Abstract
Background
Ki-67 is an established marker of cell proliferation, and the Ki-67 index correlates with the clinical course of several cancer types, including bladder cancer (BC). However, the clinicopathological and prognostic significance of Ki-67 in bladder cancer remains unclear. Therefore, we performed a systematic review and meta-analysis to clarify this relationship.
Methods
A comprehensive literature search for relevant studies published up to February 1, 2016, was performed using PubMed, Cochrane Library, Embase and ISI Web of Knowledge. The effects of Ki-67 expression on survival outcome in patients with BC and BC subtypes were evaluated. Furthermore, the relationship between Ki-67 expression and the clinicopathological features of BC were assessed.
Results
Thirty-one studies with 5147 bladder cancer patients were selected for evaluation. Ki-67 expression was significantly associated with shorter recurrence-free (HR 1.69, 95% CI: 1.33–2.14), progression-free (HR 1.89, 95% CI: 1.43–2.51), overall (HR 2.03, 95% CI: 1.31–3.16), and cancer-specific (HR 1.69, 95% CI: 1.47–1.95) survival. Moreover, whereas high expression was more common in high tumor stage, recurrence status, tumor size, there was no correlation between high Ki-67 expression and age, gender, smoking habits, and tumor number. Importantly, analysis of the different subgroups of BC suggested that significant correlations between high Ki-67 expression and survival outcome (recurrence-free/progression-free/overall/cancer-specific survival) are present only in European-American patients.
Conclusion
The present results indicate that over-expression of Ki-67 is distinctly correlated with poor patient survival. Ki-67 may serve as a valuable biomarker for prognosis in BC patients, particularly in non-Asian BC patients. The results suggest no significant association between Ki-67 expression and BC prognosis in Asian patients. Further efforts are needed to fully clarify this relationship.
Introduction
Bladder cancer (BC) is a common cancer of the urinary tract, with an estimated 429,800 new cases of BC and 165,100 deaths annually worldwide [1]. Clinically, bladder tumors are classified as non-muscle invasive bladder cancer (NMIBC)(Ta/T1) and muscle-invasive bladder cancer (MIBC)(T2-T4), NMIBC is also called superficial bladder cancer. Although many factors have been identified as risk factors for BC, such as smoking, age, obesity and diabetes, the pathogenesis of BC remains unclear [2, 3]. Cystoscopy and biopsy are the gold standards for the initial diagnosis of BC but are invasive, uncomfortable, and expensive [4, 5]. Therefore, novel biomarkers for early diagnosis, prognostic evaluation and effective treatment of BC are needed.
Ki-67 is a DNA-binding nuclear protein that is expressed throughout the cell cycle in proliferating but not quiescent (G0) cells [6]. Ki-67 is a predictive factor for tumor development, and its expression has been correlated with poor prognosis in several types of cancer [7–10]. However, the role of Ki-67 in the prognosis of BC remains controversial. Chen et al. [11] confirmed that Ki-67 was an independent predictor of tumor recurrence and progression in a study of 72 cases of NMIBC. Makboul and Gontero et al. [12, 13] demonstrated that Ki-67 was only an independent predictor of progression and not recurrence in NMIBC patients. Tanabe et al. [14] demonstrated that high Ki-67 expression status might facilitate the selection of chemoradiotherapy-based multimodal approaches in terms of prognosis and quality of life as a result of bladder preservation in MIBC patients. Studies have revealed that Ki-67 is not correlated with or an independent predictor of BC recurrence, progression, and death. For example, Acikalin et al. [15] reported that there was no correlation between Ki-67 and tumor recurrence, progression or tumor-related mortality in a study of 68 patients with stage T1 who underwent transurethral resection of the tumor.
The optimal approach to the interpretation and assessment of Ki-67 in clinical practice remains controversial among pathologists. In addition, the roles of Ki-67 expression and clinical significance in BC have not been thoroughly investigated. In this study, we performed a meta-analysis to explore the relationship between Ki-67 expression and its prognostic value in BC. This systematic review and meta-analysis was reported and performed in accordance with PRISMA guidelines (S3 Table) [16].
Materials and Methods
Study strategy
The PubMed, Cochrane, Embase and Web of Knowledge databases were searched systematically for relevant articles published up to February 1, 2016. Because the data in this study were extracted from previous studies, ethical approval from ethics committees was not required.
The search terms were ‘‘bladder,” ‘‘urothelial,” ‘‘cancer or tumor or neoplasm or carcinoma,” ‘‘expression,” ‘‘Ki-67 or Ki67 or MIB-1 or MIB 1”, and ‘‘prognosis or prognostic or outcome.” The criteria for eligibility were as follows: (1) Ki-67 expression evaluated in primary BC tissues; (2) evaluation of the relationship between Ki-67 expression and BC clinicopathological parameters and prognosis; and (3) sufficient information to estimate the hazard ratio (HR) of recurrence-free survival (RFS), progression-free survival (PFS), overall survival (OS), and cancer-specific survival (CSS) and a 95% confidence intervals (CIs). Papers containing any of the following were excluded: (1) duplicate literature or duplicate data presented at conferences; (2) reviews, no available data, or abstract only; (3) studies of cancer cell lines and animal models; and (4) insufficient data to obtain HR and its standard error. For overlapping articles, only the highest-quality and most-recent literature were retained.
Data extraction and methodological assessment
The following information was recorded for each study: the first author’s name, publication year, sample source, number of cases, median or mean of patient age, gender, cancer stage, antibody source and dilution, percentage rate of expression, and follow-up period. We preferred to collect multivariate analysis data. If data were not available, data from univariate analyses of survival outcomes were extracted. All data were extracted by two independent observers (ZMM and ZHC). The quality of the selected articles was assessed according to the Newcastle-Ottawa Scale (NOS) criteria [17]. If data could not be obtained from the literature, we regarded the related data as not available.
