Synopsis
The debate about whether asthma and chronic obstructive pulmonary disease (COPD) are distinct clinical syndromes is not new, but there has been a heightened interest in recent years in understanding the group of individuals with obstructive lung disease who appear to have elements of both asthma and COPD. These patients are of interest because of recent studies which have demonstrated increased risk for respiratory events and exacerbations in this group. We describe the clinical characteristics of this subtype of disease and suggest 4 working definitions of individuals who would fall into the asthma/COPD overlap category. These definitions shed light on the clinical and inflammatory characteristics surrounding the group with overlap syndrome in order to better understand the biologic mechanisms for their worsened outcomes. Understanding the mechanisms underlying these subtypes will hopefully lead into a better understanding of therapeutic strategies that can target specific pathobiologic pathways.
Keywords: Asthma, COPD, Overlap, Subtypes, ACOS
Introduction
The debate about the relationship between asthma and COPD on the spectrum of obstructive lung disease is far from new. The earliest and most famous example of such a debate can be found in the juxtaposition of the “Dutch” and the “British” hypotheses. Orie and colleagues from the Netherlands first described their hypothesis in 1961 that one disease termed “Chronic Nonspecific Lung Disease (CNSLD)” existed which described all individuals with asthma, chronic bronchitis and emphysema. They hypothesized that all of these individuals had shared endogenous and exogenous factors (now called gene-environment interaction) that contributed to the development of the disease. Host factors included allergic disease and bronchial hyper-responsiveness, and environmental factors included cigarette smoke and pollution.(1, 2) Conflicting with this position was the “British” hypothesis which distinguished a syndrome of chronic, irreversible airflow obstruction resulting from, most notably, exposure to smoking in susceptible individuals(3). This was thought to be separate from asthma which was related to allergic disease and airways hyper-reactivity. Patients with chronic airflow obstruction were considered to differ from those with asthma based on clinical course and pathogenesis.
Studies such as those reported by Burrows et al. in 1987,(4) which demonstrated in a large, longitudinal epidemiologic study that nonsmoking individuals with a more asthmatic, atopic phenotype had significantly less decline in FEV1 over time as well as much lower mortality rate than the group of nonatopic former and current smokers, seemed to lend substantial weight to the British hypothesis of two distinct clinical syndromes. Beyond outcome measures, other important evidence pointing to two distinct clinical syndromes drew from work that describes asthma and COPD as having distinct physiologic, inflammatory, and radiologic patterns. Asthma has been described to involve more eosinophilic inflammation(5) as opposed to the neutrophilic inflammation thought to be more dominant in individuals with COPD. Fabbri et al. also showed that in individuals with a history of asthma with a similar degree of fixed airflow obstruction and airway hyper-reactivity as a group of individuals with COPD, those with asthma had less emphysema on CT, lower residual volume and higher diffusing capacity on pulmonary function testing.(5) The patients with a history of asthma also showed more eosinophilic inflammation in blood, sputum, airway histology, and higher levels of expired nitric oxide (NO). With regards to describing a distinct COPD diagnosis, in an early paper on the subject, Vermeire and Pride proposed a COPD phenotype comprising individuals with airflow obstruction, bronchial hypersecretion, and alveolar destruction.(6) In contrast to asthma this group of patients with smoking-related airflow obstruction have neutrophil-predominant airways inflammation. (7)
Research endeavors, drug development, and clinical guidelines about COPD and asthma in the past few decades have focused on the diseases as distinct entities. However, it has always been recognized by clinicians, as evidenced by the Dutch vs British hypothesis debate, that the distinctions between COPD and asthma are less clear, with a spectrum of disease. In recent years there has been a resurgence of thought about the presence of a significant group of individuals that have attributes of both asthma and COPD, recently termed the Asthma COPD overlap syndrome (ACOS). Understanding this historical context of the Dutch vs British debate is a reminder that the idea of asthma and COPD as potentially overlapping entities is not an entirely novel perspective, but also highlights the importance of this topic given its implications for our understanding of obstructive airways disease and treatment strategies. The resurgent interest in ACOS has been kindled by the recognition by both the GINA (Asthma) and GOLD (COPD) expert panels that many patients were not adequately addressed by either group, and the acronym ACOS was put forth.
Prevalence and epidemiology of Asthma/COPD Overlap syndrome
Recent studies have focused on understanding the scope, characteristics and epidemiology of individuals with asthma/COPD overlap syndrome.(8) The estimated prevalence of the syndrome appears to vary based upon the population studied and the definitions utilized to describe the syndrome, but has been estimated between 13% and 38% of the population with obstructive lung disease.(9–21) Most estimates of prevalence of ACOS among a population of individuals with asthma appears to be slightly higher, with estimates ranging 27.1%–38%(13, 16, 18), whereas estimates of ACOS within a population of individuals with COPD appear to be slightly lower, ranging mostly from 13%–28.6%.(9–11, 14, 17, 19–21) Other than the differences in population characteristics which may account for the variation in prevalence estimates, the definitions utilized to identify individuals with ACOS also differ greatly. Several studies, particularly those in which the population studied is primarily composed of individuals with COPD, have defined ACOS as an individual with a spirometric diagnosis of COPD, most commonly employing Global Initiative of Chronic Obstructive Lung Disease (GOLD) criteria,(22) American Thoracic Society/European Respiratory Society (ATS/ERS) criteria,(23) or some slight variation of these criteria, and also reporting a diagnosis of asthma earlier in life, such as prior to the age of 40.(9, 14, 20) Others utilize functional characteristics such as bronchial hyperresponsiveness or bronchodilator reversibility in their diagnostic criteria,(11, 18, 19, 21, 24) while still others use formal criteria for asthma (e.g. Global Initiatiative for Asthma, GINA (25) or British Asthma guidelines) and COPD (e.g. ATS/ERS(23) or GOLD criteria(22)) and define overlap as meeting both sets of criteria.(15, 17, 21)
Regardless of the heterogeneity of the populations studied and the inconsistencies of the definitions of ACOS applied, the group having ACOS appear to have differing characteristics from populations having COPD or asthma alone. For the most part, individuals with ACOS are younger(14, 15) and have less cumulative smoking burden(9, 14, 15, 20) than individuals with COPD alone. Additionally, the group with ACOS have a higher body-mass index(14, 20) than counterparts with COPD or asthma alone. Inconsistent findings were a higher prevalence of women(9, 14, 17) and better lung function (as measured by FEV1)(9, 15) in the ACOS group than in those with COPD alone.
