest-only condition compared with the nest+shelter (P = 0.0007) and nest+shelter+wheel (P = 0.01) enrichment conditions (Table 2). Overall, compared with the nest-only environment, the nest+shelter and nest+shelter+wheel conditions led to more 129/Sv pups weaned and a shorter interlitter interval or time to first litter in BALB/c mice.
Table 2.
Reproductive parameters of mice according to enrichment condition
| Strain | Enrichment | No. of pups born | Pups weaned |
Weight (g) of pupsa |
Time (d) to 1st litter or interlitter interval | ||
| Total no. | % | Male | Female | ||||
| BALB/c | |||||||
| Nest (n = 119)b | 28.4 ± 1.4 | 24.8 ± 1.4 | 87.3 | 11.0 ± 0.2 | 11.0 ± 0.2 | 32.6 ± 1.0c | |
| Nest and shelter (n = 113) | 24.3 ± 1.6 | 22.6 ± 1.6 | 93.0 | 11.5 ± 0.1 | 11.4 ± 0.1 | 28.0 ± 1.1d | |
| Nest, shelter, and wheel (n = 117) | 27.0 ± 1.2 | 25.3 ± 1.3 | 93.7 | 11.6 ± 0.1 | 11.5 ± 0.1 | 29.2 ± 0.9d | |
| 129/Sv | |||||||
| Nest (n = 113) | 21.6 ± 1.4 | 16.8 ± 1.4e | 77.8 | 12.1 ± 0.2 | 12.0 ± 0.1 | 30.0 ± 1.0 | |
| Nest and shelter (n = 111) | 23.6 ± 1.5 | 21.1 ± 1.7f | 89.4 | 12.9 ± 0.2 | 12.5 ± 0.1 | 30.9 ± 1.2 | |
| Nest, shelter, and wheel (n = 115) | 23.7 ± 1.5 | 22.1 ± 1.5f | 93.2 | 12.8 ± 0.2 | 12.6 ± 0.2 | 30.1 ± 1.1 | |
Data are shown as mean ± SEM. Within each parameter, values with different letters differ significantly (P < 0.05).
At weaning (day 21).
Litter was used as the unit of measurement to compile means and SEM.
We also found overall strain-dependent differences in reproductive parameters, regardless of enrichment condition or litter parity. BALB/c mice had significantly more pups born (P = 0.0066), more pups weaned (P = 0.0009), and larger female pups at weaning (P = 0.0004), although BALB/c male pups at weaning were smaller than the 129/Sv male pups (P < 0.0001; Table 3). In the BALB/c strain, litter 1 had fewer pups than did litter 2 (P = 0.007) or litter 4 (P = 0.0007) and female mouse pups in litter 1 weighed more at weaning than did those in litters 2, 3, and 4 (P = 0.025, 0.0097, and 0.0003, respectively). In addition, the time to the first litter in the BALB/c strain was longer for litter 1 than was the interlitter interval for litters 2, 3, and 4 (P < 0.0001 for each comparison), and the interlitter interval was longer for litters 2 and 3 than for Litter 4 (P = 0.016 and 0.0002, respectively). For the 129/Sv strain, litter 1 had fewer pups than did litter 2, 3, or 4 (P < 0.0001 for each comparison) and fewer pups weaned than litters 2, 3, and 4 (P < 0.0001, P < 0.0001, P = 0.0015). In addition, the male and female 129/Sv pups in litter 1 weighed more at weaning than did those in litters 2, 3, and 4 (male pups: P = 0.0004, 0.0003, and 0.023, respectively; female pups: P < 0.0001, P < 0.0001, P = 0.004). Furthermore, similar to the findings for the BALB/c strain, the time to the first litter in 129/Sv mice was longer for litter 1 than were the interlitter intervals for litters 2, 3, and 4 (P < 0.0001 for each comparison). Among the reproductive parameters measured, fewer pups were born, female pups weighed more, and the time to first litter was longer for litter 1 compared with other litters of BALB/c mice; and fewer pups were born and weaned, male and female pup weighed more, and the time to first litter was longer for litter 1 compared with other litters of 129/Sv mice.
Table 3.
Reproductive parameters for BALB/c and 129/Sv mice according to litter parity
| Strain | Parityb | Litter size (no. of pups) | Pups weaned per litter |
Pup weight (g)a |
Time (d) to 1st litter or interlitter interval | ||
| No. | % | Male | Female | ||||
| BALB/c | |||||||
| 1 (n = 90) | 6.0 ± 0.3c | 5.6 ± 0.3 | 92.8 | 11.7 ± 0.1 | 11.6 ± 0.2c | 38.1 ± 1.0c | |
| 2 (n = 88) | 7.1 ± 0.3d | 6.6 ± 0.4 | 93.0 | 11.4 ± 0.2 | 11.2 ± 0.2d | 28.0 ± 1.2d,e | |
| 3 (n = 88) | 6.6 ± 0.3c,d | 6.1 ± 0.3 | 91.1 | 11.3 ± 0.2 | 11.4 ± 0.2d | 28.9 ± 1.1d,e | |
| 4 (n = 87) | 7.4 ± 0.3d | 6.5 ± 0.3 | 88.0 | 11.0 ± 0.2 | 11.0 ± 0.2d | 24.5 ± 0.8d | |
| 129/Sv | |||||||
| 1 (n = 89) | 4.5 ± 0.2c | 3.7 ± 0.3c | 81.7 | 13.2 ± 0.2c | 12.9 ± 0.2c | 37.9 ± 1.3c | |
| 2 (n = 88) | 6.5 ± 0.3d | 6.1 ± 0.4d | 92.7 | 12.6 ± 0.2d | 12.4 ± 0.2d | 28.4 ± 1.1d | |
| 3 (n = 83) | 6.8 ± 0.3d | 6.0 ± 0.4d | 88.2 | 12.5 ± 0.2d | 12.1 ± 0.1d | 27.0 ± 0.9d | |
| 4 (n = 79) | 6.4 ± 0.3d | 5.3 ± 0.4d | 83.2 | 12.2 ± 0.3d | 12.0 ± 0.2d | 27.4 ± 1.1d | |
Data are shown as mean ± SEM (P < 0.05- see Results text for P values). Within each parameter, values with different letters differ significantly (linear mixed model [enrichment, litter, enrichment×litter]; P < 0.05).
At weaning (day 21).
Litter was used as the unit of measurement to compile means and SEM.
