Abstract
The reliable generation of high-percentage chimeras from gene-targeted C57BL/6 embryonic stem cells has proven challenging, despite optimization of cell culture and microinjection techniques. To improve the efficiency of this procedure, we compared the generation of chimeras by using 3 different inbred, albino host, embryo-generating protocols: BALB/cAnNTac (BALB/c) donor mice superovulated at 4 wk of age, 12-wk-old BALB/c donor mice without superovulation, and C57BL/6NTac-Tyrtm1Arte (albino B6) mice superovulated at 4 wk of age. Key parameters measured included the average number of injectable embryos per donor, the percentage of live pups born from the total number of embryos transferred to recipients, and the number of chimeric pups with high embryonic-stem–cell contribution by coat color. Although albino B6 donors produced significantly more injectable embryos than did BALB/c donors, 12-wk-old BALB/c donor produced high-percentage (at least 70%) chimeras more than 2.5 times as often as did albino B6 mice and 20 times more efficiently than did 4-wk-old BALB/c donors. These findings clearly suggest that 12-wk-old BALB/c mice be used as blastocyst donors to reduce the number of mice used to generate each chimera, reduce the production of low-percentage chimeras, and maximize the generation of high-percentage chimeras from C57BL/6 embryonic stem cells.
Mouse strains carrying specific mutations at desired loci have become powerful reagents for modeling genetic disorders, understanding embryonic development, and evaluating therapeutics. With the sequencing of the C57BL/6 mouse genome and decision by the International Knockout Mouse Consortium and trans-NIH Knockout Mouse Project to provide mutated mouse C57BL/6N embryonic stem (ES) cells for every protein-coding gene, the inbred C57BL/6 strain has become very popular for the generation of new animal models, and numerous robust, germline-competent ES cell lines are available.14,18,26 Microinjection of mutant ES cells into host blastocysts produces chimeric mice, and the contribution of the ES cells to the offspring can be assessed visually according to coat color differences between ES cells and the embryo donor.12,15,18 Therefore, ES cells derived from C57BL/6 black mice are often injected in host embryos produced by albino inbred mice.
Tyrosinase-mutant albino C57BL/6 and naturally tyrosinase-deficient albino BALB/c mice have both been used to produce host embryos for injection with ES cells during the generation of chimeric mice.23,27 Both strains are often superovulated to synchronize estrus cycles and produce increased numbers of blastocysts when bred,8 however, substrain- and age-associated differences lead to variable effectiveness in generating embryos.6,8,13 Sperm abnormalities typically are relatively frequent in male BALB/c mice,10,16 but C57BL/6 substrains are notorious for perinatal mortality,25 so neither strain provides an optimally robust colony of donor mice for a transgenic facility.
Given the potential benefit of albino C57BL/6NTac-Tyrtm1Arte mice to provide host embryos, we compared outcomes for these embryos with those obtained by using the superovulated and naturally bred mature BALB/c mice typically obtained from our transgenic facility. In this study, we hypothesized that albino B6 mice are a better source for host blastocysts than are superovulated and naturally bred mature BALB/c mice in the context of generating mice with high coat-color contribution from the injected ES cells. By seeking the most efficient donor embryo protocol, we incorporated 3Rs concepts22 by reducing the total number of animals used in these procedures.
Materials and Methods
Animals.
BALB/cAnNTac and C57BL/6NTac-Tyrtm1Arte (albino B6 mice; model no. 11971, Taconic Biosciences,) mice were used in this study. The BALB/c mice originated from colonies free of minute virus of mice, mouse encephalomyelitis virus, mouse hepatitis virus, mouse parvovirus, epizootic diarrhea of infant mice virus, murine norovirus, pneumonia virus of mice, ectromelia virus, K virus, lymphocytic choriomeningitis virus, mouse adenovirus types 1 and 2, mouse cytomegalovirus, polyoma virus, reovirus 3, Sendai virus, thymic virus, lactate dehydrogenase elevating virus, β-hemolytic Streptococcus (nonGroup D) spp., Bordetella bronchiseptica, cilia-associated respiratory bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutscheri, Helicobacter spp., Klebsiella pneumoniae, Mycoplasma pulmonis, Pasteurella multocida, Pasteurella pneumotropica, Pneumocystis spp., Pseudomonas aeruginosa, Salmonella spp., Staphylococcus aureus, Streptococcus pneumoniae, Aspiculuris tetraptera, Eimeria spp., Encephalitozoon cuniculi, Entamoeba muris, Giardia muris, Hymenolepis spp., Myobia musculi, Myocoptes musculinus, Polyplax spinulosa, Psorergates simplex, Radfordia affinis, Rodentolepis spp., Spironucleus muris, Syphacia spp., and trichomonads. The albino B6 mice in this study were generously donated by Taconic Biosciences and originated from colonies free of Klebsiella oxytoca and all of the aforementioned pathogens except for Pseudomonas aeruginosa. All protocols and animals used were IACUC-approved, and mice were housed in accordance with the Guide for the Care and Use of Laboratory Animals9 in an AAALAC-accredited program at the Massachusetts Institute of Technology. All