Abstract
Background
A majority of subjects with irritable bowel syndrome (IBS) show increased behavioral and brain responses to expected and delivered aversive visceral stimuli during controlled rectal balloon distension, and during palpation of the sigmoid colon. We aimed to determine if altered brain responses to cued and uncued pain expectation are also seen in the context of a noxious somatic pain stimulus applied to the same dermatome as the sigmoid colon.
Methods
A task-dependent functional magnetic resonance imaging technique was used to investigate the brain activity of 37 healthy controls (18 females) and 37 IBS subjects (21 females) during: 1) a cued expectation of an electric shock to the abdomen versus a cued safe condition; and 2) an uncued cross-hair condition in which the threat is primarily based on context versus a cued safe condition.
Key Results
Regions within the salience, attention, default mode, and emotional arousal networks were more activated by the cued abdominal threat condition and the uncued condition than in the cued safe condition. During the uncued condition contrasted to the cued safe condition, IBS subjects (compared to healthy control subjects) showed greater brain activations in the affective (amygdala, anterior insula) and attentional (middle frontal gyrus) regions, and in the thalamus and precuneus. These disease-related differences were primarily seen in female subjects.
Conclusions & Inferences
The observed greater engagement of cognitive and emotional brain networks in IBS subjects during contextual threat may reflect the propensity of IBS subjects to overestimate the likelihood and severity of future abdominal pain.
Keywords: functional magnetic resonance imaging, pain expectations, contextual threat, irritable bowel syndrome, sex differences
Graphical Abstract
Introduction
The brain computes expected future outcomes in an uncertain world and selects optional coping strategies1 based on interactions between brain networks concerned with salience evaluation, attention and recall of past memories.2 Hypervigilance and anticipatory anxiety are often associated with prediction of future pain and can increase the subsequent subjective experience of a delivered pain stimulus,3, 4 presumably by the engagement of endogenous pain facilitation systems.1, 3, 5–7 Predictions about future pain can be manipulated by using threat designs that vary the predictability of aversive stimuli. In cued designs, there is a specific cue that gives the subject information about the probability of an aversive stimulus.6, 8–13 In uncued or contextual threat designs, there is no explicit threat cue or known threat interval, but instead the experimental environment itself is associated with aversive stimulation from prior experience.14–18 Cued expectation of painful stimuli is associated with activation in regions of the salience (insula [INS], anterior cingulate cortex [ACC]), sensorimotor and attentional control (parietal and frontal cortex) networks.19–22 The INS, amygdala and ACC play important roles in responding to an uncertain aversive stimulus and contextual threat.16, 22–24 These cued and uncued paradigms have been used to characterize brain abnormalities in subjects with anxiety disorders and post-traumatic stress disorder.1, 25, 26
Irritable bowel syndrome (IBS), a common gastrointestinal pain disorder, is characterized by chronically recurrent abdominal pain and discomfort associated with altered bowel habits. The majority of IBS subjects exhibit increased anxiety27 and gastrointestinal symptom-related worries, a measure of hypervigilance to gastrointestinal-related contexts.28 Many IBS subjects also show increased perceptual hypersensitivity to experimental visceral stimuli29–31 as well as altered responses in salience and pain processing regions both during cued expectation20, 32, 33 and during delivery of rectal pain stimuli, most consistently in INS, ACC and thalamus.34, 35 Greater engagement of an emotional arousal network including the anterior INS, amygdala and ACC has been suggested as a key neurobiological mechanism underlying hypervigilance and hypersensitivity in IBS subjects.29 Sex-related differences of brain activation have also been observed in response to experienced visceral pain and somatosensory stimuli9, 36–38 and during cued expectation of unpleasant stimuli.12, 39, 40
To examine responses to both cued and uncued threat conditions, the current study included explicit cued threat and safe conditions separated by uncued condition that can be considered in an exploratory fashion to provide contextual threat. By including three different conditions to evaluate the expectation of somatic pain stimuli (in the form of electrical shocks to the abdomen), we test two main hypotheses regarding brain responses to pain expectation: 1) Healthy controls (HCs) and IBS subjects show increased brain responses to both cued and uncued threat compared to a cued safe condition, similar to the responses previously reported for experienced experimental visceral pain (e.g. INS, thalamus, and amygdala). 2) Compared to HCs, IBS subjects have greater brain activations in regions of the salience and attentional networks as well as in sensory processing areas with the increased uncertainty of a threat. In addition, we examine whether the observed IBS-related responses differ by sex as a secondary hypothesis.
Materials and Methods
Subjects
37 right-handed IBS subjects and 37 right-handed HCs were recruited through the UCLA Digestive Disease Clinic and community advertisements. The sample of IBS subjects included 16 males and 21 females. IBS subtypes for the IBS males included 5 constipation predominant, 8 diarrhea predominant, 2 unspecified and 1 mixed. For females, subtypes included 8 constipation predominant, 3 diarrhea predominant, 2 unspecified and 8 mixed. The 37 healthy subjects included 19 males and 18 females. Exclusion criteria in all subjects comprised pregnancy, substance abuse, abdominal surgery, tobacco dependence, medications that affect the central nervous system (e.g. narcotics and opioids), oral contraceptives or psychiatric illness as determined by the Mini International Neuropsychiatric Interview.41 Diagnosis of IBS was made by a gastroenterologist or a nurse practitioner with expertise in functional gastrointestinal disorders based on the ROME III symptom criteria during a clinical examination.42 The diagnostic criteria include recurrent abdominal pain or discomfort in the past 3 months associated with two or more of the following: 1) pain/discomfort is relieved/improved by defecation 2) the onset of pain/discomfort is related to a change in frequency of stool 3) the onset of pain/discomfort is related to a change in the form (appearance) of stool. All procedures were approved by the UCLA Medical Institutional Review Board, and all subjects provided informed consent.
