Abstract
Background
Esophageal cancer is a common and aggressive malignant tumor. This study aimed to investigate the association between human papillomavirus (HPV) Types 16 and 18 and esophageal carcinoma (EC) in the world population by conducting a meta-analysis.
Materials and Methods
Computerized bibliographic and manual searches were performed to identify all eligible literatures between 1982 and 2014. PUBMED (http://www.ncbi.nlm.nih.gov/pubmed/) and CNKI (http://www.cnki.net/) were the primary sources of case-control studies, and key words used include human papillomavirus, HPV, esophageal, esophagus, cancer, carcinoma, and tumor. All searches were performed by reviewing articles and abstracts cited in the published systematic reviews and case-control studies. Prospective studies that reported relative risk (RR) estimates with 95% CIs for the association between HPV and EC were included.
Results
Thirty-three randomized studies were identified, and the main features of these trials were included in this systematic review. HPV infection rate in the EC group was 46.5%, while HPV infection rate in the control group was 26.2% (OR = 1.62; 95% CI, 1.33–1.98). In China, the merger OR value was 1.62 (95% CI: 1.26–2.07); while in the Asian region, the merger OR value was 1.63 (95% CI: 1.29–2.04). There were statistical differences in HPV testing due to different detection methods such as PCR, IHC and ISH. In the PCR detection group, the merger OR value was 1.61 (95% CI: 1.33–1.95).
Conclusions
These results indicate that HPV infection and the incidence of EC are closely associated.
Introduction
Esophageal carcinoma (EC) is the most aggressive malignant tumor of the gastrointestinal tract and the eighth most commonly occurring cancer in the world [1]. It has been well recognized that the development of EC involves multiple factors in a multistage process [2]. Alcohol, tobacco, nutritional deficiencies, infectious agents, etc. were confirmed to have a relationship to esophageal carcinogenesis [3]. However, many physical, chemical and biological factors related to EC remain unknown.
Human papillomavirus (HPV) infections, especially high-risk types 16 and 18, have recently been reported as a possible risk factor for EC. However, direct evidence of this relationship has been lacking, and results of those studies were not consistent. This study aimed to conduct a meta-analysis, and systematic review of literature to determine whether an association exists between HPV type 16 and 18 infection and EC.
Materials and Methods
Data Sources
A literature search was performed from 1982 to 2014 using PUBMED and CNKI databases without restrictions, and the following search terms were used: (human papillomavirus, HPV) and (esophageal, esophagus) and (cancer, carcinoma, tumor). Moreover, reference lists were reviewed to search for relevant studies. This systematic review was planned and reported in adherence to the standards of quality for reporting systematic reviews.
Inclusion and Validity Criteria
All searches were performed by reviewing articles and abstracts cited in the published systematic reviews and case-control studies. Inclusion criteria were as follows: (1) prospective case-control studies, (2) EC diagnosed by pathology, (3) Control group obtained from esophageal epithelial tissues of normal individuals (screening) or normal marginal tissues of EC.
Exclusion criteria
Exclusion criteria included the following: reports of poor quality, duplicate reports, inadequate information, unclear data descriptions or samples were removed, sample size less than 20, and abstracts.
Quality evaluation
The Jadad scoring method was used to evaluate several aspects of the quality of the study. Three independent researchers extracted data, blindly evaluated the quality of the literature, and analyzed the data including withdrawals and dropouts [4]. If there were differences of opinion, the cases were discussed until consensus was achieved.
Statistical Analysis
A heterogeneity test was used to select the method of data combination in the systematic review. The Cochrane’s Q-test was performed and I2 statistics were obtained, using a predefined significance threshold of 0.05.If P≥0.05, it was considered that there was no heterogeneity between studies; and a fixed effects model (FEM) was used for the analysis. If P<0.05, it was considered that heterogeneity existed between studies; and a random effects model (REM) was used after correction for analysis. If a null hypothesis, which represents that there was no heterogeneity among each study, was accepted, the Mantel-Haenszel fixed-effects model was used to calculate the combined odds ratio (OR) with 95% confidence intervals (CI) and the Forest Plot. If the null hypothesis was rejected, REM was used to calculate the combined OR with 95% CI and the Forest Plot. To detect publication bias, the asymmetry of standard error–based funnel plots was examined using the linear regression method, as suggested by Egger et al. [5]. The Stata10.0 software (Stata Corporation, College Station, Texas) was used for the statistical analysis in this study.
Subgroup analyses
EC incidence in subgroups were stratified and analyzed according to various geographical areas, the control group selection method, and various HPV detection methods.
