ABSTRACT
Objective:
To describe the clinical aspects of cases of influenza A(H1N1)pdm09 in Brazil.
Methods:
A descriptive study of cases reported in Sistema de Informação de Agravos de Notificação (SINAN), 2009-2010.
Results:
As the final classification, we obtained 53,797 (56.79%) reported cases confirmed as a new influenza virus subtype, and 40,926 (43.21%) cases discarded. Fever was the most common sign, recorded in 99.74% of the confirmed and 98.92% of the discarded cases. Among the confirmed cases, the presence of comorbidities was reported in 32.53%, and in 38.29% of the discarded cases. The case fatality rate was 4.04%; 3,267 pregnant women were confirmed positive for influenza A new viral subtype and 2,730 of them were cured. The case fatality rate of pregnant women was 6.88%.
Conclusion:
The findings suggested concern of the health system with pregnant women, and patients with comorbidities and quality of care may have favored a lower mortality. We recommend that, when caring for patients with severe respiratory symptoms, with comorbidities, or pregnant women, health professionals should consider the need for hospital care, as these factors make up a worse prognosis of infection by the pandemic influenza virus.
Keywords: Influenza A virus, H1N1 subtype, Pandemics, Epidemiology, Medical record linkage, Signs and symptoms, Comorbidity, Brazil
INTRODUCTION
Influenza or flu is present in all the world, it is highly contagious, it affects in an acute manner the respiratory system, and it presents with aggravated morbidity and mortality among the elderly, children, and among those with comorbidities.(1)
Although the clinical picture of influenza cases is unspecific, with a generally benign and self-delimited clinical evolution, there have been cases observed with severe pulmonary involvement, especially in groups at risk for influenza complications.(1)
In the year 2000, the Ministry of Health began to implement a nationwide influenza vigilance system.(2,3) Cases of seasonal influenza are not of compulsory notification in Brazil, but every suspected case of an outbreak of seasonal influenza or human influenza by a new subtype should be notified at the Sistema de Informação de Agravos de Notificação (SINAN) and be submitted to the decision-making algorithm of the International Sanitary Regulations.(4-6)
The pandemic generated a need for change in epidemiological vigilance of influenza, with monitoring of the severity of the disease and detection of changes in virulence of the virus becoming strategies of vigilance.(6,7)
In a literature review on the topic, we found no academic publications, even in governmental publications,(8–26) i.e., a consolidated description of the cases of pandemic 2009 influenza A(H1N1) during the pandemic in Brazil, reported to the Ministry of Health, and with consequent absence of knowledge in the rest of the world.
OBJECTIVE
To describe the clinical aspects of the cases of influenza A(H1N1)pdm09 notified in Brazil.
METHODS
A descriptive study was developed, using secondary data during the years 2009 and 2010. Data were obtained from the national base, the pandemic “influenza” module of SINAN, requested from the Ministry of Health, with records identified nominally. The definitions of a case of severe acute respiratory syndrome (SARS) and of SARS caused by influenza were those used by the Ministry of Health in Brazil.(1) The criteria for cases discarded of SARS caused by influenza are: case in which no infection by the influenza virus has been detected, cases in which another disease has been diagnosed, or suspect cases with an epidemiological link to cases discarded as per laboratorial findings.(1,27)
The databases were submitted to the process of double verification, using the RecLink III™ software. The Epi Info version 3.5.4. software was used for the descriptive analysis.
Ethical considerations
The project was submitted to the Scientific Research Ethics Committee of Faculdade de Medicina da Universidade de São Paulo and was approved on June 6, 2012, under official opinion number 34710, CAAE: 03608412.8.0000.0065.
RESULTS
One hundred and seventy-three 173 (0.16%) overlaps were identified and excluded. Considering the records kept, 105,054 suspect cases of influenza A(H1N1) pdm09 were notified. Of this total, 95,485 (90.9%) were notified in 2009 and 9,569 (9.1%) in 2010. At the final classification, 53,797 (51.20%) of the cases notified were classified as influenza due to the new viral subtype, 40,926 (39.00%) were discarded, 3,297 (3.10%) were caused by another infectious agent, and 7,034 (6.70%) were not classified.