mice were housed on corncob bedding, fed Prolab RMH 3000 (LabDiets, St Louis, MO), had unrestricted access to water, were cohoused, and received cotton squares as enrichment items unless otherwise noted. BALB/c females were shipped at 3 wk (n = 33) and 8 wk (n = 21) of age. Albino B6 females were shipped at 3 wk (n = 36) of age. BALB/c and Albino B6 mice were housed in 14:10 and 12:12 light:dark cycle rooms, respectively. All males were proven breeders as per cage card records, with age ranging from 3 to 13 mo and 2 to 5 mo of age for BALB/c and Albino B6, respectively. Surrogate dams were mature Crl:CD1 (ICR) mice (n = 57; weight [mean ± SEM], 33.9 ± 0.6 g) that originated from an inhouse breeding colony free of mouse hepatitis virus, mouse parvovirus, epizootic diarrhea of infant mice virus, murine norovirus, pneumonia virus of mice, ectromelia Virus, K virus, lymphocytic choriomeningitis virus, mouse adenovirus types 1 and 2, polyoma virus, reovirus 3, Sendai virus, Theiler encephalomyelitis virus, β-hemolytic Streptococcus (nonGroup D) spp., Bordetella bronchiseptica, Citrobacter rodentium, Corynebacterium kutscheri, Helicobacter spp., Klebsiella pneumoniae, Mycoplasma pulmonis, Pasteurella multocida, Pasteurella pneumotropica, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae, Aspiculuris tetraptera, Giardia muris, Myobia musculi, Myocoptes musculinus, Radfordia affinis, Spironucleus muris, Syphacia spp., and trichomonads. Colony proven-sterile vasectomized CD1 male mice were single-housed until placed with a single proestrus- or estrus-selected female CD1 mouse to generate pseudopregnant mice.
Superovulation, breeding, and embryo collection.
Each of the 3 experimental groups was assessed in 3 replicate cohorts of 4-wk-old BALB/c (n = 9, 12, and 12), 12-wk-old BALB/c (n = 7 for each replicate), and albino B6 (n = 12 for each replicate) mice. Using previously published superovulation protocols,8,13,27 4-wk old BALB/c (weight [mean ± SEM],13.5 ± 0.2 g) and 4-wk-old albino B6 (weight, 15.6 ± 0.2 g) mice were injected intraperitoneally with 5 IU pregnant mare serum gonadotropin (National Hormone and Peptide Program, Torrance, CA) intraperitoneally followed 46 to 48 h later by intraperitoneal injection of 5 IU recombinant human chorionic gonadotropin (National Hormone and Peptide Program, Torrance, CA). Each female mouse was placed in a cage with a male of the same strain; the pair was separated the following morning, and the presence (or absence) of a copulatory vaginal plug was recorded. Estrus-selected, mature 12-wk-old female BALB/c mice (weight, 20.6 ± 0.3 g) were not superovulated and were placed in cages with proven male BALB/c mice in a 1:1 male:female ratio. Host embryos were obtained from flushed mouse uteri after euthanasia by cervical dislocation performed by personnel with demonstrated training and experience in this technique. In our experience, embryos collected from BALB/c donors at precisely 3.5 d postcoitum routinely yield blastocysts that are not sufficiently expanded for microinjection—unlike those collected later in morning of the third day postcoitum. Therefore, albino B6 host embryos were collected at 3.5 d postcoitum, and BALB/c host embryos were collected 4 to 6 h after 3.5 d postcoitum. The number of embryos and their developmental stage were noted. After microscopic examination, embryos at the blastocyst stage with an intact zona pellucida were deemed injectable, whereas those at any other stage were unsuitable. All embryos were maintained in KSOM mouse embryo culture medium with amino acids and phenol red (EMD Millipore, Billerica, MA) in an incubator (Thermo Fisher Scientific, Waltham, MA) containing 5% CO2 at 37 °C until microinjection.
ES cell injection and embryo transfer.
Tissue culture was performed by the KI ES Cell and Transgenics Facility at MIT. JM8A3 ES cells did not contain a gene targeting cassette, were confirmed to be euploid by karyotyping (Cell Line Genetics, Madison, WI), and were free of rodent pathogens according to Infectious Microbe PCR Amplification testing (IDEXX Bioresearch, Columbia, MO). Aliquots of frozen ES cells were thawed from a single batch so that each injection was performed with matched cells of the same passage. Our previous gene targetings initiated in JM8A3 ES cells at passage 12 showed successful contribution of the cells to the germline of male chimeras after injection into BALB/c blastocysts. Vials from a single lot of cryopreserved JM8A3 cells (passage 14) were thawed into ES cell qualified media (recipes provided at www.komp.org and components obtained from Invitrogen [Grand Island, NY] except for leukemia inhibitory factor from EMD Millipore [Billerica, MA] and FBS from Sigma [St Louis, MO]) and grown on a fresh layer of irradiated mouse embryonic fibroblast feeder cells in a 37 °C incubator with 5% CO2. ES cells were thawed 3 d before injection and underwent daily observation and media changes to achieve optimal colony morphology. On the morning of injection, ES cells received fresh media 2 h prior to trypsinization to generate single-cell suspensions and were plated in fresh media on a gelatin-coated dish for 30 min to allow feeder cells to adhere. This step enriched the ES cell population suspended in the media before 3 × 105 suspended cells/mL were provided on ice to the microinjectionist.
