Abstract
Embryonic hair follicle (HF) induction and formation is dependent on signaling crosstalk between the dermis and specialized dermal condensates on the mesenchymal side and epidermal cells and incipient placodes on the epithelial side, but the precise nature and succession of signals remain unclear. Platelet Derived Growth Factor (PDGF) signaling is involved in the development of several organs and the maintenance of adult tissues, including HF regeneration in the hair cycle. As both PDGF receptors, PDGFRα and PDGFRβ, are expressed in embryonic dermis and dermal condensates, we explored in this study the role of PDGF signaling in HF induction and formation in the developing skin mesenchyme. We conditionally ablated both PDGF receptors with Tbx18Cre in early dermal condensates before follicle formation, and with Prx1-Cre broadly in the ventral dermis prior to HF induction. In both PDGFR double mutants, HF induction and formation ensued normally, and the pattern of HF formation and HF numbers were unaffected. These data demonstrate that mesenchymal PDGF signaling, either in the specialized niche or broadly in the dermis, is dispensable for HF induction and formation.
Background
Hair follicle (HF) induction and formation is a highly complex process controlled by successive signals between epidermal cells and incipient placodes on the epithelial side and the dermis and specialized dermal condensates (DC) as the mesenchymal counterpart (1). Several studies have identified key signaling pathways that are involved in the regulation of HF induction and formation, including Wnt, Eda/Edar/NFkB, Fgf and Shh signaling (reviewed in (1)). Platelet Derived Growth Factor (PDGF) signaling is instrumental in embryonic development and adult tissue function of several tissues, including gonads, lung, kidney, intestine, brain and skin (2). Global deletion of the PDGF receptors, PDGFRα and PDGFRβ, in knockout mice results in early embryonic lethality with specific defects suggesting unique physiological functions (2). However, both receptors mostly share overlapping expression patterns suggesting functional compensation in several tissues. In the skin, mice lacking PDGFRα exhibit strong skin defects including widespread dermal hypoplasia (3), while PDGF-A null mice show increasing loss of dermal mesenchyme and reduced hair development after birth (4). PDGF signaling was also suggested to be instrumental for HF regeneration during the hair cycle (s1). Finally, neonatal pups or embryonic skins treated with blocking antibodies against PDGFRα failed to form HFs (5, s2). In this study we determined the role of PDGF signaling in HF induction and formation with definitive genetic methods by conditionally ablating both PDGF receptors in the developing dermis and DCs.
Questions addressed
Does dermal PDGF signaling play a role during HF induction and/or formation?
Experimental design
To assess the potential involvement of dermal PDGF signaling in HF formation we ablated PDGFRα and PDGFRβ specifically in the DC at E14.5 using previously described Tbx18Cre mice (6). Prx1-Cre mice were used to ablate PDGFRs in the entire ventral dermis before DC formation (7) to test a potential role of PDGF signaling in HF induction. More detailed information is available in the supplementary materials and methods section.
Results
In the skin, previous reports have linked PDGF signaling to dermis development and the control of adult hair regeneration in the hair cycle (4, s1). To identify a potential role of this pathway in dermal condensates (DC) during embryonic HF morphogenesis, we first confirmed the expression of PDGFRα and PDGFRβ at E14.5, the beginning of HF formation after induction. Expression of both PDGF receptors was detected by qRT-PCR in the dermal compartment of E14.5 back skin (Fig. 1a). Immunofluorescence staining for both PDGFRs confirmed broad expression in the dermis and in DC cells in both dorsal and ventral skin (Fig. 1b, red). DCs were identified as GFP positive cell clusters (green) in Sox2GFP embryos (8) and staining for Edar marked HF placodes (white)(9).
Figure 1. PDGF receptors α and β are expressed in the dermis and dermal condensates of E14.5 skin and are dispensable for HF induction.
