Abstract
Despite the extensive use of doxycycline in tetracycline-inducible rodent models, little is known regarding its stability in feed or water or the most effective route or dose. We assessed the concentrations of doxycycline in reverse-osmosis–purified (RO; pH 6.0) and acidified RO (pH 2.6) water in untinted or green-tinted bottles. Doxycycline remained stable in all groups for 7 d and in acidified water in untinted bottles for 14 d. Fungal growth occurred in nonacidified water in tinted and untinted bottles by 12 and 14 d, respectively, and in tinted bottles containing acidified water on day 14, but not in untinted bottles with acidified water. Doxycycline concentrations were also assessed before and at various points after the pelleting of feed from 2 vendors. Each batch was divided for storage at 4 °C, at room temperature, or within ventilated mouse isolator cages and then sampled monthly for 6 mo. Drying caused the greatest decline in doxycycline concentration, whereas γ-irradiation plus shipping and storage condition had minimal effect. Two mouse lines with tetracycline-inducible promoters received 25, 150, or 467 µg/mL or 2 mg/mL doxycycline in water and 200 or 625 ppm in feed before analysis of GFP expression. GFP was expressed in Rosa-rtTA2 mice at 150 µg/mL, whereas Cags-rtTA3 mice required 25 µg/mL. These studies indicate that 1) doxycycline-compounded feed can be handled in the same manner as standard rodent feed, 2) tinted water bottles are not necessary for maintaining drug concentrations, and 3) concentrations lower than those used typically may be effective in lines with tetracycline-inducible promoters.
Abbreviations: RO, reverse osmosis; LC–MS/MS, liquid chromatography–tandem mass spectrometry; MFI, mean fluorescence intensity
Tetracycline (tet)-dependent regulatory systems have been commonly used in genetically modified mice since the mid1990s to study the function of genes that could not be studied by gene inactivation or transgene expression.18 These systems have been extensively reviewed.24,39,40 Doxycycline is preferable to tetracycline as an inducer in these systems due to doxycycline's high potency, superior tissue penetration, and its widespread availability.23,3 It is commonly administered in feed or drinking water.39 Despite the widespread use of doxycycline-inducible constructs, no evidence-based dose of doxycycline for most of the conditional mutant mouse models currently used nor standards for its storage and use have been published.
Some sources report that the most commonly used doxycycline concentration in feed is 200 ppm, on a dry-matter basis.39,40 However, based on our experience and the concentrations referenced in the literature, 625 ppm is used at least as frequently, if not more often.31,37,41 High doses are administered to ensure maximal induction. Although high doses may be justified for distribution into tissues with poor penetration (for example, brain)26,31, doxycycline can readily penetrate and reach adequate concentrations in most tissues.1,38,47 For example, doxycycline began to inhibit cell proliferation and colony formation in in 2 mammalian cell lines at 0.2 and 20 µg/mL.17
Doxycycline has biologic effects that extend beyond its antimicrobial activity.15,33,46 It is plausible that some of the concentrations of doxycycline commonly used, combined with its duration of administration, may have unanticipated biologic effects in vivo. Given at a dose of approximately 15 mg/kg daily for 21 d, doxycycline may inhibit tumor cell proliferation,15 and doses as low as 50 mg/kg daily (a lower dose than that achieved by using 625 ppm in feed or 2 mg/mL in drinking water) can reduce the size of abdominal aneurysms in mice.33 These findings were attributed to doxycycline's ability to inhibit matrix metalloproteinases.46
The dose–response curves published for a few specific tetracycline transactivator (tTA, ‘tet-off’) mutants indicated the minimal effective dose for those constructs was considerably lower (20 to 25 µg/mL in drinking water) than those currently used.10,22 To test the hypothesis that doxycycline doses lower than the high doses currently used (2 mg/mL in drinking water) can effectively trigger tet-inducible systems, we used 2 different conditional mouse mutants, each having a different promoter and transactivator (R26-rtTA /TG-Ren.713 and R26-CAGS-rtTA3/TG-Ren.713), to determine the minimal doxycycline dose needed for gene activation. Both mutant lines incorporated a reverse tetracycline transactivator (rtTA, ‘tet-on’) system, which are more common than are tet-on systems and are far less sensitive to doxycycline induction.3,10,14,17,22,40
In addition to differences in the dose administered, the manner in which doxycycline is handled with regard to storage and replacement varies greatly. Although various sources suggest replacing doxycycline-containing water every 3 d,10,19,22 several researchers have reported changing the water every 48 h,8 and others replace water weekly.29 By extension, it is often recommended to replace doxycycline-medicated feed at least weekly.8 Researchers often use this requirement to justify keeping water and feed hopper levels low to minimize waste, necessitating increased vigilance by animal care and investigative staff to ensure that animals always have access to sufficient food and water. Evidence-based recommendations are needed to minimize the amounts of food and water wasted during these experiments.
Tetracyclines absorb UVA light and are universally considered light-sensitive and unstable in water.11,46 These characteristics result in the need to place doxycycline solutions in light protected (tinted bottles) and to replace the water every few days.3,39 One study found that doxycycline remained stable in 37 °C water in tinted bottles for as long as 1 wk.20 Acidification of water has been reported to greatly improve doxycycline's stability.27 In light of these findings, we sought to examine the stability of doxycycline in reverse-osmosis–purified (RO) water that is acidified or nonacidified at room temperature (23 °C; the temperature at which water is commonly dispensed to mice) over a 14-d period. We selected this time frame in light of the recent trend toward replacing water bottles every 2 wk at several institutions, including ours.
Doxycycline has been shown to be widely distributed in tissues after oral administration in various species,1,2,38 and there are some data regarding plasma concentrations of doxycycline after administration to mice in water,27 but there are no reported studies on plasma concentrations when the drug is provided to mice in feed. Because the concentrations of doxycycline in commercially available feed were not developed to achieve the doses commonly administered in water, whether the 2 methods of administration are comparable is unknown. Mice, in general, consume an average of 4.5 g of feed and 6 mL of water daily.4 Using this information, we attempted to provide doxycycline in water in a way that yielded the same dose as 200- and 625-ppm doses in feed and subsequently measured the plasma concentration of doxycycline achieved in mice.
Materials and Methods
Animals.
