Abstract
The AVMA Panel on Euthanasia recommends that sensitive animals should not be present during the euthanasia of others, especially of their own species, but does not provide guidelines on how to identify a sensitive species. To determine if mice are a sensitive species we reviewed literature on empathy in mice, and measured the cardiovascular and activity response of mice observing euthanasia of conspecifics. We studied male 16-wk-old C57BL/6N mice and found no increase in cardiovascular parameters or activity in the response of the mice to observing CO2 euthanasia. Mice observing decapitation had an increase in all values, but this was paralleled by a similar increase during mock decapitations in which no animals were handled or euthanized. We conclude that CO2 euthanasia of mice does not have an impact on other mice in the room, and that euthanasia by decapitation likely only has an effect due to the noise of the guillotine. We support the conceptual idea that mice are both a sensitive species and display empathy, but under the controlled circumstances of the euthanasia procedures used in this study there was no signaling of stress to witnessing inhabitants in the room.
Because animals respond to the pheromones, odors, and vocalizations of other animals,9 those that are exposed to animals experiencing stress may react differently in test situations. For example, subsequent groups of animals may have altered test values due to increases in corticosterone, glucose, or cardiovascular parameters associated with stress induced by exposure to the odors or vocalizations of the initial group. This effect might then influence the interpretation of the experimental results. In addition, exposing animals to unnecessary stress is discouraged. One situation in which animals may express stress pheromones, odors or vocalizations is during euthanasia. The AVMA Panel on Euthanasia provides the following guidance on this subject: “distress vocalizations, fearful behavior, and release of certain odors or pheromones by a frightened animal may cause anxiety and apprehension in other animals. Therefore, for sensitive species, it is desirable that other animals not be present when individual animal euthanasia is performed.”17 This opinion is echoed in the Working Party Report in Europe: “The need to minimize fear and apprehension must be considered in determining the method of euthanasia. Distress vocalizations, fearful behavior, and release of certain odours or pheromones by a frightened animal may cause anxiety and apprehension in others. It must be remembered that many vocalizations are at high frequencies and out of the human hearing range. Therefore, whenever possible, animals should not be present during euthanasia of others, especially of their own species. This is particularly important when vocalizations or release of pheromones may occur during induction of unconsciousness.”5 The primary difference in the 2 recommendations is the AVMA specifies that only ‘sensitive species’ should not witness euthanasia.
The characteristics that define a sensitive species have not been established. One might assume that the observing animal would have a response that indicates that the animal is at least aware of the procedure. Beyond awareness, the sensitive observer might have a negative response to the event, such as altered behavior or disruption of physiologic homeostasis. Evidence of sensitivity to euthanasia of other animals has been shown in rats.25,26 In those studies, telemeterized rats were monitored as they observed the anesthesia of other rats or their euthanasia by CO2 or decapitation; heart rate increased moderately in singly and group housed female Sprague–Dawley rats exposed to decapitation.26 In comparison, singly housed (but not group housed) male Sprague–Dawley rats had significant increases in heart rate and mean arterial pressure while observing euthanasia.25 These responses were similar in magnitude to those occurring when a caretaker entered the room and performed husbandry activities.25,26 This finding suggests that the cardiovascular response and potentially the stress that is associated with this response were comparable to those that accompany routine husbandry activities.
In contrast, one study reported no adverse reactions among mice viewing the euthanasia of other mice.27 Specifically, the authors found no alterations in corticosterone levels, spleen weight, or adrenal gland weight in BALB/c/O1a male mice that were in the same room as mice being euthanized by cervical dislocation. Given the lack of response from the observing mice, the authors concluded that the euthanized mice must not be vocalizing or omitting odors.27 Despite the lack of changes in measured parameters in mice, we speculated that because rats demonstrated cardiovascular alterations, similar findings would be obtained from mice. Therefore, in the present study, we examined the cardiovascular and activity responses of mice in the same room as mice euthanized by either CO2 or decapitation. We hypothesized that mice are a “sensitive species” and would demonstrate significant increases in cardiovascular parameters and activity in response to euthanasia of other mice.
Materials and Methods
Mice.
