Abstract
Background
Time course and predictors of myocardial recovery on contemporary LVAD support is poorly defined due to limited number of recovery patients at any implanting center. This study sought to investigate myocardial recovery using multicenter data from INTERMACS.
Methods and Results
13,454 adult patients were studied. Device explant rates for myocardial recovery were 0.9% at 1-year, 1.9% at 2-year, and 3.1% at 3-year follow-up. Independent predictors of device explantation for recovery were age < 50 years (OR 2.5), non-ischemic etiology (OR 5.4), time since initial diagnosis < 2 years (OR 3.4), suboptimal HF therapy prior to implant (OR 2.2), LVEDD < 6.5 cm (OR 1.7), pulmonary systolic artery pressure < 50 mmHg (OR 2.0), BUN <30 mg/dL (OR 3.3), and axial-flow device (OR 7.6). Patients with myocarditis (7.7%), postpartum (4.4%) and adriamycin-induced cardiomyopathy (4.1%) had highest rates of device explantation for recovery. Use of neurohormonal blockers on LVAD support was significantly higher in patients who were explanted for recovery. Importantly, 9% of all LVAD patients who were not explanted for recovery have demonstrated substantial improvement in LVEF (partial recovery), and had remarkable overlap in clinical characteristic profile compared to patients who were explanted for recovery (complete recovery). Complete and partial recovery rates have declined in parallel with recent changes observed in device indications and technology.
Conclusions
Myocardial recovery is a spectrum of improvement rather than a binary clinical end-point. One in every ten LVAD patients demonstrates partial or complete myocardial recovery and should be targeted for functional assessment and optimization.
Keywords: left ventricular assist device, reverse remodeling
Left ventricular assist device (LVAD) therapy has become standard of care in patients with end-stage heart failure (HF) and increasingly being used worldwide with excellent long-term outcomes 1, 2. Although originally intended as a bridge-to-transplant device, it became evident early in the 1990s that mechanical unloading with LVAD may facilitate recovery of the failing ventricle allowing for device explant in select patients, also termed as “bridge-to-recovery” 3, 4. These clinical observations were supported by molecular studies of human myocardial samples obtained before and after LVAD support, which showed reversal and/or normalization of several components of the LV remodeling phenotype including cardiomyocyte hypertrophy, beta-receptor desensitization, cytokine activation, cytoskeletal protein disarray and deranged collagen turnover 5–9. Despite favorable changes observed in the myocardial structure and function with mechanical unloading, sustained recovery leading to device explantation occurs rare clinically and reported in less than 5% of LVAD supported patients in the current era of mechanical circulatory support therapy 10.
Although the precise mechanisms for the disconnect between molecular and structural recovery are largely unknown, growing lines of evidence suggest that a systematic, program-based approach incorporating use of guideline-directed pharmacologic therapy, serial assessment of native cardiac function by turndown echocardiograms, and individualized LVAD weaning strategies may promote myocardial recovery and lead to higher rates of clinically successful device explants 11–15. Since recovery optimization requires an active effort and resource utilization by LVAD programs , improved understanding of time-course and clinical predictors of myocardial recovery is critically important to develop appropriate patient selection and management strategies. Several studies have suggested young age, non-ischemic etiology, and short duration of heart failure as potential predictors of myocardial recovery on LVAD support 16–18. However, majority of the available data is derived from single-center studies and patients supported with pulsatile-flow LVADs, which are no longer in clinical use. Moreover, previous studies have consistently used device explantation end-point as a binary definition for recovery. This approach fails to consider the possibility that myocardial recovery may rather represent a spectrum of structural and functional improvement.
Given the limited number of recovery patients at any implanting center, significant gap in knowledge, we sought to investigate time-course and predictors of myocardial recovery on continuous-flow LVAD support using the multicenter Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS).
Methods
Data Source and Study Population
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) is a prospective registry of durable mechanical circulatory support devices implanted in the United States. In-depth description of the registry has been published and is available at http://www.INTERMACS.org. The INTERMACS protocol was approved by the National Institutes of Health, the Institutional Review Board at the Data Coordinating Center at the University of Alabama at Birmingham, and at the institutional review board of each participating hospital. All prospective implants between June 2006 and June 2015 were included in this study (Supplementary Figure 1). 14,746 adult LVAD patients (age ≥ 19 years at implant) were identified. Patients who underwent total artificial heart placement (n=325), pulsatile-flow LVAD placement (n=962), or those with prior history of heart transplantation (n=16) were excluded from the analysis. The remaining 13,454 CF-LVAD patients were included in the study. Patients who received a left ventricular assist device and a right ventricular assist device in the same operating room visit were included (n=427). Comprehensive clinical data were collected at the time of device implantation and at serial follow-up time points following CF-LVAD implantation, as previously defined.
Myocardial recovery on LVAD support was defined using two separate but interrelated criteria: (1) Complete myocardial recovery: defined as device explantation for myocardial recovery, similar to what has been traditionally used in previous studies of myocardial recovery on CF-LVAD support. Patients who had their devices removed or turned off for other reasons such as infection, device malfunction or thrombosis were excluded from this category. This definition is based on ultimate clinical outcome of the patient irrespective of the number of device implants required (per patient analysis). (2) Partial myocardial recovery: defined as demonstration of substantial improvement of the left ventricular function on CF-LVAD support (LVEF > 40% at any follow-up), yet not achieving the device explantation clinical end-point. Of 13,291 patients not reaching the device explantation end-point, 9,238 (69.5%) had reported LVEF data before and after CF-LVAD implantation. Patients with LVEF greater than 30% prior to CF-LVAD implantation (n=433) were excluded from this analysis. Remaining patients (n=8,805) were categorized into partial myocardial recovery vs. no myocardial recovery cohorts based on their highest LVEF achieved on CF-LVAD support during follow-up.
Statistical Analysis
Continuous variables were defined as mean and standard deviations and categorical variables were summarized as percentages. Baseline characteristics were compared using independent t-test for continuous variables and chi-square test for categorical variables. Cumulative incidence of complete myocardial recovery was calculated and plotted using Kaplan-Meier estimates. Patients who were transplanted or died on device support were censored from the analysis at the time of these events. Non-parametric estimate of the hazard function for complete myocardial recovery was performed using a Kernel-based approach 19, 20.
INTERMACS registry provided echocardiographic data, laboratory results, and medications used at pre-defined time points on pump support. Serial changes in these parameters were plotted for myocardial recovery cohorts. Groups were then compared cross-sectionally using ANOVA with Tukey’s post-hoc test for continuous variables and chi-square test for categorical variables. To account for potential within subject correlations, groups were also compared using mixed models for continuous variables and generalized linear models for categorical variables by adjusting time variable into ordinal. A negative binomial regression method was used to model complete and partial recovery rates over time by utilizing calendar year as a predictor of the number of recovery events. To account for biases due to varying lengths of observation time, the logarithm of events per person year was used as an offset in the negative binomial model.
Univariable predictors of complete myocardial recovery on LVAD support were identified using logistic regression. Clinical factors with a p value less than 0.10 were entered into a multivariable logistic regression model (exit criteria p> 0.05) to determine independent predictors of device explant for recovery following LVAD implantation. Correlated variables were not entered simultaneously into the multivariable model to avoid overfitting. Continuous variables were dichotomized at optimal cut-off points based on sensitivity and specificity values. A two-tailed p value ≤ 0.05 were considered statistically significant for all comparisons. Data were analyzed with the use of IBM SPSS Statistics software, version 22.0 (Armonk, NY:IBM Corp.), and R software, version 3.1.2.
