Abstract
Rodent euthanasia with CO2 by using gradual displacement of 10% to 30% of the chamber volume per minute is considered acceptable by the AVMA Panel on Euthanasia. However, whether a 50% to 100% chamber replacement rate (CRR) of CO2 is more painful or distressful than 10% to 30% CRR is unclear. Therefore, we examined physiological and behavioral parameters, corticosterone and ACTH levels, and lung histology of mice euthanized at CRR of 15%, 30%, 50%, or 100%. Adult male C57BL/6N mice were euthanized at different CO2 CRR as physiological parameters were recorded telemetrically. Video recordings were reviewed to determine when the mouse first became ataxic, when it was fully recumbent (characterized by the mouse's nose resting on the cage floor), and when breathing stopped. Overall, CO2 euthanasia increased cardiovascular parameters and activity. Specific significant differences that were associated with 50% to 100% compared with 15% to 30% CO2 CRR included an increase in systolic blood pressure per second from initiation of CO2 until ataxia, a decrease in total diastolic blood pressure until ataxia, and a decrease in total heart rate until ataxia, immobility, and death. All physiological responses occurred more rapidly with higher CRR. Activity levels, behavioral responses, plasma adrenocorticotropic hormone and corticosterone levels, and lung pathology were not different between groups. We found no physiological, behavioral, or histologic evidence that 15% or 30% CO2 CRR is less painful or distressful than is 50% or 100% CO2 CRR. We conclude that 50% to 100% CO2 CRR is acceptable for euthanizing adult male C57BL/6N mice.
Abbreviations: ACTH, adrenocorticotropic hormone; BP, blood pressure; CRR, chamber replacement rate
The AVMA Guidelines for the Euthanasia of Animals provides recommendations for the use of CO2 for rodent euthanasia.28 Providing that personnel have been trained appropriately in the technique, rodent euthanasia with CO2 by using gradual displacement of 10% to 30% of the chamber volume per minute is considered acceptable.28 The AVMA recommendations further state that the immersion of conscious animals into a container prefilled with 100% CO2 is unacceptable.28 This recommendation is partially a result of a workshop during which a consensus reached by several scientists determined that to optimize animal wellbeing during CO2 euthanasia, it is more important to avoid or minimize pain and distress than to ensure a rapid loss of consciousness.18 It is currently accepted that rapid euthanasia by using a high chamber replacement rates (CRR) of CO2 is more painful or distressful than is using a slow CRR. In fact, the use of the slow CRR is predicated on the assumption that unconsciousness is achieved prior to pain development.
Evidence to support that high flow rates of CO2 is painful is derived in part from human studies on nasal CO2 exposure.3,14,42 Contact of CO2 with the nasal mucosa leads to the production of intracellular carbonic acid, which decreases pH and thus causes pain.3 One study showed self-reported increasing pain intensity after single breaths of increasing CO2 concentrations of 50% to 100% (lower concentrations were not tested).14 Another study evaluated 7 s of nasal exposure to 35.5%, 53%, and 70% CO2 and found that pain intensity was proportional to CO2 concentration.42 Interestingly, pain intensity peaked at 3 to 4 s and then rapidly faded.42 Studies in rats show increased firing of medullary dorsal horn neurons in response to CO2 inhalation, providing further evidence of its painful effects.35 There was an increase in the response magnitude of neural firing at 25% CO2 and a near-linear increase between 37% and 87% CO2, suggesting that as CO2 increased, pain increased.35
The rationale for using a slow CRR for CO2 euthanasia assumes that rodents will be unconscious prior to the development of pain. As noted in the Newcastle report,18 determining when a rodent becomes unconscious during euthanasia is unclear and frequently relies on postural or behavioral changes. Our observations suggest that mice euthanized with low CO2 CRR have a longer period of distress prior to unconsciousness, with no clear behavioral evidence that they experience less pain, compared with mice euthanized with high CO2 CRR. This finding is consistent with a study that found that mice experienced a longer period of dyspnea during CO2 euthanasia with 20% to 30% CRR compared with 50% CRR.30 Another study evaluated CO2 euthanasia in rats, using behavioral responses and blood gas analysis to compare a prefilled chamber with a fixed flow rate of CO2 (20% CRR).20 Although differences were found in the time to ataxia, immobility, loss of pedal reflex, and respiratory arrest, the authors concluded that responses were due to the anesthetic effect of CO2 and, therefore, that no distress occurred with either method.20
In light of these observations and previous publications, we believe there is justification to reexamine the optimal CRR for CO2 euthanasia in mice, given that the combined experience (pain, fear, and dyspnea) may be less with high CRR compared with slow CRR. Therefore, to examine the effects of different CO2 CRR, we measured physiological, behavioral, and histologic parameters of mice undergoing euthanasia. We hypothesized that heart rate, blood pressure (BP), corticosterone and ACTH levels, activity, behavior, and lung histology would not differ between mice euthanized as a result of fast (50% or 100%) CO2 CRR compared with a slow (15% or 30%) CRR.
Materials and Methods
Mice.
Male C57BL/6N mice (n = 57; age, 16 wk; Taconic, Hudson, NY) were used for all procedures. Mice were individually housed in rodent shoebox cages (Allentown Caging, Allentown, NJ) on a 12:12-h light:dark cycle (lights on, 0700 to 1900). The room temperature was maintained at 23.3° C with average humidity of 55 ± 6%. Mice had unrestricted access to rodent diet (Teklad 8640, Harlan, Indianapolis, IN) and tap water in bottles and were housed on Sani-Chip bedding (Harlan) with cotton nesting squares (Ancare, Bellmore, NY) provided. Mice were free from pathogenic agents including ectromelia virus, epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, Mycoplasma pulmonis, mouse adenovirus types 1 and 2, mouse hepatitis virus, mouse parvovirus, minute virus of mice, polyoma virus, pneumonia virus of mice, reovirus type 3, Theiler murine encephalomyelitis virus, Sendai virus, endoparasites, and ectoparasites. All experimental procedures were approved by the Wright State University IACUC.
Surgery.
All surgeries were performed in a rodent-dedicated surgery suite. Mice were anesthetized with isoflurane (1% to 4%) in oxygen (induced in a chamber and maintained by mask). The neck was shaved and prepped 3 times with alternating povidone–iodine and alcohol scrubs followed by a final swab with povidone–iodine solution. The mice were continuously monitored for depth of anesthesia by assessing response to pain, movement, and respiratory rate. A 1-cm incision was made in the ventral neck and the muscle bluntly dissected to expose the carotid artery. A telemetry pressure-transmitter probe (TA11PA-C10, Data Sciences International, St Paul, MN) was inserted in the carotid artery and ligated in place. The body of the transmitter was inserted subcutaneously on the right flank and the incision closed by using 5-0 nonabsorbable black nylon monofilament suture (Arosurgical, Newport Beach, CA). Pain and discomfort were alleviated by providing carprofen (5 mg/kg SC; Penn Vet, Lancaster, PA) at the time of surgery and at 24 h postoperatively. After surgery, mice were housed singly to prevent a cage mate from disturbing the wound site and to accustom mice to single housing for individual telemetry measurements.
Telemetry measurement.