Male C57BL/6N mice (age, 16 wk; Taconic, Hudson, NY) were used for all procedures. Mice were individually housed in rodent cages (28 cm × 17.5 cm ×12 cm; Allentown Caging, Allentown, NJ) positioned on top of telemetry receiver plates. The room temperature was maintained at 23.3° C, with humidity maintained between 30% to 70%. Mice were fed Teklad 8640 rodent diet (Harlan, Indianapolis, IN) and water ad lib and were housed on Teklad Sani-chips bedding (Harlan) with cotton nesting squares (Ancare, Bellmore, NY) provided. Cage changes were performed weekly, and were not done on the day of testing. Vendor surveillance and colony sentinel monitoring results showed mice were free from pathogenic agents including ectromelia virus, epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, Mycoplasma pulmonis, mouse adenovirus strains 1 and 2, mouse hepatitis virus, mouse parvovirus, minute virus of mice, polyoma virus, pneumonia virus of mice, reovirus type 3, Theiler murine encephalomyelitis virus, Sendai virus, endoparasites, and ectoparasites. All experimental procedures were approved by the Wright State University IACUC.
Surgery.
All surgeries were performed in a rodent-dedicated surgery suite. After acclimating to the facility for 7 to 8 d, mice were anesthetized with isoflurane (1% to 4%) in 100% oxygen (induced in a chamber and maintained by mask). The neck was shaved and prepped 3 times with alternating povidone–iodine and alcohol scrubs followed by a final swab of povidone–iodine solution. The mice were monitored continually for depth of anesthesia by response to pain, observation for movement, and respiratory rate. A 1-cm incision was made in the ventral neck and the muscle bluntly dissected to expose the carotid artery. A telemetry pressure-transmitter probe (TA11PA-C10, Data Sciences International, St Paul, MN) was inserted in the carotid artery and ligated in place. The body of the transmitter was inserted subcutaneously on the right flank and the incision closed by using 5-0 nonabsorbable black nylon monofilament sutures (Arosurgical, Newport Beach, CA). Pain and discomfort were alleviated by an initial dose of carprofen (5 mg/kg SC; Penn Vet, Lancaster, PA) at the time of surgery and an additional dose at 24 h postoperatively.
Telemetry measurement.
Mice were allowed to recover for 7 to 8 d after surgery. Cages were individually placed on data acquisition receiver boards (RPC1, Data Sciences International). Radiotelemetric measurements were collected by using Ponemah software (Data Sciences International). Heart rate, systolic blood pressure, diastolic blood pressure, mean blood pressure, and activity data were collected continuously (sample rate, 1000 Hz) during both baseline and testing measurements. For data collection, the transmitters were turned on, and all personnel left the room. After at least 30 min for readings to stabilize, baseline data were collected for at least 1 h (starting at approximately 1000; lights on, 0700; lights off, 1900). On subsequent days, mice were euthanized after a similar 30-min stabilization period. This time was determined as sufficient on the basis of previous telemetry measurements to minimize the effect of the activity in the room associated with setting up the euthanasia chamber. The cages of observing mice were not manipulated at all, so the effects on these mice were limited to personnel entering the room and the noise created during the set up for the euthanasia procedure.
CO2 euthanasia.
All mice had implanted telemetry units. To minimize effects of manipulation and cage movement, all mice were euthanized in their home cage and room. The telemetry pads were placed 2 on each shelf, with 5 shelves on the rack. Therefore, observing mice were either on the same shelf or a different shelf as the euthanized mice. A total of 39 mice were euthanized in the presence of a total of 43 mice. Between 1 and 4 mice observed the euthanasia of each mouse. The mice that were observers then became euthanized mice. Each day 1 to 3 mice were euthanized between the hours of 1000 and 1300 to eliminate the effect of circadian rhythms. All mice were euthanized within 2 wks of the original telemetry implantation surgery.
To set up for euthanasia, the mouse home cage was placed in a 22-L transparent polycarbonate euthanasia chamber (44 cm × 23.5 cm × 21 cm) in the same location as the home cage had been positioned previously. The euthanasia chamber was covered with an acrylic lid with ports for gas inlet and outlet. Compressed CO2 gas was provided from a cylinder (Weiler Welding, Moraine, OH) and controlled by a CO2-specific regulator (Western Medica, Westlake, OH). Chamber air was replaced with CO2 at 4 different rates: 15%, 30%, 50%, or 100% of the chamber volume in 1 min (n = 10, 11, 9, and 9 mice euthanized in each group, respectively). Death was determined to have occurred when the blood pressure and heart rate had reached 0 according to telemetry.
Decapitation.
