ABSTRACT
During oocyte meiosis, the bipolar spindle forms in the central cytoplasm and then migrates to the cortex. Subsequently, the oocyte extrudes the polar body through two successive asymmetric divisions, which are regulated primarily by actin filaments. Myosin light chain2 (MLC2) phosphorylation plays pivotal roles in smooth muscle contraction, stress fiber formation, cell motility and cytokinesis. However, whether MLC2 phosphorylation participates in the oocyte polarization and asymmetric division has not been clarified. The present study investigated the expression and functions of MLC2 during mouse oocyte meiosis. Our result showed that p-MLC2 was localized in the oocyte cortex, with a thickened cap above the chromosomes. Meanwhile, p-MLC2 was also localized in the poles of spindle. Disruption of MLC2 activity by MLC2 knock down (KD) caused the failure of polar body extrusion. Immunofluorescent staining showed that a large proportion of oocytes arrested in telophase stage and failed to undergo cytokinesis after culturing for 12 hours. In the meantime, actin filament staining at oocyte membrane and cytoplasm were reduced in MLC2 KD oocytes. Finally, we found that the phosphorylation of MLC2 protein levels was decreased after disruption of RhoA activity. Above all, our data indicated that the RhoA-mediated MLC2 regulates the actin organization for cytokinesis during mouse oocyte maturation.
KEYWORDS: Actin, cytokinesis, MLC2, polar body extrusion, meiosis
Introduction
In mammalian oocyte meiosis, successful haploidization of maternal genome is achieved through two successive asymmetric divisions, forming a large haploid egg and two much smaller polar bodies.1 This process involves in several events which are essential for asymmetric division during oocyte maturation, including spindle organization and positioning, the establishment of cortical polarity.2 After germinal vesicle breakdown (GVBD), a meiotic spindle assembles in the central cytoplasm and then moves to the subcortical area in an actin filament dependent process; meanwhile, a thickened F-actin cap forms which is surrounded by a myosin II ring above the metaphase I (MI) spindle.3 Therefore, oocyte develops to a specialized cortical domain overlying the subcortical positioned meiotic spindle that is characterized by the actin enrichment, the cortical granules and microvilli free domain, which play key roles in the polar body extrusion.3-5
Recent studies have showed that several molecules are involved in the development and maintenance of oocyte polarity during mouse oocyte meiosis. Disruption of activities of actin nucleators Arp2/3 and Formin 2 causes aberrant actin expression and the failure of polar body extrusion.6,7 In addition, Cdc42, Ran and Rac1 are also involved in the regulation of cortical polarity during mouse oocyte maturation.8-10 Although various molecules have been proposed to contribute to cortical polarity during oocyte meiosis, the pathways and mechanisms that modulate the meiotic apparatus remain to be determined. It has been known that activated myosin II enriched at the spindle poles could pull the actin filaments and generate a force to drive the spindle migration during oocyte meiosis.11 In addition, Myosin II is activated by phosphorylation of its regulatory myosin light chain 2 (MLC2) which is crucial for the execution of cell division12. Previous study has shown that myosin light chain 2 (MLC2) is phosphorylated at Ser19, which allows myosin 2 to interact with actin, assemble an actomyosin complex and initiate contraction.13,14
Myosin light chain2 (MLC2) phosphorylation plays pivotal roles in actin/myosin motor activation to provide essential contractile forces for several cellular processes, such as cell contraction, cytokinesis, cell migration, and membrane blebbing.15-17 During the mitosis, the active myosin rapidly accumulates at the equator between two separating sister chromatids, which indicates that the phosphorylation of MLC2 is important for cytokinesis.18
It has been reported that four candidate molecules ROCK, MLCK, citron kinase and myosin phosphatase are involved in MLC phosphorylation during cytokinesis.19 Rho-kinase (ROCK) is an effector of the small GTPase RhoA and has important roles in the formation of actin fibers and actin dynamics; it is also essential for oocyte polarity establishment and oocyte meiosis.20,21 In addition, the small GTPase RhoA is a key regulator of cytoskeletal organization which regulates cell polarity, migration and division. It also suggests that RhoA regulates cytoskeleton dynamics during porcine oocyte maturation.22 However, the potential function of MLC2 phosphorylation during mouse oocyte meiosis has not been clarified.
In this study, we injected MLC2 morpholino (MO) to examine the functional roles of MLC2 during mouse oocyte meiosis. The failure of polar body extrusion and actin assembly defects indicated that MLC2 participated in oocyte maturation. Our results also demonstrated that this regulation was dependent on the activity of RhoA.