Results
Search Results
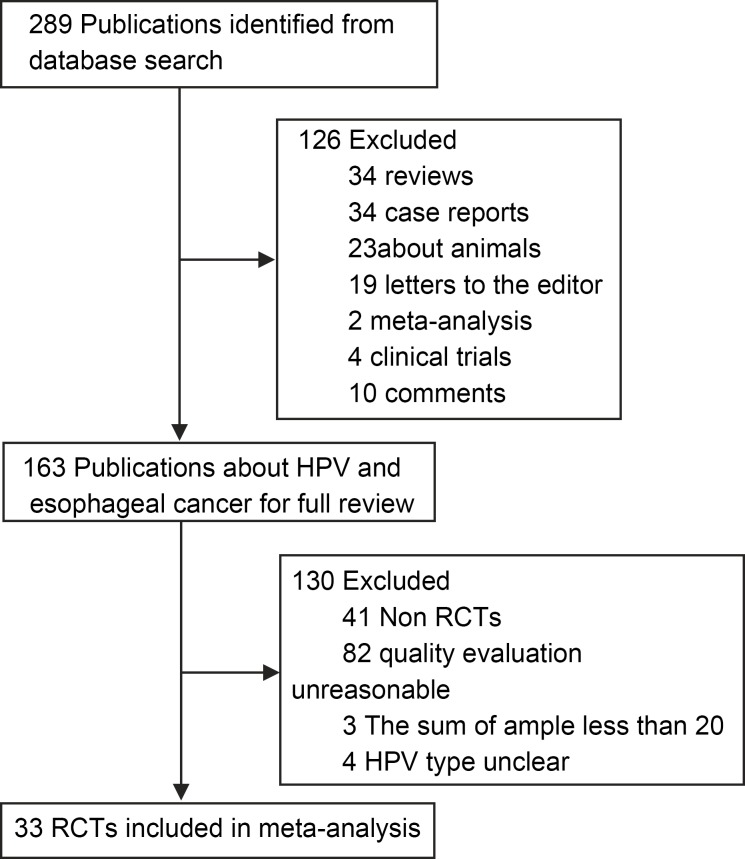
A total of 297 articles were identified by using the search criteria, and these studies were carried out from 1982 to 2014. All studies were obtained from published literature (Fig 1). Nine countries including China, Iran, Italy, Greece, Egypt, Sweden, Japan, France and Mexico were involved in the case-control studies. A total of 33 case-control studies were selected for analysis. There was no statistical significance in factors such as gender or age between the two groups. The PRISMA checklist is shown in S1 PRISMA Checklist.
Fig 1. Selection of studies for inclusion in the meta-analysis.
Information of articles
Detailed steps of the literature search are shown in Fig 1. The inclusion and exclusion of case-control studies for this systematic review are shown in the flow chart. Briefly, 33 articles matched the standard [6–38]. Among the 2,430 cases in the EC group, 1,131 were HPV positive (46.54%); while among the 3,621 cases in the control group, 977 were HPV positive (26.98%). In the control group, 14 samples came from adjacent normal tissues of gastrointestinal cancers, while 19 samples came from normal esophageal specimens. These samples were obtained during the esophageal cancer screening of healthy people living in areas of high incidence of esophageal cancer (Table 1).
Table 1. Characteristics of the 33 case-control studies included in the meta-analysis.
| Literature sources | HPV(+)/ | HPV(+)/ | Area | Incidence | Detection Method | Control selection | HPV types |
|---|---|---|---|---|---|---|---|
| cases group | control group | ||||||
| Li Y, 1991 | 12/24 | 9/24 | China | Unclear | ISH | Adjacent-normal | 16 |
| Han C, 1996 | 22/90 | 6/121 | China | High | ELISA | True-normal | 16 |
| Ren ZHP, 1996 | 35/52 | 2/30 | China | Lower | IHC | True-normal | 16,18 |
| Wang XJ, 1998 | 20/40 | 36/58 | China | High | ISH | True-normal | 16 |
| Lu LC, 1999 | 15/55 | 43/55 | China | High | ISH | Adjacent-normal | 18 |
| Cao BW 2005 | 207/265 | 203/357 | China | High | PCR | True-normal | 16, 18 |
| Liu J, 2000 | 44/60 | 23/56 | China | High | IHC | True-normal | 16,18 |
| Yu DH, 2000 | 78/112 | 42520 | China | Lower | IHC | True-normal | 16,18 |
| Kuang ZS, 2000 | 23/56 | 4/56 | China | High | PCR | Adjacent-normal | 16,18 |
| Sun LB, 2001 | 42542 | 42390 | China | Lower | PCR-RT | Adjacent-normal | 16 |
| Shen ZY, 2002 | 115/176 | 105/176 | China | High | PCR | Adjacent-normal | 6, 11, 16, 18 |
| Xu WG, 2003 | 28/40 | 10/50 | China | Unclear | ISH | True-normal | 16 |
| Zhou XB, 2003 | 31/48 | 42605 | China | High | PCR | Adjacent-normal | 16 |
| Xu CL, 2004 | 16/18 | 126/183 | China | Lower | IHC | Adjacent-normal | 16 |
| Lu HS, 2004 | 3/40 | 13/39 | China | Lower | IHC | Adjacent-normal | 16 |
| Lu XM, 2004 | 55/104 | 41/104 | China | High | PCR | Adjacent-normal | 16 |
| Chen J, 2004 | 14/30 | 7/60 | China | Lower | PCR | True-normal | 16 |
| Jiang HY, 2005 | 48/65 | 11/65 | China | Lower | IHC | Adjacent-normal | 16,18 |
| Kamangar F, 2006 | 33/99 | 106/381 | China | High | ELISA | True-normal | 16,18,73 |
| Zhou SM, 2009 | 26/82 | 10/80 | China | High | PCR | Adjacent-normal | 16 |
| Guo F,2012 | 93/300 | 61/900 | China | High | PCR | True-normal | 16,18,58 |
| Bahnassy AA, 2005 | 27/50 | 12/50 | Egypt | Unclear | PCR | Adjacent-normal | 16,18,11 |
| Benamouzig R, 1992 | 42502 | 42393 | France | Unclear | ISH | True-normal | 6, 11, 16, 18,31, 33, |
| Lyronis, 2008 | 17/30 | 42548 | Greece | Unclear | PCR | True-normal | 16,18 |
| Farhadi M, 2005 | 14/38 | 5/38 | Iran | High | PCR | True-normal | L1,18 |
| Far AE, 2007 | 33/140 | 12/140 | Iranian | Unclear | PCR | Adjacent-normal | 16.18.31.33 |
| Astori G, 2001 | 42568 | 42476 | Italy | High | PCR | Adjacent-normal | 16 |
| Tornesello ML, 2009 | 12/56 | 42609 | Italy | Unclear | PCR | True-normal | 6,16,8, 15, 20,25 |
| Khurshid A, 1998 | 17/27 | 42441 | Japan | Unclear | PCR | True-normal | 16,18,33 |
| Kawaguchi H, 2000 | 42721 | 25/58 | Japan and China | Unclear | PCR | True-normal | 16/18 |
| Dąbrowski A, 2012 | 28/56 | 4/35 | Lublin | Unclear | PCR | True-normal | 16,18 |
| Acevedo, NE, 2004 | 15/17 | 42544 | México | Unclear | PCR | True-normal | 16,11 |
| Lagergren J, 1999 | 20/193 | 61/302 | Sweden | Unclear | ELISA | True-normal | 16,18 |
Lower: lower incidence of esophageal carcinoma (EC); Higher: higher incidence of EC; PCR: polymerase chain reaction; IHC: immunohistochemistry; ISH: in situ hybridization; ELISA: enzyme-linked immunosorbent assay. Adjacent-normal: the control group was obtained from the normal marginal tissue of EC during surgery. True-normal: controls were obtained from the normal esophageal epithelial tissue.