Results
Incidence of the Complete and Partial Myocardial Recovery on CF-LVAD Support
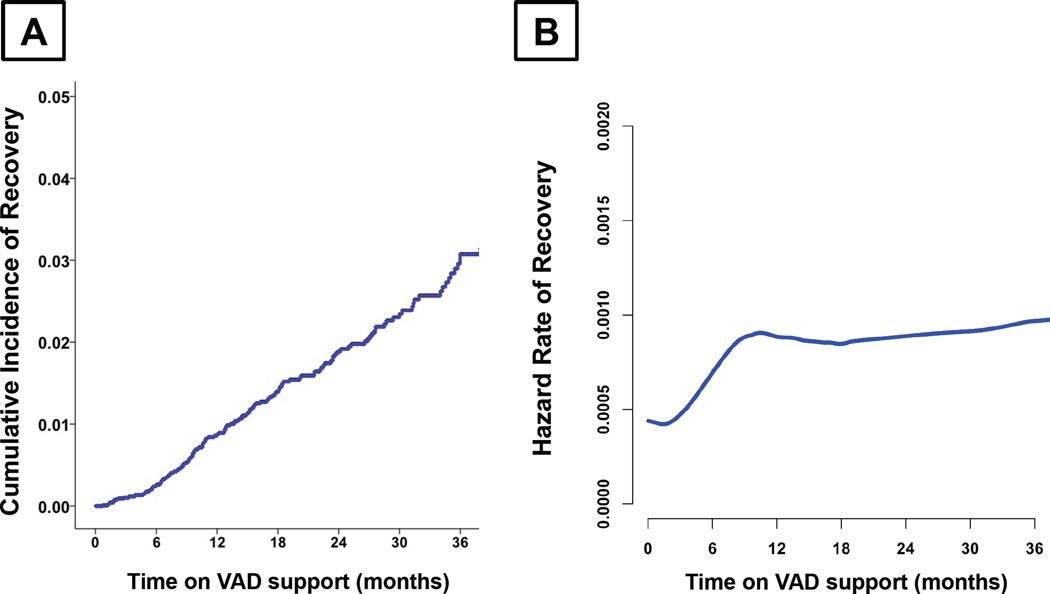
Of the 13,454 LVAD patients studied, 163 (1.2%) underwent device explantation due to ultimate recovery of the LV function. Of those, 150 patients (92.0%) recovered after the initial LVAD implantation, 12 patients (7.4%) recovered after 2nd LVAD implantation, and 1 patient (0.6%) recovered after 3rd LVAD implantation. Median support time to complete myocardial recovery from initial device implantation was 11.4 months (IQR: 19.4 months). Cumulative incidence of complete myocardial recovery after initial LVAD implantation was 0.9% at 1-year, 1.9% at 2-year, and 3.1% at 3-year follow-up (Figure 1A). Hazard rate of recovery had a linear increment within the first 6 months of device support and remained relatively constant until 36 months of device support (Figure 1B). Of 8,805 patients with LVEF less than 30% at the time of device implantation, 761 (8.6%) achieved partial myocardial recovery outcome defined as LVEF > 40% on LVAD support during any follow-up time-point. 355 patients (4.0%) had LVEF improvement to greater than 50%, and 406 patients (4.6%) had LVEF improvement to 40 – 50 %. Of note, complete and partial recovery rates observed in patients supported by pulsatile-flow LVADs in the INTERMACS registry (excluded from this contemporary analysis) were 3.1% and 17.8%, respectively, which were significantly higher than patients supported by CF-LVADs (p<0.001).
Figure 1. Complete Myocardial Recovery Leading to Continuous-Flow Left Ventricular Assist Device Explantation in the INTERMACS Registry.
A) Cumulative Incidence of Complete Myocardial Recovery B) Hazard Risk of Complete Myocardial Recovery
Clinical Characteristics of the Complete and Partial Myocardial Recovery Cohorts
Baseline characteristics of patients achieving complete and partial myocardial recovery were represented in Tables 1–4. Female gender, low body mass index, low body surface area, non-ischemic etiology of HF, shorter duration of disease, CF-LVAD type (axial vs. centrifugal-flow), implant strategy, INTERMACS profile, pre-implant ECMO use, and pre-implant mechanical ventilation were significantly associated with both complete and partial recovery (Table 1). Of note, complete myocardial recovery cohort patients were more likely to be young, use tobacco on presentation, work for income, and have a lower incidence of previous cardiac surgery, peripheral vascular disease, and chronic kidney disease.
Table 1.
Clinical Characteristics of Complete and Partial Myocardial Recovery Cohorts
| COMPLETE RECOVERY | PARTIAL RECOVERY | |||||
|---|---|---|---|---|---|---|
| Variable | Explant (n= 163, 1.2%) |
No Explant (n= 13,291, 98.8%) |
p-value | LVEF (≥40%) (n= 761, 8.6%) |
LVEF (<40%) (n= 8,044, 91.4%) |
p-value |
| Age (years) | 45.9 ± 13.8 | 57.0 ± 13.0 | <0.001 | 57.8 ± 14.1 | 56.5 ± 13.1 | 0.019 |
| Gender (Female) | 63 (38.7%) | 2824 (21.3%) | <0.001 | 296 (38.9%) | 1591 (19.8%) | <0.001 |
| Ethnicity (Hispanic) | 8 (4.9%) | 819 (6.2%) | 0.498 | 57 (7.5%) | 505 (6.3%) | 0.197 |
| BMI (kg/m2) | 27.4 ± 6.9 | 28.7 ± 6.9 | 0.017 | 27.2 ± 6.2 | 28.7 ± 6.9 | <0.001 |
| BSA (m2) | 1.97 ± 0.28 | 2.06 ± 0.29 | <0.001 | 1.95 ± 0.28 | 2.06 ± 0.29 | <0.001 |
| Heart Failure Etiology | <0.001 | <0.001 | ||||
| Ischemic | 23 (14.1%) | 6218 (46.8%) | 302 (39.7%) | 3640 (45.2%) | ||
| Non-Ischemic | 140 (85.9%) | 7073 (53.2%) | 459 (60.3%) | 4404 (54.8%) | ||
| Time since first cardiac diagnosis |
<0.001 | <0.001 | ||||
| < 1 month | 44 (28.8%) | 644 (5.0%) | 74 (10.2%) | 331 (4.3%) | ||
| 1 month – 1 year | 38 (24.8%) | 1249 (9.8%) | 106 (14.6%) | 740 (9.5%) | ||
| 1 – 2 years | 16 (10.5%) | 820 (6.4%) | 58 (8.0%) | 485 (6.2%) | ||
| > 2 years | 55 (35.9%) | 10070 (78.8%) | 487 (67.2%) | 6209 (80.0%) | ||
| CF-LVAD Type | 0.001 | <0.001 | ||||
| Axial-flow | 156 (95.7%) | 11516 (86.6%) | 721 (94.7%) | 6864 (85.3%) | ||
| Centrifugal-flow | 7 (4.3%) | 1775 (13.4%) | 40 (5.3%) | 1180 (14.7%) | ||
| Pre-implant Strategy | <0.001 | <0.001 | ||||
| Bridge to recovery | 5 (3.1%) | 50 (0.4%) | 8 (1.1%) | 27 (0.3%) | ||
| Bridge to transplant | 32 (19.6%) | 3682 (27.7%) | 158 (20.8%) | 2254 (28.0%) | ||
| Bridge to candidacy | 79 (48.5%) | 4294 (32.3%) | 241 (31.7%) | 2487 (30.9%) | ||
| Destination therapy | 46 (28.2%) | 5211 (39.2%) | 351 (46.1%) | 3246 (40.4%) | ||
| Other / Rescue | 1 (0.6%) | 54 (0.4%) | 3 (0.4%) | 30 (0.3%) | ||
| INTERMACS Profile | <0.001 | 0.005 | ||||
| Intermacs 1 | 38 (23.5%) | 1968 (14.9%) | 136 (17.9%) | 1116 (13.9%) | ||
| Intermacs 2 | 48 (29.6%) | 4922 (37.2%) | 259 (34.1%) | 3037 (37.9%) | ||
| Intermacs 3 | 56 (34.6%) | 3949 (29.8%) | 223 (29.4%) | 2520 (31.5%) | ||
| Intermacs 4–7 | 20 (12.3%) | 2391 (18.1%) | 141 (18.6%) | 1330 (16.6%) | ||
| Severity of Disease | ||||||
| ECMO | 9 (5.5%) | 316 (2.4%) | 0.009 | 28 (3.7%) | 179 (2.2%) | 0.011 |
| IABP | 20 (12.3%) | 2225 (16.7%) | 0.128 | 115 (15.1%) | 1436 (17.9%) | 0.058 |
| Mechanical ventilation |
13 (8.0%) | 504 (3.8%) | 0.006 | 41 (5.4%) | 302 (3.8%) | 0.026 |
| Dialysis | 7 (4.3%) | 331 (2.5%) | 0.144 | 23 (3.0%) | 152 (1.9%) | 0.032 |
| Comorbid Conditions | ||||||
| Previous cardiac operation |
27 (16.6%) | 4558 (34.3%) | <0.001 | 246 (32.3%) | 2609 (32.4%) | 0.951 |
