Abstract
The purpose of this study was to characterize normal human choroidal vascular development from 6–23 weeks gestation (WG). Markers of endothelial cells (EC) (CD34, CD31, vWf), angioblasts and EC (CD39), leukocytes (CD45), erythroblasts (epsilon chain of hemoglobin, Hb-e), proliferating cells (Ki67), and VEGFR-2 were employed. At 6–7 WG, many erythroblasts were observed within islands of precursor cells in the choriocapillaris layer and others were independent from the islands. Many erythroblasts (Hb-∈+) were also positive for EC markers and/or VEGFR-2. By 8–12 WG, most of the Hb-∈ cells had disappeared and vascular lumens became apparent. At 14–23 WG, some EC were proliferating on the scleral side of choriocapillaris in association with forming deeper vessels. In conclusion, embryonic choriocapillaris appears to form initially by hemo-vasculogenesis (blood vessels and blood cells form simultaneously from common precursors) while angiogenesis appears to be the mode of intermediate and large choroidal vessel development in the fetus.
Keywords: choroids, hemo-vasculogenesis, hemangioblasts, angiogenesis, vascular precursors
INTRODUCTION
The choroid is a highly vascularized and pigmented tissue embedded between the retinal pigment epithelium of the retina and the outermost portion of the eye, the sclera (Fig. 1). The choroid is separated from the retinal pigment epithelial (RPE) cells by Bruch’s membrane. The choroid has three layers of vessels: the outer layer of large vessels, arteries, and veins (Haller’s layer); a middle layer of medium-sized vessels, arterioles, and venules (Sattler’s layer); and an inner layer of capillaries adjacent to Bruch’s membrane (the choriocapillaris). These three relatively distinct layers occur only in the posterior pole, i.e., choroid posterior to peripapillary retina, the optic disc and macula (Jakobiec, 1982), while the rest of the choroid has only choriocapillaris and a deeper layer of larger blood vessels. It is a unique vascular system in that the choriocapillaris is lobular in pattern in central choroid and is fenestrated only on the retinal side of the blood vessels (Bhutto and Lutty, 2006). The diameter of the capillaries is relatively large, measuring between 15 and 30 μm. The choroidal vasculature is responsible for maintaining the metabolic and oxygen demands of the RPE cells and photoreceptors; therefore, abnormalities in this vasculature result in many kinds of congenital and adult diseases such as choroidal coloboma and age-related macular degeneration (Daufenbach et al., 1998; Lutty et al., 1999). However, our understanding of the development of this vascular system is rudimentary. Ida Mann observed choroidal vascular development using traditional histologic techniques and proposed the time course of formation of choroidal vasculature (Mann, 1928). However, in 1928 she was unable to determine which types of cells were associated with the development. Heimann (1972) observed choroidal development using an injection technique for human fetuses of 8 weeks gestation (WG) or older, when choriocapillaris lumens have already formed. The mechanisms involved in the formation of the human choroidal vasculature are still unknown.
Fig. 1.
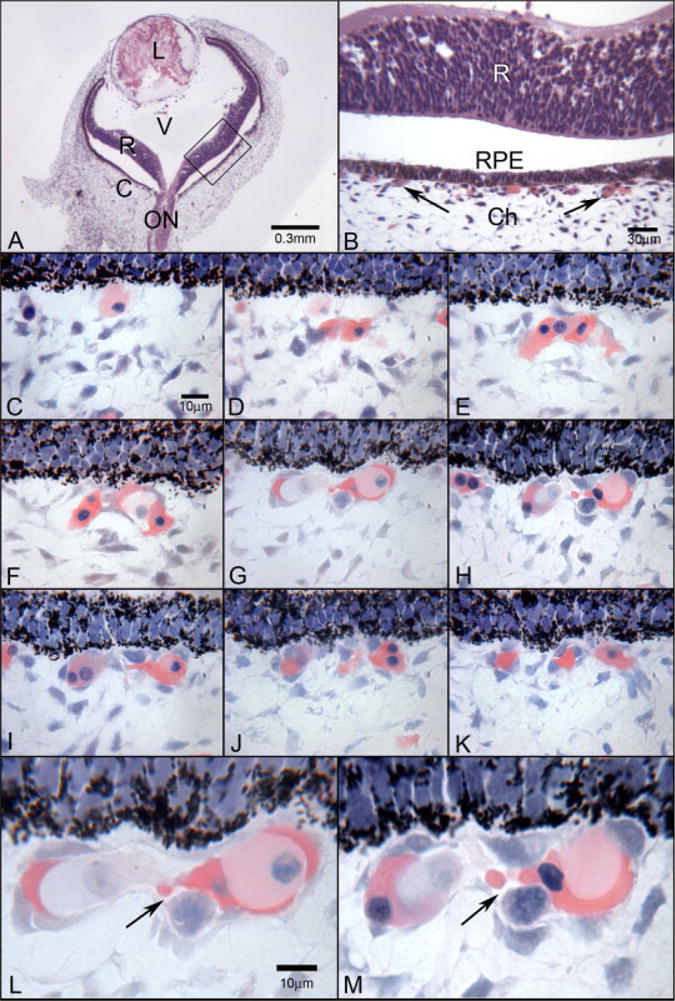
Glycol methacrylate sections from a 6-WG embryonic human eye. A: At low magnification (A), the lens (L), retina (R), optic nerve (ON), and choroid (C) are apparent. Vitreous (V) fills the chamber between lens and retina. B: Magnification of the boxed area in A, where the relationship between retina, the monolayer of retinal pigment epithelial (RPE) cells, and choroid can be seen. Arrows point to the forming choriocapillaris. The separation between retina and RPE is artifactual. A,B: Hematoxylin and eosin. Serial, Wright’s Giemsa-stained, sections of retinal pigment epithelial cells (RPE) (top in all) and developing choriocapillaris (below RPE) are shown in C–K. C: A single cell with moderately acidophilic (pink) cytoplasm is present just posterior to the pigmented RPE cells (top). D,E: Nucleated cells aggregate in this area that have intensely acidophilic cytoplasm, which is a characteristic of embryonic hemoglobin staining in erythroblasts. F–H: Mesenchymal-like cells with spindle-shaped nuclei become part of the aggregate or island of cells and in some areas appear to surround the erythroblasts while in other areas the erythroblasts form the outside of the aggregate (F). I–K: The island divides into several clusters of cells and individual erythroblasts as well. L,M: At higher magnification, the lumen-like nature of the center of the aggregate becomes more apparent and the presence of several types of cells in the center of the island is more obvious.
Vasculogenesis and angiogenesis are established as the major mechanisms for the formation of most new blood vessels. Vasculogenesis is the in situ formation of new blood vessels by differentiation and coalescence of angioblasts or vascular precursors, while angiogenesis involves migration and proliferation of endothelial cells from existing blood vessels (Risau and Flamme, 1995). A third concept for formation of vasculatures during embryonic development is that new blood vessels (vasculogenesis) and blood cells (hematopoiesis) develop simultaneously within the embryo proper from common precursors, the hemangioblast, which Sequeira Lopez et al. recently termed hemo-vasculogenesis (Sequeira Lopez et al., 2003). This occurs extra-embryonically in yolk sac before formation of blood islands (Sabin, 1917; Murray, 1932; Sabin, 2002). Ueno and Weissman demonstrated recently, however, that this is not a clonal process in yolk sac, i.e., one hemangioblast does not give rise to both lineages (Ueno and Weissman, 2006). Hemo-vasculogenesis has been observed in vitro when stem cells form embryoid bodies (Fraser et al., 2003; Zambidis et al., 2005) and in vivo in many organ systems of mouse (Sequeira Lopez et al., 2003). Whether hematopoiesis is primitive or extraembryonic, as in yolk sac, or definitive as in the aortic-gonads-mesonephros (AGM), expression of hemoglobin occurs at these sites and can be used as a marker for hematopoiesis (Tavian and Peault, 2005). Expression of the epsilon globin chain in embryonic hemoglobin (Hb-∈) occurs in primitive erythroblasts in blood from 3 to 6 WG. In vitro, Zambidis and others have used expression of epsilon globin to be an indicator of erythropoiesis in embryoid bodies (Bielinska et al., 1996; Zambidis et al., 2005).
