Abstract
Background
The association between urinary incontinence (UI) and increased mortality remains controversial. The objective of our study was to evaluate if this association exists.
Methods
We performed a systematic review and meta-analysis of observational studies comparing death rates among patients suffering from UI to those without incontinence. We searched in Medline, Embase and the Cochrane library using specific keywords. Studies exploring the post-stroke period were excluded. Hazard ratios (HR) were pooled using models with random effects. We stratified UI by gender and by UI severity and pooled all models with adjustment for confounding variables.
Results
Thirty-eight studies were retrieved. When compared to non-urinary incontinent participants, UI was associated with an increase in mortality with pooled non adjusted HR of 2.22 (95%CI 1.77–2.78). The risk increased with UI severity: 1.24 (95%CI: 0.79–1.97) for light, 1.71 (95%CI: 1.26–2.31) for moderate, and 2.72 (95%CI: 1.90–3.87) for severe UI respectively. When pooling adjusted measures of association, the resulting HR was 1.27 (95%CI: 1.13–1.42) and increased progressively for light, moderate and severe UI: 1.07 (95%CI: 0.79–1.44), 1.25 (95%CI: 0.99–1.58), and 1.47 (95%CI: 1.03–2.10) respectively. There was no difference between genders.
Conclusion
UI is a predictor of higher mortality in the general and particularly in the geriatric population. The association increases with the severity of UI and persists when pooling models adjusted for confounders. It is unclear if this association is causative or just reflects an impaired general health condition. As in most meta-analyses of observational studies, methodological issues should be considered when interpreting results.
Background
Urinary incontinence (UI) -the complaint of any involuntary loss of urine [1]- is frequent in the general population and affects men and women of all ages. It has been found in 11 to 34% of men and 13–50% of women older than 60 years (depending on the method used), and in 43–80% of all nursing home residents [2–4].
UI decreases quality of life of men and women, and has been associated with many unfavourable outcomes, including longer hospital length of stay and lower chance of regaining home after hospital discharge [5–7]. Thus, UI carries an unsuspected load on the healthcare system, with an estimate of 14 billion dollars spent each year in the United States (5 billion dollars for institutionalised citizens), and 4.6 billion Euros in France [8]. Besides, this condition will affect over 423 million people worldwide by 2018 [9].
Frail and older patients are at the highest risk for developing UI [7]. Therefore, mortality rate of patients suffering from UI is expected to be higher than the one of patients who are not suffering from this condition. UI may be a marker of an impaired general health condition and an indirect cause of death consecutive to falls, for example [10]. However the extent of the increased mortality is not clear yet, and varies across studies according to gender, as well as UI severity. Furthermore, after adjustment for the high number of comorbid conditions and disability found among urinary inctontinent patients, studies differ in their conclusions. Some find a persisting association, while others don’t. An association independent of these factors could stimulate research on UI treatment.
Thus the aim of this systematic review and meta-analysis was to determine the effect of UI on mortality, in subgroups of men and women, and according to UI severity strata. We explored all published adjusted models to determine if the association persisted after adjustment for confounders.
Subjects/Patients and Methods
We performed a systematic review and meta-analysis of all studies exploring UI and death. The study was divided into two subsets: those exploring the post-stroke period (published separately) and those in the general population. Search strategy, study selection, data extraction, and analysis were performed according to a pre-defined protocol (available on request).
Search strategy
The search strategy in Medline, Embase and the Cochrane library used predefined keywords (Search strategy in S1 File) and was limited to articles written in English, French, and Italian and published before December 7, 2014. We examined reference lists from retrieved articles, guidelines and systematic reviews and asked experts in urology and gynaecology for studies we might have missed.
Study selection and data extraction
We included retrospective and prospective studies comparing mortality rate between patients with and without UI. Urge, stress, or mixed UI had not to be the main focus of the study. Diurnal and nocturnal episodes of UI that happened at least once during the previous year were considered. UI could be diagnosed based either on caregiver records or patient’s self reporting, and as defined according to the International Continence Society by the complaint of any involuntary loss of urine [1]. Publications on the same cohort of patients were all included, but duplicate data was avoided in the meta-analyses. We excluded all case reports, studies with patients under eighteen, and articles exploring the post-stroke period.
CB and GJ independently evaluated studies for possible inclusion. Irrelevant studies were excluded based on title and abstract. Full texts were then obtained to ascertain each study’s eligibility and for data extraction. Variables extracted in all studies were: all-cause mortality of patients with and without UI, study design, population characteristics, place of living (or inclusion), variables used in adjusted models, and UI sub-type, severity, and definition. Disagreements on article inclusion and data extraction were resolved by consensus.
Quality assessment
The quality of the observational cohort studies was assessed through the Newcastle-Ottawa Quality Assessment scale (NOS) [11]. This scale explores three domains: "selection of study groups", "comparability of groups", and "ascertainment of exposure/outcome". In the "selection of study groups" domain, two points were automatically achieved in all studies ("demonstration that outcome of interest was not present at start of study" and "selection of the non exposed cohort"), one point was obtained if the cohort was not restricted to a specific sub-population (eg. post fracture), and another point if UI was diagnosed through a valid structured questionnaire. One point was given to the domain "comparability of groups" if the analyses were adjusted for age and two points if another adjustment variable was used in the models. Regarding the "ascertainment of exposure/outcome" domain, one point was attributed if the follow-up was more than one month, a second point if subjects lost were less than 10% (or if a description was provided for all patients lost to follow-up) and a third point for the assessment of outcome. Two investigators (CB, GJ) assessed study quality independently.
Pooled data analysis
Measures of association were unadjusted and adjusted hazard ratios (HR), between UI and mortality. When several HRs were reported, we kept the HR for the longest follow-up period. If published information was not sufficient to extract HR, authors were contacted. If the HR could not be obtained, we estimated it and its variance using the ratio of logarithms of event-free proportions from the proportion of death in the exposed and unexposed groups. [12] This method uses the published proportion of death, and does not consider patients lost to follow-up.
For one study [13], the unadjusted HRs were derived from the published survival curves: survival estimates were extracted from the digitalized picture of survival curves. Individual survival data were extrapolated from the sample size and the survival estimates. The estimate of HR was then obtained using a Cox regression model. The censorship rate was very low in this study and we assumed a null censorship rate to derive the HRs.
In two studies adjusted HRs were only given by gender. We assessed a global logarithm of HR as a weighted average of the gender-specific logarithm of HRs (Supplemental statistics A in S1 File). Three studies reported the ajusted HR by sub-group of severity of UI but no global HR for UI. We combined the HRs reported by severity of UI in a single global HR using a similar approach. However, in contrast to the gender-specific HRs, the severity-specific HRs from a study are not independent, as the same control participants (continent subjects) are used to calculate the HR in the different UI severity strata. Therefore, we pooled the severity-specific HRs assuming a correlation between the HRs of 0.20 (Supplemental statistics A in S1 File). The validity of this approach was tested by re-assessing global HRs–through this approach- for all studies and comparing them to the published global HR. We then explored the effect of varying the coefficient of correlation of the three considered studies on the pool estimates of adjusted HRs. The result was robust (Supplemental statistics A in S1 File).
All pooled estimates (except for those dedicated to summarize HRs for a single study) were systematically obtained using models with random effects (Der Simonian and Laird’s method). The significance level was set at 0.05 for all analyses and all statistical tests were two-sided.
We performed other meta-analyses to explore a modification of the association between UI and mortality by gender and severity of UI. For gender, we pooled the difference in logarithm of HR between men and women. By taking the exponential, we expressed results as an increase/decrease of HR in men compared with women. A positive difference means that the association between UI and mortality is stronger in men than in women. With this approach, the advantage is that we account for the fact that HR in men and women are assessed in the same studies.