R26-rtTA2/TG-Ren.713 (Rosa) and R26-CAGs-rtTA3/TG-Ren.713 (Cags) homozygous breeding pairs were generously donated (Lowe lab, Memorial Sloan Kettering Cancer Center). Offspring from each strain were backcrossed to C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) to generate heterozygotes for the experiments described. Genotypes were confirmed by PCR analysis.13 Mice were free of mouse hepatitis virus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse parvovirus, minute virus of mice, pneumonia virus of mice, reovirus type 3, Sendai virus, Theiler mouse encephalomyelitis virus, mouse adenovirus, K virus, polyoma virus, mouse cytomegalovirus, mouse thymic virus, Haantan virus, lactic dehydrogenase elevating virus, cilia-associated respiratory bacillus, Mycoplasma pulmonis, ectoparasites, and endoparasites. All mice were housed in polysulfone IVC (Thoren Caging Systems, Hazelton, PA) on autoclaved hardwood chip bedding (Sani-Chips, PJ Murphy Forest Products, Montville, NJ); γ-irradiated feed (LabDiet 5053, PMI, St Louis, MO) and RO water acidified with HCl to a pH of 2.5 to 2.8 were provided without restriction when mice were not on study. Mice were weighed at 8 to 10 wk of age (that is, when they were placed on study), on the day that doxycycline administration began, and at 6 and 13 d afterward.
Animal use was approved by the IACUC of Memorial Sloan Kettering Cancer Center, where the animal care and use program is AAALAC-accredited, and all animals are maintained and used in accordance with the recommendations provided in the Guide.21
Blood collection.
Blood was collected at the same time each day. All blood samples were collected from mice in restrainers by venipuncture of the lateral tail vein after warming under a heat lamp for 2 to 5 min. A maximum volume of 100 μL of blood was collected into a conical tube containing EDTA (Microvette 100, Kent Scientific, Torrington, CT).
Doxycycline-containing feed and water.
Commercially available, γ-irradiated rodent feed containing doxycycline, purchased from 2 manufacturers (Harlan Teklad, Indianapolis, IN, and PMI), was received within 30 d of milling. Feed containing 625 ppm doxycycline (catalog no. TD.01306, TestDiet 5053 containing 625 ppm doxycycline, PMI) were used to evaluate doxycycline stability after milling, irradiation, shipping, and storage. Feed containing 625 ppm (TD.01306) and 200 ppm (TD.00502, Harlan Teklad) doxycycline were each used to evaluate gene expression and plasma concentration in 2 genetically engineered inducible mouse strains.
For assessing the stability of doxycycline in water, we prepared 500-mL solutions containing 2 mg/mL doxycycline (D9891, Sigma Aldrich, St Louis, MO) and 5% sucrose (S9378, Sigma Aldrich) by using either RO water (pH 6.0) or HCl-acidified RO water (pH 2.7), placed 450 mL in either green-tinted or untinted, 473-mL, polysulfone bottles (Thoren Caging Systems), and analyzed as described. For studies evaluating effective doses, varying concentrations of doxycycline and sucrose were added to acidified RO water to achieve doxycycline:sucrose concentrations of 2 mg/mL:5%, 467 µg/mL:2%, 150 µg/mL:1%, and 25 µg/mL:0.23%.
Determination of doxycycline concentration.
Feed.
Pellets were ground individually in a coffee grinder (Fresh Grind, Hamilton Beach, Southern Pines, NC). Approximately 25 mg of ground pellet was placed in a 15-mL tube; 0.5 mL of a mixture of 75% water + 25% acetonitrile + 0.1% formic acid was added, and the mixture was vortexed for 5 min and then sonicated for an additional 40 min. The tube was centrifuged for 5 min at 180 x g the supernatant was transferred into a new 15-mL tube, and a portion was filtered (0.45 µm; Sun SRI, Rockwood, TN) into another tube. A 50-µL aliquot was transferred into the well of a 96-well plate for analysis by HPLC–tandem mass spectrometry (LC-MS/MS).
Plasma.
Plasma (50 µL) was added to a 1.5-mL microcentrifuge tube (Eppendorf Safe-Lock, USA Scientific, Ocala, FL), followed by 250 µL of acetonitrile. The sample was vortexed for approximately 45 s, centrifuged for 5 min at 19,445 x g, and the supernatant transferred into another microcentrifuge tube. The sample was evaporated on a centrifugal evaporator (GeneVac, Stone Ridge, NJ), and the residue was dissolved in 100 µL of solvent (75% H2O + 25% acetonitrile + 0.1% formic acid). The solution was vortexed for approximately 15 s and centrifuged for 5 min at 19,445 x g; 50 µL of the supernatant was transferred into the well of a 96-well plate for analysis by LC-MS/MS.
Water.
Each water sample (50 µL) was added to 950 µL of a mixture of 75% water + 25% acetonitrile + 0.1% formic acid (diluent) in a microfuge tube and vortexed for 10 s; 20 µL of this mixture was added to 980 µL of diluent and vortexed for 10 seconds; 50 µL of the final mixture was transferred into the well of a 96-well plate for analysis by LC-MS/MS.
Doxycycline analysis.
The concentrations of doxycycline in feed, water, and plasma were determined by using LC-MS/MS (model 6410 LC-MS/MS system, Agilent Technologies, Santa Clara, CA). Analysis was based on doxycycline (rather than its hyclate salt; 1 mg of doxycycline is equivalent to 1.154 mg of doxycycline hyclate). Analysis was performed in the multiple-reaction monitoring mode using positive-ion electrospray ionization. The HPLC separation was conducted on a 4.6 mm × 50 mm, 5 µm column (Zorbax Eclipse XDB-C18 column, Agilent Technologies), and the analyte was eluted under isocratic conditions (75% H2O+0.1% HCOOH:25% CH3CN) for 5 min at a flow rate of 0.5 mL/min. Doxycycline hyclate standards were used to generate a standard curve with a detection limit of 50 pg/mL for all types of samples collected.
Flow cytometry.
