Abstract
Many studies on the biological activities of nutmeg continue to appear in the literature. The most common targets include GIT, CNS, oxidative stress and inflammation. However, results obtained from most studies are often inconsistent due to the variability of utilized samples, lack of standardized nutmeg products or insufficient amounts of pure compounds for comprehensive follow-up investigation. To address the consistency and supply issue we utilized available technology to develop a reproducible procedure for preparation of specific extracts and isolation of the major phenolic constituents present in nutmeg kernel. A well-defined and reproducible sequence of extraction, fractionation and chromatographic purification was adopted and was guided by HPLC fingerprinting. Spectroscopic methods, mainly NMR, and mass spectrometry were utilized to identify each compound. Thirteen compounds were isolated in a pure form and identified as: elemicin (1), isoelemicin (2), myristicin (4), surinamensin (5), malabaricone C (6), 2-(3′-allyl-2′,6′-dimethoxy-phenyloxy)-1-acetoxy-(3,4-dimethoxyphenyl)-propyl ester (7), methoxylicarin A (8), licarin A (9), malabaricone B (10), licarin C (11), 5′-methoxylicarin B (12), licarin B (13), and 2-(3′-allyl-2′,6′-dimethoxy-phenyloxy)-1-methyl-5-methoxy-1,2-dihydrobenzofuran (3, a new compound). With repeated isolation runs, these pure compounds can be prepared in quantities sufficient for biological evaluation as needed. The availability of purified compounds will also allow the development of specific, accurate, and sensitive analytical procedures for pharmacokinetic studies and for quality control of nutmeg products. Both aspects are essential for nutmeg-focused drug discovery. The same approach can also be adapted to other medicinal plants of potential interest.
Keywords: Nutmeg, HPLC, MPLC flash chromatography, Phenyl propanoids, Neolignans, Benzofurans, Licarins, Malabaricones
Nutmeg kernel is the inner seed tissue of Myristica fragrans Houtt. (Myristicaceae), which is an evergreen tree indigenous to the East Indies (such as Indonesian Maluku Islands, Malaysia, Sri Lanka, Papua, and Sumatra) and is cultivated in the West Indies (such as Cuba, Dominican Republic, Grenada, and Virgin Islands), South Africa, and other tropical countries [1,2]. Nutmeg is one of the most commonly used spices in the world due to the unique taste and aroma of its volatile oil. The volatile oil (ca. 10% of seed content) consists of terpene derivatives (ca. 88% of volatile oil), such as camphene and pinene, and phenylpropanoids (ca. 8% of volatile oil), mainly myristicin, elemicin, and safrole [1,3]. Other nonvolatile secondary metabolites include diarylpropanoids (lignans), diarylalkanes (resorcinols), proanthocyanidins and catechins [1, 3]. More than 70% of the kernel's weight comprises fixed oils, lipids, pigments, starch and protein [1]. Relatively few analytical methods, utilizing GC, HPLC and HPTLC, have been reported for nutmeg [4-7]. These methods mainly focus on fingerprinting its volatile oil [5], determination of a major constituent [8,9], or detection of contaminants, such as aflatoxins [7].
In addition to its use as a kitchen spice, nutmeg has traditionally been used to increase appetite, improve digestion, and to manage such GI conditions as colic, nausea, diarrhea, and flatulence [1,3,10]. Traditional Chinese medicine, Tibetan medicine, and Ayurveda employ multi-ingredient formulas to treat neuropsychological disorders, and many formulas contain nutmeg as a common ingredient [11]. Various online documents and forums present controversial discussions on the psychotropic effects of nutmeg, which is regarded by some recreational drug users as a cheap marijuana substitute [12,13]. Different attempts to corroborate the traditional uses of nutmeg have been reported in modern literature. For example, a recent study showed nutmeg to have anti-diarrheal activity, particularly in diarrhea cases involving rotavirus [14]. In another study, adipose tissue reduction was shown in rats that were given a nutmeg extract which warrants further investigation to examine the effects of nutmeg on weight and diabetes [15]. Recent studies have also shown that nutmeg may act as a CNS stimulant, psychotropic, aphrodisiac and anti-inflammatory agent [11,16-19]. For example, Baker et al. found that nutmeg exhibited anti-inflammatory activity, through the inhibition of COX-2, resulting in decreased synthesis of prostaglandins [18]. The traditional use of nutmeg in the treatment of sexual dysfunction was tested in male rats using a 50% ethanolic extract that resulted in increased libido, and potency that was suggested to correlate with the CNS stimulating effects [19]. Both Min et al. and Cao et al. investigated the inhibitory effects of nutmeg neolignans on LPS-induced NO production [20, 21]. Compounds possessing acetylcholinesterase inhibitory activity have also been identified from M. fragrans [22]. An in vivo tetrad assay conducted in our lab with HPLC fingerprinted extracts further supported the CNS-effects of nutmeg which invoked an atypical marijuana-like effect in mice [16]. Further in vitro evaluation in our lab identified fatty acid amide hydrolase (FAAH) and monoacyl glycerol lipase (MAGL) as potential targets for nutmeg extracts with the ethyl acetate fraction being the most active (manuscript under review). It is to be noted that many of the reported studies utilized different nutmeg constituents ranging from pure compounds to extracts of various compositions of volatile oil, phenolic compounds, lipids, polysaccharides, and proteins.