For UI severity, we used two strategies. First, we stratified severity using the categories published in any articles regardless of the differences in definitions between studies. Second, we classified all articles (and severity strata, when available in articles) depending on the least number of episodes of urinary leakage needed for the diagnosis of UI. Thus we grouped monthly, weekly, or daily episodes of UI. To analyse the increase of HR with the severity of UI, we assessed for each study the difference in logarithm of HRs between two stages of severity (assuming a correlation of 0.20) and we combined these differences (Supplemental statistics B in S1 File). The results were expressed as the pooled ratio of HRs between two stages of severity of UI (exponential of the pooled difference in logarithm of HRs).
Heterogeneity was measured with I2 statistics (>75% being considered highly heterogeneous). Potential heterogeneity factors were explored by leave-one-out strategy, and sub-group analyses. Pre-specified subgroup analysis included: stratification by study design, date of publication, country, population studied (general geriatrics versus specific population), and settings. Settings were divided in community, hospital, nursing care, and a mixture of these. We stratified the pooled adjusted models into two categories: highly adjusted models (with adjustment for at least both functional status and age) and models with low adjustment variables. We tested heterogeneity across subgroups. [14]
In sensitivity analyses, we pooled published odds ratios (OR) stratified by length of follow-up (6 months, 1, 3, 5, and 10 years). We also restricted the analysis to the studies with good to fair quality in each of the three domains of the NOS.
Publication bias for each outcome was graphically explored through funnel plot and Egger's test. The trim and fill method was used to check the impact of a potential publication bias on pooled estimates. The R package “meta: Meta analysis with R”, version 1.6–1 and Review Manager of the Cochrane Library (RevMan), version 5.3 was used for these analyses.
Results
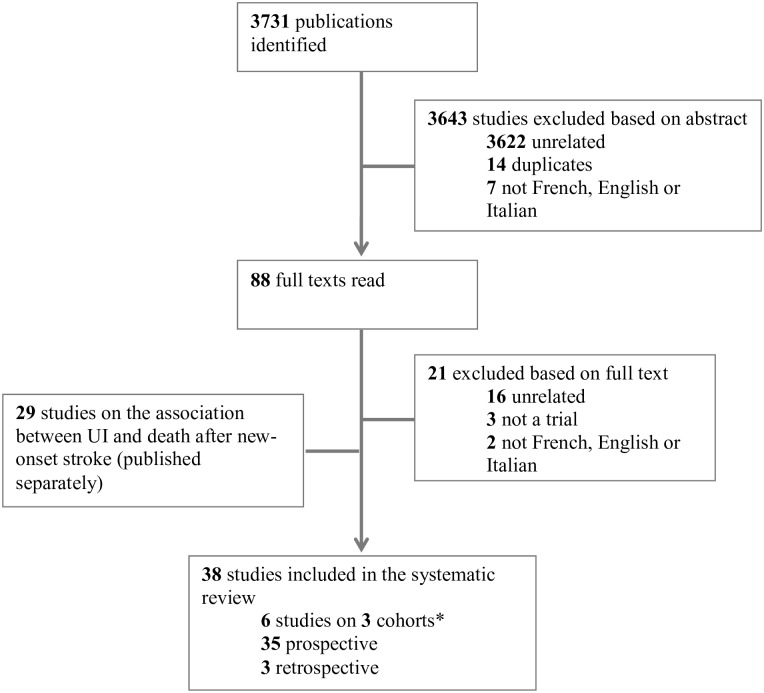
We explored 3731 citations and retrieved 38 studies (exploring 35 single cohorts) (Fig 1). Six studies reported association between UI and death at different time points at follow-up for 3 individual cohorts. The main characteristics of the studies included in the systematic review are displayed in Table 1.
Fig 1. Flow chart of identified references to retrieved studies in the systematic review.
*Three longitudinal cohorts gave multiple publications on the association between urinary incontinence and mortality, at different time points.
Table 1. Main characteristics of the studies included in the review.
| First author/ year | Design | Country | N | Follow-up (m) | Age | UI (%) | Patient | Inclusion | Men (%) | Death (%) | Association | Other association | ||
| UI | Controls | Un-adj | Adj | |||||||||||
| Anpalahan 2008 [15] | prosp | Australia | 110 | 3 | 83 | 16.4 | Geriatrics | Hospital | 31.8 | 33.3 | 3.3 | + * | + | HC(+), read(+), LOS(+) |
| Adams 2000 [16] | prosp | France | 45 | 48 | 41 | 20 | Fam amyl PNP | Community | 55.5 | 30.0 | 8.6 | + | + | - |
| Abrahamik 1993 [17] | prosp | France | 1025 | 2 | 78.1 | 41% | Geriatrics | Hospital | 43.5 | 20.2 | 10.1 | + | NA | IUC(+), LOS(+) |
| Baztan 2005 [18] | prosp | Spain | 205 | 6 | 80 | 68.6 | Geriatrics | Hospital | 39.5 | 14.9 | 3.1 | + | + | disability(+) |
| Berrios 1986 [19] | prosp | UK | 100 | 18 | 80.5 | 35 | Cognitive failure | Community | 40.8 | 68.6 | 35.4 | + | NA | - |
| Berardelli 2013 [20] | prosp | Italy | 570 | 84 | 73 + 92† | 32.1 | Geriatrics | Community | 43.3 | - | - | +/-‡ | +/-‡ | Frailty(+) |
| Bootsma 2013 [21] | prosp | Netherlands | 639 | 12 | 78.2 | 20.7 | Geriatrics | Hospital | 46.2 | 36.4 | 31.3 | +/-§ | - | HC(+), disability(+) |
| Brauer 1978 [22] | prosp | Denmark | 1486 | 24 | 80–89|| | NA | Geriatrics | HC | 34.7 | - | - | + | + | - |
| Campbell 1985¶ [23] | prosp | New Zealand | 559 | 36 | 80–84|| | 18.7 | Geriatrics | Community /HC | 35.2 | 72.5 | 34.6 | + | + | - |
| Campbell 1985¶ [24] | ||||||||||||||
| Chen 2010 [25] | prosp | Taiwan | 559 | 12 | 80.9 | 6.8 | Geriatrics | HC | 100 | 18.4 | 8.2 | +* | +* | - |
| Donaldson 1983¶ [26] | prosp | UK | 4490 | 36 | 75–84|| | NA | Geriatrics | Hospital/HC | NA | 52.7 | 42.7 | + | + | - |
| Donaldson 1980¶ [27] | 4514 | 12 | NA | |||||||||||
| Ekelund 1987 [28] | prosp | Sweden | 837 | 6 | NA | 27.9 | Geriatrics | Hospital | 37.6 | 36.9 | 19.5 | + | NA | HC(+) |
| Espallargues 2008 [29] | prosp | 6 countries | 1667 | 1 | 78.1 | 18.5 | Geriatrics | Hospital | 43.5 | - | - | + * | - * | HC(+), read(+), LOS(+) |
| Gambassi 1999 [30] | prosp | USA | 9264 | 23 | 82.1 | 60.5 | Alzheimer | HC | 30.8 | 55.5 | 41.4 | + | +/-** | - |
| Gavira 2005 [31] | prosp | Spain | 827 | 60 | 75–84|| | 39.8 | Geriatrics | Community | 41.2 | 23.0 | 20.7 | - | - | - |
| Goldfarb 1969 [32] | prosp | USA | 1280 | 84 | 75–84|| | 21 | Geriatrics | HC | 33.3 | 97.1 | 78.0 | + | NA | - |
| Herzog 1994 [33] | prosp | USA | 1956 | 72 | 60–69|| | 29.9 | Geriatrics | Community | 41.1 | 19.7 | 21.7 | - | - | - |
| Hollins 1998 [34] | prosp | UK | 2026 | 96 | NA | 39.9 | Learning disability | Community/ HC | NA | 21.1 | 8.1 | + | + | - |