Most studies show that individuals with ACOS have worse clinical outcomes than individuals with COPD alone or asthma alone. Compared to individuals with COPD alone, those with COPD and asthma have a higher risk of exacerbations, respiratory adverse events, and hospitalizations.(9, 10, 14, 17, 19, 20, 26). These patients have more dyspnea,(20) respiratory symptoms,(20, 26) physical impairment,(26) worse quality of life,(14, 15, 19, 20) and poorer disease control(19, 21) compared to those with COPD or asthma alone.
Paradoxically, several studies have demonstrated that, despite having more exacerbations and respiratory symptoms, the ACOS group has less disease severity than the group with COPD alone. One study of a large Spanish COPD cohort showed that individuals with ACOS had lower 1 year mortality than those with COPD alone.(11) Findings on lung function have been less consistent, with some observing that the group with ACOS have better lung function(9, 15) and slower decline in lung function(12) than the group with COPD alone, while others observed that lung function was lower(17, 20) in the group with ACOS. In addition to lower mortality and slower lung function decline, Fu et. al. found that the group with ACOS had slower age-related decline in exercise capacity assessed by six-minute walk test than the group with COPD alone.(24)
Despite the inconsistent findings about differences in severity of disease, there is a consistent message from the many studies performed on this subject that the group with ACOS appears to have different characteristics than those with COPD alone. For example, Hardin and colleagues found in the large COPDGene study that the group with ACOS had less emphysema but more airways wall thickness on CT scans.(14) The finding of higher wall thickness was later also demonstrated by Suzuki and colleagues when studying a smaller clinical cohort.(21)
Consensus statements
Ultimately, because of the recent interest in characterizing the group with asthma and COPD overlap in the setting of many different working definitions, there have been two recent consensus statements that aimed to better define the ACOS group.
Spanish statement
The first of these statements to be published was a consensus statement of Spanish Pulmonologists.(27) After performing a literature review, this group of experts employed a step-wise approach to reach consensus about the following topics: 1) determining a name for the syndrome, 2) agreeing on major and minor criteria for diagnosis, 3)suggesting treatment strategies, and 4) addressing gaps in understanding for future research attention. The group agreed that there was a subtype of COPD which included patients having both COPD and asthma-type characteristics, and they chose to describe this subtype as the “Mixed COPD-asthma phenotype.” Then, among several proposed criteria, they agreed upon 3 major and 3 minor criteria for identifying an individual with this subtype of disease (Table 1), agreeing that 2 major criteria or 1 major and 2 minor criteria would need to be present for an individual to qualify as having this disease subtype. These criteria include lung function measures, historical elements, as well as laboratory testing. Other criteria that were considered but did not gain enough consensus among the experts to be included were: peripheral eosinophilia, symptom variability, positive skin prick testing, elevated exhaled NO, positive methacholine testing, peak flow variability, family history of asthma or atopy, rhinitis, bronchodilator reversibility, and a positive oral corticosteroid test.
Table 1.
Major and minor criteria for “Mixed COPD/Asthma phenotype”
| Major Criteria | Minor Criteria |
|---|---|
| Positive bronchodilator testing (FEV1 increases ≥ 15% and ≥ 400mL) | Elevated total IgE level |
| Sputum eosinophilia | Personal history of atopy |
| Personal history of asthma (diagnosed before the age of 40) | Positive bronchodilator testing (FEV1 increases ≥ 12% and ≥ 200mL) on 2 or more occasions |
| To be considered as having this phenotype, the authors suggest 2 major criteria are met. Alternatively, 1 major and 2 minor criteria can be met as well. | |
Adapted from Soler-Cataluna JJ, Cosio B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Archivos de bronconeumologia 2012; 48: 331–337; with permission.
This statement also addressed treatment considerations and future research targets. Notably, 100% of the experts agreed that individuals classified as mixed COPD-asthma phenotype should have early use of inhaled corticosteroids (ICS), citing positive drug trials of ICS in individuals with COPD as well as eosinophilic airways inflammation measured by sputum eosinophilia.(28, 29). A majority of the group also agreed that ICS should be used as part of a “triple therapy” strategy (ICS, long acting beta agonist and long acting anticholinergic) in the most severe cases of asthma-COPD overlap, titrating the ICS component to symptom burden as is done with asthma, paying careful attention to the high risks associated with withdrawing the ICS component leading to higher exacerbation risk.(27)
Combined GINA/GOLD statement
The other consensus document published was the combined GINA/GOLD statement,(30) first published in 2014 and updated recently. This statement, acknowledging the difficulty of defining ACOS with the limited available research to date, was less precise about defining the syndrome, but has clearly outlined the methodology of characterizing ACOS clinically and considerations for initiation of therapy. This group defines ACOS as “characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.” Further, 5 important steps are outlined to aid in assessing individuals with respiratory symptoms in order to better characterize them as having asthma, COPD or ACOS. The first step includes taking a thorough history and exam in order to determine if the patient has chronic airways disease. Next, it is suggested that the provider review a list of characteristics more typical of asthma or COPD, and count the number of these characteristics that the patient exhibits (Table 2). If the preponderance of characteristics points to asthma or COPD, then a diagnosis of one or the other is made, otherwise a diagnosis of ACOS is considered. The characteristics include age of onset of disease, variability versus persistence of symptoms, spirometric characteristics, medical, family and past exposure history, progression of symptoms over time, and radiographic characteristics. The third step is to perform spirometry, and spirometric characteristics of asthma, COPD or ACOS are outlined, this information is suggested to be used to revise the diagnosis if needed. In step 4, therapy is commenced based upon the diagnosis. If asthma, the GINA guidelines(25) are used, if COPD, the GOLD guidelines are used,(22) and if ACOS, the group advocates starting treatment for asthma, given that ICS is an important aspect of control, and to add long acting bronchodilators if they are not already being used. Finally, in step 5, the document recommends referral for further investigation if there is continued uncertainly, the patient is unresponsive to treatment, if atypical symptoms or signs are present, or if there are further issues with tolerating or prescribing treatment such as interfering comorbidities or other issues.