Cases classified as confirmed and discarded
At final classification, we obtained 53,797 (56.79%) of the cases notified closed as influenza by a new viral subtype and 40,926 (43.21%) discarded. The incidence of pandemic influenza in the population in 2009 was 28.03/100,000 inhabitants and in 2010, it was 0.51/100,000 inhabitants.
In the evaluation of the epidemiological characteristics, among the cases confirmed, 56.73% were female, and among those discarded, this proportion was 55.48%. The mean age of the confirmed cases was 26.31 (standard deviation SD±18.10) years, and of those discarded, 26.47 (SD±21.63) years. The median age for the first group was 24 (interval: zero to 98) years, and for the second group, 25 (interval: zero to 103) years.
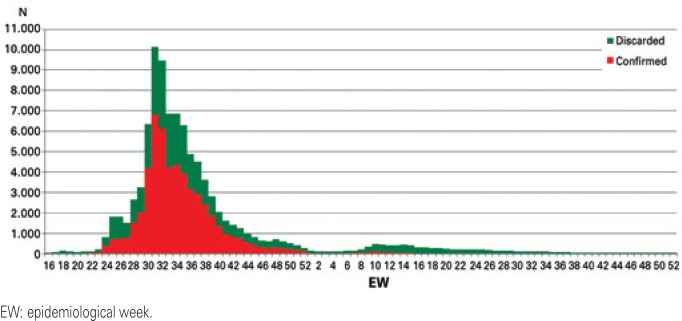
Registration of the beginning of symptoms of the first cases occurred during epidemiological week (EW) 16 of 2009. EW 31/2009 was the week with the greatest occurrence of initial symptoms (n=10,132; 10.70%) in the cases notified and confirmed (n=6,811; 12.66%) (Figure 1).
Figure 1. Distribution by epidemiological week from onset of symptoms of the cases notified with pandemic influenza A(H1N1)pdm09 as per final diagnostic classification.
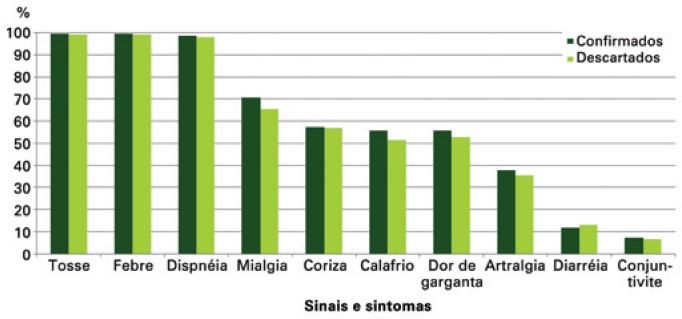
Fever was the most frequent sign, recorded in 99.74% of the confirmed cases and in 98.92% of those discarded. Cough (99.59% and 98.05%) and dyspnea (95.11% and 91.48%) were the other most frequent symptoms reported by the confirmed and discarded cases, respectively.
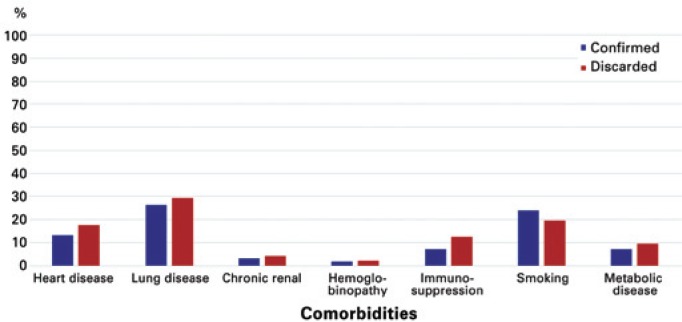
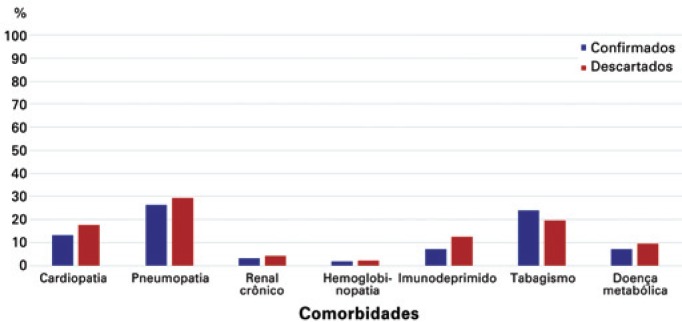
Among those confirmed, the presence of comorbidities was notified in 32.53% of cases and in 38.29% of the discarded cases. The comorbidities most frequently reported by the cases identified were chronic lung disease (27.71%), smoking (21.84%), and chronic heart disease (15.21%) (Figure 2). Other associated conditions, in addition to these reported, were notified in 58.33% of those confirmed and in 59.08% of those discarded.