A total of 9 C57BL/6 adult mice were decapitated, 4 on one day and 5 on another day, with different mice observing each day. The euthanized mice were from a different room and were being culled from the colony. A total of 8 mice observed decapitations (4 on each day). The mouse decapitator (Harvard Apparatus, Holliston, MA) was placed in the line of sight on the counter next to the shelf rack (between 100 and 170 cm from the observing mice). An individual mouse was taken from its cage, restrained, and rapidly decapitated. The body was placed adjacent to the head. The procedure was repeated until all the mice were euthanized. The mice were all euthanized in less than 4 min. To eliminate the potential for noise confounding this study, a mock decapitation cohort was studied also. For this control, no mice were involved; the guillotine was placed in the same location as for actual decapitations and the blade was raised and lowered, creating a loud noise similar to the real event. Mock decapitations took the same amount of time to perform as did actual decapitations.
Observing mice.
A total of 43 mice observed the euthanasia of other mice by CO2 or decapitation. The observing mice were left in their home cages on the shelf rack and were either on the same shelf (57 to 70 cm between cages) or an adjacent shelf (41 cm vertical distance) as the mouse being euthanized. Only mice on the same shelf could see the euthanasia. All mice were singly housed to maintain signal generation on the telemetry pads. Multiple mice observed the same euthanasia event, and some mice viewed multiple euthanasia events (total of 123 observations were recorded for CO2 euthanasia). Four mice that observed a decapitation also observed the mock decapitation.
Statistics.
SAS version 9.4 was used for all statistical analysis (Cary, NC). One-way ANOVA was conducted on data for systolic blood pressure, diastolic blood pressure, mean blood pressure, and heart rate to determine whether any of these mean values differed according to the CO2 level (15%, 30%, 50%, and 100%). Because activity is ordinal, these data were analyzed by using the Kruskal–Wallis test. Significance was determined as an α level of 0.01 for all inferences, given that 5 different outcomes were analyzed simultaneously. Because none of the values differed according to the CO2 level, the data were pooled. A t test was conducted to determine whether mean differences in the cardiovascular data were, on average, significantly different from baseline values. Activity data were evaluated by using the Wilcoxon signed-rank test. In addition, t tests were performed on the decapitation data so that all data from observing mice could be included.
Results
Observation of CO2 euthanasia.
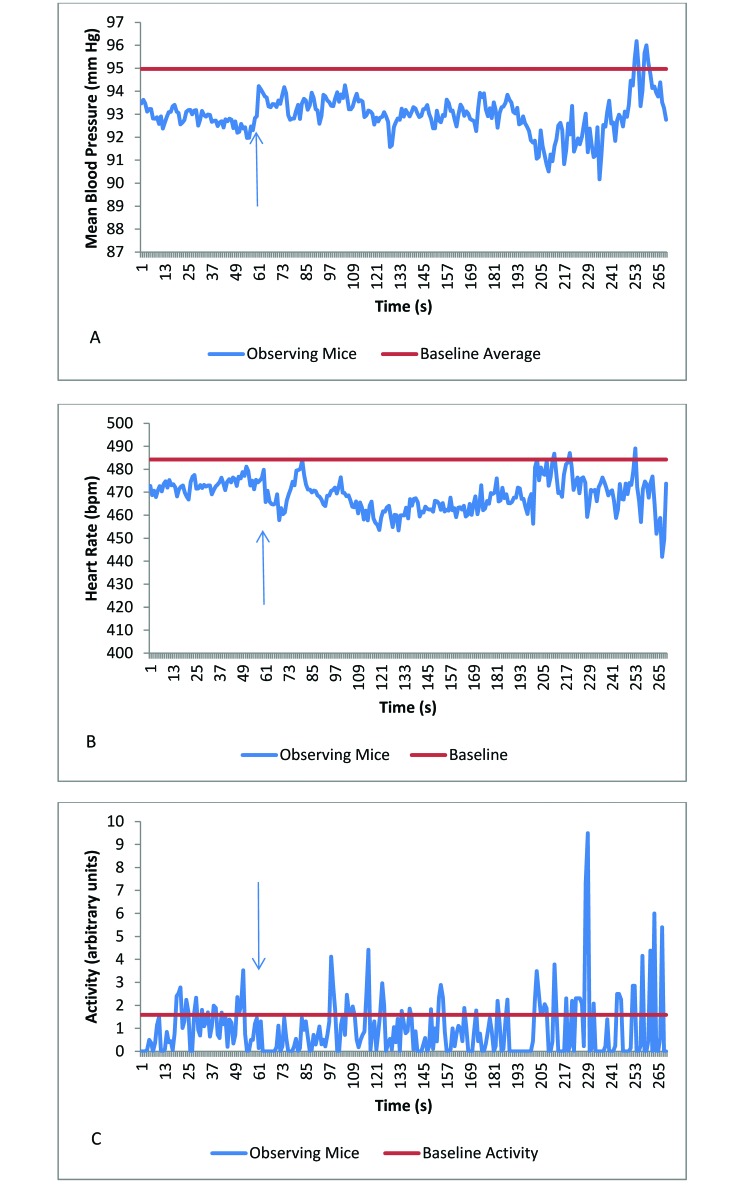
There were a total of 75 recordings from mice that observed euthanasia by CO2 (15% CO2 chamber replacement rate, n = 23; 30%, n = 27; 50%, n = 19; and 100%, n = 16). The mean cardiovascular and activity values of observing mice did not differ between baseline and observation of euthanasia at any CO2 chamber-replacement rate (Table 1). Therefore, data from all CO2 euthanasia-observation groups were combined. The mean heart rate and activity of mice observing CO2 euthanasia were significantly (P = 0.005 and P < 0.001, respectively) lower than the mean baseline readings (Table 2, Figure 1 A through C). None of the other response variables differed significantly between observation and baseline values.