Table 2.
Characteristics that support a diagnosis of asthma or COPD, grouped by category
| Characteristics favoring diagnosis of asthma | Characteristics favoring diagnosis of COPD | |
|---|---|---|
| Age | Onset <2 0 years | Onset > 40 years |
| Respiratory symptoms | Variation of symptoms with time | Symptoms persist regardless of treatment |
| Worsening of symptoms at night or in morning | Usually have daily symptoms and dyspnea with good and bad days | |
| Triggers for symptoms noted including exercise, emotion, dust or allergen exposure | Chronic bronchitis symptoms precede onset of dyspnea and not necessarily related to triggers | |
| Lung function | Variability in airflow obstruction using peak flows or spirometry | Airflow obstruction often persistent or fixed |
| Lung function between symptoms | Lung function normal between symptoms | Lung function abnormal between symptoms |
| History | Previously diagnosed by doctor with asthma | Previously diagnosed by doctor with COPD, emphysema, chronic bronchitis |
| Family history of asthma, allergic disease | Heavy exposure history common: tobacco smoke, biomass fuels | |
| Time course | Symptoms do not worsen over time, but vary seasonally or from year to year | Symptoms progress slowly over time |
| Can improve quickly and respond quickly to therapies such as ICS or bronchodilators | Symptoms often have limited response to short acting inhalers | |
| Imaging | Chest Xray usually normal | Chest Xray reveals severe hyperinflation |
| The GINA/GOLD statement notes that if three or more characteristics are present for one either asthma or COPD, it is suggested that the patient likely has that disease, however if there are a similar number of boxes checked for both, then a diagnosis of ACOS is considered. | ||
Adapted from GOLD Ga. Diagnosis of Diseases of Chronic Airflow Limitation: Asthma, COPD, and Asthma-COPD Overlap Syndrome (ACOS); 2015; with permission.
Proposed pathways to ACOS, presented as 4 phenotypes
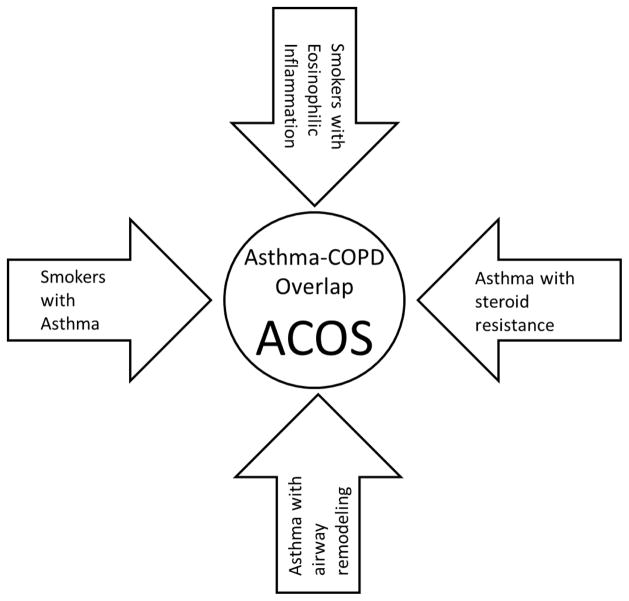
To date, studies have attempted to describe the ACOS subgroup with differing definitions as if it were a single entity. We propose that ACOS, as it is now recognized, comprises several different entities that have different clinical presentations and different pathophysiological mechanisms. Attempts at reaching consensus have been informative but more work is needed if operational definitions of ACOS can be established and validated. We describe four operational definitions of ACOS that correspond to clinically recognizable patterns. These are: 1) the smoker with airflow limitation and an eosinophilic inflammatory pattern, 2) the asthmatic who is resistant to steroid treatment and has a more neutrophilic pattern of inflammation, 3) the elderly asthmatic who has had remodeling of their airways and developed irreversible airflow obstruction, and 4) the childhood asthmatic who takes up smoking and develops irreversible airflow obstruction. (Figure 1)
Figure 1.
Four distinct pathways to Asthma-COPD Overlap Syndrome
Smokers with eosinophilic inflammation
There have been several studies which have attempted to describe the subgroup of individuals with COPD having a higher burden of eosinophilic inflammation. Bafadhel et al. investigated biomarkers during COPD exacerbations and detected three clusters or phenotypes of exacerbations including exacerbations driven by bacteria, viruses and eosinophilic airway inflammation (as detected by sputum and serum eosinophil counts). They found that 28% of exacerbations in their series were attributed to eosinophilic inflammation.(31) In addition, around 37.4% of the ECLIPSE cohort was shown to have persistent blood and sputum eosinophilia (defined as ≥2%). This subgroup was shown to have better lung function, fewer symptoms (lower SGRQ score and mMRC scores) and less progression of emphysema than the group with persistently low eosinophil counts.(32) Both of these studies are highly suggestive of a subgroup of individuals with COPD having a higher burden of eosinophilic inflammation, but also that this subgroup likely has a different pattern of disease progression and severity.