The mechanisms of vascular development can only be gleaned by using a battery of markers for vascular precursors as well as endothelial cells. One marker for both endothelial cell precursors (angioblasts) and endothelial cells is the ectoenzyme adenosine diphosphatase (ADPase). We have shown previously that resident angioblasts expressing ADPase are present well in advance of developing blood vessels in inner retina and participate in the initial formation of the fetal human (Chan-Ling et al., 2004) and newborn dog retinal vessels during vasculogenesis (McLeod et al., 1987, 1996; Lutty and McLeod, 1992). ADPase is now known to be CD39, a marker for vascular precursors (angioblasts) before, during, and after their differentiation and organization into primordial capillaries (McLeod et al., 2006). CD39 is used as an angioblast marker because there are no molecules known to date that are expressed solely by angioblasts. CD39, also called nucleoside triphosphate diphosphohydrolase-1 (NTPDase1), is the major vascular endothelial ectonucleotidase and hydrolyses nucleoside triphosphates and diphosphates, ultimately to the nucleoside analogues (Goepfert et al., 2001). In the adult vasculature, CD39 degrades ADP, eliminating the major stimulus for platelet aggregation (Marcus et al., 2005).
CD34 is a transmembrane cell surface glycoprotein, which was thought to be selectively expressed within the human and murine hematopoietic systems on stem and progenitor cells (Civin et al., 1984; Tavian et al., 1996; Labastie et al., 1998; Peichev et al., 2000). CD34 is also commonly used as a marker for endothelial cells (Fina et al., 1990). In previous studies, we found that CD34 is only expressed on endothelial cells of formed retinal vessels and not by retinal angioblasts (Chan-Ling et al., 2004).
Another marker commonly used for endothelial cells is CD31 or PECAM-1 (platelet endothelial cell adhesion molecule). CD31 is an adhesion molecule that controls the diapedesis of leukocytes and transmigration of CD34+ cells across endothelial cells (Imhof and Dunon, 1995; Yong et al., 1998). CD31’s homophilic binding contributes to maintenance of endothelial cell integrity (Newman, 1997).
Von Willebrand factor (vWf) is a large multimeric glycoprotein produced constitutively by endothelial cells and megakaryocytes (Sadler, 1998). It is stored in the Weibel Palade bodies in endothelial cells and vWf expression is necessary for formation of Weibel-Palade bodies (Michaux and Cutler, 2004). Von Willebrand factor mediates the platelet binding at sites of vascular injury, and deficiency in vWf results in the most common inherited bleeding disorder, von Willebrand disease (Sadler, 1998).
VEGF receptor 2 or flk-1 is the receptor responsible for VEGF-induced endothelial cell proliferation during angiogenesis (Witmer et al., 2003). Its importance in embryonic development is demonstrated in VEGFR-2 knockout mice in which blood island formation and vasculogenesis does not occur (Shalaby et al., 1995, 1997). The work of Cortes et al. and others suggests that the hemangioblast is VEGFR-2+ and CD34− (Cortes et al., 1999; Guo et al., 2003). Our studies (Chan-Ling et al., 2004) and unpublished results (D. S. McLeod and G. A. Lutty, 2006), demonstrate that VEGFR-2+/CD34−/CD31− cells in inner avascular fetal retina contribute to vasculogenesis. VEGFR-2 is present on many endothelial cells in adult tissues including the choriocapillaris (Blaauwgeers et al., 1999).
The purpose of this investigation was to determine the temporal and spatial relationships between cells during formation of the choroidal vasculature in the human embryo and fetus. This was accomplished using the expression of CD34, CD31, CD39, CD45, vWf, and VEGFR-2 as markers for hematopoietic cells, angioblasts, and endothelial cells. CD45, leukocyte common antigen, was used to label all hematopoietic cells except erythrocytes. The epsilon chain of embryonic hemoglobin (Hb-∈) was used as a marker for erythropoiesis and erythroblasts. The simultaneous expression of Hb-∈ and several vascular markers in cells in embryonic choroid suggests that the initial choriocapillaris develops by hemo-vasculogenesis. Proliferation (Ki67+) of endothelial cells in fetal choriocapillaris suggests that the intermediate and large blood vessels in posterior choroid develop later by angiogenesis.
RESULTS
Temporal and Spatial Relationships of Vascular Markers
Hematoxylin and eosin or Wright’s Giemsa stained serial glycol methacrylate (JB-4) sections were used to visualize the morphology in whole embryonic eyes (Fig. 1). It was apparent in these sections that a choroidal vasculature was forming posterior to the RPE monolayer (Fig. 1A,B). The high resolution afforded by these sections allowed narrow, slit-like, vascular lumens to be detected even at early time points (6–7 WG), when it was difficult to clearly identify the vascular lumens in the cryopreserved sections from the fellow eye. Wright’s Giemsa staining at 6 and 6.5 WG demonstrated many nucleated erythroblasts having intensely acidophilic cytoplasm (Fig. 1C–M). They appeared to fill the inside of developing vascular channels both at the level of the choriocapillaris layer (Fig. 1E) and in choroidal stroma (Fig. 2F). They were also present outside of the “channels” (Fig. 1C–E) and even appeared to form channels in some sections (Fig. 1F,G and L,M). Serial sections through these structures demonstrated that they were free-standing aggregates or islands of cells that were not contiguous with formed vascular lumens (Fig. 2). Choroidal stroma also had free erythroblasts, which were isolated from the island-like structures or any vascular structures at 6 WG (Fig. 2H,I). At the leading edge of apparent forming deeper, large vessels, erythroblasts were observed at the tips of these structures (Fig. 2C). Some “lumens” were forming at 6 and 6.5 WG, but some were slit-like (Fig. 1L,M) and others appeared to be more of a cavity (as in a hollow structure) than a true lumen (Fig. 2J,K), because in serial sections they were not contiguous with lumens in other vascular structures. The wall of the “lumen” was lined by cells that appeared to be erythroblasts (acidophilic cytoplasm) and prominent round basophilic nuclei in some examples (Fig. 2J,K). In other examples, the immature “lumens” were lined by endothelial cell-like cells with nucleated, highly acidophilic erythroblasts within the lumens. It was striking that not only were these “stand alone” structures (i.e., not associated with other vascular lumens or formations), but also that the erythroblast-like cells could either form or line the “lumens” as well as be within the lumens.
Fig. 2.
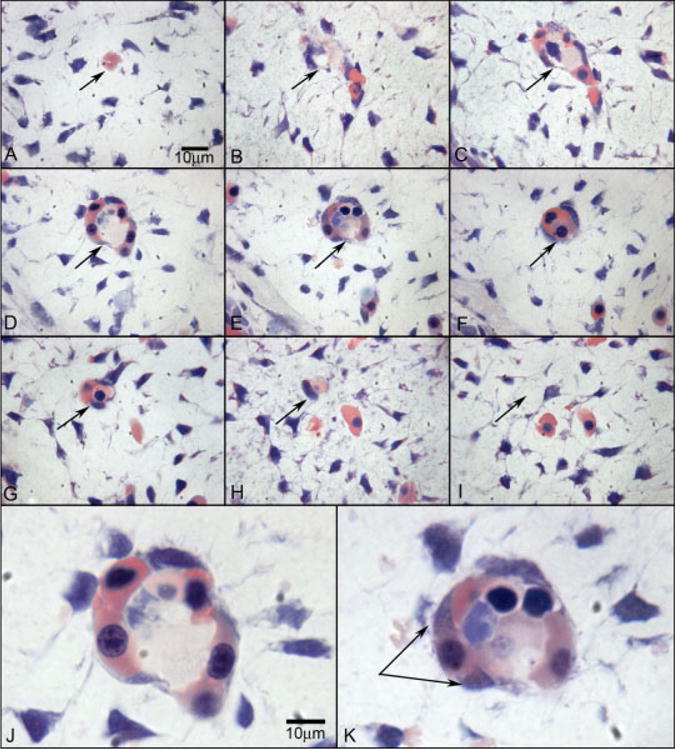
Wright’s Giemsa staining of 2.5-μm plastic sections showing an island of cells in the choroid at 6 WG. Serial sections (A–I) confirm that the structure is isolated and separated from any blood vessels at 6 WG. The arrow shows the same position in each panel. J,K: High-magnification photos of D and E, respectively. Wright’s Giemsa staining shows acidophilic cytoplasm indicative of hemoglobin in pink and basophilic nuclei in blue. One cell outside of structure (double arrow in K) shows the changing of cytoplasmic staining pattern from acidophilic into basophilic, which suggests that the cell has both the characteristics of hematopoietic cells and endothelial cells. In J, the erythroblasts appear to form the lumen.