The same person (PQ) performed all blastocyst microinjections and embryo transfer surgeries as previously described.15 An inverted microscope (ECLIPSE TE2000S, Nikon, Chiyoda, Tokyo) was used to facilitate the injection of blastocysts, which occurred in 60 mm × 15 mm culture dishes (Falcon 351007, Becton Dickinson, Franklin Lakes, NJ) containing drops of EmbryoMAX M2 medium with phenol red (EMD Millipore) covered in mineral oil (Sigma) on a cooling stage set to 4 °C. By using a standard injection needle (TransferTip [ES] modified with 20° tip angle, 15 μm inner diameter, and 1 mm flange; Eppendorf, Hauppauge, NY), each blastocyst was injected with 15 to 18 wildtype JM8A3 ES cells (Knockout Mouse Project, UC Davis, CA) as previously described.15
Pseudopregnant CD1 female mice were anesthetized with 0.2-μm–filtered 2,2,2-tribromoethanol (500 mg/kg IP) reconstituted with tert-amyl alcohol (Sigma). Once anesthetized, each animal received an application of sterile ophthalmic petroleum-based ointment (Dechra Veterinary Products, Overland Park, KS) on both eyes and a preoperative dose of meloxicam (1 mg/kg SC; Norbrook, Newry, Northern Ireland). After clipping and disinfecting of the dorsum, a surgical plane of anesthesia was confirmed by lack of the withdrawal reflex to toe pinch, and the mouse was placed in ventral recumbency under a dissecting microscope (SMZ1000, Nikon). Embryo transfer was performed as previously described.15 A total of 12 to 14 microinjected blastocysts were transferred to each uterine horn of a pseudopregnant CD1 female mouse at 2.5 d postcoitum. After replacement of the uterine horns into the abdomen, the muscle layer of peritoneal cavity was closed in a simple, interrupted fashion by using 5-0 silk suture; the skin incision was closed with surgical wound clips. Each mouse was provided with 1.0 mL of warmed subcutaneous 0.9% saline (Hospira Worldwide, Lake Forest, IL) and received thermal support operatively and postoperatively. All recipient mice recovered without complications from embryo transfer surgery, and wound clips were removed once the skin was healed.
Progeny.
Surrogate dams were cohoused with other surrogates until 4 d prior to expected parturition, when they were separated into single housing. Pups were examined visually during the first several days after parturition to determine viability, cannibalism by dam, and potential need for fostering. The coat color of 10-d-old pups was determined by 2 blinded and experienced microinjectionists (PQ and JM) according to the total percentage of the skin covered in pigmented fur; the average of these 2 scores was reported. To determine the likelihood of germline transmission of injected ES cells, transgenic facilities often assess the proportion of coat pigmentation in progeny mice, because coat-color contribution by injected ES cells correlates with genetic transmission.7
Statistics.
The sample size for each replicate of experimental group was determined by using 2 previously published reports comparing similar inbred substrains and transgenic mice, including BALB/cAnNCr and B6(Cg)-Tyrc-2J 12 and BALB/cJBomTac and C57BL/6NTac-Atm1.1ArteTyrt- m1Arte.27 Assuming that the data regarding the numbers of oocytes and embryos produced by similar inbred strain donors had the same standard deviation, we set sample sizes to obtain a 2-sided significance (α) level of 0.05 and power of 80% by using a one-way ANOVA test. Sample size was determined by using G*Power software (release 3.1.6; University of Düsseldorf, Düsseldorf, Germany). Given the assumed normal distribution of dependent variable values, homogeneity of variances, and observation independence, blastocyst data were analyzed by using one-way ANOVA and Tukey honest significant difference (HSD) posthoc testing. Given that coat-color contribution among littermates is unlikely to be distributed normally, these data were analyzed by using the Kruskal–Wallis nonparametric test and a Dunn posthoc test. Statistical analysis and the generation of associated graphs were performed by using GraphPad PRISM 5 (GraphPad Software, La Jolla, CA). Values in the text are given as mean ± SEM.
Results
Fertilization rate of host blastocyst donor mice.
A commonly used technique to assess breeding in mice is visualization of the vaginal copulatory plug, which indicates a high likelihood of obtaining fertilized embryos from donor female mice. In this study, all mated female mice except for 4 albino B6 animals had vaginal copulatory plugs. However, only 75.8% of 4-wk-old BALB/c, 85.7% of 12-wk-old BALB/c, and 94.4% of 4-wk-old albino B6 donors with copulatory plugs yielded fertilized embryos. In addition, two 4-wk-old albino B6 female mice lacked identifiable copulatory plugs but produced fertilized embryos (Table 1).