There have been other attempts to distinguish a subgroup of COPD with eosinophilic inflammation, particularly in regards to understanding whether this group has enhanced response to inhaled or oral corticosteroid treatment. Brightling et al showed that individuals with more baseline sputum eosinophilia, treated with systemic corticosteroids, showed a greater increase in FEV1 and improvement in respiratory symptoms than those with little or no eosinophilia prior to treatment.(33) Similar findings were noted for outcomes of dyspnea, quality of life and lung function in a small study of patients with severe COPD and bronchitis, of whom 40% had sputum eosinophilia prior to treatment.(34) A few studies have shown that inhaled corticosteroids have more benefit for improvement in lung function and respiratory symptoms in individuals with more sputum eosinophilia than those without.(29, 35) Going further, Siva et al tested the value of targeting eosinophilia with treatment strategies by randomizing patients to standard treatment versus standard treatment with the additional aim of reducing sputum eosinophilia. They found a modest reduction in the number of severe exacerbations experienced in the group where eosinophilia was targeted with the use of corticosteroids.(28)
Further support for this phenotype of individuals can be found in the recent work of Christenson et al, who studied airway epithelial gene expressions in asthma and COPD. They found overlap in around 100 genes altered in the airway epithelium in asthma with those upregulated in COPD, noting that the gene expression changes present in this overlap were found in large and small airways. Additionally, they went on to develop at 100-gene signature of gene expression related to Th2-related inflammation, termed the T2S score. They showed that this specific Th-2 signature was upregulated in a subgroup of COPD having eosinophilic inflammation (airways and systemic eosinophilia), as well as clinical characteristics thought to be associated with ACOS including bronchial hyperresponsiveness and treatment response to ICS.(36)
Related to this phenotype, there have been several studies which have attempted to understand the prevalence of atopy among those with COPD and how this relates to outcomes. Jamieson and colleagues found the prevalence of atopy was around 30% (measured by sensitization to a panel of common indoor and outdoor allergens) in individuals with COPD, and noted that these people had higher risk for respiratory symptoms, exacerbations and adverse outcomes.(37) Within the EUROSCOP study, Fattahi et al showed a prevalence of 18.3% of atopy in those with COPD, and noted a higher risk for respiratory symptoms in this group. They went further to show that ICS was more effective to reduce symptoms in atopic COPD patients than those without atopy,(38) echoing the results described above for individuals with COPD and sputum and serum eosinophilia, suggesting a common Th2, allergy mediated pathway for this group of patients.
Resistant asthmatic
Another subgroup of patients that has been described is a group of asthmatics who clinically appear very resistant to treatment with steroids and often have irreversible airflow obstruction. These individuals, often have elements of both asthma and COPD. Additionally, it is thought that asthmatics resistant to steroids have more neutrophil-predominant airway inflammation, which, as noted above, is traditionally thought to be the predominant type of inflammation found in individuals with COPD.(7, 39) Periostin, a marker of severe steroid-resistant asthma and CD8 driven eosinophilic inflammation, has been associated with airway remodeling and increased decline in lung function in patients with asthma.(40)
Interestingly, there have been at least a few studies which have suggested that latent infection in asthmatics is associated with the phenotype of steroid resistance. Green et al used non-culture based techniques to detect colonization with Streptococcus, Moraxella, or Haemophilus species in the sputum of over 60% of resistant asthmatics in a small cohort. They went on to show that these individuals had more sputum neutrophils than those without colonization.(41) Others have demonstrated supportive findings using mouse models of allergic airways disease and Haemophilus infection.(42) It has also been suggested that latent adenoviral infection is associated with heightened cigarette smoke induced inflammation and a pattern of steroid resistance in asthma.(43) Additionally, some animal models of latent adenovirus infection have shown more rapid progression of emphysema in the setting of cigarette smoke exposure.(44) The mechanism for the link between indolent infection and steroid resistant asthma is the activation of the innate immune system through latent bacterial or viral infections, which lead to neutrophilic inflammation that is less responsive to steroid therapy.(43)
Elderly asthmatic with irreversible airflow obstruction
Older asthmatics, particularly those with long-standing asthma do demonstrate irreversible airflow obstruction and a shift to more neutrophilic inflammation.(45) One of the earliest documented references to this group of asthmatics with only partial reversibility of airflow obstruction is found in the work of Woolcock and Read from 1968. In this work, pressure-volume curves of the lungs were estimated during an acute exacerbation and then after resolution for 10 patients with asthma from differing age categories, the youngest being 9 years old and the oldest 52. The authors found that in half of the sample, the pressure volume curves did not normalize after exacerbations and were indicative of loss of elastic recoil and hyperinflation of the lungs consistent with possible emphysema.(46) McCarthy et al noted similarly when studying a cohort of 16 stable asthmatics that nearly half had findings indicative of loss of lung elastic recoil concerning for early emphysema.(47) Another study of 18 adults with chronic persistent asthma documented loss of lung elastic recoil in a majority of these participants, which seemed to be more pronounced in the older participants compared to younger.(48) In a separate study, this group went on to study adult asthmatics based upon severity of asthma and noted that loss of lung elastic recoil was present in all participants with moderate persistent and severe persistent asthma, but less prevalent in the group with mild persistent asthma, suggesting that not only is duration but also severity of illness is a factor in the long-term impacts on lung structure and function.(49) One large longitudinal study in Europe followed individuals aged 20–44 years for 9 years, and attempted to characterize a group of individuals with airflow obstruction as asthma, COPD or ACOS. Clinically, they noted a similar profile of symptoms and history of those with asthma and those with ACOS, having similar prevalence of allergic disease, eczema and airway hyperresponsiveness. They found that those individuals with ACOS had more decline in lung function over time than those with asthma alone but less than those with COPD alone. They went on to note that those with ACOS had earlier onset, longer duration of asthma with more exposure history to cigarettes, thus leading them to the conclusion that likely those individuals with ACOS may largely constitute a group with longstanding poorly controlled asthma that have “progressed to fixed airflow obstruction,”(12) and may constitute one of the subtypes of working definitions of ACOS.