Examining the effects of enrichment and litter parity revealed several significant differences in the BALB/c strain (Table 4). The weight of the female pups at weaning was greater in the nest+shelter+wheel condition for litters 2 and 3 compared with the nest-only (P = 0.039) and nest+shelter (P = 0.037) groups for litter 2 and compared with the nest-only (P = 0.050) condition for litter 3. Only 1 significant difference between enrichment conditions emerged for litter 4: male pups in the nest+shelter+wheel condition weighed more at weaning than did those in the nest-only (P = 0.013) environment. Neither enrichment condition nor litter parity exerted significant effects in the 129/Sv strain (Table 5). In the BALB/c strain, the most interactions occurred between the nest+shelter+wheel condition and parity.
Table 4.
Reproductive parameters for BALB/c mice according to litter parity and enrichment condition
| Parity | Enrichment | No. of pups born | Pups weaned |
Pup weight (g)a |
Time (d) to 1st litter or interlitter interval | ||
| No. | % | Male | Female | ||||
| 1 | |||||||
| Nest (n = 30)b | 6.9 ± 0.5c | 6.2 ± 0.5 | 89.9 | 11.4 ± 0.3 | 11.2 ± 0.2 | 37.6 ± 1.7 | |
| Nest and shelter (n = 30) | 5.7 ± 0.5c,d | 5.5 ± 0.5 | 96.5 | 12.0 ± 0.2 | 11.6 ± 0.3 | 40.1 ± 1.8 | |
| Nest, shelter, and wheel (n = 30) | 5.4 ± 0.5d | 5.0 ± 0.5 | 92.6 | 11.4 ± 0.3 | 11.9 ± 0.3 | 36.8 ± 1.7 | |
| 2 | |||||||
| Nest (n = 30) | 7.0 ± 0.6c,d | 6.6 ± 0.6c,d | 94.3 | 11.1 ± 0.3 | 10.8 ± 0.3c | 32.5 ± 1.9c | |
| Nest and shelter (n = 29) | 6.2 ± 0.6c | 5.7 ± 0.6c | 91.9 | 11.9 ± 0.3 | 11.4 ± 0.3c | 25.0 ± 2.0d | |
| Nest, shelter, and wheel (n = 29) | 8.1 ± 0.6d | 7.5 ± 0.6d | 92.6 | 11.4 ± 0.2 | 11.4 ± 0.3d | 26.2 ± 2.0d | |
| 3 | |||||||
| Nest (n = 30) | 6.7 ± 0.5c,d | 5.7 ± 0.6c,d | 85.1 | 11.1 ± 0.2 | 11.3 ± 0.4c | 33.9 ± 1.8c | |
| Nest and shelter (n = 29) | 5.8 ± 0.6c | 5.2 ± 0.6c | 89.7 | 11.6 ± 0.4 | 11.8 ± 0.4c,d | 23.6 ± 1.8d | |
| Nest, shelter, and wheel (n = 29) | 7.4 ± 0.6d | 7.2 ± 0.6d | 97.3 | 11.2 ± 0.2 | 11.0 ± 0.2d | 28.9 ± 1.8c | |
| 4 | |||||||
| Nest (n = 29) | 8.0 ± 0.5 | 6.5 ± 0.6 | 81.3 | 10.5 ± 0.4c | 10.7 ± 0.4 | 26.4 ± 1.4 | |
| Nest and shelter (n = 29) | 7.2 ± 0.5 | 6.8 ± 0.6 | 94.4 | 10.9 ± 0.2c,d | 10.8 ± 0.2 | 22.7 ± 1.4 | |
| Nest, shelter, and wheel (n = 29) | 6.8 ± 0.5 | 6.1 ± 0.6 | 89.7 | 11.7 ± 0.2d | 11.4 ± 0.2 | 24.4 ± 1.4 | |
Data are shown as mean ± SEM. Within each parity, values with different letters differ significantly (linear mixed model [enrichment, litter, enrichment×litter]; P < 0.05).
At weaning (day 21).
Litter was used as the unit of measurement to compile means and SEM.
Table 5.
Reproductive parameters of 129/Sv mice according to litter parity and enrichment condition
| Parity | Enrichment | No. of pups born | Pups weaned |
Pup weight (g)a |
Time (d) to 1st litter or interlitter interval | ||
| No. | % | Male | Female | ||||
| 1 | |||||||
| Nest (n = 30)b | 4.2 ± 0.4 | 3.1 ± 0.5 | 73.8 | 12.4 ± 0.3 | 12.3 ± 0.3 | 34.3 ± 1.8 | |
| Nest and shelter (n = 29) | 4.5 ± 0.4 | 3.6 ± 0.5 | 80.0 | 13.3 ± 0.3 | 12.7 ± 0.2 | 42.3 ± 2.8 | |
| Nest, shelter, and wheel (n = 30) | 4.8 ± 0.4 | 4.3 ± 0.5 | 89.6 | 13.7 ± 0.3 | 13.6 ± 0.2 | 37.2 ± 2.2 | |
| 2 | |||||||
| Nest (n = 30) | 6.2 ± 0.5 | 5.9 ± 0.6 | 95.2 | 12.1 ± 0.3 | 12.0 ± 0.3 | 29.3 ± 1.7 | |
| Nest and shelter (n = 29) | 6.2 ± 0.6 | 5.4 ± 0.6 | 87.1 | 13.0 ± 0.4 | 12.9 ± 0.3 | 29.1 ± 2.2 | |
| Nest, shelter, and wheel (n = 29) | 7.2 ± 0.6 | 6.9 ± 0.6 | 95.8 | 12.6 ± 0.3 | 12.4 ± 0.3 | 26.7 ± 1.9 | |
| 3 | |||||||
| Nest (n = 28) | 6.6 ± 0.5 | 5.1 ± 0.6 | 77.3 | 12.2 ± 0.3 | 12.1 ± 0.3 | 27.6 ± 1.9 | |
| Nest and shelter (n = 27) | 7.3 ± 0.6 | 7.1 ± 0.7 | 97.3 | 12.5 ± 0.3 | 12.0 ± 0.3 | 25.0 ± 0.9 | |
| Nest, shelter, and wheel (n = 28) | 6.5 ± 0.6 | 6.0 ± 0.6 | 92.3 | 12.8 ± 0.3 | 12.4 ± 0.2 | 28.5 ± 1.9 | |
| 4 | |||||||
| Nest (n = 25) | 6.3 ± 0.6 | 3.7 ± 0.7 | 58.7 | 11.5 ± 0.6 | 11.4 ± 0.3 | 28.2 ± 2.2 | |
| Nest and shelter (n = 26) | 6.7 ± 0.5 | 6.2 ± 0.5 | 92.5 | 12.8 ± 0.3 | 12.5 ± 0.3 | 26.4 ± 1.5 | |
| Nest, shelter, and wheel (n = 28) | 6.3 ± 0.5 | 6.0 ± 0.5 | 95.2 | 12.2 ± 0.4 | 12.0 ± 0.5 | 27.6 ± 2.2 | |
Data are shown as mean ± SEM. No values differed significantly (linear mixed model [enrichment, litter, enrichment×litter]; P < 0.05).