GFP expression in peripheral blood T cells was determined using flow cytometry. Fresh whole-blood samples were depleted of RBC by incubating with 1 mL of ammonium–chloride–potassium (ACK) lysis buffer (Lonza, Walkersville, MD) at room temperature for 5 min. Samples were then centrifuged for 4 min at 667 x g and the supernatant discarded. Another 1 mL of lysis buffer was added and the sample immediately centrifuged for 4 min at 667 x g . The supernatant was again discarded. The remaining cells were resuspended in 150 μL PBS containing 1% BSA (PBS-BSA) and 0.3 μL Fc block (BioLegend, San Diego, CA). Cell suspensions were plated in 50-μL aliquots, with a replicate aliquot plated and used as an unstained control. Samples were stained by using 50 μL of a freshly prepared mix of antimouse CD45 and Thy1 antibodies (1:200 each; BioLegend) in PBS-BSA, yielding a final antibody concentration of 1:400 per test well. Samples were incubated on ice for 60 to 90 min in the dark, 100 μL PBS-BSA was then added to each well, and the plate centrifuged for 4 min at 166 x g at 4 °C. The supernatant was discarded, and 2 more wash cycles of 180 μL PBS-BSA and centrifugation were performed. Cells were suspended in 180 μL of PBS-BSA and analyzed by flow cytometry (Guava Easycyte 8HT, EMD Millipore, Billerica, MA). Data were analyzed by using FlowJo software (Tree Star, Ashland, OR). GFP-negative mouse samples were used to set the negative control gate; positive cells had a fluorescence intensity greater than the 99th percentile of the negative control cells. The mean fluorescence intensity (MFI) was used to compare groups.
Experimental plan.
Stability of doxycycline in water.
To evaluate the extent to which room lighting conditions (750 ± 110 lx during lights on; 12:12-h light:dark cycle) affect doxycycline in water bottles within mouse cages, samples were collected from green-tinted polysulfone bottles and standard, untinted polysulfone bottles (Thoren Caging Systems). On day 0, doxycycline-containing water was prepared by using either RO or acidified RO water, as described. Each solution was divided among 3 tinted and 3 untinted bottles. A 1-mL sample was taken from each bottle before its placement into a cage. Thereafter, a 1-mL water sample was collected from each bottle 7 and 14 d after placement or earlier (if the water became unfit for consumption; for example, presence of mold or precipitates). Water samples were stored at –80 °C until analyzed.
Stability of doxycycline in feed during milling and after storage.
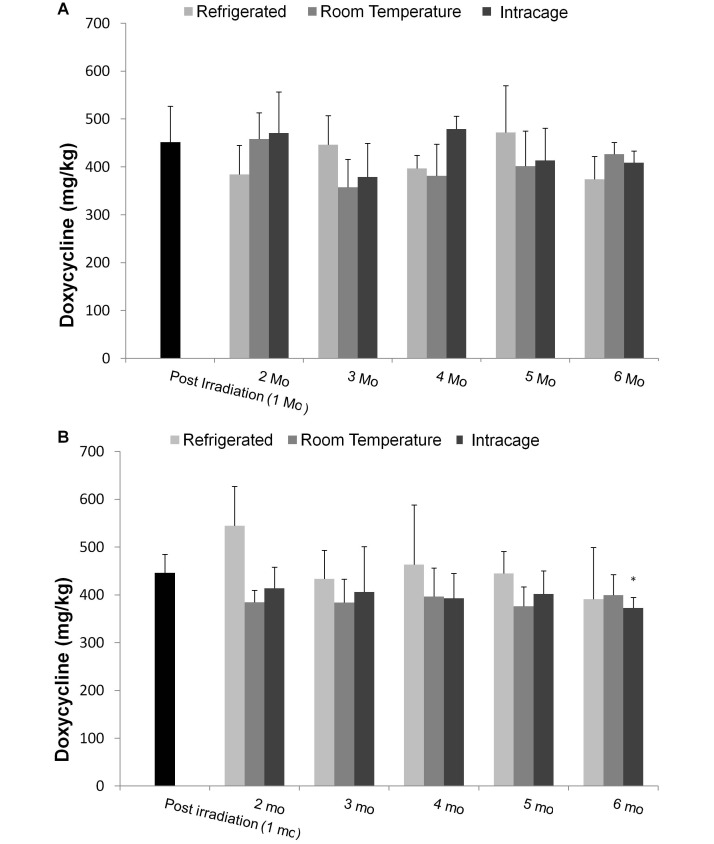
The doxycycline concentrations in 2 commercially available (vendor A: Harlan Teklad, Madison, WI; vendor B: Purina Mills International, Richmond, IN) high-dose doxycycline diets (625 ppm; 5 pellets or equivalent volume per sample) collected during 5 stages of production (meal, wet pellet, dried pellet, and before and after γ-irradiation) and monthly until 6 mo after milling were analyzed by LC-MS/MS. Postirradiation samples were collected on receipt of the diet (approximately 1 mo after milling), at which time each batch of feed was divided and stored at 4 °C (refrigerated), 22 °C (room temperature), or within animal cages. The first monthly storage samples were evaluated beginning 2 mo after milling. For intracage storage, feed was placed into mouse cages within small, ventilated aluminum containers that fit within the food hopper (Figure 1) but were not accessible to the mice. Cages contained 4 or 5 mice of both strains and sexes that were 7 to 10 wk of age at the beginning of the study. To demonstrate that the doxycycline was biologically active at the last time point, feed collected for the last intracage doxycycline concentration (6 mo after milling date and stored inside a cage with animals for 5 mo) analysis was fed to 10 (5 male and 5 female) naïve Rosa mice. Blood was collected just prior to the provision of doxycycline diet and again after receiving the doxycycline diet for 13 d. Samples were analyzed for GFP expression by flow cytometry as described. Once collected, all feed samples were stored at –80 °C until analyzed. During the production phases, feed samples were collected by each manufacturer and shipped immediately. The samples were then stored at –80 °C upon receipt.
Figure 1.
To ensure that the same group of pellets was sampled each month, aluminum containers containing feed were placed in feed hoppers, without restricting mice's access to feed for consumption.
Doses of doxycycline.
The effective doses of doxycycline in both feed and water were determined by measuring GFP expression by flow cytometry. To determine efficiency of feed compared with water as vehicles, doses in water that were comparable to those commercially available in feed (200 and 625 ppm) were calculated based on published consumption averages for 10-wk-old C57BL/6J mice (4.5 g feed and 6 mL water per mouse daily);3 the calculated comparable low dose in water was 150 µg/mL, and the high dose was 467 µg/mL. Two additional doses in water were chosen to assess doses above and below those readily available in feed: the ultralow dose (25 µg/mL) was chosen based on doses published for tTA systems,10,40 and the ultrahigh dose (2 mg/mL) is the dose cited most often in the literature.39,40
Doxycycline dose required to stimulate GFP expression.