| Holroyd-Leduc 2004 [35] | prosp | USA | 6506 | 24 | 77 | 14.8 | Geriatrics | Community | 37 | 10.9 | 8.7 | + | - | HC(-), disability(+) |
| First author/ year | Design | Country | N | Follow-up (m) | Age | UI (%) | Patient | Inclusion | Men (%) | Death (%) for | Association | Other association | ||
| UI | Controls | Unadj | Adj | |||||||||||
| John 2014 [6] | retro | Switzerland | 699 | 36 | 80 | 27.8 | Home care services | Community | 24.6 | 24.9 | 12.8 | + | + | HC(-), read(-) LOS(+) |
| Johnson 2000 [13] | prosp | USA | 3485 | 36 | 75–84|| | 28.7 | Geriatrics | Community | 51.5 | - | - | + | +/-§§ | - |
| Kohn 1991 [36] | prosp | Israel | 188 | 60 | 82.2 | 30.1 | Geriatrics | Hospital | 42.1 | 95.9 | 70.8 | + | NA | - |
| Koyano 1986 [37] | prosp | Japan | 2567 | 60 | 72.4 | 8.8 | Geriatrics | Community | 47.6 | 57.2 | 15.4 | + | NA | disability(+) |
| Krumholz 2001 [20] | prosp | USA | 103164 | 12 | 76.8 | 22.7 | Myocardial infarct | Hospital | 50.1 | 45.7 | 14.6 | + | + | - |
| Landi 2012 [38] | prosp | Italy | 2787 | 12 | 80.4 | 54.9 | Geriatrics | Community | 39.8 | - | - | + | NA | Low BMI(+) |
| Luk 2013 [39] | prosp | Hong Kong | 312 | 12 | 88 | 99 | Cognitive failure | HC | 22.8 | 34.3 | 33.3 | - | NA | - |
| Min 2009 [40] | prosp | USA | 649 | 60 | 82 | 35.9 | Geriatrics | Community | 37.2 | - | - | + | NA | - |
| Nakanishi 1999 [41] | prosp | Japan | 1405 | 42 | 65–74|| | 11.9 | Geriatrics | Community | 40.1 | 35.3 | 11.1 | +‡‡ | +‡‡ | - |
| Nuotio 2009¶ [42] | prosp | Finland | 398 | 72 | 70–79|| | 31.9 | Geriatrics | Community /HC | 43.5 | 42.5 | 25.9 | + | - | - |
| Nuotio 2002¶ [43] | 1052 | 120 | 73.3 | 5.6 | 49.8 | 78.0 | 47.8 | + | +/-†† | - | ||||
| Pagliacci 2007 [44] | prosp | Italy | 511 | 48 | 41.9 | NA | Spinal cord injury | Hospital | 80 | - | - | +‡‡ | +‡‡ | Read(+),LOS(+) complication(+) |
| Sorbye 2013 [45] | prosp | Norway | 331 | 12 | 84.2 | 49.2 | Hip fracture | Hospital | 20.2 | 20.2 | 10.1 | + | NA | HC (+), disability (+), IUC(+), fall(+) |
| Thom 1997 [5] | retro | USA | 5986 | 108 | 75–79|| | 6.1 | Geriatrics | Community | 49.8 | 40.9 | 25.5 | + | +/-†† | HC (+), read(+) |
| Tilvis 1995 [46] | prosp | Finland | 649 | 60 | 79.7 | 19.3 | Geriatrics | Hospital/HC | 26.3 | 39.5 | 22.7 | + | - | HC (-) |
| Venkatsen 1990 [22] | prosp | UK | 73 | 1.5 | 79 | 7 | Pneumonia >65y | Hospital | 52.1 | - | - | + | NA | - |
| Zweig 1990 [21] | retro | USA | 133 | 1 | 80 | 44.4 | Pneumonia >60y | Hospital | 45 | 20.3 | 12.2 | + | - | - |
* Urinary incontinence along with other geriatric symptoms.
† Two cohorts;
‡ association found among sever UI;
§ association found at three month not 12 month;
|| mode;
¶ same cohort published in two articles;
** only for moderate dementia;
†† association found for men, not women;
‡‡ for bowel and urinary loss of control.
§§ depending on the adjusted model considerate.
BMI: body mass index; fam amyl PNP: familial amyloidoic polyneuropathy; HC: home care; read: hospital readmission; IUC: indwelling urinary catheters; LOS: length of hospital stay; NA: not assessed; UI: urinary incontinence; unadj and adj: association between urinary incontinence and death unadjusted or adjusted for confounders; prosp: prospective study; retro: retrospective study; read: hospital readmission; >65y: patients older than 65 years old.
This review included 158 456 patients from nineteen countries. The prevalence of UI ranged from 5.6% to 99%, but the proportion of incontinent patients was unknown in four studies [17, 22,44,47]. The selected studies mainly explored the effect of UI in the general geriatric population. Nine articles assessed this effect on more specific populations such as individuals affected by cognitive failure [19], Alzheimer’s disease [30], spinal cord injury [44], post myocardial infarction [20], learning disability [34], pneumonia [21,22], post surgery after a hip fracture [45] and familial amyloid polyneuropathy after liver transplantation [16]. Time to follow-up ranged from four weeks [21,29] to ten years [43]. Twelve studies included hospitalized patients, 13 explored participants in the community, five among home care patients, and five studies among a mixture of settings (hospitalized and home care patients [26,46] or community and home care [23,34,42]).
Seven studies [15,22,29,38,40,44,47] included in the review could not be incorporated in the meta-analysis as part of the data on UI and mortality was lacking. The authors of those studies were unreachable or the databases were no longer available.
Unadjusted association between UI and death
All but three [31,33,48] out of 38 studies (92.1%) found a positive association between UI and death in unadjusted analysis. However some studies considered the association between death and UI along with other geriatric symptoms [15,25,29] or together with faecal incontinence [41,44]. The association was true only for severe UI in the study by Berardelli et al., and only found at three months (not afterwards) in the study by Bootsma et al.
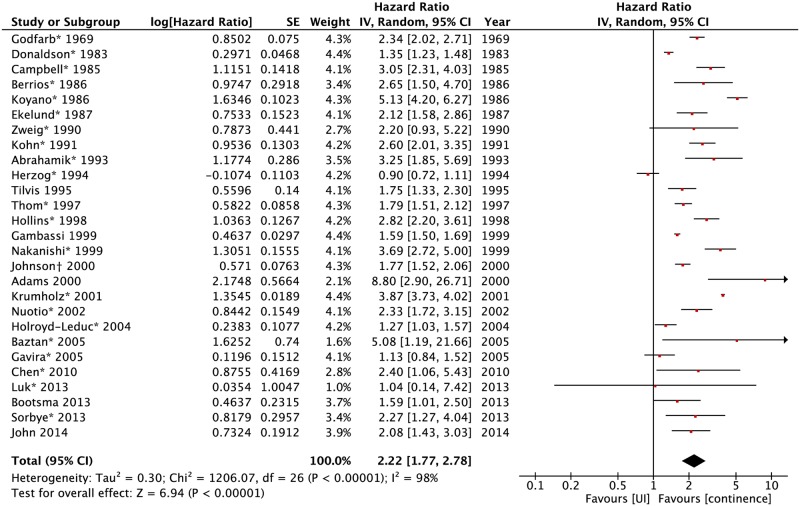
Unadjusted survival analysis was available in 5 studies (Fig 2). Estimated HR from published proportions of death was calculated for 21 studies. HR could be estimated from the Kaplan-Meier curve for one study [13]. The resulting pooled HR was 2.22 (95%CI: 1.77–2.78).