Table 1.
Summary of study groups
| n | No. (%) of donors with copulatory plugs | No. (%) of donors fertilized | No. of fertilized embryos | No. of injectable blastocysts | No. of blastocysts injected | No. of pups born | No. of chimeras born | No. of female chimeras born | No. of male chimeras born | No. of male chimeras with % chimerism by coat color |
||||
| 100% | 70% to 100% | 40% to 70% | 40% or less | |||||||||||
| 4-wk-old BALB/c, superovulated donors | 33 | 33 (100.0) | 25 (75.8) | 374 | 218 | 209 | 29 | 9 | 2 | 7 | 0 | 4 | 2 | 11 |
| 12 wk-old BALB/c, nonsuperovulated donors | 21 | 21 (100.0) | 18 (85.7) | 149 | 120 | 112 | 74 | 52 | 3 | 49 | 2 | 42 | 6 | 9 |
| 4-wk-old albino B6, superovulated donors | 36 | 32 (88.9) | 34 (94.4) | 651 | 418 | 253 | 47 | 26 | 9 | 17 | 0 | 10 | 2 | 14 |
Fertilized donors are female mice with fertilized embryos (at any stage) isolated at time of euthanasia. Numbers of fertilized embryos include 2-cell, 4-cell, 8-cell, morula, and blastocyst stage embryos. Numbers of injectable blastocysts are those blastocysts with an intact zona pellucida. Numbers of blastocysts injected indicates those blastocysts that underwent ES cell injection and were transferred into pseudopregnant recipient mice
Production of host embryos.
The quantity and expected morphology of embryos produced from matings are important qualities of donors. Because not all mice with copulatory plugs produced fertilized embryos, embryo quantity and expected morphology were assessed in all donor mice and analyzed with regard to fertilization status. Among mice with fertilized embryos, the average number of fertilized embryos per mouse was 15.6 ± 1.8 for 4-wk-old BALB/c mice, 9.3 ± 0.8 for 12-wk-old BALB/c mice, and 19.1 ± 1.7 for 4-wk-old albino B6 mice, with 8.4 ± 1.5, 6.7 ± 1.1, and 12.7 ± 1.5 injectable embryos per mouse, respectively (Table 1). Albino B6 mice produced significantly (P < 0.05) more fertilized embryos than did mice in both BALB/c groups and more injectable blastocysts than did 12-wk old BALB/c mice. As expected, the superovulated 4-wk-old BALB/c mice produced significantly (P < 0.05) more oocytes and embryos than did 12-wk old BALB/c mice. In addition, donors from all groups produced fertilized embryos isolated at various stages, as was indicated by the greater number of fertilized embryos than injectable blastocysts (Table 1).
Success rates regarding live pups.
As described earlier, CD1 recipients were implanted with ES-cell–injected blastocysts and underwent pregnancy and parturition. The numbers of live pups generated from these procedures were analyzed. The overall live pup success rate was calculated by dividing the number of live births (not including neonatal pup mortality within the first 3 postnatal days) by the total number of blastocysts transferred to all recipients and multiplying by 100%. The recipient live pup success rate was calculated by dividing the corrected number of live births (as for the overall rate) by the number of blastocysts transferred into the recipient and multiplying by 100%. Therefore, using data present in Table 1, the overall live pup success rate for 4- and 12-wk-old BALB/c and 4-wk-old albino B6 blastocysts microinjected and transferred (n = 209, 112, and 253, respectively) is 15%, 66%, and 22%, respectively. The recipient live pup success rate for 4- and 12-wk-old BALB/c and 4-wk-old albino B6 blastocysts microinjected and transferred to recipient mice (n = 16, 10, and 19, respectively) was 24%, 74%, and 26%, respectively. The differences between these 2 rates are due to the failure of transferred embryos to result in pregnancy in some recipients. Injected blastocysts from 12-wk-old BALB/c mice produced significantly (P < 0.001) more pups and higher success rates than did those from either 4-wk-old mouse group (Figure 1).
Figure 1.