Mice were allowed to recover for 1 wk after surgery. They were individually housed on data-acquisition receiver boards (RPC1, Data Sciences International) to ensure signal integrity. Radiotelemetric measurements were collected by using Ponemah software (Data Sciences International). Heart rate, BP, and activity data were collected continuously (sample rate, 1000 Hz) during both baseline and testing measurements. For data collection, the transmitters were turned on, and all personnel left the room. After allowing at least 30 min for readings to stabilize, baseline data were collected for at least 1 h (starting at approximately 1000). On subsequent days, mice were euthanized after a similar 30-min stabilization period. The stabilization period was used to minimize the effect of movement of the cage on the cardiovascular parameters and mouse activity.
Euthanasia.
To avoid effects of handling, all mice were euthanized in their home cages in the holding room. To eliminate the effect of circadian rhythms, 1 to 3 mice were euthanized daily between 1000 and 1300. To set up, the mouse home cage (5.8 L, Allentown Caging) was placed in a 22-L transparent polycarbonate shoebox euthanasia chamber (44 cm × 23.5 cm × 21 cm) in the same location where the home cage was located previously. The euthanasia chamber was covered with an acrylic lid, with ports for gas inlet and outlet. Compressed CO2 gas was provided from a cylinder (Weiler Welding, Moraine, OH) and controlled by a CO2-specific regulator (Western Medica, Westlake, OH). Chamber air was replaced with CO2 at 4 different rates—15%, 30%, 50%, or 100% of the chamber volume per minute; each group comprised 12 mice, except the 50% CRR group had 13 mice. CO2 concentration was measured every 15 s during the euthanasia procedure by using an indoor-air–quality meter (CM-0003, CO2meter.com, Ormond Beach, FL) with accuracy of ±30 ppm and ±5% of the measured value. CO2 levels in the home cage were measured at approximately 2 cm above the bottom of the cage. Mice were monitored until 30 s after complete cessation of heart beat and blood pressure. Only one person was in the room during testing and remained silent throughout the procedure.
Behavior.
Mice were video recorded using a Logitech C920 camera (Newark, CA) during the euthanasia procedures. Videos were analyzed blinded to group for activity (hopping, running, sedentary, standing/rearing), breathing pattern (normal, sniffing, labored breathing, terminal gasping), ataxia, face wiping, grooming, recumbency or cessation of walking, and loss of muscle tone/nose resting on the bedding. Time to the initiation of the activity and number of occurrences were recorded.
Air exposure.
A control study to determine the effect of noise and air movement on the mice was conducted in 28 mice. Similar to the euthanasia procedure, mice in their home cage were moved into the euthanasia chamber. However, only air from a compressed cylinder (Weiler Welding) was forced into the chamber. We tested 3 rates (15%, 30%, and 50% Air CRR; equipment was not available to do a 100% Air CRR). The heart rate, blood pressure, activity and behavior of the mice in response to the air and noise were recorded for 5 min. After at least 30 min for stabilization, these mice were subsequently euthanized by CO2.
Corticosterone and ACTH measurements.
Immediately after euthanasia, blood was collected by cardiocentesis into either heparin (corticosterone assays) or EDTA (adrenocorticotropic hormone [ACTH] assays) collection tubes. Blood samples were centrifuged at 385 × g for 15 min at room temperature, and plasma removed. Plasma samples for corticosterone analysis were stored at –18 °C until assay. Corticosterone levels were determined by using a standard enzyme immunoassay kit (Arbor Assays, Ann Arbor, MI). Briefly, 5 µL of dissociation reagent was mixed with 5 µL plasma and then incubated for 5 min at room temperature. This solution was diluted 1:50 with assay buffer and used in the assay according to manufacturer's instructions; the intra-assay coefficient of variation was 4.46%. Plasma samples for ACTH analysis were stored at –80 °C until analysis by using a commercially available kit (ImmunChem Double Antibody ACTH radioimmunoassay, MP Biomedicals, Santa Ana, CA). Plasma samples were diluted 1:4 with assay diluent before being processed according to the manufacturer's instructions; the intraassay coefficient of variation was 4.86%.
Histology.
Lungs were inflation-fixed with 10% neutral buffered formalin, harvested from each mouse after euthanasia, processed through a gradient of alcohols and xylene, embedded in paraffin, cut at 5 μm, and stained with hematoxylin and eosin. Lungs were examined for acute hemorrhagic lesions, congestion, and perivascular edema. Changes were scored on a scale of 0 to 3: 0, normal; 1, mild change; 2, moderate change; and 3, severe change. Scoring was performed blind to method of euthanasia.
Statistical analysis.
The area under the curve (AUC) and the per-second average AUC above baseline values were analyzed for heart rate, systolic BP, diastolic BP, and mean BP for 12 or 13 mice in each group. Three time points were used to estimate conclusion of the potentially painful experience: when ataxia was first evident, when the mouse stopped moving and rested its head on the ground (nose down), and when the heart stopped beating (this occurred within a few seconds of the cessation of regular breathing as defined by taking a breath at least once every 2 seconds). These 3 time points were individually analyzed because the time until unconsciousness (and absence of pain perception) during the euthanasia procedure could not be determined unequivocally.
All analyses were done using SAS version 9.4 (SAS Institute, Cary, NC). Analysis of covariance (ANCOVA) was used for all analyses, to control for the baseline measurements. The initial analysis used one-way multivariate ANOVA with the factor CO2 concentration having 4 levels (15%, 30%, 50%, and 100%). The subsequent analysis compared the estimated curves from the telemetry data. Functional data analysis used the information in the slopes and curves, as reflected in their derivatives. Because 4 comparisons were made, a Bonferroni correction was applied to control for inflated type I error, yielding a cut-off of 0.0125 for the level of significance for the cardiovascular tests to have an overall level of significance of α = 0.05. The activity outcome and pathology data were ordinal and there are 4 levels of the treatment, so the nonparametric Kruskal–Wallis test was used for all comparisons. For behavioral analyses, the Fisher Exact test was performed due to small counts in some cells. Log rank tests were used for the time-to-peak analyses.
Results
CO2 data.
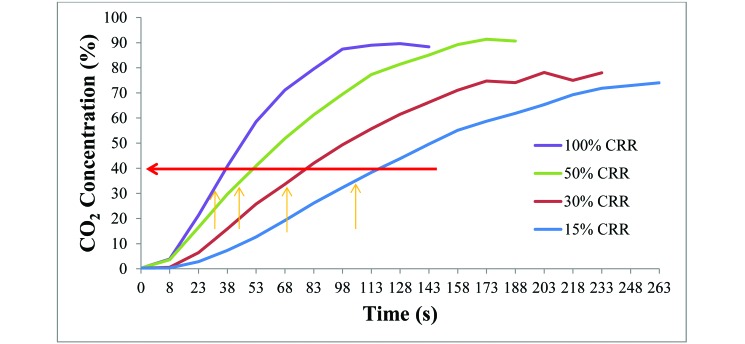
CO2 input into the cage within the euthanasia chamber showed a near-linear increase after a short lag, presumably due to initial mixing of the CO2 in the chamber and the time it took to reach the sensor within the home cage (Figure 1). As expected, higher CO2 CRR led to more rapid increases in the CO2 concentration within the chamber.
Figure 1.
CO2 concentration in the home cage with different CO2 chamber replacement rates (CRR). The red arrow is the level at which pain occurs. Each of the yellow arrows represents the point of full recumbency (nose down) of the mice at the respective CO2 CRR.
Baseline radiotelemetric data.