Fig 2. Forest plot of unadjusted HR of death for urinary incontinence.
* Estimated from the ratio of logarithms of event-free proportions from the published proportion of death in the exposed and unexposed groups. † HR could be estimated from the Kaplan-Meier curve for one study UI: urinary incontinence.
In a sensitivity analysis, a pooled analysis was done computing all OR published, stratified by their follow-up period (Fig A in S2 File). The result was statistically significant for all periods.
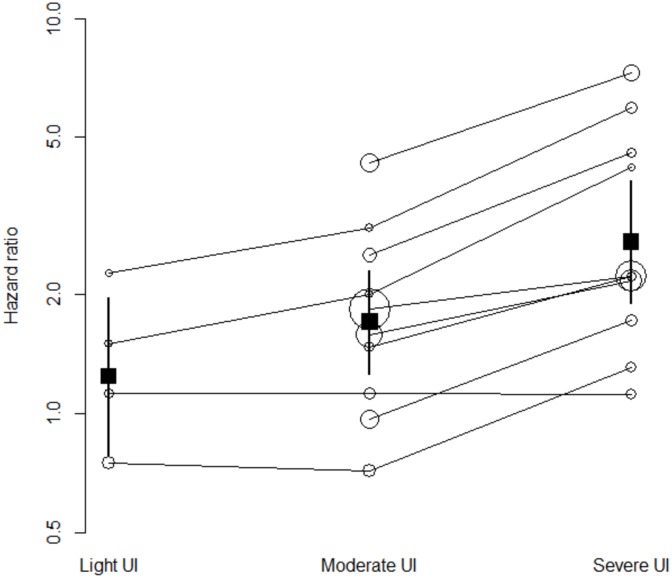
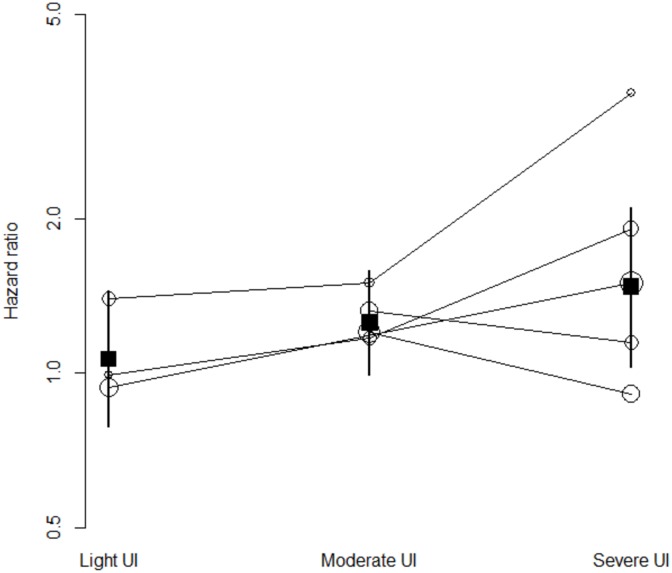
The association between UI and time to death increased gradually with the severity of leakage, when stratified according by published categories (light, moderate, severe) (Fig 3) or extracted frequencies of leakage episodes (Fig B in S2 File). In the analyses conducted with all studies, the pooled HRs were 1.24 (95%CI 0.79–1.97; I2 = 71%), 1.71 (95%CI 1.26–2.31; I2 = 92%), and 2.72 (95%CI 1.90–3.87; I2 = 92%) for light, moderate, and severe UI respectively (Fig 3). Four studies reported HRs for both light and severe UI. The pooled ratio of HRs was 1.83 (95%CI 1.16–2.89). Ten studies reported HRs for both moderate and severe UI. The pooled ratio of HRs was 1.47 (95%CI 1.28–1.69). Thus the HR in patients with severe UI was approximately 1.8 times the HR in patients with light UI and 1.5 times the HR in patients with moderate UI and those differences were statistically significant. Four studies reported HRs for both light and moderate UI. The pooled ratio of HRs was 1.08 (95%CI: 0.81–1.44): the HR was slightly greater in patients with moderate UI than in patients with light UI, but the difference was not statistically significant.
Fig 3. Unadjusted HRs of studies (white circles) and pooled HRs (black boxes) of death for urinary incontinence by (published) UI severity.
Circle-sizes are inversely proportional to studies' standard error. UI severity subgroups are bounded by solid lines. UI: urinary incontinence.
In seven studies, association between UI and death could be stratified by gender and another study included only men [25]. Although the HR was slightly higher for men 2.23 (95%CI: 1.45–3.42) compared to women 2.01 (95%CI: 1.19–3.38), there was no statistical difference in the logarithmic of HR between genders (Fig C in S2 File).
Adjusted association between UI and death
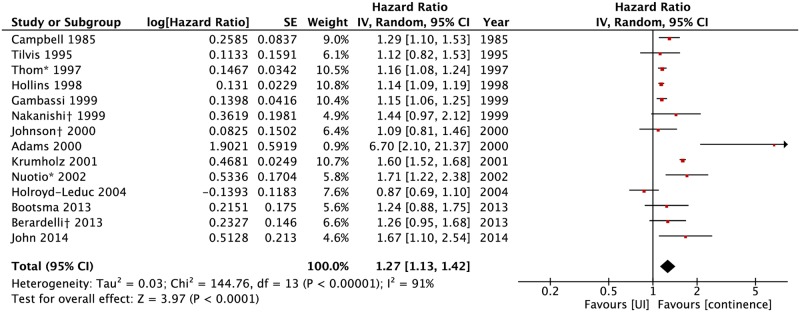
Twelve studies (31.6%) included in the review gave no adjusted results and eight (21.0%) showed no association between UI and death after adjustment for different confounders. Sixteen studies (42.2%) still showed a positive association in adjusted models (Table 1). Adjusted HRs were available in fourteen studies (Fig 4). UI was associated with death with a pooled HR of 1.27 (95%CI: 1.13–1.42).
Fig 4. Forest plot of adjusted HR of death for urinary incontinence.
Adjusted HR was published by gender (*) or severity of UI subgroups (†) only. A summarized HR was obtained through a meta-analysis of all subgroups for each of those studies. UI: urinary incontinence.
The association between UI and time to death increased gradually with the severity of leakage, when stratified according to published categories (Fig 5), or estimated frequencies of leakage episodes (Fig D in S2 File). In the analyses conducted with all studies, the pooled HRs were 1.07 (95%CI 0.79–1.44; I2 = 0%), 1.25 (95%CI 0.99–1.58; I2 = 0%), and 1.47 (95%CI 1.03–2.10; I2 = 61%) for light, moderate, and severe UI respectively (Fig 5). Three studies reported HRs for both light and severe UI. The pooled ratio of HRs was 1.79 (95%CI 1.23–2.61): the HR in patients with severe UI was approximately 1.8 times the HR in patients with light UI and the difference was statistically significant. Four studies reported HRs for both moderate and severe UI and two studies reported HRs for both light and moderate UI. The pooled ratio of HRs were 1.12 (95%CI 0.71–1.75) and 1.13 (95%CI 0.63–2.01), respectively: the HR was slightly greater in patients with severe UI than in patients with moderate UI, and in patients with moderate UI compared to patients with light UI, but those differences were not statistically significant.
Fig 5. Adjusted HRs (white circles) and pooled HRs (black boxes) of death for urinary incontinence by (published) UI severity.
Circle-sizes are inversely proportional to studies' standard error. UI severity subgroups are bounded by solid lines. UI: urinary incontinence.
In three studies, association between UI and death could be stratified by gender. The HRs for men and women were 1.50 (95%CI: 1.01–2.22) and 1.17 (95%CI: 1.00–1.37), respectively. There was no statistical difference between genders (Fig E in S2 File).