The antibodies used for vascular markers, CD39, VEGFR-2, CD31, and CD34, had comparable localization in developing choriocapillaris at 6 WG (Fig. 3A–D). The hematopoietic marker CD45 was present in a few scattered cells in choroid, some of which were associated with developing choriocapillaris (Fig. 3H–J). CD39 was also present in retinal angioblasts in the avascular embryonic human retina (data not shown) (Chan-Ling et al., 2004; McLeod et al., 2006). VEGFR-2 was also present in cells in inner retina (data not shown) as well as in the sheet of cells under the RPE layer. All four endothelial cell markers stained the sheet of cells (Fig. 3A–D), which represented the developing choriocapillaris, and a few large vessels in posterior pole between 6 and 12 WG (Fig. 3F). Some lumens were observed in cryosections, but they were broad, almost lacunae-like. After 14 WG, both choriocapillaris and large vessels were stained with the vascular markers, but lumens became more apparent in choriocapillaris, and deeper large vessels became more numerous with increasing age (Fig. 3E–G).
Fig. 3.
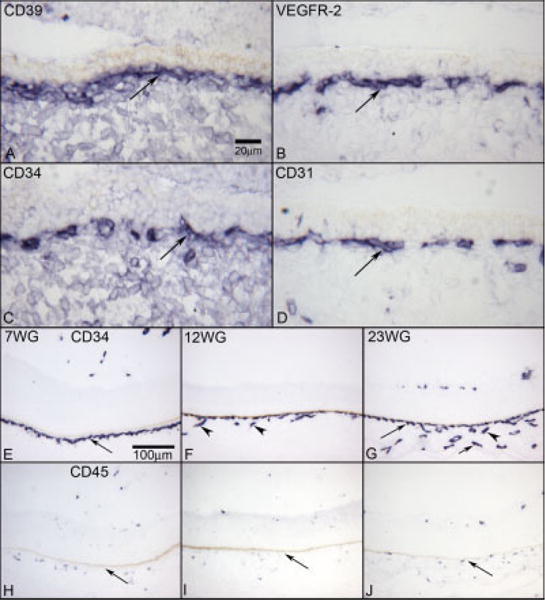
Comparison of vascular markers (A–D), and choroidal vascular development with CD34 labeling (E–G) and the presence of CD45 (H–J) by age. CD39 (A) shows a layer of positive cells in the periphery of choriocapillaris (long arrow) at 6 WG. VEGFR-2 (B), CD34 (C), and CD31 (D) show the similar staining of the choriocapillaris layer (long arrow). At 7 WG (E), CD34-positive cells are only in the choriocapillaris layer (long arrow). At 12 WG (F), CD34 shows deeper vessels forming (arrowheads). At 23 WG (G), CD34 shows the three vascular layers that are present in adult choriocapillaris (long arrow), Sattler’s layer (arrowhead), and the outermost large vessels of Haller’s layer (short arrow). CD45 is localized to single cells associated with developing choriocapillaris and larger choroidal vessels at 7 (H), 12 (I), and 23 (J) WG. The RPE cells are present above the choriocapillaris in all plates, but the pigment has been bleached. APase reaction product in all.
Evidence for Hemo-Vasculogenesis in Choroid Between 6 and 7 Weeks of Gestation
In cryopreserved tissue, Hb-∈ localization was combined with CD31 on 6 and 7 WG specimens to determine if the cells that had acidophilic cytoplasm and large nuclei in JB-4 sections were erythroblasts (expressed Hb-∈). If the same cells expressed vascular markers and Hb-∈, this would suggest common precursors for erythroblasts and endothelial cells and that the vasculature had formed by hemo-vasculogenesis. CD31+/Hb-∈+ cells were observed in the forming choriocapillaris at 6–7 weeks gestation (Fig. 4). Some single cells within the choroidal stroma were also double labeled for Hb-∈ and CD31 at 6 WG (Fig. 4A–D). Some CD31+/Hb-∈+ appeared to line the lumen-like formations while others were present within the lumens (Fig. 4A–D), although the latter cells had reduced expression of CD31. There were also examples where CD31+/Hb-∈+ appeared to be budding from the developing choriocapillaris at 7 WG (Fig. 4E–H).
Fig. 4.
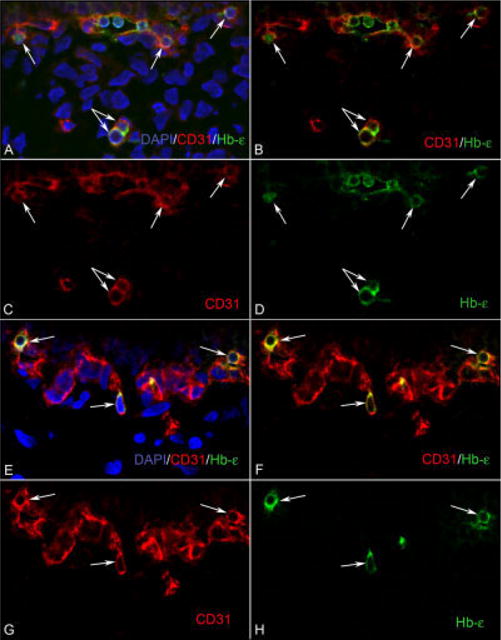
The developing choroidal vasculature contains CD31/Hb-∈ double positive cells. A–D: At 6 WG, clusters of CD31/Hb-∈ (red/green) positive cells (arrows) are visible in the choriocapillaris layer, whereas in the choroidal stroma, there are isolated cells (double arrow) that are also double labeled. E–H: At 7 WG, there are isolated cells (arrows) that are CD31/Hb-∈ positive, which appear attached to the choriocapillaris. These CD31/Hb-∈-positive cells have characteristics of both hematopoietic and endothelial cells. B and F are merged images of the single color images C, D, and G, H, respectively. A and E are images showing nuclear counter staining with DAPI (blue) merged with B and F, respectively.
At 6 and 6.5 WG, double labeling of Hb-∈ and VEGFR-2 was also performed. As with CD31, cells expressing Hb-∈ also expressed VEGFR-2 (6 WG, Fig. 5). VEGFR-2+/Hb-∈+ cells were part of the island-like formations in developing choriocapillaris (Fig. 5, top), and there were also single cells double-labeled in choroidal stroma. The majority of the cells in developing choriocapillaris expressed VEGFR-2 while Hb-∈ was present in a select subset of VEGFR-2+ cells. At 6.5 WG, labeling of Hb-∈ was combined with Ki67 to determine if erythroblasts proliferate. Few Hb-∈+ cells were double labeled with Ki67 anywhere in the choroid (results not shown).
Fig. 5.
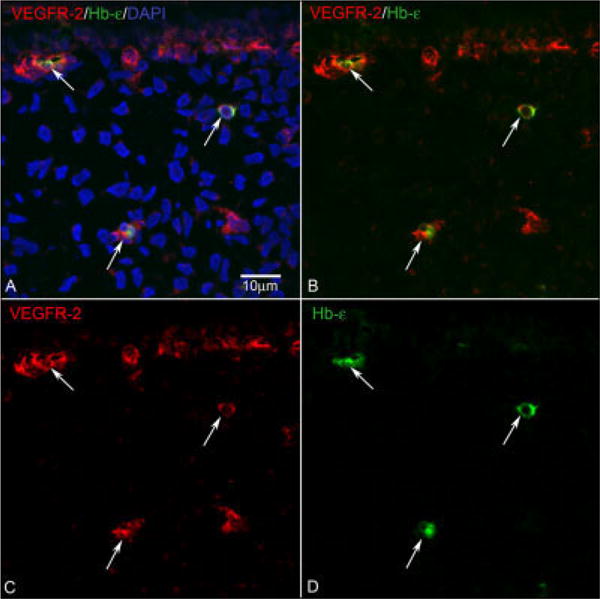
VEGFR-2/Hb-∈ double labeling. At 6 WG (A–D), isolated cells (arrows in the middle and bottom) that were double stained by VEGFR-2 (C, Red) and Hb-∈ (D, Green) were found in the choroidal stroma. More VEGFR-2+/Hb∈+ cells (arrow at top) were located in the choriocapillaris layer. B is a merged image of single images in C and D. A shows nuclear counterstaining (blue) combined with both colors.
Triple labeling was used to determine if a more definitive endothelial cell marker like vWf was present in cells expressing Hb-∈ and the hematopoietic and endothelial cell marker CD34 (Fig. 6). VWf is found only on endothelial cells and megakaryocytes and, therefore, is perhaps the most definitive marker for endothelial cells employed in these studies. VWf expression was confined to the developing choriocapillaris and not present in individual cells in stroma. Many cells in developing choriocapillaris expressed both vWf and CD34, but very few cells expressed Hb-∈ as well (arrow in Fig. 6A–H). Cells expressing both Hb-∈ as well as CD34 but not vWf were more abundant (Fig. 6A–H). Upon closer inspection, the Hb-∈ and vWf appeared to be present in different parts of the double-labeled cells (Fig. 6J). Hb-∈ expression declined dramatically at 7.5 WG compared to prior ages (Fig. 7).