Childhood asthmatic who smokes and develops COPD
The final proposed subtype includes individuals who have asthma from childhood or early adulthood, then later develop fixed airflow obstruction and COPD-type features due to long-term primary smoking exposure. In one study of a Finnish primary care cohort, among 190 current and former smokers with a history of asthma but without a previous diagnosis of COPD, 27.4% had fixed airflow obstruction, consistent with a possible ACOS picture. Age over 60 and higher burden of smoking history (over 20 pack-years) were associated with being part of the overlap group in this study.(16) A similar study of 256 Korean patients with asthma (defined by bronchodilator reversibility or positive methacholine challenge) found that 38% had incompletely reversible airflow obstruction on two separate occasions at least 3 months apart. This group was noted to be older, higher proportion male, and have more current and former smokers than the group with asthma alone. Interestingly, the overlap group in this study had lower peripheral eosinophil count but higher total IgE level.(18)
This subtype is not only described in the context of studies of adult asthmatics, but is also captured in studies of adults with a primary diagnosis of COPD who self-report a diagnosis of asthma from childhood or early adulthood. In the COPDGene study, Hardin et al defined ACOS as a report of doctor diagnosis of asthma before the age 40. Studies that describe ACOS in the framework of populations with asthma show the ACOS group to be older, higher proportion of males, with a higher burden of smoke exposure than the group with asthma alone. In contrast, ACOS patients derived from a group of COPD patients as described by Hardin tend to be younger, with a higher proportion of females, and a significantly lower burden of smoking history than those with COPD alone. The ACOS group was also noted to have less emphysema and significantly higher FEV1/FVC ratio than the group with COPD alone, despite having more respiratory symptoms and exacerbation events.(14) Taking this and other similar studies into account, it appears that the smoking asthmatic ACOS group is a hybrid of both asthma and COPD with clinical features and inflammatory pathways of both diseases. These patients appear to have a much higher burden of respiratory symptoms and healthcare use than the group with either disease alone.
Other considerations
Genetic overlap
Another topic that has been studied with regard to asthma and COPD overlap is understanding shared genetic loci of both diseases to help better elucidate the overlap phenotype. Hardin et al performed an adjusted genome-wide analysis comparing ACOS cases to COPD controls in Non-Hispanic Whites and African Americans.(14) They found that no SNPs reached the predetermined significance threshold but there were several loci that were close, including the CSMD1 gene on chromosome 8 and a variant on the SOX5 gene on chromosome 12, both among Non-Hispanic Whites. Though they tested several known asthma and COPD SNPs for associations with overlap syndrome, no SNPs that were significant in their associations after applying multiple testing thresholds. The CSMD1 gene was found to be associated with emphysema findings radiographically, while the SOX5 gene has thought to be involved in lung development. It was postulated from the associations with SOX5 in ACOS that participants with ACOS might have started with asthma and then developed fixed airways obstruction due to abnormalities in lung development.(14) Related to this, Christenson et al studied airway epithelial gene expression in asthma and COPD and found around 100 common genes that are upregulated in both asthma and COPD. They additionally noted a specific gene expression signature in a subgroup of individuals with COPD having eosinophilic inflammation, thought to have a clinical picture consistent with ACOS. These individuals interestingly had higher treatment response to ICS.(36) Smolonska and colleagues conducted a genome-wide association study for both asthma and COPD that suggested the common presence of two single nucleotide polymorphisms (SNPs) in genes DDX1, and COMMD10 that both participate in the NF-kappa-β inflammatory pathway, but the findings could not be replicated in other cohorts.(50)
Therapeutic options/targets
As noted above, there have been several studies which have attempted to understand whether the ACOS group is more responsive to corticosteroids, either inhaled or systemic. These studies have mostly targeted individuals with COPD having serum or sputum eosinophilia. (28, 33–35) For the most part, it has been strongly suggested that individuals with ACOS, particularly those in the category of COPD with eosinophilic inflammation, have an accentuated treatment response to ICS compared to COPD in general.(29, 35) For this reason the GINA/GOLD combined statement on ACOS has suggested considering ICS therapy early in the group with ACOS,(30) though stronger evidence based on randomized clinical trials is still needed.
Given the studies which have shown a higher degree of eosinophilia and allergic, Th2-type inflammation in individuals with ACOS compared to those with COPD alone,(31, 32) there has also been some interest in whether this subgroup of individuals would be responsive to monoclonal antibody therapy such as mepolizumab or benralizumab. Brightling et. al. recently reported the results of a Phase 2a trial of benralizumab versus placebo therapy in individuals with moderate to severe COPD with history of exacerbation having sputum eosinophil count of 3% or higher.(51) Benralizumab is an anti-IL5 receptor alpha antibody which has previously been shown to reduce sputum eosinophils in asthmatics.(52)The authors found that although overall exacerbation rate was not different between the placebo and treatment group, subgroup analyses among individuals with serum eosinophils higher than 200 or 300 cells per microliter showed a numerical but not statistically significant decrease in exacerbations, lung function (FEV1), and disease status in the treatment group compared to placebo.(51) Mepolizumab, an anti-IL5 monoclonal antibody, has been studied in severe eosinophilic asthma and found to reduce exacerbations and improve asthma control.(53) This drug has also been considered for the treatment of COPD and is currently under investigation in individuals with COPD having peripheral blood eosinophilia and eosinophilic bronchitis (NCT02105961, NCT01463644). Omalizumab, a monoclonal anti-IgE therapy shown to improve outcomes in severe allergic asthma,(54) has not yet been studied in individuals with COPD having elevated IgE and allergic features, however as more becomes known about ACOS and the phenotypes of individuals included in this group, it is possible this therapy could also be studied in this context.