At weaning (day 21).
Litter was used as the unit of measurement to compile means and SEM.
First-generation reproductive parameters.
In the BALB/c strain, one breeding pair derived from each type of natal housing condition was removed from the study, due to loss of either the male or female of the pair after pairing at the Charles River Laboratories facility. In addition, one BALB/c breeding pair from the nest+shelter+wheel natal enrichment condition did not produce a litter after 88 d of pairing and was removed from the study at that time. In the 129/Sv strain, 3 breeding pairs were nonproductive (no litters during 62 to 69 d of pairing)—one from the nest+shelter enrichment condition and 2 from the nest+shelter+wheel condition—and were removed from study at that time. In addition, for the 129/Sv strain, no litter 2 was born within the time limits of the study from 1 cage for the nest+shelter natal enrichment condition and for 1 cage among the group raised under the nest+shelter+wheel condition.
The generational study revealed no significant differences in comparisons according to enrichment condition in the natal cage (Table 6). Litter parity comparisons were omitted because, except for the number of pups born and the interlitter interval, these data were collected for litter 1 only.
Table 6.
Reproductive parameters of offspring across 1 or 2 litters
| Litter 1 |
|||||||||
| Strain | Pups weaned |
Pup weight (g)a |
Litter 2 |
||||||
| Enrichment | No. of pups born | No. | % | Male | Female | Time to 1st litter (d) | No. of pups born | Interlitter interval (d) | |
| BALB/c | |||||||||
| Nest (n = 29)b | 5.7 ± 0.3 | 5.3 ± 0.4 | 93.0 | 10.5 ± 0.4 | 10.3 ± 0.3 | 28.0 ± 1.5 | 5.7 ± 0.5 | 27.5 ± 1.8 | |
| Nest and shelter (n = 29) | 6.5 ± 0.4 | 5.9 ± 0.5 | 90.8 | 11.1 ± 0.3 | 10.9 ± 0.4 | 25.8 ± 0.7 | 6.7 ± 0.5 | 28.4 ± 1.6 | |
| Nest, shelter, and wheel (n = 26) | 6.4 ± 0.4 | 6.4 ± 0.4 | 100.0 | 10.3 ± 0.2 | 10.3 ± 0.2 | 27.0 ± 1.2 | 6.6 ± 0.6 | 30.9 ± 2.0 | |
| 129/Sv | |||||||||
| Nest (n = 30) | 4.6 ± 0.4 | 3.8 ± 0.5 | 82.6 | 11.5 ± 0.5 | 11.6 ± 0.9 | 30.9 ± 2.2 | 6.8 ± 0.4 | 29.5 ± 1.5 | |
| Nest and shelter (n = 29) | 5.2 ± 0.5 | 4.7 ± 0.5 | 90.4 | 10.9 ± 0.3 | 10.6 ± 0.3 | 30.8 ± 2.4 | 6.8 ± 0.4 | 27.9 ± 1.3 | |
| Nest, shelter, and wheel (n = 28) | 5.4 ± 0.4 | 4.6 ± 0.5 | 85.2 | 11.7 ± 0.5 | 11.5 ± 0.4 | 31.9 ± 2.4 | 7.0 ± 0.5 | 26.5 ± 1.2 | |
Data are shown as mean ± SEM; no values differed significantly.
Pup weights were analyzed by using a covariate of weaning day to account for different weaning days (days 17–23).
Litter was used as the unit of measurement to compile means and SEM.
Phenotypes and behavioral testing of offspring.
Body weight.
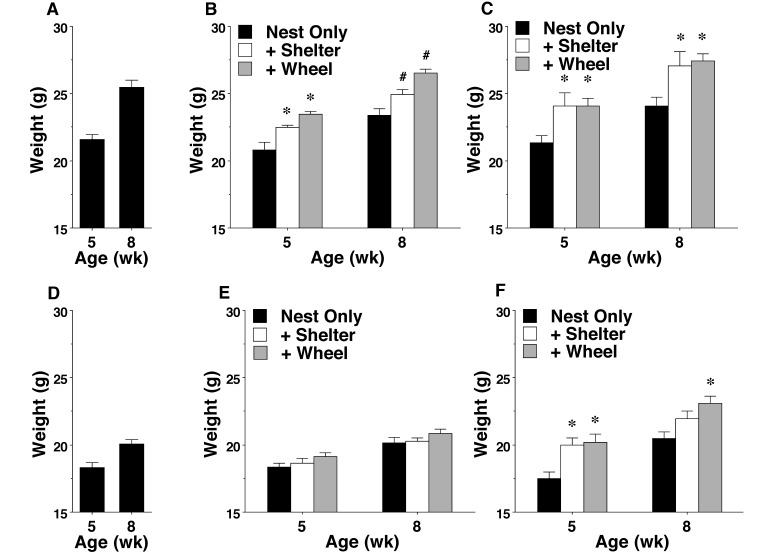
Previous studies have shown that cage enrichment leads to increased body weight in C57BL/6 and 129S6/SvEv/Tac mice.7,36 In the present study, both the nest+shelter and nest+shelter+wheel conditions led to significant increases in the weight of male BALB/c and 129/Sv mice (main effect of enrichment condition: F2,58 = 13.07, P < 0.0001; main effect of strain: F1,58 = 4.75, P = 0.0334). In addition, significant differences in weight were observed in the 129/Sv, but not the BALB/c, female groups (main effect of enrichment condition: F2,61 = 6.86, P = 0.0021; main effect of strain: F1,61 = 6.81, P = 0.0114; Figure 2).
Figure 2.
