Abstract
Tritrichomonas muris is occasionally identified during routine fecal screening of laboratory mice. Frequently, entire racks are affected, and because no effective treatment is available, culling of affected mice and rederivation by embryo transfer have been suggested. The current study evaluated whether treatment with ronidazole, a nitroimidazole efficacious against T. fetus infections in cats, combined with limited culling was effective against T. muris in laboratory mice (Mus musculus). A subset (n = 39) of mice were treated with ronidazole (400 mg/L in drinking water) for 15 d, after which 6 of the mice still shed T. muris. Consequently all mice in the affected rack received ronidazole (500 mg /L in drinking water) for 25 d. All mice were retested by using pooled samples, and those positive for T. muris (except for a valuable breeding pair) were culled. The remaining mice continued to receive ronidazole for another 17 d. At the end of the treatment period, all mice were tested (days 60 and 81) and were shown to be negative for T. muris. Over the following year, sentinel mice from the rack were tested every 3 mo and remained negative for tritrichomonads by fecal smear. Thus, a combination of limited culling and treatment with ronidazole in the drinking water successfully cleared research mice of infection with T. muris.
Tritrichomonads are common parasites that have been described in many species.3 For example, Tritrichomonas fetus occurs in the reproductive tract of cattle, where it can lead to stillbirths and female infertility.12 More recently, T. fetus has been described in the gastrointestinal tract of cats.11 Whether T. fetus in cattle and T. fetus in cats are the same organism or merely closely related remains a topic of debate.13,16,19 T. fetus is now recognized as one of the most common parasites in the gastrointestinal tract of cats and often leads to large-bowel diarrhea (that is, the feces often contain mucous and fresh blood, defecation is associated with urgency, and fecal leakage can be seen).5,17,18,21 T. muris (Figure 1) occurs in the gastrointestinal tract of mice and, although the pathogenetic effects are unclear (that is, affected but otherwise normal mice usually do not show any clinical signs of gastrointestinal disease, but immunocompromised mice might demonstrate gastrointestinal disease), T. muris is considered to be highly transmissible (that is, often times an entire rack of laboratory mice is affected), and culling or rederivation through embryo transfer has been recommended to eradicate the organism from the colony.7,9 When an infection involves a colony of standard laboratory mice, culling is not associated with any major costs or delays in research projects, because replacement mice are routinely available. However, genetically modified mice are often very valuable when only few breeding pairs exist worldwide, and successfully crossbreeding several strains may take several months. Therefore, culling of entire racks of genetically modified mice might represent considerable burdens in terms of cost and research time.
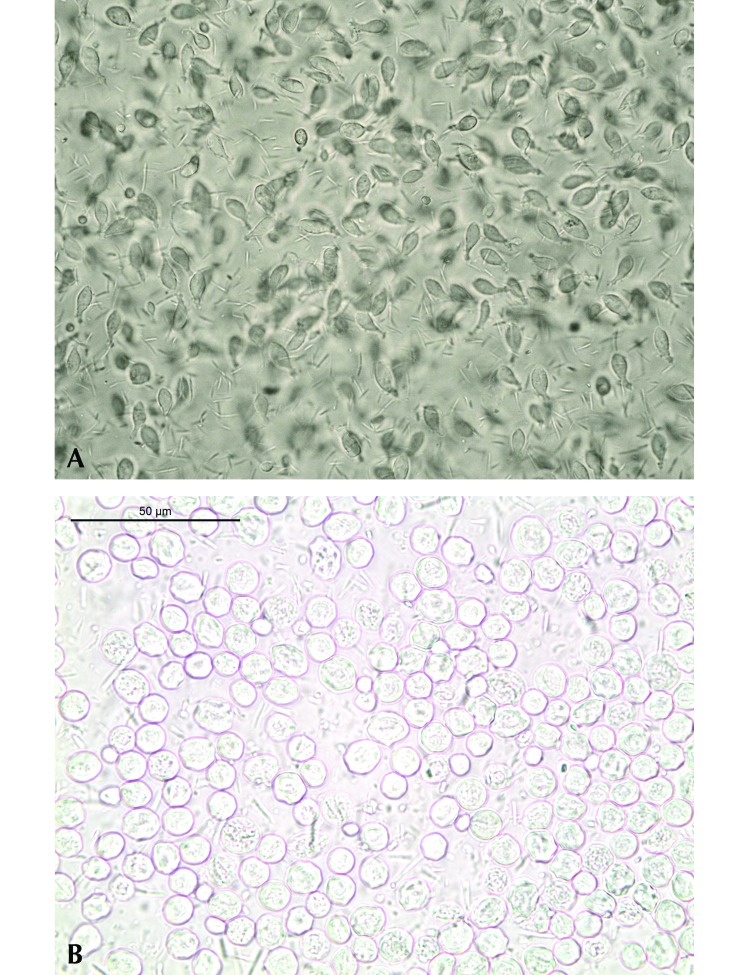
Figure 1.