As demonstrated above, nutmeg contains a complex mixture of secondary metabolites with potential for drug discovery. Thus, there is a need to have reliable access to as many of these compounds as possible, in a pure form, to allow for further investigation of their biological activities in a consistent and reliable manner. Such access would also avail these compounds for use as analytical standards in pharmacokinetic studies and quality control of nutmeg products. Towards this goal, we have developed a reproducible procedure for isolation, purification and identification of the major non-polymeric phenolic constituents of nutmeg. A schematic representation of this procedure is shown in Figure 1. At its core, our approach adapts current technology to traditional phytochemical methods of medicinal plant investigation. A pivotal component of the procedure is a qualitative HPLC method that was developed and optimized to guide the isolation/purification process from beginning to end.
Figure 1.
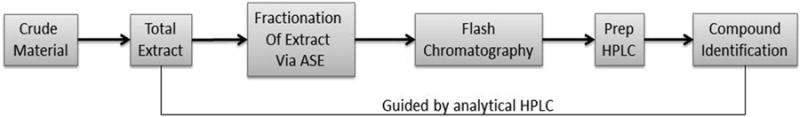
Workflow schematic of the HPLC-guided isolation of non-polymeric phenolic compounds of nutmeg kernel.
As a result of applying the HPLC-guided procedure to nutmeg kernel, twelve known compounds were isolated in various yields and were identified as: elemicin (1), isoelemicin (2), myristicin (4), surinamensin (5), malabaricone C (6), 2-(3′-allyl-2′,6′-dimethoxy-phenyloxy)-1-acetoxy-(3,4-dimethoxyphenyl)-propyl ester (7), methoxylicarin A (8), licarin A (9), malabaricone B (10), licarin C (11), 5′-methoxylicarin B (12), and licarin B (13). Another isolated compound was identified as 2-(3′-allyl-2′,6′-dimethoxy-phenyloxy)-1-methyl-5-methoxy-1,2-dihydrobenzofuran (3) and is being reported for the first time in the literature. Our rational approach towards isolating these compounds is described in this paper.
As shown in Figure 2, HPLC fingerprints of all purchased nutmeg products showed significant overlap with varying levels of the major markers. Thus, only one product was chosen for the isolation procedure (Mond Trading). Choice was based on product cost, availability and preference kernel over powder. From 370 g of the freshly powdered of nutmeg kernel, 52.2 g of DTE was obtained after initial extraction with MeOH, freeze defatting and concentration.
Figure 2.
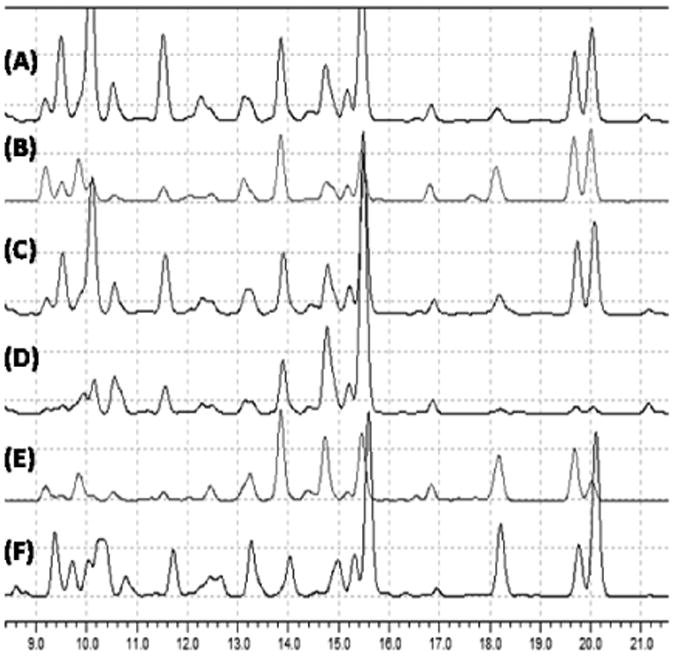
HPLC fingerprints of the total methanolic extracts of nutmeg samples obtained from different vendors; (A) Archer Farms powder, (B) Archer Farms whole, (C) McCormick powder, (D) Habash whole, (E) Mond powder, (F) Sharp Labs whole.