Ten mice (5 male and 5 female) from each of 2 conditionally mutant strains were placed into 1 of 6 doxycycline treatment groups: 25, 150, or 467 µg/mL or 2 mg/mL in water or 200 or 625 ppm in feed. When mice were 8 to 10 wk of age, a baseline blood sample was analyzed by flow cytometry for GFP expression (day –6). Doxycycline was administered in either food or water beginning on day 0 and ending on day 13. Blood for immediate analysis was collected on days 2, 6, and 13. Feed and water were replaced every 7 d.
Plasma concentration of doxycycline.
To determine the plasma concentrations achieved with various doses of doxycycline, groups of 10 mice (equal representation of Rosa and Cags) were assigned to receive 25, 150, or 467 µg/mL or 2 mg/mL in water or 200 or 625 ppm in feed. Blood was sampled as described for GFP expression analysis and then immediately centrifuged to separate plasma. Plasma samples were stored at –80 °C until analysis. Feed and water were replaced every 7 d.
Statistical analysis.
Analysis was performed by using computational software (SAS version 9, SAS Institute, Cary, NC). All analyzed variables (6 mo time point under all storage conditions compared with baseline, day 7, and final water samples in untinted compared with tinted bottles for each water type; plasma concentrations achieved in different dosage groups; and MFI for each dosage group compared with negative controls and baseline values) were continuous, and differences between groups were evaluated by using the Wilcoxon rank sum test or Kruskall–Wallis test. P values less than or equal to 0.05 were considered significant.
Results
Animal weights.
All animals gained weight during the 2 wk on study, with male mice weighing approximately 5 g more than female mice by the end of the study (data not shown). Mice that received 2 mg/mL doxycycline gained the least amount of weight by the end of the experiment (mean ± 1 SD, 0.9 ± 0.7 g), and those in the 150-μg/mL group gained the most weight (1.5 ± 0.7 g).
Stability of doxycycline in water.
The doxycycline concentrations measured in water are summarized in Table 1. Concentrations in untinted bottles with acidified and nonacidified water at day 7 were the same as those measured on day 0 and then declined to 94% and 88%, respectively, of the initial concentration by day 14. Tinted bottles with acidified and nonacidified water had average day-14 doxycycline concentrations that were 98% and 95%, respectively, of that measured on day 0; these declined further to 85% and 84%, respectively, on the last day of collection. Due to fungal contamination, tinted bottles with nonacidified water were evaluated on day 12 rather than 14. This same contamination was seen, although to a much lesser extent, in tinted bottles containing acidified water and in untinted bottles with nonacidified water. No contamination was grossly visible in the untinted bottles with acidified water. Percentage change in doxycycline concentration on day 7 did not differ between tinted and untinted bottles; day 14 values were not compared, due to fungal growth. Comparing the day 0 doxycycline concentrations with the final concentrations in each group revealed that doxycycline concentrations only remained stable for 14 d when in acidified water in untinted bottles.
Table 1.
Quantities of doxycycline under various conditions
| Day | Concentration (mg/mL; mean ± 1 SD) | % Recovered | |
| Untinted bottle, acidified water | |||
| 0 | 1.5 ± 0.1 | 100 | |
| 7 | 1.5 ± 0.1 | 100 | |
| 14 | 1.4 ± 0.2 | 93.8b | |
| Untinted bottle, nonacidified water | |||
| 0 | 1.5 ± 0.0 | 100 | |
| 7 | 1.5 ± 0.0 | 100 | |
| 14 | 1.3 ± 0.2 | 88 | |
| Tinted bottle, acidified water | |||
| 0 | 1.6 ± 0.1 | 100 | |
| 7 | 1.6 ± 0.1 | 98 | |
| 14 | 1.4 ± 0. 2 | 85 | |
| Tinted bottle, nonacidified water | |||
| 0 | 1.7 ± 0.2 | 100 | |
| 7 | 1.6 ± 0.1 | 95 | |
| 12a | 1.4 ± 0.1 | 84 | |
This sample was collected on day 12 due to the appearance of fungal growth.
This group is the only one that demonstrated no significant decrease in doxycycline concentration over 14 d.
Stability of doxycycline in feed.
Pellet drying had the greatest effect on the doxycycline concentration. Measured concentrations in meal averaged 562 ± 130 ppm in feed A and 535 ± 110 ppm in feed B, and dropped to 463 ± 27 ppm and 412 ± 75 ppm, respectively, after drying (Figure 2). Doxycycline concentration did not differ between any of the 3 storage conditions for feed produced by either vendor at any of the time points or between the 6- and 1-mo samples of feed A. When comparing 1- and 6-mo samples of feed B, those stored refrigerated or at room temperature did not differ, but the doxycycline concentrations in the cage-stored samples were significantly (P = 0.02) lower at 6 mo than 1 mo. In addition, the MFI of mice given feed A from the last intracage storage time point was similar to that of mice fed fresh, refrigerated feed.
Figure 2.
The measured concentration of doxycycline in feed from (A) vendor A and (B) vendor B during storage under 3 conditions (refrigerated, room temperature, and intracage). Mean concentrations of doxycycline in feed (n = 5 pellets) were measured by using LC-MS/MS. Bars represent 1 SD. The postirradiation (1 mo) sample served as the baseline for comparison over time. *, P < 0.05 when compared with baseline.
Doxycycline dose needed to stimulate GFP expression.