Study quality/risk of bias
Quality scale
The NOS is shown in Table A in S2 File. Quality was mainly limited by poor definition of exposure (UI) and comparability of groups. Many studies defined UI based on unspecified personal patient information, carer report [27,34,46,47,49], or medical records [5,20,21,26]. Only 19 reports were based on more reliable sources like specific questionnaires [41,42,50,13,17,40] or pre-existing scales such as the modified Barthel index [18,39], or the Minimum Data Set [6,30,38,45]. Information on the diagnosis of UI was lacking in many articles. In eight studies patients with indwelling urinary catheters were classified as being incontinent of urine [6,17,18,23,24,31,45,50]. However, except for five studies [13,15,28,39,49], most of the other studies gave no information regarding that consideration. Comparability was limited when none or low adjustment was reported.
Sources of heterogeneity
The results of stratified subgroup analysis are shown in Table 2. No statistically significant differences were found between subgroups that were observed both in unadjusted and adjusted models. For the unadjusted pool analysis, the country where the study took place explained part of the heterogeneity. In unadjusted models, the published HR subgroup represented 23.8% of the total population and had only mild heterogeneity (I2 48%). The variables of adjustment included in the models varied greatly, but could be regrouped into categories (Table B in S2 File). The high heterogeneity found in unadjusted models (I2 98%) decreased marginally in adjusted ones (I2 88%), particularly in the subgroup of highly adjusted models (I2 47%), but the subgroup difference was not statistically different.
Table 2. Subgroup analyses.
| Unadjusted analysis | Adjusted analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Factors | N studies | Pooled HR | Within strata | Between strata comparison | N studies | Pooled HR | Within strata | Between strata comparison |
| Publication year | ||||||||
| Less than 10 y | 7 | 1.77 (1.29 to 2.42) | 0.0004 | 3 | 1.34 (1.08 to 1.65) | 0.0070 | ||
| 10 to 20 y | 10 | 2.29 (1.59 to 3.30) | <0.0001 | 0.45 | 9 | 1.26 (1.09 to 1.46) | 0.0020 | 0.88 |
| More than 20 y | 10 | 2.30 (1.61 to 3.29) | <0.0001 | 2 | 1.25 (1.09 to 1.45) | 0.0002 | ||
| Study' continent | ||||||||
| America (North) | 7 | 1.83 (1.16 to 2.91) | <0.0001 | 0.02 | 5 | 1.17 (0.96 to 1.43) | 0.1300 | 0.56 |
| Asia/Oceania | 6 | 3.31 (2.44 to 4.49) | <0.0001 | 2 | 1.32 (1.13 to 1.53) | 0.0003 | ||
| Europe | 15 | 2.07 (1.75 to 2.45) | <0.0001 | 7 | 1.35 (1.11 to 1.65) | 0.0020 | ||
| Population studied | ||||||||
| General geriatrics | 18 | 2.06 (1.66 to 2.56) | <0.0001 | 0.42 | 9 | 1.19 (1.07 to 1.32) | 0.0010 | 0.19 |
| Other | 9 | 2.53 (1.62 to 3.97) | <0.0001 | 5 | 1.40 (1.12 to 1.75) | 0.0030 | ||
| Setting | ||||||||
| Hospital inpatients | 8 | 2.60 (1.92 to 3.52) | <0.0001 | 2 | 1.49 (1.20 to 1.86) | 0.0003 | ||
| Community | 10 | 2.07 (1.43 to 3.00) | <0.0001 | 7 | 1.23 (1.01 to 1.49) | 0.0400 | ||
| Home care | 4 | 1.94 (1.39 to 2.71) | <0.0001 | 0.61 | 1 | 1.15 (1.06 to 1.25) | 0.0008 | 0.16 |
| Mix | 5 | 2.14 (1.47 to 3.13) | <0.0001 | 4 | 1.24 (1.08 to 1.43) | 0.0030 | ||
| Follow-up | ||||||||
| <1 y | 4 | 2.38 (1.85 to 3.05) | <0.0001 | 0 | - | - | ||
| 1–5 y | 14 | 2.21 (1.57 to 3.10) | <0.0001 | 0.79 | 10 | 1.30 (1.09 to 1.55) | 0.0040 | 0.23 |
| >5 y | 9 | 2.06 (1.47 to 2.88) | <0.0001 | 4 | 1.16 (1.10 to 1.23) | <0.0001 | ||
| Design | ||||||||
| Prospective | 13 | 2.20 (1.71 to 2.82) | <0.0001 | 0.68 | 12 | 1.26 (1.10 to 1.45) | 0.0009 | 0.85 |
| Retrospective | 3 | 2.05 (1.63 to 2.57) | <0.0001 | 2 | 1.31 (0.94 to 1.84) | 0.1200 | ||
| Adjustment level* | ||||||||
| Low | - | - | - | - | 6 | 1.31 (1.09 to 1.57) | 0.0040 | 0.54 |
| High | - | - | - | 8 | 1.22 (1.06 to 1.40) | 0.0070 | ||
* Highly adjusted models are those with adjustment for at least both functional status and neurological deficit.
HR: hazard ratio; NOQ scale: Newcastle-Ottawa Quality Assessment scale; UIC: urinary indwelling catheters; y: years
Risk of publication bias
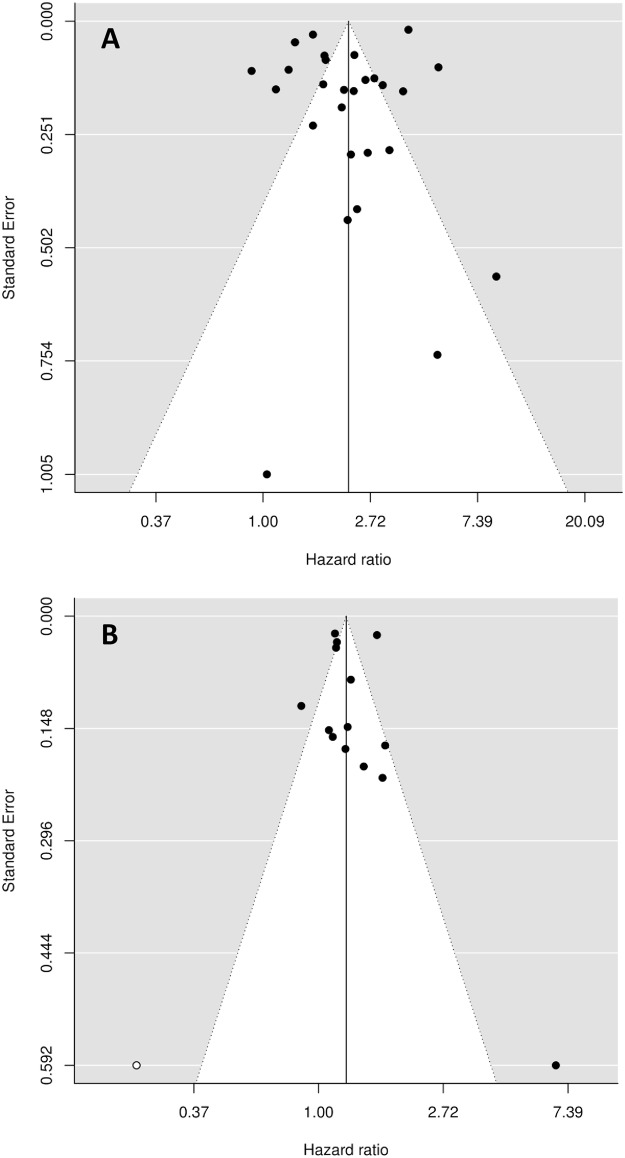
Funnel plots showed no obvious publication bias and Egger test failed to detect heterogeneity (Fig 6). However, for the adjusted association between UI and death, the Fill and Trim method detected one missing study on the left part of the funnel plot. When adding this hypothetical study, the pooled HR was not affected: 1.25 (95%CI 1.11–1.40).