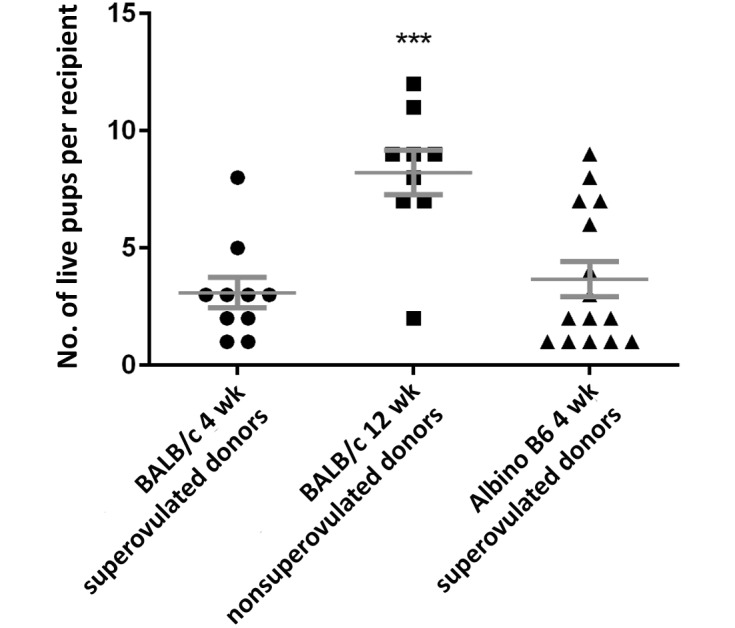
Number of live pups per recipient. Each data point represents the number of 3-d-old pups from each litter of the experimental group. Data are given as mean ± SEM. ***, Significant (P < 0.001) difference between 12-wk-old BALB/c mice and both 4-wk-old groups.
Success rates regarding chimeric pups.
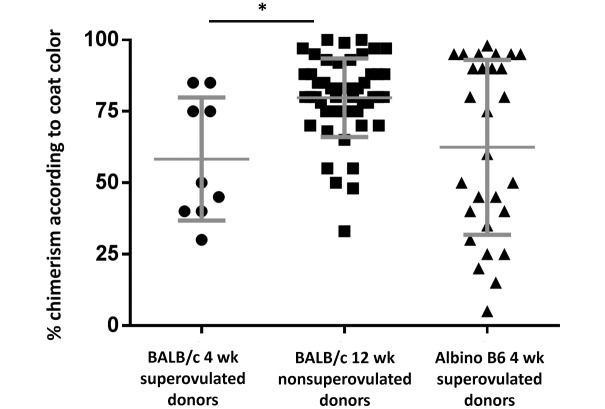
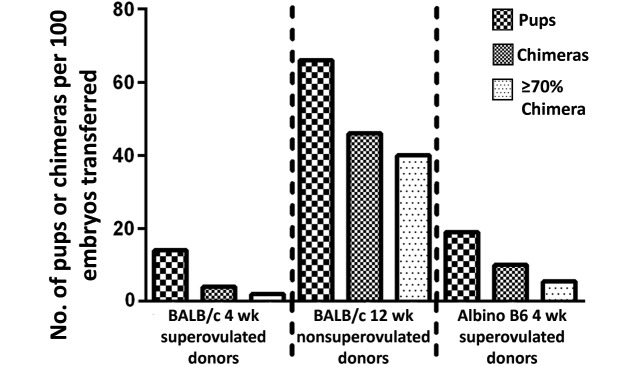
Our assessment of successful incorporation of the injected ES cells was based on the success rates of generating mice with mixed coat color (chimeras) and on the proportion of coat color contribution, as previously discussed. Of all blastocysts from 4- and 12-wk-old BALB/c and 4-wk-old albino B6 mice that were injected and transferred into recipients, 14%, 66%, and 19%, respectively, resulted in live pups, with 4%, 46%, and 10%, respectively, generating chimeric pups. Of the live pups born from the blastocysts of 4- and 12-wk-old BALB/c and 4-wk-old albino B6 mice, 31% (9 of 29), 70% (52 of 74), and 55% (26 of 47), respectively, were chimeras (Table 1). The average coat color contribution for pups born from injected blastocysts from 4- and 12-wk-old BALB/c and 4-wk-old albino B6 mice was 58%, 80%, and 62%, respectively (Figure 2). Injected blastocysts from the 12-wk-old BALB/c mice yielded significantly (P < 0.05) higher chimerism than did those from 4-wk old BALB/c animals (Figure 2). Overall efficiency, determined by dividing the number of pups that were at least 70% chimeric by the total number of injected blastocysts implanted into all recipients, was 1.9% for 4-wk-old BALB/c donors, 40.2% for 12-wk-old BALB/c mice, and 5.5% for albino B6 donors (Figure 3). Given JM8A3 ES cells demonstrate an XY karyotype,18 male pups were predominant in the current study, with blastocysts from 4- and 12-wk old BALB/c and 4-wk old albino B6 mice generating 58% (17 of 29), 77% (57 of 73), and 55% (26 of 47) male progeny among all live pups, respectively. Chimeric male mice accounted for 78%, 94%, and 65% of the chimeras generated from injected blastocysts from 4- and 12-wk old BALB/c and 4-wk old albino B6 mice, respectively. Blastocysts from 12-wk-old BALB/c mice ultimately provided the highest numbers of live pups, male chimeras, and male pups with high chimerism according to coat color contribution. The overall efficiency of blastocysts from albino B6 female donors was higher than that of blastocysts produced by 4-wk-old BALB/c mice, but albino B6 donors also generated a higher proportion of female pups than did the 4-wk-old BALB/c donors.
