Abstract
Variations in adrenal and gonadal hormone profiles have been linked to increased rates of oppositional defiant disorder (ODD) and conduct disorder (CD). These relationships suggest that certain hormone profiles may be related to how well children respond to psychological treatments for ODD and CD. The current study assessed whether pre-treatment profiles of adrenal and gonadal hormones predicted response to psychological treatment of ODD and CD. One hundred five children, 6 – 11 years old, participating in a randomized, clinical trial provided samples for cortisol, testosterone, dehydroepiandrosterone, and androstenedione. Diagnostic interviews of ODD and CD were administered up to three years post-treatment to track treatment response. Group-based trajectory modeling identified two trajectories of treatment response: 1) a High-response trajectory where children demonstrated lower rates of an ODD or CD diagnosis throughout follow-up, and 2) a Low-response trajectory where children demonstrated higher rates of an ODD or CD diagnosis throughout follow-up. Hierarchical logistic regression predicting treatment response demonstrated that children with higher pre-treatment concentrations of testosterone were four times more likely to be in the Low-response trajectory. No other significant relationship existed between pre-treatment hormone profiles and treatment response. These results suggest that higher concentrations of testosterone are related to how well children diagnosed with ODD or CD respond to psychological treatment over the course of three years.
Keywords: oppositional defiant disorder, conduct disorder, testosterone, cortisol, treatment response
Introduction
Oppositional defiant disorder (ODD) and conduct disorder (CD) are among the most commonly diagnosed psychiatric disorders in childhood and adolescence (Maughan, Rowe, Messer, Goodman, & Meltzer, 2004; Roberts, Roberts, & Xing, 2007). Recent prevalence estimates for ODD and CD in nationally representative samples range from 1.3% – 6.9% and 0.5% – 4.5%, respectively, with rates generally higher for males than females (Lahey, et al., 2000; Roberts, et al., 2007; Simonoff, et al., 1997). ODD and CD are also among the most common disorders referred for outpatient treatment (APA, 2000; Hill & Maughan, 2001; Kazdin, 1995). Not only are these disorders common in clinical and non-clinical populations, they tend to co-occur especially as youth get older. For instance, Maughan and colleagues (Maughan, et al., 2004) estimate that there is a 60% co-morbidity among ODD and CD for adolescents aged 13 to 15 years compared to only 12% for children aged five to seven. The high rate of co-morbidity of these two disorders has even led to a trend of viewing ODD and CD as a single clinical category.
Psychological treatments for ODD and CD, including cognitive-behavior therapy (Kazdin, Siegel, & Bass, 1992), parent-training (Foote, Schuhmann, Jones, & Eyberg, 1998; Webster-Stratton, Hibbs, & Jensen, 2005), and family therapy (Henggeler, Schoenwald, Borduin, Rowland, & Cunningham, 1998; Sexton, Alexander, & Lebow, 2005) provide considerable benefit to children, adolescents, and their families. Meta-analyses on the short-term effects of these treatments report mean effect sizes ranging from .34 – .86 (Kaminski, Valle, Filene, & Boyle, 2008; McCart, Priester, Davies, & Azen, 2006; Serketich & Dumas, 1996; Weisz, Weiss, Han, Granger, & Morton, 1995). However, research on the long-term effects of these interventions is mixed. Some studies report maintenance of treatment gains shortly after completing treatment (Reid, Webster-Stratton, & Hammond, 2003) and over long-term follow-up (Kolko, et al., 2009; Long, Forehand, Wierson, & Morgan, 1994) whereas other studies report significant declines as early as one to two years later (Eyberg, et al., 2001; Hemphill & Littlefield, 2001). Identifying factors associated with the long-term effectiveness of these interventions would enhance the overall understanding of ODD and CD while informing the design and implementation of future interventions.
The current report focuses on pre-treatment hormone profiles as factors related to the long-term effectiveness of treatment for ODD and CD. Although the exact physiological mechanisms are still unknown, there may be several pathways relating hormone profiles to ODD and CD in children and adolescents. Children with ODD and CD may experience hypoactivity in endocrine functioning which promotes engagement in sensation-seeking behavior, such as aggression or fire-setting, as a way to reduce the aversiveness associated with this hypoactivity (van Goozen, Fairchild, Snoek, & Harold, 2007). Indeed, a considerable amount of research supports this inverse relationship between hypoactive endocrine functioning and increased ODD and CD symptoms and diagnoses. Lower concentrations of cortisol are related to increased aggression, ODD and CD diagnoses in both boys and girls (Pajer, Gardner, Rubin, Perel, & Neal, 2001; Shirtcliff, Granger, Booth, & Johnson, 2005), although this relationship is not always consistent (van Bokhoven, et al., 2005). But perhaps most convincingly is that low concentrations of cortisol can have a longitudinal relationship with aggressive ODD and CD symptoms from two to five years later (McBurnett, Lahey, Rathouz, & Loeber, 2000; Shoal, Giancola, & Kirillova, 2003). Such a long-term relationship between cortisol hypoactivity and ODD and CD could also relate to how well individual children respond to current interventions for these disorders.