Table 1.
Cardiovascular and activity (mean ± 1 SD) of mice watching the euthanasia of other mice
| CO2 concentration used for euthanasia |
||||||
| Baseline | 15% | 30% | 50% | 100% | P | |
| Systolic blood pressure (mm Hg) | 107.2 ± 5.9 | 102.7 ± 6.8 | 109.0 ± 9.6 | 107.0 ± 7.4 | 105.8 ± 12.8 | 0.11 |
| Diastolic blood pressure (mm Hg) | 81.9 ± 6.9 | 76.9 ± 7.1 | 81.5 ± 9.9 | 80.6 ± 8.0 | 80.0 ± 13.0 | 0.37 |
| Mean blood pressure (mm Hg) | 94.8 ± 5.6 | 90.2 ± 6.4 | 95.7 ± 9.0 | 94.1 ± 7.0 | 93.3 ± 12.6 | 0.18 |
| Heart rate (bpm) | 484.7 ± 30.8 | 465.5 ± 44.7 | 467.5 ± 49.4 | 467.8 ± 59.4 | 470.1 ± 66.1 | 0.99 |
| Activity (arbitrary units) | 1.64 ± 1.61 | 0.35 ± 1.35 | 1.03 ± 2.80 | 1.00 ± 3.42 | 1.99 ± 6.66 | 0.30 |
Responses did not differ between CO2 concentrations.
Table 2.
Responses of mice observing CO2euthanasia relative to baseline values
| Difference from baseline value | P | |
| Systolic blood pressure (mm Hg) | −1.10 ± 9.4 | 0.29 |
| Diastolic blood pressure (mm Hg) | −2.21 ± 9.6 | 0.04 |
| Mean blood pressure (mm Hg) | −1.57 ± 9.0 | 0.11 |
| Heart rate (bpm) | −17.03 ± 53.5 | 0.005 |
| Activity (arbitrary units) | −0.57 ± 3.7 | < 0.001 |
Heart rate and activity were decreased significantly compared with baseline levels.
Figure 1.
(A) Mean blood pressure, (B) heart rate, and (C) activity in mice observing the euthanasia of other mice by using 15%, 30%, 50%, or 100% CO2. Baseline data are presented as the average of a 30-min period; data for the last minute prior to decapitation or mock decapitation are included also. The arrow identifies the time when CO2 euthanasia was initiated. The heart rate and activity of the mice that observed CO2 euthanasia were decreased compared with baseline values.
Observation of decapitation.