Questionnaires
Questionnaires were completed before scanning to determine IBS symptom classification, severity, duration of symptoms, and abdominal sensation during the past week [UCLA Bowel Symptom Questionnaire, BSQ];43 levels of anxiety and depression [Hospital Anxiety Depression Scale, HAD];44 as well as 21 point verbal descriptor anchored numerical rating scales for intensity and unpleasantness of pain associated with the abdominal stimulus.45
Pain threshold assessment procedure
Individual pain threshold was assessed before imaging data acquisition. Abdominal electrical stimulation was performed using two electrode stimulation pads placed on the left side of subjects’ lower abdomen in the region overlaying the sigmoid colon. Transcutaneous electrical stimulation to the abdomen was delivered with a Digitimer constant-current stimulator (model DS7A; Digitimer). Each stimulus consisted of a pulse train lasting 750ms with 2ms pulse width and frequency of 37 Hz. Shock level for the abdominal stimulation was individually set based on an ascending method of limits work-up procedure. The work-up began with a mild current intensity of 0.5 mA and increased 0.5 mA steps until subjects described the stimulation as ‘aversive but tolerable’. After a brief rest period, a test stimulus at the aversive but tolerable threshold was given to subjects. Immediately after the stimulation, subjects were asked to rate the maximum intensity and unpleasantness of the pain they just felt using the Gracely Pain Scales.45
Expectation of abdominal pain paradigm
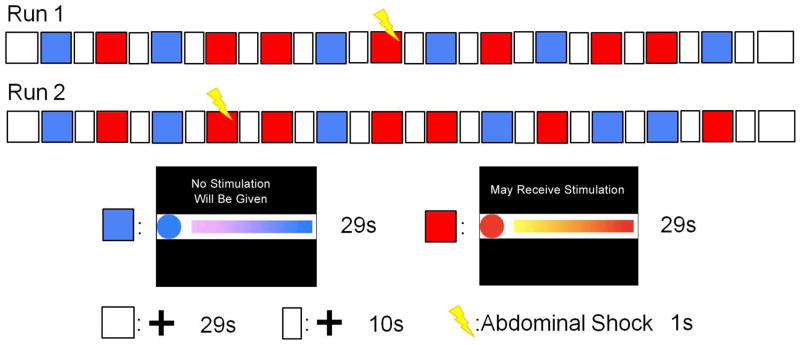
Before the anticipation of abdominal pain experimental paradigm began, subjects were told that they would see three types of visual images. The first type of image was an animation that included a blue circle with a colored bar moving to the right of the blue circle. The moving bar was elongating while simultaneously adding gradient colors, from purple to blue, indicating how much time was left in this condition (Figure 1). In addition, a specific sentence “No Stimulation Will Be Given” was shown on the screen in this condition. Before and during this cued safe condition, the subjects were specifically told that they would not receive an electrical stimulation through the electrodes attached to their abdomen. The second cued period was signaled by an animation that included a red circle and a colored bar moving to the right of the red circle. The moving bar was elongating while simultaneously adding gradient colors, from yellow to red, indicating how much time was left in this condition (Figure 1). In addition, a specific sentence “May Receive Stimulation” was presented on the screen in this condition. During this cued threat condition, the subjects were specifically informed that they may receive an electrical stimulation at any time, the magnitude of which would match the level that they previously described as aversive but tolerable during the pain thresholding procedure. The third image was a stationary cross-hair shown in the middle of the screen with a white background. Before the imaging acquisition began, the subjects were told that stimulation would not occur during the cross-hair periods but were asked to focus on the cross-hair. During the actual condition, two electrodes were attached to the subjects’ lower abdomen and there was neither an ongoing explicit safety cue nor a specific sentence shown on the screen. In addition, there was not a moving bar to indicate how much time was left in this condition. Although set up to serve as uncued periods to separate the cued conditions, we hypothesize that these cross-hair periods represent a contextual threat due to the prior history of shock and the continued presence of the abdominal electrodes, coupled with an absence of explicit ongoing cues for either safety or imminent shock or period duration. These periods will therefore be referred to as uncued or contextual threat periods below. The anticipation of abdominal pain procedure included two separate scanning runs. Each run contained seven cued threat and six cued safe conditions, and each of these conditions lasted 29 seconds. A 10-second cross-hair condition was in between each cued condition. At the beginning and end of each run, a 29 second cross-hair period was also presented. Although subjects were told that they would receive an electrical stimulation at any time during the cued threat condition, in fact, the one-second shock was only delivered once in each run. For run 1, the shock was delivered at the twelfth second of the forth cued threat condition; for run 2, the shock was administered at the fifth second of the second cued threat condition. The presentations of conditions and timings were generated by E-Prime v2 (Psychology Software Tools, Inc, USA) and are shown in Figure 1.
Figure 1. The experimental paradigm of anticipation to abdominal shock.
There were two scanning runs, each run contained six cued safe conditions (lasted 29 second), seven cued threat conditions (lasted 29 seconds) and a shock within a threat condition. At the start and end of each run as well as between each 29-second condition, a crosshair with white background was presented for 29 seconds and 10 seconds, respectively. The blue block represents a cued safe condition with a description, a blue circle and a moving colored blue bar indicating how much time is left. The red block represents a cued threat condition with a description, a red circle and a moving colored red bar also indicating how much time is left in this trial. The white block represents a cross-hair condition.
Functional magnetic resonance imaging (fMRI) data acquisition
Brain activity during the task was measured using a Siemens 3 Tesla Trio scanner with echo planar sequence, repetition time: 2000ms, echo time: 28ms, flip angle: 77 degrees, slice thickness: 4mm, and 40 slices obtained with whole-brain coverage. For registration purposes, high resolution structural images were collected with standard T1-weighted magnetization-prepared rapid acquisition gradient echo, repetition time: 2200ms, echo time: 3.26ms, flip angle: 9 degrees, slice thickness: 1 mm, 176 slices, 256 × 256 voxel matrices, and 1.0×1.0×1.0 mm voxel size.
fMRI preprocessing
Prior to statistical analysis, all imaging data was preprocessed using Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Centre for the Study of Cognitive Neurology, London, UK). Images were first converted from DICOM into NIFTI format followed by slice timing correction to adjust for differences in slice acquisition times. Realignment was performed to estimate the 6 variable parameters of rigid body transformation and control for superfluous motion. High resolution T1-weighted magnetization-prepared rapid acquisition gradient echo images were used to align functional images for normalization into the standard Montreal Neurological Institute brain space (2mm isotropic). All images were smoothed with 8mm full width of the kernel at half its maximum height.