Another pathway being explored is the positive relationship between increased concentrations of gonadal and adrenal androgens and ODD and CD. Gonadal steroid hormones are androgens produced during sexual maturation and are believed to excite physiological and behavioral activity making it more likely that children and adolescents engage in disruptive behaviors to reduce these agitated states. The most potent androgen, testosterone, has been correlated with increased aggressive behavior seen in ODD and CD (Olweus, Mattsson, Schalling, & Low, 1988; Pajer, et al., 2006; Scerbo & Kolko, 1994), although this association is not consistent (Constantino, et al., 1993). Studies have also begun to examine whether adrenal androgens, such as dehydroepiandrosterone (DHEA) and androstenedione, serve as biomarkers of ODD and CD. Emerging research has shown that higher concentrations of DHEA and androstenedione are associated with conduct problems in children ages 9 to 14 (Dmitrieva, Oades, Hauffa, & Eggers, 2001; Dorn, et al., 2009). Taken together, research on gonadal and adrenal androgens suggests that these hormones may be related to disruptive behaviors in ODD and CD.
If certain hormone profiles are associated with increased ODD and CD symptoms, then they may also be linked to prolonged ODD and CD diagnoses during and after treatment. This can determine how well these children ultimately respond to existing treatments for ODD and CD. However, few studies have examined whether adrenal and gonadal hormones are related to the success of psychological treatment for ODD and CD, and among those that have, cortisol has received the most attention. In a study of 22 children undergoing psychological treatment for ODD and CD, Van de Weil and colleagues (2004) found that lower concentrations of resting cortisol was associated with higher pre-treatment behavior problems but did not predict a child’s response to treatment. While informative, this study employed a small sample size, did not include females and did not examine the effects of multiple hormone profiles on treatment response. Thus, it remains unclear whether pre-treatment concentrations of cortisol or other hormones are related to how well children and adolescents with ODD and CD respond to treatment. Identifying such a relationship could help fill the gaps in our understanding of the relationship between hormone profiles and treatment response and in explaining the mixed, long-term efficacy for current behavioral interventions for ODD and CD. Based on this previous research, we hypothesized: 1) lower concentrations of cortisol at pre-treatment would increase the risk of treatment non-response for ODD and CD, and 2) higher concentrations of testosterone, DHEA, and androstenedione would increase the risk of treatment-non-response for ODD and CD.
Method
Sample
All methods and procedures were approved by the local Institutional Review Board. Parental consent and child assent was obtained after reviewing all procedures with the family and before proceeding with any part of the study. Children included in the current study are part of a larger randomized clinical trial examining the long-term effects of psychological treatment for ODD and CD. Two multiple-gate screening phases were used to determine the sample for the clinical trial. First, a clinic screen was conducted by phone or interview to obtain information regarding the child’s psychiatric diagnosis, behavioral problems, and treatment needs. Second, a formal diagnostic assessment was conducted to determine the presence of ODD and CD. Inclusion criteria were: 1) males or females aged 6–11 years, 2) residence with at least one parent or guardian, 3) intellectual level no more than two standard deviations below age norms, and 4) an ODD or CD diagnosis. Exclusion criteria were: 1) concurrent individual or family participation in a treatment program for ODD or CD, 2) current psychosis, bipolar disorder, and major depressive disorder marked by significant vegetative signs, substance abuse, or eating disorder, or 3) suicidality with a plan or homicidality.
Children eligible for the current study (N = 177) were selected from the intervention arms of the larger clinical trial (Kolko, Dorn, Bukstein, & Burke, 2008; Kolko, et al., 2009). Participants were randomly assigned to one of two experimental treatments: one delivered in a community-based format (COMM) and the other in a clinic-based format (CLIN). The COMM and CLIN treatments were similar in content and techniques used to achieve a family’s goals for treating ODD and CD. Both treatments used cognitive-behavioral skills training, psychiatric consultation, parent management training, family therapy, school consultation, and peer interventions but differed in the ecological contexts in which they were delivered. Children randomized to the COMM condition received treatment in the family’s home, school, and/or community settings. Children randomized to the CLIN condition received outpatient treatment in a traditional clinic setting with phone consultation given to teachers and parents to address disruptive behaviors. The majority of services provided to children in the COMM and CLIN conditions were parent management training, cognitive-behavioral therapy, and family therapy. A third treatment condition was included as a non-randomized, treatment as usual (TAU) condition to reflect the current standard of care in the community. TAU participants had the option of receiving traditional psychological treatment for ODD and CD that included psychodynamic, parent-training, family systems or cognitive-behavioral therapy at an outpatient community mental health center. These participants were recruited using flyers and postings and were screened for study eligibility using the same inclusion/exclusion criteria. The average length of treatment ranged from 20–25 weeks across the three treatment conditions.
Since funding for the collection of hormone samples was secured after the initiation of the clinical trial, 52 study participants had completed their pre-treatment evaluation without providing saliva samples and are therefore not included in the current analyses. Of the remaining 125 participants the following were excluded from analysis: 11 using steroid medications (topical, inhaled, or oral) in the last two weeks, and nine unable/unwilling to provide saliva samples at pre-treatment. The remaining 105 participants included in this study did not differ significantly from those who were excluded on any demographic or pre-treatment variable. The mean age for these 105 participants at pre-treatment was 8.90 (SD = 1.73) years with the average participant coming from a low to moderate socioeconomic background. Consistent with other samples studying ODD and CD, there was a majority of males in this study. The racial make-up was predominately Caucasian and African-American children (see Table 1).
Table 1.