Tritrichomonas muris. (A) Trophozoites within the content of the large intestine of a laboratory mouse; magnification, 400×. (B) Pseudocysts in a direct wet (saline) fecal smear of an infected laboratory mouse in the present study; magnification, 630×.
When T. fetus was first identified in cats with chronic diarrhea in the United States, the infection was considered to be untreatable.2,6,8 Several broad-spectrum antiparasitic agents and antimicrobials were tested for efficacy and, although some decreased the number of organisms shed, none was efficacious in eradicating T. fetus infections in cats.1,8 However, one group of researchers then tested the nitroimidazole ronidazole, which had previously been used as an antiparasitic agent in pigeons but was not approved for use in cats.4 Ronidazole proved effective at a dosage of 30 mg/kg q 12 h orally for 14 d. Although ronidazole was efficacious in eradicating T. fetus from many cats, treatment was associated with side effects, namely neurologic signs, when higher-than-recommended doses were used.15 As a result, the recommended dosage was decreased to 15 mg/kg every 12 h or 30 mg/kg every 24 h orally for 14 d. This dosage appears to be effective in most cats infected with T. fetus, and neurologic side effects have not been reported at these dosages.
To our knowledge, ronidazole has not previously been used in laboratory mice. The primary goal of this study was to determine whether limited culling and treatment with ronidazole might rescue a valuable colony of genetically modified mice from T. muris infection.
Case Report
The sentinel mice on one rack of a valuable colony of genetically modified mice housed at the facilities of the Center for Preclinical Research (Klinikum rechts der Isar, Technical University of Munich, Munich, Germany) were identified to be positive for a tritrichomonad infection on the basis of fecal smears.20 The mice were of various ages and both sexes and represented a variety of genetic backgrounds (NMRI-nu+/–; p62 −/−; p65 lox/lox; Krasmut/+; p48Cre/+, p65lox/lox; p48 Cre/+, Krasmut/+; p65lox/lox Lys Cre; p48Cre/+, p65lox/lox, Krasmut/+; p48Cre/+, p65lox/lox, Krasmut/+, p16lox/lox). Mice were housed in IVC (Tecniplast, Buguggiate, Italy), with steam-sterilization of all cages, bedding, enrichment, water, feed, and miscellaneous materials. Access to the facility was key-chip–controlled, and personal protective clothing, including face mask and gloves, was mandatory. Light management consisted of a 12:12-h light:dark cycle with transition phases. According to the FELASA guidelines for health monitoring of mouse, rat, hamster, guinea pig, and rabbit colonies in breeding and experimental units, sentinel mice were used.14 In addition to T. muris, the target rack was shown to be positive for murine norovirus and Helicobacter spp.
To confirm infection, several mice (n = 40) from different cages of the rack were tested by using a real-time PCR assay for the detection of T. muris (IDEXX Laboratories, Ludwigsburg, Germany). Briefly, total nucleic acid was extracted from feces by using the QIAamp DNA Blood BioRobot MDx kit (Qiagen, Venlo, Netherlands) on an automated Qiagen platform (BioRobot MDx, Qiagen) according to the manufacturer's instructions with slight modifications. Real-time PCR analysis at IDEXX Laboratories was performed by using the LightCycler 480 system (Roche Diagnostics, Basel, Switzerland) with proprietary forward and reverse primers and a hydrolysis probe. The target gene for T. muris detection was the 18S rRNA-Gen gene. This PCR assay has been shown to have a level of detection of 10 DNA copies. All mice tested were shown to be positive for T. muris, and in a pilot study (Figure 2 A), 39 mice (1 mouse was removed from the colony because it had reached the endpoint of the primary research study) in 14 cages were treated with 400 mg/L ronidazole (Ridzol 10% Bt, Dr Hesse Tierpharma, Hohenlockstedt, Germany) in autoclaved drinking water for 15 d. Because nitroimidazoles are generally considered to be poorly palatable, water consumption was monitored daily by using a permanent marker to record the meniscus of the water level in the water bottle and estimating whether each adult animal had consumed at least approximately 5 mL during a 24-h period; when water intake was considered insufficient, a teaspoon of dextrose (approximately 2 g; Dextropur, Dextro Energy, Germany) was added to the drinking water containing the ronidazole to improve palatability. Mice were observed daily for any potential grossly visible side effects of ronidazole treatment; but neurologic examinations were not performed. In addition, all mice were weighed repeatedly (days 1, 2, 3, 4, 7, 9, 11, 14, and 16).