| Tobacco use, history | 24 (33.3%) | 2343 (30.1%) | 0.552 | 118 (28.4%) | 1541 (30.1%) | 0.467 |
| Tobacco use, current | 9 (12.5%) | 337 (4.3%) | 0.001 | 16 (3.8%) | 221 (4.3%) | 0.652 |
| Severe diabetes | 6 (8.3%) | 771 (9.9%) | 0.656 | 46 (11.1%) | 486 (9.5%) | 0.294 |
| Peripheral vascular disease |
0 (0.0%) | 380 (4.9%) | 0.050 | 16 (3.8%) | 242 (4.7%) | 0.415 |
| Chronic Kidney Disease |
4 (5.6%) | 1727 (22.2%) | <0.001 | 87 (20.9%) | 1114 (21.7%) | 0.697 |
| Major stroke | 0 (0.0%) | 297 (3.8%) | 0.116 | 16 (3.8%) | 188 (3.7%) | 0.852 |
| Pulmonary Disease | 7 (9.7%) | 734 (9.4%) | 0.933 | 38 (9.1%) | 481 (9.4%) | 0.867 |
| Social risk factors | ||||||
| Education Level | 0.372 | 0.683 | ||||
| None | 0 (0.0%) | 21 (0.2%) | 2 (0.4%) | 8 (0.1%) | ||
| Grade School | 1 (0.9%) | 336 (3.4%) | 22 (3.9%) | 203 (3.4%) | ||
| High School | 55 (48.7%) | 4451 (45.0%) | 261 (46.0%) | 2719 (45.0%) | ||
| College & Tech School |
36 (31.9%) | 2690 (27.2%) | 153 (26.9%) | 1615 (26.7%) | ||
| Associate / Bachelor | 13 (11.5%) | 1645 (16.6%) | 85 (15.0%) | 1007 (16.7%) | ||
| Post-grad degree | 8 (7.1%) | 752 (7.6%) | 45 (7.9%) | 492 (8.1%) | ||
| Working for income | 51 (34.2%) | 2058 (16.9%) | <0.001 | 130 (18.1%) | 1262 (17.1%) | 0.475 |
| Center Volume | 0.196 | 0.158 | ||||
| 1 – 10 implants | 11 (6.7%) | 1210 (9.2%) | 73 (9.7%) | 710 (8.9%) | ||
| 11– 20 implants | 34 (20.9%) | 1996 (15.2%) | 120 (16.9%) | 1200 (15.0%) | ||
| 21– 30 implants | 26 (16.0%) | 2659 (20.2%) | 167 (22.1%) | 1538 (19.3%) | ||
| 31– 50 implants | 51 (31.3%) | 3917 (29.8%) | 226 (30.0%) | 2526 (31.7%) | ||
| > 50 implants | 41 (25.2%) | 3350 (25.5%) | 168 (22.3%) | 2006 (25.1%) | ||
| Concomitant Surgery | 96 (58.9%) | 7927 (59.7%) | 0.845 | 413 (54.3%) | 4813 (59.8%) | 0.003 |
| Duration on device support (months) |
16.6 ± 12.2 | 16.5 ± 15.5 | 0.892 | 24.1 ± 17.5 | 17.3 ± 15.1 | <0.001 |
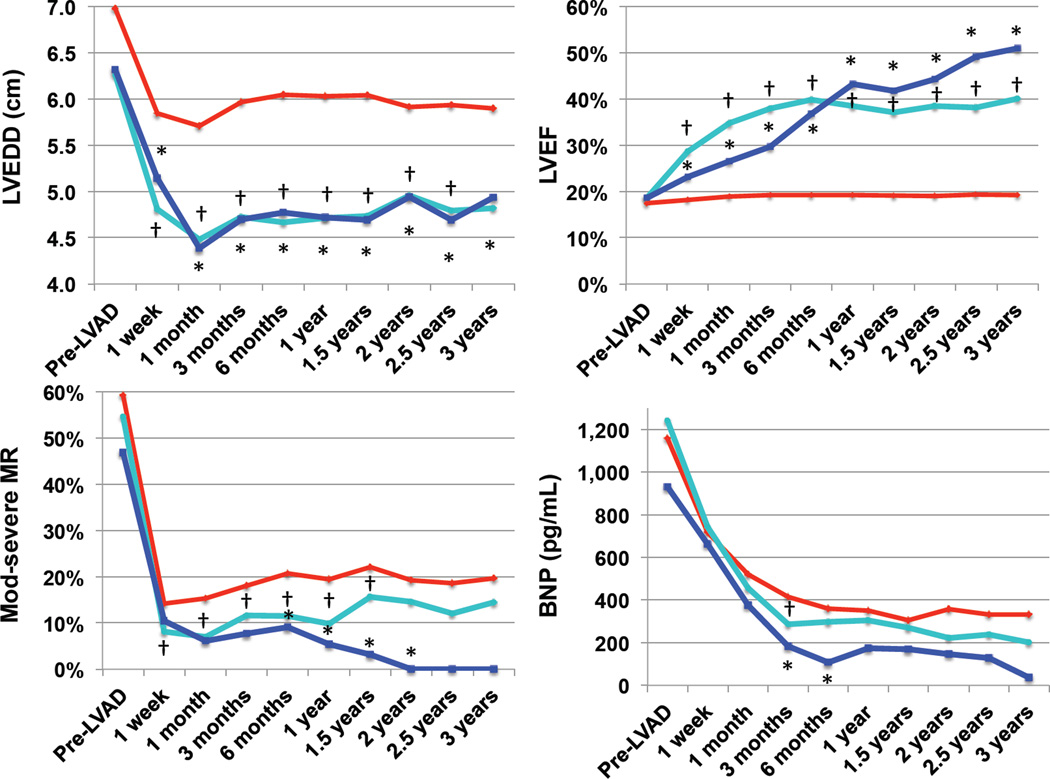
When hemodynamic parameters were analyzed, complete and partial recovery cohort patients had significantly lower pulmonary artery systolic, diastolic, and capillary wedge pressures prior to device implantation compared to non-recovery cohorts (Table 2). Pre-implant left ventricular end-diastolic diameter (LVEDD) was significantly lower in both complete and partial myocardial recovery cohorts. Partial recovery cohort patients had a significantly higher baseline LVEF and lower incidence of severe mitral insufficiency, however these factors were not significant for the complete myocardial recovery cohort patients.
Table 2.
Hemodynamic and Echocardiographic Parameters at the time of LVAD Implantation
| COMPLETE RECOVERY | PARTIAL RECOVERY | |||||
|---|---|---|---|---|---|---|
| Variable | Explant (n= 163, 1.2%) |
No Explant (n= 13,291, 98.8%) |
p-value | LVEF (≥40%) (n= 761, 8.6%) |
LVEF (<40%) (n= 8,044, 91.4%) |
p-value |
| Hemodynamics | ||||||
| Heart Rate (per min) | 96.4 ± 19.0 | 88.3 ± 17.6 | <0.001 | 89.9± 17.2 | 88.8 ± 17.5 | 0.084 |
| Systolic BP (mmHg) | 101.9 ± 15.5 | 104.4 ± 16.1 | 0.050 | 105.8 ± 16.4 | 104.2 ± 15.8 | 0.006 |
| Diastolic BP (mmHg) | 65.9 ± 12.4 | 64.2 ± 11.5 | 0.061 | 64.3 ± 11.9 | 64.3 ± 11.3 | 0.981 |
| RA mean (mmHg) | 11.6 ± 7.7 | 13.5 ± 8.4 | 0.041 | 13.1 ± 8.5 | 13.6 ± 8.6 | 0.243 |
| PA systolic (mmHg) | 40.9 ± 13.2 | 50.4 ± 14.7 | <0.001 | 46.8 ± 14.8 | 50.6 ± 14.4 | <0.001 |
| PA diastolic (mmHg) | 21.4 ± 8.7 | 25.2 ± 8.8 | <0.001 | 23.3 ± 8.7 | 25.2 ± 8.7 | <0.001 |
| PCWP mean (mmHg) | 22.2 ± 8.5 | 24.7 ± 9.0 | 0.017 | 22.7 ± 8.4 | 24.8 ± 9.0 | <0.001 |
| Cardiac Output (L/min) | 4.07 ± 1.40 | 4.19 ± 1.44 | 0.383 | 4.21 ± 1.55 | 4.19 ± 1.44 | 0.679 |
| Echocardiogram | ||||||
| LVEDD (cm) | 6.32 ± 1.05 | 6.84 ± 1.12 | <0.001 | 6.26 ± 0.97 | 6.98 ± 1.08 | <0.001 |
| LVEF (%) | 18.6 ± 7.1 | 18.6 ± 6.1 | 0.918 | 18.6 ± 4.8 | 17.5 ± 4.3 | <0.001 |
| RV Function | 0.371 | 0.342 | ||||
| Normal | 28 (31.8%) | 1995 (25.3%) | 130 (27.4%) | 1302 (23.9%) | ||
| Mild | 17 (19.3%) | 2046 (25.9%) | 125 (26.4%) | 1447 (26.5%) | ||
| Moderate | 29 (33.0%) | 2476 (31.3%) | 143 (30.2%) | 1748 (32.0%) | ||
| Severe | 14 (15.9%) | 1382 (17.5%) | 76 (16.0%) | 957 (17.5%) | ||
| Mitral regurgitation | 0.249 | 0.016 | ||||
| None | 12 (8.2%) | 886 (7.5%) | 60 (8.1%) | 522 (6.7%) | ||
| Mild | 60 (41.1%) | 4053 (34.4%) | 276 (37.3%) | 2659 (34.0%) | ||
| Moderate | 39 (26.7%) | 3984 (33.9%) | 254 (34.3%) | 2686 (34.3%) | ||
| Severe | 35 (24.0%) | 2842 (24.2%) | 150 (20.3%) | 1956 (25.0%) | ||
| Tricuspid regurgitation | ||||||
| None | 18 (12.4%) | 1153 (9.9%) | 0.464 | 70 (9.5%) | 693 (8.9%) | 0.848 |
| Mild | 67 (46.2%) | 5340 (45.9%) | 326 (44.5%) | 3567 (46.1%) | ||
| Moderate | 47 (32.4%) | 3668 (31.5%) | 240 (32.7%) | 2496 (32.2%) | ||
| Severe | 13 (9.0%) | 1484 (12.7%) | 97 (13.2%) | 988 (12.8%) | ||
Common pre-implant laboratory abnormalities for the complete and partial recovery cohorts included lower blood urea nitrogen and lower serum albumin, potentially reflecting superior renal function and acuity of presentation in these cohorts (Table 3). Complete myocardial recovery was associated with higher hemoglobin, elevated platelet counts, lower serum creatinine, and a decreased BNP levels. On the other hand partial recovery was associated with increased serum Na, lower pre-albumin, lower hemoglobin and higher pro-BNP levels.