Fig. 6.
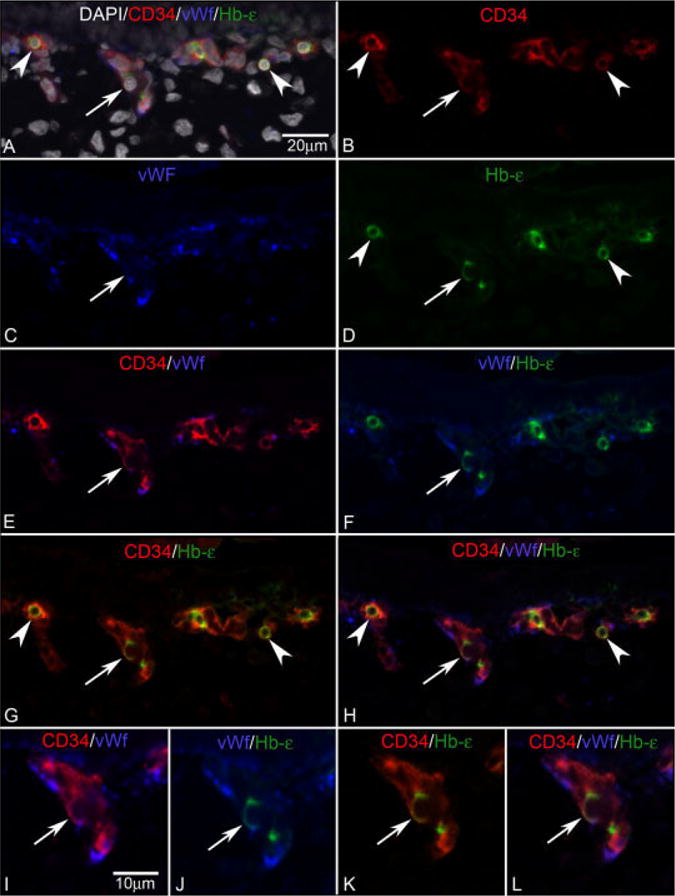
CD34/vWf/Hb-∈ triple labeling. A: Merged image of all colors with DAPI nuclear counter-staining (gray). B: CD34 staining in red. C: vWf staining in blue. D: Hb-∈ staining is green. At 6 WG (A–L), double-labeled cells (G, arrowhead) for CD34 (B, arrowhead) and Hb-∈ (D, arrowhead) are observed in the choroid. Some of CD34+/Hb-∈+ are triple-labeled cells (H, arrow) for CD34 (B, arrow), vWf (C, arrow), and Hb-∈ (D, arrow). I–L: A triple-stained cell is shown at high magnification (arrow) within the vascular structure. E,I: Merged images of CD34 (red) and vWf (blue). F,J: Merged images of vWf (blue) and Hb-∈ (green). G,K: Merged images of CD34 (red) and Hb-∈ (green). H,L: Merged images of CD34 (red), vWf (blue), and Hb-∈ (green). Arrows show the same cell in A–L.
Fig. 7.
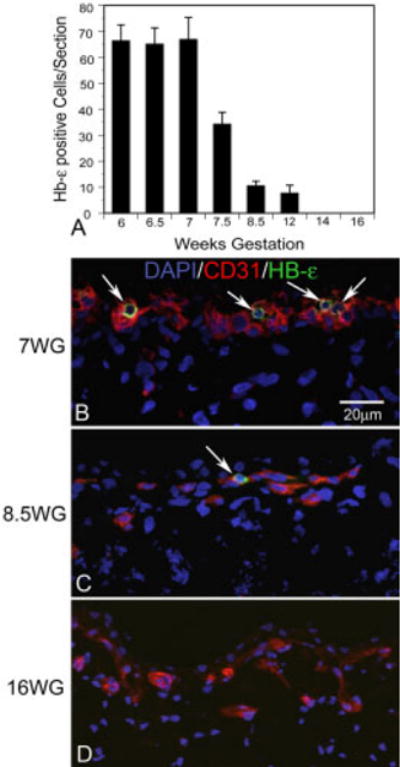
Quantification of Hb-∈ positive cells in the choroids and CD31/Hb-∈ double staining with age. A: After 7 WG, the number of Hb-∈-positive cells decreased rapidly and Hb-∈-positive cells were gone by 14 WG. B: At 7 WG, Hb-∈-positive, round shaped cells (arrows), were common in the choroids examined. C: At 8.5 WG, the number of Hb-∈-positive cells decreased and the morphology was smaller and more oval. D: At 16 WG, there were no detectable Hb-∈-positive cells in the sections examined.
Double labeling with CD34 and Ki67 was also performed on tissues from embryonic eyes at 6 WG and 7 WG. Some cells were double labeled with CD34 and Ki67 in the posterior pole, but few cells were double stained for CD34 and Ki67 in the periphery (6 WG; Fig. 8A,B). This suggested that angiogenesis, which requires proliferation, was not occurring in the peripheral developing choriocapillaris.
Fig. 8.
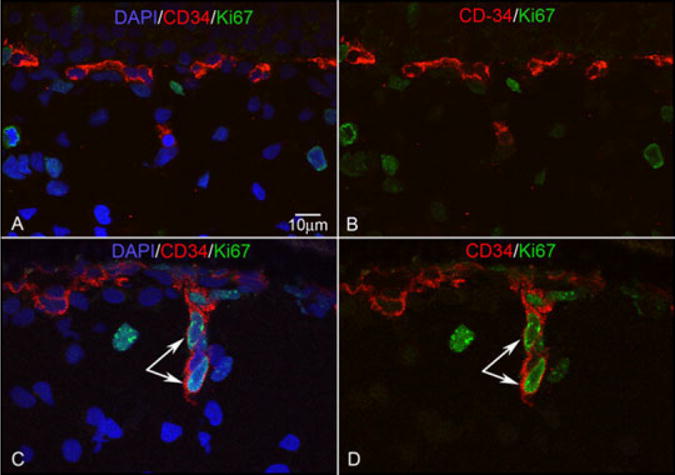
CD34/Ki67 double staining. CD34 reaction is shown in red, Ki67 reaction is shown in green. CD34+/Ki67+ cells are not observed at 6 WG (A,B), while CD34+/Ki67+ cells (arrow) appear to be budding from choriocapillaris to form a diving vessel at 12 WG (C,D). B and D are merged images of CD34 (red) and Ki67 (green). A and C are the result of merging B and D with nuclear counterstaining shown in blue.
In the JB-4 sections at 7.5 WG, there were some vascular cavities in the choriocapillaris that were very large and lacunae-like in the peripheral choroid (data not shown). However, anti-CD39 labeled flatmounts provided a different impression of choriocapillaris (Fig. 9A). At 5.5 WG, few formed lumens were apparent in the flat perspective but the impression was that the choriocapillaris was a sheet of CD39+ cells. By 7.5 WG (Fig. 9B), blood vessels were present but they appeared to be filled primarily with CD39+ cells.
Fig. 9.
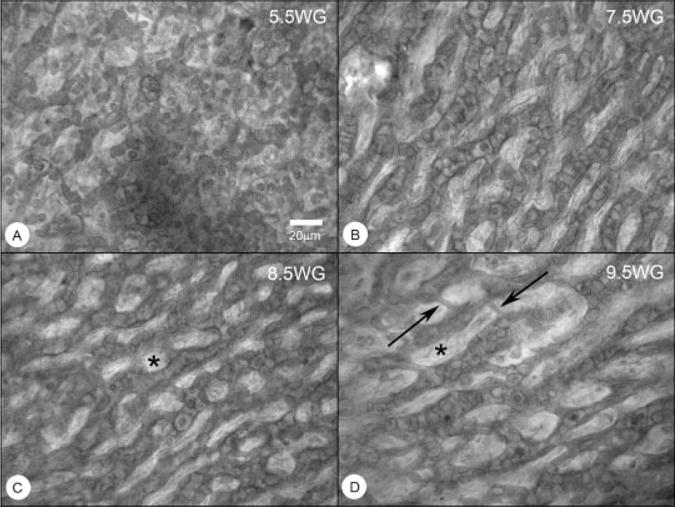
Flatmounts of CD39 labeled choroids from 5.5, 7.5, 8.5, and 9.5 WG. The choriocapillaris at 5.5 WG is very cellular with only a few vascular segments and no obvious pattern. At 7.5 WG, wide vascular segments are apparent but not contiguous and the “segments” are filled with CD39+ cells, perhaps representing components of the vascular islands. At 8.5 WG, a choriocapillaris-like pattern of choroidal capillaries is present. At 9.5 WG, the pattern appears to be stretched, having wider intercapillary septa (*) and undergoing remodeling (narrow vascular segments, arrows).