Heterogeneity test
Thirty-three tests for heterogeneity (i = 94.78, P<0.001) revealed that there was heterogeneity between studies and between subgroups. Therefore, overall and subgroup analyses were corrected using REM; and the method of DerSimonian-Laird as used to merge data, and calculate ORs and 95% CIs. Egger’s regression analysis was used to more objectively evaluate publication bias (Table 2).
Table 2. Quality assessment and subgroup analysis of HPV infection and esophageal carcinoma.
| Group | No. of Studies | Heterogeneity of ORs | Model used | Egger's test | ||||
|---|---|---|---|---|---|---|---|---|
| χ2 (df) | P | OR (95% CI) | χ2 | P | 95% CI | |||
| ALL | 33 | 78.4 | <0.001 | 1.45 (1.34–1.57) | REM | 2.05 | 0.058 | -0.01–2.64 |
| Area | ||||||||
| High | 13 | 31.93 (11) | <0.001 | 2.96 (2.01–4.34) | REM | 1.71 | 0.173 | -0.61–3.03 |
| Lower | 7 | 56.21 (7) | <0.001 | 3.57 (1.15–11.12) | REM | 0.37 | 0.443 | -2.90–5.68 |
| China | 21 | 31.93 (12) | <0.001 | 2.96 (2.01–4.35) | REM | 1.69 | 0.130 | -0.46–3.33 |
| Non-China | 12 | 56.21 (8) | <0.001 | 3.57 (1.15–11.13) | REM | 0.37 | 0.558 | -4.35–2.49 |
| Asia | 24 | 31.93 (13) | <0.001 | 2.96 (2.01–4.36) | REM | 1.73 | 0.090 | -0.24–0.017 |
| Non-Asia | 9 | 56.21 (9) | <0.001 | 3.57 (1.15–11.14) | REM | 0.37 | 0.091 | -0.67–7.04 |
| Method | ||||||||
| PCR | 18 | 20.39 (10) | 0.030 | 3.49 (2.49–4.90) | REM | 1.25 | 0.012 | 0.91–2.38 |
| IHC | 6 | 38.43 (5) | <0.001 | 4.76 (1.36–16.63) | REM | 0 | 0.659 | -5.02–7.10 |
| ELSIA | 4 | 20.02 (2) | <0.001 | 0.90 (0.13–6.48) | REM | 0 | 0.666 | -74.37–81.47 |
| ISH | 5 | 19.03(2) | <0.001 | 0.90 (0.15–6.42) | REM | 0 | 0.254 | -4.30–11.12 |
| Self | ||||||||
| Self | 14 | 56.09 (9) | <0.001 | 2.18 (1.02–4.66) | REM | 0.89 | 0.200 | -0.80–3.41 |
| NO | 19 | 36.69 (9) | <0.001 | 4.58 (2.69–7.80) | REM | 2.33 | 0.102 | -0.32–3.22 |
PCR: polymerase chain reaction; IHC: immunohistochemistry; ELISA: enzyme-linked immunosorbent assay; ISH: in situ hybridization; REM: random effects model; OR: odds ratio; CI: confidence interval.