Fig 6. Funnel plot for unadjusted (panel A) or adjusted (panel B) HR of death.
Panel A: With The Trim and Fill approach, no missing study was detected. Panel B: With The Trim and Fill approach, one missing study was detected on the left part of the funnel plot (white dot).
Sensitivity analyses
The pooled published ORs gave the same results as the pooled estimated HRs. Taking out all studies one by one did not alter the direction of the association. However in adjusted analysis, the study by Krumholz et al. explained part of the heterogeneity as the I2 decreased from 91% to 55% without changing the extent of the association. This large-scale prospective study explores a specific population of elderly patients after heart infarct.
When restricting the analysis to studies with good to fair quality in each of the three domains of the NOS, no differences were noted for most of the adjusted and unadjusted pool estimates (Table C in S2 File). However, the pooled unadjusted HR dropped to 1.85 (95%CI: 1.33–2.58), when studies with poor quality in the domain of “comparability of groups” were excluded. When studies with poor quality in the domain of “selection of study groups" were excluded, the pooled adjusted HR was 1.17 (95%CI: 1.04–1.32).
Discussion
Our study confirms that UI is associated with a higher risk of death in the general (geriatric) population. The association is also seen in a broad range of specific conditions (e.g.: myocardial infarct, hip fracture, or cognitive impairment) and in all settings (hospitalized patients, nursing home residents, and patients living in the community). The risk increases with the severity and number of events of urinary leakage, exists for men and women, and persists in adjusted survival regression models.
Only four studies retrieved in this systematic review gave the cause of death and no assumption could be made for a different cause in the subgroup of UI patients [16,22,34,39]. Pneumonia represented 20–65% of the deaths. However these studies were not representative of the general population and death might be bound to the disease/condition itself (eg infection after immunosuppressant [16], or pneumonia for patients with neurologic impairment [22,34,39]).
The association between UI and death is probably multifactorial. On one hand, risk factors for the development of UI by themselves have a negative impact on survival. Indeed this meta-analysis shows that the association is closely tied to age, comorbid conditions, and disability, since the pooled HR of 2.2 is reduced to 1.3 when pooling adjusted models. For this reason, unadjusted HR should not be interpreted on its own and studies exploring UI and death should report adjusted HR. On the other hand, UI gives rise to multiple unfavourable outcomes [7], such as increased risk of falls and related injuries [10,51,52], depression [53], and infections [54–56]. Infections affect 20% of patients with UI and cause a mortality rate of 0.3%. Falls and depression increase mortality by 15% and 17% respectively [57]. However, the exact interaction between fall, depression, infection, and UI is difficult to assess and must have multiple interconnections, since all of those symptoms are frequent in the general elderly population and share many confounding factors. Thus, mortality is probably not entirely explained by those conditions.
New evidence and understanding of the pathophysiology of UI have gone way beyond the simple “mechanical” model of UI. Higher intakes of some micronutrients such as calcium [58], vitamin B12, and Zinc [59], as well as the total energy consumption, and saturated (opposed to polyunsaturated) fat are associated with UI [59,60]. Indeed, the most promising gene associated with lower urinary tract symptoms is a variant of the vitamin D receptor [61]. Vitamin D and calcium have both been extensively studied and associated with death [62–64]. Finally, recurrent infections, and/or a specific microbiota in the bladder of UI patients [65–67] might trigger a systemic mechanism. The genetics, the microbiota, and the nutritional theories are promising and could offer a perspective for future research to find the missing rational link between UI and death.
A causal association is supported by the dose response observed across studies. Nevertheless, meta-analysis of observational studies always face methodological limits. The remaining effect after adjustment for confounding factors (like age, disability and comorbid conditions) may be explained by a persisting confounding effect (under adjustment), or a publication bias (only significant adjusted models, or only models where the UI is associated with death are published). In favour of the last two hypotheses is the fact that the effect of published models is close to each other, independently of the number of the variables used to adjust. Published models would be those with a maximum covariate and persistent positive effect (or with only a small loss of statistical significance). Furthermore, not all adjusted models included all-important confounding factors. To overcome this hypothesis would necessitate a meta-analysis of individual data and/or to adjust all models with the same confounding factors. The second argument for bias unrecognized by standard evaluation is the fact that one third of studies gave no adjusted models.
To our knowledge, there are no studies exploring specific UI treatments with drugs or surgery using mortality as the main outcome. Future studies exploring the decreased mortality after UI treatment would strengthen the causal hypothesis. A relative drawback comes from the fact that some interventions to reduce UI (weight loss/bariatric surgery or treatments addressing the disability) are also prone to affect mortality [68,69]. Causal or not, the association between UI and death is strong, and could offer–by simply UI and its severity assessment- an overall mortality risk indicator. Unfortunately, UI is still often overlooked with only around half of UI patients seeking medical help [70].
The strength of our study is the use of HR (reported, calculated or estimated) as an effect estimate to pool results. Many published models report ORs, but this measure of association overestimates the real ratio of incidence, especially when the event is frequent (more than 10%) or follow-up is long, and is limited to the specific time-point considered (e.g. at 1 month post inclusion) [12]. The two strategies of UI severity stratification make the dose-response more reliable. Nevertheless, this meta-analysis has several limitations. Firstly, the definition of UI used is inconsistent across studies and often based on unreliable sources (eg medical chart or not specified). Secondly, most pooled HRs were estimated from the proportion of deaths. This measure does not take into account the loss to follow-up. However, the sensitivity analysis pooling published OR from a given proportion of deaths gave similar results. Thirdly, we could not stratify the analyses on UI subtype (urge, stress and mixed type incontinence), which have different risk factors and possibly different impacts on mortality.
Conclusion
UI is a predictor of higher mortality in the general and particularly in the geriatric population. The association increases with the severity of UI and persists when pooling models adjusted for confounders. UI being a widely spread disorder, more attention should be given to the elderly in terms of its screening and treatment.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
We gratefully acknowledge Aleksandra Porowska and Gaël Gosteli for the advices on the final manuscript, and Ezgi Dilek Demirtas and Joël Spaltenstein for their correction of the English manuscript.