Results
Subcellular distribution of p-MLC2 during oocyte meiosis
We first examined the p-MLC2 distribution during mouse oocyte maturation by immunofluorescent staining. Our result showed that p-MLC2 was present in the oocyte at different developmental stages. At GV stage, p-MLC2 was primarily distributed at the cortex of oocytes. As oocyte enters into metaphase, the meiotic spindle formed in the center of oocyte, and p-MLC2 accumulated in the cortical cap domain overlying the chromosomes; meanwhile, p-MLC2 also concentrated at the poles of oocyte spindle. During telophase stage, p-MLC2 formed a myosin ring, which may play a key role in the contractile ring formation. When oocyte entered into metaphase II (MII) stage, p-MLC2 again concentrated in the oocyte cortical cap domain and spindle poles (Fig. 1A). Our result also showed that p-MLC2 was co-localized with actin in the oocyte membrane (Fig. 1B). The dynamic distribution pattern indicated that p-MLC2 plays essential roles in oocyte meiosis, which might be related to actin filaments.
Figure 1.
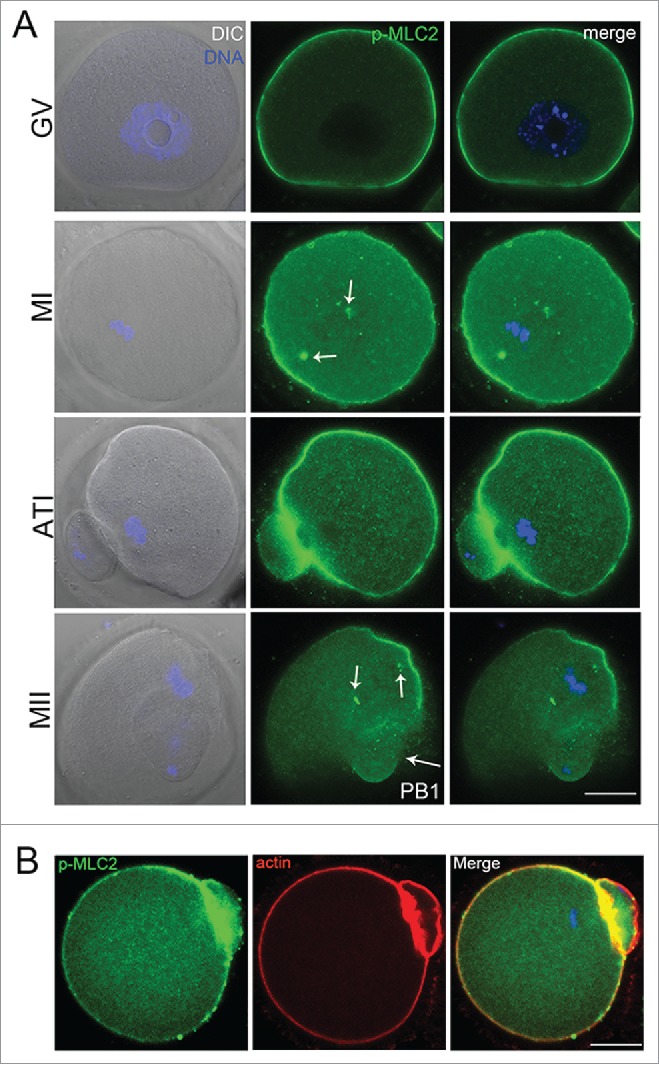
(A) Confocal imaging analysis of the p-MLC2 localization during mouse oocyte meiotic maturation. Oocyte at GV, metaphase I (MI), telophase (ATI) and metaphase II stages were immunolabeled with p-MLC2 antibody. (B) p-MLC2 was co-localized with actin in oocyte membrane. Arrows showed that the localization of p-MLC2 at the spindle poles. Blue: chromatin; Green: p-MLC2; Red: actin. Bar = 20 μm.
MLC2 knockdown (KD) blocks polar body extrusion in mouse oocytes
To examine the functional roles of MLC2 during oocyte meiotic maturation, we employed injection with MLC2 morpholino into GV oocytes. As shown in Figure 2A, p-MLC2 expression was significantly reduced after MLC2 morpholino injection, which was verified by western blot analysis (1 vs 0.55 ± 0.14, p < 0 .05). Our results showed that after MLC2 knockdown (KD), oocytes failed to extrude the first polar body. The rate of polar body extrusion was significantly reduced compared with controls (76.2 ± 1.62% vs 52.5 ± 2.10%, p < 0 .05), indicating that MLC2 participated in the oocyte meiotic process. We also injected p-MLC2 antibody to confirm this. Similarly, the p-MLC2 antibody injection also decreased the polar body extrusion compared with controls (77.8 ± 2.14% vs 54.7 ± 0.95%, p < 0 .05) (Fig. 2B).
Figure 2.