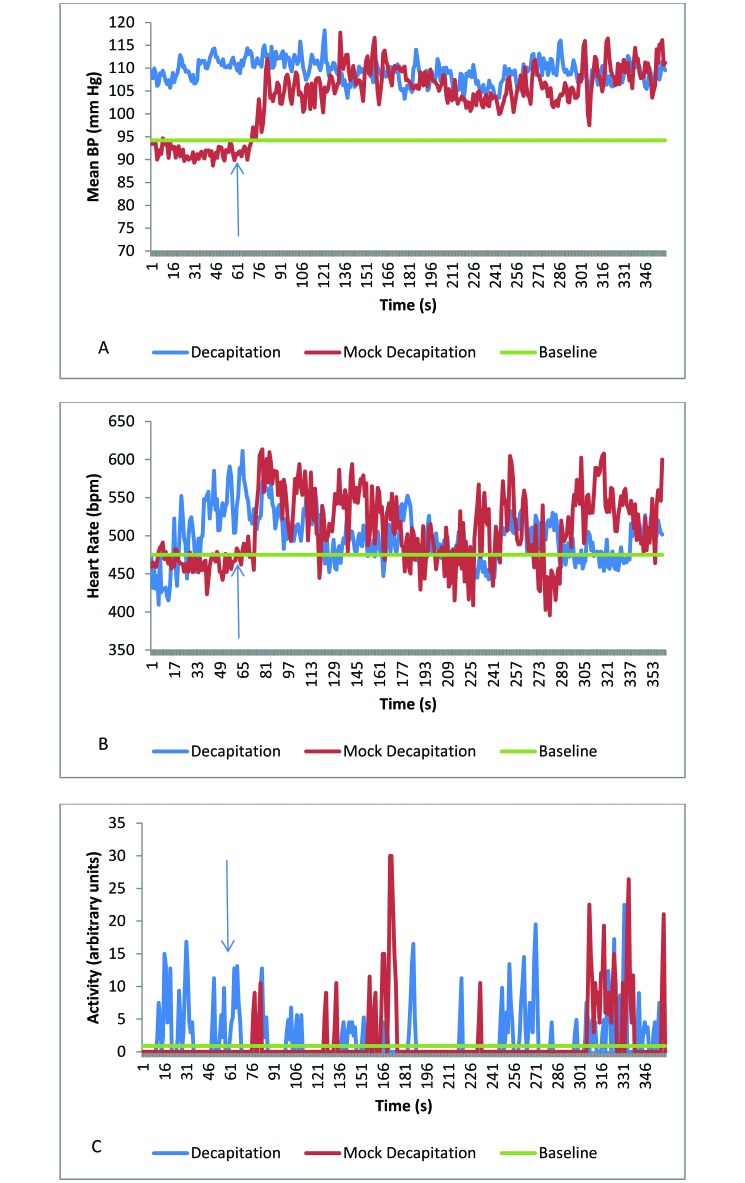
Mice were decapitated on a countertop next to the housing rack. The average mean, systolic, and diastolic blood pressures of decapitation-observing mice were all higher during euthanasia than at baseline (P ≤ 0.004 for all comparisons; Table 3, Figure 2 A through C). Similar increases (P ≤ 0.008) occurred in mice observing mock decapitation, during which the guillotine was opened and closed only, with no mice handled or euthanized. Values did not differ between observation of actual and mock decapitation (Table 3). However, during the minute immediately prior to observing decapitation, 2 of the 8 mice were active, whereas all 4 mice were at rest (inactive) prior to viewing mock decapitation. This difference in activity led to differences in the heart rate and mean blood pressure (Figure 2) at the initiation of the mock and actual decapitation procedures.
Table 3.
Responses of mice observing euthanasia by decapitation or mock decapitation
| Decapitation |
Mock decapitation |
|||||
| Baseline (mean) | Mean | P (baseline) | Mean | P (baseline) | P (decapitation) | |
| Systolic blood pressure (mm Hg) | 106.6 | 125.2a | <0.001 | 120.8a | <0.001 | 0.051 |
| Diastolic blood pressure (mm Hg) | 81.6 | 94.3a | 0.004 | 90.5a | 0.008 | 0.073 |
| Mean blood pressure (mm Hg) | 94.2 | 110.0a | 0.001 | 105.9a | 0.003 | 0.063 |
| Heart rate (bpm) | 475.4 | 497.2 | 0.159 | 516.7 | 0.012 | 0.932 |
| Activity (arbitrary units) | 0.9 | 1.8 | 0.148 | 2.0 | 0.625 | 0.999 |
Responses of observing mice did not differ between actual and mock decapitation.
Significantly different from baseline value.
Figure 2.
(A) Mean blood pressure, (B) heart rate, and (C) activity in mice to observing actual and mock decapitation. Baseline data are presented as the average of a 30-min period; data for the last minute prior to decapitations or mock decapitation are included also. The arrow identifies the time when the first decapitation or mock decapitation was conducted. The responses of observing mice did not differ between decapitation and mock decapitation.