Radiotelemetry was used to measure baseline heart rate, BP, and activity. Baseline data collected from the mice did not differ significantly between the 4 groups (Table 1). The average increase in these values was used to determine the change from baseline for each mouse when exposed to CO2.
Table 1.
Baseline data (mean ± 1 SD) of mice in each group (n≥ 12 per group)
| Chamber replacement rate (per minute) |
|||||
| 15% | 30% | 50% | 100% | Pa | |
| Heart rate (bpm) | 471 ± 33.9 | 493 ± 32.2 | 478 ± 23.7 | 489 ± 29.2 | 0.25 |
| Baseline systolic blood pressure (mm Hg) | 105 ± 6.6 | 109 ± 7.3 | 107 ± 4.0 | 108 ± 5.5 | 0.49 |
| Diastolic blood pressure (mm Hg) | 83.6 ± 7.8 | 82.5 ± 4.5 | 79.8 ± 7.3 | 82.7 ± 7.1 | 0.54 |
| Mean arterial blood pressure (mm Hg) | 94.5 ± 6.6 | 95.9 ± 5.1 | 93.4 ± 5.0 | 96.0 ± 5.6 | 0.62 |
| Activity (arbitrary units) | 1.2 ± 1.2 | 2.5 ± 2.1 | 1.3 ± 1.1 | 1.2 ± 1.6 | 0.24 |
ANOVA group significance level
Radiotelemetric and behavioral data in response to air flow.
The telemetric responses of mice exposed to air from a cylinder at 15%, 30%, or 50% CRR showed no significant differences when compared with each other. The pooled response compared with baseline showed significant (P < 0.0001) increases in all cardiovascular parameters but no significant difference in activity (P = 0.88; Figure 2). Although not mirrored in the activity telemetric data, the behavioral responses of the mice clearly indicated that they were aware of the air flow. Initial responses to the air input included both sniffing and exploratory behavior within the nest (movement) and occurred earliest in the 50% air CRR group (Table 2). Additional reactions to the air input occurred in some mice from all groups and included leaving the nest and rearing. Other behaviors occurred less frequently (data not shown), including tail raising or twitching, burying the head in the nest, face wiping, and grooming. There were no significant differences in the incidence of these activities between groups.
Figure 2.
Average (A) heart rate, (B) mean blood pressure, and (C) activity during the introduction of compressed air into the euthanasia chamber at 15%, 30%, and 50% chamber volume per minute.
Table 2.
Behavioral responses of mice exposed to air at different chamber replacement rates
| Chamber replacement rate (per minute) |
||||
| 15% (n = 8) | 30% (n = 7) | 50% (n = 13) | Pa | |
| Latency to sniffing (s; mean ± 1 SD) | 80.3 ± 24.4 | 36.3 ± 29.3 | 10.4 ± 10.1 | 0.0001 |
| Latency to movement (s; mean ± 1 SD) | 69.6 ± 58.8 | 22.6 ± 27.8 | 8.3 ± 10.0 | 0.003 |
| Proportion (%) of mice that left nest | 12.5 | 28.6 | 50.0 | 0.20 |
| Proportion (%) of mice that reared | 12.5 | 57.1 | 50.0 | 0.14 |
ANOVA group significance level
Time to endpoint measurements during CO2 euthanasia.
The average time to ataxia, no further movement (nose down), and cessation of heart beat (death) was significantly shorter as CO2 CRR was increased (P < 0.0001 for all comparisons except 50% compared with 100% until ataxia [P = 0.0078] and nose down [P = 0.0041] and 15% compared with 30% until death [P = 0.0012]; Table 3)
Table 3.
Time (s; mean ± 1 SD) until indicated behavior during euthanasia at different CO2CRR. Mean± SD.
| Chamber replacement rate (per minute) |
|||||
| 15% | 30% | 50% | 100% | Pa | |
| Sniffing | 23.4 ± 15.0 | 9.2 ± 4.5 | 6.7 ± 4.3 | 5.3 ± 3.9 | <0.0001 |
| Moved out of nest | 66.4 ± 27.1 | 36.7 ± 15.5 | 25.5 ± 8.3 | 20.9 ± 6.6 | <0.0001 |
| Ataxia | 79.4 ± 13.4 | 49.6 ± 8.8 | 31.7 ± 5.3 | 24.7 ± 4.8 | <0.0001 |
| Nose down | 106 ± 17.7 | 69.4 ± 6.4 | 45.5 ± 13.6 | 32.6 ± 5.5 | <0.0001 |
| Death | 204 ± 32.3 | 159 ± 22.8 | 102 ± 14.4 | 69.3 ± 6.8 | <0.0001 |
n ≥ 9 for all data except ataxia in 100% CRR group (n = 7 ; because some mice did not move, ataxia could not be evaluated).
ANOVA group significance level
Cardiovascular response to CO2 euthanasia.
Mice undergoing CO2 euthanasia demonstrated an initial rise in heart rate followed by a decline and then a secondary rise followed by a slow decline to 0 (Figure 3 A). Interestingly, the trough between the first and second peaks was approximately when mice became ataxic. In contrast, the mean, diastolic, and systolic BP measurements initially increased and then slowly declined to 0 (Figure 3 B). The peak in BP most closely paralleled the time when the mice moved out of the nest, occurring a few seconds prior to the movement. The total and average heart rate, systolic BP, diastolic BP, and mean BP were evaluated over the total time until ataxia. The total measures each represent the total AUC above baseline values. The per-second measures represent the given measurement divided by the duration of the respective trial, thus adjusting for the increased duration of the euthanasia procedure with lower CO2 CRR.
Figure 3.
Average (A) heart rate, (B) mean blood pressure, and (C) activity in response to euthanasia with 15%, 30%, 50%, and 100% CO2 CRR. No activity occurred after 197 s.
In the first analysis, the data until the mice were ataxic were examined. For this analysis it was necessary to perform log transformations on 6 of the 8 dependent variables (total and average heart rate, total systolic BP, diastolic BP, and total and average mean BP). According to a P value of 0.01 or lower, the mean AUC of total heart rate, total diastolic BP, and average systolic BP measured until ataxia showed strong evidence of difference among the levels of CO2 (Table 4). None of the other dependent variables showed evidence of a statistically significant relationship with the CO2 concentration. The Tukey studentized range test indicated that the total heart rate until ataxia at 50% CO2 CRR was significantly lower than the mean AUC above baseline of total heart rate until ataxia for 15% and 30% CO2 CRR (P = 0.0016 and 0.0085, respectively). Although the overall difference in average systolic BP was significant, none of the pairwise comparisons were below the cutoff of α = 0.0125 (P = 0.0127 for 15% compared with 100% CRR and P = 0.0183 for 15% compared with 50% CO2 CRR). Even though an overall difference was detected for total diastolic BP, no 2 individual levels were significantly different from each other (P = 0.0130 for 15% compared with 50% CRR).
Table 4.