The relationship of HPV types 16 and 18 and EC
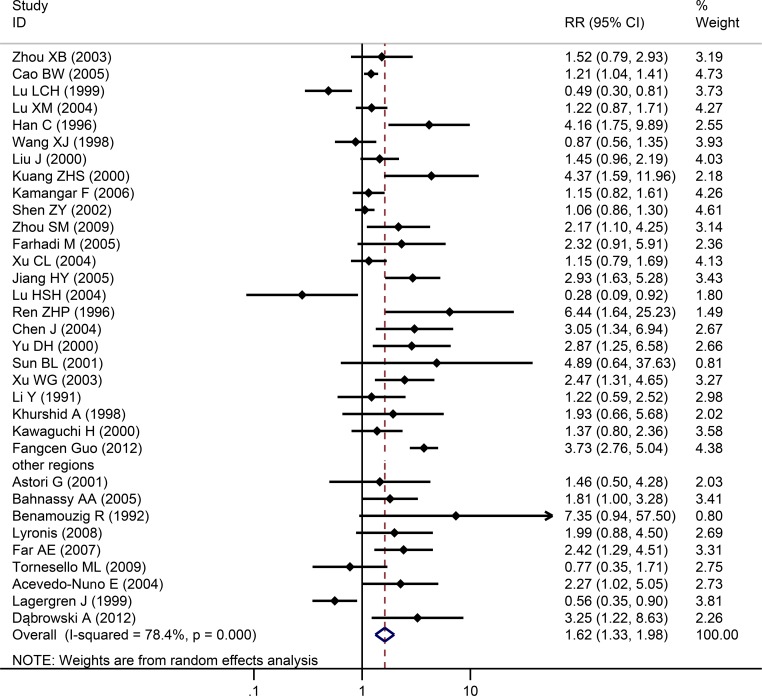
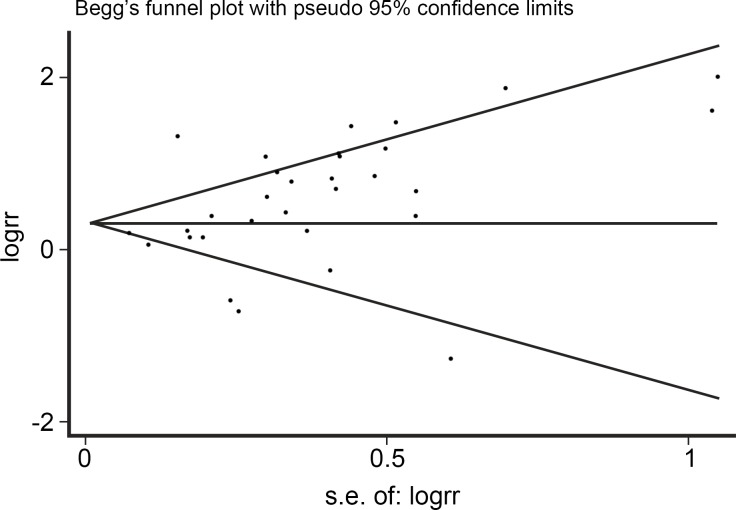
Among the 33 studies, infection rate in the EC group was 46.5%, while infection rate in the control group was 27.0%. Heterogeneity test revealed that there was heterogeneity (χ2 = 78.4, P<0.001) with a P<0.05 for the Q-test. A REM analysis was applied. The combined effect of these test results revealed an association between HPV infection and EC. From the independent OR and synthetic OR of the 33 researches, HPV infection was found to be closely associated with EC. The merger OR value was 1.62; 95% CI of 1.33–1.981.57, Fig 2. The accuracy of each study as argument, with OR/SE as the dependent variable, had a 95% CI of -0.0111784–2.63736 (P = 0.058). Therefore, there was no significant bias in the publications, there was no substantive effect on the synthetic OR, and the conclusion was reliable (Fig 3).
Fig 2. Individual trial and overall risk ratios of the association between HPV (types 16 and 18) infection and esophageal carcinoma.
Fig 3. Begg’s funnel plot on studies of HPV infection and esophageal carcinoma.
Subgroup systematic review
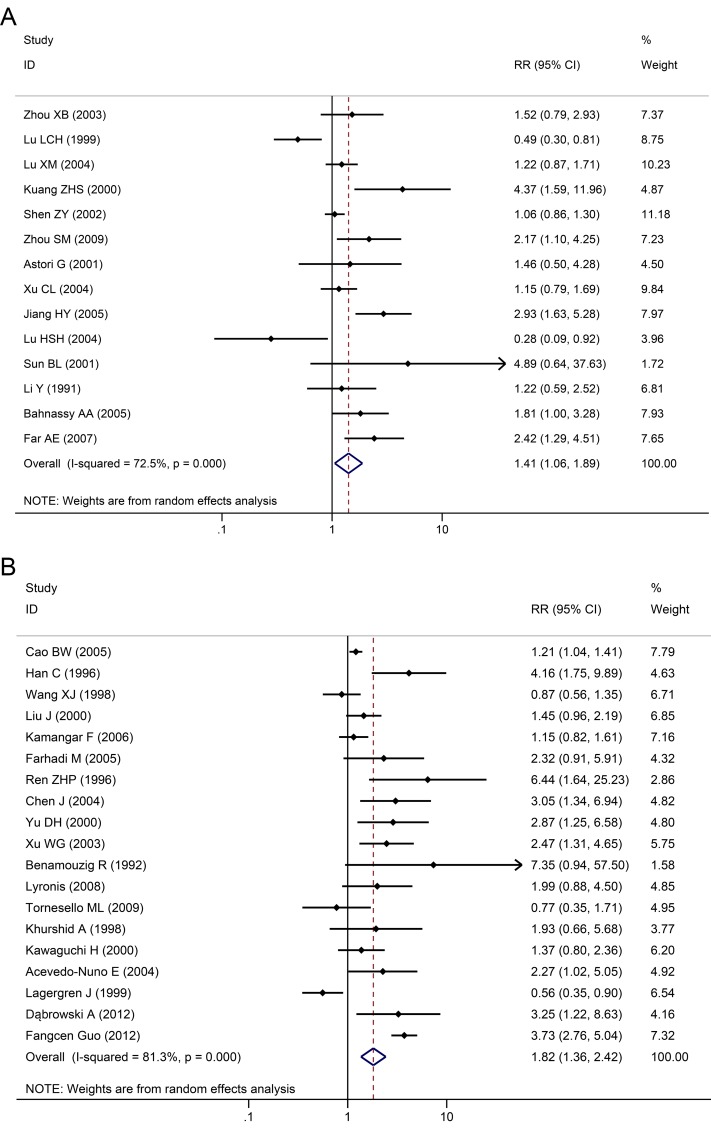
EC incidence in subgroups were stratified and analyzed according to various geographical areas (Fig 4A–4C), the control group selection method (Fig 5), and various HPV detection methods.
Fig 4. Individual trial and overall risk ratios of relationships between HPV infection and esophageal carcinoma in various geographical areas.