Successive solvent extraction of DTE resulted in three fractions: hexane (11.5 g), EtOAc (17.6 g), and MeOH (19.3 g). The EtOAc fraction showed an HPLC fingerprint (Figure 3A) of the major UV-active compounds of the total extract (Figure 2) and based on our recent work, it was most active in both FAAH and MAGL inhibition assays. The EtOAc fraction was thus chosen for further isolation procedures. Repeated MPLC of an aliquot of the EtOAc fraction (7.9 g) resulted in 12 sub-fractions (75% recovery, Figure 3B) whose TLC and HPLC fingerprints were aligned to generate a ‘roadmap’ to aid subsequent HPLC purification of the major constituent(s) present in each fraction (Figure 4B). The number of prominent HPLC peaks indicated at least a matching number of compounds in the EtOAc fraction distributed in sub-fractions obtained by MPLC (Figure 4B). Minor constituents could also be detected and were marked for possible isolation. All peaks were labeled in numerical order based on HPLC order of elution (Figure 3A and 4A).
Figure 3.
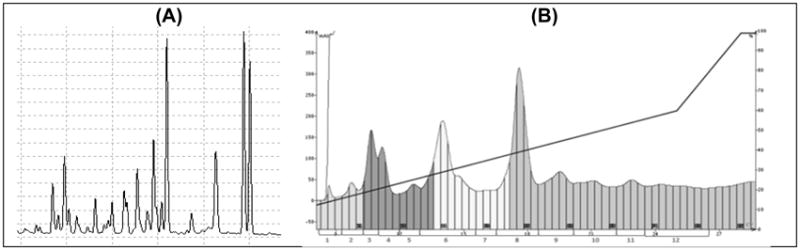
Chromatographic profiles of the EtOAc fraction of nutmeg; (A) HPLC fingerprint; and (B) MPLC flash chromatogram with marked subfractions for further purification.
Figure 4.
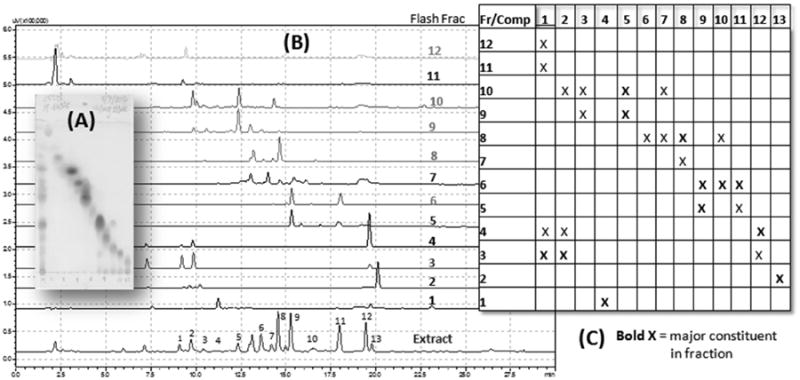
The ‘roadmap’ of isolation of nutmeg compounds present in the ethyl acetate fraction. (A) TLC, and (B) HPLC profiles of flash chromatography fractions; (C) target compounds in each fraction.
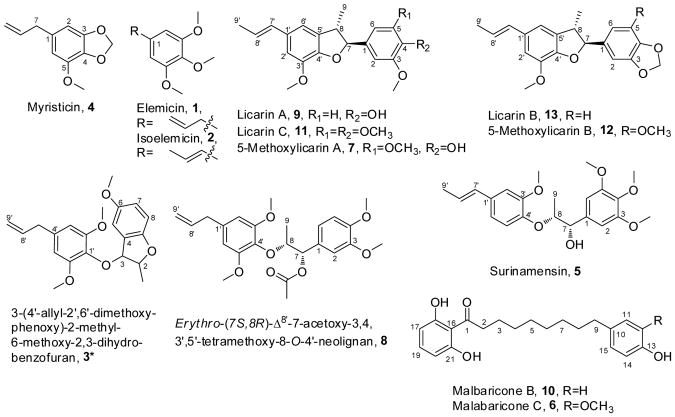
Repeated preparative HPLC of individual flash sub-fractions resulted in the isolation of 13 pure compounds that were identified by NMR and MS data analysis (Figure 5). Twelve are known phenolic constituents of nutmeg comprising phenylpropanoids, neolignans and diarylnonanoids. One compound was identified as a new phenylpropanoid dimer, 3, and is reported here for the first time. Significant steps of its structure elucidation are summarized below.
Figure 5.
Chemical structures of major and minor constituents isolated from the ethyl acetate fraction of nutmeg kernel. *New compound.