Cardiovascular values (mean ± 1 SD; n≥ 12 mice per group) for total AUC above baseline for time until ataxia at various CO2CRR
| Chamber replacement rate (per minute) |
|||||
| 15% | 30% | 50% | 100% | Pb | |
| Total heart rate (sum of heart beats)a | 4632 ± 3195 | 3104 ± 1606 | 1476 ± 566 | 2156 ± 783 | 0.001 |
| Heart rate (beats per second)a | 58.4 ± 38.4 | 65.2 ± 39.6 | 46.1 ± 14.0 | 86.6 ± 23.5 | 0.04 |
| Total systolic blood pressure (sum of mm Hg)a | 1262 ± 621 | 1131 ± 381 | 853 ± 308 | 728 ± 167 | 0.27 |
| Systolic blood pressure (mm Hg) per second | 16.0 ± 7.7 | 23.2 ± 8.5 | 27.8 ± 10.9 | 30.0 ± 7.2 | 0.007 |
| Total diastolic blood pressure (sum of mm Hg)a | 1049 ± 517 | 879 ± 423 | 695 ± 302 | 540 ± 192 | 0.008 |
| Diastolic blood pressure (mm Hg) per second | 13.3 ± 6.3 | 17.8 ± 8.8 | 22.7 ± 10.2 | 22.0 ± 7.9 | 0.12 |
| Total mean blood pressure (sum of mm Hg) | 1131 ± 559 | 985 ± 389 | 746 ± 297 | 636 ± 172 | 0.08 |
| Mean blood pressure (mm Hg) per second | 14.4 ± 6.8 | 20.1 ± 8.4 | 24.4 ± 10.2 | 26.1 ± 6.7 | 0.02 |
Data were transformed for analysis
ANOVA group significance level
In addition, total AUC above baseline until nose down and until death was evaluated for the same 8 dependent variables. For analysis of the time to nose down it was necessary to perform log transformations on the same 6 dependent variables as for the time until ataxia. For time until death, data for total and average heart rate and total systolic, diastolic, and mean BP were log-transformed to meet model assumptions. On the basis of P values of less than 0.01 and 0.0001, respectively, the mean AUC of total heart rate for both time to nose down and time to death showed strong evidence of differing among the levels of CO2 (Tables 5 and 6). The Tukey studentized range test showed that the total heart rate until nose down was lower for both 50% and 100% CO2 CRR compared with 15% CRR (P < 0.0001 and P = 0.0061, respectively) and lower for 50% CO2 CRR compared with 30% CO2 CRR (P = 0.0007). The total heart rate until death was lower for both 50% and 100% CO2 CRR than 15% CO2 CRR (P = 0.0029 and 0.0003, respectively) and 30% CO2 CRR (P = 0.0009 and <0.0001, respectively). None of the other dependent variables showed evidence of a statistically significant relationship with the level of CO2 CRR.
Table 5.
Cardiovascular values (mean ± 1 SD; n≥ 12 mice per group) for total AUC above baseline for time until nose down at various CO2CRR
| Chamber replacement rate (per minute) |
|||||
| 15% | 30% | 50% | 100% | Pb | |
| Total heart rate (sum of heart beats)a | 6079 ± 3746 | 4075 ± 2342 | 1835 ± 843 | 2078 ± 964 | <0.01 |
| Heart rate (beats per second)a | 57.7 ± 34.5 | 57.5 ± 32.4 | 40.0 ± 15.5 | 63.6 ± 25.4 | 0.04 |
| Total systolic blood pressure (sum of mm Hg)a | 1520 ± 955 | 1377 ± 716 | 1003 ± 438 | 744 ± 216 | 0.67 |
| Systolic blood pressure (mm Hg) per second | 13.4 ± 9.7 | 20.6 ± 10.1 | 25.3 ± 12.0 | 23.7 ± 7.2 | 0.03 |
| Total diastolic blood pressure (sum of mm Hg)a | 1437 ± 907 | 1320 ± 732 | 1008 ± 559 | 717 ± 268 | 0.03 |
| Diastolic blood pressure (mm Hg) per second | 13.6 ± 8.3 | 18.4 ± 11.0 | 23.0 ± 11.0 | 21.8 ± 7.5 | 0.16 |
| Total mean blood pressure (sum of mm Hg) | 1466 ± 921 | 1350 ± 689 | 1007 ± 522 | 719 ± 235 | 0.44 |
| Mean blood pressure (mm Hg) per second | 13.9 ± 8.5 | 19.3 ± 10.2 | 23.2 ± 11.1 | 22.2 ± 7.0 | 0.11 |
Data were transformed for analysis
ANOVA group significance level
Table 6.
Cardiovascular values (mean ± 1 SD; n≥ 12 mice per group) for total AUC above baseline for time until death at various CO2CRR
| Chamber replacement rate (per minute) |
|||||
| 15% | 30% | 50% | 100% | Pb | |
| Total heart rate (sum of heart beats)a | 10549 ± 5884 | 8569 ± 4060 | 4159 ± 1888 | 3354 ± 1892 | <0.0001 |
| Heart rate (beats per second)a | 51.7 ± 7.9 | 54.9 ± 26.0 | 41.8 ± 20.0 | 47.8 ± 27.0 | 0.23 |
| Total systolic blood pressure (sum of mm Hg)a | 1862 ± 1280 | 1816 ± 706 | 1315 ± 702 | 845 ± 319 | 0.13 |
| Systolic blood pressure (mm Hg) per second | 9.0 ± 6.0 | 11.7 ± 5.2 | 12.7 ± 5.4 | 11.9 ± 4.4 | 0.24 |
| Total diastolic blood pressure (sum of mm Hg)a | 2082 ± 1527 | 2091 ± 1023 | 1481 ± 859 | 931 ± 374 | 0.02 |
| Diastolic blood pressure (mm Hg) per second | 10.0 ± 6.9 | 13.5 ± 7.2 | 14.2 ± 7.1 | 13.13 ± 5.11 | 0.51 |
| Total mean blood pressure (sum of mm Hg) | 1891 ± 1339 | 1874 ± 832 | 1316 ± 741 | 851 ± 339 | 0.08 |
| Mean blood pressure (mm Hg) per second | 9.1 ± 6.1 | 12.1 ± 5.9 | 12.7 ± 5.9 | 12.0 ± 4.6 | 0.27 |
Data were transformed for analysis
ANOVA group significance level
The time to achieve peak values for heart rate, systolic BP, diastolic BP, and mean BP were analyzed to assess the relationship between time to occurrence and CO2 CRR. Because time until an event was a parameter of interest, log-rank tests were conducted for each outcome (Table 7). Because the P value for each outcome was less than 0.01, pairwise log-rank tests were performed to determine where any differences might lie. To control for inflated type I error from multiple comparisons, the α level was adjusted down to 0.0083. The mice exposed to 15% CO2 CCR had longer times to peak heart rate, systolic BP, diastolic BP, and mean BP than did those in the 50% CO2 CCR group (P < 0.0001, P < 0.0001, P = 0.0018, and P = 0.0004, respectively). The 15% CO2 CCR mice had longer times to peak systolic BP, diastolic BP, and mean BP than did the 100% CO2 CCR mice (P < 0.0001, P = 0.0009, and P = 0.0003, respectively). The 30% CO2 CCR mice had longer times to peak heart rate and systolic BP than did those exposed to 50% CO2 CCR (P = 0.0009 and P < 0.0001, respectively). The 30% CO2 CCR group had longer times to peak systolic BP, diastolic BP, and mean BP than did the 100% CO2 CCR mice (P < 0.0001, P = 0.0008, and P < 0.0001, respectively). No other significant differences were detected.
Table 7.