Discussion
Several studies provide evidence that rodents respond to alterations in their environment through pheromones, odors, vocalization, and sight.2,3,8,18,21,24,28-30 Pheromones and odors affect reproduction and sexual behavior2,21,29,30 and can trigger aversion.3,8,18,24,28 Mice that observed other mice experience painful injections,24 restraint,22 transport,7 shocks,3,12 or surgical procedures28 demonstrated responses including increased freezing behavior, corticosterone elevation, increased sniffing frequency, and movement away from the source of odors coming into the cage. In one study, surgically anosmic mice did not show aversion in response to stressed C57BL/6J mice.24 In contrast, mice in chambers adjacent to CD1 mice undergoing an abdominal injection of acetic acid or formalin footpad injection had greater pain responses when a cage mate was injected simultaneously and only when the response of the other mouse was visible to the bystander mouse—vocalization or odors did not contribute to the increased response. In another study, pain hyperalgesia was present despite a clear absence of imitation.16
Compared with mice, rats appear to respond to vocalizations more readily.20 Rats produce 22-kHz ultrasonic vocalizations when exposed to aversive situations, whereas mice do not produce similar vocalization under similar conditions.15,23 These ultrasonic calls elicit freezing and defensive behaviors in other rats in the room.14,31 The response of the bystander rat depends on activation of the medial geniculate nucleus of the thalamus, thus demonstrating that vocalizations are the main factor that stimulates the fear response in bystanders.14 These previous mouse and rat studies clearly demonstrate the ability to communicate stressful events among rodents in the absence of physical contact but do leave in question the respective contributions of olfaction, vision, and hearing to the responses.
Housing condition has been shown to modify the stress response in rodents. In one study, mice were exposed to rats to determine the effect of housing different species in the same room.6 BALB/c male mice were housed either singly or in groups at 30 to 100 cm from rats for 7 to 14 d; the mice were housed in opaque cages so that only auditory and olfactory cues were present. Whereas group-housed mice had no increase in excitatory junction currents (a relative measure of neurotransmitter release) from the vas deferens, single-housed mice had a significant increase in both the amplitude and success rate of the excitatory junction currents.6 These findings are similar to those of a study in which rats displayed increased stress-like responses when housed singly than in a group.25 These findings demonstrate that cohousing of rodents can ameliorate the stress induced by external cues in the room.
In addition to the reactions of mice to various activities involving other mice, awareness of the possibility that rodents experience and display empathy is increasing. Empathy refers to an animal's ability to respond to the feelings of another animal. Originally thought to be unique to higher primates, considerable accumulating evidence suggests that empathy extends to other mammalian species, including rodents.4,11,13,16,19,20 For example, evidence supporting empathy and emotional contagion in mice was obtained on the basis of their familiarity with the mouse that experienced a stressor. Specifically, observing mice had increased responses when they had been cohoused for 14 or 21 d with the subject mouse, yet the observer mice did not respond when the subject mouse was a stranger.16 Similar results were obtained in another study in which the shocking of cage mates elicited freezing behavior in observer mice, but unfamiliar mice did not elicit this behavior.10 These studies establish a firm basis that mice exhibit empathy.
The current study examined the cardiovascular and activity reactions of C57BL/6N male mice to 2 different euthanasia methods. For both methods, we attempted to eliminate extraneous effectors that might directly affect the responses of the mice. Given the generally poor vision of mice, they likely were unable to see the euthanasia procedure.1 Contributing to the inability to see were the long distance between the euthanasia setup and the cages containing observing mice, the cage walls (although not opaque), and in some cases the stainless steel shelf between cages. Therefore, observing mice likely did not obtain any visual signals regarding euthanasia. The CO2 euthanasia method took as long as 3 min to complete and was amenable to the release of pheromones, odors, and vocalizations. Nevertheless, the cardiovascular and activity parameters measured in the mice in the adjacent cages remained at background levels. In fact, the observer mice primarily rested or slept in a nest during the euthanasia events. In contrast, the mice that underwent rapid decapitation vocalized in response to restraint, and odors including those of urine, feces, and blood likely were detectable by the observer mice. In a previous study, mice in chambers with a connecting tube for odor transmission but no visibility of other mice had no aversive responses to the urination or defecation of a mouse in the opposite cage.24 We therefore concluded that any response of the observer mice was attributable to the release of pheromones, blood, or vocalizations by the decapitated mice. The observing mice responded to the decapitation with increases in all cardiovascular parameters and activity. However, decapitation was associated with considerable noise during the operation of the guillotine and, albeit to a lesser extent, raising the lid of the cage to pick up a mouse. The noise from the guillotine might also have included ultrasonic sounds from the metal-on-metal movement. To test whether the response of the observing mice was related to the noise of the procedure, we also measured responses to mock decapitations. The observing mice had a similar response to the mock decapitation event as they did to decapitation euthanasia, with elevations in both cardiovascular parameters and activity. These findings suggest that the response to the guillotine-operation noise may be driving the euthanasia-associated differences in the cardiovascular values and activity or that the noise is masking any response by the observing mice.