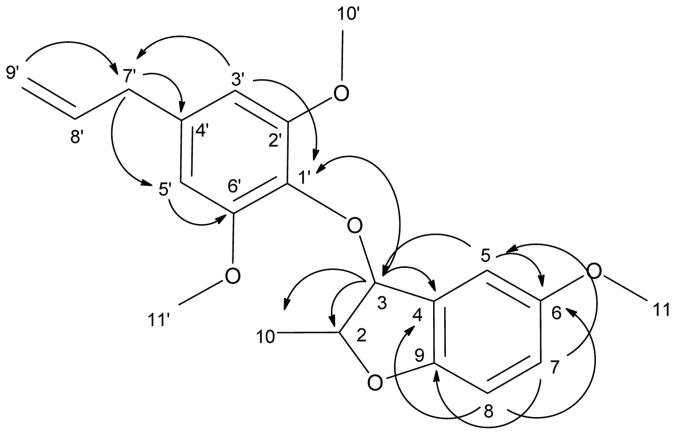
For compound 3, HRMS indicated a molecular formula of C21H24O5 suggesting a substituted phenylpropanoid dimer with 10 double-bond equivalents. HMQC and DEPT135 showed 4 methyl (1 CH3 & 3 OCH3), 2 methylene (one of which is olefinic), and 8 methine carbons (5 of which are aromatic/olefinic), and 7 quaternary carbons (by subtraction from the 13C spectrum, 5 of which are oxygenated). 1H and 13C NMR spectra indicated that one part of the molecule was an isoelemicin residue. Identity of the isoelemicin residue was confirmed by HMBC correlations between an olefinic methylene proton (δ 5.05) and the methylene carbon (δ 40.2) suggested a propenyl residue attached to a benzene ring, as shown by long-range couplings between the same carbon (δ 40.2) and two aromatic protons (δ 6.56). The two aromatic protons also exhibited long-range couplings with O-substituted aromatic carbons (δ 153.6 × 2 and δ 133.5). Two of the O-substituted carbons were coupled with methoxy protons (δ 3.83). The lack of coupling between the aromatic protons suggested a symmetric aromatic system with two m-substituted methoxy groups. Analysis of another set of HMBC correlations showed a second benzofuran aromatic system with one methoxy substituent whose protons (δ 3.78) were coupled with an aromatic O-substituted carbon (δ 147.2) and three aromatic protons in a 1,2,4 substitution pattern (δ 6.73, 6.67 and 6.94, respectively). The δ 6.94 proton showed long-range correlations with two O-substituted methine carbons (C-2 and C-3 at δ 82.1 and 72.9, respectively) belonging to the dihydrofuran ring. Further COSY and HMBC correlations established a methyl substituent (H-9) on C-2 (δ 1.00). The two ring systems were joined by an ether bridge between C-3 and C-1′ as supported by an HMBC correlation between H-2 and C-1′. Major HMBC correlations are shown in Figure 6. Thus, compound 3 was identified as 3-(4′-allyl-2′,6′-dimethoxy-phenyloxy)-2-methyl-6-methoxy-2,3-dihydrobenzofuran and is reported for the first time in the literature. Absolute stereochemistry at C-2 and C-3 is yet to be determined.
Figure 6.
Significant HMBC correlations of compound 3.
New compounds isolated from nutmeg continue to be reported. For example, Min et al. identified one new compound, (8R,8′S)-7′-(3′,4′-methylenedioxyphenyl)-8,8′-dimethyl-7-(3,4-dihydr-oxyphenyl)-butane, in a group of isolated lignans evaluated for inhibition of NO production [20]. More recently, Cao et al. isolated five new neolignans that were also evaluated against the same target [32]. In our case, we were able to isolate another new phenylpropanoid dimer from nutmeg in the course of this phytochemical study. The developed procedure was consistent on repeated application and chromatograms obtained from analytical HPLC, flash chromatography, as well as preparative HPLC were highly reproducible. An essential component of the ‘roadmap’ is HPLC fingerprinting, which provided an initial overall profile of the extract to direct the purification of individual compounds from their respective MPLC sub-fractions and to verify their identity after purification. The utilization of automation and programmed methods is another key factor for the reproducibility of the overall procedure. Thus, the need to follow a standard operating procedure becomes more significant. This approach may also be utilized with other plant extracts after specific alterations to analytical methods, such as solvents, detection and type of columns, depending on the chemical profile/constituents of each plant. Compounds consistently isolated using this standard procedure may be used (i) for biological evaluation, (ii) as starting material for chemical derivatization or (iii) as authentic standards for quantitative analytical methods for product quality control and/or pharmacokinetic studies. Our lab is currently pursuing a number of these leads. The ‘roadmap’ approach can also provide shortcuts to specific compounds of interest (depending on nature of investigation) without the need to isolate the remaining ones. However, some of the limitations of this procedure include instrument capacity thresholds and the need to run multiple batches of the successive extraction and isolation steps in order to obtain sufficient quantities of target compounds especially minor constituents. Also, some of the compounds identified in the HPLC fingerprint could not be isolated in a pure form because of lack of column resolution or potential instability. Minor modifications are currently being applied to our procedure to enhance its ability to resolve this issue.
In summary, medicinal plant extracts comprise complex mixtures of secondary metabolites, many of which may be valuable for drug discovery and/or phytochemical profiling and quality control. Isolation and purification of plant secondary metabolites is a challenging task especially with the level of complexity encountered with these types of samples and the skill needed to perform isolation procedures. In an attempt to overcome such a challenge, a reproducible HPLC-guided isolation/purification procedure was developed that is relatively fast and more approachable by less experienced personnel. The procedure was applied to nutmeg which has a complex profile of phenolic secondary metabolites with potential medicinal value. A total of 13 compounds were isolated in varying yields and, among these, one new compound has been identified and is reported for the first time. The isolated compounds are utilized in our lab as standards for development of quantitative analytical methods and as leads for biological evaluation in a number of assays. The reported approach has also been adapted for use with other medicinal plants currently being investigated in our lab.