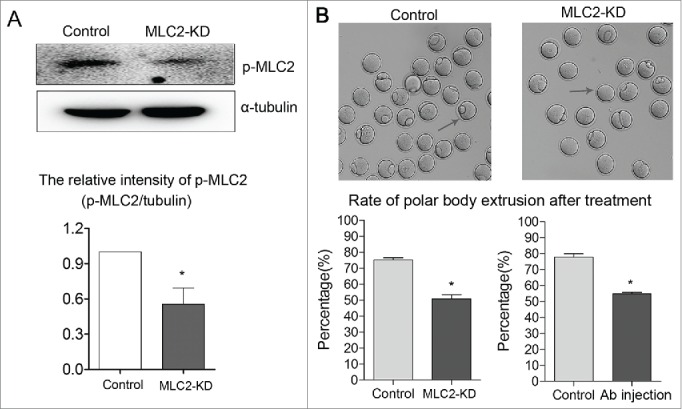
Effect of MLC2 KD on mouse oocyte maturation. Fully grown oocytes microinjected with MLC2-MO were arrested at GV stage with milrinone for 22–24 h to block mRNA translation, washed in milrinone-free medium, and then cultured for 12 h to examine the rate of polar body extrusion. (A) p-MLC2 expression was significantly reduced after MLC2 morpholino injection by protein gel blot. (B) Polar body extrusion failure after MLC2 morpholino injection. The rate of polar body extrusion was decreased after MLC2 morpholino injection or antibody (Ab) injection. Images were acquired with a camera on a stereomicroscope. Arrows showed that the control oocytes extruded the polar body while the treated oocytes failed. *, significant difference (p < 0.05).
MLC2 KD causes the failure of cytokinesis in oocyte meiosis
We tried to find the cause of polar body extrusion defect, and we focused on the earlier process: cytokinesis. Cytokinesis begins at anaphase with the assembly of transient structure called the contractile ring. Previous study showed that MLC2 phosphorylation controls the assembly of the actomyosin contractile apparatus and its contractility.19 Therefore, MLC2 phosphorylation might be related to the contractile ring formation during mouse oocyte meiosis. After culturing for 12h, more than half of oocytes in control group extruded the polar body and arrested at the MII stage. In contrast, some oocytes in MLC2-KD group arrested at telophase I and failed to extrude the polar body (Fig. 3). After culturing for 12 hours, the rate of telophase I-arrested oocytes was significantly higher than that of controls (4.7 ± 2.42% vs18.4 ± 0.89%, p < 0 .05), the MI arrested oocytes was no significantly increased compared with controls (Control: 20.6 ± 4.5, MLC2 KD: 28.1 ± 3.9, p>0 .05). In addition, the MII arrested oocytes was significantly decreased compared with controls (Control: 74.1 ± 3.5, MLC2 KD: 53.0 ± 4.7, p < 0 .05). This result indicated that depletion of MLC2 affects the completion of cytokinesis and the final polar body extrusion.
Figure 3.
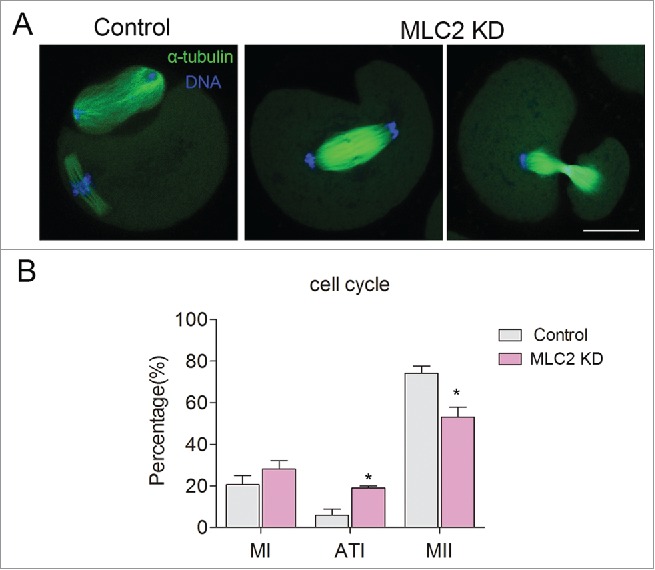
Effect of MLC2 KD on mouse oocyte cytokinesis. (A) In control group, the oocytes extruded the first polar body after culturing for 12h, however, in MLC2 KD oocytes, the oocytes arrested occurred during telophase I. Green: α-tubulin, blue: DNA. (B) The rate of telophase I oocyte was significantly increased after MLC2 KD. *, significant difference (p < 0.05), Bar = 20 μm.
MLC2-KD affects actin distribution and oocyte cortical polarity
To examine the cause of cytokinesis failure, we examined the actin filament by fluorescent staining in MI oocytes. As shown in Figure 4A, the oocyte chromosome migrated to the cortex and formed actin cap in the later of MI, however, in the MLC2-KD group, the oocyte could not formed actin cap. After culturing for 12h, most of oocytes formed small polar bodies and spindles were located under the region of cortex where the actin cap had formed. However, in MLC2-KD group, the oocyte could not formed actin cap.