Time (s; mean ± 1 SD, n≥ 12 per group) to peak cardiovascular values during euthanasia at different CO2CRR
| Chamber replacement rate (per minute) |
|||||
| 15% | 30% | 50% | 100% | Pa | |
| Heart rate (bpm) | 52.0 ± 48.1 | 25.1 ± 20.3 | 7.4 ± 6.8 | 22.7 ± 25.8 | <0.01 |
| Systolic blood pressure (mm Hg) | 51.5 ± 21.4 | 37.4 ± 22.5 | 17.6 ± 6.7 | 17.5 ± 4.3 | <0.01 |
| Diastolic blood pressure (mm Hg) | 57.2 ± 27.5 | 52.5 ± 20.6 | 31.2 ± 13.8 | 23.1 ± 6.6 | <0.01 |
| Mean blood pressure (mm Hg) | 53.0 ± 24.1 | 37.0 ± 23.3 | 20.7 ± 9.4 | 17.2 ± 5.6 | <0.01 |
ANOVA group significance level
We then analyzed whether peak values for heart rate, systolic BP, diastolic BP, and mean BP showed any relationship to the level of CO2 CRR (Table 8). There were no significant differences in the mean peaks for the cardiovascular parameters.
Table 8.
Peak physiological values (mean ± 1 SD, n≥ 12 per group) during euthanasia at different CO2CRR
| Chamber replacement rate (per minute) |
|||||
| 15% | 30% | 50% | 100% | Pa | |
| Heart rate (bpm) | 678 ± 71.5 | 661 ± 43.2 | 640 ± 39.5 | 709 ± 93.7 | 0.06 |
| Systolic blood pressure (mm Hg) | 140 ± 14.2 | 151 ± 15.3 | 146 ± 12.8 | 152 ± 6.3 | 0.12 |
| Diastolic blood pressure (mm Hg) | 116 ± 10.5 | 118 ± 10.01 | 115 ± 7.76 | 116 ± 4.0 | 0.93 |
| Mean blood pressure (mm Hg) | 125 ± 11.0 | 131 ± 12.2 | 128 ± 11.0 | 131 ± 4.3 | 0.53 |
| Activity (arbitrary units) | 130 ± 80.0 | 118 ± 51.1 | 68.8 ± 32.7 | 55.3 ± 23.1 | 0.004 |
ANOVA group significance level
Behavioral responses to different CO2 CRR.
The amount of activity (total and average) and the time spent moving (total and average) were compared across the 4 levels of CO2 (Figure 3 C). Activity ceased during the nose-down stage, therefore no further measurements occurred after this point. Because none of the P values were less than 0.025 (Tables 9 and 10), there was insufficient evidence to suggest a difference in amount of activity or time active by the mice among the CO2 levels. Values for the amount and duration of activity during the nose-down stage were near significance, therefore a simple linear regression analysis was performed to see how each changed over the 4 levels of CO2 CRR. For the activity during the nose-down stage, the natural logarithm of the mean activity level decreased (P = 0.03), on average, by 0.051 for every liter per minute increase in CO2 CRR. For the duration of activity during the nose-down phase, the total time spent moving decreased (P = 0.01), on average, by 0.48 s for every liter per minute increase in CO2 CRR.
Table 9.
Activity (arbitrary units; mean ± 1 SD, n≥ 12 per group) during euthanasia
| Chamber replacement rate (per minute) |
|||||
| 15% | 30% | 50% | 100% | Pa | |
| Total activity during ataxia | 458 ± 605 | 334 ± 297 | 168 ± 157 | 148 ± 81.3 | 0.30 |
| Activity per second during ataxia | 6.5 ± 9.8 | 7.0 ± 5.7 | 5.3 ± 4.4 | 6.2 ± 3.5 | 0.56 |
| Total activity during nose down | 627 ± 673 | 574 ± 382 | 273 ± 185 | 181 ± 110 | 0.03 |
| Activity per second during nose down | 6.1 ± 6.6 | 8.4 ± 5.9 | 5.7 ± 3.4 | 5.7 ± 3.4 | 0.49 |
ANOVA group significance level
Table 10.
Duration of activity (s; mean ± 1 SD, n≥ 12 per group) during euthanasia
| Chamber replacement rate (per minute) |
|||||
| 15% | 30% | 50% | 100% | Pa | |
| Total duration of activity during ataxiab | 10.4 ± 10.2 | 6.8 ± 6.1 | 4.0 ± 3.6 | 4.0 ± 2.2 | 0.10 |
| Average duration of activity during ataxia (per second) | 0.14 ± 0.16 | 0.14 ± 0.11 | 0.13 ± 0.11 | 0.17 ± 0.10 | 0.93 |
| Total duration of activity during nose downb | 12.7 ± 11.4 | 10.9 ± 7.0 | 6.9 ± 4.4 | 4.7 ± 3.1 | 0.06 |
| Average activity during nose down (per second) | 0.12 ± 0.11 | 0.16 ± 0.11 | 0.15 ± 0.09 | 0.15 ± 0.09 | 0.85 |
ANOVA group significance level
Data were transformed for analysis
Behavioral assessment included video recording of the mice and evaluation of whether mice wiped their face, reared, groomed, jumped, or walked during the course of the euthanasia procedure. None of the mice jumped, wiped its face, buried its nose, held its breath, or demonstrated any other evidence of pain except for labored breathing or gasping and moving. All mice were dyspneic. A single mouse (30% CRR group) reared during the euthanasia procedure; no other mice in any other group exhibited this behavior. There was no significant difference between groups in the incidence of any of these behaviors (P ≥ 0.48).
Histology.
Histologic analysis of the incidence and severity of pulmonary perivascular edema, congestion, and alveolar hemorrhage revealed mild or moderate changes in 75% of mice (Table 11). There were no significant differences between the 4 groups.
Table 11.
Histological lesions (score; mean ± 1 SD) associated with CO2euthanasia
| Chamber replacement rate (per minute) |
|||||
| 15% (n =14) | 30% (n =14) | 50% (n =18) | 100% (n =14) | Pa | |
| Perivascular edema | 1.14 ± 0.86 | 1.14 ± 0.77 | 0.89 ± 0.90 | 0.79 ± 0.57 | 0.49 |
| Congestion | 0.14 ± 0.36 | 0 ± 0 | 0.17 ± 0.38 | 0.21 ± 0.42 | 0.38 |
| Hemorrhage | 0.29 ± 0.61 | 0.07 ± 0.26 | 0.06 ± 0.24 | 0.07 ± 0.26 | 0.42 |
Lesions were scored according to the following scale: 0, no lesion; 1, mild lesion; 2, moderate lesion; and 3, severe lesion
ANOVA group significance level
Corticosterone and ACTH assays.
There were no significant differences in plasma corticosterone or ACTH levels between the different CO2 CRR (P = 0.22 and 0.07, respectively; Table 12).
Table 12.
Corticosterone and ACTH levels during CO2euthanasia
| Chamber replacement rate (per minute) |
|||||
| 15% | 30% | 50% | 100% | Pa | |
| Corticosterone (ng/mL) | 40.4 ± 25.3 | 15.7 ± 9.7 | 32.1 ± 20.9 | 30.8 ± 30.1 | 0.22 |
| ACTH (pg/mL) | 812 ± 592 | 258 ± 174 | 501 ± 228 | 342 ± 196 | 0.07 |
Group sizes for CRR of 15%, 30%, 50%, and 100% are 8, 9, 10, and 9 mice for corticosterone and 4, 6, 6, and 5 mice for ACTH, respectively
ANOVA group significance level
Discussion
This study is the first describing the cardiovascular effects of CO2 euthanasia on mice. Although all CO2 CRR conditions were associated with significant changes in various parameters from their baseline values, most parameters did not differ significantly between different CO2 CRR. Mice exposed to 50% or 100% CO2 CRR had significantly greater increases in the average systolic BP prior to ataxia than did mice exposed to 15% CO2 CRR. This result may reflect the prolonged time until the initial response in the 15% CO2 CRR group. However, for several parameters, the values were lower in the mice exposed to the faster CO2 CRR. These decreases might be attributed to the associated shorter time until death.