Table 3.
Laboratory Values at the time of LVAD Implantation
| COMPLETE RECOVERY | PARTIAL RECOVERY | |||||
|---|---|---|---|---|---|---|
| Variable | Explant (n= 163, 1.2%) |
No Explant (n= 13,291, 98.8%) |
p-value | LVEF (≥40%) (n= 761, 8.6%) |
LVEF (<40%) (n= 8,044, 91.4%) |
p-value |
| Sodium (mEq/L) | 135.4 ± 4.8 | 134.8 ± 4.8 | 0.123 | 135.5 ± 4.6 | 134.7 ± 4.8 | <0.001 |
| Potassium (mEq/L) | 4.05 ± 0.48 | 4.07 ± 0.50 | 0.580 | 4.06 ± 0.49 | 4.07 ± 0.49 | 0.880 |
| BUN (mg/dL) | 21.4 ± 11.8 | 29.8 ± 18.4 | <0.001 | 27.6 ± 15.9 | 29.6 ± 18.4 | 0.001 |
| Creatinine (mg/dL) | 1.16 ± 0.59 | 1.41 ± 0.71 | <0.001 | 1.37 ± 0.82 | 1.39 ± 0.64 | 0.500 |
| Albumin (g/dL) | 3.23 ± 0.75 | 3.40 ± 0.66 | 0.002 | 3.33 ± 0.60 | 3.40 ± 0.67 | 0.005 |
| Pre-Albumin (mg/dL) | 18.6 ± 7.0 | 18.6 ± 7.4 | 0.998 | 17.9 ± 7.2 | 18.7 ± 7.4 | 0.041 |
| Bilirubin, tot (mg/dL) | 1.46 ± 2.26 | 1.41 ± 1.83 | 0.743 | 1.35 ± 1.61 | 1.39 ± 1.54 | 0.459 |
| AST (u/L) | 87.0 ± 221.8 | 61.6 ± 239.3 | 0.188 | 76.4 ± 309.7 | 56.3 ± 206.4 | 0.091 |
| ALT (u/L) | 97.2 ± 143.4 | 71.4 ± 233.8 | 0.170 | 87.9 ± 323.0 | 68.5 ± 231.8 | 0.117 |
| WBC Count (×1000/uL) | 9.45 ± 3.8 | 8.58 ± 4.1 | 0.006 | 8.38 ± 4.0 | 8.52 ± 3.9 | 0.336 |
| Hemoglobin (g/dL) | 11.8 ± 2.0 | 11.3 ± 2.1 | 0.007 | 11.1 ± 2.0 | 11.4 ± 2.1 | 0.001 |
| Platelets (×1000/uL) | 198.9 ± 85.1 | 197.5 ± 80.8 | 0.824 | 196.3 ± 88.4 | 198.5 ± 79.3 | 0.502 |
| INR | 1.27 ± 0.42 | 1.33 ± 0.43 | 0.133 | 1.31 ± 0.41 | 1.32 ± 0.43 | 0.554 |
| Pro-BNP (pg/mL) | 4642.4 ± 7323.3 | 6787.2 ± 7887.3 | 0.153 | 9880.8 ± 11664.4 | 6617.3 ± 7737.5 | 0.003 |
| BNP (pg/mL) | 926.7± 860.9 | 1169.9 ± 1097.6 | 0.024 | 1240.9 ± 1149.5 | 1157.4 ± 1086.1 | 0.199 |
Utilization of HF therapies prior to CF-LVAD implantation was summarized in Table 4. As shown, both complete and partial myocardial recovery patients were less likely to be on a beta-blocker, angiotensin receptor blocker, and aldosterone blocker compared to no-recovery cohorts. In addition, utilization of intracardiac defibrillator (ICD) and/or cardiac resynchronization therapy (CRT) devices was significantly lower in both partial and complete myocardial recovery cohorts. Interestingly, only 32.5% of complete recovery patients were on optimal HF therapy – defined as use of at least 2 neurohormonal blockers in combination with an ICD and/or CRT device – as compared to 47.5% of partial recovery patients and 62.0% of no-recovery patients (p<0.001).
Table 4.
Utilization of Heart Failure Therapies Prior to LVAD Implantation
| COMPLETE RECOVERY | PARTIAL RECOVERY | |||||
|---|---|---|---|---|---|---|
| Variable | Explant (n= 163, 1.2%) |
No Explant (n= 13,291, 98.8%) |
p-value | LVEF (≥40%) (n= 761, 8.6%) |
LVEF (<40%) (n= 8,044, 91.4%) |
p-value |
| Medical Therapy | ||||||
| Beta-blocker | 0.008 | 0.038 | ||||
| Current Use | 72 (47.4%) | 7148 (55.4%) | 404 (55.0%) | 4287 (54.7%) | ||
| Prior Use | 31 (20.4%) | 2944 (22.8%) | 153 (20.8%) | 1906 (24.3%) | ||
| No | 49 (32.2%) | 2803 (21.7%) | 177 (24.1%) | 1642 (21.0%) | ||
| ACE inhibitors | 0.197 | 0.369 | ||||
| Current Use | 55 (35.7%) | 3783 (30.5%) | 212 (29.4%) | 2294 (30.5%) | ||
| Prior Use | 23 (14.9%) | 2468 (19.9%) | 142 (19.7%) | 1601 (21.3%) | ||
| No | 76 (49.4%) | 6155 (49.6%) | 366 (50.8%) | 3620 (48.2%) | ||
| ARB | 0.013 | 0.453 | ||||
| Current Use | 12 (7.8%) | 1165 (9.6%) | 59 (8.5%) | 704 (9.6%) | ||
| Prior Use | 2 (1.3%) | 851 (7.0%) | 47 (6.8%) | 544 (7.4%) | ||
| No | 139 (90.8%) | 10060 (83.3%) | 590 (84.8%) | 6055 (82.9%) | ||
| Aldosterone blocker | 0.030 | <0.001 | ||||
| Current Use | 52 (34.2%) | 5334 (42.5%) | 266 (37.5%) | 3343 (43.9%) | ||
| Prior Use | 17 (11.2%) | 1704 (13.6%) | 87 (12.3%) | 1105 (14.5%) | ||
| No | 83 (54.6%) | 5501 (43.9%) | 356 (50.2%) | 3161 (41.5%) | ||
| Device Therapy | ||||||
| ICD | 68 (37.4%) | 10854 (79.7%) | <0.001 | 503 (66.4%) | 6727 (84.0%) | <0.001 |
| CRT | 11 (14.1%) | 2348 (29.6%) | 0.003 | 100 (21.7%%) | 1641 (31.8%) | <0.001 |
| Optimal HF therapy* | 51 (32.5%) | 7789 (59.7%) | <0.001 | 355 (47.5%) | 4923 (62.0%) | <0.001 |
Defined as prior or current use of at least 2 neurohormonal blocking agents from 3 major drug classes (beta-blockers, ACE inhibitors/ARB, and Aldosterone antagonists) in combination with an ICD and/or CRT device.