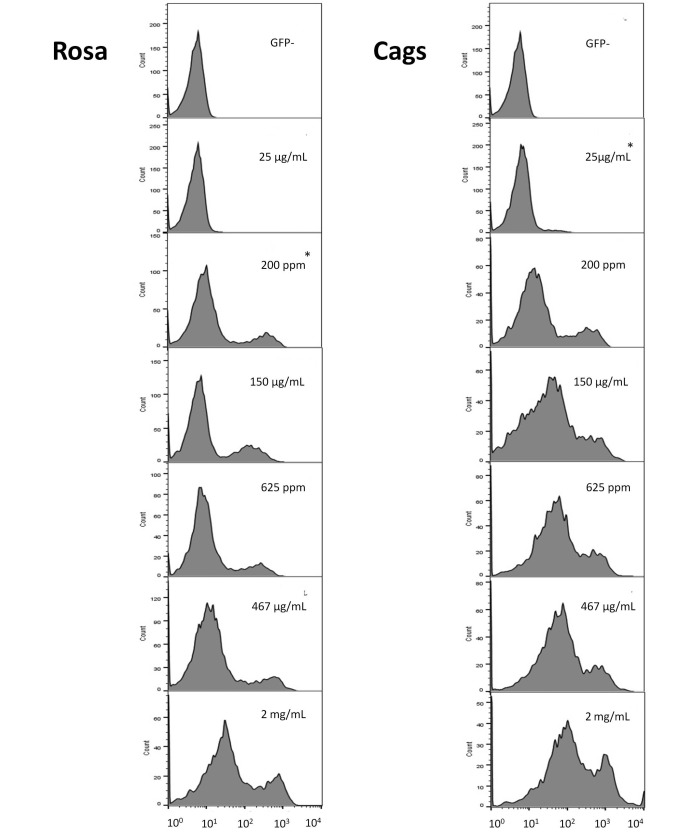
For all groups, T-cell MFI values were similar between days 6 and 13 (Figure 3). All doxycycline doses resulted in greater (P ≤ 0.003) GFP expression at day 13 in Cags mice compared with GFP−/− controls. In the Rosa line, the 25-µg/mL dose did not result in a difference in GFP expression as compared with that of negative controls. GFP expression in the 2-mg/mL group of the Cags line was not significantly different from that in either the 625-ppm or the 467-µg/mL group. Rosa mice displayed only a marginally significant (P = 0.05) difference when comparing the 2-mg/mL group with the 467-µg/mL dosage group, whereas other doses induced a much greater difference (P ≤ 0.008) in the level of expression.
Figure 3.
The number of T cells (y axis) and the respective mean fluorescence intensity (MFI) expressed as log10 (x axis) for the Rosa (left) and Cags (right) promoters. MFI was determined by using flow cytometry and gating on the T-cell population. From top to bottom: GFP-negative population (included to show where the histogram lies in the absence of GFP expression), GFP expression at 25 µg/mL doxycycline, 200 ppm, 150 µg/mL, 625 ppm, 467 µg/mL, and 2 mg/mL. Data of a representative sample from each group on day 13 are shown. As the histogram shifts to the right, GFP expression increases. *, Lowest concentration of doxycycline that yields MFI values higher (P < 0.05) than GFP- negative controls.
In the Cags line, MFI did not differ between mice given 625 ppm and 467 μg/mL doxycycline but did differ (P = 0.03) between the 200-ppm and 150-μg/mL groups. The opposite was observed for the Rosa line: MFI differed (P = 0.03) between mice given feed containing 625 ppm compared with water containing 467 μg/mL.
Plasma concentrations.
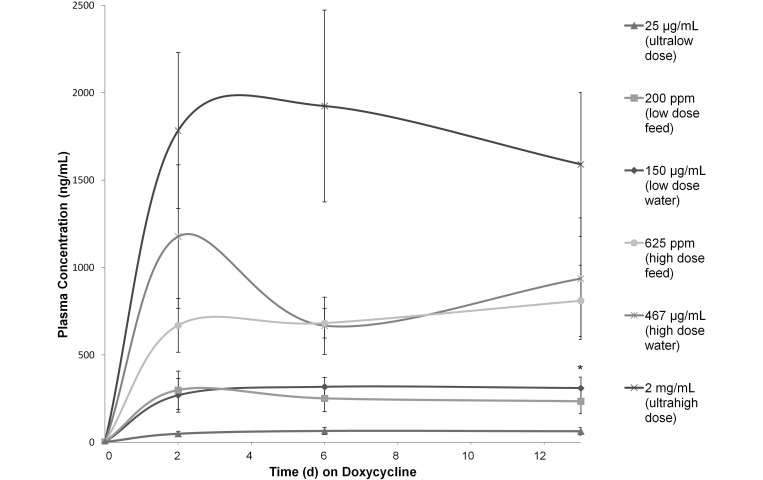
For all groups except the 467-µg/mL and 2-mg/mL groups, plasma concentrations increased rapidly after the initiation of doxycycline treatment (day 0) and then plateaued between days 2 and 6 (Figure 4). The plasma doxycycline concentration in the 467-µg/mL group dropped (albeit nonsignificantly) between days 2 and 6 and in the 2-mg/mL group between days 6 and 13. On day 13, the mean plasma doxycycline concentrations were: 64.3 ± 22 ng/mL with 25 μg/mL in water, 235.1 ± 70.1 ng/mL with 200 ppm in feed, 311.1 ± 63.4 ng/mL with 150 μg/mL in water, 810.7 ± 204.3 ng/mL with 625 ppm in feed, 937.3 ± 347.4 ng/mL with 467 μg/mL in water, and 1591.5 ± 411.4 ng/mL with 2 mg/mL in water.
Figure 4.
Plasma doxycycline concentration (ng/mL; mean ± 1 SD; n = 10 per time point) after administration of doxycycline in feed or water. *, The administration of comparable doses in feed and water yielded a significant (P < 0.05) difference for the low-dose groups only.
When the plasma concentrations achieved with each feed dose were compared with their expected comparable doses in water, there was no significant difference between the high-dose groups, but the low-dose feed group yielded lower (P = 0.01) plasma concentrations than those in the low-dose water group.
Additional findings.
Soon after initiation of the study, mice on the high and ultrahigh doses of doxycycline appeared to consume less feed and water than did other groups. At that point, water intake was measured by using a graduated cylinder, and feed was weighed at each blood collection time point. Only the 2-mg/mL dose group showed a significantly (P = 0.002) lower daily consumption of water than that of controls on regular water. According to the average measured water consumption, actual dose of doxycycline consumed daily by the different water-dosed groups was approximately 113 μg (25-μg/mL group), 660 μg (150 μg/mL), 2 mg (467 μg/mL), and 6.4 mg (2 mg/mL). Animals on 625-ppm feed showed a trend (P = 0.07) toward lower feed consumption, compared with those receiving either untreated or 200-ppm feed.