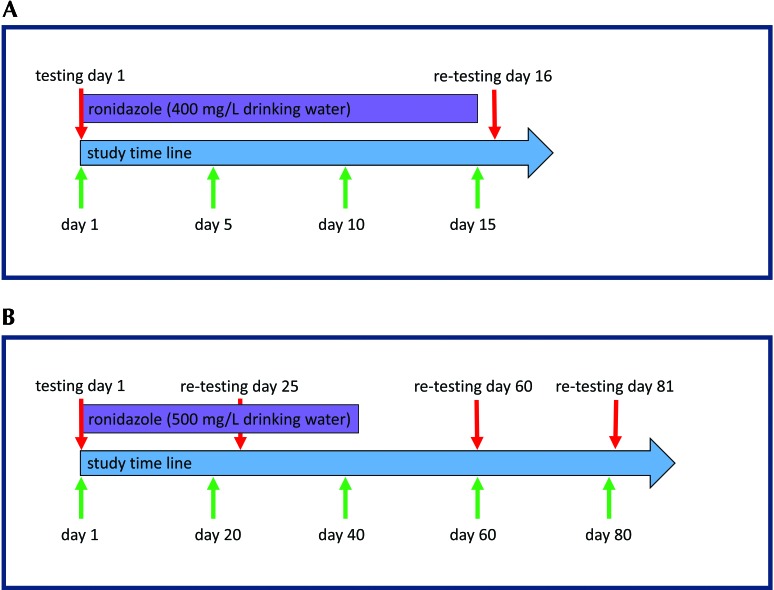
Figure 2.
(A) Experimental protocol for the pilot study. On day 1 of the pilot study, a subset of 39 affected mice began to receive ronidazole; 34 of these mice were retested for T. muris on day 16. (B) Experimental protocol for the second treatment period. On day 1, 49 mice began to receive ronidazole. All mice housed in the rack were retested on days 25 (n = 79), 60 (n = 36), and 81 (n = 46).
None of the mice showed any overt adverse side effects during the pilot treatment period. Furthermore, several breedings took place during the treatment period; breeding success did not appear to be affected by ronidazole treatment but was not studied in detail. However, because water consumption was considered insufficient in 6 of the 14 cages, dextrose was added to the ronidazole-spiked drinking water. After addition of dextrose to the drinking water, water consumption was considered to be adequate for all cages. The median body weight of the mice differed significantly (day 1, 27.4 g; day 2, 27.0; day 3, 27.3 g; day 4, 27.7 g; day 7, 27.7 g; day 9, 27.7 g; day 11, 27.6 g; day 14, 27.6 g; day 16, 26.8 g; P value [Friedman test], <0.0001; Prism, Graph Pad, La Jolla, CA). Dunn's multiple comparison testing revealed significant differences in body weight between baseline (day 1) and days 11 and 14, suggesting an overall weight gain in the mice during the treatment period. Due to their genotype, a few mice (n = 5) were removed from the colony during the current study period because they had reached the end point for their primary study.
On day 16, fecal samples were collected from all remaining mice (n = 34); 28 of these mice tested negative for T. muris by real-time PCR analysis after ronidazole treatment. However, the remaining 6 mice were positive for T. muris shedding by real-time PCR; 2 of these positive mice were housed individually, 3 positive mice shared their cage with a negative mouse, and the remaining positive mouse was housed with a negative mouse. All 6 of these positive mice were culled.