When specific HF etiologies were investigated, highest rates of myocardial recovery leading to device explantation were observed in patients with myocarditis (7.7%), followed by those with postpartum cardiomyopathy (4.4%) and adriamycin-induced dilated cardiomyopathy (4.1%) (Supplementary Figure 2). Similarly, highest rates of partial recovery were observed in patients with adriamycin-induced dilated cardiomyopathy (22.1%), followed by postpartum cardiomyopathy (17.3%), and valvular heart disease (12.8%).
Time Course of Reverse Remodeling on LVAD Support
Time-dependent changes in mean LVEDD, mean LVEF (%), mitral regurgitation, and serum BNP levels in complete and partial myocardial recovery patients were summarized in Figure 2. As shown, both cohorts demonstrated an early reduction in LVEDD following CF-LVAD insertion, which remained stable throughout the follow-up period. Prevalence of moderate-to-severe mitral insufficiency and serum BNP levels decreased in all patients, however the reduction was more significant in complete and partial myocardial recovery patients at multiple time points. Mixed model analysis confirmed significant reduction in LVEDD and improvement in LVEF in myocardial recovery groups, accounting for potential within subject correlations (p<0.001).
Figure 2. Indices of Reverse Remodeling in Myocardial Recovery Cohorts.
A) Change in LVEDD over time B) Change in LVEF over time C) Change in prevalence of moderate-to-severe MR over time D) Change in serum BNP levels over time. Complete myocardial recovery cohort (device explantation) represented in blue, partial myocardial recovery cohort represented in turquoise, no-recovery cohort represented in red. * p <0.05 Complete vs. no-recovery, † p <0.05 Partial vs. no-recovery
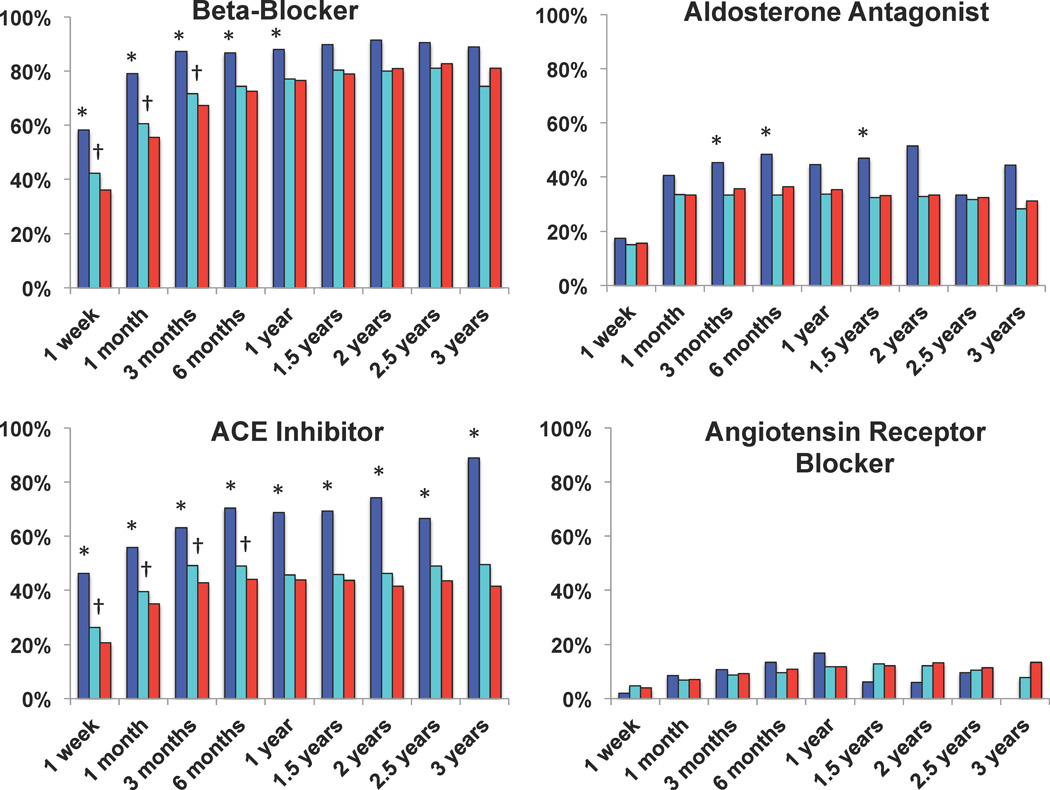
Utilization of neurohormonal blocking agents including beta-blocker, ACE inhibitors, and aldosterone antagonists was significantly higher in the complete recovery cohort compared to no-recovery cohort (Figure 3). Partial recovery cohort also had increased use of beta-blockers and ACE inhibitors, primarily limited to the first 6 months of CF-LVAD support, and to a much lower extent as compared to the complete recovery cohort, suggesting underutilization of reverse remodeling therapies in this cohort. Generalized linear model analysis confirmed higher beta-blocker and ACE-inhibitor use in myocardial recovery cohorts, accounting for potential within subject correlations (p<0.001).
Figure 3. Utilization of Neurohormonal Blocker Therapy on Continuous-Flow Left Ventricular Assist Device Support.
Complete myocardial recovery cohort (device explantation) represented in blue, partial myocardial recovery cohort represented in turquoise, no-recovery cohort represented in red. * p <0.05 Complete vs. no-recovery and Complete vs. partial recovery , † p <0.05 Partial vs. no-recovery
Predicting Complete and Partial Myocardial Recovery: INTERMACS Recovery Risk Model
Two independent risk prediction models were developed to predict complete myocardial recovery and partial myocardial recovery (Table 5). Pre-implant clinical factors independently associated with complete myocardial recovery (device explantation) were young age, non-ischemic etiology, shorter duration of cardiac disease, suboptimal HF therapy, small LV size, absence of pulmonary hypertension, low blood urea nitrogen, and use of axial-flow device. Pre-implant clinical factors independently associated with partial myocardial recovery included female gender, low body surface area, non-ischemic etiology, short duration of cardiac disease, suboptimal HF therapy, small LV size, higher baseline LVEF, absence of pulmonary hypertension, absence of hyponatremia, low serum blood urea nitrogen, and use of axial-flow pump. Since CF-LVAD device design was a significant factor for both complete and partial myocardial recovery with highest odds ratios, we further investigated reverse remodeling patterns between the two device types, which demonstrated a significantly lower LVEDD, higher LVEF, lower incidence of moderate-to-severe mitral regurgitation, and lower BNP in patients supported with axial-flow devices (Supplementary Figure 3).
Table 5.