Statistical analysis
The statistical analysis was conducted using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) and STATA 14.0 (Stata Corporation, TX). HRs and 95% CIs were used to evaluate the relationships between Ki-67 expression and RFS, PFS, OS, and CSS rates. ORs (odds ratios) and 95% CIs were used to estimate the relationships between Ki-67 expression and clinicopathological parameters, including age, sex status, tumor stage, recurrence status, tumor number, and tumor size. The statistical significance of the pooled ORs and HRs was evaluated by the Z test. Heterogeneity among the studies was evaluated with Cochran’s Q test and I2 tests [18]. When the I2 statistic results were 0–50%, a fixed-effect model was used to calculate parameters. If the I2 statistic results were 50–100%, a random-effects model was considered more appropriate than a fixed-effects model. A p value < 0.05 was considered statistically significant. Funnel plots and Begg’s test were used to evaluate potential publication bias [19].
Results
Study characteristics
Our search strategy initially identified 412 articles. Following deduplication (n = 60), the two reviewers independently screened the identified titles and abstracts. After manually screening the titles and abstracts, 22 studies were excluded because they were case reports (n = 2), review articles (n = 6), conference abstracts (n = 4), meta-analysis (n = 2) or studies irrelevant to the human studies (n = 8). Seven articles were ultimately excluded due to overlap with previously reported studies (n = 4). Thus, 31 articles published from 2001 to 2016 were included in the final meta-analysis [20–50] (Fig 1).
Fig 1. Flow chart shows study selection procedure.
The main characteristics of the 31 studies included in our meta-analysis are presented in S1 Table. Of the 31 studies, 5 were conducted in America, five in Germany, five in China, three in Greece, three in Spain, three in Korea, two in Italy, two in Japan, and one each in Portugal, Switzerland, and the UK. In 5 of the 31 studies, patients received intravesical BCG therapy. The follow-up period of the studies ranged from 2 months to 124 months. The age of the patients ranged from 21 to 97 years, and the overall proportion of males was 80.33%.
Positive/high Ki-67 expression was defined by immunohistochemistry (IHC) using different antibodies and cut-off values (range, 5–55%) (S2 Table).
Of the 31 studies, 23 provided HRs and 95% CI values directly. Six papers provided the relative risk (RR), and two articles provided OR values, which we used to estimate HR. Of the 31 studies, a significant association between high Ki-67 expression and poor RFS, PFS, OS and CSS was demonstrated in five [22, 26, 34, 35, 48], five [31, 36, 43, 46, 48], six [20, 24, 28, 29, 31, 42] and seven studies [27, 30, 33, 34, 42, 46, 47], respectively. Of the literature, eleven, five, three and two studies linking Ki-67 expression with poor RFS [21, 25, 37–41, 43–45], PFS[21, 25, 41, 44, 49], OS [23, 32, 41] and CSS [43, 50], respectively, lacked statistical significance.
Correlation of high Ki-67 expression with RFS in bladder cancer
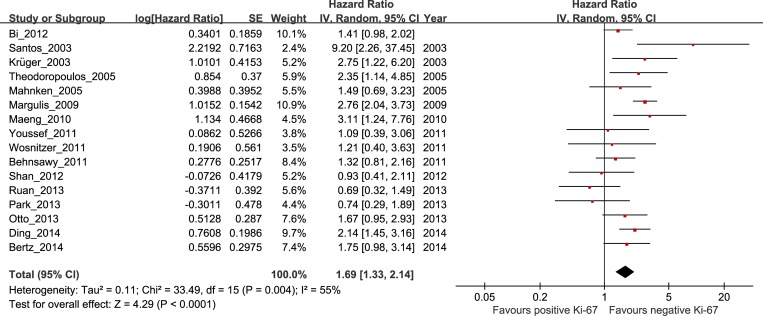
Of the 16 studies investigating the association between Ki-67 expression and RFS, 7 involved Asian patients (n = 2163), and 9 involved non-Asian patients (n = 610). The overall HR for BC patients was 1.69 (95% CI 1.33–2.14, P < 0.0001, n = 2773), with significant heterogeneity (I2 = 55%, P = 0.004; Fig 2 and Table 1). Subgroup analyses indicated that the risk was significant in non-Asian patients (HR 2.23, 95% CI 1.82–2.73, P < 0.00001) with heterogeneity (I2 = 33%, P = 0.16), but not in Asian patients (HR 1.36, 95% CI 0.97–1.90, P = 0.07), with significant heterogeneity (I2 = 55%, P = 0.04).
Fig 2. The hazard ratio (HR) of Ki-67 expression associated with RFS in all bladder cancer patients.
Table 1. Results of subgroup analysis of the association between Ki-67 expression and RFS/PFS/OS/CSS of bladder cancer.
| Outcome | Studies (n) | Patients | HR | 95%CI | P value | Model | Heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| Chi2, I2, P value | ||||||||
| RFS | All study | 16 | 2773 | 1.69 | 1.33–2.14 | 0.000 | Random | 33.49, 55%, 0.004 |
| Asian | 7 | 2163 | 1.36 | 0.97–1.90 | 0.07 | Random | 13.37, 55%, 0.04 | |
| Non-Asian | 9 | 610 | 2.23 | 1.82–2.73 | 0.000 | Fixed | 11.85, 33%, 0.16 | |
| Stage T1 | 6 | 927 | 1.45 | 1.09–1.93 | 0.01 | Fixed | 8.58, 42%, 0.13 | |
| Stage Ta-T1 | 6 | 774 | 1.99 | 1.54–2.57 | 0.000 | Fixed | 9.26, 46%, 0.10 | |