Further interpretation of the results of this study is complex. Identifying pain or distress and the level of each in mice during euthanasia is a critical component. Objective measures that may indicate pain include altered activity, changes in food and water consumption, protection or guarding of injured areas, and so forth. However, most of these signs are used predominantly for identifying chronic pain and have not been validated for assessing pain associated with euthanasia. Pain and distress cause increased levels of circulating catecholamines, with subsequent increases in heart rate and blood pressure.22,23,25,33,36 However, there are mixed reports on the efficacy of using heart rate as an indicator of pain intensity.26,40 In one study, men but not women had a strong correlation coefficient between pain intensity or unpleasantness and heart rate.40 Other recent studies have examined the use of heart rate variability as a marker of pain.26 A recent review concluded that there are currently no validated objective markers of nociception or pain recommended for clinical use in humans, although the authors noted that the analysis of heart rate variability could potentially be developed for this purpose.11
Physiological events not reflective of pain or distress also might affect cardiovascular parameters. Indeed, decades of research data have characterized the normal physiologicalresponses of mammals to elevated CO2 and hypoxia.13,21,34 High CO2 and accompanying hypercapnia and hypoxia have profound effects on respiration rates and cardiovascular parameters that are mediated by peripheral17 and central31 chemoreceptors. Low oxygen concentration in the chamber occurred much later than did the increased CO2 (data not shown). Therefore, the changes in cardiovascular parameters we observed in the current study likely arose due to a combination of pain or distress and the normal physiological response to elevated environmental CO2 and consequential hypercapnia. Because most of the cardiovascular parameters did not differ between the different CO2 CRR, one conclusion is that there is no significant difference in pain or distress among conditions. However, with these multiple confounding factors and given the lack of clarity of whether levels of pain or distress correlate with the increased cardiovascular values, further research into this area is warranted.
The heart-rate curves during euthanasia showed a biphasic peak (Figure 3 A). We speculate that the first heart rate peak increase is likely due to fear, dyspnea, and perhaps the stimulation of peripheral chemoreceptors subsequent to hypercapnia and associated blood pH alterations. The second peak we attribute directly to hypercapnia, hypoxia, and blood acidity due to increased CO2 levels, because the mice are likely unconscious during this time. This biphasic peak in our mice contrasts with a study in Sprague–Dawley rats, which showed a single peak followed by a rapid decline in the pulse rate during CO2 euthanasia.39 This disparity may be due to species-specific differences or differences in methodology. In the referenced study,39 rats were transferred to the euthanasia chamber immediately prior to euthanasia—a practice that might have affected cardiovascular parameters. In our study, we acclimated the mice to the euthanasia chamber for 30 min, thus reducing the effect of this potential confounder.
Similar to cardiovascular increases, levels of ACTH and corticosterone rise when animals are in distress or pain.12 However, unlike cardiovascular responses, there is evidence of graduated elevations in these hormones to different levels of pain and distress.19,24 Physiological levels of ACTH can increase 10 to 50 fold, depending on the nature of the stressor.2,4,9 This variable response does not translate to corticosteroid levels, given that only marginal increases in ACTH are required to reach peak corticosteroid secretory rates.24 Saturation of the corticosteroid response occurs at about 300 pg/mL of ACTH. At higher ACTH levels, the duration of the adrenocortical response increases whereas peak corticosteroid levels do not.24 Measurement of corticosterone is sufficiently sensitive for quantifying the effects of mild stressors, as shown by exposing mice to increasingly novel situations.19 In contrast, for highly stressful or painful situations, rats exposed to electrical shocks did not have a variable response,16 therefore, once a threshold level of ACTH is achieved, corticosterone levels likely plateau. In fact, corticosterone levels in the current study did not differ significantly among mice in the different groups, nor were they significantly different from normal levels observed in B6D2F1 mice.7 This is not surprising given that previous rodent studies indicate that approximately 4 min is required for corticosterone levels to increase in response to a stressful event.10,15,37 In the present study, death occurred in less than 237 s in all but one mouse (247 s), and blood was collected immediately after death. The results from our study also parallel those of a previous study41 in which corticosterone levels did not differ between mice euthanized with CO2 at 20% and 100%.
In light of the delayed response of corticosterone, we believe that ACTH may be a better indicator of differences in stress in these studies. Because ACTH levels increase within seconds of a painful or distressful insult,38 ACTH might be elevated in association with rapid euthanasia procedures. In fact, another study reported an elevation in ACTH in all CO2 CRR groups compared with unstressed mice.7 However, no significant differences between the groups were present, suggesting that the intensity of distress or pain did not vary with different CO2 CRR. Alternatively, this result might indicate that ACTH levels are an ineffective method of quantifying pain or distress in mice.
The current study revealed significant increases in the frequency of lung perivascular edema, hemorrhage, and congestion resulting from euthanasia with CO2. We found no evidence of significant differences in histologic parameters due to CO2 CRR. This finding contrasts with a previous study, which demonstrated increased histologic damage in rats exposed to lower CO2 CRR (comparisons done between 60% to 100%).14 The difference in results might be explained by inadequate sample size in the previous study (no statistical analysis, n = 2 per group), different species, and evaluation of high CO2 CRR only (we evaluated 15% to 100% CRR). In the current study, we found no variations in pathologic lung lesions in mice euthanized with different CO2 CRR. Although this finding indicates that lung histology is not useful when making CO2 CRR recommendations, the lack of difference supports the use of both high and low CRR, and recommendations should be based on other physiological and behavioral evidence of pain or distress.
Our study found that all mice showed investigative and avoidance responses, including sniffing and movement out of the nest, to the different CO2 CRR. Several articles conclude that high CO2 CRR is more painful or distressful than low CO2 CRR, on the basis of aversion or avoidance to CO2 concentrations exceeding 15% (mice8,18,27 and rats8,14,18,27,32). The aversion studies show that all CO2 CRR tested are aversive (that is, animals will always leave the chamber). Moreover, the CO2 concentration at which rats and mice leave (about 12% to 15%) is similar regardless of CRR. These results provide no basis for concluding that higher or lower CRR are more humane. Although mice euthanized with 50% or 100% CO2 CRR sniffed earlier and moved earlier, all mice in the 15% and 30% CO2 CRR groups also exhibited these behaviors in our study. The performance of a behavior at an earlier time does not equate to a higher level of pain, and this finding does not substantiate that there was more pain associated with the 50% and 100% CO2 CRR than with the lower CRR. Significant differences in time to exhibit other behaviors occurred as well with differing CRR. Times to ataxia, nose down, and death were significantly different among groups, with higher CO2 CRR resulting in shorter latency time for each behavior. The unequivocal time point of death occurred between 69 and 204 s after beginning CO2 exposure, depending on the CRR. However, we believe that when a mouse loses sentience would be a more meaningful time point to examine, in that beyond this time, the capacity to suffer is lost.29 According to our cardiovascular data, we believe that loss of awareness or consciousness occurs soon after the mouse becomes ataxic, that is, 25 to 79 s after the beginning of CO2 exposure. When the time period prior to sniffing is eliminated, the time until first sign of investigative behavior—and the time frame of potential suffering—is reduced to 20 to 56 s. During euthanasia, only one mouse reared, and none of the mice shook its head or wiped its face. These results parallel those in BALB/c mice euthanized with CO2.27 Interestingly in the cited study, mice showed less rearing in response to moderate and high CO2 CRR compared with air but did not have a similar decrease with slow CO2 CRR and exhibited increased wiping of the face with slow, moderate, and high CO2 CRR.27 These findings highlight a potential difference between mice and rats in regard to CO2 sensitivity and require further experimentation to determine the cause of the differing behavioral responses.