Statistical analysis
The experiment was analyzed in a block design. The general linear model was applied in SPM8 to determine brain activity during the three conditions (cued threat, cued safe and uncued threat). At the subject-level, regressors for the three conditions were convolved with a canonical hemodynamic response function. In avoidance of contamination from the brain responses to the shocks, the two cued threat periods with shocks were excluded from our analysis. Motion realignment parameters were also included as covariates. Individual brain responses for the cued threat condition and the uncued condition were determined by contrasting the estimated parameters with the cued safe condition (i.e. cued threat vs. cued safe and uncued threat vs. cued safe, respectively). The first level contrast maps (one map was cued threat vs. cued safe and the other map was uncued threat vs. cued safe) were used as the dependent variable in second level whole brain group analyses. Using the full factorial model option in SPM8, we specified group (male HCs, female HCs, male IBS and female IBS) as a factor and age, anxiety and depression scores as covariates. The cerebellum was excluded using an explicit mask. To examine the overall effect of the experimental conditions across groups, global conjunction analysis was performed for the contrasts of cued threat vs. cued safe and uncued threat vs. cued safe. To examine the main aim, linear contrasts were specified to test for main effects of disease (IBS vs. HCs). For the secondary hypothesis, linear contrasts were specified to examine whether the disease effect differed by sex (female IBS vs. female HCs and male IBS vs. male HCs). Whole brain statistical parametric maps were first thresholded at voxelwise p=0.005 uncorrected. To account for multiple comparisons and avoid Type I error, Monte Carlo simulation implemented in AlphaSim program (http://afni.nimh.nih.gov) was performed at 10,000 iterations. Statistical significance (p<0.05, corrected) was achieved with a minimum cluster size of 120 contiguous voxels.
Clinical characteristics analysis
Specifying group as a four level factor (female HCs, male HCs, female IBS, male IBS), a one way ANOVA and post-hoc comparisons were performed to test for group differences in age, anxiety and depression, and abdominal pain ratings using SPSS version 22 software. Post hoc comparisons included female HCs vs. male HCs, female HCs vs. female IBS, male HCs vs. male IBS, and female IBS vs. male IBS. All p values were corrected for multiple comparisons using FDR correction at 5%.46, 47 Independent sample t-tests were performed to examine BSQ measures between male IBS and female IBS subjects.
Results
Clinical characteristics
Detailed clinical and psychological characteristics of the four groups are summarized in Table 1. No significant differences between the four groups were observed in terms of age, abdominal pain threshold, pre- and post-abdominal pain intensity and unpleasantness. However, significant group differences were observed for anxiety and depression symptom scores (F=5.96, p=.001 and F=5.5, p=.002, respectively). Post-hoc analysis revealed that IBS subjects had significantly greater anxiety and depression scores than HCs (t=3.94, q<.001 and t=3.58, q<.001, respectively, corrected). In addition, amongst women, female IBS subjects showed significantly greater anxiety and depression scores than female HCs (t=3.87, q<.001 and t=3.66, q<.001, respectively, corrected). However, no significant IBS-related difference was observed in anxiety and depression scores in males (t=1.65, q=.215 for anxiety and t=1.26, q=.214 for depression, corrected). There were no significant differences in anxiety, depression and bowel symptom scores between male and female IBS subjects.
Table 1.
Clinical characteristics
| HCM | HCF | IBSM | IBSF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | F/t | Sig. | |
| Age | 19 | 36.37 | 12.12 | 18 | 30.11 | 10.99 | 16 | 33.69 | 11.71 | 21 | 31 | 9.39 | 1.24 | .302 |
| Anxiety1 | 19 | 3.63 | 2.14 | 18 | 2.11 | 1.64 | 16 | 5.75 | 5.12 | 21 | 6.9 | 5.02 | 5.96 | .001 |
| Depression1 | 19 | 1.74 | 1.45 | 18 | .67 | 1.14 | 16 | 2.56 | 2.25 | 21 | 3.14 | 2.67 | 5.5 | .002 |
| Overall bowel symptoms2 | 15 | 10.6 | 3.52 | 21 | 8.1 | 4.84 | 1.71 | .097 | ||||||
| Abdominal pain3 | 16 | 10.13 | 3.9 | 21 | 7.86 | 4.21 | 1.68 | .103 | ||||||
| Abdominal discomfort4 | 16 | 9.25 | 5.71 | 21 | 10.86 | 5.98 | .83 | .414 | ||||||
| Duration of symptoms5 | 12 | 13.58 | 9.03 | 19 | 11.26 | 8.96 | .7 | .49 | ||||||
| Threshold6 | 19 | 3.37 | 1.74 | 18 | 3.11 | 1.45 | 16 | 3.91 | 2.37 | 21 | 2.79 | 1.63 | 1.22 | .309 |
| Pre intensity7 | 19 | 12.16 | 4.03 | 18 | 12.33 | 2.93 | 16 | 12.31 | 2.6 | 21 | 12.33 | 3.44 | .01 | .998 |
| Pre unpleasant7 | 19 | 9.16 | 3.79 | 18 | 9.33 | 2.2 | 16 | 9.81 | 2.14 | 21 | 10.43 | 2.64 | .82 | .485 |
| Post intensity8 | 19 | 11.16 | 3.89 | 18 | 11.78 | 3.49 | 16 | 12.31 | 2.75 | 21 | 11.29 | 4.33 | .35 | .79 |
| Post unpleasant8 | 19 | 9.05 | 3.54 | 18 | 9.72 | 2.87 | 16 | 10.13 | 2.5 | 21 | 10.29 | 3.26 | .6 | .614 |
F/t: F values and t values from ANOVA and t-tests for four and two group comparisons, respectively.