Figure 4.
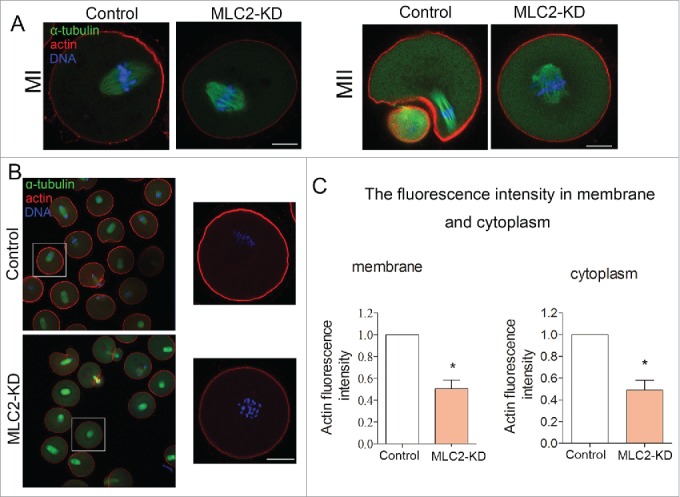
Effect of MLC2 KD on actin dynamics during mouse oocyte meiosis. (A) At MI and MII stages, actin cap formed in the control group, whereas there was no actin cap formed in MLC2 KD treatment oocytes. Red, actin; Green, spindle; Blue, chromatin. Bar = 20 μm. (B) Actin expression in oocyte membrane and cytoplasm after MLC2 KD treatment. (C) Average actin fluorescence intensity in mouse oocyte membrane and cytoplasm. Red: actin; Blue: chromatin. Bar = 20 μm.*, significant difference (p < 0.05).
Next we determined the actin distribution after MLC2 knockdown treatment. The result showed that the actin filament staining was decreased during oocyte maturation in either oocyte membrane or cytoplasm (Fig. 4B). Meanwhile, we quantitatively analyzed the fluorescence intensity of actin in the control and knockdown oocytes. As shown in Figure 4C, the actin fluorescence intensity was significantly reduced compared to that in control oocyte of membrane after MLC2 knockdown (1 vs 0.50 ± 0.08, p < 0 .05). Similarly, the fluorescence intensity of cytoplasmic actin was also lower than that of control oocytes after MLC2 knockdown (1 vs 0.49 ± 0.09, p < 0 .05). Therefore, our results indicated that MLC2 is involved in the actin assembly during mouse oocyte meiosis, which further affects the polar body extrusion.
MLC2 functions in the RhoA-MLC2 signaling pathway in mouse oocyte meiosis
To investigate the possible signaling pathway of MLC2 for actin assembly during mouse oocyte meiosis, we investigated the expression of p-MLC2 after disruption of RhoA activity. As shown in Figure 5A, immunofluorescent staining revealed that the pole localization of p-MLC2 was disrupted and the expression of p-MLC2 also decreased after RhoA inhibitor (Rhosin) treatment. Western blot analysis also showed that p-MLC2 protein expression was significantly reduced after treatment with RhoA inhibitor. The relative intensity (p-MLC2/α-tubulin) also significantly reduced compare to that in control group (1 vs 0.28 ± 0.08, p < 0 .05) (Fig. 5B). Our result indicated that the RhoA-MLC2 signaling pathway is involved in the mouse oocyte meiosis.
Figure 5.
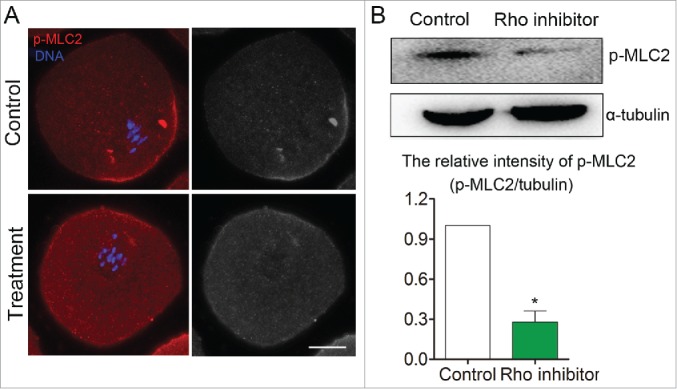
Disruption of RhoA activity caused MLC2 protein phosphorylation level decreased during mouse oocyte meiosis. (A) Immunofluorescent staining result showed that MLC2 phosphorylation level was reduced after disruption of RhoA activity. (B) Western blot result also showed MLC2 phosphorylation level was significantly decreased after disruption of RhoA activity. Red: p-MLC2; Blue: chromatin. Bar = 20 μm.*, significant difference (p < 0.05).