Clearly CO2 exposure can cause pain in rodents; for example, previous studies have found that nociceptors in rats are stimulated at CO2 levels above 37%.30,35 We further analyzed our data to determine the chamber CO2 concentration at the onset of recumbency (nose down) in mice, which occurs after the loss of ataxia (potentially the same time point as for loss of consciousness). On average, mice in all 4 CO2 experimental groups began to be recumbent when the CO2 concentration in the chamber had reached 30% to 35% (Figure 1). If consciousness is lost at the onset of recumbency or earlier, then mice may not experience pain associated with any of the CO2 CRR euthanasia scenarios we evaluated. Therefore, it may be inaccurate to conclude that higher CO2 CRR cause more pain than do lower CRR if the mouse has lost sentience before painful levels of CO2 are reached. The time at which loss of consciousness occurs should be defined before additional conclusions regarding pain perception at high CO2 CRR are made.
Our study has several limitations and strengths. We believe it is critical to maximize the likelihood that the responses by the mice were due only to the CO2 CRR and not to transport, handling, or exposure to novel situations—all of which are distressful. Therefore, we euthanized the mice in their home cage after allowing at least 30 min for acclimation, and remaining silent throughout the procedure. Because of these experimental features, we feel confident that the results we obtained are truly indicative of the CO2 euthanasia experience but might differ from those of mice that are moved, cohoused with other mice, or experience another distressful event during the euthanasia process.
Even though we took steps to avoid extraneous effects not due to CO2, it is clear that just turning a gas on itself was responsible for some of the changes in the values, given that air from a compressed cylinder led to altered behavioral and cardiovascular values. We were unable to determine whether this effect was due to the noise or the change in air flow associated with gas input. In addition, this study assessed only a single sex, age, and strain of mice. Other strains of mice may respond differently to CO2 euthanasia, given that strain-specific effects occur with low-level exposures to hypoxia (10% oxygen) and hypercapnia (5% to 10% CO2).1,6 Furthermore, studies have shown sex-specific effects regarding the link between heart rate and pain, suggesting that analysis of sex is important.40 Another limitation is that, using our measurement techniques, we were unable to distinguish between pain, fear, and dyspnea. Finally, we were unable to definitively identify the time during the euthanasia procedure that mice became unconscious. Given the decreases in the BP and heart rate curves, we suspect that unconsciousness occurred soon after ataxia developed. Future analyses of mouse euthanasia can use this clinical sign as a likely point at which mice may be losing awareness.
Euthanasia by inhalation of CO2 gas caused changes in heart rate, blood pressure, lung histology, and behavior in all groups of mice. Some changes may be indicative of pain or distress, whereas others may simply be caused by hypoxic–hypercapnic physiology. Few differences among groups emerged, with the notable exception of the time necessary for certain behaviors to occur. We believe that the time frame for potential suffering is brief and directly related to the CO2 CRR. Higher CRR shorten periods of potential suffering, but we were unable to determine whether pain was associated with any of the CO2 CRR. Another consideration are studies in humans, which show a rapid diminution of pain (although not the increased distress) associated with CO2 inhalation.42 Response times in the detection of CO2 range between 0.7 to 1.9 s,5,42 peak at 3 to 4 s, and rapidly diminish within 5 to 7 s.42 This response is much quicker compared with the natural buffering from other agents.42 If rapid diminishment of pain also occurs in mice, then this effect would further invalidate the concept of increased pain with faster CRR. However, air hunger is a significant stressor and cause of fear, such that prolonged exposure to high CO2 and the associated dyspnea become significant problems. The amount of time to loss of sentience therefore would be the major parameter affected with different CO2 CRR and should be considered when making decisions regarding CRR.
Given the absence of significant effects on most of the parameters examined, we contend that there is no clear physiological, behavioral, or histologic evidence of improved welfare when using currently recommended (15% or 30%) CO2 CRR for the euthanasia of adult male C57BL/6 mice rather than higher CRR. In fact, because of the longer time until loss of sentience and death, and prolonged period of dyspnea30 with slow CO2 CRR, a faster (50% to 100%) CO2 chamber replacement rate may be more humane. The evidence of dyspnea and distress with all CO2 CRR make it critical to also compare current results with other AVMA-approved euthanasia techniques.
Acknowledgments
The project described was supported by the Grants for Laboratory Animal Science (GLAS) from the American Association for Laboratory Animal Science. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- 1.Adachi T, Ogawa H, Okabe S, Kitamuro T, Kikuchi Y, Shibahara S, Shirato K, Hida W. 2006. Mice with blunted hypoxic ventilator response are susceptible to respiratory disturbance during hypoxia. Tohoku J Exp Med 209:125–134. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi AM, Steenbergen HL, van de Poll NE, Farabollini F. 1994. Sex-dependent effects of restraint on nociception and pituitary-adrenal hormones in the rat. Physiol Behav 55:789–793. [DOI] [PubMed] [Google Scholar]
- 3.Anton F, Euchner I, Handwerker HO. 1992. Psychophysical examination of pain induced by defined CO2 pulses applied to the nasal mucosa. Pain 49:53–60. [DOI] [PubMed] [Google Scholar]
- 4.Axelrod J, Reisine TD. 1984. Stress hormones: their interaction and regulation. Science 224:452–459. [DOI] [PubMed] [Google Scholar]
- 5.Cain WS, Murphy CL. 1980. Interactions between chemoreceptive modalities of odour and irritation. Nature 284:255–257. [DOI] [PubMed] [Google Scholar]
- 6.Campen MJ, Tagaito Y, Jenkins TP, Balbir A, O'Donnell CP. 2005. Heart rate variability responses to hypoxic and hypercapnic exposures in different mouse strains. J Appl Physiol(1985) 99:807–813. [DOI] [PubMed] [Google Scholar]
- 7.Chesnokova V, Auernhammer CJ, Melmed S. 1998. Murine leukemia inhibitory factor gene disruption attenuates the hypothalamo-pituitary-adrenal axis stress response. Endocrinology 139:2209–2216. [DOI] [PubMed] [Google Scholar]
- 8.Conlee KM, Stephens ML, Rowan AN, King LA. 2005. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim 39:137–161. [DOI] [PubMed] [Google Scholar]