Hospital Anxiety and Depression;
BSQ overall symptoms in the past week (0–20; 0: no symptoms; 20: the most intense symptoms imaginable);
BSQ abdominal pain in the past week (0–20; 0: no pain; 20: the most intense pain imaginable);
BSQ discomfort in the past week (0–20; 0: no sensation; 20: the most intense sensation imaginable);
BSQ duration in years, derived from onset of symptom;
Averaged abdominal stimulation threshold (mA) which was described as aversive but tolerable;
Pre abdominal threat of pain intensity and unpleasantness (for intensity of pain: range 0–20, with 0 representing no sensation and 18 signifying extremely intense; for unpleasantness of pain: range 0–20, with 0 representing neutral unpleasantness and 17 signifying very intolerable unpleasantness);
Post abdominal threat of pain intensity and unpleasantness (0–20); Statistically significant p<0.05; SD: standard deviation
Brain responses associated with cued pain expectation and contextual threat
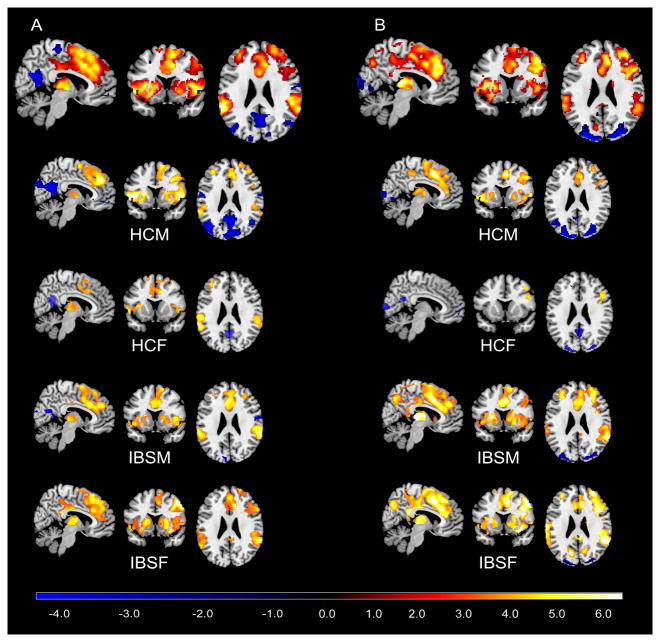
For the contrast of cued threat versus cued safe, the conjunction analysis showed significant brain responses bilaterally in the dorsal medial frontal cortex (BA8/32), middle frontal gyrus (MFG) (BA46/45/10), inferior parietal lobe, ACC, mid cingulate cortex, posterior cingulate cortex (PCC), anterior INS, supplementary motor area, thalamus, basal ganglia, amygdala, supramarginal gyrus, occipital cortex as well as the right precentral gyrus and right precuneus (Figure 2A and Table S1). The same brain regions were activated in response to the uncued condition compared to cued safe condition, with additional activation in the postcentral gyrus (Figure 2B and Table S2) supporting the hypothesis of the cross-hair representing an uncued contextual threat.
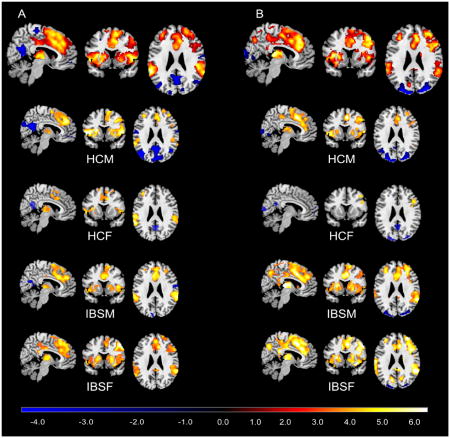
Figure 2. Brain activation maps of conjunction analysis.
(A) Brain responses associated with the contrast of cued threat versus cued safe. (B) Brain responses associated with contextual threat. All significantly statistical results (p<.05, corrected) were overlapped on a MRIcron ch2better template. The color bar indicates Z-scores. HCM: healthy male subjects; HCF: healthy female subjects; IBSM: male IBS subjects; IBSF: female IBS subjects.
Disease-related differences in brain responses associated with cued and uncued pain expectation
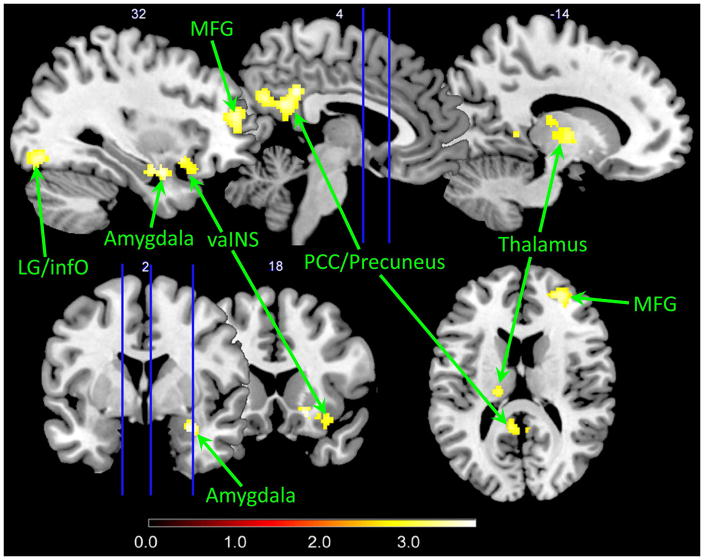
During the uncued condition contrasted to the cued safe condition, IBS subjects (compared to HCs) showed significantly greater brain responses in the right amygdala, right ventral anterior INS, right MFG (BA46 extending ventrally to BA10), right inferior occipital cortex, left thalamus, PCC and precuneus, regardless of sex (Table 2 and Figure 3). It is worth noting that the greater brain responses in the PCC/precuneus for the IBS subjects versus HCs contrast were due specifically to negative activation in HCs and positive activation in IBS subjects (Figure 2B). In the contrast of cued threat versus cued safe, IBS subjects had greater activation in the left inferior occipital cortex compared to HCs (Table 2). There was no significantly greater response in HCs compared to IBS subjects in the two contrasts.
Table 2.