A small subset of animals (4 female Rosa mice in the 2-mg/mL dose group and 3 female Rosa controls) were submitted for complete necropsy to further investigate the decreased food and water intake. Tissues were evaluated microscopically by a board-certified veterinary pathologist. There were no significant findings in the control animals. In contrast, 3 of 4 the mice from the 2-mg/mL group showed a moderately distended cecum on gross examination. Histologically, these animals also displayed multifocal, moderate, neutrophilic gastritis.
Discussion
Doxycycline has been used to manipulate gene expression for over 20 y, yet recommendations for its handling and storage have not been investigated. This study revealed that protecting doxycycline solutions from light at the levels in mouse cages in a vivarium setting is unnecessary. Our findings did not substantiate the need to replace doxycycline-containing feed weekly and indicate that storing irradiated doxycycline-containing feed at room temperature is adequate. Even though the ultrahigh dose we used here was required to obtain maximal gene expression in Rosa mice, maximal expression is not necessary in all mouse models or constructs. Until recently, Rosa had been the most commonly used ubiquitous promoter, but this frequency is changing with the advent of more potent promoters, such as Cags.9,34,35 Therefore, using a lower dose of doxycycline likely is more appropriate, given that doing so likely would avoid the potential for adverse effects that may be associated with the administration of high doses.
Although tinted bottles has been recommended protect doxycycline solutions from light,39,40 our results show that the light levels present within the animal cage do not significantly alter the stability of doxycycline solutions for at least 7 d; in fact, doxycycline was more stable in untinted bottles. This outcome may be due in part to the finding that the green-tinted bottles seemed to promote the growth of fungi, even in acidified water. Accordingly, weekly doxycycline water changes may be appropriate when acidified RO water is used. Although we did not assess the use of amber-colored bottles, our results show that untinted polysulfone bottles are a viable option for delivering doxycycline solutions. A previous study assessing doxycycline stability in water evaluated only low therapeutic concentrations that did not require the addition of sucrose to enhance palatability.27 In that study, doxycycline concentrations declined over a 7-d period in both acidified and nonacidified tap water, in contrast to what we observed.27 One explanation might be our use of RO water, given that tap water might contain minerals or other substances that could accelerate the degradation of doxycycline.11,44,47
Prior studies showed that, in our hands, there is considerable variability in drug levels between individual feed pellets as a result of inadequate mixing of the diet prior to pelleting28,36. These studies also revealed that this variability did not translate to significant variability in the resulting plasma concentrations of the compounded drug.28,36 We noted increasing variability in the concentration of doxycycline in sera with increasing doxycycline concentrations. Because this trend also occurred in doxycycline-treated water, which is presumed to be a homogenous mixture, this finding likely was not due to variation of doxycycline concentrations in the pellets. A more plausible explanation is variability between individual mice regarding tolerance to the bitter taste of doxycycline. With both delivery methods, as the concentration of doxycycline increases, small differences in consumption would translate to greater differences in the ingested dose.
We underestimated the degree to which doxycycline degrades during feed processing. Therefore the doses administered in feed and the expected comparable doses in water were quite different, at least in the low-dose groups. However, the decreased water consumption in the high-dose water group, although not statistically significant, was sufficient to result in the ingested dose approximating the dose administered in feed. We also found that differences in the plasma concentration of doxycycline did not consistently translate to biologic significance, as evidenced by GFP expression, which may be due to a plateau in effect in most cases.
Our findings support our hypothesis that doxycycline feed can be handled similarly to standard feed, because doxycycline concentration did not differ between feed stored at room temperature or when refrigerated. Furthermore at 6 mo after milling, no difference in doxycycline concentrations were detected between any of the storage conditions. When comparing the 6-mo samples with the postirradiation feed B samples, the intracage pellets had a significantly lower concentration of doxycycline. We suspect that this difference was a function of sampling, because the measured doxycycline varied between feed samples. In addition, the intracage condition represents an extreme that would not be replicated in practice, because fresh feed would be added periodically to replenish that consumed.
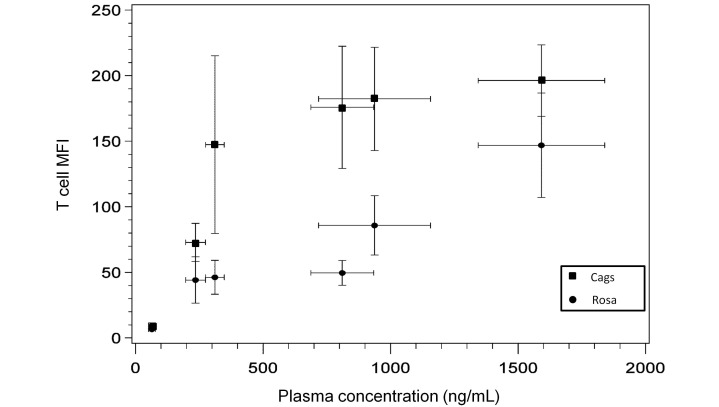
The current study replicates previous findings: the Rosa promoter is far less sensitive to doxycycline than is Cags (Figure 5)9,13,32,34 .In addition, these constructs use different transactivators, and rtTA3 is more sensitive than is rtTA2.12 Because these 2 lines had such different properties, we analyzed them separately, although T cells are expected to have fairly uniform GFP expression under both promoters. To minimize animal numbers, we attempted to isolate T cells from peripheral blood by using survival blood collection. As leukocytes mature and divide, some lose GFP expression.6,42,43,45 Peripheral blood contains a high proportion of terminally differentiated cells, perhaps explaining some of the mosaic effect we noted, as well as the difference in maximal expression we measured as compared with that in other studies evaluating thymic, splenic, or bone marrow cells.6,34
Figure 5.
T-cell mean fluorescence intensity (MFI) in Rosa and Cags mice (n = 10) at various doxycycline plasma concentrations on day 13. Data points represent the mean GFP expression and mean plasma concentration measured for each of the 6 dose groups (from lowest to highest plasma doxycycline concentration achieved at doses of 25 µg/mL, 200 ppm, 150 µg/mL, 625 ppm, 467 µg/mL, and 2 mg/mL) in this study. Bars indicate 95% confidence intervals.