4A: China, 4B: non-China and 4C: Asia/non-Asia.
Fig 5. Individual trial and overall risk ratios of relationships between HPV infection and esophageal carcinoma compared to various controls.
There were 21 case-control studies in China. The merger OR value was 1.62 (95% CI: 1.26–2.07) including high incidence areas for EC such as the Taihang, Qinling and Dabie mountain ranges, as well as the northeast of Sichuan, east of Xinjiang, east of Fujian and east of Guangdong provinces. No publication bias (Egger’s test, P>0.05) was found, indicating that the results had good reliability. In other regions (outside China) such as Egypt, France, Greece, Iran and other countries, merger OR value was 1.80 (95% CI: 1.16–2.79); and there was also no publication bias (Egger’s test, P>0.05). In Asian countries such as China, Japan and India, the merger OR value was 1.63 (95% CI: 1.29–2.04); and there was no publication bias (Egger’s test, P>0.05). In non-Asian countries such as Egypt, France, Greece, Iran, Italy, Mexico and Sweden, merger OR value was 1.64 (95% CI: 1.01–2.67); and there was no publication bias (Egger’s test, P>0.05). In addition, results were same in both high risk and low risk areas. In high risk areas, merger OR value was 1.29 (95% CI: 1.05–1.59); while in low risk areas, merger OR value was 2.06 (95% CI: 1.08–3.94). (Fig 4A–4C)
In the control patient group (healthy individuals), merger OR value was 1.41 (95% CI: 1.06–1.89); while in the control tissue group (normal tissues from EC patients), merger OR value was 1.82 (95% CI: 1.36–2.42, Fig 5).
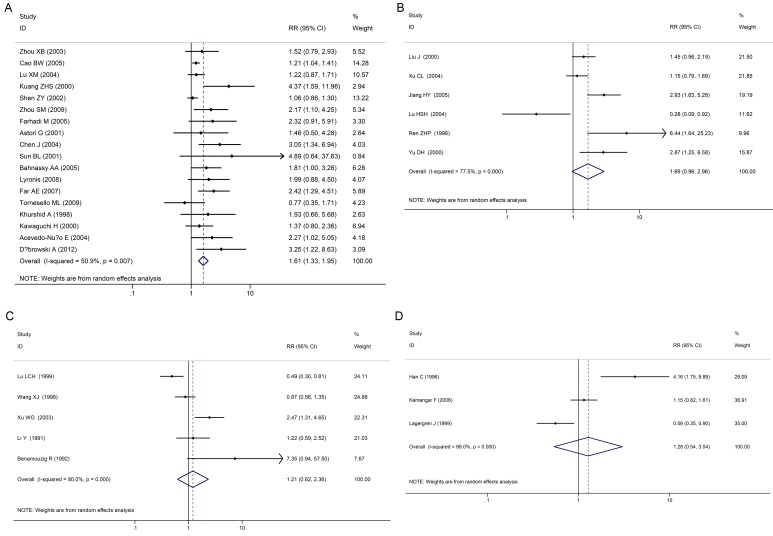
Various HPV detection methods were summarized and analyzed: 18 cases were detected by PCR, 6 cases were detected by ISH, 6 cases were detected by IHC, and 3 cases were detected by ELISA. In the PCR group, merger OR value was 1.61 (95% CI: 1.33–1.95); in the ISH group, merger OR value was 1.21 (95% CI: 0.62–2.36); and in the IHC group, merger OR value was 1.69 (95% CI: 0.96–2.96). An opposite result was acquired in the ELISA group, where the merger OR value was 1.28 (95% CI: 0.54–3.04; Fig 6A–6D).
Fig 6. Individual Trial and Overall Risk Ratios of Relationships between HPV Infection and EC using Various Detection Methods.
(6A:PCR, 6B IHC, 6C:ISH, 6D:ELISA).
Discussion
EC is the fifth most common cancer in developing countries, and the eighth most common cancer worldwide[39]. Areas of high prevalence for EC are mainly located in developing countries, and there are obvious regional differences. [40] The world’s highest areas of incidence are located in Asia, which is called, the "EC belt" [41]. ECs vary greatly by geographic distribution, in which there is a higher incidence in China, America and the eastern Himalayas [42]. However, the incidence of esophageal cancer is low in developed Western countries.
There are several proposed risk factors for EC including eating habits, tobacco, alcohol, pollution, genetic factors, infection of HPV viruses and EBV (Epstein-Barr) virus [43], family history (immediate blood relatives within three generations), etc. In developed countries, tobacco and alcohol is a major factor [42–44]. However, it is different in countries with high incidence of EC, as few cases are attributed to smoking or alcohol consumption there [45].
Currently, the role of HPV infection in esophageal cancer is unclear. Many studies from Africa and China have shown that HPV infections were associated with esophageal cancer. However, in areas with lower prevalence of HPV, there was no decrease in risk of EC [46–49]. The differences in the results of the studies may be due to: 1. Differences in race, living habits, environmental factors that lead to HPV infection. 2. Differences in research design, detection means, methods, statistical analysis. The aim of this study was to perform a comprehensive analysis of the data to determine the relationship between EC and HPV.
In order to explain the association between HPV and EC, we reviewed many articles and selected only case-control studies for analysis. To our knowledge, this is the first study that conducted a systematic review of the relationship between HPV infection (types 16 and 18) and EC worldwide. Results of the comprehensive evaluation of this final systematic review revealed that there was a significant association between HPV infection and EC risk with an integrated OR = 1.62 (95% CI: 1.33–1.98). In META analysis, the presence or absence of heterogeneity directly affected the results of the statistics. Therefore, this study was conducted to strictly evaluate heterogeneity.