Body weights (mean ± SEM) in mice from different housing conditions. The results from B6 (A, male; D, female) mice are included as examples of typical weights in wild-type mice. (B) BALB/c and (C) 129/Sv male mice. (E) BALB/c and (F) 129/Sv female mice. Value differed significantly (P < 0.05) from that for (*) the nest-only group or (#) both other enrichment conditions.
Elevated plus maze testing.
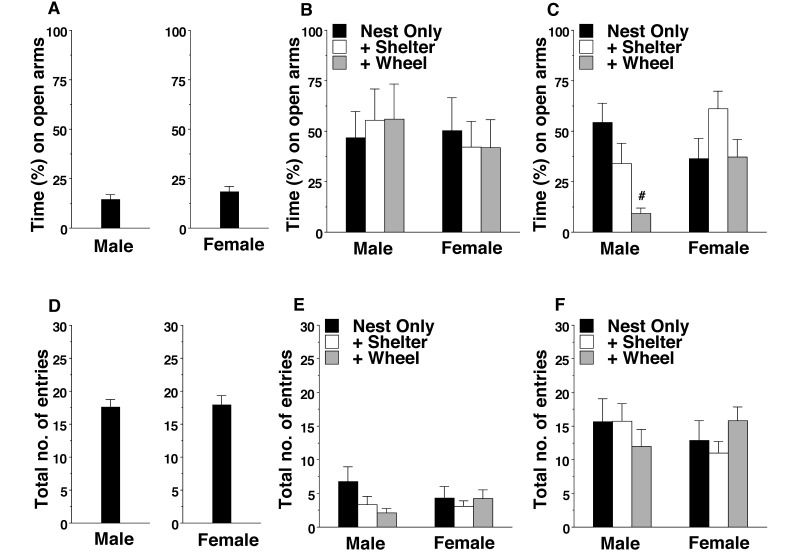
Wild-type mice typically demonstrate a strong preference for the closed arms of the maze18, as clearly evident in both the male and female B6 mice in the current study (Figure 3 A). In contrast, BALB/c mice from all enrichment conditions showed a lack of discrimination between the open and closed arms (Figure 3 B). Examination of the data indicated this result in the BALB/c mice was due to low exploration and the frequent occurrence of immobility (that is, ‘freezing), suggesting high anxiety during the test. A 2-way ANOVA confirmed the lack of sex- or enrichment-associated effects in the BALB/c mice, but 129/Sv mice yielded a different result (main effect of enrichment condition: F2,63 = 4.96, P = 0.01; enrichment × sex interaction: F2,63 = 4.44, P = 0.0158). In the 129/Sv strain, male mice housed with a wheel demonstrated a strong preference for the closed arms (Figure 3 C), similar to the percentage of time that B6 mice spent in the open arms.
Figure 3.
Time spent in (%) and total entries into the open arms of an elevated plus maze. Data are shown as the mean ± SEM for a 5-min test. (A and D) The results from B6 mice are included as examples of the typical strong preference of wild-type mice for the closed arms. (B) BALB/c and (C) 129/Sv male mice. (E) BALB/c and (F) 129/Sv female mice. Data from a BALB/c female mouse in the nest-only group that jumped off of the maze during the test are missing. #, Value significantly (P < 0.05) different from those for both other enrichment conditions.
The number of arm entries provided an index of activity during the elevated plus maze test (Figure 3 D through E). The BALB/c and 129/Sv mice had markedly different levels of activity (main effect of strain: F1,118 = 64.32, P < 0.0001), with fewer arm entries by BALB/c mice. Neither enrichment condition nor sex had significant effects on the number of arm entries.
Open field test.
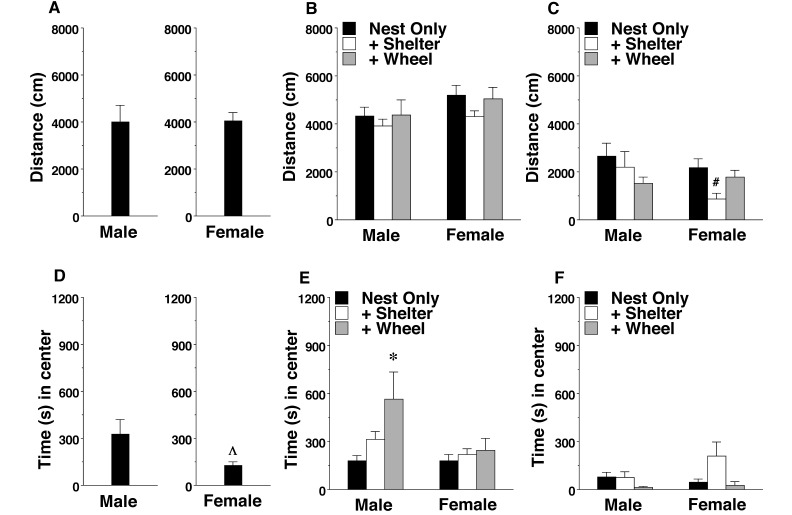
In the 1-h open field test, 129/Sv mice had generally low levels of activity, measured as distance traveled (Figure 4 A through C). A 3-way ANOVA confirmed significant main effects of strain (F1,119 = 127.56, P < 0.0001) and enrichment condition (F2,119 = 3.54, P = 0.0322) and a 2-way interaction between strain and sex (F1,119 = 6.02, P = 0.01) on distance traveled. Posthoc analyses indicated that, among 129/Sv female mice, the group housed in nest+shelter cages explored less of the novel open field during the session than did their counterparts in other environments.
Figure 4.
Distance traveled and center time in a novel open field. Data are shown as the mean ± SEM for a 1-h test. (A and D) The results from B6 mice are included as examples of the typical activity and exploration of wild-type mice. (B) BALB/c and (C) 129/Sv male mice. (E) BALB/c and (F) 129/Sv female mice. Value differed significantly (P < 0.05) from the mean for (Λ) male B6 mice, (*) the nest-only group, or (#) both other enrichment conditions.