The absence of any clear evidence of stress-like responses to either euthanasia techniques is counter to our hypothesis. The strong historical evidence of a response in rodents to stressed cohorts led us to expect that mice observing euthanasia would display similar responses.3,7,12,22,24,28 The results of the current study agree with previous findings that similarly showed no significant effect of euthanasia in the housing room.27 The mice in our study responded differently from rats, which showed a mild response to euthanasia.26 A previous study found a significant response to a mock decapitation in one experimental group, but in general the heart rate elevation to mock decapitation was lower than that during actual decapitation.26 Although we found a significant decrease in heart rate and activity in mice observing CO2 euthanasia, we believe that these decreases were artefactual, given that the baseline data were contaminated by short periods of activity in some mice. That is, during activity, the heart rate and blood pressure of mice increase, thus increasing the ‘resting’ baseline cardiovascular values. In addition, we do not consider that the reduction in activity was due to freezing behavior, because a fear-induced increase in cardiovascular values likely would accompany this absence of activity. Observer mice typically rested in the nest while other mice were euthanized. We believe that including the complete baseline values was more important than selecting specific times when the mice were still to adequately capture any alterations associated with observing a euthanasia event.
Speculating on the possible causes for the absence of a response in our experiments yielded a few important variables that need further investigation. First, the observing mice likely were unable to visualize the euthanasia procedure due to distance and the visual obstruction of the cage walls and shelf rack. Second, all of the mice studied were strangers to each other; none had been housed together previously. As previously demonstrated,16 both of these conditions were necessary for alterations in behavior to occur in mice that observed other mice that were in pain. Finally, it is likely that during the brief time needed to perform each euthanasia, signals from the euthanized mice might not have been transmitted, or did not elicit a stress response to the euthanasia by ultrasonic vocalization (not typical in mice), audible vocalization, emission of an odor, or pheromone. This final possibility seems unlikely because the mice audibly vocalized during scruffing for the decapitation, and we interpreted this vocalization as a response to a stressful situation.
We conclude that CO2 euthanasia of mice does not affect other mice in the room as long as the euthanized mice are not visible to the bystander mice and that euthanasia by decapitation likely only has an effect due to the noise of the guillotine. Acoustically shielding the guillotine may diminish the cardiovascular alterations that occur in mice in response to observing this type of euthanasia. We support the conceptual ideas that mice are both a sensitive species and display empathy, but under the controlled conditions of the euthanasia procedures used in the current study, there was no signaling of the stressful condition to the mice that witnessed these procedures.
Acknowledgment
The project described was supported by the Grants for Laboratory Animal Science (GLAS) from the American Association for Laboratory Animal Science. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
References
- 1.Baker M. 2013. Neuroscience: through the eyes of a mouse. Nature 502:156–158. [DOI] [PubMed] [Google Scholar]
- 2.Beynen AC. 1992. Communication between rats of experiment-induced stress and its impact on experimental results. Anim Welf 1:153–159. [Google Scholar]
- 3.Carr WJ, Roth P, Amore M. 2013. Responses of male mice to odors from stressed s nonstressed males and females. Psychon Sci 25:275–276. [Google Scholar]
- 4.Chen Q, Panksepp JB, Lavis GP. 2009. Empathy is moderated by genetic background in mice. PLoS One. 4:e4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Close B, Banister K, Baumans V, Bernoth EM, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H, Morton D, Warwick C. 1996. Recommendations for euthanasia of experimental animals: Part 1. DGXI of the European Commission Lab Anim 30:293–316. [DOI] [PubMed] [Google Scholar]