| Stage Ta/1-T4 | 4 | 1072 | 1.56 | 0.91–2.66 | 0.11 | Random | 12.52, 76%, 0.006 | |
| UBC | 14 | 2560 | 1.79 | 1.40–2.28 | 0.000 | Random | 28.88, 55%, 0.007 | |
| SCC | 1 | 152 | 1.09 | 0.39–3.08 | 0.87 | Fixed | - | |
| BCG | 5 | 522 | 1.63 | 1.20–2.23 | 0.002 | Fixed | 4.42, 10%, 0.35 | |
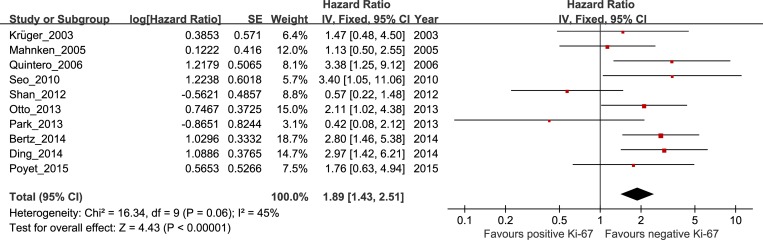
| PFS | All study | 10 | 1694 | 1.89 | 1.43–2.51 | 0.000 | Fixed | 16.34, 45%, 0.06 |
| Asian | 4 | 618 | 1.35 | 0.48–3.82 | 0.57 | Random | 11.41, 74%, 0.01 | |
| Non-Asian | 6 | 1076 | 2.05 | 1.45–2.89 | 0.000 | Fixed | 4.33, 0%, 0.50 | |
| Stage T1 | 5 | 799 | 1.78 | 1.22–2.60 | 0.003 | Fixed | 6.42, 38%, 0.17 | |
| Stage Ta-T1 | 4 | 799 | 2.80 | 1.75–4.49 | 0.000 | Fixed | 1.04, 0%, 0.79 | |
| Stage Ta-T4 | 1 | 96 | 0.57 | 0.22–1.49 | 0.253 | Fixed | - | |
| OS | All study | 9 | 1159 | 2.03 | 1.31–3.16 | 0.002 | Random | 40.37, 80%, 0.000 |
| Asian | 2 | 241 | 2.97 | 0.19–47.15 | 0.44 | Random | 12.21, 92%, 0.0005 | |
| Non-Asian | 7 | 918 | 1.96 | 1.26–3.06 | 0.003 | Random | 27.12, 78%, 0.0001 | |
| Stage Ta-T1 | 4 | 638 | 2.76 | 1.81–4.20 | 0.001 | Fixed | 1.05, 0%, 0.79 | |
| Stage T2-T4 | 1 | 82 | 2.33 | 0.99–5.43 | 0.05 | Fixed | - | |
| Stage Ta/1-T4 | 4 | 439 | 1.40 | 0.82–2.40 | 0.22 | Random | 15.33, 80%, 0.002 | |
| CSS | All study | 9 | 2528 | 1.69 | 1.47–1.95 | 0.000 | Fixed | 10.42, 23%, 0.24 |
| Asian | 1 | 103 | 1.58 | 0.56–4.47 | 0.38 | Fixed | - | |
| Non-Asian | 8 | 2425 | 1.69 | 1.47–1.95 | 0.000 | Fixed | 10.41, 33%, 0.17 | |
| Stage T1 | 3 | 695 | 2.86 | 1.16–7.02 | 0.02 | Random | 4.95, 60%, 0.08 | |
| Stage Ta-T1 | 1 | 192 | 3.46 | 1.22–9.80 | 0.01 | Fixed | - | |
| Stage T2-T4 | 1 | 73 | 4.70 | 1.14–19.28 | 0.032 | Fixed | - | |
| Stage Ta/1-T4 | 5 | 1641 | 1.61 | 1.39–1.87 | 0.000 | Fixed | 0.73, 0%, 0.95 |
BCG: bacillus Calmette-Guerin; CSS: cancer-specific survival; Fixed: Fixed, Inverse Variance model; HR: hazard ratio; I2: I-squared; OS: overall survival; PFS: progression-free survival; Random: Random, I-V heterogeneity model; RFS: recurrence-free survival; SCC: squamous cell carcinoma; UBC: urothelial bladder cancer.
Next, subgroups including tumor stage (six studies for stage T1, six for stages Ta-T1, and four for stages Ta/1-T4) and type of BC (14 studies for urothelial bladder cancer and 1 for squamous cell carcinoma) were analyzed. The analyses indicated that high Ki-67 expression was associated with shorter RFS in stage T1 and stages Ta-T1 patients (HR 1.45, 95% CI 1.09–1.93, P = 0.01; and HR 1.99, 95% CI 1.54–2.57, P < 0.00001, respectively) with heterogeneity (I2 = 42%, P = 0.13; and I2 = 46%, P = 0.10, respectively), but no association with shorter RFS was observed in patients in stages Ta/1-T4 (HR 1.56, 95% CI 0.91–2.66, P = 0.11). Moreover, our analyses revealed that Ki-67 expression was associated with shorter RFS in urothelial bladder cancer (HR 1.79, 95% CI 1.40–2.28, P < 0.00001). No significant association was observed between Ki-67 expression and squamous cell carcinoma (HR 1.09, 95% CI 0.39–3.08, P = 0.87). Furthermore, Ki-67 expression was an independent prognostic factor for BC treated with BCG therapy (HR, 1.63; 95% CI, 1.20–2.23; P = 0.002) (Table 1).
Relationships between Ki-67 expression and RFS in bladder cancer using different cut-off values
Subgroup analysis demonstrated that the relationship between Ki-67 expression and RFS was not significant using different Ki-67 cut-off values (10%, 25%, 50%). The pooled HRs and 95% CIs were as follows: 1.56 (95% CI 1.13–2.16) vs. 1.68 (95% CI 1.27–2.21) for a cut-off value of 10%, 1.61(95% CI 1.16–2.22) vs. 1.97 (95% CI 1.48–2.62) for a cut-off value of 25%, and 1.65 (95% CI 1.27–2.15) vs. 1.99 (95% CI 1.14–3.49) for a cut-off value of 50% (S1–S3 Figs and S2 Table).
Correlation between high Ki-67 expression and PFS in bladder cancer
The pooled HR and 95% CI for RFS provided in ten studies was 1.89, 95% CI 1.43–2.51, P < 0.0001, with heterogeneity (I2 = 45%, P = 0.06; Fig 3 and Table 1). The risk was significant in non-Asian patients but not in Asian patients, and the combined HRs and 95% CIs were as follows: HR 2.05, 95% CI 1.45–2.89, P < 0.0001; and HR 1.35, 95% CI 0.48–3.82, P = 0.57, respectively. Further subgroup analysis indicated that the risk was higher in the very early stage (stages Ta-T1) compared with stage T1, with the following combined HRs and 95% CIs: HR 2.80, 95% CI 1.75–4.49, P < 0.00001; HR 1.78, 95% CI 1.22–2.60, P = 0.003, respectively. But no significant association with PFS was observed in patients in stages Ta-T4, and the combined HRs and 95% CIs were as follows: HR 0.57, 95% CI 0.22–1.49, P = 0.253.