During a second treatment cycle (Figure 2 B), the entire rack (that is, all mice that had been part of the pilot project as well as those that had not previously been treated, thus comprising 49 mice in 19 cages) was treated with 500 mg/L ronidazole in the drinking water for 42 d. During this treatment period, approximately 8 g/L dextrose was added to all drinking water. Because some mice had litters during the treatment period and others were removed because they lacked the desired genotype or had reached a primary study endpoint, the number of mice varied throughout the treatment period; only 9 mice were part of the colony for the entire duration of the second study period. Pooled fecal samples (by cage, representing 5 to 12 mice per sample) were collected on days 25 (79 mice total), 60 (36 mice total), and 81 (46 mice total). For collection of the pooled sample, all mice in each cage were removed individually, and fecal material from each mouse was collected into the same 1.5-mL tube and carefully mixed by using the wooden end of a sterile cotton swab. Once again, no overt side effects were observed in any of the mice. Real-time PCR testing on day 25 showed that 1 of the 6 group samples was positive for T. muris. This positive group sample represented 11 mice from 3 cages; 9 of these mice (that is, all of those from 2 cages) were culled on day 29 because retesting all 9 animals was considered economically unfeasible. The remaining 2 positive mice comprised a valuable breeding pair, which were retested, shown to be negative for T. muris shedding, and therefore retained. Pooled samples from all remaining mice were retested on days 60 and 81; none of those group samples tested positive for T. muris. Dirty-bedding sentinel mice (approximately 1 tablespoon of soiled bedding from each occupied cage in the rack was added to the 4 sentinel cages twice each week) were evaluated and remained negative for tritrichomonad infection according to standard fecal smears every 3 mo for 1 y after completion of the current study.20
Discussion
This report is the first description of the management of T. muris infestation in a colony of laboratory mice by using a combination of culling and pharmacotherapy. This method proved to be effective and allowed us to spare most of the valuable colony. Treatment with ronidazole at 400 or 500 mg/L drinking water was not associated with any obvious adverse effects in any of the mice, and body weights did not decrease significantly. Instead, body weights were significantly higher on days 11 and 14 than on day 1, suggesting an overall weight gain in the mice. This result probably reflects the fact that several mice enrolled in this study were not fully grown at the time of enrollment and thus continued to gain weight. These findings suggest that mice tolerated the dosages of ronidazole used here well and that higher dosages might also be acceptable. The starting dose we chose was slightly lower than that used routinely in birds (500 mg ronidazole/L of drinking water), because data regarding the therapeutic dosing of mice were unavailable and because we wanted to be conservative in our approach so that we avoided gross adverse side effects in this valuable breeding colony. Although management with ronidazole proved to be effective in the majority of mice, underlying effects on the primary research projects cannot be excluded.
One limitation of the current study was the lack of a control group. However, the colony had been under surveillance for T. muris infection before the trial began, and sentinels were consistently positive for T. muris. Therefore, spontaneous clearance of the organism from the colony seems unlikely. In addition, because not all mice were euthanized at the completion of the study, microscopic side effects of ronidazole treatment might have been missed.
Ronidazole has been described as embryotoxic and teratogenic and to lead to infertility.10 In addition, there are anecdotal reports of ronidazole being mutagenic and carcinogenic in rodents. Furthermore, ronidazole is a broad-spectrum antibiotic and thus might alter the intestinal microbiota. Many of the mice were used in studies of pancreatic adenocarcinoma or acute pancreatitis, both of which might be affected by alterations in the intestinal microbiota. However, some of the mice were used as breeding animals. Ronidazole's effects on the microbiota are likely temporary and thus unlikely to carry over to the F1 or F2 generation. Therefore, although the potential effects of ronidazole on experimental outcomes must be considered carefully, long-term effects on the breeding colony are unlikely and, especially in a valuable population of genetically modified mice, rescue therapy with ronidazole appears warranted to avoid rederivation by embryo transfer. This benefit maybe especially important when embryo transfer is cost-prohibitive or when persons skilled in the technique are unavailable.