Figure 2.
Percentage chimerism according to coat color. Each data point represents a single chimeric pup born in the respective experimental group. Coat color was determined at 10 d of age. Data are presented as mean ± SEM. *, Significant (P < 0.05) difference between groups.
Figure 3.
Mean numbers of pups, chimeras, and chimeric pups with greater than 70% chimerism per 100 embryos transferred to recipients.
Discussion
We determined the efficiencies of generating chimeric offspring by using blastocysts from female donors treated with 3 different protocols. The number of host blastocysts needed for ES cell injection is often dictated by experimental needs. Producing too few host blastocysts can negatively affect the success of an experiment, whereas producing too many blastocysts wastes animal and personnel resources.
Multiple efficiency parameters can be used to assess the effectiveness of these donor-embryo–generating protocols. Regardless of female mice with no evidence of a vaginal copulatory plug, more albino B6 mice were fertilized than were those from either BALB/c experimental group. Other substrains of BALB/c mice have shown decreased fertility, with the incidence of abnormal sperm head morphology significantly greater in BALB/cAnN and BALB/cByJ than BALB/cA.16 When comparing the reproductive physiology of BALB/c mice, it is important to consider the variability in parameters of various substrains. Other differences between BALB/c substrains have been noted; for example, superovulated BALB/cJ mice produce significantly fewer oocytes but result in significantly more live pup births than do BALB/cByJ mice.1
We hypothesized that the protocol using donor blastocysts from superovulated albino B6 mice would yield more high-percentage chimeric pups relative to either BALB/c protocol. Although albino B6 mice produced more blastocysts than did both BALB/c mouse groups, the live-pup– and chimera-generating efficacies of the blastocysts from the 12-wk-old BALB/c mice far surpassed those of the younger, superovulated donors. Ultimately, our data do not support our hypothesis. However, blastocysts from albino B6 mice generated more chimeras and had higher efficiencies than did those from 4-wk-old BALB/c mice.
Although blastocyst quantity is an important efficiency parameter, blastocyst quality is important also. We did not directly measure blastocyst quality in this study, and this assessment should be considered for future work and done by using established strategies.2,19,21 However, blastocysts from 12-wk-old BALB/c mice had a much higher rate of live pups than did both 4-wk-old groups. The effects of superovulation on oocyte and embryo quality have been documented.2,15 Although it is common to obtain immature unfertilized oocytes from mated CD1 mice that are superovulated by using pregnant mare serum gonadotropin and human chorionic gonadotropin,24 overdose superovulation can result in reduced blastocyst-stage development in oocytes fertilized in vitro3 and decreased blastocyst quality.2 In addition, one study demonstrated a decreased rate of blastocyst development, a reduced implantation rate, increased fetal mortality, and decreased live fetus weight from embryos derived from superovulated C57BL/6J relative to unstimulated mice.4 In our current study, there were no significant differences in live pup success rate between the 2 superovulated groups. However, we observed significant differences in multiple parameters, including embryo production and ability to generate chimeras between superovulated and nonsuperovulated BALB/c mice. This difference is biased by the age of the mice used. It is well established in the literature that superovulation of younger, prepubertal mice yields more ovulated oocytes and embryos than does superovulation of older, postpubertal mice,5,11,13,17,20 causing younger mice to be used preferentially by transgenic programs to generate high quantities of oocytes after superovulation. We suspect that older superovulated female BALB/c mice yield fewer oocytes than do the younger animals in this study, but the data we collected cannot be used to make that determination. Therefore, the increased chimera-producing efficiency of embryos from older BALB/c mice compared with that of the younger cohort likely resulted due to either age or superovulation treatment or the combination of these factors.
Regarding chimerism, our study demonstrates that blastocysts from 12-wk-old BALB/c mice yield significantly more chimeras with a high coat-color contribution from injected JM8A3 ES cells than do those from 4-wk-old BALB/c and albino B6 mice. We defined high chimerism as those pups with 70% or greater coat-color contribution from ES cells, consistent with previously published thresholds for determining germline transmission.7,12 Pups born from the blastocysts of 12-wk-old BALB/c mice were 8-fold and 20-fold more often above 70% chimerism according to coat-color contribution of injected ES cells than were those from 4-wk-old albino B6 and BALB/c mice, respectively. We extrapolated the reported data to determine that, to achieve a single pup that is at least 70% chimeric according to coat color by using JM8A3 ES cells, at least 50, 3, or 18 blastocysts from 4- or 12-wk-old BALB/c or 4-wk-old albino B6 donors, respectively, must be injected and transferred to a recipient. Therefore, according to the calculation regarding the generation of blastocysts, each highly chimeric pup requires 6.2, 0.4, or 1.4 donors when they are 4- or 12-wk-old BALB/c or 4-wk-old albino B6 mice. When including the data regarding female mice with copulatory plugs, we extrapolate that each highly chimeric pup requires 9.7 of the 4-wk-old BALB/c mice, 0.5 of 12-wk-old BALB/c, and 1.4 of albino B6 mice. Therefore, we recommend considering the needs of the transgenic program when determining the numbers of donors needed for microinjection sessions. In our experience, using 8 to 10 plugged nonsuperovulated 12-wk old BALB/c female mice or 8 to 12 plugged superovulated C57BL/6NTac animals yields at least 30 or 50 injectable, good-quality blastocysts, respectively. We do not routinely use superovulated 4-wk-old BALB/c female mice as host blastocyst donors. The described efficiency parameters are important features in guiding transgenic facility managers in using the presented data for their respective programs to generate donor blastocysts for the production of chimeras. Transgenic facility managers should weigh these efficiencies against the financial costs associated with these protocols, including those for purchasing novel transgenic mice and the maintenance per diems for colonies of mature BALB/c mice.