Sample Characteristics by Treatment Group at Pre-treatment
| Variable | COMM Mean (SD) or n n = 39 |
CLIN Mean (SD) or n n = 37 |
TAU Mean (SD) or n n = 29 |
|
|---|---|---|---|---|
| Sex | ||||
| Male (N = 80) | 30 | 29 | 21 | |
| Female (N = 25) | 9 | 8 | 8 | |
| Ethnic Group | ||||
| Caucasian (N = 55) | 18 | 19 | 18 | |
| African-American (N = 43) | 17 | 16 | 10 | |
| Hispanic (N = 1) | 1 | 0 | 0 | |
| Bi-racial (N = 6) | 3 | 2 | 1 | |
| Age (in years) | 8.72 (1.75) | 8.84 (1.69) | 9.22 (1.75) | |
| Socioeconomic statusa | 37.62 (10.42) | 35.78 (12.78) | 28.93 (10.51)* | |
| Number of ODD/CD symptoms | 7.44 (1.97) | 8.16 (2.01) | 7.90 (3.06) | |
| Number of treatment sessions | 21.44 (6.17)** | 14.27 (6.69)** | 4.32 (3.26)** | |
| ODD and CD Diagnoses | ||||
| ODD (N = 82) | 32 | 31 | 19 | |
| CD (N = 23) | 7 | 6 | 10 | |
| Co-morbid ADHD | 25 | 26 | 24 | |
| Raw Hormone Concentrations | ||||
| Mean Cortisol (µg/dL) | 0.15 (0.15) | 0.13 (.04) | 0.13 (0.05) | |
| Diurnal Change in Cortisol (µg/dL) | 0.67 (1.65) | 0.38 (0.29) | 0.30 (0.35) | |
| Pooled Testosterone (pg/mL) | 36.16 (17.03) | 38.56 (20.69) | 56.23 (26.20)* | |
| Dehydroepiandrosterone (pg/mL) | 38.59 (16.94) | 44.55 (30.55) | 37.02 (19.75) | |
| Androstenedione (pg/mL) | 73.79 (51.16) | 74.91 (43.08) | 113.18 (62.15)** | |
Note.
p < .001;
= p < .01.
= Based on Hollingshead criteria (range: 14–60).
Procedure
Data were collected at five different evaluations: pre-treatment, post-treatment, and one-year, two-year and three-year follow-up. Master’s level clinicians completed each treatment evaluation with the parent/guardian and child after obtaining consent/assent. Participants completed questionnaires and a semi-structured, diagnostic interview at each evaluation. Participants were given $10 for completing an evaluation with each child and parent informant having the benefit of treatment services at no cost. All participants received an additional $10 for providing saliva samples.
Saliva samples were collected at two different times during each evaluation. Participants were instructed not to brush their teeth or eat two hours before each collection and to swish their mouth with water prior to passively drooling into collection tubes. Trident® sugarless gum was used to stimulate saliva, consistent with previous studies using this method of saliva sampling (Granger, Schwartz, Booth, & Arentz, 1999). Sample 1 was collected 15 minutes after arrival to the evaluation and following the consent process. Average time of day for collecting Sample 1 at the pre-treatment evaluation was 12:00 pm (SD = 2.5 hours). Sample 2 was collected immediately following completion of the diagnostic interview which lasted on average 69 minutes (SD = 30 minutes). Two additional saliva samples were collected to assess diurnal change in cortisol concentrations. Children and caregivers were given materials and instructed to provide saliva samples immediately before bedtime, Sample 3, and immediately upon wakening, Sample 4. At bedtime, children were instructed not to eat or drink anything two hours prior to sampling. In the morning, children were instructed to provide the sample immediately upon awakening and prior to eating, drinking or brushing their teeth. All samples were stored at −80° C.
Measures
Schedule for Affective Disorders and Schizophrenia for School-Aged Children for DSM-IV, Present and Lifetime (K-SADS-PL)
The K-SADS-PL diagnostic interview is a well-established and widely used instrument with known reliability and validity (Kaufman, et al., 1997). Diagnostic impressions and total symptom count were derived from interviews with the child and caregiver. Inter-rater reliabilities (n = 71) obtained from the larger clinical trial were moderate-to-high for ODD (k = .79) and CD (k = .74). A new variable, ODD/CD diagnosis, was created to reflect the presence of either ODD or CD diagnosis at any treatment evaluation (Either ODD or CD Diagnosis = 1, Neither ODD nor CD Diagnosis = 0).
Cortisol
Samples were assayed in duplicate using a highly-sensitive enzyme immunoassay (Salimetrics, State College, PA). The test has a lower limit of sensitivity of <.003 µg /dl and average intra-and inter-assay coefficients of variation 3.35% – 3.65% and 3.75% – 6.41%, respectively. Values from matched serum and saliva samples collected by Salimetrics showed the expected strong linear relationship, r = .91, p < .0001.
Testosterone
Samples were assayed in duplicate using a highly-sensitive enzyme immunoassay. The test has a lower limit sensitivity of < 1 pg/mL with average intra-and inter-assay coefficients of variation ranging from 2.5% – 6.7% and 5.6% – 14.05%, respectively. The reported serum-saliva correlation was r = 0.96, p < .0001.