Multivariable Predictors of Complete and Partial Recovery on LVAD Support
| Parameter | Estimate | SE | p-value | OR (95% CI) |
|---|---|---|---|---|
| COMPLETE RECOVERY | ||||
| Age < 50 years | 0.914 | 0.305 | 0.003 | 2.493 (1.371 – 4.534) |
| Non-ischemic etiology | 1.688 | 0.336 | <0.001 | 5.407 (2.799 – 10.444) |
| Time since cardiac diagnosis < 2 yrs | 1.224 | 0.245 | <0.001 | 3.400 (2.105 – 5.492) |
| Sub-optimal HF therapy (pre-LVAD) | 0.806 | 0.243 | 0.001 | 2.239 (1.392 –3.602) |
| LVEDD < 6.5 cm | 0.519 | 0.224 | 0.021 | 1.681 (1.083 – 2.610) |
| PASP < 50 mmHg | 0.696 | 0.258 | 0.007 | 2.005 (1.210 – 3.323) |
| BUN < 30 mg/dL | 1.193 | 0.343 | 0.001 | 3.297 (1.684 – 6.457 |
| Axial-flow device | 2.034 | 0.592 | 0.001 | 7.643 (2.395 – 24.396) |
| Hosmer-Lemeshow p = 0.910 | ||||
| PARTIAL RECOVERY | ||||
| Female gender | 0.531 | 0.116 | <0.001 | 1.700 (1.355 – 2.134) |
| Body surface area < 2.0 m2 | 0.393 | 0.108 | <0.001 | 1.482 (1.200 – 1.830) |
| Non-ischemic etiology | 0.392 | 0.111 | <0.001 | 1.480 (1.190 – 1.840) |
| Time since cardiac diagnosis < 2 yrs | 0.448 | 0.115 | <0.001 | 1.566 (1.249 – 1.963) |
| Sub-optimal HF therapy (pre-LVAD) | 0.281 | 0.107 | 0.009 | 1.325 (1.074 – 1.643) |
| LVEDD < 6.5 cm | 0.993 | 0.108 | <0.001 | 2.700 (2.118– 3.337) |
| LVEF (20 – 30% vs. <20%) | 0.512 | 0.110 | <0.001 | 1.668 (1.346 – 2.068) |
| PASP < 50 mmHg | 0.319 | 0.105 | 0.002 | 1.376 (1.120 – 1.690) |
| Na > 135 mEq/L | 0.210 | 0.105 | 0.046 | 1.234 (1.004 – 1.517) |
| BUN < 30 mg/dL | 0.282 | 0.112 | 0.012 | 1.326 (1.065 – 1.651) |
| Axial-flow device | 1.417 | 0.208 | <0.001 | 4.124 (2.744 – 6.198) |
| Hosmer-Lemeshow p = 0.686 | ||||
Changing Trends in Myocardial Recovery
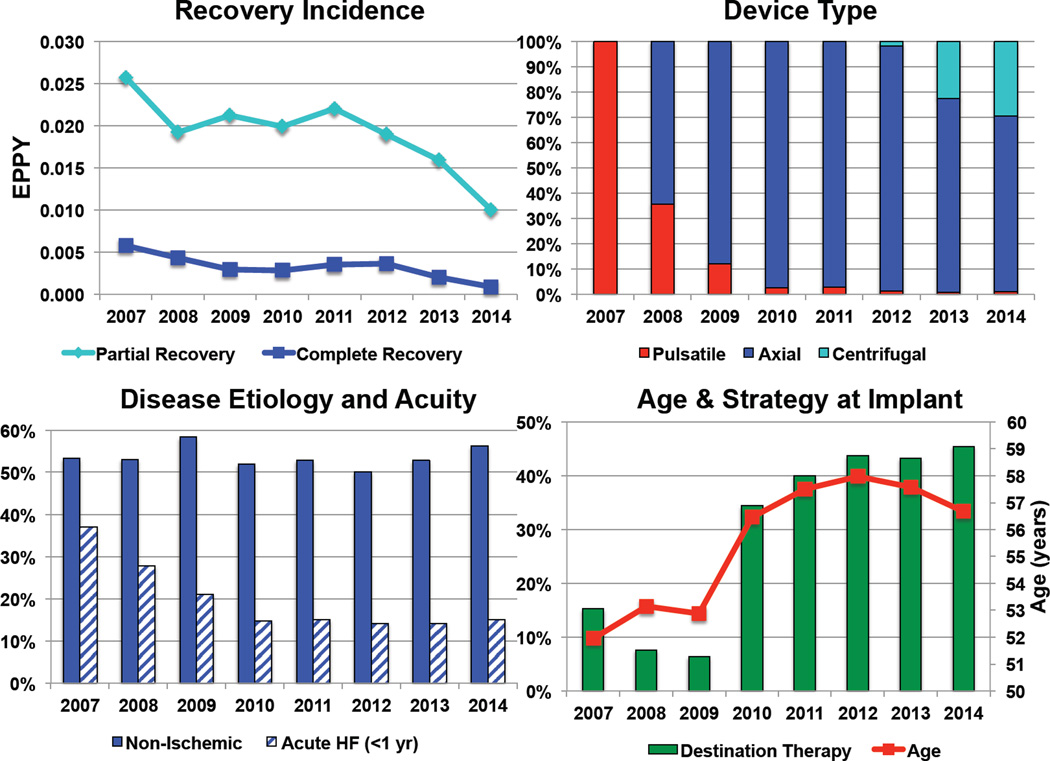
Since the analysis extends over a lengthy period in which device indications and utilization patterns were rapidly changing, we analyzed myocardial recovery trends in the INTERMACS registry from 2007 through 2014 (Figure 4). Complete and partial myocardial recovery rates were significantly decreased in this time period in a time-dependent manner. This was accompanied by a significant change in device technology with early predominance of pulsatile-flow pumps and more recent introduction of centrifugal-flow CF-LVADs. Although the percentage of patients with non-ischemic etiology remained relatively stable, prevalence of patients with acute presentation have declined significantly. In 2010, there was a steep increase in prevalence of destination therapy patients coinciding with the FDA approval of CF-LVAD technology for this indication, and a parallel increase in mean age at device implantation.
Figure 4. Changing Trends in LVAD Induced Myocardial Recovery: INTERMACS Registry Data.
A) Change in incidence of partial and complete myocardial recovery over time, EPPY: events per patient year B) Change in LVAD type used over time C) Change in etiology and acuity of HF in LVAD recipients over time D) Change in mean age and device strategy at the time of device implantation over time, p <0.05 for all trends.
Discussion
This study evaluated the time-course and clinical risk factors associated with complete and partial myocardial recovery in patients supported with contemporary LVADs, in an era in which application of this technology is rapidly expanding to broader HF populations. Our principal findings are as follows: (1) Complete myocardial recovery leading to device explantation is rare (1.2%) in the current mechanical circulatory support device era, but not uncommon even beyond the first year of device support; (2) 8.6% of patients exhibit substantial improvement of LV function (partial myocardial recovery) on long-term CF-LVAD support (3) Patients achieving complete versus partial myocardial recovery on device support have a remarkable overlap in clinical characteristics suggesting that myocardial recovery represents a spectrum of improvement rather than a binary phenomenon (4) Patient-related (age, etiology of HF, duration of disease, LV size, renal function) and treatment-related (device type, use of neurohormonal blockade) factors appear to be associated with recovery on CF-LVAD support; (5) Recent changes in device indications and technology have contributed to the lower rates of myocardial recovery observed. Taken together, these findings suggest that substantially higher number of LVAD patients than previously reported improve their myocardial function while on device support. Such patients might be most likely to benefit from targeted approaches and treatment strategies to enhance myocardial recovery.
Single-center studies using a combinatorial approach to promote recovery through prospective assessment of native cardiac function, use of guideline-directed medical therapy, and individualized device weaning strategies reported substantially higher rates of complete myocardial recovery than reported in this study, highlighting the importance and the pro-active role of LVAD programs in achieving this favorable outcome 11–14, 16, 17. Most effective approach reported to date remains the Harefield protocol, which combines mechanical unloading with a novel two-stage pharmacologic strategy using neurohormonal blockers followed by addition of beta-2-agonist to prevent myocardial atrophy once the maximal regression in the LVEDD had been observed 11, 12. 60% (12 out of 20) of patients with chronic non-ischemic cardiomyopathy supported with CF-LVAD were successfully explanted using this protocol, however results from the multicenter Harefield Recovery Protocol Study (HARPS) were not replicated in the United States 12. Moreover, “real world” data from this study and other multicenter registries demonstrate substantially lower rates of myocardial recovery observed in LVAD patients 18, 21. Although many reasons can account for the variability observed; differences in patient selection, pharmacologic management, assessment of cardiac function on pump support, speed optimization protocols, and device explantation criteria used are potential contributors to this discrepancy. Therefore, low nationwide incidence of myocardial recovery could reflect the fact that many programs may not have protocols in place to prospectively screen and promote recovery in LVAD supported patients.
A notable finding of this study was characterization of patients exhibiting substantial recovery of the LV function on device support while not achieving device explantation end-point, termed as “partial myocardial recovery” cohort. Interestingly, patients with partial myocardial recovery had remarkable overlap in clinical risk profile compared to those achieving complete recovery and device explantation, suggesting that myocardial recovery is rather a continuum of functional and clinical improvement. This observation suggests that the number of patients who can potentially benefit from prospective functional assessment and optimization of therapy to achieve recovery is substantially higher than previously anticipated. Although the precise reasons for lack of complete recovery in this cohort are unknown, underutilization of neurohormonal blocker agents on device support is a potentially contributing factor, as shown in this study.
Prospective single-center studies demonstrated that reverse remodeling is generally complete by 6 months of LVAD support 12, 14. However, data from current analysis have shown that risk of complete myocardial recovery and device explantation is relatively constant within the first 3 years of LVAD support. Moreover, we observed a time-dependent gradual increase in mean LVEF of complete myocardial recovery cohort, which continued to increase during the follow-up duration. This finding suggests that myocardial recovery can be successfully achieved even much later in the course of LVAD support and lack of early functional improvement should not preclude a patient from ongoing recovery assessments and therapies.