Fig 3. The hazard ratio (HR) of Ki-67 expression associated with PFS in all bladder cancer patients.
Correlation of high Ki-67 expression with OS and CSS in bladder cancer
The pooled HR for OS provided in nine studies indicated that Ki-67 expression was associated with worse survival in BC patients (HR = 2.03, 95% CI 1.31–3.16; P = 0.002), with heterogeneity (I2 = 80%, P < 0.0001; S4 Fig and Table 1). Subgroup analysis demonstrated that the risk was significant in non-Asian patients but not in Asian patients, and the combined HRs and 95% CIs were as follows: HR 1.96, 95% CI 1.26–3.06, P = 0.003; and HR 2.97, 95% CI 0.19–47.15, P = 0.44, respectively. Next, subgroups including tumor stage (four studies for stages Ta-T1, one for stages T2-T4, and four for stages Ta/1-T4) were analyzed. The analyses indicated that high Ki-67 expression was associated with shorter OS in stages Ta-T1 patients (HR 2.76, 95% CI 1.81–4.20, P = 0.001) with heterogeneity (I2 = 0%, P = 0.79), but no association with shorter OS was observed in patients in stages T2-T4 and stages Ta/1-T4 (HR 2.33, 95% CI 0.99–5.43, P = 0.05; and HR 1.40, 95% CI 0.82–2.40, P = 0.22, respectively).
Similarly, the pooled HR for CSS provided in nine studies indicated that Ki-67 expression was associated with worse survival in BC patients (HR = 1.69, 95% CI 1.47–1.95; P < 0.0001), with heterogeneity (I2 = 23%, P = 0.24; S5 Fig and Table 1). Subgroup analysis demonstrated the risk was significant in non-Asian patients but not in Asian patients, and the combined HRs and 95% CIs were as follows: HR 1.69, 95% CI 1.47–1.95, P < 0.0001; and HR 1.58, 95% CI 0.56–4.47, P = 0.38, respectively. Next, subgroups including tumor stage (three studies for stage T1, one for stages Ta-T1, one for stages T2-T4, and five for stages Ta/1-T4) were analyzed. The analyses indicated that high Ki-67 expression was associated with shorter OS in stage T1, stages Ta-T1, stages T2-T4, and stages Ta/1-T4 patients (HR 2.86, 95% CI 1.16–7.02, P = 0.02; HR 3.46, 95% CI 1.22–9.80, P = 0.01; HR 4.70, 95% CI 1.14–19.28, P = 0.032; and HR 1.61, 95% CI 1.39–1.87, P < 0.00001, respectively).
Relationships between Ki-67 expression and clinicopathological parameters
In this meta-analysis, the relationships between clinicopathological characteristics such as age, gender, smoking habits, tumor stage, recurrence status, tumor number, and tumor size and elevated Ki-67 expression were compared on the basis of 31 studies. The results of the meta-analysis revealed significant associations between high Ki-67 expression and higher tumor stage (Ta vs. T1; Ta/1 vs. T2-4), recurrence status, and larger tumor size. The combined ORs and 95% CIs were as follows: OR 0.29, 95% CI 0.19–0.42, P < 0.00001; OR 0.30, 95% CI 0.09–1.02, P = 0.05; OR 0.43, 95% CI 0.20–0.90, P = 0.02; and OR 1.80, 95% CI 1.26–2.56, P = 0.001, respectively. However, significant associations between Ki-67 and age, gender, smoking habits, and tumor number were not observed in BC patients. The combined ORs and 95% CIs were as follows: OR 1.02, 95% CI 0.41–2.54, P = 0.97; OR 1.09, 95% CI 0.83–1.43, P = 0.55; OR 1.28, 95% CI 0.86–1.89, P = 0.22; and OR 1.28, 95% CI 0.60–2.77, P = 0.52, respectively (Table 2).
Table 2. Results of subgroup analysis of the association between Ki-67 expression and clinicopathological parameters.
| Outcome of interest | Studies (n) | Patients | OR | 95%CI | P value | Model | Heterogeneity |
|---|---|---|---|---|---|---|---|
| Chi2, I2, P value | |||||||
| Age (≥65 vs. <65) | 2 | 293 | 1.02 | 0.41–2.54 | 0.97 | Random | 2.03, 51%, 0.15 |
| Gender (Male vs. Female) | 6 | 1551 | 1.09 | 0.83–1.43 | 0.55 | Fixed | 3.59, 0%, 0.61 |
| Asian | 3 | 522 | 0.89 | 0.56–1.43 | 0.63 | Fixed | 1.75, 0%, 0.42 |
| Non-Asian | 3 | 1029 | 1.20 | 0.86–1.68 | 0.29 | Fixed | 0.87, 0%, 0.65 |
| Smoke habits (Smoke vs. Non-smoke) | 1 | 588 | 1.28 | 0.86–1.89 | 0.22 | Fixed | - |
| Ta vs. T1 | 4 | 570 | 0.29 | 0.19–0.42 | 0.000 | Fixed | 3.20, 6%, 0.36 |
| Ta-1 vs. T2-4 | 3 | 1010 | 0.30 | 0.09–1.02 | 0.05 | Random | 12.97, 85%, 0.002 |
| Recurrence vs. No recurrence | 6 | 897 | 0.43 | 0.20–0.90 | 0.02 | Random | 18.64, 73%, 0.002 |
| Multiple vs. Single | 3 | 522 | 1.28 | 0.60–2.77 | 0.52 | Random | 6.28, 68%, 0.04 |
| Tumor size (<3 vs. ≥3cm) | 4 | 686 | 1.80 | 1.26–2.56 | 0.001 | Fixed | 3.46, 13%, 0.33 |
Fixed: Fixed, Inverse Variance model; I2: I-squared; OR: odd ratio; Random: Random, I-V heterogeneity model.