DHEA
Samples were assayed using a highly-sensitive enzyme immunoassay. The test has a lower limit of sensitivity of 5 pg/mL and intra-and inter-assay coefficients of variation ranging from 5.3% – 5.8% and 7.9% – 8.5%, respectively. The reported serum-saliva correlation was r = 0.86, p < .0001.
Androstenedione
Samples were assayed using a radioimmunoassay kit from ICN Pharmaceuticals, Inc. to determine concentrations of androstenedione. The detection limit was 0.05 ng/mL and inter-assay coefficients of variation ranged between 2.4–2.5%. Part way through the clinical trial, the ICN lab closed and was no longer able to assay concentrations of androstenedione. A second company, Salimetrics, was solicited to assay the remaining samples. The test at Salimetrics was an enzyme immunoassay with a lower limit of sensitivity of 5 pg/mL and intra-and inter-assay coefficients of variation that range between 1.5% – 7.5% and 3.8 – 8.5%, respectively with a serum-saliva correlation of r = 0.77, p < .0001. The majority (67%) of pre-treatment androstenedione concentrations were assayed with the ICN Pharmaceuticals kit. A random sample of 30 pre-treatment samples was selected and rerun with the methodology of Salimetrics. Regression modeling was then applied to values of androstenedione to ensure consistency between the results obtained from the different companies.
Data Pre-processing and Analytic Strategy
Saliva was assayed to establish individual concentrations from Samples 1 and 2 that were then averaged to derive a mean value of cortisol. A mean value was obtained to avoid potential sampling bias in any single sample of cortisol given its ultradian rhythm and initial sensitivity to the research setting. The difference in Samples 3 and 4 (Difference = Morning Cortisol collected in Sample 4 subtracted from Evening Cortisol collected in Sample 3) was used to reflect diurnal change in cortisol, a measure previously linked to behavior problems in children and adolescents (Susman, et al., 2007). Saliva from Samples 1 and 2 was pooled and assayed to derive collective values for testosterone, DHEA and androstenedione. Raw values for each hormone collected at pre-treatment were log-transformed to reduce skewness and kurtosis. Using a winsorizing procedure to minimize bias due to outliers, extreme values for each hormone were reassigned a value two standard deviations away from each hormone’s respective mean. Commensurate with acceptable levels (e.g., Kertes & Gunnar, 2004), an average five percent of hormone data were reassigned values of two standard deviations.
The data analytic strategy consisted of a two-step process to test the study’s hypotheses. The first step used group-based trajectory modeling via SAS PROC TRAJ (Jones, Nagin, & Roeder, 2001) to identify distinct treatment response trajectories of ODD/CD diagnosis and their corresponding trends (intercept, linear, quadratic or cubic). One advantage of group-based trajectory modeling is that it uses empirically derived methods for identifying the optimal number of trajectories for a particular outcome as determined by model fit indicators such as the Bayesian Information Criterion (BIC). A second advantage of group-based trajectory modeling is that the analysis identifying the optimal number of trajectories yields posterior probabilities that are used to determine how well an individual remains in a respective trajectory group over time. These probabilities are also used to categorically assign each participant to a particular trajectory and these assignments can then be used as outcome variables in subsequent statistical models. As such, the second step in the data analytic strategy involved using the categorical assignments to a treatment response trajectory obtained from the group-based model as the dependent variable in a hierarchical logistic regression equation including all hormones assessed at pre-treatment as predictors.
Results
Preliminary Data Analysis
Differences between the three treatment groups were assessed to identify important covariates that might affect treatment response. A multivariate analysis of variance indicated significant differences between the treatment groups on socioeconomic status (SES), F(2, 101) = 4.83, p < .01, f2 = .10, and number of treatment sessions received, F(2, 101) = 72.12, p < .001, f2 = 1.43. To control for their effect on treatment response, SES and number of treatment sessions were statistically controlled when deriving the number of treatment response trajectories. A multivariate analysis of covariance controlling for the time of day saliva was sampled revealed significant differences between the three treatment groups for testosterone, F(3, 64) = 3.91, p < .05, f2 = .18, and androstenedione, F(3, 64) = 3.03, p < .05, f2 = .14, with results indicating that the TAU condition had significantly higher concentrations of each of these two hormones (see Table 1). A dummy coded variable, ‘TAU membership’ (COMM+CLIN = 0, TAU = 1), was created to control for group differences in hormone concentrations in the logistic regression model when predicting treatment response. There were no significant differences between groups on age, number of males and females in each group, ODD and CD symptom severity or co-morbid ADHD diagnoses.