References
- 1.Carvalho KP, Gadelha APR. 2007. Effects of 3 benzimidazoles on growth, general morphology and ultrastructure of Tritrichomonas foetus. FEMS Microbiol Lett 275:292–300. [DOI] [PubMed] [Google Scholar]
- 2.Foster DM, Gookin JL, Poore MF, Stebbins ME, Levy MG. 2004. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc 225:888–892. [DOI] [PubMed] [Google Scholar]
- 3.Frey CF, Muller N. 2012. Tritrichomonas–systematics of an enigmatic genus. Mol Cell Probes 26:132–136. [DOI] [PubMed] [Google Scholar]
- 4.Gookin JL, Copple CN, Papich MG, Poore MF, Stauffer SH, Birkenheuer AJ, Twedt DC, Levy MG. 2006. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med 20:536–543. [DOI] [PubMed] [Google Scholar]
- 5.Gookin JL, Levy MG, Law JM, Papich MG, Poore MF, Breitschwerdt EB. 2001. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res 62:1690–1697. [DOI] [PubMed] [Google Scholar]
- 6.Gookin JL, Stauffer SH, Coccaro MR, Poore MF, Levy MG, Papich MG. 2007. Efficacy of tinidazole for treatment of cats experimentally infected with Tritrichomonas foetus. Am J Vet Res 68:1085–1088. [DOI] [PubMed] [Google Scholar]
- 7.Kashiwagi A, Kurosaki H, Luo H, Yamamoto H, Oshimura M, Shibahara T. 2009. Effects of Tritrichomonas muris on the mouse intestine: a proteomic analysis. Exp Anim 58:537–542. [DOI] [PubMed] [Google Scholar]
- 8.Kather EJ, Marks SL, Kass PH. 2007. Determination of the in vitro susceptibility of feline Tritrichomonas foetus to 5 antimicrobial agents. J Vet Intern Med 21:966–970. [DOI] [PubMed] [Google Scholar]
- 9.Koyama T, Endo T, Asahi H, Kuroki T. 1987. Life cycle of Tritrichomonas muris. Zentralbl Bakteriol Mikrobiol Hyg A 264:478–486. [DOI] [PubMed] [Google Scholar]
- 10.Kozhukharov E, Donev B, Stoianov KH. 1985. [[Research on Pharmachim's ronidazole for its antifertility, embryotoxic and teratogenic action]] Vet Med Nauki 22:76–82. [Article in Bulgarian]. [PubMed] [Google Scholar]
- 11.Levy MG, Gookin JL, Poore M, Birkenheuer AJ, Dykstra MJ, Litaker RW. 2003. Tritrichomonas foetus and not Pentatrichomonas hominis is the etiologic agent of feline trichomonal diarrhea. J Parasitol 89:99–104. [DOI] [PubMed] [Google Scholar]
- 12.Rae DO, Crews JE. 2006. Tritrichomonas foetus. Vet Clin North Am Food Anim Pract 22:595–611. [DOI] [PubMed] [Google Scholar]
- 13.Reinmann K, Muller N, Kuhnert P, Campero CM, Leitsch D, Hess M, Henning K, Fort M, Muller J, Gottstein B, Frey CF. 2012. Tritrichomonas foetus isolates from cats and cattle show minor genetic differences in unrelated loci ITS-2 and EF-1α. Vet Parasitol 185:138–144. [DOI] [PubMed] [Google Scholar]
- 14.FELASA Working Group on Revision of Guidelines for Health Monitoring of Rodents and Rabbits; Mähler Convenor M, Berard M, Feinstein R, Gallagher A, Illgen-Wilcke B, Pritchett-Corning K, Raspa M. 2014. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim 48:178–192. [DOI] [PubMed] [Google Scholar]
- 15.Rosado TW, Specht A, Marks SL. 2007. Neurotoxicosis in 4 cats receiving ronidazole. J Vet Intern Med 21:328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slapeta J, Craig S, McDonell D, Emery D. 2010. Tritrichomonas foetus from domestic cats and cattle are genetically distinct. Exp Parasitol 126:209–213. [DOI] [PubMed] [Google Scholar]
- 17.Stockdale H, Givens MD, Dykstra CC, Blagburn BL. 2009. Tritrichomonas foetus infections in surveyed pet cats. Vet Parasitol 160:13–17. [DOI] [PubMed] [Google Scholar]
- 18.Tolbert MK, Gookin J. 2009. Tritrichomonas foetus: a new agent of feline diarrhea. Compend Contin Educ Vet 31:374–381. [PubMed] [Google Scholar]
- 19.Walden HS, Dykstra C, Dillon A, Rodning S, Givens D, Bird R, Newton J, Lindsay D. 2013. A new species of Tritrichomonas (Sarcomastigophora: Trichomonida) from the domestic cat (Felis catus). Parasitol Res 112:2227–2235. [DOI] [PubMed] [Google Scholar]
- 20.Wolf D, Vrhovec M, Failing K, Rossier C, Hermosilla C, Pantchev N. 2014. Diagnosis of gastrointestinal parasites in reptiles: comparison of 2 coprological methods. Acta Vet Scand 56:44–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaeger MJ, Gookin JL. 2005. Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats. Vet Pathol 42:797–804. [DOI] [PubMed] [Google Scholar]