In addition to activity, the time spent in the center region was recorded (Figure 4 D through E). In the B6 strain, the male mice generally had greater exploration of the center regions than did female mice (F1,18 = 4.50, P = 0.0482). A comparison of the BALB/c and 129/Sv mice confirmed a significant main effect of strain (F1,119 = 34.29, P < 0.0001), an enrichment condition × strain interaction (F2,119 = 6.02, P = 0.0032), and a strain × sex interaction (F1,119 = 6.15, P = 0.0145). Examination of the data suggested these significant effects were due primarily to a subset of mice that remained in the center regions for extended amounts of time during the initial intervals of the session. For example, during the first 5 min, 10 mice stayed in the center for more than 4 min; 3 of these 10 mice were BALB/c male mice housed in cages containing wheels, and 5 of the 10 mice were 129/Sv female mice housed in nest+shelter cages. These results suggest that mice from these 2 enrichment groups were more likely to freeze in a novel environment than were mice from nest-only cages.
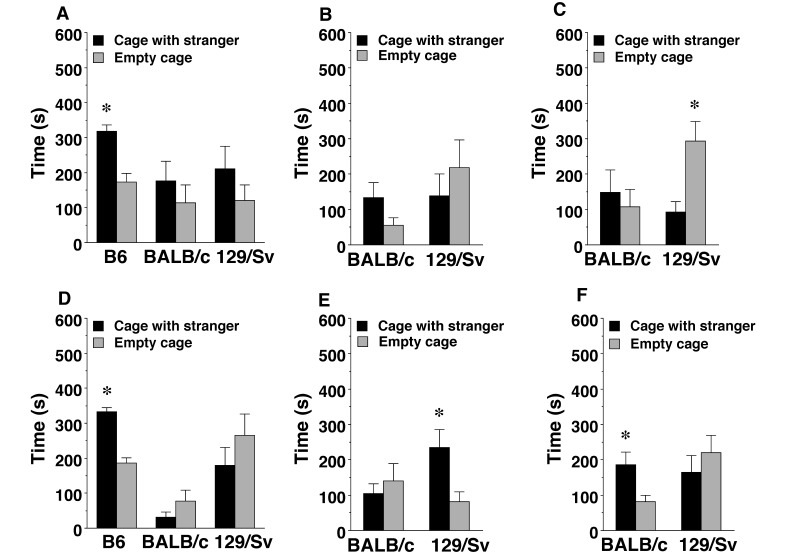
Social approach test.
In line with previous reports,18,26 the present study found strong social preference in male and female B6 mice but not in BALB/c and 129/Sv mice housed in nest-only cages (Figure 5 A and D). Exposure to nest+shelter or nest+shelter+wheel enrichment did not increase sociability in male mice from either the BALB/c or 129/Sv strains (Figure 5 B and C); in fact, 129/Sv male mice from the nest+shelter+wheel group demonstrated overt social avoidance (F1,11 = 9.60, P = 0.0101). Among female mice, exposure to more complex enrichment environments had strain-dependent prosocial effects (Figure 5 E and F). In particular, significant social preference emerged in the female BALB/c mice from the nest+shelter+wheel cages (F1,9 = 9.76, P = 0.0122) and in the 129/Sv female mice from the nest+shelter cages (F1,13 = 6.79, P = 0.0218).
Figure 5.
Sociability in a 3-chamber choice task. Data are shown as the mean ± SEM for a 10-min test. The results from B6 mice are included as examples of positive sociability in male and female wild-type mice. (A–C) Male mice with (A) nest only, (B) nest and shelter, or (C) nest, shelter, and wheel. (D–F) Female mice with (D) nest only, (E) nest and shelter, or (F) nest, shelter, and wheel. *, Values significantly (P < 0.05) different within group between the empty cage side and stranger cage side.
Marble burying.
Caging environment did not have a significant effect on digging behavior in the marble-burying test. All of the experimental groups buried 11 to 16 marbles during the 30-min assay (data not shown). A 3-way ANOVA indicated significant main effects of strain (F1,118 = 17.26, P < 0.0001) and sex (F1,118 = 5.64, P = 0.0191) but not enrichment condition on the number of marbles buried by BALB/c and 129/Sv mice.
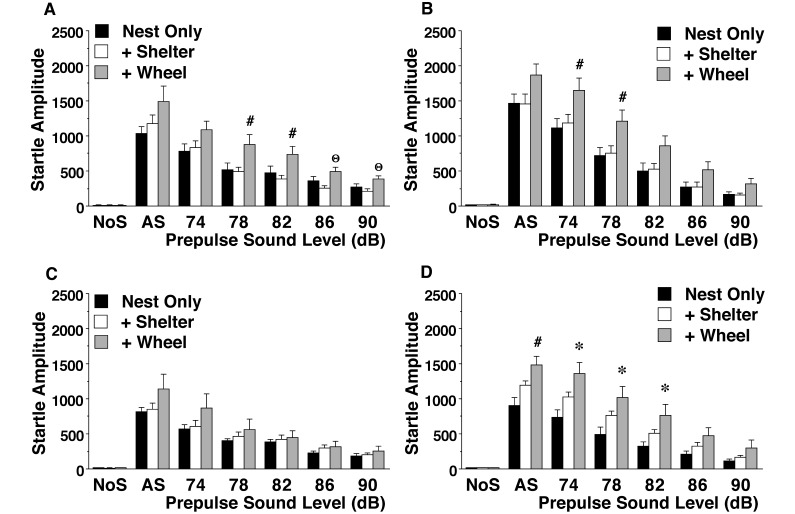
Acoustic startle test.
Enrichment condition had highly significant effects on the magnitude of startle amplitudes after the presentation of acoustic stimuli (Figure 6). Specifically, BALB/c male mice and 129/Sv female and male mice raised in the nest+shelter+wheel cages had higher startle amplitudes than did groups housed under the nest-only or nest+shelter conditions. Repeated-measures ANOVA revealed significant effects of enrichment condition (main effect: F2,119 = 12.63, P < 0.0001; condition × prepulse level interaction: F12,714 = 8.26, P < 0.0001) and main effects of strain (F1,119 = 11.20, P = 0.0011) and sex (F1,119 = 9.21, P = 0.003). It was noteworthy that the enrichment condition × prepulse level interaction nearly achieved significance (F12,174 = 1.80, P = 0.0507) in the BALB/c female group.
Figure 6.
Magnitude of acoustic startle responses. Trials included no-stimulus (NoS) controls and acoustic startle alone (AS; 120 dB) presentation. Data are shown as mean ± SEM. (A) Male BALB/c mice. (B) Male 129/Sv mice. (C) Female BALB/c mice. (D) Female 129/Sv mice. Value differed significantly (P < 0.05) from that for (*) the nest-only group, (Θ) the nest and shelter group, or (#) both other housing conditions.