Disease-related differences in brain responses
| Contrast | H | Region | Voxels | Z | x | y | z |
|---|---|---|---|---|---|---|---|
| cross-hair vs. cued safe
| |||||||
| IBS>HC | PCC/precuneus | 500 | 3.35 | 4 | −42 | 34 | |
| R | lingual/Inf occipital | 319 | 3.44 | 24 | −84 | −2 | |
| R | amygdala/vaINS | 235 | 3.46 | 30 | 2 | −22 | |
| 2.93 | 32 | 14 | −14 | ||||
| R | MFG (BA46/10) | 206 | 3.4 | 30 | 48 | 16 | |
| L | thalamus | 178 | 3.1 | −18 | −22 | 14 | |
|
| |||||||
| cued threat vs. cued safe
| |||||||
| IBS>HC | L | Inf occipital | 294 | 3.37 | −28 | −94 | −6 |
MNI coordinates (x,y,z) for peak voxels or suprathreshold voxels; Z: Z-values of peak voxels or suprathreshold voxels within the significant clusters (voxel threshold p<.005; cluster-corrected p<.05); H: hemisphere; L: left; R: right; vaINS: ventral anterior insula; PCC: posterior cingulate cortex; Lingual: lingual gyrus; Inf occipital: inferior occipital cortex; IBS: patients with irritable bowel syndrome; HC: healthy controls
Figure 3. Disease-related differences in brain responses during contextual threat.
Significantly greater responses were observed in patients with IBS compared to HCs (p<.05, corrected). All significantly statistical results (p<.05, corrected) were overlapped on a MRIcron ch2better template. The color bar indicates Z-scores. MFG: middle frontal gyrus; vaINS: ventral anterior insula; PCC: posterior cingulate cortex; LG: lingual gyrus; infO: inferior occipital cortex
Sex-related differences in brain responses associated with contextual threat and cued pain expectation
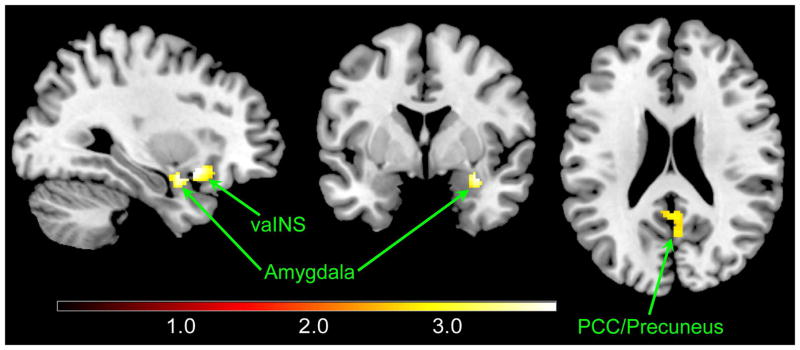
Female IBS subjects compared to female HCs showed greater brain signals in the right amygdala, right ventral anterior INS, PCC and precuneus in response to the uncued contextual threat (Table 3 and Figure 4). These differences were not observed when comparing male IBS subjects with male HCs.
Table 3.
Sex-related differences in brain responses
| Contrast | H | Region | Voxels | Z | x | y | z |
|---|---|---|---|---|---|---|---|
| cross-hair vs. cued safe
| |||||||
| IBSF>HCF | PCC/precuneus | 278 | 3.69 | 2 | −40 | 32 | |
| R | amygdala/vaINS | 246 | 4.08 | 32 | 14 | −12 | |
MNI coordinates (x,y,z) for peak voxel; Z: Z-values of peak voxels within the significant clusters (voxel threshold p<.005; cluster-corrected p<.05); H: hemisphere; R: right; PCC: posterior cingulate cortex; vaINS: ventral anterior insula; HCF: healthy females; IBSF: female IBS patients
Figure 4. Different brain responses associated to sex.
Significantly greater activations were shown in female IBS patients compared to female HCs in response to the cross-hair versus the cued safe. All significantly statistical results (p<.05, corrected) were overlapped on a MRIcron ch2better template. The color bars indicate Z-scores. vaINS: ventral anterior insula; PCC: posterior cingulate cortex
Discussion
The current study examined brain responses to cued and uncued abdominal threat in HCs and IBS subjects, and characterized disease and sex-related differences in these responses. As hypothesized, brain regions associated with salience, attention, cognitive evaluation and sensory processing were activated during both the cued and uncued threat conditions compared to the cued safe condition. However, disease-related differences in the brain responses were mainly seen in the contrast of uncued threat versus cued safe, where IBS subjects showed greater activations in affective (amygdala, INS), sensory (thalamus), attentional (MFG) and self-referential (precuneus) regions. In addition, these greater brain responses were primarily observed between female IBS subjects and female HCs.
Brain responses associated with cued pain expectation
The conjunction analysis findings during cued expectation of an aversive stimulus versus the cued safe condition were consistent with previous reports from studies using an analogous paradigm of pain expectation.12, 13, 19, 48 Confirming previously reported similarities of brain activation patterns during both delivered and expected aversive stimuli,13, 49 most of the regions activated during cued abdominal pain expectation in the current study have been found to be activated during experimental visceral pain paradigms.35 The reasons underlying these similarities are not known. However, as stimulus ratings differ significantly between the actual and the expected pain stimuli,39 one may speculate that the similar activation of the INS and ACC seen under both conditions is more reflective of the engagement of the salience network, rather than to activation of a “pain matrix” related to processing of aversive sensory information.
Brain responses associated with uncued threat
When the potential for an aversive stimulus application becomes ambiguous, and the timulus is not paired with a specific cue, the brain defaults to a strategy of making predictions about the stimulus based on the context in which a previous stimulus has been experienced.15, 50, 51 Such an ambiguous environmental state can be conceptualized as an uncertain threat about the pending event.52, 53 Studies have suggested that ambiguity regarding type or occurrence of a stimulus can lead to heightened behavioral and brain responses especially in subjects with anxiety.26, 53–55 When faced with such an ambiguous situation, the brain will respond with enhanced attention and attempt to predict likely outcomes.1, 56 The regions involved in this threat assessment include the INS, amygdala, striatum, ACC, thalamus, frontal and parietal cortex1, 24, 52, which were also observed in the current study. These activated brain regions are therefore consistent with our hypothesis that the period with a cross-hair created a contextual threat.