Discussion
Asymmetric division is a unique characteristic of meiotic division in mammalian oocytes, and eventually produces oocyte with the high polarity. Our present study investigated the possible functions and mechanisms of myosin light chain 2 (MLC2) during mouse oocyte meiotic maturation. The results showed that disruption of MLC2 activity affected actin assembly and following polar body extrusion. Furthermore, the regulation of MLC2 on actin cytoskeleton integrity was dependent on the RhoA activity during mouse oocyte meiosis.
It is well known that MLC2 phosphorylation involves in cell polarity and cell cytokinesis in somatic cell, however, its localization and roles in mouse oocyte has remained unclear. Our results showed that p-MLC2 accumulated at the oocyte cortex and the poles of spindle, which was consistent with previous research.23 Previous studies showed that the actin filament close to spindle poles was pulled to spindle poles and accumulated around the spindle poles during oocyte meiosis. Also, a dynamic meshwork of actin filaments extended from the spindle poles to the cortex and myosin pulled on these actin to move the spindle during oocyte meiosis.11,24 Therefore, the localization pattern of p-MLC2 in mouse oocyte suggested that MLC2 phosphorylation was involved in the actin dynamics during oocyte meiosis. To confirm our hypothesis, we disrupted the MLC2 activity by morpholino injection, which led to the failure of oocyte polar body extrusion and the increased rate of arrested telophase I. These results were somewhat expected, because disruption of MLC2 phosphorylation caused multipolar spindles and cytokinesis failure in cancer cells.25 It was also reported that expression of unphosphorylated MLC2 in mammalian cells caused failure of cytokinesis.26 Thus, our result suggested that MLC2 phosphorylation may positively affect polar body extrusion in mouse oocytes meiosis.
To determine the possible reason of polar body extrusion defects after MLC2 depletion, we examined actin filament distribution, which plays essential roles in oocyte polarity formation and cytokinesis.27 During mouse oocyte meiotic maturation, actin was required for the chromosome migration,9,28 cortical spindle anchorage,29,30 cortex development and polarity establishment 2,31 and first polar body extrusion.6,27 Additionally, some actin nucleation factors such as Arp2/3 complex,6 Formin2 7,29 and Spire1/2 32 were involved in the actin organization during mouse oocyte maturation. Previous work showed that myosin II was activated by phosphorylation of its regulatory MLC at Ser19/Thr18, and the phosphorylation at MLC Ser19 was critical for actin assembly.12,33 It was also reported to be involved in several cellular processes due to its motor on actin/myosin activation, such as cell contraction, cytokinesis, cell migration, and membrane blebbing. Thus, we hypothesized that the dynamic changes of actin might be related to MLC phosphorylation in mouse oocytes.
Actin filament staining in MLC2 knockdown oocytes was significantly decreased in oocyte cytoplasm and membrane compared to that in control oocytes, confirmed by the fluorescence intensity analysis. Our results suggested that MLC2 phosphorylation participated in polar body extrusion mediated by its regulation of actin assembly. Recent evidence has documented that the Rho-induced activation of ROCK promoted actin stress fiber formation by directly phosphorylating MLC in some cells.34 Based on this fact, we hypothesized that MLC2 phosphorylation might participate in the actin-mediated oocyte meiosis in conjunction with RhoA. Our recent work demonstrated that both RhoA and ROCK participated in oocyte cytokinesis and spindle migration by mediating actin organization during oocyte meiosis.20,30 The decreased p-MLC2 expression after RhoA inhibitor treatment confirmed that MLC2 phosphorylation regulated actin organization through RhoA-MLC2 signaling pathway during mouse oocyte meiosis.
In conclusion, our results indicated that MLC2 phosphorylation regulates actin-based cytokinesis in mouse oocytes, and this regulation may be mediated via a RhoA-MLC2-actin pathway.
Materials and methods
Antibodies and chemicals
A rabbit monoclonal anti-p-Myosin Light Chain2 antibody and anti-α-tubulin were from Cell Signaling Technology (Danvers, MA); Phalloidin-TRITC and a mouse monoclonal anti-α-tubulin-FITC antibody were from Sigma (St Louis, MO). Alexa Fluor 488, 594 antibodies were from Invitrogen (Carlsbad, CA).
Oocyte harvest and culture
All procedures with mice were conducted according to the Animal Research Institute Committee guidelines of Nanjing Agriculture University, China. The experimental protocols were approved by Nanjing Agriculture University Animal Research Institute Committee. Mice were housed in a temperature controlled room with appropriate dark-light cycles, fed a regular diet, and maintained under the care of the Laboratory Animal Unit, Nanjing Agricultural University, China. Germinal vesicle-intact oocytes were harvested from ovaries of 4–6 week-old ICR mice and cultured in M16 medium (Sigma, MO) under paraffin oil at 37°C in a 5% CO2 atmosphere. Oocytes were removed from culture at different times for microinjection, immunofluorescent staining and protein gel blot.