Despite the differences in startle magnitude, levels of prepulse inhibition were similar across enrichment conditions in the 2 strains (data not shown). Repeated-measures ANOVA did not reveal any significant effects for housing or sex, but an interaction between strain and prepulse sound level emerged (F4,476 = 29.51, P < 0.0001). Therefore, the different enrichment environments did not influence sensorimotor gating and the ability to inhibit startle responses.
Discussion
The effects of individual enrichment scenarios on reproductive parameters depended on mouse strain and litter parity. The time to first litter might have been affected by several other factors other than the enrichment condition, including the change to a breeder–production diet, differences in the time to sexual maturity between strains and individual mice, and the stage of estrous cycle at time of pairing. In general in both strains, litter 1 had fewer pups born (and weaned for 129/Sv), higher weaning weights, and longer time to first litter (compared with interlitter intervals for the other litters) than did some or all of the other 3 litters. The parental mice were bred at 6 wk of age, and we might have seen different measures for litter 1 had the females been bred at 7 or 8 wk of age. Even though this timing could have been influenced by other factors, we included this information in the data and analyses to provide a more complete timeline for the litter production—for example, across both strains when paired at 6 wk of age, time to first litter was 4 to 16 d longer than were the interlitter intervals.
Although it was unsurprising to find that both strains showed differences between the first and subsequent litters, the interaction between enrichment condition and litter parity in some cases was unexpected. Litter parity in mice affects breeding success parameters when primiparous and multiparous female are compared, with first litters tending to have fewer pups with lower body weights.14,22 Recently, researchers found strain-dependent differences in the effects of litter parity on fecundity, in that primiparous litter loss was greater in B6 mice than in BALB/c mice; however, loss of the entire litter, rather than survival to weaning of individual pups, was recorded in the previous study.35 In contrast, most reports on the effects of litter parity focus on differences between the first litter and subsequent several litters (and before the decline in fertility that occurs with the age of the dam). Initially, breeding success is expected to increase over the next 2 or 3 litters after the first litter is born, but then breeding success declines due to increasing age and the stress of continuous pregnancy in the female mice. In the current study, we show that, although the main difference in litter parity remained between litter 1 and subsequent litters, additional differences between litters 2, 3, and 4 occurred.
Our previous work36 suggested that the presence of nesting material had the greatest effect on reproductive success and that the addition of single, other enrichment items did not have robust effects. In the current study, we tried to optimize the likelihood of identifying beneficial enrichment conditions by comparing a typical condition (cotton square only) with a superior nest condition (comprising a paper hut for shelter and additional nesting substrate) as well as this superior nest condition with the addition of an exercise wheel. Our purpose was to determine whether one or both of these relatively low-cost sets of enrichment items achieved significant improvements over standard mouse housing conditions. Regarding enrichment conditions irrespective of litter parity, 129/Sv mice weaned significantly fewer pups in the nest-only condition than in the other 2 conditions and BALB/c mice had a longer interlitter interval under the nest-only condition. Therefore, according to those 2 parameters, both the nest+shelter and nest+shelter+wheel conditions were better than the nest-only condition in both strains. This outcome is somewhat similar to that of a study that showed strain-associated differences in breeding performance indices in response to which nesting material was used.6 In particular, F1 mice reared in 3 different enrichment paradigms demonstrated no reproductive effects due to the enrichment in the natal cage that carried over to the adult breeding mice. This finding is unlike a study16 that did find a generational difference in enrichment effect; however, the cited study compared the behavior of socially enriched and socially impoverished male mice rather than the effects of particular items of environmental enrichment on reproduction.
The interaction between litter parity and enrichment condition was limited to BALB/c mice. A study examining reproductive parameters and enrichment over 2 litters in B6 mice found a significant effect of parity on pup survival rate and average pup weight between litters 1 and 2 but no significant interaction of parity and enrichment condition;27 those results are similar to our findings in the 129/Sv strain. Although the interaction was not consistent across parities in our study, in general, several reproductive parameters were improved in the nest+shelter+wheel condition compared with the nest-only condition.
In addition to examining reproductive parameters, our present study addressed whether enriched housing conditions led to alterations in strain phenotypes. One reason for selecting BALB/c and 129/Sv was that these strains have behavioral profiles divergent from C57BL/6 (B6), a strain often used as a ‘wild-type’ standard for inbred mouse strains.18,26 In particular, B6 can serve as a highly sociable control for mouse models of autism, schizophrenia, and other human disorders characterized by social deficits. Our results showed that exposure to a wheel, in addition to other enrichment items, had significant effects in several different behavioral assays. In the elevated plus maze, male 129/Sv mice from the nest+shelter+wheel condition showed a strong preference for the closed arms, similar to that of the B6 mice. In contrast, the BALB/c and 129/Sv mice from the other housing conditions failed to demonstrate an overt arm preference, possibly due to increased freezing in the open arms of the maze. In a 1-h open field test, male BALB/c mice from the nest+shelter+wheel condition spent significantly more time in the center region without an overall change in total distance traveled. Thus, in the male mice, exposure to the nest+shelter+wheel items led to profiles of behavior that were either more comparable to that of the wild-type strain (B6) or indicative of less anxiety without alterations in general activity during the tests.
An enriched environment leads to increased sociability in NMRI mice.17 In the present study, the nest+shelter+wheel housing condition had divergent effects on social approach, dependent on strain and sex. In the 3-chamber choice task, exposure to the nest+shelter+wheel condition had prosocial effects in BALB/c female mice but led to an unusual, overt social avoidance in 129/Sv male mice. These types of divergent effects of the nest+shelter+wheel enrichment condition did not occur in the acoustic startle test. In that assay, the nest+shelter+wheel condition was associated with significantly higher startle amplitudes in all of the groups except BALB/c female mice, which showed a strong trend for increased startle magnitudes. At the same time, enrichment had no effect on levels of prepulse inhibition. A similar pattern of increased acoustic startle responses without concomitant changes in prepulse inhibition has been reported in rats reared with cage enrichment after weaning.33 In the present study, access to the wheel might have led to an enhanced physical condition, with better ability to produce the large-scale muscular reflexes measured as startle responses. The higher amplitudes did not appear to be due to increased body weight in the nest+shelter+wheel groups, given that the groups provided enrichment without the wheels did not demonstrate the same changes despite having similar weights.