Experimental
Plant material, chemicals and reagents
Six nutmeg products were obtained in whole kernel or powdered form from the following suppliers: Archer Farms, Minneapolis, MN (Target Brands whole and powder), Habash Trading, Bedford Park, IL (whole), McCormick & Co., Hunt Valley, MD (McCormick Gourmet powder), Mond Trading, Toronto, ON (whole), and Sharp Labs, Park Orange, FL (whole).
Instruments
Successive solvent extraction was performed on an ASE 150 instrument (Thermo Scientific,) with the following settings for each solvent: number of static cycles, 3; static time, 10 min; temperature, 50°C; purging time, 100 sec; rinse volume: 60%. Approximately 120 mL were collected for each solvent. Analytical HPLC and molecular weight determinations were performed on an LC-MS instrument equipped with UV and MS detectors (LCMS-2020, Shimadzu, Japan) using an RP column (HyPurity®, C18, 3 μm, 150 × 4.6 mm, Thermo Scientific) with a SecurityGuard cartridge (C18, 4.0 × 3.0 mm, Phenomenex, Torrance, CA). Solvents: acetonitrile (solvent A) and 0.1% aqueous formic acid. Gradient: 40% solvent A 2 min, to 80% in 22 min, to 100% in 0.01 min, at 100% for 2 min, to 40% in 0.01 min, maintain at 40% for 4 min (total run time 30 min); flow rate, 1 mL/min; detection, 270 nm; injection volume, 10 μL. Preparative HPLC was performed on a Prominence system (Shimadzu) equipped with UV detector using an RP column (Luna®, C18, 5 μ, 250 × 1.0 mm, Phenomenex). Solvents: acetonitrile (solvent A) and 0.1% aqueous formic acid. Two different gradients were used depending on the complexity of each fraction. Gradient I: 40% solvent A 2 min, to 100% in 30 min, at 100% for 2.0 min, to 40% in 0.01 min, maintain at 40% for 6 min (total run time 40 min). Gradient II: 40% solvent A 40 min, to 70% in 30 min, to 100% in 0.01 min, at 100% for 4.0 min, to 40% in 0.01 min, maintain at 40% for 6 min (total run time 80 min). Flow rate, 5 mL/min; detection, 270 nm; injection volume, 500 μL. Both HPLC systems were controlled by LabSolutions® software (Shimadzu) running under Microsoft Windows 7. Medium pressure flash liquid chromatography (MPLC) of the EtOAc fraction was performed on an Isolera™ One system (Biotage, Sweden) using prepacked 50 g HP-silica column and a linear gradient of EtOAc in n-hexane (0-60% in 30 min). Flow rate, 25 mL/min; detection at 270 nm; sample size, 500 mg dissolved in 2 mL EtOAc applied to a 10 g silica cartridge and dried before inserting on column; fraction volume, 22 mL with similar fractions pooled and concentrated at 45°C under reduced pressure. NMR analysis was performed on a JNM-ECS400 spectrometer (JEOL, Peabody, MA) at 400 and 100 MHz for 1H and 13C experiments, respectively. Chemical shifts were recorded in ppm (δ unit) relative to TMS. HRMS data for compound 3 were recorded on a TOF 6224 accurate-mass LCMS (Agilent, Santa Clara, CA). Thin-layer chromatography was utilized as a secondary fingerprinting technique and was performed on silica gel 60 plates (precoated F254, Merck, Darmstadt, Germany) with n-hexane-EtOAc (65:35, v:v) as a developing solvent and detection at 254 nm followed by dipping in 10% methanolic H2SO4 and heating at 100°C.
Preparation and fingerprinting of total extracts
A preliminary small-scale extraction was conducted for each nutmeg product as follows: 5 g was ground in a coffee blender (or used as is for purchased powder), soaked in 50 mL methanol (MeOH), ultrasonicated for 30 min and filtered. The solid material was re-extracted twice using the same procedure and all filtrates were combined. The filtered extracts were stored overnight at -20°C and the solid lipid precipitate was subsequently filtered out. The defatted filtrates were fingerprinted by HPLC (Figure 2). One product (Mond nutmeg) was selected for scaled up extraction using 370 g of sample soaked in MeOH (1000 mL × 3). The combined filtrates were concentrated at 45°C under reduced pressure in a rotary evaporator to produce a defatted total extract (DTE).
Preparation of solvent fractions
Four batches of successive solid phase extractions were performed with n-hexane, EtOAc and MeOH in a programmable ASE 150 unit. Each batch used 12.0 g of DTE thoroughly mixed with 24 g silica to form a homogenous flowing powder. The DTE-coated silica was packed in a 100 mL stainless steel cylinder which was inserted in the ASE 150 and extracted with each of the above mentioned solvents starting with n-hexane. A thin layer of Ottawa sand was packed above and below the sample to enhance solvent flow through the cylinder. After each solvent extraction run, the extract was removed, the following solvent introduced and the extraction program restarted. All solvent fractions were concentrated under vacuum at 45°C in a rotary evaporator then moved to pre-weighed capped vials.