Abbreviations
- HR
hazard ratio
- NOS
the Newcastle-Ottawa Quality Assessment scale
- OR
odds ratio
- UI
urinary incontinence
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187(1):116–26. [DOI] [PubMed] [Google Scholar]
- 2.Markland AD, Richter HE, Fwu CW, Eggers P, Kusek JW. Prevalence and trends of urinary incontinence in adults in the United States, 2001 to 2008. J Urol 2011;186(2):589–93. 10.1016/j.juro.2011.03.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedretdinova D, Fritel X, Panjo H, Ringa V. Prevalence of Female Urinary Incontinence in the General Population According to Different Definitions and Study Designs. Eur Urol 2015. [DOI] [PubMed] [Google Scholar]
- 4.Jerez-Roig J, Santos MM, Souza DL, Amaral FL, Lima KC. Prevalence of urinary incontinence and associated factors in nursing home residents. Neurourol Urodyn 2014. [DOI] [PubMed] [Google Scholar]
- 5.Thom DH, Haan MN, Van Den Eeden SK. Medically recognized urinary incontinence and risks of hospitalization, nursing home admission and mortality. Age Ageing 1997;26(5):367–74. [DOI] [PubMed] [Google Scholar]
- 6.John G, Gerstel E, Jung M, Dallenbach P, Faltin D, Petoud V, et al. Urinary incontinence as a marker of higher mortality in patients receiving home care services. BJU Int 2014;113(1):113–9. 10.1111/bju.12359 [DOI] [PubMed] [Google Scholar]
- 7.Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Comorbidities and personal burden of urgency urinary incontinence: a systematic review. Int J Clin Pract 2013;67(10):1015–33. 10.1111/ijcp.12164 [DOI] [PubMed] [Google Scholar]
- 8.Faltin DL. [Epidemiology and definition of female urinary incontinence]. J Gynecol Obstet Biol Reprod (Paris) 2009;38(8 Suppl):S146–52. [DOI] [PubMed] [Google Scholar]
- 9.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 2011;108(7):1132–8. 10.1111/j.1464-410X.2010.09993.x [DOI] [PubMed] [Google Scholar]
- 10.Brown JS, Vittinghoff E, Wyman JF, Stone KL, Nevitt MC, Ensrud KE, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc 2000;48(7):721–5. [DOI] [PubMed] [Google Scholar]
- 11.Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7(27):iii–x, 1–173. [DOI] [PubMed] [Google Scholar]
- 12.Perneger TV. Estimating the relative hazard by the ratio of logarithms of event-free proportions. Contemp Clin Trials 2008;29(5):762–6. 10.1016/j.cct.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 13.Johnson TM 2nd, Bernard SL, Kincade JE, Defriese GH. Urinary incontinence and risk of death among community-living elderly people: results from the National Survey on Self-Care and Aging. J Aging Health 2000;12(1):25–46. [DOI] [PubMed] [Google Scholar]
- 14.Borenstein M. Introduction to meta-analysis. Chichester, U.K: John Wiley & Sons, 2009. [Google Scholar]
- 15.Anpalahan M, Gibson SJ. Geriatric syndromes as predictors of adverse outcomes of hospitalization. Intern Med J 2008;38(1):16–23. [DOI] [PubMed] [Google Scholar]
- 16.Adams D, Samuel D, Goulon-Goeau C, Nakazato M, Costa PM, Feray C, et al. The course and prognostic factors of familial amyloid polyneuropathy after liver transplantation. Brain 2000;123 (Pt 7):1495–504. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamik A, Leblond JB, Perilliat I, Henry O, Loison M, De Madet M, et al. [Evaluation and clinical management of urination disorders in 1,025 patients in a geriatric department]. Ann Med Interne (Paris) 1993;144(2):92–6. [PubMed] [Google Scholar]
- 18.Baztan JJ, Arias E, Gonzalez N, Rodriguez de Prada MI. New-onset urinary incontinence and rehabilitation outcomes in frail older patients. Age Ageing 2005;34(2):172–5. [DOI] [PubMed] [Google Scholar]
- 19.Berrios GE. Urinary incontinence and the psychopathology of the elderly with cognitive failure. Gerontology 1986;32(2):119–24. [DOI] [PubMed] [Google Scholar]
- 20.Krumholz HM, Chen J, Chen YT, Wang Y, Radford MJ. Predicting one-year mortality among elderly survivors of hospitalization for an acute myocardial infarction: results from the Cooperative Cardiovascular Project. J Am Coll Cardiol 2001;38(2):453–9. [DOI] [PubMed] [Google Scholar]
- 21.Zweig S, Lawhorne L, Post R. Factors predicting mortality in rural elderly hospitalized for pneumonia. J Fam Pract 1990;30(2):153–9. [PubMed] [Google Scholar]
- 22.Venkatesan P, Gladman J, Macfarlane JT, Barer D, Berman P, Kinnear W, et al. A hospital study of community acquired pneumonia in the elderly. Thorax 1990;45(4):254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell AJ, Diep C, Reinken J, McCosh L. Factors predicting mortality in a total population sample of the elderly. J Epidemiol Community Health 1985;39(4):337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell AJ, Reinken J, McCosh L. Incontinence in the elderly: prevalence and prognosis. Age Ageing 1985;14(2):65–70. [DOI] [PubMed] [Google Scholar]
- 25.Chen LK, Peng LN, Lin MH, Lai HY, Hwang SJ, Lan CF. Predicting mortality of older residents in long-term care facilities: comorbidity or care problems? J Am Med Dir Assoc;11(8):567–71. 10.1016/j.jamda.2009.11.012 [DOI] [PubMed] [Google Scholar]
- 26.Donaldson LJ, Jagger C. Survival and functional capacity: three year follow up of an elderly population in hospitals and homes. J Epidemiol Community Health 1983;37(3):176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donaldson LJ, Clayton DG, Clarke M. The elderly in residential care: mortality in relation to functional capacity. J Epidemiol Community Health 1980;34(2):96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekelund P, Rundgren A. Urinary incontinence in the elderly with implications for hospital care consumption and social disability. Arch Gerontol Geriatr 1987;6(1):11–8. [DOI] [PubMed] [Google Scholar]
- 29.Espallargues M, Philp I, Seymour DG, Campbell SE, Primrose W, Arino S, et al. Measuring case-mix and outcome for older people in acute hospital care across Europe: the development and potential of the ACMEplus instrument. QJM 2008;101(2):99–109. 10.1093/qjmed/hcm136 [DOI] [PubMed] [Google Scholar]
- 30.Gambassi G, Landi F, Lapane KL, Sgadari A, Mor V, Bernabei R. Predictors of mortality in patients with Alzheimer's disease living in nursing homes. J Neurol Neurosurg Psychiatry 1999;67(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavira Iglesias F, Caridad YOJM, Guerrero Munoz JB, Lopez Perez M, Romero Lopez M, Pavon Aranguren MV. [Five-year follow-up of urinary incontinence in older people in a Spanish rural population]. Aten Primaria 2005;35(2):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldfarb AI. Predicting mortality in the institutionalized aged. A seven-year follow-up. Arch Gen Psychiatry 1969;21(2):172–6. [DOI] [PubMed] [Google Scholar]
- 33.Herzog AR, Diokno AC, Brown MB, Fultz NH, Goldstein NE. Urinary incontinence as a risk factor for mortality. J Am Geriatr Soc 1994;42(3):264–8. [DOI] [PubMed] [Google Scholar]
- 34.Hollins S, Attard MT, von Fraunhofer N, McGuigan S, Sedgwick P. Mortality in people with learning disability: risks, causes, and death certification findings in London. Dev Med Child Neurol 1998;40(1):50–6. [PubMed] [Google Scholar]
- 35.Holroyd-Leduc JM, Mehta KM, Covinsky KE. Urinary incontinence and its association with death, nursing home admission, and functional decline. J Am Geriatr Soc 2004;52(5):712–8. [DOI] [PubMed] [Google Scholar]
- 36.Kohn D, Sinoff G, Strulov A, Ciechanover M, Wei JY. Long-term follow-up of patients aged 75 years and older admitted to an acute care hospital in Israel. Aging (Milano) 1991;3(3):279–85. [DOI] [PubMed] [Google Scholar]
- 37.Koyano W, Shibata H, Haga H, Suyama Y. Prevalence and outcome of low ADL and incontinence among the elderly: five years follow-up in a Japanese urban community. Arch Gerontol Geriatr 1986;5(3):197–206. [DOI] [PubMed] [Google Scholar]