- 6.D'Arbe M, Einstein R, Lavidis NA. 2002. Stressful animal housing conditions and their potential effect on sympathetic neurotransmission in mice. Am J Physiol Regul Integr Comp Physiol 282:R1422–R1428. [DOI] [PubMed] [Google Scholar]
- 7.de Laat JMT, van Tintelen G, Beynen AC. 1989. Transportation of rats affects behavior of nontransported rats in the absence of physical contact (preliminary communication). Z Versuchstierkd 32:235–237. [PubMed] [Google Scholar]
- 8.Dua JK, Dobson MJ. 1974. Role of olfactory cues in acquisition and extinction of avoidance. J Exp Psychol 103:461–465. [Google Scholar]
- 9.Fox MW. 1986. Social influences and pheromones. p 47–54. In: Laboratory animal husbandry: ethology, welfare and experimental variables. Albany (NY): State University of New York Press. [Google Scholar]
- 10.Gonzalez-Liencres C, Juckel G, Tas C, Friebe A, Brune M. 2014. Emotional contagion in mice: The role of familiarity. Behav Brain Res 263:16–21. [DOI] [PubMed] [Google Scholar]
- 11.Guzmán YF, Tronson NC, Guedea A, Huh KH, Gao C, Radulovic J. 2009. Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav Brain Res 201:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iimori K, Tanaka M, Kohno Y, Ida Y, Nakagawa R, Hoaki Y, Tsuda A, Nagasaki N. 1982. Psychological stress enhances noradrenaline turnover in specific brain regions in rats. Pharmacol Biochem Behav 16:637–640. [DOI] [PubMed] [Google Scholar]
- 13.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. 2010. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci 13:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim EJ, Kim ES, Covey E, Kim JJ. 2010. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One 5:e15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knutson B, Burgdorf J, Pansepp J. 2002. Ultrasonic vocalizations as indices of affective state in rats. Psychol Bull 128:961–977. [DOI] [PubMed] [Google Scholar]
- 16.Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. 2006. Social modulation of pain as evidence for empathy in mice. Science 312:1967–1970. [DOI] [PubMed] [Google Scholar]
- 17.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R. [Internet]. 2013. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition [Cited 25 May 2016]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf.
- 18.Müller-Velten HG. 1966. [Uber den angstgeruch bei der hausmaus (Mus musculus L.).] Z Vgl Physiol 52:401–429. [Article in German]. [Google Scholar]
- 19.Panksepp JB, Lahvis GP. 2011. Rodent empathy and affective neuroscience. Neurosci Biobehav Rev 35:1864–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panksepp J, Panksepp JB. 2013. Toward a cross-species understanding of empathy. Trends Neurosci 36: 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkes AS, Bruce HM. 1961. Olfactory stimuli in mammalian reproduction. Science 134:1049–1054. [DOI] [PubMed] [Google Scholar]
- 22.Pitman DL, Ottenweller JE, Natleson BH. 1988. Plasma corticosterone levels during repeated presentation of 2 intensities of restraint stress: chronic stress and habituation. Physiol Behav 43:47–55. [DOI] [PubMed] [Google Scholar]
- 23.Portfors CV. 2007. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci 46:28–34. [PubMed] [Google Scholar]
- 24.Rottman SJ, Snowdon CT. 1972. Demonstration and analysis of an alarm pheromone in mice. J Comp Physiol Psychol 81:483–490. [DOI] [PubMed] [Google Scholar]
- 25.Sharp J, Zammit T, Azar T, Lawson D. 2002. Does witnessing experimental procedures produce stress in male rats? Contemp Top Lab Anim Sci 41:8–12. [PubMed] [Google Scholar]
- 26.Sharp J, Zammit T, Azar T, Lawson D. 2003. Are “by-stander” female Sprague-Dawley rats affected by experimental procedures? Contemp Top Lab Anim Sci 42:19–27. [PubMed] [Google Scholar]
- 27.Tuli JS, Smith JA, Morton DB. 1995. Corticosterone, adrenal and spleen weight in mice after tail bleeding, and its effect on nearby animals. Lab Anim 29:90–95. [DOI] [PubMed] [Google Scholar]
- 28.Valenta JG, Rigby MK. 1968. Discrimination of the odor of stressed rats. Science 161:599–601. [DOI] [PubMed] [Google Scholar]
- 29.Van Der Lee S, Boot LM. 1955. Spontaneous pseudopregnancy in mice. Acta Physiol Pharmacol Neerl 4:442–444. [PubMed] [Google Scholar]
- 30.Whitten WK. 1958. Modification of the oestrous cycle of the mouse by external stimuli associated with the male; changes in the oestrous cycle determined by vaginal smears. J Endocrinol 17:307–313. [DOI] [PubMed] [Google Scholar]
- 31.Wöhr M, Schwarting RKW. 2008. Ultrasonic calling during fear conditioning in the rat: no evidence for an audience effect. Anim Behav 76:749–760. [Google Scholar]