MLC2 morpholino (MO) injection
For MLC2 knock-down in mouse oocyte, MLC2 morpholino 5′-GTG TCT GAG GCC AGT TGC CAC CTCT-3′ (Gene Tools, Philomath, OR)) was diluted with reagent grade water (Sigma) to give a 1 mM stock concentration. Each fully grown GV oocyte was microinjected with 5–10 pl of MLC2 morpholino using an Eppendorf FemtoJet (Eppendorf AG, Hamburg, Germany) under an inverted microscope (Olympus IX71 Japan). After injection, oocytes were cultured in M16 medium that contained 5 μM milrinone for 24 h, and then washed three times (2 min each wash) in fresh M16 medium. Oocytes were then transferred to fresh M16 medium and cultured under paraffin oil at 37°C in a 5% CO2 atmosphere. Each control oocyte was microinjected with 5–10 pl of MO standard control (5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′). For p-MLC2 antibody injection, each fully grown GV oocyte was microinjected with 5–10 pl of p-MLC2 antibody, and then cultured in fresh M16 medium.
Rhosin (RhoA inhibitor) treatment
RhoA inhibitor, Rhosin was employed to inhibit the activity of intracellular RhoA during oocytes meiosis. Rhosin was diluted to 50 mM stock solution in dimethylsulfoxide (DMSO) and stored at −20°C. Rhosin was diluted in M16 medium to concentration of 100μM for the experiment.
Confocal microscopy
For single staining of p-MLC2, actin and spindle, oocytes were fixed in 4% paraformaldehyde in PBS at room temperature for 30 min and then transferred to a membrane permeabilization solution (0.5% Triton X-100) for 20 min. After 1 h in blocking buffer (1% BSA-supplemented PBS), oocytes were incubated at 4°C overnight with rabbit anti-p-MLC2 (1:100), 10 μg/ml of Phalloidin-TRITC. After three washes in wash buffer (0.1% Tween 20 and 0.01% Triton X-100 in PBS), oocytes were labeled with Alexa Fluor 488 goat-anti-rabbit IgG (1:100; for p-MLC2 staining) at room temperature for 1 h. Samples were co-stained with Hoechst 33342 for 10 min. For double staining of spindle and actin, oocytes were stained with anti-α-tubulin-FITC antibody three hours in room temperature and then labeled with Phalloidin-TRITC for 1h, washed three times in PBS containing 0.1% Tween 20 and 0.01% Triton X-100 for 2 min, and stained with Hoechst 33342 (10 μg/ml in PBS) for 10 min.
Samples were mounted on glass slides and examined with a confocal laser-scanning microscope (Zeiss LSM 700 META). At least 30 oocytes were examined for each experimental group.
Western blot analysis
A total of 150 mouse oocytes were placed in Laemmli sample buffer (SDS sample buffer and 2-Mercaptoethanol) and heated at 100°C for 5 min. Proteins were separated by SDS-PAGE and then electrophoretically transferred to polyvinylidene fluoride membranes. After transfer, membranes were blocked in PBST (PBS containing 0.1% Tween 20) containing 5% non-fat milk for 1 h, followed by incubation at 4°C overnight with a rabbit monoclonal anti-p-MLC2 (1:1000) and a rabbit monoclonal anti-α-tubulin antibody (1:1000; for p-MLC2, incubation buffer was 5% BSA in PBST). After washing 3 times in PBST (10 min each), membranes were incubated at 37°C for 1 h with HRP conjugated Pierce Goat anti-Rabbit IgG (1:1000). Finally, membranes were washed 3 times in TBST and then the membranes were visualized using chemiluminescence reagent (Millipore, Billerica, MA). This experiment was repeated at least 3 times using different samples.
Fluorescence and western band intensity analysis
Fluorescence intensity was assessed using Image J software (NIH). For fluorescence intensity analysis, samples of control and treated oocytes were mounted on the same glass slide. And we used the same parameters to normalize across replicates. After immunofluorescent staining, the average fluorescence intensity per unit area within the region of interest (ROI) of immunofluorescence images was examined. Independent measurements using identically sized ROIs were taken for the cell cytoplasm. When calculating the fluorescence intensity, we ignored the abnormal ones (little oocytes with extreme strong or weak). Average values of all measurements were used to determine the final average intensities for control and treated oocytes.
For quantification of the western blot results, intensity values of bands were measured using the Image J. Three different replicates were used for the analysis.
Statistical analysis
At least three biological replicates were used for each analysis. Each replicate was done by an independent experiment at the different time. Results are given as means ± SEM. Statistical comparisons were made using analysis of variance (ANOVA) and differences between treatments groups were assessed with Duncan's multiple comparisons test. A p-value of < 0.05 was considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Yu Zhang for providing the RhoA inhibitor. We also thank Teng Wang and Yue Zhang for the technical assistance and helpful discussions.