HPV infection rates may be related to geographical location. Therefore, we preformed a subgroup analysis according to geographical location worldwide. The stratification study revealed that regardless of whether the studies were in China, Asia, outside of China, or outside of Asia, or in high or low risk areas, HPV infection was associated with EC. In the current research, most studies from low-risk areas also had an association between HPV and EC, and these correlations were stronger than high-risk areas; which was different from other reports. A possible reason was that in high-risk areas, EC occurred due to other reasons such as eating habits, tobacco, alcohol, pollution, genetic factors and HPV infection; thus, the influence of HPV decreased [50]. In low-risk areas, HPV infection appears to be the main cause.
Although most of the esophageal cancer risk factors have been determined, there may still be unknown confounders that may interfere with the results. The literature included case-control studies that were inevitably affected by a variety of bias.
However, due to the current study design, many kinds of bias were possible. (1) Most studies detected HPV from tumor tissues and tissues around tumors that could have been infected with HPV. (2) This systematic review included many studies from all over the world. The variable HPV prevalence could have been related to the various geographic regions studied.
The choice of the control group may have also affected the results, because the healthy controls group revealed that HPV was associated more closely with EC. Cancer adjacent tissues as controls also confirmed a link between HPV and EC, but with a weaker correlation than the cancer tissue itself. This may be related to the HPV infection of the cancer tissue in the body or contamination of samples. There are many methods for detecting HPV, and there was no uniform detection method used in these studies. The researchers did not make use the same HPV detection methods used such as PCR, ELISA, ISH and IHC; although some of the early HPV detection techniques have since been abandoned. Therefore, these results are likely to have some bias, and PCR seems to be the most accurate monitoring method [51].
The current result was the same as that reported by Zhang et al. [48,52] except that their study was limited to a Chinese population, and used a PCR detection method for HPV16. Zhang et al. found that there was a relatively high level of HPV 16 prevalence in Chinese patients with esophageal cancer, and concluded that HPV-16 infection may be a risk factor for esophageal cancer. The current research included the entire world population, and all methods to detect HPV16. Using a subgroup analysis, we obtained results similar to those of Zhang et al. While, there was the limitation that the analyses did not distinguish between the two primary histologic forms of esophageal cancer: squamous cell carcinoma and adenocarcinoma. From our research, squamous cell carcinoma were more likely linked to HPV, adenocarcinoma was fewer than squamous cell carcinoma. This may be related to its biological characteristics.
Conclusions
These results indicate that HPV is closely associated with EC in China, Asia, and all over the world. In investigating the association between HPV and EC, the selection of the control group is important in order to avoid interfering factors. This systematic review provides epidemiologic evidence to support the association between HPV infection and EC. Multi-center studies on regional incidences with strict control of false positives and false negatives would be needed to confirm the association of HPV and EC. If confirmed, HPV testing may be useful in groups at high risk for EC; while HPV vaccine might be useful as a primary prevention measure.
Guarantor of the article: Bangwei Cao, PhD and Bing Liu
Supporting Information
(DOC)
Abbreviations
- HPV
human papillomavirus
- EC
esophageal carcinoma
- RR
risk ratio
- CIs
confidence intervals
- PCR
polymerase chain reaction
- ISH
in situ hybridization
- ICH
immunohistochemistry
- ELISA
enzyme-linked immunosorbent assay
- FEM
fixed effects model
- REM
random effects model
- OR
odds ratio
- EBV
Epstein-Barr
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Administration of Hospitals’ Youth Programme, code: QML20150107 (to Jing Wang); The traditional Chinese medicine science and technology development fund project of Beijing (QN2015-10) (to Jing Wang); The Research Foundation of Beijing Friendship Hospital (yyqdkt2014-10) (to Jing Wang); The Capital Medical and Health Development Fund (2016) (to Jing Wang).
References
- 1.Parkin DM. The role of cancer registries in cancer control. Int J ClinOncol 2008, 13:102–111. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A1, Polineni R, Hussein Z, Vigoda I, Bhagat TD, Bhattacharyya S, et al. Role of epigenetic alterations in the pathogenesis of Barrett's esophagus and esophageal adenocarcinoma. Int J ClinExpPathol. 2012;5(5):382–96. [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology. 2015,149(2):302–317. 10.1053/j.gastro.2015.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ZurHausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nature Reviews Cancer 2002, 2:342–350. [DOI] [PubMed] [Google Scholar]