Disease-related differences in brain responses
Although similar brain regions were activated during the cued and uncued pain expectation, disease-related group differences in degree of activation were mainly observed when brain responses during the uncued condition were contrasted with those during the cued safe condition. There are several possible reasons to explain these findings. In the current study, the intensity of the abdominal shock stimuli was adjusted to the individual pain threshold, which did not show significant differences between IBS subjects and HCs. It is therefore possible that during the cued pain expectation, the brains of both IBS subjects and HCs compute a similar salience regarding the somatic pain experience, resulting in similar expectancy and perception of threat. Similar observations with electric stimulation of the gut have previously been reported.57 During the cross-hair periods without strong cues, the IBS subjects show significant differences in the affective and attentional brain regions likely due to greater affect in an ambiguous environment with the lack of predictive cues.22 This interpretation is consistent with findings in subjects with anxiety disorder who showed significantly greater startle responses and activation of the amygdala and anterior INS during an unpredictable threat condition, but not under a predictable threat condition.1, 25, 26, 58 The amygdala and ventral anterior INS are key regions in interoception, emotion, pain and salience processing59, 60 and the anterior INS has also been shown to be involved in outcome prediction.61 Consistent with such a role in the prediction of future events, both brain regions were also found to be activated during expectation of unpredictable negative events.16, 54, 62 In summary, considerable evidence supports the concept that the amygdala and anterior INS responses in IBS subjects during unpredictable threat are a reflection of altered salience and emotional arousal network engagement in IBS subjects.1, 63
In addition to the anterior INS and amygdala, we observed greater activation of the PCC and precuneus in IBS subjects compared to HCs during contextual threat. Some studies showed that during pain expectation, the precuneus and PCC were deactivated in HCs64 and exhibited decreased activation associated with expected pain intensity.49 People with anxiety had increased precuneus activity during reward anticipation, suggesting that these subjects were unable to disengage from self-attention, leading to increased self-focused hypervigilance.65 When viewed together, these reports are in line with the current findings that IBS subjects showed activation of the PCC and precuneus while HCs showed deactivation of these regions in response to contextual threat. The salience network has been suggested to play a role in switching between the default mode network and task-related networks.66, 67 Along with these findings, we speculate that IBS subjects have altered salience network function, affecting the regulation of PCC and precuneus activity68 during contextual threat.
IBS subjects also showed heightened MFG activation during contextual threat. The MFG is involved in decision making69 and self-generated attention processes.70 Activation of the MFG has been linked to attentional processes related to pain71, 72 and to unpredictable pain expectation.22 Healthy subjects showed robust activation in the MFG during expectation of heat pain,49 electric shocks,73 and laser pain,74 and these responses were positively correlated with the magnitude of anticipated pain intensity.75 When viewed together we speculate that the observed greater activation of the MFG in IBS subjects during contextual threat reflects greater allocation of attentional resources to the task, and that this abnormality may be related to the observed alterations in salience network engagement.76, 77
Greater activity in the thalamus was also observed in IBS subjects. Activation of thalamus has previously been reported in HCs in response to pain anticipation which was associated with emotional arousal and vigilance.78 A recent study in IBS subjects showed activation of the thalamus during both rectal distension and expectation of such stimuli.20 Although the role(s) of thalamus in IBS is still unclear, recent evidence has identified microstructural alterations in thalamic connections with the ACC and INS, and these alterations were associated with symptom severity.79
Sex-related differences in brain responses
In response to contextual threat, female IBS subjects showed greater amygdala, anterior INS, PCC and precuneus activity compared to female HCs, while no such differences were observed between male IBS subjects and male HCs. These sex-specific disease differences have also been demonstrated in our two previous resting-state fMRI studies in which female IBS subjects showed altered dynamics of the amygdala80 and altered functional connectivity between anterior INS and precuneus68 relative to female HCs, while these differences were not seen in males. Interestingly, female IBS subjects also had higher anxiety and depression symptom scores compared to female HCs, and these differences were not significant in males. Although we have controlled for symptoms of anxiety and depression in our analysis, it is possible that female IBS subjects had other affective characteristics (e.g. pain catastrophizing) contributing to the differences in brain responses.
Limitations
Due to sample size constraints, we were not able to do a complete analysis of sex effects and instead focused only on whether the disease effect differed by sex for areas found to be different in the primary group comparison. To more comprehensively understand sex-related differences, future studies are needed with increased samples of both IBS males and females and matched HCs.
Another limitation concerns the exploratory nature of the cross-hair periods as a contextual threat condition. The primary design was that of cued safe and cued threat periods separated by uncued periods with a simple cross-hair display. Although not a standard design for a contextual threat study which often manipulate contextual cues (e.g. taking off and putting on the shock electrodes), we hypothesize that the limited specific information that subjects received during the cross-hair periods, coupled with the same environmental context where the subject had previously experienced the shock, as well as the continued presence of the electrodes attached to the abdomen81 justify conceptualizing the cross-hair periods as incorporating a significant component of contextual threat. Although the results fit well with this hypothesis, further studies that manipulate contextual variables are needed to confirm the findings of altered IBS responses to contextual threat.
It should also be noted that the current study was designed to minimize conditioned responses and conditioning related to placebo/nocebo responses and was targeted to elucidating the mechanism of anxiety rather than fear. We therefore used a fewer number and greater temporal ambiguity of shock/cue pairings compared with standard conditioning studies.82, 83
Conclusions
The findings of this study emphasize an IBS-related abnormality in brain responses in the context of ambiguous expectation of an abdominal somatic pain stimulus. The findings suggest a central role of stimulus appraisal and salience detection in IBS related brain and presumably symptom responses. While both groups had brain responses to the cued pain expectation which presumably carries similar salience for both groups, the IBS subjects showed relatively larger responses in brain regions involved in salience detection, emotional arousal and self-consciousness during contextual threat. We speculate that these brain findings are related to hypervigilance and heightened reactivity particularly to the uncertainty of threat previously reported in IBS84–87 and anxiety disorders.1, 7, 26 Future studies that directly manipulate contextual cues and predictability will be important to follow up on these findings and compare these results across other chronic pain conditions.
Supplementary Material
Table S1. Brain responses associated with cued threat.
Table S2. Brain responses associated with contextual threat.
Key Messages.
The aim of this study was to determine if uncertainty related to the future visceral events influences brain responses in subjects with IBS.
The blood-oxygen-level dependent responses to cued and uncued expectation of abdominal pain contrasted with cued safe conditions were compared between 37 healthy control subjects and 37 subjects with IBS.
IBS subjects differ from healthy control subjects when there was no specific cue about the occurrence of abdominal pain stimulus, showing increased brain responses in multiple affective, sensory and cognitive brain regions.