Publication bias
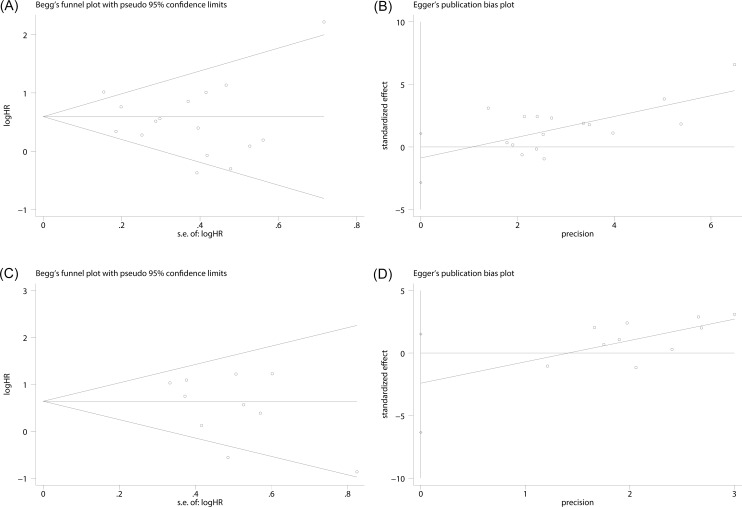
Publication bias was conducted by Begg’s test for RFS and PFS of bladder carcinoma, with P values of 0.964 and 0.152, respectively. (Fig 4A and 4C). Quantitative assessment by Egger’s test for RFS and PFS suggested that our analyses were stable (P = 0.350, P = 0.195) (Fig 4B and 4D).
Fig 4. Funnel plots were used to evaluate publication bias on RFS and PFS.
(A) Begg’s test was not significant intending no significant bias was observed on RFS. (B) Egger’s test was not significant intending no significant bias was observed on RFS. (C) It showed no publication bias on PFS in Begg’s test, (D) It showed no publication bias on PFS in Egger’s test.
Discussion
Increasing evidence indicates that BC genomes exhibiting the most complex alterations are associated with a high Ki-67 proliferation index [51]. Pichu et al. [52] reported that in BC cells, prior exposure to anti-Ki-67 siRNA induces tumor cells to undergo curcumin-induced growth arrest and apoptosis by non-p53 and non-p21-dependent signaling pathways, which may be useful for gene therapy. Wang et al. [53] reported that the combined effects of TP53 and Ki-67 revealed predictive value for NMIBC recurrence. However, the relationship between Ki-67 and outcome remains unclear, and the roles and clinical significance of Ki-67 expression in BC have not been thoroughly investigated [54].
In the present study, the analyses of the pooled data indicated that (1) BC patients with high Ki-67 expression had a lower survival rate; (2) high Ki-67 expression was associated with the more aggressive clinical stage and larger tumor size in BC patients; (3) aberrant Ki-67 expression was higher in recurrent BC than in non-recurrent BC; (4) Ki-67 expression was not strongly associated with age, gender, and tumor number in BC patients; (5) a strong relationship between poor prognostic indicators and Ki-67 expression was established only for European-American patients. The correlation between Ki-67 expression and survival outcome (RFS/PFS/OS/CSS) did not reach statistical significance in Asian patients. Our study provides insights on the results of individual studies focused on the hypothesis that Ki-67 is a prognostic factor for BC and suggests that adjuvant therapy may be helpful in the high-risk subgroup of patients. Although further validation and investigation are needed, these data provide new insights on the biological aggressiveness of BC in Asian versus in non-Asian patients.
The biological mechanism of Ki-67 explains its prognostic significance in BC. Ki-67 is an index of cell proliferation and a measure of cell growth fraction during the G1, S, G2 and M stages of the cell cycle and is widely applied in immunohistochemistry (IHC) to estimate the activities of cell proliferation in many cancers. Some researches investigated the relationships between the Ki-67 and distant metastases [55, 56]. They found that Ki-67 expression was upregulated in the transforming growth factor-β1 (TGF-β1) treated tumors, and TGF-β1 promotes EMT (epithelial-to-mesenchymal transition), migration, and invasion in bladder cancer cells [57]. Furthermore, it was showed that highly Ki-67 may induce EMT by increasing the expression of vimentin, which enhances cancer cell invasion and metastatic [58].
The present meta-analysis is the first study to systematically evaluate the associations between Ki-67 expression and clinicopathological features and prognostic factors in BC. Ki-67 can be considered an oncogene, and its activation may contribute to tumor progression and poor prognosis. Based on this meta-analysis, we suggest that Ki-67 expression in BC tends to indicate a poor prognosis.
Several limitations of this study must be acknowledged. In the included studies, the antibodies used to detect Ki-67 expression were not identical (anti-Ki67 mAb and anti-MIB-1 mAb). The definitions of the cut-off value also differed. Clinical factors such as race, age, and the use of different chemotherapies in each study may also be sources of bias. Non-English studies, unpublished studies, and studies that did not provide sufficient data to calculate HRs were not included in the assessment of the predictive value of Ki-67 for survival. These approaches may have produced errors due to the inclusion of inaccurate readings. Finally, although we included 31 studies comprising 5147 cases in this meta-analysis, few studies were categorized for subgroup analysis, and several survival subgroup analyses data lack. Therefore, more well-designed and large-scale trials are needed to confirm these findings.
In conclusion, our meta-analysis confirmed the significant associations between Ki-67 expression and clinicopathological features and prognostic factors in BC. Although subgroup analysis indicated no significant association between Ki-67 expression and BC prognosis in Asian patients. Our meta-analysis demonstrates that Ki-67 has a detrimental effect on clinicopathological features and recurrence status in BC. Therefore, Ki-67 could serve as an independent prognostic factor of RFS, PFS, OS and CSS in European-American patients. Ki-67 may be a novel candidate for BC genotyping and an indicator for predicting the prognosis of BC patients.
Supporting Information
HR of Ki-67 expression associated with RFS in all BC patients subgroup. Abbreviations: HR, hazard ratio; RFS, recurrence-free survival; BC, bladder cancer.
(TIF)
. HR of Ki-67 expression associated with RFS in all BC patients subgroup. Abbreviations: HR, hazard ratio; RFS, recurrence-free survival; BC, bladder cancer.
(TIF)
. HR of Ki-67 expression associated with RFS in all BC patients subgroup. Abbreviations: HR, hazard ratio; RFS, recurrence-free survival; BC, bladder cancer.
(TIF)
Abbreviations: HR, hazard ratio; OS, overall survival; BC, bladder cancer.