As expected, a doxycycline dose (150 μg/mL) markedly lower than that typically used yielded significant GFP expression in Cags mice and reached maximal expression with 625 ppm in feed. In contrast, Rosa mice only achieved maximal expression on 2 mg/mL doxycycline in water. A dose between 467 μg/mL and 2 mg/mL in water would likely have been sufficient for maximal expression, given that small changes in doxycycline dose can result in marked differences in gene expression.8,39,45 Similarly, a dose between 150 μg/mL in water and 625 ppm in feed may have been sufficient for maximal expression in Cags mice.
No animals appeared clinically dehydrated in this study; however, mice given the 2-mg/mL dose drank less than did other dosage groups, despite the high level of sucrose we added in an attempt to mask the bitter taste of doxycycline. A study that used the same dose administered in water with lower concentrations of sucrose similarly reported dehydration.8 This effect is an important consideration for tetracycline-inducible models that are likely to develop debilitation (for example, cancer models), given that high doxycycline doses can have clinical implications.7
Although doxycycline has a wide safety margin and even though toxicity has not been described in mice, toxicity has been documented in various animal species.5,7,16 The affected organs vary by species, with cardiac toxicity in rats16 and cattle7 and ulcerative gastritis reported in dogs.4 The few mice that were screened for potential side effects in our study prevents comparison of these data. However, higher doses of doxycycline might induce gastrointestinal inflammation, which, had it occurred, might have contributed to the reduced food and water consumption noted anecdotally in the 467-μg/mL doxycycline groups. Further study is required to ascertain whether the 467-μg/mL dose consistently induces gastritis, as this effect might confound data interpretation. Another point of interest that is beyond the scope of the current study is the effect of various doxycycline doses on the microbiome.
Acknowledgments
We acknowledge and thank Lukas Dow for his generosity in providing animals with which to establish our colony as well as his advice and training on performing GFP analysis. We also thank the members of the Laboratory of Comparative Pathology, particularly Desiree Powell, Carmela Bacani, Aziz Toma, Jacqueline Candelier, John Sibley, Antonio Bravo, and Lena Rodriguez, for their immense patience and technical assistance. This study was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265. [DOI] [PubMed] [Google Scholar]
- 2.Anadón A, Martinez-Larrañaga MR, Diaz MJ, Bringas P, Fernandez MC, Fernandez-Cruz ML, Iturbe J, Martinez MA. 1994. Pharmacokinetics of doxycycline in broiler chickens. Avian Pathol 23:79–90. [DOI] [PubMed] [Google Scholar]
- 3.A-Mohammadi S, Alvarez-Vallina L, Ashworth LJ, Hawkins RE. 1997. Delay in resumption of the activity of tetracycline-regulatable promoter following removal of tetracycline analogues. Gene Ther 4:993–997. [DOI] [PubMed] [Google Scholar]
- 4.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. 2002. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 32:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banga HS, Deshmukh S, Brar RS, Gadhave PD, Chavhan SG, Sandhu HS. 2010. A case of intranasal hemangioma and concurrent tetracycline-induced ulcerative gastritis in dogs. Toxicol Int 17:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biankin SA, Collector MI, Biankin AV, Brown LJ, Kleeberger W, Devereux WL, Zahnow CA, Baylin SB, Watkins DN, Sharkis SJ, Leach SD. 2007. A histological survey of green fluorescent protein expression in ‘green’ mice: implications for stem cell research. Pathology 39:247–251. [DOI] [PubMed] [Google Scholar]
- 7.Brihoum M, Amory H, Desmecht D, Cassart D, Deleuze S, Rollin F. 2010. Descriptive study of 32 cases of doxycycline-overdosed calves. J Vet Intern Med 24:1203–1210. [DOI] [PubMed] [Google Scholar]
- 8.Cawthorne C, Swindell R, Stratford IJ, Dive C, Welman A. 2007. Comparison of doxycycline delivery methods for tet-inducible gene expression in a subcutaneous xenograft model. J Biomol Tech 18:120–123. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CM, Krohn J, Bhattacharya S, Davies B. 2011. A comparison of exogenous promoter activity at the ROSA26 locus using a PhiC31 integrase mediated cassette exchange approach in mouse ES cells. PLoS One 6:e23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Kelz MB, Zeng G, Sakai N, Steffen C, Shockett PE, Picciotto MR, Duman RS, Nestler EJ. 1998. Transgenic animals with inducible, targeted gene expression in brain. Mol Pharmacol 54:495–503. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Li H, Wang Z, Tao T, Hu C. 2011. Photoproducts of tetracycline and oxytetracycline involving self-sensitized oxidation in aqueous solutions: effects of Ca2+ and Mg2+. J Environ Sci (China) 23:1634–1639. [DOI] [PubMed] [Google Scholar]
- 12.Das AT, Zhou X, Vink M, Klaver B, Verhoef K, Marzio G, Berkhout B. 2004. Viral evolution as a tool to improve the tetracycline-regulated gene expression system. J Biol Chem 279:18776–18782. [DOI] [PubMed] [Google Scholar]
- 13.Dow LE, Premsrirut PK, Zuber J, Fellmann C, McJunkin K, Miething C, Park Y, Dickins RA, Hannon GJ, Lowe SW. 2012. A pipeline for the generation of shRNA transgenic mice. Nat Protoc 7:374–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duerr J, Gruner M, Schubert SC, Haberkorn U, Bujard H, Mall MA. 2011. Use of a new-generation reverse tetracycline transactivator system for quantitative control of conditional gene expression in the murine lung. Am J Respir Cell Mol Biol 44:244–254. [DOI] [PubMed] [Google Scholar]
- 15.Duivenvoorden WCM, Popović SV, Lhoták Š, Seidlitz E, Hirte HW, Tozer RG, Singh G. 2002. Doxycycline decreases tumor burden in a bone metastasis model of human breast cancer. Cancer Res 62:1588–1591. [PubMed] [Google Scholar]
- 16.El-Neweshy MS. 2013. Experimental doxycycline overdose in rats causes cardiomyopathy. Int J Exp Pathol 94:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ermak G, Cancasci VJ, Davies KJA. 2003. Cytotoxic effect of doxycycline and its implications for tet-on gene expression systems. Anal Biochem 318:152–154. [DOI] [PubMed] [Google Scholar]