Choriocapillaris at 8 to 12 Weeks of Gestation
Cells expressing CD31, CD34, and CD39 were apparent immediately posterior to the RPE layer from 8–12 WG (Fig. 3F). Most Hb-∈+ cells had disappeared at 8.5 WG (Fig. 7). Some cells in the developing choriocapillaris at 8.5 and 12 WG were double labeled with CD34 and Ki67 in the posterior pole choroid, but only a few double-stained cells were observed in periphery at 12 WG (Fig. 8). The proliferating cells (CD34+/Ki67+) at 12 WG were located on the scleral side of choriocapillaris. This apparent budding from choriocapillaris appeared to represent the initial stage of new intermediate choroidal vessel formation at 12 WG and perhaps, in some cases, the anastomosis of choriocapillaris with deeper large choroidal vessels already formed. This proliferation of vascular cells continued to be present throughout fetal developmental stages. Formed lumens could be detected in choriocapillaris at 12 WG in cryopreserved tissue (results not shown).
A more lobular pattern that is a characteristic of choriocapillaris (McLeod and Lutty, 1994) was becoming apparent at 8.5 and 9.5 WG, but no true lobules were observed and the overall impression was a chicken-wire-like redundant pattern (Fig. 9C,D). Sections of CD39-stained choroids after embedding in JB-4 demonstrated some formed lumens at 10 WG and many free CD39+ cells as well. By 12 WG, choriocapillaris in all areas had lumen, but in the periphery the monolayered choriocapillaris had wide lacunae-like lumen (data not shown). From the posterior pole to the equator, large choroidal vessels (potential arteries and veins) were forming at 12 WG, as shown in the through-focus images in Figure 10A,B. The pattern at this age was also not as dense as observed in adult humans (McLeod and Lutty, 1994), suggesting that the number of choriocapillaris channels would need to be increased as the eye grows. Free CD39+ cells were still present in choroid at the level of the choriocapillaris and below (Fig. 10C). Three layers of choroidal vessels were observed at 12 WG (Fig. 10C) only in the presumed macula. The lobular pattern that is a characteristic of adult choroid was still not present in the posterior pole at this time.
Fig. 10.
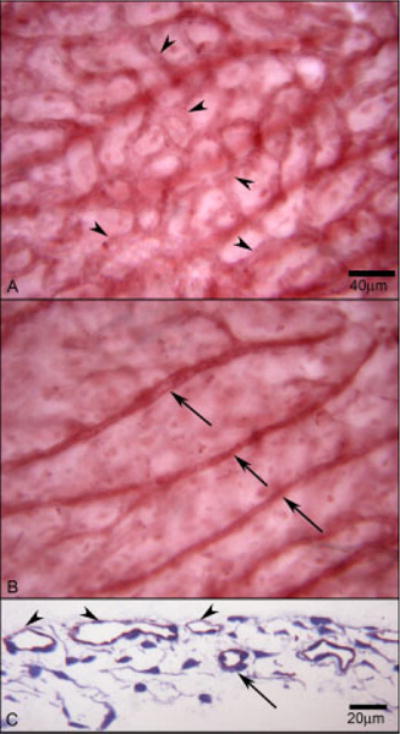
An area in the posterior pole of a whole-mounted choroid stained with CD39. At 12 WG, the flat-mounted choroid shows choriocapillaris in the superficial layer (arrowheads) (A) and large vessels in the deeper layer (arrows) (B). C: A cross-section of the area shows these two layers of blood vessels, choriocapillaris (arrowhead) and deeper vessels (arrow). Red APase CD39 reaction product with toluidine blue counterstain in C.
Choroidal Vasculature Between 14 and 23 Weeks of Gestation
No Hb-∈ expressing cells were present in choroid between 14–23 WG (Fig. 7). Double labeling of CD34 and Ki67 demonstrated that some choroidal endothelial cells were proliferating at these ages (results not shown). These proliferating cells were located on the scleral side of choriocapillaris at 14 and 16 WG, while some of these proliferating cells were also located in the RPE side of choriocapillaris in the periphery at 20 WG and 23 WG. Budding from choriocapillaris was observed during formation of intermediate choroidal vessels. The formation of the large- (presumably arteries and veins) and medium-sized choroidal vessels (presumably arterioles and venules) progressed centrifugally, starting in posterior choroid around the optic nerve and progressing toward the peripheral choroid posterior to the ora serrata. By 23 WG, the deeper blood vessels were starting to form in the far periphery and three layers were present in the central choroid (Fig. 3G). From 14 through 23 WG, obvious lumens of choriocapillaris and large vessels could be observed throughout choroid.
DISCUSSION
The existence of a common precursor for endothelial and hematopoietic cells, the hemangioblast, has been hypothesized for many years (Sabin, 1917; Murray, 1932) and recent studies have demonstrated they are present intra- and extra-embryonically in mouse (Ogawa et al., 2001; Orkin and Zon, 2002; Ueno and Weissman, 2006). Recently, Sequeira Lopez and associates reported simultaneous generation of endothelial and hematopoietic cells in several tissues of the mouse embryo and applied the term hemo-vasculogenesis to the process (Sequeira Lopez et al., 2003). The current study suggests that hemangioblasts are present intra-embryonically in developing choroid and documents apparent hemo-vasculogenesis in embryonic human choroid using Wright’s Giemsa staining and immunolocalization of Hb-∈ in the same cells that are positive for CD31, CD34, VEGFR-2, and vWf, standard markers for endothelial cells in adult tissue. However, CD31, CD34, and VEGFR-2 have been used as markers for hematopoietic precursors and VEGFR-2 is a marker for hemangioblasts (Forrai and Robb, 2003), while vWF is almost a definitive marker for endothelial cells, since the only other cell type expressing it is megakaryocytes. It was striking that a few cells were expressing Hb-∈, CD34, and vWf, further suggesting the same precursors were capable of erythropoiesis, hematopoiesis, and vasculogenesis, the definition of hemo-vasculogenesis. In the fetal period, hemo-vasculogenesis was complete and new blood vessels appeared to form by angiogenesis since endothelial cells were proliferating. However, there were free CD39+ cells present in choroid at the level of the choriocapillaris (Fig. 10), so it is possible that these cells participate in vasculogenesis, which expands the vasculature to achieve adult vascular density as the eye enlarges.
Choroidal Vascular Development With Age
Prior studies of the choroid in human embryos and fetuses defined the anatomical events resulting in a choroidal vasculature. Ida Mann described the choriocapillaris as endothelial tubes and said that fetal erythrocytes were present (Mann, 1928). Her drawings, however, do not clearly depict the erythrocytes inside of tubes. The ultrastructural study of Sellheyer and Spitznas clearly show erythroblasts, mesenchymal cells, and endothelial cells at 6.5 WG (Sellheyer, 1990). Only slit-like lumens were present and the “endothelial cells” were very large and highly vesiculated. Mann (1928), Sellheyer and Spitznas (1988), and Heimann (1972), who used latex casts, observed only a choriocapillaris until around the end of the 8th WG. However, our study suggests that the lumens Mann reported may not have been endothelial tubes but rather hollow aggregates or islands of precursors, which were shown by serial sectioning in the current study to not be connected to patent blood vessels. Furthermore, the erythrocytes were actually erythroblasts forming in those islands of cells. At 12 WG, the prior studies observed large and intermediate blood vessels in choroid (Mann, 1928; Heimann, 1972; Sellheyer and Spitznas, 1988). So our anatomical results are consistent with the prior observations but the previous studies do not definitively identify the cells involved, their relationship to vascular precursors, or the mechanism by which the vessels assemble.