Determination of Treatment Response Trajectories
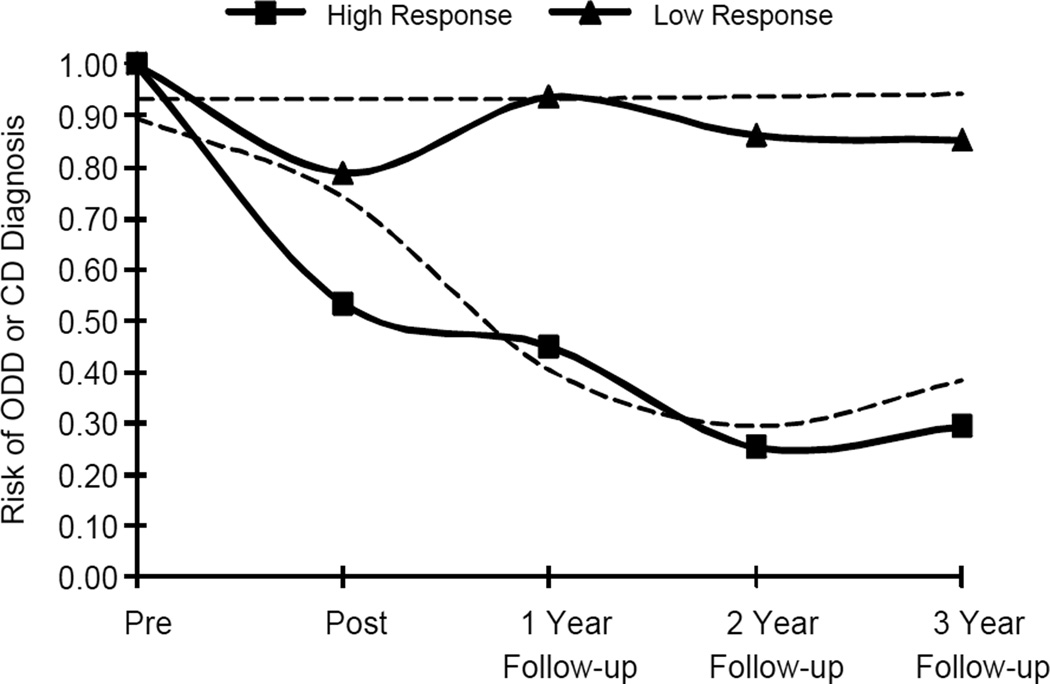
The optimal number of treatment response trajectories and corresponding trends were identified using a semi-parametric logit model of ODD/CD diagnosis at each of five time points from pre-treatment throughout the three-year follow-up. A two-group trajectory model yielded the best fit to the observed data with a BIC of −286.69. Convergence for a three-group model could not be achieved and so the two trajectory model was retained. Since previous research has linked age and sex with varying rates of ODD and CD, these variables were included as covariates in a preliminary trajectory model along with socioeconomic status and total number of treatment sessions. Neither age nor sex was significantly related to either treatment response trajectory and so were removed from further analysis. Results of the final logit model that included SES and the number of treatment sessions as covariates resulted in an improved model fit, BIC = −248.43, with a High-response trajectory following a quadratic trend and a Low-response trajectory following an intercept-only trend (see Figure 1). Mean posterior probabilities of membership in the High-response (n = 56) and Low-response trajectories (n = 49) were .92 and .89, respectively, and indicated good classification into the trajectory groups. There were 24 participants from the COMM condition, 15 participants from the CLIN condition, and 10 participants from the TAU condition assigned to the Low-response trajectory group. Significant differences in ODD/CD diagnoses between treatment response trajectories emerged as early as the post-treatment evaluation, χ2(105) = 10.53, p = .001.
Figure 1.
Treatment Response Trajectories over 3-Year Follow-up. Semi-parametric logit model of treatment response trajectories based on the presence of an oppositional defiant disorder (ODD) or conduct disorder (CD) diagnosis. Solid lines represent observed values and dashed lines represent predicted probabilities.
Pre-treatment Hormone Concentrations and Treatment Response
The time of day saliva samples were collected was not related to treatment response at the bivariate level (r = .03, p = .80) and therefore was not entered as a covariate in the logistic regression model. Hierarchical logistic regression then tested whether pre-treatment hormone concentrations predicted treatment response (High-response trajectory = 0, Low-response trajectory = 1). TAU membership was entered into the logistic regression equation as a covariate at Level 1. Hormones were then entered as predictors at Level 2. Table 2 presents results from the logistic regression. Higher concentrations of testosterone at pre-treatment significantly increased risk of being assigned to the Low-response trajectory, b = 1.49, p < .05. The corresponding odds ratio of 4.44 indicates that a one standard deviation increase in log-transformed, pre-treatment concentrations of testosterone was associated with a four-fold increase in risk of being assigned to the Low-response trajectory. This suggests that higher concentrations of testosterone at baseline were significantly associated with a poorer response to psychological treatment of ODD and CD. Although not significant at the traditional .05 level, diurnal change in cortisol concentrations was marginally related to the Low-response trajectory, b = 0.49, p = .10. Contrary to expectations, there were no significant relationships between concentrations of mean cortisol, DHEA or androstenedione with treatment response. In a post-hoc test, we assessed whether these findings maintained when using only the sub-sample of children diagnosed with ODD. Results indicated that there were no significant predictors of ODD trajectories once children diagnosed with CD were removed from the analysis.
Table 2.
Pre-treatment Variables Predicting Treatment Response Trajectory
| Baseline Variable | b | SE | Wald χ2 | OR | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Step 1 | |||||||
| TAU Membership | .73 | 0.56 | 1.69 | 0.50 | 0.21 | 1.21 | |
| Step 2 | |||||||
| TAU Membership | −1.09 | 0.75 | 2.09 | 0.34 | 0.08 | 1.47 | |
| Mean Cortisol | 0.98 | 0.85 | 1.31 | 2.66 | 0.50 | 14.16 | |
| Diurnal Cortisol Change | 0.49 | 0.30 | 2.59† | 1.63 | 0.90 | 2.95 | |
| Pooled Testosterone | 1.49 | 0.75 | 3.95* | 4.44 | 1.02 | 19.27 | |
| Dehydroepiandrosterone | −1.03 | 0.75 | 1.89 | 0.36 | 0.08 | 1.55 | |
| Androstenedione | −0.68 | 0.69 | 0.97 | 0.51 | 0.13 | 1.96 | |
Note. R2= .14 (Cox & Snell), .19 (Nagelkerke). Model χ2(8) = 8.11.