We previously found that enriched housing leads to increased inhibitory control over exploratory behavior in C57BL/6Tac mice.36 In the present study, 129/Sv male mice from the nest+shelter+wheel condition spent less time on the open arms of the elevated plus maze and less time in proximity to a stranger mouse during the social test, which might be viewed as greater control over risk-taking behavior, than did their counterparts in other environments. Other studies have found that an enriched environment leads to decreased ‘risky’ exploration by C57BL/6 mice in elevated plus maze and open field tests.2,39 However, one report indicated that enriched housing led to increased center time in an open field in B6 mice, compared with decreased center time in 129 mice, supporting the present findings of divergent enrichment effects, dependent on strain, in specific behavioral tasks.3 Furthermore, enrichment has been shown to increase aggression in a strain-dependent manner,8,28 an outcome that might have detrimental consequences for social dynamics in the home-cage setting. Overall, the results suggest that enrichment can both increase and decrease anxiety-like behavior and sociability, thus complicating generalizations regarding possible beneficial behavioral effects of enrichment on mouse wellbeing.
In contrast, in terms of reproductive parameters, both the nest+shelter and nest+shelter+wheel conditions were better than was the nest-only condition in both strains (for 2 or 3 of the reproductive parameters). However, litter parity had more effects than did enrichment condition, and interaction between litter parity and enrichment condition occurred in the BALB/c mice. Generationally, any effects of the natal cage environment on reproduction did not persist from the offspring to the adult cage. This result might mean that whatever enrichment is in the cage currently overshadows any former effect from the natal cage.
Although we expected an effect of litter parity in terms of a difference between litter 1 and the other litters, we did not expect significant differences between later litters, at least until the dams reached 6 mo of age or more, when fertility might decline. We also did not expect the interaction between litter parity and enrichment condition. It would have been interesting to examine the question of litter parity differences in the behavioral testing as well. Although the analysis of parity effects on strain behavioral profiles was beyond the scope of this project, future studies might examine the question of litter parity on behavioral measures by weaning from different litters for behavioral testing to compare the results of mice from different parities in the same enrichment condition.
In the generational study, we investigated the possible long-term effects of an early enriched environment on the breeding success of mice after their transfer to a different setting. After raising the offspring in the described enrichment conditions, we sent mice to a colleague at a commercial facility (Charles River Laboratories) for evaluation under different housing and enrichment conditions. We found no evidence for persistent effects of the natal cage enrichment on adult reproductive success after the stresses of transfer and introduction to a novel housing environment. This result may be very good news for investigators who obtain mice from vendors, where the mice likely are raised in cage environments that differ from those at the investigator's institution. However, because the generational study only involved breeding for a total of 2 litters, we might not have detected differences that emerged in later litters. Neither the offspring shipped for breeding at the commercial facility nor their progeny underwent behavioral testing. Perhaps differences in reproductive parameters due to the natal enrichment of the F1 mice might have emerged as behavioral differences in the F2 mice.
In conclusion, we found strain- and sex-dependent differences in tested behavioral measures of mice raised in different enrichment conditions. In addition, litter parity had greater effects on reproductive parameters measured than did the enrichment condition, and this effect was not solely due to differences between the first compared with subsequent litters. The interaction between parity and enrichment condition appeared to be strain-dependent, given that it occurred only in the BALB/c strain of mice. We found no generational effect of natal cage enrichment in the reproductive parameters measured.
Acknowledgments
We thank Donna Webb for her work in colony management of the mice and in data collection for this project and also Amy Davidson, Allison Leclerc, and Rosa Edsall for their assistance with the project. We thank Kara Saddoris, Viktoriya Nikolova, and Natallia Riddick for their expert assistance in conducting the behavioral tests and Rachel Kloss for statistical advice. We thank the husbandry staff of the Division of Laboratory Animal Medicine at UNC for their patience and precision with a challenging cage-change protocol. This project was supported by funds from the Division of Laboratory Animal Medicine (University of North Carolina, Chapel Hill) and by the Mouse Behavioral Phenotyping Core of the Carolina Institute for Developmental Disabilities (University of North Carolina, Chapel Hill; NIH/NICHD U54-HD079124). Charles River Laboratories supported this project by providing the initial breeder pairs of mice. At Charles River, excellent husbandry was provided by Geomaris Maldonado and Marie Heyer.
References
- 1.Bayne K, Wurbel H. 2014. The impact of environmental enrichment on the outcome variability and scientific validity of laboratory animal studies. Rev Sci Tech 33:273–280. [DOI] [PubMed] [Google Scholar]
- 2.Branchi I, Alleva E. 2006. Communal nesting, an early social enrichment, increases the adult anxiety-like response and shapes the role of social context in modulating the emotional behavior. Behav Brain Res 172:299–306. [DOI] [PubMed] [Google Scholar]
- 3.Coke-Murphy C, Buendia MA, Saborido TP, Stanwood GD. 2014. Simple shelter-style environmental enrichment alters behavior in mice. Transl Neurosci 5:185–196. [Google Scholar]
- 4.Forsyth N, Young G, Mench J. 2007. Effects of cage size and enrichment on aggression and stereotypic behavior in 3 strains of laboratory mice. J Am Assoc Lab Anim Sci 46:93–94. [Google Scholar]
- 5.Gaskill BN, Winnicker C, Garner JP, Pritchett-Corning KR. 2013. The naked truth: breeding performance in nude mice with and without nesting material. Appl Anim Behav Sci. 143: 110–116. [Google Scholar]