Choriocapillaris Forms by Hemo-Vasculogenesis
The first time points examined in the current study were 5.5 WG, when sheets of CD39+ cells were present in a flat mount (Fig. 9A), and 6 WG when the sheet of cells was labeled with vascular markers (CD31, CD34, CD39, and VEGFR-2) in cross-sections of developing choriocapillaris (Fig. 3A–D). It was striking in specimens embedded in JB-4 that the sheet contained cells that had extremely acidophilic cytoplasm as observed in erythroblasts, which are present in the embryo at this stage. We observed many nucleated erythroblasts surrounded by loosely arranged apparent endothelial cells of the choriocapillaris. Recently, Sequira Lopez and associates provided evidence that erythroblasts and endothelial cells are generated simultaneously and likely derived from common precursor cells in mouse embryo (Sequeira Lopez et al., 2003) but their analysis did not prove the origin of both cell types was clonal. The precursors (VEGFR-2+) aggregate, then differentiate into erythroblasts and endothelial cells. Once the lumens form, erythroblasts are separated from endothelial cells and could be observed in the newly formed lumens. Furthermore, they demonstrated that this phenomenon occurs throughout the mouse embryo and they called this process hemo-vasculogenesis, the process by which vasculogenesis, erythropoiesis, and hematopoiesis occur simultaneously in island-like structures and perhaps from common precursors.
There are several lines of evidence that suggest that the embryonic human choriocapillaris forms by hemo-vasculogenesis. First, in JB-4 serial sections in the current study, island of precursors were observed, which were not associated with formed blood vessels when the tissue was serially sectioned. Cells in these islands appeared to be erythroblasts with Giemsa staining; they had large nuclei with acidophilic cytoplasm. Furthermore, they were both in cavities made within the islands and, in other examples, they formed the walls of the cavities; i.e., they were not always in the presumptive lumen. In fact, individual erythroblasts were observed in choroidal stroma as well. Confirmation that these cells were erythroblasts was achieved by double staining with endothelial cell markers, hematopoietic cell markers, and Hb-∈. Hb-∈ is one of the chains of embryonic hemoglobin and has been used recently to demonstrate erythroblast differentiation in embryoid bodies formed from embryonic stem cells in vitro (Zambidis et al., 2005). Double labeling with vascular markers (CD31, CD34, and VEGFR-2) and Hb-∈ demonstrated that cells within the islands of precursors where choriocapillaris was forming and even some single cells in choroidal stroma were double labeled between 6 and 7 WG. The fact that they had Hb-∈, endothelial cell, and hematopoietic markers of varying degree suggests that endothelial cells, erythropoietic cells, and hematopoietic cells in the initial choriocapillaris may have common precursors, i.e., hemo-vasculogenesis appears to be the way by which the initial choriocapillaris develops. One difference between the current observations on human embryonic choriocapillaris and the prior study in mouse by Sequira Lopez is that they never observed erythroblasts forming the wall of the cavities nor did they observe vesicles or vacuoles inside the clusters of erythroblasts (Fig. 2). Sequira Lopez found that mouse erythroblasts were generated after lumens or cavities were formed (Sequeira Lopez et al., 2003). This was similar to the Florence Sabin’s description of blood islands in yolk sac, hematopoietic cells in the center, and endothelial cells forming the lumen (Sabin, 1917). However, Ferkowicz and Yoder have found that primitive erythropoiesis is initiated extra-vascularly (Ferkowicz and Yoder, 2005). In the study of Zambidis et al. (2005) on human embryoid bodies, they found erythroblasts make hollow chambers as we observed in choroid, as well as develop within endothelial cell–lined chambers.
Angiogenesis Involved in Large Choroidal Blood Vessel Development
A few large vessels were already forming in the posterior pole at the same embryonic time points as choriocapillaris. Those vessels had packed erythroblasts in their lumens, like the forming choriocapillaris, and were free-standing structures. Therefore, some arteries and veins may develop by the same process as choriocapillaris, hemo-vasculogenesis (results not shown). The number of those vessels was not nearly enough to form all of the large and intermediate choroidal blood vessels found in adult. Ki67+ cells seemed to be associated with the development of intermediate and large choroidal vessels as development progressed. To confirm whether endothelial cells were proliferating, double staining for CD34 and Ki67 was done on older specimens and it was apparent that endothelial cells were proliferating, especially on the scleral side of choriocapillaris and at the tips of diving vessels at 12 WG and older (Fig. 7C,D). Our results showed more proliferating cells are in the posterior pole than in the periphery. The progression of intermediate and large vessel formation suggested that the deeper larger blood vessels developed centrifugally from the optic nerve region. Since Ki67 expression was associated with endothelial cells at vessel tips, the development of most large choroidal vessels and perhaps anastomosis of choriocapillaris with large and intermediate choroidal blood vessels appears to be accomplished by angiogenesis. With age, the number of Ki67+ cells increased throughout the choriocapillaris and may represent angiogenesis that is required to increase the density of the choriocapillaris as the globe expands. The density of capillaries at 12 WG (Fig. 10A) is quite low compared to the adult choriocapillaris (McLeod and Lutty, 1994).
It is interesting that the fetal human retinal vasculature only forms after 12 WG. It forms by vasculogenesis, the differentiation and coalescence of CD39+ angioblasts without proliferation of the angioblasts (McLeod et al, 2006). The vascular markers employed in this study, CD34, CD31, and VWf, were only observed in endothelial cells in formed retinal blood vessels, and not associated with angioblasts. Therefore, the choroidal and retinal vasculatures in embryonic and fetal humans form by unique and different processes, which may help explain their distinct differences in the adult human.
In summary, islands or aggregates of precursors as found in yolk sac appear to be the origin of the first blood vessels in human embryonic choroid. This process, hemo-vasculogenesis, is responsible for formation of blood cells as well as endothelial cells from common precursors, as observed in embryoid bodies formed from human embryonic stem cells in vitro (Zambidis et al., 2005). This study is the first to suggest in human that hemangioblasts are present intra-embryonically and that they appear to be the common precursor that participates in hemo-vasculogenesis. This in situ assembly of choriocapillaris explains how the choriocapillaris can form without any apparent feeding arterioles or draining venules. The lobular pattern of adult choriocapillaris may be a consequence of development from islands of precursors where capillaries are formed independent of flow and without guidance of neurons or matrix. This is as opposed to human retina where a mesh-like capillary system forms initially by vasculogenesis (Chan-Ling et al., 2004; McLeod et al., 2006) but it is remodeled in part by blood flow and in part by neuronal development and tissue needs, resulting in an end-arterial vasculature. There are free CD39+ cells, presumably angioblasts, present during fetal development that may contribute to expansion of the choriocapillaris by vasculogenesis. The choriocapillaris later appears to bud by angiogenesis forming anastomoses with intermediate and larger choroidal blood vessels. In the specimens used in this study, an adult density and pattern of choriocapillaris was never achieved, but the lobular system may not form completely until arterioles that feed the choriocapillaris and venules that drain this system are well established. The current study demonstrates for the first time the embryonic development of an ocular vasculature by hemo-vasculogenesis, which may contribute to the early development and unique attributes of the human choroidal vasculature, which is responsible for the development, health, and function of the retinal photoreceptors.
EXPERIMENTAL PROCEDURES
Age Determination of Human Embryos and Fetuses and Tissue Preparation for Cryopreservation
Human eyes, ranging in age from 5.5 to 23 WG, were provided by Advanced Bioscience Resources, Inc (Alameda, CA). Utilization of this human tissue was in accordance with the Declaration of Helsinki with approval of the JCCI at Johns Hopkins University School of Medicine. The gestational age of each embryo or fetus was determined by the fetal foot size or sonography. The gestational age of the 5.5-WG specimen was estimated based on the last menstrual period and sonography, but this may not be exact. During development, the first 8 weeks of gestation are considered the embryonic period and thereafter is considered fetal. The eyes and how they were processed are listed in Table 1. After enucleation, the eyes were immediately fixed at room temperature for 1 hr with 2% paraformaldehyde in 0.1 M sodium phosphate buffer with 5% sucrose. The eyes were then washed in 0.1 M sodium phosphate buffer with 5% sucrose and shipped at 4°C to the Wilmer Ophthalmological Institute. Young eyes (6–9 WG) were processed and embedded in toto. For older eyes (9–23 WG), the anterior segments were removed and coulottes of retinal/choroidal/scleral tissue were processed. All tissue was washed (30 min/wash) at room temperature in 0.1 M sodium phosphate buffer with increasing concentrations of sucrose: a 2:1 solution of 5% sucrose: 20% sucrose; a 1:1 solution; and finally a 1:2 mixture (Lutty et al., 1993). The eyecups or whole eyes were held at room temperature for 2 hr in 20% sucrose in 0.1 M sodium phosphate buffer. The tissues were placed in flat embedding molds with an embedding solution consisting of a 2:1 mixture of 20% sucrose in 0.1M sodium phosphate buffer:OCT compound and infiltrated for 30 min at room temperature as reported previously (Lutty et al., 1993). After photographic documentation, the quadrants or whole eyes were then frozen in isopentane cooled with dry ice.