This study represents the largest myocardial recovery risk prediction analysis performed to date. Previous single-center and multi-center studies have also reported factors associated with LVAD-induced myocardial recovery (Supplemental Table 1a). It is important to note that the majority of these studies were conducted on patients with pulsatile-flow devices, were statistically underpowered and primarily focused on device explantation as the recovery end-point. Yet, recovery patients reported in these cohorts were predominantly young (mean age range: 27 – 50) with non-ischemic etiology (range: 60–100%, mean: 94%), thus consistent with our findings. Only few studies to date investigated potential predictors of recovery by comparing patients who recovered vs. who did not. Risk factors identified in at least 2 independent studies were limited to young age, non-ischemic etiology, and short duration of heart failure (Supplemental Table 1b) 16–18. Our findings confirm and extend these original observations. Focusing on factors common to both complete and partial recovery; we identified suboptimal pre-implant heart failure therapy, small left ventricle, absence of pulmonary hypertension, preserved renal function, and use of axial-flow device as novel independent predictors of myocardial recovery. Patients who have not been exposed to neurohormonal blockade and/or CRT prior to device implantation are potentially more likely to respond to these therapies following CF-LVAD implantation, which may explain the higher recovery rates observed in patients who are not on optimized HF therapy. Concomitant kidney disease may potentially interfere with the recovery process by reducing diuretic response leading to incomplete unloading of the failing ventricle as well as creating challenges for use or titration of neurohormonal blocking agents, particularly ACE inhibitors, angiotensin receptor blockers, and aldosterone antagonists. LV chamber size at the time of LVAD implantation may indicate extent of LV remodeling and reflect chronicity of structural changes in the myocardium. Pulmonary hypertension may reflect presence of underlying pulmonary parenchymal disease and/or chronically remodeled pulmonary vasculature due to long-standing HF. Interestingly, large clinical studies assessing predictors of CRT (cardiac resynchronization therapy) response in less sick heart failure populations also demonstrated non-ischemic etiology, recent onset of symptoms, small LV size/volume as significant predictors, suggesting presence of a common recovery pathway in heart failure irrespective of the platform used 22. Specifically, patients with adriamycin-induced heart failure had the highest chances of partial myocardial recovery with nearly one in every four patients exhibiting substantial improvement in LVEF during LVAD support. Interestingly, LVAD unloading leads to significant improvements in mitochondrial structure and function, which is the target organelle for adriamycin, a widely used chemotherapeutic agent associated with cardiotoxicity 23, 24. Myocarditis and peripartum cardiomyopathy were among other specific heart failure etiologies that were associated with significantly higher rates of myocardial recovery. Finally, a much higher percentage of patients who achieved complete myocardial recovery received neurohormonal blocking agents on LVAD support as compared to those with partial or no recovery, highlighting importance of medical therapy in promoting recovery outcome. Future studies will be required to investigate whether patients who achieved only partial recovery on LVAD support could be further improved to complete recovery and device explantation with augmentation of neurohormonal blocker therapy.
A novel finding of this study was the significant association between device type and myocardial recovery outcomes. Compared to those supported with pulsatile-flow LVADs, patients supported with continuous-flow LVADs had significantly lower rates of myocardial recovery, a finding consistent with a previous single-center report 25. Pulsatile-flow LVADs may unload of the failing ventricle more effectively than continuous-flow LVADs as evidenced by greater improvements in LV systolic and diastolic function and greater reduction in circulating levels of BNP, TIMP4, and MMP9 in patients supported with these older-generation pumps 26. In addition, continuous-flow devices are typically operated on lower speed settings in an attempt to maintain aortic valve opening and pulsatility, and such low speed operation may lead to ineffective unloading of the failing heart. An observation, even more relevant in the current era of device support, is the higher incidence of complete (1.3% vs. 0.4%) and partial (9.5% vs. 3.3%) myocardial recovery in patients supported with axial compared to centrifugal continuous-flow pumps. Notable physiological differences exist between the two pumps designs including increased flow pulsatility and afterload sensitivity (flat pressure-head vs. flow curves) seen in centrifugal pumps, which may in turn lead to differential patterns of unloading and reduction in wall stress in the failing heart. A recent in-vitro study suggested inadequate hemodynamic unloading of the centrifugal-flow pumps using an experimental mock-circulatory loop model 27, which was further supported by the clinical observations from RAMP studies demonstrating a relatively flat LVEDD slope in patients supported by a centrifugal-flow pumps 28–30. These findings were challenged by a recent in-vivo study of bovine model of ischemic HF as well as a clinical hemodynamic RAMP study, both of which suggested comparable unloading between the two pump designs. Nevertheless, future prospective studies will be required to confirm association between different device physiologies with reverse remodeling, which may have strong clinical implications for device selection in patients who have higher likelihood of recovery. It will be interesting to see whether newer device designs that implementing artificial pulse technology may lead to higher recovery rates in LVAD patients.
Study Limitations
Although INTERMACS represent high-quality registry dataset, limitations inherent to retrospective data analysis apply to current study. Since only FDA approved devices are included in the INTERMACS, selection of device type was potentially biased by approval status at the time of implantation. Proper assessment of native cardiac function on LVAD support requires speed adjustment to ensure minimal device support (i.e. 6,000 rpm in patients with Heartmate II LVAD), however weaning echocardiogram data and device speed settings at the time of follow-up echocardiograms were not available in the INTERMACS registry. Paired echocardiogram data was unavailable in a significant number of patients, which could introduce potential bias due to missing data. Previous studies have shown a substantial risk of recurrent of heart failure, cardiac transplantation, and mortality in patients following LVAD explantation for myocardial recovery. Unfortunately, post-explant data was not available in the INTERMACS registry. Our analysis identified device type as a potential predictor of myocardial recovery. This finding needs to be interpreted with caution, as the primary intention of this study was not to compare differences in outcomes or unloading patterns between different device types. Moreover, device selection is non-randomized in the INTERMACS registry, which may affect our findings regarding device type. Finally, we were unable to perform cellular, molecular, or genetic correlates of myocardial recovery since analysis of cardiac tissue was not available in the INTERMACS registry.
In conclusion, although myocardial recovery leading to device explantation is rare, one in every ten LVAD patients exhibit substantial improvement in LV function while on device support. Efforts should be maximized to properly identify individuals with high-likelihood of recovery on LVAD support, systematically monitor native cardiac function, and optimize reverse remodeling therapy to achieve best possible outcomes.
Supplementary Material
CLINICAL PERSPECTIVE.
Mechanical unloading with continuous-flow left ventricular assist devices (CF-LVADs) lead to favorable changes in the structure and function of the failing ventricle termed as “reverse remodeling”. Despite these improvements, complete myocardial recovery leading to device explantation occurs rarely in the current era of mechanical circulatory support. Using the multicenter INTERMACS dataset, we investigated incidence and predictors of myocardial recovery in patients supported with durable contemporary CF-LVADs. We found that incidence of complete myocardial recovery was 1% at 1 year and 3% at 3 years; suggesting that myocardial recovery occurs beyond the first year of device support. More importantly, 9% of patients supported with CF-LVADs exhibited significant improvement in cardiac function (LVEF > 40%) on device support, termed as “partial myocardial recovery”. Clinical characteristics of patients with complete and partial myocardial recovery showed a remarkable overlap, suggesting that myocardial recovery represents a spectrum of improvement rather than a binary phenomenon. Clinical and molecular studies focusing on patients with partial myocardial recovery may provide insights into mechanisms of reverse remodeling, despite the low incidence of device explantation observed in the current era. Taken together, these findings suggest that a substantially higher number of CF-LVAD patients than previously reported exhibit functional improvement on device support and can be targeted for myocardial recovery. Patients who are likely to recover based on their risk profile may benefit from prospective recovery assessment by serial echocardiograms, guideline-directed neurohormonal blockade, and individualized weaning strategies.
Acknowledgments
We would like to thank the Intermacs investigators, coordinators, and participating institutions for the data they have provided for this registry. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs) or the National Institutes of Health. This study was supported by Lisa and Mark Schwartz and the Program to Reverse Heart Failure at New York Presbyterian Hospital/Columbia University.
Sources of Funding
Data collection for this work was funded in whole or in part with Federal Funds from the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201100025C.
