Abstract
This assay is used to count the number of cells that have undergone apoptosis. Apoptosis will be detected by initially staining the cells with Annexin V and propidium Iodide solution followed by flow cytometry analysis. It is based on the principle that normal cells are hydrophobic in nature as they express phosphatidyl serine in the inner membrane (side facing the cytoplasm) and when the cells undergo apoptosis, the inner membrane flips to become the outer membrane, thus exposing phosphatidyl serine. The exposed phosphatidyl serine is detected by Annexin V, and propidium iodide stains the necrotic cells, which have leaky DNA content that help to differentiate the apoptotic and necrotic cells.
Materials and Reagents
Annexin V FLUOS staining kit (F. Hoffmann-La Roche, catalog number: 11858777001)
The kit contains ready-to-use Annexin-V-FLUOS solution, propidium iodide solution, and incubation buffer
Trypsin
NaCl
KCl
Na2HPO4
KH2PO4
PBS buffer (pH 7.4) (see Recipes)
Equipment
Flow cytometer
Centrifuge
T25 culture flask
Procedure
Seed cells (1 × 106 cells) in a T25 culture flask (in triplicate for experiments) and three T25 culture flask for control (unstained, Annexin only, and propidium iodide only).
After 48 h incubation, collect the supernatant (floating apoptotic cells) and trypsinize the adherent cells (~2 × 106 cells) from each T25 flask (combine respective floating and trypsinized cells resulting in six tubes).
Wash the collected cells twice with PBS and centrifuge (670 × g, 5 min, RT).
-
Re-suspend each pellet (~2 × 106 cells) in PBS (400 µl).
For experimental cells (Triplicate) - (400 µl of cells + 100 µl of incubation buffer with 2 µl of Annexin [1 mg/ml] and 2µl of propidium iodide [1 mg/ml]).
For control cells
Control 1: (unstained) - (without any stain (400 µl of cells + 100 µl of incubation buffer)
Control 2: (Annexin V only) - (400 µl of cells + 100 µl of incubation buffer with 2 µl of Annexin (1 mg/ml))
Control 3: (propidium iodide only) - (400 µl of cells + 100 µl of incubation buffer with 2 µl of propidium iodide (1 mg/ml))
-
Analyze the cells using a flow cytometry without washing the cells
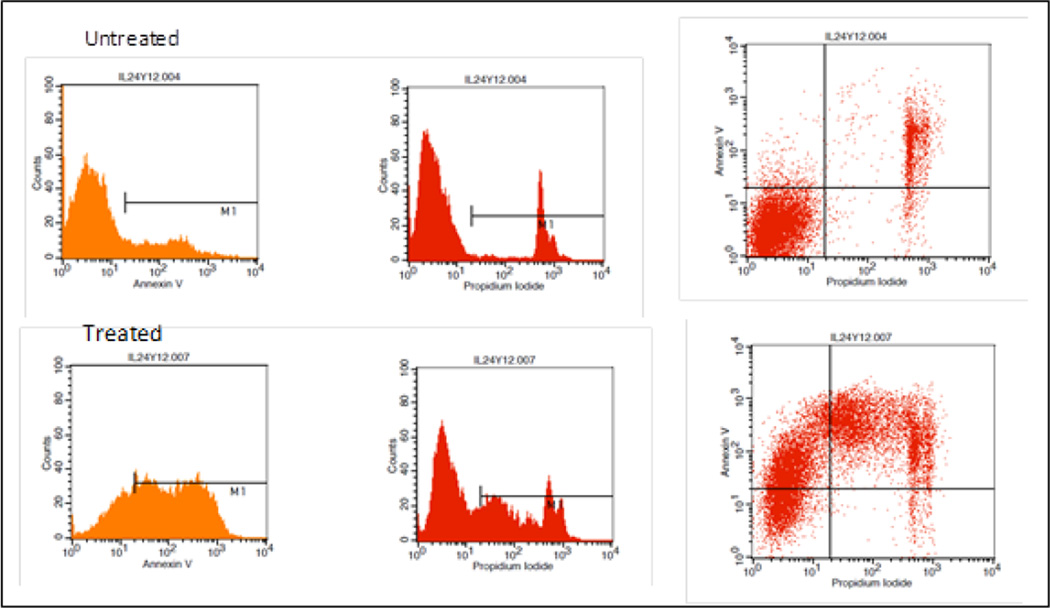
Cells that were propidium iodide (PI) negative and Annexin V negative are considered healthy, cells, PI negative and Annexin V positive cells are considered apoptotic, and cells that are positive to both PI and Annexin V considered necrotic (Figure 1).
Figure 1.
Recipes
-
PBS buffer (pH 7.4)
8 g NaCl
0.2 g KCl
2.3 g Na2HPO4
0.22 g KH2PO4
In 800 ml of distilled H2O adjust the pH to 7.4.
Acknowledgments
The authors thank Dr. Palanimuthu Ponnusamy Moorthy and Dr. Dhanya Haridas for critical reading of the apoptosis experimental protocol. We also thank Philip Hexley and Victoria Smith, Flow Cytometry Research Facility at UNMC, for their support.
References
- 1.Lakshmanan I, Ponnusamy MP, Das S, Chakraborty S, Haridas D, Mukhopadhyay P, Lele SM, Batra SK. MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene. 2012;31(7):805–817. doi: 10.1038/onc.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]