TABLE 1.
Processing of Eyes
| Age (WG) | Cryo | Whole mount | Whole eye |
|---|---|---|---|
| 5.5 | 1 | ||
| 6 | 1 | 1 | |
| 6.5 | 1 | 1 | |
| 7 | 2 | ||
| 7.5 | 3 | 2 | 1 |
| 8 | 2 | ||
| 8.5 | 2 | 1 | |
| 9 | 2 | ||
| 9.5 | 1 | ||
| 12 | 2 | 2 | |
| 14 | 1 | 2 | |
| 16 | 1 | ||
| 20 | 2 | ||
| 22 | 1 | 1 | |
| 23 | 1 | 1 | |
| Total | 19 | 13 | 3 |
Immunohistochemistry on Cryopreserved Tissue
Streptavidin alkaline phosphatase (APase) immunohistochemistry was performed on eight-micron-thick sections of cryopreserved tissue using a nitroblue tetrazolium (NBT) system recently developed by Bhutto et al. (2004). The sections were incubated overnight at 4°C with one of the following primary antibodies: mouse anti-CD34 (1:800; Signet Laboratory); mouse anti-CD31 (1:1,000; Dako); mouse anti-CD39 (1:400; Chemicon); mouse anti-CD45 (1:800, Chemicon); mouse anti-Ki67 (1:1,000; Zymed); and mouse anti-VEGFR-2 (1:600; Imclone Systems). After washing in TBS, sections were incubated for 30 min at room temperature with the appropriate biotinylated secondary antibodies diluted 1:500 (Kirkegaard and Perry, Gaithersburg, MD). Finally, sections were incubated with streptavidin APase (1:500; Kirkegaard and Perry), and APase activity was developed with a 5-bromo-4-chloro-3-indoyl phosphate (BCIP)-NBT kit (Vector Laboratories, Inc.), yielding a blue reaction product.
For qualitative assessment of immunohistochemistry at the level of choroid-Bruch’s membrane-RPE complex, the removal of melanin pigment was desirable. Melanin in RPE was bleached by a technique recently developed by Bhutto et al. (2004) after streptavidin APase immunohistochemistry. When bleaching was complete, coverslips were mounted with Kaiser’s glycerogel mounting medium without counterstaining.
Double Labeling for Immunofluorescence on Cryopreserved Tissue
Double labeling with immunofluorescence was performed on cryopreserved tissue sections. Sections were permeabilized with absolute methanol at −20°C and blocked with 2% normal goat serum in TBS. The sections were incubated for 2 hr at room temperature with a mixture of mouse anti-CD34 (1:800; Signet Laboratory) and rabbit anti-Ki67 (1:50; Zymed). After they were washed in TBS, sections were incubated for 30 min at room temperature with the mixed secondary antibodies:goat anti-mouse conjugated with Cy3 diluted 1:500 and goat anti-rabbit conjugated with FITC diluted 1:100 (Jackson Immuno Research). Finally, sections were mounted in DAPI counterstaining medium (Vector Laboratories, Inc.). Sections were then observed with a Zeiss Meta 510 confocal microscope (Carl Zeiss).
Double Labeling With Hb-∈ and Vascular Markers on Cryopreserved Tissue
For double labeling for Hb-∈ and endothelial cell markers, cryosections were additionally postfixed at room temperature overnight with 2% paraformaldehyde in TBS before starting the immunofluorescence technique. After washing in TBS, the sections were processed as previously stated. The sections were incubated for 2 hr at room temperature with one of the following primary antibodies: mouse anti-human CD34 (1:800); mouse anti-CD31 (1:1,000); mouse anti-Ki67 (1: 100); or mouse anti-VEGFR-2 (1:100). After washing in TBS, sections were incubated for 30 min at room temperature with the goat anti-mouse secondary antibodies conjugated with Cy3 (Jackson Immuno Research) diluted 1:500. After washing in TBS, the sections were incubated for 2 hr at room temperature with goat anti-Hb-∈ (1:500; Cortex Biochem), which is commercially pre-conjugated with FITC. Finally, sections were washed in TBS and mounted in DAPI counter-staining medium (Vector Laboratories, Inc.).
Triple Labeling With Hb-∈ and Vascular Markers on Cryopreserved Tissue
Triple labeling for epsilon globin (Hb-∈) and vascular markers was accomplished by post-fixing cryosections with 2% paraformaldehyde at room temperature overnight in TBS before immunostaining. After washing in TBS, the sections were incubated for 2 hr at room temperature with a mixture of mouse anti-CD34 (1:800) and rabbit anti-von Willebrand factor (1: 2,500). After washing in TBS, sections were incubated for 30 min at room temperature with the mixed secondary antibodies: goat anti-mouse conjugated with Cy3 diluted 1:500 and goat anti-rabbit conjugated with Alexa Fluor 633 (Molecular Probes, Carlsbad, CA) diluted 1:200. After washing in TBS, the sections were incubated for 2 hr at room temperature with goat anti-Hb-∈ (1:500; Cortex Biochem) directly conjugated to FITC. Finally, sections were washed in TBS and mounted in DAPI counterstaining medium (Vector Laboratories, Inc.).
Confocal Microscopy
Tissue sections with fluorescent secondary antibodies and DAPI counterstain were examined with a Zeiss 510 META confocal microscope. Excitation wavelengths included 405, 488, and 532 nm for the DAPI, FITC, and Cy3 respectively. The tissues were analyzed with either 5, 10, 20, 40, or 63× objectives. Multispectral confocal microscopy was used as a validation tool for intracellular localization of antigens. Since multiple fluorescent colors were used to label various antigens and nuclei, it was necessary to use a multispectral confocal microscope that could separate the color optical overlaps in order to determine true co-localization and reveal autofluorescence.
Flat-Embedding Whole Choroids
For flat-embedding choroids, after the anterior segments were removed from other eyes, the retinas were separated from the RPE, and then the choroids were separated from the sclera. The RPE were removed in 0.1% EDTA/TBS. After washing in TBS, the choroids were permeabilized in 1%Triton/TBS for 30 min and processed as published previously (Otsuji et al., 2002). The choroids were then incubated for 72 hr at 4°C with mouse anti-CD39 (1:10; Chemicon). After they were washed in 1%Triton/TBS, choroids were incubated for 48 hr at 4°C with the biotinylated goat anti-mouse antibody diluted 1:100 (Kirkegaard and Perry, Gaithersburg, MD). After washing in 1%Triton/TBS, the choroids were incubated for 24 hr at 4°C with streptavidin APase (1:100; Kirkegaard and Perry). Finally, APase activity was developed with HistoMark Red kit (Kirkegaard and Perry), yielding a red reaction product.
Tissue Preparation of Whole Eyes and Immunohistochemistry on Whole Choroids
To visualize the choroidal vasculature in two dimensions (as a whole mount and in cross-section), the whole choroids were embedded in JB-4 (Polysciences, Inc.) after APase immunohistochemistry as just mentioned (Otsuji et al., 2002). In brief, they were postfixed flat in 25% Karnovsky’s fixative overnight at 4°C, washed in 0.1 M cacodylate buffer with 5% sucrose, and then dehydrated in 30, 50, 70, 80, 90, and 95% ethanol. They were then infiltrated and embedded in JB-4. Sections 2.5 microns thick were cut with a dry glass knife on a Sorvall MT-2 ultramicrotome (Sorvall, Norwalk, CT) and dried on glass slides before staining. These sections were stained by the following stains: thionin counterstain for nuclei; hematoxylin and eosin; and Wright’s Giemsa for hemoglobin.
JB-4 (glycol methacrylate, Polysciences, Warrington, PA) embedded whole eyes for serial sectioning or choroids for flat-embedding (Table 1) were prepared as follows. After fixation for 1 hr with 2% paraformaldehyde and shipping overnight at 4°C in buffer, the eyes were washed in TBS. Whole eyes were refixed for 48 hr in 25% Karnovsky’s glutaraldehyde/paraformaldehyde fixative at 4°C and then washed in 0.1 M cacodylate buffer with 5% sucrose. Whole eyes were embedded in JB-4 in a similar fashion except all dehydration and infiltration times were extended to 30 min in each concentration of ethanol.