MPLC of EtOAc fraction
An aliquot of the EtOAc fraction (7.9 g) was divided into 500 mg samples and each was subjected to flash MPLC (Isolera 1 System, Biotage, Sweden) under the following conditions: cartridge, silica HP 50 g; gradient, EtOAc (A) in n-hexane, 0% (A) for 198 mL to equilibrate, 0% (A) for 264 mL, 0% to 60% (A) for 1386 mL, 60% (A) to 100% (A) for 198 mL, and staying at 100% (A) for 66 mL; flow rate, 50 mL/min; detection, 270 nm; fraction volume, 22 mL. Twelve sub-fractions were collected by combining eluates corresponding to each major peak of the generated chromatogram. Each sub-fraction was concentrated using the rotary evaporator, transferred into pre-weighed capped vials and dried at 45°C in a Centrifan PE-T evaporator (kd Scientific, Holliston, MA). HPLC analysis was performed on each sub-fraction and compared with the total fingerprint to identify the major constituent(s) in each sub-fraction.
Isolation of pure compounds
A 10% solution of each MPLC sub-fraction was prepared in MeOH and successive preparative HPLC purification was performed with 500 μL sample injection per run. Based on sub-fraction complexity, gradient I was used for sub-fractions 1-5 & 11-12, while gradient II was used for fractions 6-10. Flow rate, 5 mL/min; detection, 270 nm; manual collection of individual peaks into labeled test tubes. Similar fractions combined from separate runs were concentrated at 45°C in a Centrifan concentrator in capped pre-weighed labeled vials. Purity of each sample was verified by analytical HPLC before obtaining NMR data. The isolated compounds were as follows:
Elemicin (1) [23]
Yield: 55 mg.
MS: m/z = 209 [M+H]+ (calcd for C12H17O3: 209).
Isoelemicin (2) [24]
Yield: 22 mg.
MS: m/z = 209 [M+H]+ (calcd for C12H17O3: 209).
3-(4′-Allyl-2′,6′-dimethoxy-phenyloxy)-1-methyl-5-methoxy-1,2-dihydrobenzofuran (3)
Yield: 35 mg.
1H NMR (acetone-d6) δ: 1.00 (3H, d, J=6.41 Hz, H-10), 3.34 (2H, d, J = 6.87 Hz, H-7′), 3.78 (3H, s, H-11), 3.83 (6H, s, H-10′,11′), 4.71 (1H, d, J = 3.21 Hz, H-3), 4.29 (1H, dq, J = 6.41,3.21 Hz, H-2), 6.56 (2H, s, H- 3′,5′), 6.94 (1H, d, J = 1.37 Hz, H-5), 6.73 (1H, d, J = 8.24 Hz, H-8), 5.01 (1H, dd, J = 10.07,1.37 Hz, H-9′cis), 5.09 (1H, dd, J = 16.94,1.37 Hz, H-9′trans), 5.97 (1H, m, H-8′), 6.68 (1H, dd, J = 8.24,1.83 Hz, H-7).
13C NMR (acetone-d6) δ: 12.6 (C-10), 40.2 (C-7′), 55.4 (C-11), 55.6 (C-10′,11′), 72.9 (C-3), 82.1 (C-2), 105.7 (C-3′,5′), 109.6 (C-5), 114.5 (C-8), 115.2 (C-9′), 118.6 (C-7), 132.4 (C-4), 133.5 (C-1′), 136.0 (C-4′), 137.7 (C-8′), 145.4 (C-9), 147.2 (C-6), 153.6 (C-2′,6′).
HRMS: m/z = 357.1694 [M+H]+ (calcd. for C21H25O5: 357.1702).
Myristicin (4) [25]
Yield: 10 mg.
MS: m/z = 193 [M+H]+ (calcd for C11H13O3: 193).
Surinamensin (5) [26]
Yield: 10 mg.
MS: m/z = 371 [M+H-H2O]+ (calcd for C22H27O5: 371).
Malabaricone C (6) [27]
Yield: 22 mg.
MS: m/z = 359 [M+H]+ (calcd for C21H27O5: 359).
5-Methoxylicarin A (odoratisol A, 7) [28]
Yield: 12 mg.
MS: m/z = 357 [M+H]+ (calcd for C21H25O5: 357).
Erythro-(7S,8R)-Δ8′-7-acetoxy-3,4,3′,5′-tetramethoxy-8-O-4′-neolignan (8) [29]
Yield: 18 mg.
MS: m/z = 431 [M+H]+ (calcd for C24H31O7: 431).
(1)
Licarin A (dehydrodiisoeugenol (9) [30]
Yield: 10 mg.
MS: m/z = 327 [M+H]+ (calcd for C20H23O4: 327).
Malabaricone B (10) [27]
Yield: 15 mg.
MS: m/z = 343 [M+H]+ (calcd for C21H27O4: 343).