Asthmatics who smoke, or who have a substantial smoking history, are typically excluded from studies of asthma. Thus, there is a paucity of data on this ACOS subgroup. However, one well-designed trial in 39 smoking asthmatics suggested that they had a blunted response to corticosteroids, but a better response to the leukotriene modifier montelukast than non-smoking asthmatics.(55) Thus, this ACOS subgroup may have a different therapeutic response than those with COPD and eosinophilia. It has been suggested that the steroid resistance in smokers with asthma and COPD is the consequence of impaired histone deacetylase activity that can be restored by low dose theophylline.(56) Accordingly, pending evidence from trials in this subgroup, theophylline may be a useful adjunct to inhaled corticosteroids.
Future Considerations/Summary
The concept of asthma and COPD belonging to a single continuum of airways disease is far from new, however the debate over this subject has evolved over the past decades. In the past several years there is an emerging base of literature which has strongly suggested that a group of individuals exists with a clinical syndrome intermediate between asthma and COPD, and that such individuals have features of both diseases. Importantly, this group appears in some studies to have worse symptom outcomes as well as higher risk for respiratory events and exacerbations. There have been attempts to better characterize this population with regards to biomarkers, genetic signature, and clinical as well as CT phenotypes with the ultimate goal of being able to better prescribe and tailor therapy to this group, however the evidence is still emerging in order to better reach these goals. Based on the available evidence, however, we do not think that ACOS can be defined as a single entity, but rather a cluster of different subtypes that likely have different mechanisms of disease and require somewhat different approaches to treatment. We outline above four proposed subgroups of patients who can be considered for inclusion in the asthma/COPD overlap syndrome. These subgroups appear to have distinct natural histories, clinical features, and inflammatory mechanisms. Additionally, we hope such a characterization will lend itself to a better understanding of possible treatments that can be rigorously tested as sub-populations of the asthma/COPD overlap syndrome.
Key points.
It is increasingly recognized that there is a group of individuals having characteristics of both chronic obstructive pulmonary disease and asthma.
This group is thought to have characteristics of both diseases and appears to have a different clinical phenotype and might be at higher risk for respiratory events and exacerbations as well as have heightened symptoms.
Understanding this subgroup of individuals is important in understanding the mechanisms for adverse outcomes and determining if specialized treatments have utility.
Patients with Asthma-COPD overlap syndrome are a heterogeneous group including patients with COPD and eosinophilia, smoking asthmatics, long-standing asthmatics with airway remodeling, and steroid resistant asthmatics with neutrophilic inflammation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nirupama Putcha, Email: Nputcha1@jhmi.edu, Johns Hopkins University School of Medicine, Division of Pulmonary and Critical Care Medicine, 5501 Hopkins Bayview Circle, JHAAC 3B.22, Baltimore, MD 21224, 410-550-9932.
Robert A. Wise, Email: rwise@jhmi.edu, Johns Hopkins University School of Medicine, Division of Pulmonary and Critical Care Medicine, 5501 Hopkins Bayview Circle, JHAAC 4B.74, Baltimore, MD 21224, 410-550-0545.
References
- 1.Orie NG, Sluiter HJ, De Vries K, Tammeling GJ, Witkop J. The host factor in bronchitis. Bronchitis, Royal Vangorcum. 1961:43–59. [Google Scholar]
- 2.Sluiter HJ, Koeter GH, de Monchy JG, Postma DS, de Vries K, Orie NG. The Dutch hypothesis (chronic non-specific lung disease) revisited. The European respiratory journal. 1991;4:479–489. [PubMed] [Google Scholar]
- 3.Fletcher C, Peto R. The natural history of chronic airflow obstruction. British medical journal. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burrows B, Bloom JW, Traver GA, Cline MG. The course and prognosis of different forms of chronic airways obstruction in a sample from the general population. The New England journal of medicine. 1987;317:1309–1314. doi: 10.1056/NEJM198711193172103. [DOI] [PubMed] [Google Scholar]
- 5.Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, Ligabue G, Ciaccia A, Saetta M, Papi A. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2003;167:418–424. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- 6.Vermeire PA, Pride NB. A “splitting” look at chronic nonspecific lung disease (CNSLD): common features but diverse pathogenesis. The European respiratory journal. 1991;4:490–496. [PubMed] [Google Scholar]
- 7.Keatings VM, Barnes PJ. Granulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjects. American journal of respiratory and critical care medicine. 1997;155:449–453. doi: 10.1164/ajrccm.155.2.9032177. [DOI] [PubMed] [Google Scholar]
- 8.Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald JM. Asthma and COPD Overlap Syndrome (ACOS): A Systematic Review and Meta Analysis. PloS one. 2015;10:e0136065. doi: 10.1371/journal.pone.0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrecheguren M, Roman-Rodriguez M, Miravitlles M. Is a previous diagnosis of asthma a reliable criterion for asthma-COPD overlap syndrome in a patient with COPD? International journal of chronic obstructive pulmonary disease. 2015;10:1745–1752. doi: 10.2147/COPD.S87025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung WS, Lin CL, Kao CH. Comparison of acute respiratory events between asthma-COPD overlap syndrome and COPD patients: a population-based cohort study. Medicine. 2015;94:e755. doi: 10.1097/MD.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosio BG, Soriano JB, Lopez-Campos JL, Calle-Rubio M, Soler-Cataluna JJ, de-Torres JP, Marin JM, Martinez-Gonzalez C, de Lucas P, Mir I, Peces-Barba G, Feu-Collado N, Solanes I, Alfageme I, Casanova C. Defining the Asthma-COPD overlap syndrome in a COPD cohort. Chest. 2015 doi: 10.1378/chest.15-1055. [DOI] [PubMed] [Google Scholar]