= p < .05;
= p = .10.
Discussion
The current study is novel in that it: (1) included multiple hormones associated with ODD and CD, (2) incorporated an adequate sample size that included both males and females, and (3) employed a three year follow-up assessing the long-term response to treatment. This study also addresses a gap in the research literature by evaluating the response to psychological treatment for ODD and CD based on each participant’s hormone profile prior to intervention. The major finding from this study is that testosterone significantly predicted how well children responded to the psychological treatments in this study. Higher concentrations of testosterone at pre-treatment were associated with a poorer response to treatment, as indicated by the presence of either an ODD or CD diagnosis throughout a three-year follow-up period. Prior research (Pajer, et al., 2006; Scerbo & Kolko, 1994) has shown an association between higher concentrations of testosterone and higher rates of aggression and antisocial behavior, common symptoms associated with ODD and CD. Thus, not only is testosterone associated with higher rates of aggression in clinical populations, but it also appears that higher concentrations are associated with the degree to which children benefit from treatment. The unique contribution of this study is that it extends previous cross-sectional research on testosterone and disruptive behaviors with an application to longitudinal, treatment outcome research.
There were no other significant relationships between the other hormones assessed and treatment response. While difficult to interpret null findings, other studies have failed to find a relationship between resting cortisol concentrations and how well individuals responded to treatment (van De Wiel, et al., 2004). There was also no association between pre-treatment concentrations of DHEA or androstenedione and treatment response. Although there have been links between higher concentrations of each of these hormones and aggression (Dmitrieva, et al., 2001; Dorn, et al., 2009), adrenal androgens were not related to longer-term treatment outcome in this study. As this study is among the first to examine whether adrenal and gonadal hormones are related to treatment response, additional research is needed to draw stronger conclusions about profiles of cortisol, DHEA, and androstenedione and treatment outcome for ODD and CD.
There are several limitations to this study. Although both sexes are included, the sample of girls is relatively small and thus sex differences could not be adequately examined. However, there were no associations between sex and any of the hormone concentrations or treatment response trajectory. This appears due to the small number of females in this study and the younger ages of all participants. Future studies with larger samples of girls are needed to examine this issue. Only children 6–11 years of age and diagnosed with either ODD or CD were eligible for the clinical trial. Even with the three-year follow-up of ODD and CD after treatment, it is not appropriate to generalize findings to older children diagnosed with ODD or CD. Because the hormone component of this study merged with an ongoing clinical trial not all participants in the clinical trial are included in this study. Further, the non-randomized TAU condition was significantly different in initial testosterone and androstenedione concentrations, SES and the number of treatment sessions received. Although these differences were statistically controlled, future research should consider randomizing participants to such a condition in order to further rule-out differences on these parameters. The range in times when saliva samples were collected is also a limitation. Since the hormone component merged with an existing clinical trial, procedures for collecting samples were dictated largely by the procedures of the clinical trial. Collecting samples at the same time of day across participants is important given diurnal variations in hormones. For instance, samples collected in the evening yield significantly lower concentrations of cortisol than samples collected in the morning. Lower concentrations are a known correlate of ODD and CD. This has the potential to influence predictions of treatment response, although the influence of sampling time was not significantly related to the outcomes in this study. In addition, the study design precluded the use of a stressor paradigm that would allow for an assessment of how cortisol reactivity is related to treatment outcome. The effects of puberty were also not assessed in the larger trial. While we do not expect that our results would diminish due to the effects of puberty, future research will need to more closely examine the influence of stage or timing of puberty on hormones that are related to treatment response. Finally, results of a post-hoc analysis assessing whether hormones predicted ODD diagnosis over time yielded non-significant parameter estimates. While this could indicate that pre-treatment hormones may be more closely related to CD status, the reduced sample size resulting from the removal of children with CD may have also limited the power to detect a relationship between pre-treatment hormones and ODD status. Further study with larger samples of ODD and CD are needed to more adequately assess any unique relationship between pre-treatment hormones and these individual disorders.