Although superovulation leads to significantly more blastocysts from albino B6 mice than from nonsuperovulated 12-wk-old BALB/c mice, live pup and chimera efficiencies were significantly more robust for blastocysts from nonsuperovulated 12-wk-old BALB/c mice. In addition, albino B6 mouse blastocysts demonstrate a high efficiency for producing pups with 70% chimerism, above that of 4-wk-old BALB/c blastocysts. As previously discussed, the use of albino B6 mice as blastocyst donors provides advantages over BALB/c mice, in that albino B6 mice respond well to superovulation and are better breeders.9,13
The biggest limitation to our study is that we did not confirm germline transmission in the chimeras generated from these experiments. Confirming germline transmission is a major goal of this technology. Despite this drawback, coat-color contribution remains widely used and helpful predictor of germline transmission.12,15,18 Furthermore, we might have been able to achieve increased efficiencies by modifying our microinjection procedures. Previous studies have used 10 to 12 ES cells in albino B6 host blastocsyts,27 in contrast to the 15 to 18 ES cells that we used in our current study and that is used routinely in our transgenic facility.15 We also were limited in the availability of the BALB/c male mice used in this experiment; we suspect that we might have observed increased fertility and subsequently higher-quality blastocysts if we had used younger, mature male BALB/c mice exclusively.
This report is the first to perform a side-by-side comparison of 3 different protocols, including the novel C57BL/6NTac-Tyrtm1Arte mouse mutant, for producing host blastocysts for creating genetically engineered mice through the introduction of ES cells. Our analyses support the use of mature, 12-wk-old BALB/c or superovulated albino B6 mice for the generation of host blastocysts. The adoption of these donor blastocyst protocols depend on the transgenic facility and experimental needs. To use mature BALB/c female mice for donor blastocysts, a facility must maintain a population of mice that is much larger than the minimum, given that natural estrus cycling must be accommodated, and the necessary size of the colony may mean that some animals are not used. If a facility cannot maintain a large BALB/c colony, albino B6 mice may be a viable option for the generation of donor blastocysts, given that this strain yielded higher fertility and increased numbers of blastocysts relative to the BALB/c experimental groups.
Acknowledgments
We are very grateful for the generous gift of the albino B6 mice from Taconic (facilitated by Dr. Megan MacBride). Numerous people provided critical support for this project, including Alexandre N Viana, Amanda Murphy, John M Mkandawire, Allan Discua, Tony Chavarria, Noranne Enzer, Matt Demers, Sarah Elmiligy, Tsetan Wangchuk, Chris Wilber, Alyssa Pappa, and the veterinary, administrative, and husbandry staff at the MIT Division of Comparative Medicine.