These findings are consistent with the concept that when faced with making predictions about the occurrence of abdominal pain in an uncertain context, IBS subjects overestimate the likelihood of such an occurrence and engage brain networks involved in affective and sensory processing.
Acknowledgments
This study was supported by NIH grants P50 DK064539 (EAM), R01 DK048351 (EAM), and P30 DK041301 (ER). Pilot scans were provided by the Ahmanson-Lovelace Brain Mapping Center, UCLA. The authors would like to acknowledge Cathy Liu, Connor Fling and Kareem Hamadani for editorial assistance.
Abbreviations
- ACC
anterior cingulate cortex
- fMRI
functional magnetic resonance imaging
- HCs
healthy controls
- INS
insula
- IBS
irritable bowel syndrome
- MFG
middle frontal gyrus
- PCC
posterior cingulate cortex
Footnotes
Conflict of interest statement: The authors declare no competing financial interests.
Author Contributions:
Manuscript Preparation and Critical Revisions: JYH, BN, JSL, LAK, AG, and EAM
Study Design: BN, JSL, KT, and EAM
Data Acquisition: JYH, JS, NH, CAM, and SRS
Funding: LAK, JSL, KT, BN, and EAM
Data Analysis and Interpretation: JYH, JSL, BN and EAM
References
- 1.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it’s not what you thought it was. Am Psychol. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22:3206–14. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci. 2006;26:4437–43. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12:306–13. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44:975–81. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–81. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 9.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, Ruby K, Mayer EA. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R268–76. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- 10.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–77. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schunck T, Erb G, Mathis A, Jacob N, Gilles C, Namer IJ, Meier D, Luthringer R. Test-retest reliability of a functional MRI anticipatory anxiety paradigm in healthy volunteers. J Magn Reson Imaging. 2008;27:459–68. doi: 10.1002/jmri.21237. [DOI] [PubMed] [Google Scholar]
- 12.Kano M, Farmer AD, Aziz Q, Giampietro VP, Brammer MJ, Williams SC, Fukudo S, Coen SJ. Sex differences in brain response to anticipated and experienced visceral pain in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2013;304:G687–99. doi: 10.1152/ajpgi.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seifert F, Schuberth N, De Col R, Peltz E, Nickel FT, Maihofner C. Brain activity during sympathetic response in anticipation and experience of pain. Hum Brain Mapp. 2013;34:1768–82. doi: 10.1002/hbm.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grillon C, Baas JM, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biol Psychiatry. 2006;60:752–9. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 15.Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology (Berl) 2008;199:421–37. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20:929–40. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–24. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz A, Merikangas K, Swendsen H, Cui L, Heaton L, Grillon C. Measuring anxious responses to predictable and unpredictable threat in children and adolescents. J Exp Child Psychol. 2011;110:159–70. doi: 10.1016/j.jecp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaguez L, Coen S, Gregory LJ, Amaro E, Jr, Altman C, Brammer MJ, Bullmore ET, Williams SC, et al. Brain response to visceral aversive conditioning: a functional magnetic resonance imaging study. Gastroenterology. 2005;128:1819–29. doi: 10.1053/j.gastro.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 20.Larsson MB, Tillisch K, Craig AD, Engstrom M, Labus J, Naliboff B, Lundberg P, Strom M, et al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142:463–472. e3. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidel EM, Pfabigan DM, Hahn A, Sladky R, Grahl A, Paul K, Kraus C, Kublbock M, et al. Uncertainty during pain anticipation: The adaptive value of preparatory processes. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32:1804–14. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci Lett. 2008;430:92–7. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci. 2008;28:6211–9. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons AN, Flagan TM, Wittmann M, Strigo IA, Matthews SC, Donovan H, Lohr JB, Paulus MP. The effects of temporal unpredictability in anticipation of negative events in combat veterans with PTSD. J Affect Disord. 2013;146:426–32. doi: 10.1016/j.jad.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 28.Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- 29.Keszthelyi D, Troost FJ, Masclee AA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G141–54. doi: 10.1152/ajpgi.00060.2012. [DOI] [PubMed] [Google Scholar]
- 30.Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26(Suppl 3):119–21. doi: 10.1111/j.1440-1746.2011.06640.x. [DOI] [PubMed] [Google Scholar]
- 31.Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386–94. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–59. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–43. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheehan J, Gaman A, Vangel M, Kuo B. Pooled analysis of brain activity in irritable bowel syndrome and controls during rectal balloon distension. Neurogastroenterol Motil. 2011;23:336–46. e158. doi: 10.1111/j.1365-2982.2010.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benson S, Kattoor J, Kullmann JS, Hofmann S, Engler H, Forsting M, Gizewski ER, Elsenbruch S. Towards understanding sex differences in visceral pain: enhanced reactivation of classically-conditioned fear in healthy women. Neurobiol Learn Mem. 2014;109:113–21. doi: 10.1016/j.nlm.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Kern MK, Jaradeh S, Arndorfer RC, Jesmanowicz A, Hyde J, Shaker R. Gender differences in cortical representation of rectal distension in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1512–23. doi: 10.1152/ajpgi.2001.281.6.G1512. [DOI] [PubMed] [Google Scholar]
- 38.Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: a retrospective study of single-trial fMRI data. Neuroimage. 2008;39:1867–76. doi: 10.1016/j.neuroimage.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 39.Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum Brain Mapp. 2009;30:689–98. doi: 10.1002/hbm.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benson S, Kotsis V, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER, Elsenbruch S. Behavioural and neural correlates of visceral pain sensitivity in healthy men and women: does sex matter? Eur J Pain. 2012;16:349–58. doi: 10.1002/j.1532-2149.2011.00027.x. [DOI] [PubMed] [Google Scholar]
- 41.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 42.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–90. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96:3341–7. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- 44.Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–4. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- 45.Heft MW, Gracely RH, Dubner R, McGrath PA. A validation model for verbal description scaling of human clinical pain. Pain. 1980;9:363–73. doi: 10.1016/0304-3959(80)90050-0. [DOI] [PubMed] [Google Scholar]