Licarin C (11) [31]
Yield: 26 mg.
MS: m/z = 371 [M+H]+ (calcd for C22H27O5: 371).
5-Methoxylicarin B (12) [21]
Yield: 34 mg.
MS: m/z = 355 [M+H]+ (calcd for C21H23O5: 355).
(2)
Licarin B (13) [30]
Yield: 50 mg.
MS: m/z = 325 [M+H]+ (calcd for C20H21O4, M.W. 325).
Acknowledgments
This project was supported by the National Institute on Drug Abuse of the National Institutes of Health (NIDA-NIH) under Award Number R24DA036410. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors wish to thank Dr Nataliya Sidelnikova and Ms Trijaye McDowell for their assistance during earlier phases of the project.
References
- 1.Khan IA, Abourashed EA, editors. Leung's Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 3rd. Wiley; Hoboken, NJ: 2010. pp. 467–470. [Google Scholar]
- 2.Asgarpanah J, Kazemivash N. Phytochemistry and pharmacologic properties of Myristica fragrans Houtt.: A review. African Journal of Biotechnology. 2012;11:12787–12793. [Google Scholar]
- 3.Fetrow CW, Avila JR. Complementary & Alternative Medicines. Vol. 762 Springhouse; Springhouse, Pennsylvania: 1999. [Google Scholar]
- 4.Du SS, Yang K, Wang CF, You CX, Geng ZF, Guo SS, Deng ZW, Liu ZL. Chemical constituents and activities of the essential oil from Myristica fragrans against cigarette beetle Lasioderma serricorne. Chemistry & Biodiversity. 2014;11:1449–1456. doi: 10.1002/cbdv.201400137. [DOI] [PubMed] [Google Scholar]
- 5.Ehlers D, Kirchhoff J, Gerard D, Quirin KW. High-performance liquid chromatography analysis of nutmeg and mace oils produced by supercritical CO2 extraction - comparison with steam-distilled oils - comparison of East Indian, West Indian and Papuan oils. International Journal of Food Science Technology. 1998;33:215–223. [Google Scholar]
- 6.Pandey R, Rameshkumar KB, Kumar B. Ultra high performance liquid chromatography tandem mass spectrometry method for the simultaneous determination of multiple bioactive constituents in fruit extracts of Myristica fragrans and its marketed polyherbal formulations using a polarity switching technique. Journal of Separation Science. 2015;38:1277–1285. doi: 10.1002/jssc.201401297. [DOI] [PubMed] [Google Scholar]
- 7.Tripathi IP, Dwivedi N. Pharmacognostical standardization of nutmeg seeds (Myristica fragrans Houtt.) – a traditional medicine. International Journal of Pharmaceutical Sciences and Research. 2015;6:3096–3102. [Google Scholar]
- 8.Dighe V, Charegaonkar G. HPTLC analysis of myristicin and safrole in seed powder of Myristica fragrans Houtt. Journal of Planar Chromatography. 2009;22:445–448. [Google Scholar]
- 9.Li F, Yang XW. Analysis of anti-inflammatory dehydrodiisoeugenol and metabolites excreted in rat feces and urine using HPLC-UV. Biomedical Chromatography. 2012;26:703–707. doi: 10.1002/bmc.1717. [DOI] [PubMed] [Google Scholar]
- 10.Jellin JM, Gregory PJ, Batz F, Hitchens K. Pharmacist's Letter/Prescriber's Letter Natural medicines Comprehensive Database. 5th. Vol. 2071 Therapeutic Research Faculty; Stockton, CA: 2003. [Google Scholar]
- 11.Antonio RL, Kozasa EH, Galduroz JC, Dawa, Dorjee Y, Kalsang T, Norbu T, Tenzin T, Rodrigues E. Formulas used by Tibetan doctors at Men-Tsee-Khang in India for the treatment of neuropsychiatric disorders and their correlation with pharmacological data. Phytotherapy Research. 2013;27:552–563. doi: 10.1002/ptr.4749. [DOI] [PubMed] [Google Scholar]
- 12.Weil AT. The use of nutmeg as a psychotropic agent. Bulletin on Narcotics. 1966;18:15–23. [Google Scholar]
- 13.Nagano I. Do you know about the narcotic effects of nutmeg? 2009 http://www.alternet.org/story/140480/. Last accessed on 8/23/2015.