This work was supported by NIH grants T32-OD010978, P30-ES002109, and P30-CA14051
References
- 1.Byers SL, Payson SJ, Taft RA. 2006. Performance of 10 inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology 65:1716–1726. [DOI] [PubMed] [Google Scholar]
- 2.Dehghan T, Mozdarani H, Khoradmehr A, Kalantar SM, Bakhshandeh M, Bouzarjomehri F, Kalantar SM, Sepehr Javan M. 2014. Evaluation of embryo quality after concurrent use of ovarian stimulating hormones and γ-irradiation. Iran J Reprod Med 12:573–580. [PMC free article] [PubMed] [Google Scholar]
- 3.Edgar DH, Whalley KM, Mills JA. 1987. Effects of high-dose and multiple-dose gonadotropin stimulation on mouse oocyte quality as assessed by preimplantation development following in vitro fertilization. J In Vitro Fert Embryo Transf 4:273–276. [DOI] [PubMed] [Google Scholar]
- 4.Ertzeid G, Storeng R. 2001. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod 16:221–225. [DOI] [PubMed] [Google Scholar]
- 5.Gates AH, Bozarth JL. 1978. Ovulation in the PMSG-treated immature mouse: effect of dose, age, weight, puberty, season, and strain (BALB/c, 129, and C129F1 hybrid). Biol Reprod 18:497–505. [DOI] [PubMed] [Google Scholar]
- 6.Golkar-Narenji A, Gourabi H, Eimani H, Barekati Z, Akhlaghi A. 2012. Superovulation, in vitro fertilization (IVF) and in vitro development (IVD) protocols for inbred BALB/cJ mice in comparison with outbred NMRI mice. Reprod Med Biol 11:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen GM, Markesich DC, Burnett MB, Zhu Q, Dionne KM, Richter LJ, Finnell RH, Sands AT, Zambrowicz BP, Abuin A. 2008. Large-scale gene trapping in C57BL/6N mouse embryonic stem cells. Genome Res 18:1670–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institut Clinique de la Souris. [Internet] 2015. ICS Standard operating procedure for EUCOMM mice production, 1–4. [Cited 13 November 2015]. Available at: www.knockoutmouse.org/kb/file/28/
- 9.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 10.Kishikawa H, Tateno H, Yanagimachi R. 1999. Chromosome analysis of BALB/c mouse spermatozoa with normal and abnormal head morphology. Biol Reprod 61:809–812. [DOI] [PubMed] [Google Scholar]
- 11.Lane M, Gardner DK. 2004. Preparation of gametes, in vitro maturation, in vitro fertilization, and embryo recovery and transfer, p 24–40. In: Gardner DK, Lane M, Watson AJ. A laboratory guide to the mammalian embryo. New York (NY): Oxford University Press. [Google Scholar]
- 12.Lee AYF, Evans K, Willis B, Lloyd KCK. 2013. Combining sperm plug genotyping and coat color chimerism predicts germline transmission. Transgenic Res 22:1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo C, Zuñiga J, Edison E, Palla S, Dong W, Parker-Thornburg J. 2011. Superovulation strategies for 6 commonly used mouse strains. J Am Assoc Lab Anim Sci 50:471–478. [PMC free article] [PubMed] [Google Scholar]
- 14.Mouse Genome Sequencing Consortium, Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigó R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. 2002. Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562. [DOI] [PubMed] [Google Scholar]
- 15.Nagy A, Gertsenstein M, Vintersten K, Behringer R. 2003. Production of chimeras, p 461. In: Manipulating the mouse embryo: a laboratory manual, 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. [Google Scholar]
- 16.Ohta H, Sakaide Y, Wakayama T. 2008. Age- and substrain-dependent sperm abnormalities in BALB/c mice and functional assessment of abnormal sperm by ICSI. Hum Reprod 24: 775–781. [DOI] [PubMed] [Google Scholar]
- 17.Ozgunen KT, Erdogan S, Mazmanoglu N, Pamuk I, Logoglu G, Ozgunen T. 2001. Effect of gonadotrophin dose on oocyte retrieval in superovulated BALB/c mice. Theriogenology 56:435–445. [DOI] [PubMed] [Google Scholar]
- 18.Pettitt SJ, Liang Q, Rairdan XY, Moran JL, Prosser HM, Beier DR, Lloyd K, Bradley A, Skarnes WC. 2009. Agouti C57BL/6N embryonic stem cells for mouse genetic resources. Nat Methods 6:493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prados FJ, Debrock S, Lemmen JG, Agerholm I. 2012. The cleavage stage embryo. Hum Reprod 27:i50–i71. [DOI] [PubMed] [Google Scholar]
- 20.Pritchett KR, Taft RA. 2007. Reproductive biology of the laboratory mouse, p 91–121. In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL. The mouse in biomedical research, 2nd ed., vol 3 Burlington (MA): Academic Press. [Google Scholar]
- 21.Rødgaard T, Heegaard PMH, Callesen H. 2015. Noninvasive assessment of in vitro embryo quality to improve transfer success. Reprod Biomed Online 31:585–592. [DOI] [PubMed] [Google Scholar]
- 22.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London (UK): Methuen [Google Scholar]
- 23.Schuster-Gossler K, Lee AW, Lerner CP, Parker HJ, Dyer VW, Scott VE, Gossler A, Conover JC. 2001. Use of coisogenic host blastocysts for efficient establishment of germline chimeras with C57BL/6J ES cell lines. Biotechniques 31:1022–1024. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Ock SA, Chian RC. 2006. Effect of gonadotrophin stimulation on mouse oocyte quality and subsequent embryonic development in vitro. Reprod Biomed Online 12:304–314. [DOI] [PubMed] [Google Scholar]
- 25.Weber EM, Algers B, Hultgren J, Olsson IAS. 2013. Pup mortality in laboratory mice—infanticide or not? Acta Vet Scand 55:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Weyden L, White JK, Adams DJ, Logan DW. 2011. The mouse genetics toolkit: revealing function and mechanism. Genome Biol 12:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zevnik B, Uyttersprot NC, Perez AV, Bothe GWM, Kern H, Kauselmann G. 2014. C57BL/6N albino/agouti mutant mice as embryo donors for efficient germline transmission of C57BL/6 ES cells. PLoS One 9:e90570. [DOI] [PMC free article] [PubMed] [Google Scholar]