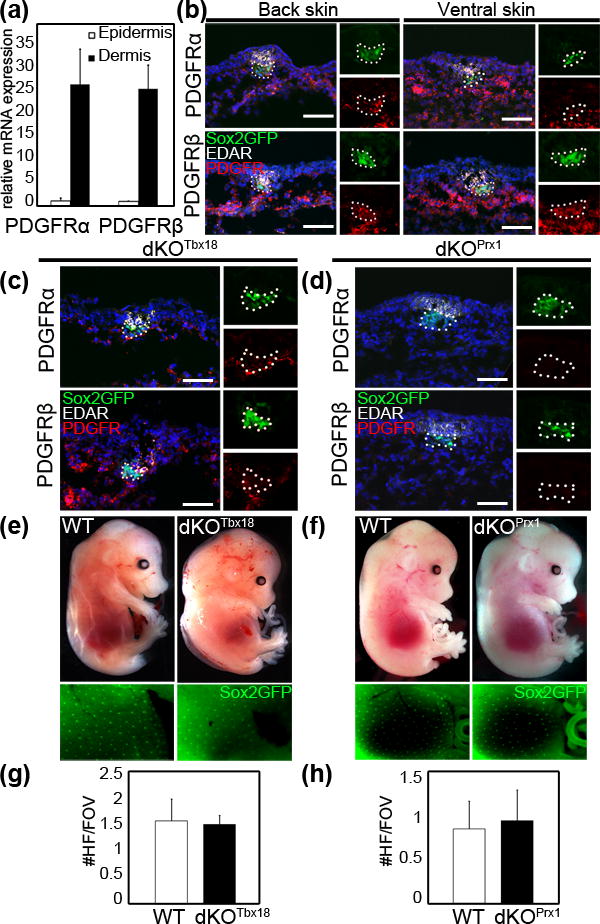
(a) qRT-PCR of FACS sorted cells from E14.5 back skin shows high PDGFRα and PDGFRβ expression in the dermis. (b) Immunofluorescence staining for PDGFRα and PDGFRβ demonstrating widespread expression in back and ventral skin at E14.5. Note that both PDGFRs are also expressed in GFP+ dermal condensates (DCs) of Sox2GFP+ mice. (c) Immunofluorescence staining of E14.5 dKOTbx18 back skin shows efficient PDGFRα and PDGFRβ ablation in Sox2GFP+ DCs. (d) Immunofluorescence staining of E14.5 dKOPrx1 ventral skin shows efficient ablation of PDGFRα and PDGFRβ in the entire dermis including Sox2GFP+ DCs. (e) E14.5 WT and dKOTbx18 show a comparable Sox2GFP+ DC pattern. (f) E14.5 WT and dKOPrx1 show a similar Sox2GFP+ DC pattern. (g–h) Quantification of HFs per field of view (FOV), assessed by staining for placode marker EDAR. HFs form in similar numbers in E14.5 dKOTbx18 (g) and dKOPrx1 (h) skin compared to controls (n≥3, ≥20 FOVs for each). Dapi (blue) highlighted nuclei. Scale bar = 50μm.
Next to explore the functional role of PDGF signaling during HF induction and formation, we ablated both PDGF receptors by crossing PDGFRαfl/fl;PDGFRβfl/fl double-floxed mice (s3, s4) with two different Cre lines in a Sox2GFP background: Tbx18Cre for ablation specifically in DCs at E14.5 in the back skin (6), and Prx1-Cre for knockout in the entire ventral dermis at E11.5 prior to HF induction (7). Efficient double knockout (dKO) gene ablation of both PDGFRα/β with Tbx18Cre (dKOTbx18) and Prx1-Cre (dKOPrx1) was confirmed by immunofluorescence at E14.5 (Fig. 1c, d). Some dKOTbx18 embryos presented a hemorrhage and edema phenotype (Fig. 1e, Fig. S1) as previously described for PDGFRα or PDGFRβ single null mutants (3, s5). Sox2GFP positive DCs were detectable in a similar pattern in dKO and WT controls after both broad dermal and DC-specific ablation (Fig. 1e, f). Likewise, staining for placode marker Edar (Fig. 1c, d) and subsequent quantification of formed placodes showed similar numbers in dKOTbx18 back skin (Fig. 1g) and dKOPrx1 ventral skin (Fig. 1h) compared to WT (Fig. 1b). Taken together, these data demonstrate that PDGF signaling is dispensable for HF induction, i.e. specification of placodes and DCs.
To assess a potential role of PDGF signaling in the DC and DP in HF downgrowth and formation we examined later stages of HF morphogenesis in dKOTbx18 and dKOPrx1 mutant skin. Analysis of E18.5 Hematoxylin/Eosin stained sections of dKOTbx18 back skin revealed normal HF formation without apparent morphological changes (Fig. 2a). Immunofluorescence staining for PDGFRα and β confirmed efficient ablation of both receptors in the entire dermis (Fig. S2a) as Tbx18Cre displays widespread dermal Cre activity after E16.5 (6). ITGA8+ DCs and DPs in developing HFs were identified (s6), confirming formation of 1st, 2nd and 3rd wave HFs in both WT and dKOTbx18 E18.5 back skins (Fig. S2a). Quantification of total HF numbers revealed no significant difference between WT and dKOTbx18 embryos (Fig. 2b), although dKOTbx18 skin had slightly fewer 3rd wave HFs than WT control (Fig. S2b). This minor difference might be due to broad ablation of PDGF signaling, but is likely caused by the onset of embryonic lethality, as E18.5 was the latest point we obtained dKOTbx18 mice (Fig. S2c). Thickness measurements of dKOTbx18 skin revealed significantly thinner dermis in double mutants compared to WT controls (Fig. 2c), which is consistent with similar observations in PDGF-A null mutants (4). dKOPrx1 mutants on the other hand were viable and developed normally. Analysis of ventral skin sections at P0 confirmed that both PDGFRs were absent (Fig. S2d). In this knockout model of broad dermal PDGFR ablation Hematoxylin/Eosin staining demonstrated normal HF formation in dKOPrx1 skin (Fig. 2d), and quantification of HF numbers showed no significant difference between dKOPrx1 and controls (Fig. 2e). As with dKOTbx18 embryos, the thickness of the dermis was strongly decreased in dKOPrx1 compared to WT (Fig. 2f). Taken together, both PDGFR double-mutant models demonstrate that dermal PDGF signaling is not required for HF formation and maturation.