- 9.Cook DM, Kendall JW, Greer MA, Kramer RM. 1973. The effect of acute or chronic ether stress on plasma activity ACTH concentration in the rat. Endocrinology 93:1019–1024. [DOI] [PubMed] [Google Scholar]
- 10.Coover GD, Heybach JP, Lenz J, Miller JF. 1979. Corticosterone “Basal levels” and response to ether anesthesia in rats on a water deprivation regimen. Physiol Behav 22:653–656. [DOI] [PubMed] [Google Scholar]
- 11.Cowen R, Stasiowska MK, Laycock H, Bantel C. 2015. Assessing pain objectively: the use of physiological markers. Aneasthesia. 70:828–847. [DOI] [PubMed] [Google Scholar]
- 12.Dallman MR, Hellhammer D. 2011. Regulation of the hypothalamo–pituitary–adrenal axis, chronic stress, and energy: the role of brain networks. p 11–36. In: Contrada RJ, Baum A. The handbook of stress science, biology, psychology, and health. New York (NY): Springer Publishing. [Google Scholar]
- 13.Daly Mdeb.1997. Peripheral arterial chemoreceptors and respiratory-cardiovascular integration. Oxford (UK): Clarendon Press. [Google Scholar]
- 14.Danneman PJ, Stein S, Walshaw SO. 1997. Humane and practical implications of using CO2 mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci 47:376–385. [PubMed] [Google Scholar]
- 15.Davidson JM, Jones LE, Levine S. 1968. Feedback regulation of adrenocorticotropin secretion in “basal” and “stress” conditions: acute and chronic effects of intrahypothalamic corticoid implantation. Endocrinology 82:655–663. [DOI] [PubMed] [Google Scholar]
- 16.Friedman SB, Ader R, Grota LJ, Larson T. 1967. Plasma corticosterone response to parameters of electric shock stimulation in the rat. Psychosom Med 29:323–328. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez C, Almaraz L, Obeso A, Rigual R. 1994. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74:829–898. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins P, Playle L, Golledge H, Leach M, Banzett R, Coenen A, Cooper J, Danneman P, Flecknell P, Kirkden R, Niel L, Raj M. 2006. Newcastle consensus meeting on CO2 euthanasia of laboratory animals. Presented at the University of Newcastle upon Tyne, UK, 27–28 February 2006. [Cited 26 May 2016]. Available at: www.nc3rs.org.uk/downloaddoc.asp?id=416&page=292&skin=0. [Google Scholar]
- 19.Hennessy MB, Levine S. 1978. Sensitive pituitary-adrenal responsiveness to varying intensities of psychologic stimulation. Physiol Behav 21:295–297. [DOI] [PubMed] [Google Scholar]
- 20.Hewett TA, Kovacs MS, Artwohl JE, Bennett BT. 1993. A comparison of euthanasia methods in rats, using CO2 in prefilled and fixed flow rate filled chambers. Lab Anim Sci 43:579–582. [PubMed] [Google Scholar]
- 21.Heymans C, Neil E. 1958. Reflexogenic areas of the cardiovascular system. London (UK): Churchill. [DOI] [PubMed] [Google Scholar]
- 22.Janig W. 1985. Systemic and specific autonomic reactions in pain: efferent, afferent and endocrine components. Eur J Anaesthesiol 2:319–346. [PubMed] [Google Scholar]
- 23.Janig W. 1995. The sympathetic nervous system in pain. Eur J Anaesthesiol Suppl 10:53–60. [PubMed] [Google Scholar]
- 24.Keller-Wood ME, Shinsako J, Dallman MF. 1983. Integral as well as proportional adrenal responses to activityH. Am J Physiol 245:R53–R59. [DOI] [PubMed] [Google Scholar]
- 25.Klabunde RE. 2012. Neurohumoral control of the heart and circulation, p 136–137. In: Cardiovascular Physiology Concepts, 2nd edition. Baltimore (MD): Lippincott Williams and Wilkins. [Google Scholar]
- 26.Koenig J, Jarczok MN, Ellis RJ, Hillecke TK, Thayer JF. 2014. Heart rate variability and experimentally induced pain in healthy adults: a systematic review. Eur J Pain 18:301–314. [DOI] [PubMed] [Google Scholar]
- 27.Leach MC, Bowell VA, Allan TF, Morton DB. 2002. Aversion to gaseous euthanasia agents in rats and mice. Comp Med 52:249–257. [PubMed] [Google Scholar]
- 28.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R, [Internet] 2013. AVMA Guidelines for the euthanasia of animals: 2013 edition [Cited 26 May 2016].Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf.
- 29.Mellor DJ, Diesch TJ, Gunn AJ, Bennet L. 2007. Fetal ‘awareness’ and ‘pain’: What precautions should be taken to safeguard fetal welfare during experiments? Proceedings of the 6th world congress on alternatives and animal use in the life sciences. 21–25 August 2007. Alternatives to animal testing and experimentation: AATEX.14:79–83. [Google Scholar]
- 30.Moody CM, Chua B, Weary DM. 2014. The effect of CO2 flow rate on the euthanasia of laboratory mice. Lab Anim 48:298–304. [DOI] [PubMed] [Google Scholar]
- 31.Nattie E, Li A. 2012. Central chemoreceptors: locations and functions. Compr Physiol 2:221–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niel L, Stewart SA, Weary DM. 2008. Effect of flow-rate on aversion to gradual-fill CO2 exposure in rats. Appl Anim Behav Sci 109:77–84. [Google Scholar]
- 33.Nijsen MJ, Ongenae NG, Coulie B, Meulemans AL. 2003. Telemetric animal model to evaluate visceral pain in the freely moving rat. Pain 105:115–123. [DOI] [PubMed] [Google Scholar]
- 34.O'Regan RG, Majcherczyk S. 1982. Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. J Exp Biol 100:23–40. [DOI] [PubMed] [Google Scholar]
- 35.Peppel P, Anton F. 1993. Responses of rat medullary dorsal horn neurons following intranasal noxious chemical stimulation: effects of stimulus intensity, duration, and interstimulus interval. J Neurophysiol 70:2260–2275. [DOI] [PubMed] [Google Scholar]
- 36.Rietmann TR, Stauffacher M, Bernasconi P, Auer JA, Weishaupf MA. 2004. The association between heart rate, heart rate variability, endocrine and behavioral pain measures in horses suffering from laminitis. J Vet Med A Physiol Pathol Clin Med 51:218–225. [DOI] [PubMed] [Google Scholar]
- 37.Riley V, Fitzmaurice MA, Spackman DH. 1981Psychoneuroimmunologic factors in neoplasia: studies in animals. p 31–102. In: Ader R. Psychoneuroimmunology. New York (NY): Academic Press. [Google Scholar]
- 38.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89. [DOI] [PubMed] [Google Scholar]
- 39.Smith W, Harrap SB. 1997. Behavioural and cardiovascular responses of rats to euthanasia CO2 gas. Lab Anim 31:337–346. [DOI] [PubMed] [Google Scholar]
- 40.Tousignant-Laflamme Y, Rainville P, Marchand S. 2005. Establishing a link between heart rate and pain in healthy subjects: a gender effect. J Pain. 6:341 –347. [DOI] [PubMed] [Google Scholar]
- 41.Valentine H, Williams WO, Maurer KJ. 2012. Sedation or inhalant anesthesia before euthanasia with CO2 does not reduce behavioral or physiological signs of pain and stress in mice. J Am Assoc Lab Anim Sci 51:50–57. [PMC free article] [PubMed] [Google Scholar]
- 42.Wise PM, Wysocki CJ, Radil T. 2003. Time-intensity ratings of nasal irritation from CO2. Chem Senses 28:751–760. [DOI] [PubMed] [Google Scholar]