(TIF)
Abbreviations: HR, hazard ratio; CSS, cancer-specific survival; BC, bladder cancer.
(TIF)
(DOCX)
(DOCX)
(DOC)
(RAR)
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No.81372733/H1619), the National Science Foundation of China (No. 81302240), the Fundamental Scientific Research Fund for Colleges and Universities Directly Under the Ministry of Education (No. lzujbky-2014-165), and the Twelfth Five-Year National Science and Technology Support Program projects (2012BAI10B01).
Data Availability
The data in this study were extracted from previous studies, a list of which is included in the Supporting Information.
Funding Statement
This study was supported by the National Natural Science Foundation of China (grant no. 81372733/H1619), the National Science Foundation of China (no. 81302240), the Fundamental Scientific Research Fund for Colleges and Universities Directly Under the Ministry of Education (no. lzujbky-2014-165), and the Twelfth Five-Year National Science and Technology Support Program projects (2012BAI10B01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011; 306:737–45. 10.1001/jama.2011.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014; 507:315–22. 10.1038/nature12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotan Y, Svatek RS, Sagalowsky AI. Should we screen for bladder cancer in a high-risk population?: A cost per life-year saved analysis. Cancer. 2006; 107:982–90. 10.1002/cncr.22084 [DOI] [PubMed] [Google Scholar]
- 5.Avritscher EB, Cooksley CD, Grossman HB, Sabichi AL, Hamblin L, Dinney CP, et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006; 68:549–53. 10.1016/j.urology.2006.03.062 [DOI] [PubMed] [Google Scholar]
- 6.Schluter C, Duchrow M, Wohlenberg C, Becker MH, Key G, Flad HD, et al. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993; 123:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krabbe LM, Bagrodia A, Haddad AQ, Kapur P, Khalil D, Hynan LS, et al. Multi-institutional validation of the predictive value of Ki-67 in patients with high grade urothelial carcinoma of the upper urinary tract. J Urol. 2015; 193:1486–93. 10.1016/j.juro.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 8.Shui R, Yu B, Bi R, Yang F, Yang W. An interobserver reproducibility analysis of Ki67 visual assessment in breast cancer. PLoS One. 2015; 10:e0125131 10.1371/journal.pone.0125131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen S, Zhou W, Li CM, Hu J, Hu XM, Chen P, et al. Ki-67 as a prognostic marker in early-stage non-small cell lung cancer in Asian patients: a meta-analysis of published studies involving 32 studies. BMC Cancer. 2015; 15:520 10.1186/s12885-015-1524-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gayed BA, Youssef RF, Bagrodia A, Darwish OM, Kapur P, Sagalowsky A, et al. Ki67 is an independent predictor of oncological outcomes in patients with localized clear-cell renal cell carcinoma. BJU Int. 2014; 113:668–73. 10.1111/bju.12263 [DOI] [PubMed] [Google Scholar]
- 11.Chen JX, Deng N, Chen X, Chen LW, Qiu SP, Li XF, et al. A novel molecular grading model: combination of Ki67 and VEGF in predicting tumor recurrence and progression in non-invasive urothelial bladder cancer. Asian Pac J Cancer Prev. 2012; 13:2229–34. [DOI] [PubMed] [Google Scholar]
- 12.Makboul R, Refaiy AE, Badary FA, Abdelkawi IF, Merseburger AS, Mohammed RA. Expression of survivin in squamous cell carcinoma and transitional cell carcinoma of the urinary bladder: a comparative immunohistochemical study. Korean J Urol. 2015; 56:31–40. 10.4111/kju.2015.56.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gontero P, Gillo A, Fiorito C, Oderda M, Pacchioni D, Casetta G, et al. Prognostic factors of 'high-grade' Ta bladder cancers according to the WHO 2004 classification: are these equivalent to 'high-risk' non-muscle-invasive bladder cancer? Urol Int. 2014; 92:136–42. 10.1159/000351961 [DOI] [PubMed] [Google Scholar]
- 14.Tanabe K, Yoshida S, Koga F, Inoue M, Kobayashi S, Ishioka J, et al. High Ki-67 Expression Predicts Favorable Survival in Muscle-Invasive Bladder Cancer Patients Treated With Chemoradiation-Based Bladder-Sparing Protocol. Clin Genitourin Cancer. 2015; 13:e243–51. 10.1016/j.clgc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 15.Acikalin D, Oner U, Can C, Acikalin MF, Colak E. Predictive value of maspin and Ki-67 expression in transurethral resection specimens in patients with T1 bladder cancer. Tumori. 2012; 98:344–50. 10.1700/1125.12403 [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 18.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005; 21:3672–3. 10.1093/bioinformatics/bti536 [DOI] [PubMed] [Google Scholar]
- 19.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006; 295:676–80. 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 20.Kamai T, Takagi K, Asami H, Ito Y, Oshima H, Yoshida KI. Decreasing of p27(Kip1)and cyclin E protein levels is associated with progression from superficial into invasive bladder cancer. Br J Cancer. 2001; 84:1242–51. 10.1054/bjoc.2000.1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruger S, Thorns C, Stocker W, Muller-Kunert E, Bohle A, Feller AC. Prognostic value of MCM2 immunoreactivity in stage T1 transitional cell carcinoma of the bladder. Eur Urol. 2003; 43:138–45. [DOI] [PubMed] [Google Scholar]
- 22.Santos LL, Amaro T, Pereira SA, Lameiras CR, Lopes P, Bento MJ, et al. Expression of cell-cycle regulatory proteins and their prognostic value in superficial low-grade urothelial cell carcinoma of the bladder. Eur J Surg Oncol. 2003; 29:74–80. [DOI] [PubMed] [Google Scholar]