This study is among the first accounts of the relationship between pre-treatment concentrations of adrenal and gonadal hormones associated with response to psychological treatment for ODD and CD. There are several strengths of the current study. First, hormones included cortisol, testosterone, DHEA, and androstenedione and were obtained from children enrolled in a larger randomized clinical trial examining the effectiveness of psychological treatments for ODD and CD. Second, ODD and CD diagnoses were made using gold-standard methods and were assessed for three consecutive years following completion of psychological treatment. Finally, the sample size is large compared to published studies and included both boys and girls. Results are based on the predictive relationships between pre-treatment hormone concentrations and the trajectory of treatment response while controlling for relevant demographic and treatment-related variables. These findings lend importance to the evaluation of whether psychological treatments can alter pre-treatment hormone profiles to produce a more beneficial treatment response. Studies are emerging that have examined whether psychological treatments alter hormone concentrations, in particular cortisol, for children receiving treatment (Fisher, Stoolmiller, Gunnar, & Burraston, 2007) and in cortisol stress reactivity in populations at-risk for antisocial behavior (Brotman, et al., 2007). To our knowledge there have been no published reports of the effects of psychological treatments on concentrations of testosterone, DHEA, or androstenedione, an important area for future research. Additional research in this area has important implications for identifying potential physiological mechanisms and processes of change associated with psychological treatment of ODD and CD as well as the further enhancement of these interventions. Until then, we feel the current data offer some important implications for clinical intervention. While the type and setting of treatment varied across the groups evaluated in this study, the predominant clinical strategies used were parent management training, cognitive-behavioral therapy, and family therapy. Using these strategies with an early focus on aggressive behaviors and interaction patterns may increase a child’s chances of responding to treatment for ODD and CD. The CLIN condition had the highest number of children in the High response trajectory. This suggests that treatment delivered in traditional clinic settings in a relatively brief length of time can be helpful in reducing risk for ODD or CD. Booster sessions may be another therapeutic option to consider in the long-term treatment of ODD and CD as they may reduce the risk of continued ODD or CD across different developmental stages. They may also help maintain treatment gains for those children initially benefitting from treatment.
Acknowledgments
This manuscript was supported by grants from the National Institutes of Nursing Research (R01NR07615), Mental Health (R01MH57727), and Diabetes and Digestive and Kidney Diseases (T32DK063929). We thank Dr. James Peugh for providing his statistical expertise to the data analysis in this study.
Contributor Information
Chad E. Shenk, Email: chad.shenk@cchmc.org, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., MLC 3015, Cincinnati, OH 45229.
Lorah D. Dorn, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., MLC 3015, Cincinnati, OH 45229
David J. Kolko, Western Psychiatric Institute & Clinic, University of Pittsburgh School of Medicine, Pittsburgh, PA
Elizabeth J. Susman, The Pennsylvania State University, University Park, PA
Jennie G. Noll, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., MLC 3015, Cincinnati, OH 45229
Oscar G. Bukstein, University of Texas Health Science Center, Houston, TX
References
- APA. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Brotman LM, Gouley KK, Huang K-Y, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Archives of General Psychiatry. 2007;64(10):1172–1179. doi: 10.1001/archpsyc.64.10.1172. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Grosz D, Saenger P, Chandler DW, Nandi R, Earls FJ. Testosterone and aggression in children. Journal of the American Academy of Child & Adolescent Psychiatry. 1993;32(6):1217–1222. doi: 10.1097/00004583-199311000-00015. [DOI] [PubMed] [Google Scholar]
- Dmitrieva T, Oades R, Hauffa B, Eggers C. Dehydroepiandrosterone sulphate and corticotropin levels are high in young male patients with conduct disorder: Comparisons for growth factors, thyroid and gonadal hormones. Neuropsychobiology. 2001;43(3):134–140. doi: 10.1159/000054881. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Kolko DJ, Susman EJ, Huang B, Stein H, Music E, Bukstein OG. Salivary gonadal and adrenal hormone differences in boys and girls with and without disruptive behavior disorders: Contextual variants. Biological Psychology. 2009;81(1):31–39. doi: 10.1016/j.biopsycho.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyberg SM, Funderburk BW, Hembree-Kigin TL, McNeil CB, Querido JG, Hood KK. Parent-child interaction therapy with behavior problem children: One and two year maintenance of treatment effects in the family. Child & Family Behavior Therapy. 2001;23(4):1–20. [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8–10):892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote RC, Schuhmann EM, Jones ML, Eyberg SM. Parent-child interaction therapy: A guide for clinicians. Clinical Child Psychology and Psychiatry. 1998;3(3):361–373. [Google Scholar]
- Granger DA, Schwartz EB, Booth A, Arentz M. Salivary testosterone determination in studies of child health and development. Hormones and Behavior. 1999;35(1):18–27. doi: 10.1006/hbeh.1998.1492. [DOI] [PubMed] [Google Scholar]
- Hemphill SA, Littlefield L. Evaluation of a short-term group therapy program for children with behavior problems and their parents. Behaviour Research and Therapy. 2001;39(7):823–841. doi: 10.1016/s0005-7967(00)00058-9. [DOI] [PubMed] [Google Scholar]
- Henggeler SW, Schoenwald SK, Borduin CM, Rowland MD, Cunningham PB. Multisystemic Treatment of Antisocial Behavior in Children and Adolescents. New York, NY: Guilford Press; 1998. [Google Scholar]
- Hill J, Maughan B. Conduct disorders in childhood and adolescence. New York, NY: Cambridge University Press; 2001. [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–393. [Google Scholar]