- 5.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997, 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Huang GQ, Xiao HY, Hong YF, Mao T, Deng WJ. Esophageal tissue adjacent to carcinoma and the presence of human papilloma virus DNA. Huaxi medical university journals 1991,22:157–160. (in Chinese) [Google Scholar]
- 7.Han C, Qiao G, Hubbert NL, Li L, Sun C, Wang Y, et al. Serologic association between human papillomavirus type 16 infection and esophageal cancer in Shaanxi Province, China. Journal of the National Cancer Institute 1996, 88:1467–1471. [DOI] [PubMed] [Google Scholar]
- 8.Ren ZHP, Shi Z, Chen WL. HPV16, 18E6 protein and p21 ras p53 expression in esophageal cancer tissues. Chinese journal of clinical and experimental pathology 1996,12:203–205. (in Chinese) [Google Scholar]
- 9.Wang XJ, Wang CJ, Wang XH, Tao DM, Xiao HZ. Etiologic Relationship between Human Papillomavirus and Esophageal Cancer. Chinese Journal of Clinical Oncology 1996,23:761–764(in Chinese) [Google Scholar]
- 10.Dąbrowski A, Kwaśniewski W, Skoczylas T, Bednarek W, Kuźma D, Goździcka-Józefiak A. Incidence of human papilloma virus in esophageal squamous cell carcinoma in patients from the Lublin region. World J Gastroenterol. 2012,18(40):5739–44. 10.3748/wjg.v18.i40.5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu LC, Shen ZY, You SJ, Shen J, Cai WY. Detection of human papillomavirus(HPV) on EC by PCR and in-situ hybridization. Chinese Journal of Cancer1999,2:162–164(in Chinese) [Google Scholar]
- 12.Jie Liu, Qin Su, Wei Zhang. Relationship between HPV-E6 P53 protein and esophageal squamous cell carcinoma.World Journal of Gastroenterology 2000,8:494–496.(in Chinese) [Google Scholar]
- 13.Yu DH, Wang P, Yao M, Wan M. The correlation of helicobacter pyloril-forminfection and HPV16, 18 infection in human esophageal cancer. Chinese Journal of Zoonoses 2000,16:48–50. (in Chinese) [Google Scholar]
- 14.Shengli H, Zhang J, Jing L, Qi J. Expression of Bcl-2 and MDM-2, and HPV-16 Infection Rate in Esophageal Carcinoma and in Its Pre-cancerous Lesion. Journal of Ningxia Medical College. 200426, 85–87(in Chinese) [Google Scholar]
- 15.Kuang ZS, Tang CZ, Shen ZY. HPV16, 18 detection in esophageal cancer. Guangdong Medicine 2000,4:305–306(in Chinese) [Google Scholar]
- 16.Sun LB, Xu HL. Esophageal FHIT gene abnormalities and HPV infection Relationship. Gastroenterology2001,6: 5(in Chinese) [Google Scholar]
- 17.Shen ZY, Xu LY, Li EM, Cai WJ, Shen J, Chen MH, et al. The multistage process of carcinogenesis in human esophageal epithelial cells induced by human papillomavirus. Oncology reports 2004, 11:647–654. [PubMed] [Google Scholar]
- 18.Xu W, Zhang L, Lu Z, Li J, Ke Y, Xu G. Detection of human papillomavirus type 16 E6 mRNA in carcinomas of upper digestive tract. Zhonghuayixuezazhi 2003, 83:1910. [PubMed] [Google Scholar]
- 19.Zhou X-B, Guo M, Quan L-P, Zhang W, Lu Z-M, Wang Q-H, et al. Detection of human papillomavirus in Chinese esophageal squamous cell carcinoma and its adjacent normal epithelium. World Journal of Gastroenterology 2003, 9:1170–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu CL, Qian XL, Zhou XS, Zhao QZ, Li YC. Expression of HPV 16-E6 and E7 oncoproteins in squamous cell carcinoma tissues of esophageal cancer and non-cancer tissues. Chinese Journal of cancer 2004,23:165–168. (in Chinese) [PubMed] [Google Scholar]
- 21.Chang F, Syrjänen S, Shen Q, Cintorino M, Santopietro R, Tosi P, et al. Human papillomavirus involvement in esophageal carcinogenesis in the high-incidence area of China: a study of 700 cases by screening and type-specific in situ hybridization. Scandinavian journal of gastroenterology 2000, 35:123–130. [DOI] [PubMed] [Google Scholar]
- 22.Cao Bangwei, Yu Jinglin, He Huan, Li Shentao, Zhao Yuliang. Meta analysis on the relationship between HPV infection and esophageal cancer in Chinese population. Journal of Capital Medical University. 2010,3:258–263(in Chinese) [Google Scholar]
- 23.Lu X-M, Zhang YM, Lin RY, Liang XH, Zhang YL, Wang X, et al. p53 polymorphism in human papillomavirus-associated Kazakh's esophageal cancer in Xinjiang, China. World Journal of Gastroenterology 2004, 10:2775–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Zhou Y, Li J, Wu P. Effect of HPV16 infection on the EC and the gene expressions of P53 and P16. Chinese Journal of laboratory diagnosis 2004,8:182–185. (in Chinese) [Google Scholar]
- 25.Jiang HY, Feng DY, Yan YH, Zheng H. The Relation of Human Papillomavirus(HPV) and Esophageal Squamous Cell Carcinogenesis. Journal of clinical research 2005,22:1061–1063. [Google Scholar]
- 26.Kamangar F, Qiao YL, Schiller JT, Dawsey SM, Fears T, Sun XD, et al. Human papillomavirus serology and the risk of esophageal and gastric cancers: Results from a cohort in a high‐risk region in China. International journal of cancer 2006, 119:579–584. [DOI] [PubMed] [Google Scholar]
- 27.Zhou SM, Sheyhidin I, Yang T, Zhang LW, Hasim A, Lu XM, et al. Relationship between human papillomavirus 16 and esophageal squamous cell carcinoma in Uygur population in Xinjiang Uygur Autonomous Region.World Chinese Journal of Digestology 2009, 17:3214–3217 [Google Scholar]
- 28.Liao P, Qin J, Zeng T, Li F, Cai J, He L. A 1: 2 matched case-control study on the interaction of HPV16E6 and HLA-DR9 allele to esophageal cancer in Kazakh ethnicity, Xinjiang. Zhonghualiuxingbingxuezazhi = Zhonghualiuxingbingxuezazhi 2009, 30:951. [PubMed] [Google Scholar]