- 23.Gakiopoulou-Givalou H, Nakopoulou L, Panayotopoulou EG, Zervas A, Mavrommatis J, Giannopoulos A. Non-endothelial KDR/flk-1 expression is associated with increased survival of patients with urothelial bladder carcinomas. Histopathology. 2003; 43:272–9. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, Quintero A, Merlo F, Requena MJ, et al. Prognostic factors in survival of patients with stage Ta and T1 bladder urothelial tumors: the role of G1-S modulators (p53, p21Waf1, p27Kip1, cyclin D1, and cyclin D3), proliferation index, and clinicopathologic parameters. Am J Clin Pathol. 2004; 122:444–52. 10.1309/LTFU-3UUM-BY09-5HUM [DOI] [PubMed] [Google Scholar]
- 25.Mahnken A, Kausch I, Feller AC, Kruger S. E-cadherin immunoreactivity correlates with recurrence and progression of minimally invasive transitional cell carcinomas of the urinary bladder. Oncol Rep. 2005; 14:1065–70. [PubMed] [Google Scholar]
- 26.Theodoropoulos VE, Lazaris AC, Kastriotis I, Spiliadi C, Theodoropoulos GE, Tsoukala V, et al. Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU Int. 2005; 95:425–31. 10.1111/j.1464-410X.2005.05314.x [DOI] [PubMed] [Google Scholar]
- 27.Weiss C, Rodel F, Wolf I, Papadopoulos T, Engehausen DG, Schrott KM, et al. Combined-modality treatment and organ preservation in bladder cancer. Do molecular markers predict outcome? Strahlenther Onkol. 2005; 181:213–22. 10.1007/s00066-005-1417-4 [DOI] [PubMed] [Google Scholar]
- 28.Mylona E, Magkou C, Gorantonakis G, Giannopoulou I, Nomikos A, Zarogiannos A, et al. Evaluation of the vascular endothelial growth factor (VEGF)-C role in urothelial carcinomas of the bladder. Anticancer Res. 2006; 26:3567–71. [PubMed] [Google Scholar]
- 29.Galmozzi F, Rubagotti A, Romagnoli A, Carmignani G, Perdelli L, Gatteschi B, et al. Prognostic value of cell cycle regulatory proteins in muscle-infiltrating bladder cancer. J Cancer Res Clin Oncol. 2006; 132:757–64. 10.1007/s00432-006-0123-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilmy M, Campbell R, Bartlett JM, McNicol AM, Underwood MA, McMillan DC. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic infiltration and COX-2 expression and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer. 2006; 95:1234–8. 10.1038/sj.bjc.6603415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quintero A, Alvarez-Kindelan J, Luque RJ, Gonzalez-Campora R, Requena MJ, Montironi R, et al. Ki-67 MIB1 labelling index and the prognosis of primary TaT1 urothelial cell carcinoma of the bladder. J Clin Pathol. 2006; 59:83–8. 10.1136/jcp.2004.022939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yurakh AO, Ramos D, Calabuig-Farinas S, Lopez-Guerrero JA, Rubio J, Solsona E, et al. Molecular and immunohistochemical analysis of the prognostic value of cell-cycle regulators in urothelial neoplasms of the bladder. Eur Urol. 2006; 50:506–15; discussion 15. 10.1016/j.eururo.2006.03.027 [DOI] [PubMed] [Google Scholar]
- 33.Shariat SF, Bolenz C, Godoy G, Fradet Y, Ashfaq R, Karakiewicz PI, et al. Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J Urol. 2009; 182:78–84; discussion 10.1016/j.juro.2009.02.125 [DOI] [PubMed] [Google Scholar]
- 34.Margulis V, Lotan Y, Karakiewicz PI, Fradet Y, Ashfaq R, Capitanio U, et al. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst. 2009; 101:114–9. 10.1093/jnci/djn451 [DOI] [PubMed] [Google Scholar]
- 35.Maeng YH, Eun SY, Huh JS. Expression of fibroblast growth factor receptor 3 in the recurrence of non-muscle-invasive urothelial carcinoma of the bladder. Korean J Urol. 2010; 51:94–100. 10.4111/kju.2010.51.2.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo HK, Cho KS, Chung J, Joung JY, Park WS, Chung MK, et al. Prognostic value of p53 and Ki-67 expression in intermediate-risk patients with nonmuscle-invasive bladder cancer receiving adjuvant intravesical mitomycin C therapy. Urology. 2010; 76:512 e1-7. 10.1016/j.urology.2010.04.040 [DOI] [PubMed] [Google Scholar]
- 37.Behnsawy HM, Miyake H, Abdalla MA, Sayed MA, Ahmed Ael F, Fujisawa M. Expression of cell cycle-associated proteins in non-muscle-invasive bladder cancer: correlation with intravesical recurrence following transurethral resection. Urol Oncol. 2011; 29:495–501. 10.1016/j.urolonc.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 38.Wosnitzer MS, Domingo-Domenech J, Castillo-Martin M, Ritch C, Mansukhani M, Petrylack DP, et al. Predictive value of microtubule associated proteins tau and stathmin in patients with nonmuscle invasive bladder cancer receiving adjuvant intravesical taxane therapy. J Urol. 2011; 186:2094–100. 10.1016/j.juro.2011.06.051 [DOI] [PubMed] [Google Scholar]
- 39.Youssef RF, Shariat SF, Kapur P, Kabbani W, Ghoneim T, King E, et al. Expression of cell cycle-related molecular markers in patients treated with radical cystectomy for squamous cell carcinoma of the bladder. Hum Pathol. 2011; 42:347–55. 10.1016/j.humpath.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 40.Bi J, Chen X, Zhang Y, Li B, Sun J, Shen H, et al. Fascin is a predictor for invasiveness and recurrence of urothelial carcinoma of bladder. Urol Oncol. 2012; 30:688–94. 10.1016/j.urolonc.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 41.Shan GY, Zhang Z, Chen QG, Yu XY, Liu GB, Kong CZ. Overexpression of RIN1 associates with tumor grade and progression in patients of bladder urothelial carcinoma. Tumour Biol. 2012; 33:847–55. 10.1007/s13277-011-0311-1 [DOI] [PubMed] [Google Scholar]
- 42.Oderda M, Ricceri F, Pisano F, Fiorito C, Gurioli A, Casetta G, et al. Prognostic factors including Ki-67 and p53 in Bacillus Calmette-Guerin-treated non-muscle-invasive bladder cancer: a prospective study. Urol Int. 2013; 90:184–90. 10.1159/000343431 [DOI] [PubMed] [Google Scholar]