Wright’s Giemsa Staining
Wright’s Giemsa staining was performed on JB-4 sections (2.5 microns thick), using Wright’s Giemsa stain solution (Polysciences, Inc. Warrington, PA) for 10 min at room temperature, followed by a 10-sec differentiation with 70% ethyl alcohol. This yielded a pink reaction product in the acidophilic cytoplasm of erythroblasts. They were then washed in double distilled water and dried. Finally, coverslips were applied with Permount mounting medium (Polysciences, Inc., Warrington, PA).
Acknowledgments
This work was supported in part by NIH-EY-016151 (G.L.), EY01765 (Wilmer), the Altsheler-Durell Foundation, and a gift from the Himmelfarb Family Foundation in the name of Morton Goldberg, M.D. Takuya Hasegawa was a Bausch and Lomb Japan Research Fellow.
References
- Bhutto IA, Lutty GA. The vasculature of choroid. In: Schepro D, editor. Microvasculature research: biology and pathology. San Diego: Elsevier; 2006. pp. 369–374. [Google Scholar]
- Bhutto IA, Kim SY, McLeod DS, Merges CA, Fukai N, Olsen BR, Lutty GA. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:1544–1552. doi: 10.1167/iovs.03-0862. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Narita N, Heikinheimo M, Porter SB, Wilson DB. Erythropoiesis and vasculogenesis in embryoid bodies lacking visceral yolk sac endoderm. Blood. 1996;88:3720–3730. [PubMed] [Google Scholar]
- Blaauwgeers HG, Holtkamp GM, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, Van Hinsbergh VW, Schlingemann RO. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Am J Pathol. 1999;155:421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Ling T, McLeod DS, Hughes S, Baxter L, Chu Y, Hasegawa T, Lutty GA. Astrocyte-endothelial cell relationships during human retinal vascular development. Invest Ophthalmol Vis Sci. 2004;45:2020–2032. doi: 10.1167/iovs.03-1169. [DOI] [PubMed] [Google Scholar]
- Civin CI, Strauss LC, Brovall C, Fackler M, Schwartz J, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- Cortes F, Debacker C, Peault B, Labastie M. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mech Dev. 1999;83:161–164. doi: 10.1016/s0925-4773(99)00030-1. [DOI] [PubMed] [Google Scholar]
- Daufenbach D, Ruttum MS, Pulido J, Keech RV. Chorioretinal colobomas in a pediatric population. Ophthalmology. 1998;105:1455–1458. doi: 10.1016/S0161-6420(98)98028-9. [DOI] [PubMed] [Google Scholar]
- Ferkowicz M, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Fina L, Molgaard H, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland D, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- Forrai A, Robb L. The hemangioblast: between blood and vessels. Cell Cycle. 2003;2:86–90. [PubMed] [Google Scholar]
- Fraser S, Yamashita J, Jakt L, Okada M, Ogawa M, Nishikawa S, Nishikawa S. In vitro differentiation of mouse embryonic stem cells: hematopoietic and vascular cell types. Methods Enzymol. 2003;365:59–72. doi: 10.1016/s0076-6879(03)65004-4. [DOI] [PubMed] [Google Scholar]
- Goepfert C, Sundberg C, Sevigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- Guo H, Fang B, Liao L, Zhao Z, Liu J, Chen H, Hsu S, Cui Q, Zhao R. Hemangioblastic characteristics of fetal bone marrow-derived Flk1(+)CD31(−)CD34(−) cells. Exp Hematol. 2003;31:650–658. doi: 10.1016/s0301-472x(03)00087-0. [DOI] [PubMed] [Google Scholar]
- Heimann K. The development of the choroid in man. Ophthal Res. 1972;3:257–273. [Google Scholar]
- Imhof B, Dunon D. Leukocyte migration and adhesion. Adv Immunol. 1995;58:345–416. doi: 10.1016/s0065-2776(08)60623-9. [DOI] [PubMed] [Google Scholar]
- Jakobiec FA. Ocular anatomy, embryology, and teratology. Philadelphia: Harper and Row; 1982. [Google Scholar]
- Labastie M, Cortes F, Romeo PH, Dulac C, Peault B. Molecular identity of hematopoietic precursor cells emerging in the human embryo. Blood. 1998;92:3624–3635. [PubMed] [Google Scholar]
- Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35–42. [PubMed] [Google Scholar]
- Lutty GA, McLeod DS. A new technique for visualization of the human retinal vasculature. Arch Ophthalmol. 1992;110:267–276. doi: 10.1001/archopht.1992.01080140123039. [DOI] [PubMed] [Google Scholar]
- Lutty GA, Merges C, Threlkeld AB, Crone S, McLeod DS. Heterogeneity in localization of isoforms of TGF-b in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci. 1993;34:477–487. [PubMed] [Google Scholar]
- Mann IC. The development of the human eye. Cambridge: Cambridge University Press; 1928. [Google Scholar]
- Marcus AJ, Broekman M, Drosopoulos J, Olson KE, Islam N, Pinsky D, Levi R. Role of CD39 (NTPDase-1) in thromboregulation, cerebroprotection, and cardioprotection. Semin Thromb Hemost. 2005;31:234–246. doi: 10.1055/s-2005-869528. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Lutty GA. High resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994;35:3799–3811. [PubMed] [Google Scholar]
- McLeod DS, Lutty GA, Wajer SD, Flower RW. Visualization of a developing vasculature. Microvasc Res. 1987;33:257–269. doi: 10.1016/0026-2862(87)90021-5. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Brownstein R, Lutty GA. Vaso-obliteration in the canine model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 1996;37:300–311. [PubMed] [Google Scholar]
- McLeod DS, Hasegawa T, Prow T, Merges C, Lutty GA. The initial fetal human retinal vasculature develops by vasculogenesis. Dev Dyn. 2006;235:3336–3347. doi: 10.1002/dvdy.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaux G, Cutler DF. How to roll an endothelial cigar: the biogenesis of Weibel-Palade bodies. Traffic. 2004;5:69–78. doi: 10.1111/j.1600-0854.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- Murray PD. The development in vitro of blood of the early chick embryo. Proc Royal Acad Soc Lond. 1932;111:497–521. [Google Scholar]
- Newman P. The biology of PECAM-1. J Clin Invest. 1997;100:S25–29. [PubMed] [Google Scholar]
- Ogawa M, Fraser S, Fujimoto T, Endoh M, Nishikawa S, Nishikawa S. Origin of hematopoietic progenitors during embryogenesis. Int Rev Immunol. 2001;20:21–44. doi: 10.3109/08830180109056721. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon L. Hematopoiesis and stem cells: plasticity versus developmental heterogeneity. Nat Immunol. 2002;3:323–328. doi: 10.1038/ni0402-323. [DOI] [PubMed] [Google Scholar]
- Otsuji T, Mcleod DS, Hansen B, Lutty GA. Immunohistochemical staining and morphometric analysis of the monkey choroidal vasculature. Exp Eye Res. 2002;75:201–208. doi: 10.1006/exer.2002.2020. [DOI] [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Ann Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Sabin FR. Origin and development of the primitive vessels of the chick and pig. Carnegie Contrib Embryol. 1917;6:61–124. [Google Scholar]
- Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living. J Hematother Stem Cell Res. 2002;11:5–7. doi: 10.1089/152581602753448496. [DOI] [PubMed] [Google Scholar]
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- Sellheyer K. Development of the choroid and related structures. Eye. 1990;4:255–261. doi: 10.1038/eye.1990.37. [DOI] [PubMed] [Google Scholar]
- Sellheyer K, Spitznas M. The fine structure of the developing human choriocapillaris during the first trimester. Graefe Arch Clin Exp Ophthalmol. 1988;226:65–74. doi: 10.1007/BF02172721. [DOI] [PubMed] [Google Scholar]
- Sequeira Lopez ML, Chernavvsky DR, Nomasa T, Wall L, Yanagisawa M, Gomez RA. The embryo makes red blood cell progenitors in every tissue simultaneously with blood vessel morphogenesis. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1126–1137. doi: 10.1152/ajpregu.00543.2002. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi T, Gertsenstein M, Wu X, Breitman M, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Ho J, Stanford W, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Tavian M, Peault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- Tavian M, Coulombel L, Luton D, Clemente H, Dieterlen-Lievre F, Peault B. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood. 1996;87:67–72. [PubMed] [Google Scholar]
- Ueno H, Weissman I. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Yong K, Watts M, Shaun Thomas N, Sullivan A, Ings S, Linch DC. Transmigration of CD34+ cells across specialized and nonspecialized endothelium requires prior activation by growth factors and is mediated by PECAM-1 (CD31) Blood. 1998;91:1196–1205. [PubMed] [Google Scholar]
- Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]