- 12.de Marco R, Marcon A, Rossi A, Anto JM, Cerveri I, Gislason T, Heinrich J, Janson C, Jarvis D, Kuenzli N, Leynaert B, Probst-Hensch N, Svanes C, Wjst M, Burney P. Asthma, COPD and overlap syndrome: a longitudinal study in young European adults. The European respiratory journal. 2015 doi: 10.1183/09031936.00008615. [DOI] [PubMed] [Google Scholar]
- 13.Harada T, Yamasaki A, Fukushima T, Hashimoto K, Takata M, Kodani M, Okazaki R, Takeda K, Watanabe M, Kurai J, Shimizu E. Causes of death in patients with asthma and asthma-chronic obstructive pulmonary disease overlap syndrome. International journal of chronic obstructive pulmonary disease. 2015;10:595–602. doi: 10.2147/COPD.S77491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardin M, Cho M, McDonald ML, Beaty T, Ramsdell J, Bhatt S, van Beek EJ, Make BJ, Crapo JD, Silverman EK, Hersh CP. The clinical and genetic features of COPD-asthma overlap syndrome. The European respiratory journal. 2014;44:341–350. doi: 10.1183/09031936.00216013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauppi P, Kupiainen H, Lindqvist A, Tammilehto L, Kilpelainen M, Kinnula VL, Haahtela T, Laitinen T. Overlap syndrome of asthma and COPD predicts low quality of life. The Journal of asthma : official journal of the Association for the Care of Asthma. 2011;48:279–285. doi: 10.3109/02770903.2011.555576. [DOI] [PubMed] [Google Scholar]
- 16.Kiljander T, Helin T, Venho K, Jaakkola A, Lehtimaki L. Prevalence of asthma-COPD overlap syndrome among primary care asthmatics with a smoking history: a cross-sectional study. NPJ primary care respiratory medicine. 2015;25:15047. doi: 10.1038/npjpcrm.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MA, Noh CS, Chang YJ, Hong YK, Lee JS, Lee SW, Lee SD, Oh YM. Asthma and COPD overlap syndrome is associated with increased risk of hospitalisation. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19:864–869. doi: 10.5588/ijtld.14.0327. [DOI] [PubMed] [Google Scholar]
- 18.Lee HY, Kang JY, Yoon HK, Lee SY, Kwon SS, Kim YK, Rhee CK. Clinical characteristics of asthma combined with COPD feature. Yonsei medical journal. 2014;55:980–986. doi: 10.3349/ymj.2014.55.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menezes AM, Montes de Oca M, Perez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, Muino A, Jardim JR, Valdivia G, Talamo C. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145:297–304. doi: 10.1378/chest.13-0622. [DOI] [PubMed] [Google Scholar]
- 20.Miravitlles M, Soriano JB, Ancochea J, Munoz L, Duran-Tauleria E, Sanchez G, Sobradillo V, Garcia-Rio F. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status Respiratory medicine. 2013;107:1053–1060. doi: 10.1016/j.rmed.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Tada Y, Kawata N, Matsuura Y, Ikari J, Kasahara Y, Tatsumi K. Clinical, physiological, and radiological features of asthma-chronic obstructive pulmonary disease overlap syndrome. International journal of chronic obstructive pulmonary disease. 2015;10:947–954. doi: 10.2147/COPD.S80022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(GOLD) GIfCOLd. Global Strategy for Diagnosis, Management, and Prevention of COPD. 2014 http://www.goldcopd.org/
- 23.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schunemann H, Wedzicha W, MacDonald R, Shekelle P. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of internal medicine. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 24.Fu JJ, Gibson PG, Simpson JL, McDonald VM. Longitudinal changes in clinical outcomes in older patients with asthma, COPD and asthma-COPD overlap syndrome. Respiration; international review of thoracic diseases. 2014;87:63–74. doi: 10.1159/000352053. [DOI] [PubMed] [Google Scholar]
- 25.From the Global Strategy for Asthma Management and Prevention. 2015 [Google Scholar]
- 26.de Marco R, Pesce G, Marcon A, Accordini S, Antonicelli L, Bugiani M, Casali L, Ferrari M, Nicolini G, Panico MG, Pirina P, Zanolin ME, Cerveri I, Verlato G. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PloS one. 2013;8:e62985. doi: 10.1371/journal.pone.0062985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soler-Cataluna JJ, Cosio B, Izquierdo JL, Lopez-Campos JL, Marin JM, Aguero R, Baloira A, Carrizo S, Esteban C, Galdiz JB, Gonzalez MC, Miravitlles M, Monso E, Montemayor T, Morera J, Ortega F, Peces-Barba G, Puente L, Rodriguez JM, Sala E, Sauleda J, Soriano JB, Viejo JL. Consensus document on the overlap phenotype COPD-asthma in COPD. Archivos de bronconeumologia. 2012;48:331–337. doi: 10.1016/j.arbres.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, Monteiro W, Berry M, Parker D, Wardlaw AJ, Pavord ID. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. The European respiratory journal. 2007;29:906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 29.Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. The European respiratory journal. 2006;27:964–971. doi: 10.1183/09031936.06.00072105. [DOI] [PubMed] [Google Scholar]
- 30.GOLD Ga. Diagnosis of Diseases of Chronic Airflow Limitation: Asthma, COPD, and Asthma-COPD Overlap Syndrome (ACOS) 2015. [Google Scholar]
- 31.Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, Lindblad K, Patel H, Rugman P, Dodson P, Jenkins M, Saunders M, Newbold P, Green RH, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. American journal of respiratory and critical care medicine. 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 32.Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. The European respiratory journal. 2014;44:1697–1700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 33.Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, Pavord ID. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet (London, England) 2000;356:1480–1485. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 34.Pizzichini E, Pizzichini MM, Gibson P, Parameswaran K, Gleich GJ, Berman L, Dolovich J, Hargreave FE. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. American journal of respiratory and critical care medicine. 1998;158:1511–1517. doi: 10.1164/ajrccm.158.5.9804028. [DOI] [PubMed] [Google Scholar]