- 29.Bahnassy AA, Zekri ARN, Abdallah S, El‐Shehaby AM, Sherif GM. Human papillomavirus infection in Egyptian esophageal carcinoma: Correlation with p53, p21waf, mdm2, C‐erbB2 and impact on survival. Pathology international 2005, 55:53–62. [DOI] [PubMed] [Google Scholar]
- 30.Benamouzig R, Pigot F, Quiroga G, Validire P, Chaussade S, Catalan F, et al. Human papillomavirus infection in esophageal squamous‐cell carcinoma in western countries. International journal of cancer 1992, 50:549–552. [DOI] [PubMed] [Google Scholar]
- 31.Lyronis ID, Baritaki S, Bizakis I, Krambovitis E, Spandidos DA. K-ras mutation, HPV infection and smoking or alcohol abuse positively correlate with esophageal squamous carcinoma. Pathology & Oncology Research 2008, 14:267–273. [DOI] [PubMed] [Google Scholar]
- 32.Farhadi M, Tahmasebi Z, Merat S, Kamangar F, Nasrollahzadeh D, Malekzadeh R. Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. World J Gastroenterol 2005, 11:1200–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Astori G, Merluzzi S, Arzese A, Brosolo P, dePretis G, Maieron R, et al. Detection of human papillomavirus DNA and p53 gene mutations in esophageal cancer samples and adjacent normal mucosa. Digestion 2001, 64:9–14. [DOI] [PubMed] [Google Scholar]
- 34.Tornesello ML, Monaco R, Nappi O, Buonaguro L, Buonaguro FM. Detection of mucosal and cutaneous human papillomaviruses in oesophagitis, squamous cell carcinoma and adenocarcinoma of the oesophagus. Journal of Clinical Virology 2009, 45:28–33. 10.1016/j.jcv.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 35.Khurshid A, Kazuya N, Hanae I, Manabu I. Infection of human papillomavirus (HPV) and Epstein-Barr virus (EBV) and p53 expression in human EC. J Pak Med Assoc 1998,48:138–142. [PubMed] [Google Scholar]
- 36.Kawaguchi H, Ohno S, Araki K, Miyazaki M, Saeki H, Watanabe M, et al. p53 polymorphism in human papillomavirus-associated esophageal cancer. Cancer research 2000, 60:2753–2755. [PubMed] [Google Scholar]
- 37.Acevedo-Nuno E, Gonzalez-Ojeda A, Vazquez-Camacho G, MA AB-PL, Moreno-Villa H, Montoya-Fuentes H. Human papillomavirus DNA and protein in tissue samples of oesophageal cancer, Barrett's oesophagus and oesophagitis. Anticancer research 2004, 24:1319–1324. [PubMed] [Google Scholar]
- 38.Lagergren J, Wang Z, Bergström R, Dillner J, Nyrén O. Human papillomavirus infection and esophageal cancer: a nationwide seroepidemiologic case-control study in Sweden. Journal of the National Cancer Institute 1999, 91:156–162. [DOI] [PubMed] [Google Scholar]
- 39.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians 2005, 55:74–108. [DOI] [PubMed] [Google Scholar]
- 40.Syrjänen K. Geographic origin is a significant determinant of human papillomavirus prevalence in oesophageal squamous cell carcinoma: systematic review and meta-analysis. Scand J Infect Dis 2013;45:1–18. 10.3109/00365548.2012.702281 [DOI] [PubMed] [Google Scholar]
- 41.Mosavi-Jarrahi A, Mohagheghi MA. Epidemiology of esophageal cancer in the high-risk population of iran. Asian Pac J Cancer Prev 2006, 7(3):375–380. [PubMed] [Google Scholar]
- 42.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. International journal of cancer 2001, 94:153–156. [DOI] [PubMed] [Google Scholar]
- 43.Syrjänen K. HPV infections and oesophageal cancer. Journal of clinical pathology 2002, 55:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan Y, Yuan JM, Wang R, Gao YT, Yu MC. Alcohol, Tobacco and Diet in Relation to Esophageal Cancer: The Shanghai Cohort Study. Nutr Cancer. 2008; 60(3): 354–363. 10.1080/01635580701883011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown LM, Hoover R, Silverman D, Baris D, Hayes R, Swanson GM, et al. Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. American journal of epidemiology 2001, 153:114–122. [DOI] [PubMed] [Google Scholar]
- 46.Chang F, Syrjänen S, Shen Q, Cintorino M, Santopietro R, Tosi P, et al. Human papillomavirus involvement in esophageal carcinogenesis in the high-incidence area of China: a study of 700 cases by screening and type-specific in situ hybridization. Scandinavian journal of gastroenterology 2000, 35:123–130. [DOI] [PubMed] [Google Scholar]
- 47.Syrjänen K. HPV infections and oesophageal cancer. Journal of clinical pathology 2002, 55:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang SK, Guo LW, Chen Q, Zhang M, Liu SZ, Quan PL, et al. The association between human papillomavirus 16 and esophageal cancer in Chinese population: a meta-analysis.BMC Cancer. 2015,15,99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lagergren J, Wang Z, Bergström R, et al. Human Papillomavirus Infection and Esophageal Cancer: a Nationwide Seroepidemiologic Case-Control Study in Sweden. J Natl Cancer Inst. 91(2): 156–162. [DOI] [PubMed] [Google Scholar]
- 50.Brewster DH, Nowell SL, Clark DN. Risk of oesophageal cancer among patients previously hospitalised with eating disorder. Cancer Epidemiol. 2015,39(3):313–20. 10.1016/j.canep.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Georgantis G, Syrakos T, Agorastos T, Miliaras S, Gagalis A, Tsoulfas G, et al. Detection of human papillomavirus DNA in esophageal carcinoma in Greece. World J Gastroenterol. 2015,21(8):2352–7. 10.3748/wjg.v21.i8.2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang SK, Guo LW, Chen Q, Zhang M, Liu SZ, Quan PL, et al. Prevalence of human papillomavirus 16 in esophageal cancer among the Chinese population: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15(23):10143–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.