- 38.Landi F, Liperoti R, Lattanzio F, Russo A, Tosato M, Barillaro C, et al. Effects of anorexia on mortality among older adults receiving home care: an observation study. J Nutr Health Aging;16(1):79–83. [DOI] [PubMed] [Google Scholar]
- 39.Luk JKH, Chan WK, Ng WC, Chiu PKC, Ho C, Chan TC, et al. Mortality and health services utilisation among older people with advanced cognitive impairment living in residential care homes. Hong Kong Med J;19(6):518–24. 10.12809/hkmj133951 [DOI] [PubMed] [Google Scholar]
- 40.Min L, Yoon W, Mariano J, Wenger NS, Elliott MN, Kamberg C, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc 2009;57(11):2070–6. 10.1111/j.1532-5415.2009.02497.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakanishi N, Tatara K, Shinsho F, Murakami S, Takatorige T, Fukuda H, et al. Mortality in relation to urinary and faecal incontinence in elderly people living at home. Age Ageing 1999;28(3):301–6. [DOI] [PubMed] [Google Scholar]
- 42.Nuotio M, Luukkaala T, Tammela TL, Jylha M. Six-year follow-up and predictors of urgency-associated urinary incontinence and bowel symptoms among the oldest old: a population-based study. Arch Gerontol Geriatr 2009;49(2):e85–90. 10.1016/j.archger.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 43.Nuotio M, Tammela TL, Luukkaala T, Jylha M. Urgency and urge incontinence in an older population: ten-year changes and their association with mortality. Aging Clin Exp Res 2002;14(5):412–9. [DOI] [PubMed] [Google Scholar]
- 44.Pagliacci MC, Franceschini M, Di Clemente B, Agosti M, Spizzichino L. A multicentre follow-up of clinical aspects of traumatic spinal cord injury. Spinal Cord 2007;45(6):404–10. [DOI] [PubMed] [Google Scholar]
- 45.Sorbye LW, Grue EV. Hip fracture and urinary incontinence—use of indwelling catheter postsurgery. Scand J Caring Sci 2013;27(3):632–42. 10.1111/j.1471-6712.2012.01075.x [DOI] [PubMed] [Google Scholar]
- 46.Tilvis RS, Hakala SM, Valvanne J, Erkinjuntti T. Urinary incontinence as a predictor of death and institutionalization in a general aged population. Arch Gerontol Geriatr 1995;21(3):307–15. [DOI] [PubMed] [Google Scholar]
- 47.Brauer E, Mackeprang B, Bentzon MW. Prognosis of survival in a geriatric population. Scand J Soc Med 1978;6(1):17–24. [DOI] [PubMed] [Google Scholar]
- 48.Luk JK, Chan WK, Ng WC, Chiu PK, Ho C, Chan TC, et al. Mortality and health services utilisation among older people with advanced cognitive impairment living in residential care homes. Hong Kong Med J 2013;19(6):518–24. 10.12809/hkmj133951 [DOI] [PubMed] [Google Scholar]
- 49.Bootsma AM, Buurman BM, Geerlings SE, de Rooij SE. Urinary incontinence and indwelling urinary catheters in acutely admitted elderly patients: relationship with mortality, institutionalization, and functional decline. J Am Med Dir Assoc;14(2):147.e7–12. [DOI] [PubMed] [Google Scholar]
- 50.Berardelli M, De Rango F, Morelli M, Corsonello A, Mazzei B, Mari V, et al. Urinary incontinence in the elderly and in the oldest old: correlation with frailty and mortality. Rejuvenation Res 2013;16(3):206–11. 10.1089/rej.2013.1417 [DOI] [PubMed] [Google Scholar]
- 51.Oliver D, Daly F, Martin FC, McMurdo ME. Risk factors and risk assessment tools for falls in hospital in-patients: a systematic review. Age Ageing 2004;33(2):122–30. [DOI] [PubMed] [Google Scholar]
- 52.Hunter KF, Voaklander D, Hsu ZY, Moore KN. Lower urinary tract symptoms and falls risk among older women receiving home support: a prospective cohort study. BMC Geriatr 2013;13:46 10.1186/1471-2318-13-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farage MA, Miller KW, Berardesca E, Maibach HI. Psychosocial and societal burden of incontinence in the aged population: a review. Arch Gynecol Obstet 2008;277(4):285–90. [DOI] [PubMed] [Google Scholar]
- 54.Brogan E, Langdon C, Brookes K, Budgeon C, Blacker D. Can't swallow, can't transfer, can't toilet: factors predicting infections in the first week post stroke. J Clin Neurosci 2015;22(1):92–7. 10.1016/j.jocn.2014.05.035 [DOI] [PubMed] [Google Scholar]
- 55.Beeckman D, Van Lancker A, Van Hecke A, Verhaeghe S. A systematic review and meta-analysis of incontinence-associated dermatitis, incontinence, and moisture as risk factors for pressure ulcer development. Res Nurs Health 2014;37(3):204–18. 10.1002/nur.21593 [DOI] [PubMed] [Google Scholar]
- 56.Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA 2014;311(8):844–54. 10.1001/jama.2014.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zivin K, Yosef M, Miller EM, Valenstein M, Duffy S, Kales HC, et al. Associations between depression and all-cause and cause-specific risk of death: a retrospective cohort study in the Veterans Health Administration. J Psychosom Res 2015;78(4):324–31. 10.1016/j.jpsychores.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 58.Maserejian NN, Giovannucci EL, McVary KT, McKinlay JB. Intakes of vitamins and minerals in relation to urinary incontinence, voiding, and storage symptoms in women: a cross-sectional analysis from the Boston Area Community Health survey. Eur Urol 2011;59(6):1039–47. 10.1016/j.eururo.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dallosso H, Matthews R, McGrother C, Donaldson M. Diet as a risk factor for the development of stress urinary incontinence: a longitudinal study in women. Eur J Clin Nutr 2004;58(6):920–6. [DOI] [PubMed] [Google Scholar]
- 60.Maserejian NN, Giovannucci EL, McVary KT, McGrother C, McKinlay JB. Dietary macronutrient and energy intake and urinary incontinence in women. Am J Epidemiol 2010;171(10):1116–25. 10.1093/aje/kwq065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cartwright R, Mangera A, Tikkinen KA, Rajan P, Pesonen J, Kirby AC, et al. Systematic review and meta-analysis of candidate gene association studies of lower urinary tract symptoms in men. Eur Urol 2014;66(4):752–68. 10.1016/j.eururo.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asemi Z, Saneei P, Sabihi SS, Feizi A, Esmaillzadeh A. Total, dietary, and supplemental calcium intake and mortality from all-causes, cardiovascular disease, and cancer: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 2015;25(7):623–34. 10.1016/j.numecd.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 63.Zittermann A, Prokop S. The role of vitamin D for cardiovascular disease and overall mortality. Adv Exp Med Biol 2014;810:106–19. [DOI] [PubMed] [Google Scholar]
- 64.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2014;1:CD007470 10.1002/14651858.CD007470.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brubaker L, Wolfe AJ. The new world of the urinary microbiota in women. Am J Obstet Gynecol 2015;213(5):644–9. 10.1016/j.ajog.2015.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pearce MM, Zilliox MJ, Rosenfeld AB, Thomas-White KJ, Richter HE, Nager CW, et al. The female urinary microbiome in urgency urinary incontinence. Am J Obstet Gynecol 2015;213(3):347.e1–47.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pearce MM, Hilt EE, Rosenfeld AB, Zilliox MJ, Thomas-White K, Fok C, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio 2014;5(4):e01283–14. 10.1128/mBio.01283-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Subak LL, King WC, Belle SH, Chen JY, Courcoulas AP, Ebel FE, et al. Urinary Incontinence Before and After Bariatric Surgery. JAMA Intern Med 2015;175(8):1378–87. 10.1001/jamainternmed.2015.2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Subak LL, Wing R, West DS, Franklin F, Vittinghoff E, Creasman JM, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med 2009;360(5):481–90. 10.1056/NEJMoa0806375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duralde ER, Walter LC, Van Den Eeden SK, Nakagawa S, Subak LL, Brown JS, et al. Bridging the Gap: Determinants of Undiagnosed or Untreated Urinary Incontinence in Women. Am J Obstet Gynecol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.