Author Contributions
XD SCS conceived and designed the experiments; XD JL performed the experiments; XD SCS analyzed the data; JL contributed reagents/materials/analysis tools; XD SCS wrote the paper.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20140030), the Fundamental Research Funds for the Central Universities (KJQN201402, KJJQ201501), China; and the Biogreen 21 Program (PJ011126), RDA, Republic of Korea.
References
- 1.Brunet S, Verlhac MH. Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update 2011; 17:68-75; PMID:20833637; http://dx.doi.org/ 10.1093/humupd/dmq044 [DOI] [PubMed] [Google Scholar]
- 2.Yi K, Li R. Actin cytoskeleton in cell polarity and asymmetric division during mouse oocyte maturation. Cytoskeleton (Hoboken) 2012; 69:727-37; PMID:22753278; http://dx.doi.org/ 10.1002/cm.21048 [DOI] [PubMed] [Google Scholar]
- 3.Deng M, Kishikawa H, Yanagimachi R, Kopf GS, Schultz RM, Williams CJ. Chromatin-mediated cortical granule redistribution is responsible for the formation f the cortical granule-free domain in mouse eggs. Dev Biol 2003; 257:166-76; PMID:12710965; http://dx.doi.org/ 10.1016/S0012-1606(03)00045-9 [DOI] [PubMed] [Google Scholar]
- 4.Longo FJ, Chen DY. Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev Biol 1985; 107:382-94; PMID:4038667; http://dx.doi.org/ 10.1016/0012-1606(85)90320-3 [DOI] [PubMed] [Google Scholar]
- 5.Maro B, Johnson MH, Webb M, Flach G. Mechanism of polar body formation in the mouse oocyte: an interaction between the chromosomes, the cytoskeleton and the plasma membrane. J Embryol Exp Morphol 1986; 92:11-32; PMID:3723057 [PubMed] [Google Scholar]
- 6.Sun SC, Wang ZB, Xu YN, Lee SE, Cui XS, Kim NH. Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PLoS One 2011; 6:e18392; PMID:21494665; http://dx.doi.org/ 10.1371/journal.pone.0018392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leader B, Lim H, Carabatsos MJ, Harrington A, Ecsedy J, Pellman D, Maas R, Leder P. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol 2002; 4:921-8; PMID:12447394; http://dx.doi.org/ 10.1038/ncb880 [DOI] [PubMed] [Google Scholar]
- 8.Na J, Zernicka-Goetz M. Asymmetric positioning and organization of the meiotic spindle of mouse oocytes requires CDC42 function. Curr Biol 2006; 16:1249-54; PMID:16782018; http://dx.doi.org/ 10.1016/j.cub.2006.05.023 [DOI] [PubMed] [Google Scholar]
- 9.Deng M, Suraneni P, Schultz RM, Li R. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev Cell 2007; 12:301-8; PMID:17276346; http://dx.doi.org/ 10.1016/j.devcel.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 10.Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell 2007; 12:309-17; PMID:17276347; http://dx.doi.org/ 10.1016/j.devcel.2006.12.010 [DOI] [PubMed] [Google Scholar]
- 11.Schuh M, Ellenberg J. A new model for asymmetric spindle positioning in mouse oocytes. Curr Biol 2008; 18:1986-92; PMID:19062278; http://dx.doi.org/ 10.1016/j.cub.2008.11.022 [DOI] [PubMed] [Google Scholar]
- 12.Moussavi RS, Kelley CA, Adelstein RS. Phosphorylation of vertebrate nonmuscle and smooth muscle myosin heavy chains and light chains. Mol Cell Biochem 1993; 127-128:219-27; PMID:7935353; http://dx.doi.org/ 10.1007/BF01076773 [DOI] [PubMed] [Google Scholar]
- 13.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 1996; 271:20246-9; PMID:8702756; http://dx.doi.org/ 10.1074/jbc.271.34.20246 [DOI] [PubMed] [Google Scholar]
- 14.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al.. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996; 273:245-8; PMID:8662509; http://dx.doi.org/ 10.1126/science.273.5272.245 [DOI] [PubMed] [Google Scholar]
- 15.Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol 1999; 147:1023-38; PMID:10579722; http://dx.doi.org/ 10.1083/jcb.147.5.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 1997; 272:12257-60; PMID:9139666; http://dx.doi.org/ 10.1074/jbc.272.19.12257 [DOI] [PubMed] [Google Scholar]
- 17.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 2001; 3:346-52; PMID:11283607; http://dx.doi.org/ 10.1038/35070019 [DOI] [PubMed] [Google Scholar]