- 6.Gaskill BN, Pritchett-Corning KR, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. 2013. Energy reallocation to breeding performance through improved nest building in laboratory mice. PLoS One 8:e74153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinla I, Leidmaa E, Visnapuu T, Philips MA, Vasar E. 2014. Enrichment and individual housing reinforce the differences in aggressiveness and amphetamine response in 129S6/SvEv and C57BL/6 strains. Behav Brain Res 267:66–73. [DOI] [PubMed] [Google Scholar]
- 8.Howerton CL, Garner JP, Mench JA. 2008. Effects of a running wheel-igloo enrichment on aggression, hierarchy linearity, and stereotypy in group-housed male CD-1 (ICR) mice. Appl Anim Behav Sci 115:90–103. [Google Scholar]
- 9.Hunt C, Hambly C. 2006. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group housed males. Physiol Behav 87:519–526. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson EK, Avery AC, Vandewoude S. 2012. Environmental enrichment during rearing alters corticosterone levels, thymocyte numbers, and aggression in female BALB/c mice. J Am Assoc Lab Anim Sci 51:18–24. [PMC free article] [PubMed] [Google Scholar]
- 11.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 12.Kaliste EK, Mering SM, Huuskonen HK. 2006. Environmental modification and agonistic behavior in NIH/S male mice: nesting material enhances fighting but shelters prevent it. Comp Med 56:202–208. [PubMed] [Google Scholar]
- 13.Kedia S, Chattarji S. 2014. Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J Neurosci Methods 233:150–154. [DOI] [PubMed] [Google Scholar]
- 14.Krackow S, Gruber F. 1990. Sex-ratio and litter size in relation to parity and mode of conception in 3 inbred strains of mice. Lab Anim 24:345–352. [DOI] [PubMed] [Google Scholar]
- 15.Martin B, Golden E, Carlson OD, Egan JM, Mattson MP, Maudsley S. 2008. Caloric restriction: impact upon pituitary function and reproduction. Ageing Res Rev 7:209–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mashoodh R, Franks B, Curley JP, Champagne FA. 2012. Paternal social enrichment effects on maternal behavior and offspring growth. Proc Natl Acad Sci USA 109 Suppl 2:17232–17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesa-Gresa P, Perez-Martinez A, Redolat R. 2013. Environmental enrichment improves novel object recognition and enhances agonistic behavior in male mice. Aggress Behav 39:269–279. [DOI] [PubMed] [Google Scholar]
- 18.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. 2007. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav Brain Res 176:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicol CJ, Brocklebank S, Mendl M, Sherwin CM. 2008. A targeted approach to developing environmental enrichment for 2 strains of laboratory mice. Appl Anim Behav Sci 110:341–353. [Google Scholar]
- 20.Olsson IAS, Dahlborn K. 2002. Improving housing conditions for laboratory mice: a review of ”environmental enrichment”. Lab Anim 36:243–270. [DOI] [PubMed] [Google Scholar]
- 21.Peters AG, Bywater PM, Festing MFW. 2002. The effect of daily disturbance on the breeding performance of mice. Lab Anim 36:188–192. [DOI] [PubMed] [Google Scholar]
- 22.Reading AJ. 1966. Effects of parity and litter size on birth weight of inbred mice. J Mammal 47:111–114. [PubMed] [Google Scholar]
- 23.Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson RT, Churchill GA, Schweitzer IB, Myers DD. 2001. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim 35:58–73. [DOI] [PubMed] [Google Scholar]
- 24.Richter SH, Garner JP, Wurbel H. 2009. Environmental standardization: Cure or cause of poor reproducibility in animal experiments? Nat Methods 6:257–261. [DOI] [PubMed] [Google Scholar]
- 25.Richter SH, Garner JP, Zipser B, Lewejohann L, Sachser N, Touma C, Schindler B, Chourbaji S, Brandwein C, Gass P, van Stipdonk N, van der Harst J, Spruijt B, Voikar V, Wolfer DP, Wurbel H. 2011. Effect of population heterogenization on the reproducibility of mouse behavior: a multi-laboratory study. PLoS One 6:e16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. 2006. A mouse model system for genetic analysis of sociability: C57BL/6J compared with BALB/cJ inbred mouse strains. Biol Psychiatry 59:415–423. [DOI] [PubMed] [Google Scholar]
- 27.Shair HN, Nunez Y, Osman MM. 2011. Enrichment materials do not negatively affect reproductive success and offspring survival and weight in mice. Lab Anim(NY) 41:14–19. [DOI] [PubMed] [Google Scholar]
- 28.Swetter BJ, Karpiak CP, Cannon JT. 2011. Separating the effects of shelter from additional cage enhancements for group-housed BALB/cJ mice. Neurosci Lett 495:205–209. [DOI] [PubMed] [Google Scholar]
- 29.The Jackson Laboratory. 2014Breeding performance survey, females of 35 commonly used strains of JAX mice. MPD:31406. Mouse Phenome Database website. [Cited 29 April 2015]. Available at: http://Phenome.jax.org.
- 30.Toth LA, Kregel K, Leon L, Musch TI. 2011. Environmental enrichment of laboratory rodents: the answer depends on the question. Comp Med 61:314–321. [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai PP, Pachowsky U, Stelzer HD, Hackbarth H. 2002. Impact of environmental enrichment in mice. 1: effect of housing conditions on body weight, organ weights and haematology in different strains. Lab Anim 36:411–419. [DOI] [PubMed] [Google Scholar]
- 32.Van Loo PLP, Kruitwagen CLJJ, Koolhaas JM, Van de Weerd HA, Van Zutphen LFM, Baumans V. 2002. Influence of cage enrichment on aggressive behaviour and physiological parameters in male mice. Appl Anim Behav Sci 76:65–81. [Google Scholar]
- 33.Varty GB, Paulus MP, Braff DL, Geyer MA. 2000. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol Psychiatry 47:864–873. [DOI] [PubMed] [Google Scholar]
- 34.Võikar V, Kõks S, Vasar E, Rauvala H. 2001. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav 72:271–281. [DOI] [PubMed] [Google Scholar]
- 35.Weber EM, Algers B, Wurbel H, Hultgren J, Olsson IA. 2012. Influence of strain and parity on the risk of litter loss in laboratory mice. Reprod Domest Anim 48:292–296. [DOI] [PubMed] [Google Scholar]
- 36.Whitaker J, Moy SS, Godfrey V, Nielsen J, Bellinger D, Bradfield J. 2009. Effects of cage size and enrichment on reproductive performance and behavior in C57BL/6Tac mice. Lab Anim (NY) 38:24–34. [DOI] [PubMed] [Google Scholar]
- 37.Whitaker J, Moy SS, Saville BR, Godfrey V, Nielsen J, Bellinger D, Bradfield J. 2007. The effect of cage size on reproductive performance and behavior of C57BL/6 mice. Lab Anim (NY) 36:32–39. [DOI] [PubMed] [Google Scholar]
- 38.Wolfer DP, Litvin O, Morf S, Nitsch RM, Lipp HP, Wurbel H. 2004. Laboratory animal welfare: cage enrichment and mouse behaviour. Nature 432:821–822. [DOI] [PubMed] [Google Scholar]
- 39.Zhu SW, Yee BK, Nyffeler M, Winblad B, Feldon J, Mohammed AH. 2006. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res 169:10–20. [DOI] [PubMed] [Google Scholar]