- 46.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. [Google Scholar]
- 47.Benjamini Y, Hochberg Y. On the Adaptive Control of the False Discovery Rate in Multiple Testing with Independent Statistics. Journal of Educational and Behavioral Statistics. 2000;25:60–83. [Google Scholar]
- 48.Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, Harris RE, Edwards RR, et al. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014;66:203–12. doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102:12950–5. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whalen PJ. Fear, Vigilance, and Ambiguity: Initial Neuroimaging Studies of the Human Amygdala. Current Directions in Psychological Science. 1998;7:177–188. [Google Scholar]
- 51.Bouton ME. Context, Ambiguity, and Classical Conditioning. Current Directions in Psychological Science. 1994;3:49–53. [Google Scholar]
- 52.Bach DR, Dolan RJ. Knowing how much you don’t know: a neural organization of uncertainty estimates. Nat Rev Neurosci. 2012;13:572–86. doi: 10.1038/nrn3289. [DOI] [PubMed] [Google Scholar]
- 53.Lake JI, Labar KS. Unpredictability and uncertainty in anxiety: a new direction for emotional timing research. Front Integr Neurosci. 2011;5:55. doi: 10.3389/fnint.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O’Daly O. Anterior insula responds to temporally unpredictable aversiveness: an fMRI study. Neuroreport. 2014;25:596–600. doi: 10.1097/WNR.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinz A, Smolka MN, Braus DF, Wrase J, Beck A, Flor H, Mann K, Schumann G, et al. Serotonin transporter genotype (5-HTTLPR): effects of neutral and undefined conditions on amygdala activation. Biol Psychiatry. 2007;61:1011–4. doi: 10.1016/j.biopsych.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 56.Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn Sci. 2003;7:197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 57.Azpiroz F. Gastrointestinal perception: pathophysiological implications. Neurogastroenterol Motil. 2002;14:229–39. doi: 10.1046/j.1365-2982.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- 58.Gorka SM, Nelson BD, Phan KL, Shankman SA. Insula response to unpredictable and predictable aversiveness in individuals with panic disorder and comorbid depression. Biol Mood Anxiety Disord. 2014;4:9. doi: 10.1186/2045-5380-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502–11. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–52. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Luthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–66. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: the value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ter Minassian A, Ricalens E, Humbert S, Duc F, Aube C, Beydon L. Dissociating anticipation from perception: Acute pain activates default mode network. Hum Brain Mapp. 2013;34:2228–43. doi: 10.1002/hbm.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maresh EL, Allen JP, Coan JA. Increased default mode network activity in socially anxious individuals during reward processing. Biol Mood Anxiety Disord. 2014;4:7. doi: 10.1186/2045-5380-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–5. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong JY, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, Stains J, Heendeniya N, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci. 2014;34:14252–9. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fleck MS, Daselaar SM, Dobbins IG, Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb Cortex. 2006;16:1623–30. doi: 10.1093/cercor/bhj097. [DOI] [PubMed] [Google Scholar]
- 70.Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philos Trans R Soc Lond B Biol Sci. 2007;362:887–99. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunckley P, Aziz Q, Wise RG, Brooks J, Tracey I, Chang L. Attentional modulation of visceral and somatic pain. Neurogastroenterol Motil. 2007;19:569–77. doi: 10.1111/j.1365-2982.2007.00908.x. [DOI] [PubMed] [Google Scholar]
- 72.Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, Chang L. Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness. Neuroscience. 2005;133:533–42. doi: 10.1016/j.neuroscience.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 73.Drabant EM, Kuo JR, Ramel W, Blechert J, Edge MD, Cooper JR, Goldin PR, Hariri AR, et al. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage. 2011;55:401–10. doi: 10.1016/j.neuroimage.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AK. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lobanov OV, Zeidan F, McHaffie JG, Kraft RA, Coghill RC. From cue to meaning: brain mechanisms supporting the construction of expectations of pain. Pain. 2014;155:129–36. doi: 10.1016/j.pain.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiech K, Tracey I. Pain, decisions, and actions: a motivational perspective. Front Neurosci. 2013;7:46. doi: 10.3389/fnins.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Hum Brain Mapp. 2011;32:1836–46. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Porro CA, Cettolo V, Francescato MP, Baraldi P. Functional activity mapping of the mesial hemispheric wall during anticipation of pain. Neuroimage. 2003;19:1738–47. doi: 10.1016/s1053-8119(03)00184-8. [DOI] [PubMed] [Google Scholar]
- 79.Ellingson BM, Mayer E, Harris RJ, Ashe-McNally C, Naliboff BD, Labus JS, Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013;154:1528–41. doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang Z, Ashe-McNalley C, Stains J, Heendeniya N, et al. Patients with Chronic Visceral Pain Show Sex-Related Alterations in Intrinsic Oscillations of the Resting Brain. J Neurosci. 2013;33:11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lanzetta JT, Orr SP. Excitatory strength of expressive faces: effects of happy and fear expressions and context on the extinction of a conditioned fear response. J Pers Soc Psychol. 1986;50:190–4. doi: 10.1037//0022-3514.50.1.190. [DOI] [PubMed] [Google Scholar]
- 82.Au Yeung ST, Colagiuri B, Lovibond PF, Colloca L. Partial reinforcement, extinction, and placebo analgesia. Pain. 2014;155:1110–7. doi: 10.1016/j.pain.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agren T, Engman J, Frick A, Bjorkstrand J, Larsson EM, Furmark T, Fredrikson M. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012;337:1550–2. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- 84.Mayer EA, Bushnell MC. Functional pain syndromes : presentation and pathophysiology. Seattle: IASP Press; 2009. International Association for the Study of Pain. [Google Scholar]
- 85.Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut. 2011;60:1589–99. doi: 10.1136/gutjnl-2011-300253. [DOI] [PubMed] [Google Scholar]
- 86.Tkalcic M, Domijan D, Pletikosic S, Setic M, Hauser G. Attentional biases in irritable bowel syndrome patients. Clin Res Hepatol Gastroenterol. 2014;38:621–8. doi: 10.1016/j.clinre.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Kilpatrick LA, Ornitz E, Ibrahimovic H, Treanor M, Craske M, Nazarian M, Labus JS, Mayer EA, et al. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS) Biol Psychol. 2010;84:272–8. doi: 10.1016/j.biopsycho.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Brain responses associated with cued threat.
Table S2. Brain responses associated with contextual threat.