- Kaminski JW, Valle LA, Filene JH, Boyle CL. A meta-analytic review of components associated with parent training program effectiveness. Journal of Abnormal Child Psychology. 2008;36(4):567–589. doi: 10.1007/s10802-007-9201-9. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Conduct disorders in childhood and adolescence. 2nd. London: Sage; 1995. [Google Scholar]
- Kazdin AE, Siegel TC, Bass D. Cognitive problem-solving skills training and parent management training in the treatment of antisocial behavior in children. Journal of Consulting and Clinical Psychology. 1992;60(5):733–747. doi: 10.1037//0022-006x.60.5.733. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR. Evening activities as a potential confound in research on the adrenocortical system in children. Child Development. 2004;75(1):193–204. doi: 10.1111/j.1467-8624.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- Kolko D, Dorn L, Bukstein O, Burke J. Clinically referred ODD children with or without CD and healthy controls: Comparisons across contextual domains. Journal of Child & Family Studies. 2008;17(5):714–734. [Google Scholar]
- Kolko D, Dorn L, Bukstein O, Pardini D, Holden E, Hart J. Community vs. clinic-based modular treatment of children with early-onset ODD or CD: A clinical trial with 3-year follow-up. Journal of Abnormal Child Psychology. 2009;37(5):591–609. doi: 10.1007/s10802-009-9303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Schwab-Stone M, Goodman SH, Waldman ID, Canino G, Rathouz PJ, Miller TL, Dennis KD, Bird H, Jensen PS. Age and gender differences in oppositional behavior and conduct problems: A cross-sectional household study of middle childhood and adolescence. Journal of Abnormal Psychology. 2000;109(3):488–503. [PubMed] [Google Scholar]
- Long P, Forehand R, Wierson M, Morgan A. Does parent training with young noncompliant children have long-term effects? Behaviour Research and Therapy. 1994;32(1):101–107. doi: 10.1016/0005-7967(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Maughan B, Rowe R, Messer J, Goodman R, Meltzer H. Conduct disorder and oppositional defiant disorder in a national sample: Developmental epidemiology. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2004;45(3):609–621. doi: 10.1111/j.1469-7610.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57(1):38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McCart M, Priester P, Davies W, Azen R. Differential effectiveness of behavioral parent-training and cognitive-behavioral therapy for antisocial youth: A meta-analysis. Journal of Abnormal Child Psychology. 2006;34(4):527–543. doi: 10.1007/s10802-006-9031-1. [DOI] [PubMed] [Google Scholar]
- Olweus D, Mattsson A, Schalling D, Low H. Circulating testosterone levels and aggression in adolescent males: A causal analysis. Psychosomatic Medicine. 1988;50(3):261–272. doi: 10.1097/00006842-198805000-00004. [DOI] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry. 2001;58(3):297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Pajer K, Tabbah R, Gardner W, Rubin R, Czambel RK, Wang Y. Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology. 2006;31(10):1245–1256. doi: 10.1016/j.psyneuen.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Reid MJ, Webster-Stratton C, Hammond M. Follow-up of children who received the Incredible Years intervention for oppositional-defiant disorder: Maintenance and prediction of 2-year outcome. Behavior Therapy. 2003;34(4):471–491. [Google Scholar]
- Roberts RE, Roberts CR, Xing Y. Rates of DSM-IV psychiatric disorders among adolescents in a large metropolitan area. Journal of Psychiatric Research. 2007;41(11):959–967. doi: 10.1016/j.jpsychires.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: Relationship to aggressive, hyperactive, and internalizing behaviors. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33(8):1174–1184. doi: 10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Serketich WJ, Dumas JE. The effectiveness of behavioral parent training to modify antisocial behavior in children: A meta-analysis. Behavior Therapy. 1996;27(2):171–186. [Google Scholar]
- Sexton TL, Alexander JF, Lebow JL. Handbook of Clinical Family Therapy. Hoboken, NJ US: John Wiley & Sons Inc.; 2005. Functional family therapy for externalizing disorders in adolescents. [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17(1):167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, Kirillova GP. Salivary cortisol, personality, and aggressive behavior in adolescent boys: A 5-year longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(9):1101–1107. doi: 10.1097/01.CHI.0000070246.24125.6D. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Meyer JM, Silberg JL, Maes HH, Loeber R, Rutter M, Hewitt JK, Eaves LJ. The Virginia Twin Study of Adolescent Behavioral Development: Influence of age, sex, and impairment on rates of disorder. Archives of General Psychiatry. 1997;54(9):801–808. doi: 10.1001/archpsyc.1997.01830210039004. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Developmental Psychology. 2007;43(4):811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- van Bokhoven I, Van Goozen SH, van Engeland H, Schaal B, Arseneault L, Seguin JR, Nagin DS, Vitaro F, Tremblay RE. Salivary cortisol and aggression in a population-based longitudinal study of adolescent males. Journal of Neural Transmission. 2005;112(8):1083–1096. doi: 10.1007/s00702-004-0253-5. [DOI] [PubMed] [Google Scholar]
- van De Wiel NMH, van Goozen SHM, Matthys W, Snoek H, van Engeland H. Cortisol and treatment effect in children with disruptive behavior disorders: A preliminary study. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(8):1011–1018. doi: 10.1097/01.chi.0000126976.56955.43. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133(1):149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C, Hibbs ED, Jensen PS. Psychosocial Treatments for Child and Adolescent Disorders: Empirically-based Strategies for Clinical Practice. 2nd. Washington, DC US: American Psychological Association; 2005. The Incredible Years: A training series for the prevention and treatment of conduct problems in young children. [Google Scholar]
- Weisz JR, Weiss B, Han SS, Granger DA, Morton T. Effects of psychotherapy with children and adolescents revisited: A meta-analysis of treatment outcome studies. Psychological Bulletin. 1995;117(3):450–468. doi: 10.1037/0033-2909.117.3.450. [DOI] [PubMed] [Google Scholar]