Figure 2. PDGF signaling in the dermis and DCs is not required for HF formation.
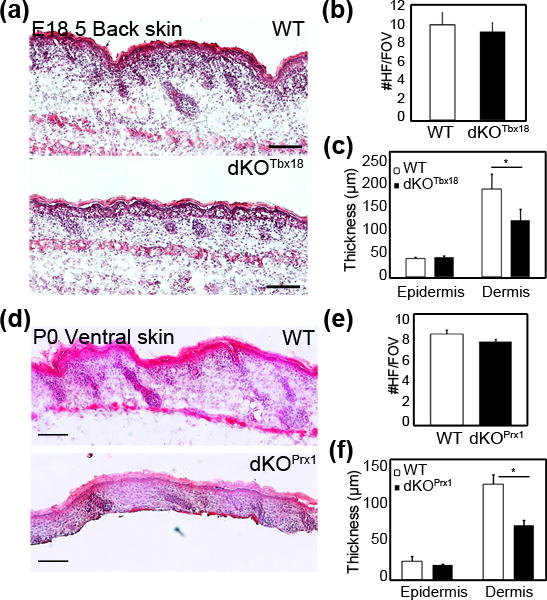
(a) Hematoxylin/eosin staining of E18.5 WT and dKOTbx18 back skin sections. (b) Quantification of total HFs per field of view (FOV; n=3, ≥50 FOVs for each). Comparable HF numbers in E18.5 WT and dKOTbx18 back skin. (c) Thickness measurement of E18.5 back skin (n=3, ≥30 FOVs for each). dKOTbx18 dermis is significantly thinner than WT. (d) Hematoxylin/eosin staining of E18.5 WT and dKOPrx1 ventral skin sections. (e) Quantification of total HFs per field of view (n=2, ≥20 FOVs for each). WT and dKOPrx1 show comparable HF numbers. (f) Thickness measurement of E18.5 ventral skin of WT and dKOPrx1 (n=2, ≥30 FOVs for each). Mutant dermis is significantly thinner than WT. *p<0.05 using Student t test. Scale bar = 100μm.
Conclusions
PDGF signaling has been involved in many developmental processes and was shown to be crucial for maintenance of different adult tissues. Previous reports suggested that activation of this pathway should be important for HF morphogenesis and regeneration. To specifically address its role in HF morphogenesis, we ablated both PDGFRs broadly in the dermis prior to HF induction and in DCs during 1st wave HF formation. We found that dermal PDGF signaling is not required for HF induction nor subsequent HF downgrowth and formation. Lastly we confirmed that dermal ablation of this pathway leads to a thinner dermis. Taken together, these results highlight the involvement of PDGF signaling in dermal development but show that it is dispensable for HF morphogenesis. The importance of PDGF signaling during HF regeneration remains to be assessed.
Supplementary Material
Acknowledgments
Many thanks to colleagues for sharing mice: Philippe Soriano (PDGFRα and PDGFRβ floxed), Larysa Pevny (Sox2GFP) and Chen-Leng Cai (Tbx18Cre). We thank the personnel of the Flow Cytometry Core Facility. A.R. was supported by Fondation pour la Recherche Médicale and Prix Claude Rozé/CECED. M.R. was supported by NIH/NIAMS grants (R01AR059143; R01AR063151) and the Irma T. Hirschl Trust fellowship.
Footnotes
Author contributions
A.R. and M.R. conceived and designed experiments, A.R., R.S. and M.T. performed experiments, C.C. contributed essential reagents and tools, A.R. and M.R. analyzed and interpreted data, A.R. prepared figures, and A.R. and M.R. wrote the manuscript.
Conflict of interests
The authors declare no conflict of interest.
References
- 1.Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012:917–927. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 3.Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson L, Bondjers C, Betsholtz C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 1999;126:2611–2621. doi: 10.1242/dev.126.12.2611. [DOI] [PubMed] [Google Scholar]
- 5.Gao J, DeRouen MC, Chen CH, et al. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 2008;22:2111–2124. doi: 10.1101/gad.1689908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grisanti L, Clavel C, Cai X, et al. Tbx18 targets dermal condensates for labeling, isolation, and gene ablation during embryonic hair follicle formation. J Invest Dermatol. 133:344–353. doi: 10.1038/jid.2012.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 8.Clavel C, Grisanti L, Zemla R, et al. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell. 23:981–994. doi: 10.1016/j.devcel.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sennett R, Rezza A, Dauber KL, Clavel C, Rendl M. Cxcr4 is transiently expressed in both epithelial and mesenchymal compartments of nascent hair follicles but is not required for follicle formation. Exp Dermatol. 23:748–750. doi: 10.1111/exd.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