- 14.Goncalves JLS, Lopes RC, Oliviera DB, Costa SS, Miranda MM, Romanos MT, Santos NS, Wigg MD. In vitro anti-rotavirus activity of some medicinal plants used in Brazil against diarrhea. Journal of Ethanopharmacology. 2005;99:403–407. doi: 10.1016/j.jep.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen PH, Le TV, Kang HW, Chae J, Kim SK, Kwon KI, Seo DB, Lee SJ, Oh WK. AMP-activated protein kinase (AMPK) activators from Myristica fragrans (nutmeg) and their anti-obesity effect. Bioorganic & Medicinal Chemistry Letters. 2010;20:4128–4131. doi: 10.1016/j.bmcl.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 16.El-Alfy AT, Wilson L, Elsohly MA, Abourashed E. Towards a better understanding of the psychopharmacology of nutmeg: Activities in the mouse tetrad assay. Journal of Ethnopharmacology. 2009;126:280–286. doi: 10.1016/j.jep.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagaraju B, Sahar SH, Ali B, Kushnoor NZ, Zahra A, Zothanmawia C, Surendranatha A. Anxiolytic effect of Myristica fragrans. International Journal of Phytotherapy Research. 2013;3:1–7. [Google Scholar]
- 18.Baker I, Chohan M, Opara EI. Impact of cooking and digestion, in vitro, on the antioxidant capacity and anti-inflammatory activity of cinnamon, clove and nutmeg. Plant Foods for Human Nutrition. 2013;68:364–369. doi: 10.1007/s11130-013-0379-4. [DOI] [PubMed] [Google Scholar]
- 19.Tajuddin AS, Latif A, Qasmi IA, Amin KM. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg) BMC Complementary and Alternative Medicine. 2005;5:1–7. doi: 10.1186/1472-6882-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min BS, Cuong TD, Hung TM, Min BK, Shin BS, Woo MH. Inhibitory effect of lignans from Myristica fragrans on LPS-induced NO production in RAW264.7 cells. Bulletin of the Korean Chemical Society. 2011;32:4059–4062. [Google Scholar]
- 21.Cao GY, Yang XW, Xu W, Li F. New inhibitors of nitric oxide production from the seeds of Myristica fragrans. Food and Chemical Toxicology. 2013;62:167–171. doi: 10.1016/j.fct.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 22.Cuong TD, Hung TM, Han HY, Roh HS, Seok JH, Lee JK, Jeong JY, Choi JS, Kim JA, Min BS. Potent acetylcholinesterase inhibitory compounds from Myristica fragrans. Natural Product Communications. 2014;9:499–502. [PubMed] [Google Scholar]
- 23.Medina AL, Lucero ME, Holguin FO, Estell RE, Posakony JJ, Simon J, O'Connell MA. Composition and antimicrobial activity of Anemopsis californica leaf oil. Journal of Agricultural and Food Chemistry. 2005;53:8694–8698. doi: 10.1021/jf0511244. [DOI] [PubMed] [Google Scholar]
- 24.Grice ID, Rogers KI, Griffiths LR. Isolation of bioactive compounds that relate to the anti-platelet activity of Cymbopogon ambigus. Evidence-Based Complementary and Alternative Medicine. 2011;2011:1–8. doi: 10.1093/ecam/nep213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen PH, Kang HW, Le TVT, Chae J, Kim SK, Kwon KI, Lim SI, Oh WK. Simple process for the decrease of myristicin content from Myristica fragrans (nutmeg) and its activity with AMP-activated protein kinase (AMPK) Journal of Food Biochemistry. 2011;35:1715–1722. [Google Scholar]
- 26.Francis KS, Suresh E, Nair MS. Chemical constituents from Myristica fragrans fruit. Natural Product Research. 2014;28:1664–1668. doi: 10.1080/14786419.2014.934236. [DOI] [PubMed] [Google Scholar]
- 27.Hou JP, Wu H, Wang Y, Weng XC. Isolation of some compounds from nutmeg and their antioxidant activities. Czech Journal of Food Science. 2012;30:164–170. [Google Scholar]
- 28.Giang PM, Son PT, Matsunami K, Otsuka H. New neolignans and lignans from Vietnamese medicinal plant Machillus odoratissima Nees. Chemical & Pharmaceutical Bulletin. 2006;54:380–383. doi: 10.1248/cpb.54.380. [DOI] [PubMed] [Google Scholar]
- 29.Duan L, Tao HW, Hao XJ, Gu QQ, Zhu WM. Cytotoxic and antioxidative phenolic compounds from the traditional Chinese medicinal plant, Myristica fragrans. Planta Medica. 2009;75:1241–1245. doi: 10.1055/s-0029-1185506. [DOI] [PubMed] [Google Scholar]
- 30.Leon-Diaz R, Meckes M, Said-Fernandez S, Molina-Salinas GM, Vargas-Villarreal J, Torres J, Luna-Herrera J, Jimenez-Arellanes A. Antimycobacterial neolignans isolated from Aristolochia taliscana. Memorias Do Instituto Oswaldo Cruz. 2010;105:45–51. doi: 10.1590/s0074-02762010000100006. [DOI] [PubMed] [Google Scholar]
- 31.Aiba CJ, Gottlieb OR, Pagliosa FM, Yoshida M, Magalhaes MT. Neolignans from Nectandra miranda. Phytochemsitry. 1977;16:745–748. [Google Scholar]
- 32.Cao GY, Xu W, Yang XW, Gonzalez FJ, Fei L. New neolignans from the seeds of Myristica fragrans that inhibit nitric oxide production. Food Chemistry. 2015;173:231–237. doi: 10.1016/j.foodchem.2014.09.170. [DOI] [PMC free article] [PubMed] [Google Scholar]