Footnotes
Disclosures
Dr. Naka received consulting fees from Thoratec. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. 17
References
- 1.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. The New England journal of medicine. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH HeartMate III. Advanced heart failure treated with continuous-flow left ventricular assist device. The New England journal of medicine. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Frazier OH. First use of an untethered, vented electric left ventricular assist device for long-term support. Circulation. 1994;89:2908–2914. doi: 10.1161/01.cir.89.6.2908. [DOI] [PubMed] [Google Scholar]
- 4.Levin HR, Oz MC, Chen JM, Packer M, Rose EA, Burkhoff D. Reversal of chronic ventricular dilation in patients with end-stage cardiomyopathy by prolonged mechanical unloading. Circulation. 1995;91:2717–2720. doi: 10.1161/01.cir.91.11.2717. [DOI] [PubMed] [Google Scholar]
- 5.Bruckner BA, Stetson SJ, Perez-Verdia A, Youker KA, Radovancevic B, Connelly JH, Koerner MM, Entman ME, Frazier OH, Noon GP, Torre-Amione G. Regression of fibrosis and hypertrophy in failing myocardium following mechanical circulatory support. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2001;20:457–464. doi: 10.1016/s1053-2498(00)00321-1. [DOI] [PubMed] [Google Scholar]
- 6.Klotz S, Barbone A, Reiken S, Holmes JW, Naka Y, Oz MC, Marks AR, Burkhoff D. Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. Journal of the American College of Cardiology. 2005;45:668–676. doi: 10.1016/j.jacc.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Torre-Amione G, Stetson SJ, Youker KA, Durand JB, Radovancevic B, Delgado RM, Frazier OH, Entman ML, Noon GP. Decreased expression of tumor necrosis factor-alpha in failing human myocardium after mechanical circulatory support : A potential mechanism for cardiac recovery. Circulation. 1999;100:1189–1193. doi: 10.1161/01.cir.100.11.1189. [DOI] [PubMed] [Google Scholar]
- 8.Vatta M, Stetson SJ, Jimenez S, Entman ML, Noon GP, Bowles NE, Towbin JA, Torre-Amione G. Molecular normalization of dystrophin in the failing left and right ventricle of patients treated with either pulsatile or continuous flow-type ventricular assist devices. Journal of the American College of Cardiology. 2004;43:811–817. doi: 10.1016/j.jacc.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 9.Klotz S, Danser AH, Foronjy RF, Oz MC, Wang J, Mancini D, D'Armiento J, Burkhoff D. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. Journal of the American College of Cardiology. 2007;49:1166–1174. doi: 10.1016/j.jacc.2006.10.071. [DOI] [PubMed] [Google Scholar]
- 10.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A, Yacoub MH. Left ventricular assist device and drug therapy for the reversal of heart failure. The New England journal of medicine. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 12.Birks EJ, George RS, Hedger M, Bahrami T, Wilton P, Bowles CT, Webb C, Bougard R, Amrani M, Yacoub MH, Dreyfus G, Khaghani A. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation. 2011;123:381–390. doi: 10.1161/CIRCULATIONAHA.109.933960. [DOI] [PubMed] [Google Scholar]
- 13.Frazier OH, Baldwin AC, Demirozu ZT, Segura AM, Hernandez R, Taegtmeyer H, Mallidi H, Cohn WE. Ventricular reconditioning and pump explantation in patients supported by continuous-flow left ventricular assist devices. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:766–772. doi: 10.1016/j.healun.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drakos SG, Wever-Pinzon O, Selzman CH, Gilbert EM, Alharethi R, Reid BB, Saidi A, Diakos NA, Stoker S, Davis ES, Movsesian M, Li DY, Stehlik J, Kfoury AG, Investigators U. Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. Journal of the American College of Cardiology. 2013;61:1985–1994. doi: 10.1016/j.jacc.2013.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel SR, Saeed O, Murthy S, Bhatia V, Shin JJ, Wang D, Negassa A, Pullman J, Goldstein DJ, Maybaum S. Combining neurohormonal blockade with continuous-flow left ventricular assist device support for myocardial recovery: a single-arm prospective study. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:305–312. doi: 10.1016/j.healun.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Simon MA, Kormos RL, Murali S, Nair P, Heffernan M, Gorcsan J, Winowich S, McNamara DM. Myocardial recovery using ventricular assist devices: prevalence, clinical characteristics, and outcomes. Circulation. 2005;112:I32–I36. doi: 10.1161/CIRCULATIONAHA.104.524124. [DOI] [PubMed] [Google Scholar]
- 17.Dandel M, Weng Y, Siniawski H, Potapov E, Drews T, Lehmkuhl HB, Knosalla C, Hetzer R. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation. 2008;118:S94–S105. doi: 10.1161/CIRCULATIONAHA.107.755983. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DJ, Maybaum S, MacGillivray TE, Moore SA, Bogaev R, Farrar DJ, Frazier OH HeartMate IICI. Young patients with nonischemic cardiomyopathy have higher likelihood of left ventricular recovery during left ventricular assist device support. Journal of cardiac failure. 2012;18:392–395. doi: 10.1016/j.cardfail.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Hess KR, Serachitopol DM, Brown BW. Hazard function estimators: a simulation study. Stat Med. 1999;18:3075–3088. doi: 10.1002/(sici)1097-0258(19991130)18:22<3075::aid-sim244>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.muhaz: Hazard Function Estimation in Survival Analysis. [computer program]. R package version1.2.6. 2014 http://CRANR-projectorg/package=muhaz.
- 21.Pan S, Aksut B, Wever-Pinzon OE, Rao SD, Levin AP, Garan AR, Fried JA, Takeda K, Hiroo T, Yuzefpolskaya M, Uriel N, Jorde UP, Mancini DM, Naka Y, Colombo PC, Topkara VK. Incidence and predictors of myocardial recovery on long-term left ventricular assist device support: Results from the United Network for Organ Sharing database. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1624–1629. doi: 10.1016/j.healun.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Ellenbogen KA, Huizar JF. Foreseeing super-response to cardiac resynchronization therapy: a perspective for clinicians. Journal of the American College of Cardiology. 2012;59:2374–2377. doi: 10.1016/j.jacc.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 23.Gupte AA, Hamilton DJ, Cordero-Reyes AM, Youker KA, Yin Z, Estep JD, Stevens RD, Wenner B, Ilkayeva O, Loebe M, Peterson LE, Lyon CJ, Wong ST, Newgard CB, Torre-Amione G, Taegtmeyer H, Hsueh WA. Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ Cardiovasc Genet. 2014;7:266–276. doi: 10.1161/CIRCGENETICS.113.000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 25.Krabatsch T, Schweiger M, Dandel M, Stepanenko A, Drews T, Potapov E, Pasic M, Weng YG, Huebler M, Hetzer R. Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile-flow systems? The Annals of thoracic surgery. 2011;91:1335–1340. doi: 10.1016/j.athoracsur.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Kato TS, Chokshi A, Singh P, Khawaja T, Cheema F, Akashi H, Shahzad K, Iwata S, Homma S, Takayama H, Naka Y, Jorde U, Farr M, Mancini DM, Schulze PC. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circulation Heart failure. 2011;4:546–553. doi: 10.1161/CIRCHEARTFAILURE.111.962142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senage T, Fevrier D, Michel M, Pichot E, Duveau D, Tsui S, Trochu JN, Roussel JC. A mock circulatory system to assess the performance of continuous-flow left ventricular assist devices (LVADs): does axial flow unload better than centrifugal LVAD? ASAIO journal. 2014;60:140–147. doi: 10.1097/MAT.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, Latif F, Restaino SW, Mancini DM, Flannery M, Takayama H, John R, Colombo PC, Naka Y, Jorde UP. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. Journal of the American College of Cardiology. 2012;60:1764–1775. doi: 10.1016/j.jacc.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uriel N, Levin AP, Sayer GT, Mody KP, Thomas SS, Adatya S, Yuzefpolskaya M, Garan AR, Breskin A, Takayama H, Colombo PC, Naka Y, Burkhoff D, Jorde UP. Left Ventricular Decompression During Speed Optimization Ramps in Patients Supported by Continuous-Flow Left Ventricular Assist Devices: Device-Specific Performance Characteristics and Impact on Diagnostic Algorithms. Journal of cardiac failure. 2015;21:785–791. doi: 10.1016/j.cardfail.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Sauer AJ, Meehan K, Gordon R, Abicht T, Rich JD, Anderson AS, Yancy C, McGee EC., Jr Echocardiographic markers of left ventricular unloading using a centrifugal-flow rotary pump. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33:449–450. doi: 10.1016/j.healun.2013.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.