- 18.Gossen M, Bujard H. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89:5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunther EJ, Belka GK, Wertheim GBW, Wang J, Hartman JL, Boxer RB, Chodosh LA. 2002. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J 16:283–292. [DOI] [PubMed] [Google Scholar]
- 20.Honnorat-Benabbou VC, Lebugle AA, Sallek B, Duffaut-Lagarrigue D. 2001. Stability study of tetracyclines with respect to their use in slow release systems. J Mater Sci Mater Med 12: 107–110. [DOI] [PubMed] [Google Scholar]
- 21.Institute for Laboratory Animal Research. 2010. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 22.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lűbbert H, Bujard H. 1996. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA 93:10933–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krueger C, Pfleiderer K, Hillen W, Berens C. 2004. Tetracycline derivatives: alternative effectors for Tet transregulators. Biotechniques 37:546–550. [DOI] [PubMed] [Google Scholar]
- 24.Lewandoski M. 2001. Conditional control of gene expression in the mouse. Natl Rev Genet 2:743–755. [DOI] [PubMed] [Google Scholar]
- 25.Liang Y, Denton MB, Bates RB. 1998. Stability studies of tetracycline in methanol solution. J Chromatogr A 827:45–55. [DOI] [PubMed] [Google Scholar]
- 26.Mansuy IM, Bujard H. 2000. Tetracycline-regulated gene expression in the brain. Curr Opin Neurobiol 10:593–596. [DOI] [PubMed] [Google Scholar]
- 27.Marx JO, Vudathala D, Murphy L, Rankin S, Hankenson FC. 2014. Antibiotic administration in the drinking water of mice. J Am Assoc Lab Anim Sci 53:301–306. [PMC free article] [PubMed] [Google Scholar]
- 28.McIntyre AR, Lipman NS. 2007. Amoxicillin-clavulanic acid and trimethoprim-sulfamethoxazole in rodent feed and water: effects of compounding on antibiotic stability. J Am Assoc Lab Anim Sci 46:26–32. [PubMed] [Google Scholar]
- 29.Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, Innocent N, Cardiff RD, Schnall MD, Chodosh LA. 2002. Conditional activitation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell 2:451–461. [DOI] [PubMed] [Google Scholar]
- 30.Moutier R, Tchang F, Caucheteux SM, Kanellopoulos-Langevin C. 2003. Placental anomalies and fetal loss in mice, after administration of doxycycline in food for tet-system activation. Transgenic Res 12:369–373. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura JL, Garcia E, Pieper RO. 2008. S6K1 plays a key role in glial transformation. Cancer Res 68:6516–6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyabi O, Naessens M, Haigh K, Gembarska A, Goossens S, Maetens M, De Clercq S, Drogat B, Haenebalcke L, Bartunkova S, De Vos I, De Craene B, Karimi M, Berx G, Nagy A, Hilson P, Marine JC, Haigh JJ. 2009. Efficient mouse transgenesis using Gateway-compatible ROSA26 locus targeting vectors and F1 hybrid ES cells. Nucleic Acids Res 37:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, Baxter BT. 2002. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg 35:923–929. [DOI] [PubMed] [Google Scholar]
- 34.Premsrirut PK, Dow LE, Kim SY, Camiolo M, Malone CD, Miething C, Scuoppo C, Zuber J, Dickins RA, Kogan SC, Shroyer KR, Sordella R, Hannon GJ, Lowe SW. 2011. A rapid and scalable system for studying gene function in mice using conditional RNA interference. Cell 145:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren BZ, Lahn BT. 2010. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS One 5:e10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricart Arbona RJ, Lipman NS, Riedel ER, Wolf FR. 2010. Treatment and eradication of murine fur mites: I. toxicologic evaluation of ivermectin-compounded feed. J Am Assoc Lab Anim Sci 49:564–570. [PMC free article] [PubMed] [Google Scholar]
- 37.Saini Y, Harkema JR, LaPres JJ. 2008. HIF1α is essential for normal intrauterine differentiation of alveolar epithelium and surfactant production in the newborn lung of mice. J Biol Chem 283:33650–33657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saivin S, Houin G. 1988. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet 15:355–366. [DOI] [PubMed] [Google Scholar]
- 39.Saunders TL. 2010. Inducible transgenic mouse models. Methods Mol Biol 693:103–115. [DOI] [PubMed] [Google Scholar]
- 40.Schönig K, Bujard H. 2003. Generating conditional mouse mutants via tetracycline-controlled gene expression. Methods Mol Biol 209:69–104. [DOI] [PubMed] [Google Scholar]
- 41.Sotillo R, Hernando E, Díaz-Rodríguez E, Teruya-Feldstein J, Cordón-Cardo C, Lowe SW, Benezra R. 2007. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 11:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takiguchi M, Dow LE, Prier JE, Carmichael CL, Kile BT, Turner SJ, Lowe SW, Huang DCS, Dickins RA. 2013. Variability of inducible expression across the hematopoietic system of tetracycline transactivator transgenic mice. PLoS One 8:e54009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Overstraeten-Schlögel N, Delgaudine M, Beguin Y, Gothot A. 2009. Limitations of the use of GFP transgenic mice in bone marrow transplantation studies. Leuk Lymphoma 47:1392–1393. [DOI] [PubMed] [Google Scholar]
- 44.Werner JJ, Arnold WA, McNeill K. 2006. Water hardness as a photochemical parameter: tetracycline photolysis as a function of calcium concentration, magnesium concentration, and pH. Environ Sci Technol 40:7236–7241. [DOI] [PubMed] [Google Scholar]
- 45.Wörtge S, Eshkind L, Cabezas-Wallscheid N, Lakaye B, Kim J, Heck R, Abassi Y, Diken M, Sprengel R, Bockamp E. 2010. Tetracycline-controlled transgene activation using the ROSA26-iM2-GFP knock-in mouse strain permits GFP monitoring of doxycycline-regulated transgene-expression. BMC Dev Biol 10:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zakeri B, Wright GD. 2008. Chemical biology of tetracycline antibiotics. Biochem Cell Biol 86:124–136. [DOI] [PubMed] [Google Scholar]
- 47.Zhanel GG, Homenuik K, Nichol K, Noreddin A, Vercaigne L, Embil J, Gin A, Karlowsky JA, Hoban DJ. 2004. The glycyclines: a comparative review with the tetracyclines. Drugs 64:63–88. [DOI] [PubMed] [Google Scholar]