- 35.Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, Berry M, Parker D, Monteiro W, Pavord ID, Bradding P. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60:193–198. doi: 10.1136/thx.2004.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, Lenburg ME, Spira A, Woodruff PG. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease American journal of respiratory and critical care medicine. 2015;191:758–766. doi: 10.1164/rccm.201408-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamieson DB, Matsui EC, Belli A, McCormack MC, Peng E, Pierre-Louis S, Curtin-Brosnan J, Breysse PN, Diette GB, Hansel NN. Effects of allergic phenotype on respiratory symptoms and exacerbations in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2013;188:187–192. doi: 10.1164/rccm.201211-2103OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fattahi F, ten Hacken NH, Lofdahl CG, Hylkema MN, Timens W, Postma DS, Vonk JM. Atopy is a risk factor for respiratory symptoms in COPD patients: results from the EUROSCOP study. Respiratory research. 2013;14:10. doi: 10.1186/1465-9921-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsoumakidou M, Tzanakis N, Kyriakou D, Chrysofakis G, Siafakas NM. Inflammatory cell profiles and T-lymphocyte subsets in chronic obstructive pulmonary disease and severe persistent asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2004;34:234–240. doi: 10.1111/j.1365-2222.2004.01858.x. [DOI] [PubMed] [Google Scholar]
- 40.Kanemitsu Y, Ito I, Niimi A, Izuhara K, Ohta S, Ono J, Iwata T, Matsumoto H, Mishima M. Osteopontin and periostin are associated with a 20-year decline of pulmonary function in patients with asthma. American journal of respiratory and critical care medicine. 2014;190:472–474. doi: 10.1164/rccm.201403-0562LE. [DOI] [PubMed] [Google Scholar]
- 41.Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, Carroll MP, Bruce KD, Howarth PH. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PloS one. 2014;9:e100645. doi: 10.1371/journal.pone.0100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Essilfie AT, Simpson JL, Dunkley ML, Morgan LC, Oliver BG, Gibson PG, Foster PS, Hansbro PM. Combined Haemophilus influenzae respiratory infection and allergic airways disease drives chronic infection and features of neutrophilic asthma. Thorax. 2012;67:588–599. doi: 10.1136/thoraxjnl-2011-200160. [DOI] [PubMed] [Google Scholar]
- 43.Hogg JC. Role of latent viral infections in chronic obstructive pulmonary disease and asthma. American journal of respiratory and critical care medicine. 2001;164:S71–75. doi: 10.1164/ajrccm.164.supplement_2.2106063. [DOI] [PubMed] [Google Scholar]
- 44.Vitalis TZ, Kern I, Croome A, Behzad H, Hayashi S, Hogg JC. The effect of latent adenovirus 5 infection on cigarette smoke-induced lung inflammation. The European respiratory journal. 1998;11:664–669. [PubMed] [Google Scholar]
- 45.Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, Falsey AR, Mathur SK, Ramsdell JW, Rogers L, Stempel DA, Lima JJ, Fish JE, Wilson SR, Boyd C, Patel KV, Irvin CG, Yawn BP, Halm EA, Wasserman SI, Sands MF, Ershler WB, Ledford DK. Asthma in the elderly: Current understanding and future research needs--a report of a National Institute on Aging (NIA) workshop. The Journal of allergy and clinical immunology. 2011;128:S4–24. doi: 10.1016/j.jaci.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woolcock AJ, Read J. The static elastic properties of the lungs in asthma. The American review of respiratory disease. 1968;98:788–794. doi: 10.1164/arrd.1968.98.5.788. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy DS, Sigurdson M. Lung elastic recoil and reduced airflow in clinically stable asthma. Thorax. 1980;35:298–302. doi: 10.1136/thx.35.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gelb AF, Zamel N. Unsuspected pseudophysiologic emphysema in chronic persistent asthma. American journal of respiratory and critical care medicine. 2000;162:1778–1782. doi: 10.1164/ajrccm.162.5.2001037. [DOI] [PubMed] [Google Scholar]
- 49.Gelb AF, Licuanan J, Shinar CM, Zamel N. Unsuspected loss of lung elastic recoil in chronic persistent asthma. Chest. 2002;121:715–721. doi: 10.1378/chest.121.3.715. [DOI] [PubMed] [Google Scholar]
- 50.Smolonska J, Koppelman GH, Wijmenga C, Vonk JM, Zanen P, Bruinenberg M, Curjuric I, Imboden M, Thun GA, Franke L, Probst-Hensch NM, Nurnberg P, Riemersma RA, van Schayck CP, Loth DW, Brusselle GG, Stricker BH, Hofman A, Uitterlinden AG, Lahousse L, London SJ, Loehr LR, Manichaikul A, Barr RG, Donohue KM, Rich SS, Pare P, Bosse Y, Hao K, van den Berge M, Groen HJ, Lammers JW, Mali W, Boezen HM, Postma DS. Common genes underlying asthma and COPD? Genome-wide analysis on the Dutch hypothesis. The European respiratory journal. 2014;44:860–872. doi: 10.1183/09031936.00001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brightling CE, Bleecker ER, Panettieri RA, Jr, Bafadhel M, She D, Ward CK, Xu X, Birrell C, van der Merwe R. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. The Lancet Respiratory medicine. 2014;2:891–901. doi: 10.1016/S2213-2600(14)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, Busse WW, Wenzel S, Wu Y, Datta V, Kolbeck R, Molfino NA. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. The Journal of allergy and clinical immunology. 2013;132:1086–1096. e1085. doi: 10.1016/j.jaci.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, Chanez P. Mepolizumab treatment in patients with severe eosinophilic asthma. The New England journal of medicine. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 54.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, van As A, Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. The Journal of allergy and clinical immunology. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 55.Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, Deykin A, DiMango E, Fish JE, Ford JG, Israel E, Kiley J, Kraft M, Lemanske RF, Jr, Leone FT, Martin RJ, Pesola GR, Peters SP, Sorkness CA, Szefler SJ, Wechsler ME, Fahy JV. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. American journal of respiratory and critical care medicine. 2007;175:783–790. doi: 10.1164/rccm.200511-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. The Journal of allergy and clinical immunology. 2013;131:636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]