- 18.Matsumura F, Ono S, Yamakita Y, Totsukawa G, Yamashiro S. Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis of cultured cells. J Cell Biol 1998; 140:119-29; PMID:9425160; http://dx.doi.org/ 10.1083/jcb.140.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol 2005; 15:371-7; PMID:15935670; http://dx.doi.org/ 10.1016/j.tcb.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 20.Duan X, Liu J, Dai XX, Liu HL, Cui XS, Kim NH, Wang ZB, Wang Q, Sun SC. Rho-GTPase effector ROCK phosphorylates cofilin in actin-meditated cytokinesis during mouse oocyte meiosis. Biol Reprod 2014; 90:37; PMID:24429217; http://dx.doi.org/ 10.1095/biolreprod.113.113522 [DOI] [PubMed] [Google Scholar]
- 21.Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist 2007; 13:454-69; PMID:17901255; http://dx.doi.org/ 10.1177/1073858407303611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Duan X, Cao R, Liu HL, Cui XS, Kim NH, Rui R, Sun SC. Small GTPase RhoA regulates cytoskeleton dynamics during porcine oocyte maturation and early embryo development. Cell Cycle 2014; 13:3390-403; PMID:25485583; http://dx.doi.org/ 10.4161/15384101.2014.952967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang QX, Zhang QH, Qi ST, Wang ZW, Hu MW, Ma XS, Zhu MS, Schatten H, Wang ZB, Sun QY. Deletion of Mylk1 in oocytes causes delayed morula-to-blastocyst transition and reduced fertility without affecting folliculogenesis and oocyte maturation in mice. Biol Reprod 2015; 92:97; PMID:25761595; http://dx.doi.org/ 10.1095/biolreprod.114.122127 [DOI] [PubMed] [Google Scholar]
- 24.Bezanilla M, Wadsworth P. Spindle positioning: actin mediates pushing and pulling. Curr Biol 2009; 19:R168-9; PMID:19243693; http://dx.doi.org/ 10.1016/j.cub.2008.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q, Sahasrabudhe RM, Luo LZ, Lewis DW, Gollin SM, Saunders WS. Deficiency in myosin light-chain phosphorylation causes cytokinesis failure and multipolarity in cancer cells. Oncogene 2010; 29:4183-93; PMID:20498637; http://dx.doi.org/ 10.1038/onc.2010.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komatsu S, Yano T, Shibata M, Tuft RA, Ikebe M. Effects of the regulatory light chain phosphorylation of myosin II on mitosis and cytokinesis of mammalian cells. J Biol Chem 2000; 275:34512-20; PMID:10944522; http://dx.doi.org/ 10.1074/jbc.M003019200 [DOI] [PubMed] [Google Scholar]
- 27.Sun QY, Schatten H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 2006; 131:193-205; PMID:16452714; http://dx.doi.org/ 10.1530/rep.1.00847 [DOI] [PubMed] [Google Scholar]
- 28.Li H, Guo F, Rubinstein B, Li R. Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat Cell Biol 2008; 10:1301-8; PMID:18836438; http://dx.doi.org/ 10.1038/ncb1788 [DOI] [PubMed] [Google Scholar]
- 29.Dumont J, Million K, Sunderland K, Rassinier P, Lim H, Leader B, Verlhac MH. Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev Biol 2007; 301:254-65; PMID:16989804; http://dx.doi.org/ 10.1016/j.ydbio.2006.08.044 [DOI] [PubMed] [Google Scholar]
- 30.Zhong ZS, Huo LJ, Liang CG, Chen DY, Sun QY. Small GTPase RhoA is required for ooplasmic segregation and spindle rotation, but not for spindle organization and chromosome separation during mouse oocyte maturation, fertilization, and early cleavage. Mol Reprod Dev 2005; 71:256-61; PMID:15791586; http://dx.doi.org/ 10.1002/mrd.20253 [DOI] [PubMed] [Google Scholar]
- 31.Yi K, Rubinstein B, Li R. Symmetry breaking and polarity establishment during mouse oocyte maturation. Philos Trans R Soc Lond B Biol Sci 2013; 368:20130002; PMID:24062576; http://dx.doi.org/ 10.1098/rstb.2013.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfender S, Kuznetsov V, Pleiser S, Kerkhoff E, Schuh M. Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr Biol 2011; 21:955-60; PMID:21620703; http://dx.doi.org/ 10.1016/j.cub.2011.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholey JM, Taylor KA, Kendrick-Jones J. Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature 1980; 287:233-5; PMID:6893621; http://dx.doi.org/ 10.1038/287233a0 [DOI] [PubMed] [Google Scholar]
- 34.Hirano K, Derkach DN, Hirano M, Nishimura J, Kanaide H. Protein kinase network in the regulation of phosphorylation and dephosphorylation of smooth muscle myosin light chain. Mol Cell Biochem 2003; 248:105-14; PMID:12870661; http://dx.doi.org/ 10.1023/A:1024180101032 [DOI] [PubMed] [Google Scholar]