- 43.Otto W, Denzinger S, Fritsche HM, Burger M, Rossler W, Bertz S, et al. Introduction and first clinical application of a simplified immunohistochemical validation system confirms prognostic impact of KI-67 and CK20 for stage T1 urothelial bladder carcinoma: single-center analysis of eight biomarkers in a series of three hundred six patients. Clin Genitourin Cancer. 2013; 11:537–44. 10.1016/j.clgc.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 44.Park J, Song C, Shin E, Hong JH, Kim CS, Ahn H. Do molecular biomarkers have prognostic value in primary T1G3 bladder cancer treated with bacillus Calmette-Guerin intravesical therapy? Urol Oncol. 2013; 31:849–56. 10.1016/j.urolonc.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 45.Ruan J, Wei B, Xu Z, Yang S, Zhou Y, Yu M, et al. Predictive value of Sox2 expression in transurethral resection specimens in patients with T1 bladder cancer. Med Oncol. 2013; 30:445 10.1007/s12032-012-0445-z [DOI] [PubMed] [Google Scholar]
- 46.Bertz S, Otto W, Denzinger S, Wieland WF, Burger M, Stohr R, et al. Combination of CK20 and Ki-67 immunostaining analysis predicts recurrence, progression, and cancer-specific survival in pT1 urothelial bladder cancer. Eur Urol. 2014; 65:218–26. 10.1016/j.eururo.2012.05.033 [DOI] [PubMed] [Google Scholar]
- 47.Wang LC, Xylinas E, Kent MT, Kluth LA, Rink M, Jamzadeh A, et al. Combining smoking information and molecular markers improves prognostication in patients with urothelial carcinoma of the bladder. Urol Oncol. 2014; 32:433–40. 10.1016/j.urolonc.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 48.Ding W, Gou Y, Sun C, Xia G, Wang H, Chen Z, et al. Ki-67 is an independent indicator in non-muscle invasive bladder cancer (NMIBC); combination of EORTC risk scores and Ki-67 expression could improve the risk stratification of NMIBC. Urol Oncol. 2014; 32:42 e13-9. 10.1016/j.urolonc.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 49.Poyet C, Buser L, Roudnicky F, Detmar M, Hermanns T, Mannhard D, et al. Connexin 43 expression predicts poor progression-free survival in patients with non-muscle invasive urothelial bladder cancer. J Clin Pathol. 2015; 68:819–24. 10.1136/jclinpath-2015-202898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Zhou M, Feng C, Gao P, Ding G, Zhou Z, et al. Prognostic value of Ki67 and p63 expressions in bladder cancer patients who underwent radical cystectomy. Int Urol Nephrol. 2016; 48:495–501. 10.1007/s11255-015-1197-4 [DOI] [PubMed] [Google Scholar]
- 51.Schepeler T, Lamy P, Hvidberg V, Laurberg JR, Fristrup N, Reinert T, et al. A high resolution genomic portrait of bladder cancer: correlation between genomic aberrations and the DNA damage response. Oncogene. 2013; 32:3577–86. 10.1038/onc.2012.381 [DOI] [PubMed] [Google Scholar]
- 52.Pichu S, Krishnamoorthy S, Shishkov A, Zhang B, McCue P, Ponnappa BC. Knockdown of Ki-67 by dicer-substrate small interfering RNA sensitizes bladder cancer cells to curcumin-induced tumor inhibition. PLoS One. 2012; 7:e48567 10.1371/journal.pone.0048567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Feng C, Ding G, Ding Q, Zhou Z, Jiang H, et al. Ki67 and TP53 expressions predict recurrence of non-muscle-invasive bladder cancer. Tumour Biol. 2014; 35:2989–95. 10.1007/s13277-013-1384-9 [DOI] [PubMed] [Google Scholar]
- 54.Bryan RT, Zeegers MP, James ND, Wallace DM, Cheng KK. Biomarkers in bladder cancer. BJU Int. 2010; 105:608–13. 10.1111/j.1464-410X.2009.08880.x [DOI] [PubMed] [Google Scholar]
- 55.Lan YJ, Chen H, Chen JQ, Lei QH, Zheng M, Shao ZR. Immunolocalization of vimentin, keratin 17, Ki-67, involucrin, beta-catenin and E-cadherin in cutaneous squamous cell carcinoma. Pathol Oncol Res. 2014; 20:263–6. 10.1007/s12253-013-9690-5 [DOI] [PubMed] [Google Scholar]
- 56.da Silva SD, Morand GB, Alobaid FA, Hier MP, Mlynarek AM, Alaoui-Jamali MA, et al. Epithelial-mesenchymal transition (EMT) markers have prognostic impact in multiple primary oral squamous cell carcinoma. Clin Exp Metastasis. 2015; 32:55–63. 10.1007/s10585-014-9690-1 [DOI] [PubMed] [Google Scholar]
- 57.Islam SS, Mokhtari RB, Noman AS, Uddin M, Rahman MZ, Azadi MA, et al. Sonic hedgehog (Shh) signaling promotes tumorigenicity and stemness via activation of epithelial-to-mesenchymal transition (EMT) in bladder cancer. Mol Carcinog. 2016; 55:537–51. 10.1002/mc.22300 [DOI] [PubMed] [Google Scholar]
- 58.Yu JQ, Zhou Q, Zheng YF, Bao Y. Expression of Vimentin and Ki-67 Proteins in Cervical Squamous Cell Carcinoma and their Relationships with Clinicopathological Features. Asian Pac J Cancer Prev. 2015; 16:4271–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HR of Ki-67 expression associated with RFS in all BC patients subgroup. Abbreviations: HR, hazard ratio; RFS, recurrence-free survival; BC, bladder cancer.
(TIF)
. HR of Ki-67 expression associated with RFS in all BC patients subgroup. Abbreviations: HR, hazard ratio; RFS, recurrence-free survival; BC, bladder cancer.
(TIF)
. HR of Ki-67 expression associated with RFS in all BC patients subgroup. Abbreviations: HR, hazard ratio; RFS, recurrence-free survival; BC, bladder cancer.
(TIF)
Abbreviations: HR, hazard ratio; OS, overall survival; BC, bladder cancer.
(TIF)
Abbreviations: HR, hazard ratio; CSS, cancer-specific survival; BC, bladder cancer.
(TIF)
(DOCX)
(DOCX)
(DOC)
(RAR)
Data Availability Statement
The data in this study were extracted from previous studies, a list of which is included in the Supporting Information.