Figure 2. Distribution of comorbidities of notified cases, as per final diagnostic classification.
Among those confirmed, 65.85% used the clinical-epidemiological criterion for the final case classification and 34.15% the laboratorial criterion. To discard the cases, these proportions were 79.36% and 19.57%, respectively. In cases that led to death, 73.27% were confirmed by the laboratory.
We highlight that 3.93% of cases classified as discarded also had samples processed by reverse transcription polymerase chain reaction (RT-PCR) with positive results for influenza due to a new viral subtype. Moreover, 21.20% of negative cultures in the cases classified as confirmed and 3.82% of positive cultures among those classified as discarded stood out.
The chest X-ray results presented most frequently with interstitial infiltrate, 57.41% among the cases confirmed and 54.23% among the discarded, followed by results considered normal in 23.26% of those confirmed and 18.66% of those discarded. Consolidation was present in 3,968 (9.90%) of the cases notified, in which 1,533 were confirmed and 2,435 discarded.
As to progression, 46.42% of the cases confirmed and 70.38% of the discarded were admitted to hospital.
In the analysis of evolution of the cases, 47,643 (93.77%) of those confirmed for influenza by a new viral subtype evolved to cure. The mortality rate (2,176/53,797) was 4.04%.
Pregnant women
We obtained as final classification for the pregnant women, 3,267 (53.57%) of the cases notified confirmed as influenza by a new viral subtype and 2,820 (46.33%) discarded. The incidence of influenza by new viral subtype in pregnant women in 2009 was 97.02/100,000 inhabitants and in 2010, 4.26/100,000 inhabitants.
The mean age of the confirmed cases was 25.32 (SD±6.42) years and of the discarded, 25.89 (SD±6.42) years. The median age for both groups was 25 (interval: 10 to 49) years. The age mode for confirmed cases was 21 years and for the discarded cases, 23 years.
In the evaluation of gestational age, most of the pregnant women confirmed, 1,288 (39.42%), were in the second trimester of gestation, followed by 1,249 (38,23%) in the third gestational trimester. Among those discarded, 1,125 (39.89%) were in the second trimester and 1,018 (36.10%) in the third. Gestational age was unknown for 105 (3.21%) of the confirmed pregnant women and 84 (2.98%) of those discarded.
The record of the beginning of the first symptoms occurred in EW 17 of 2009. EW 31 and 32/2009 were the weeks with greatest occurrence of onset of symptoms, with 538 and 418 cases notified confirmed and 320 and 324 discarded, respectively.
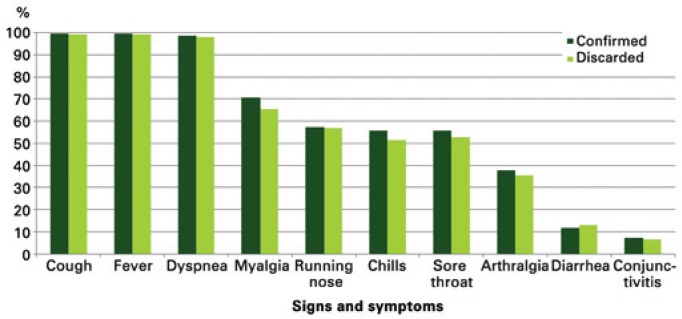
Cough (99.47%), fever (99.39%), and dyspnea (98.27%) were the most frequent signs and symptoms among those notified. Myalgia (68.14%), runny nose (57.13%), sore throat (54.25%), and chills (53.76%) were the symptoms most often reported (Figure 3).
Figure 3. Proportion of signs and symptoms of pregnant women reported as per final diagnostic classification.
Among those confirmed, the presence of comorbidities was reported in 35.48% of the cases and among 39.01% of the cases discarded.
Among the pregnant women, 56.29% were confirmed and 25.39% were discarded due to laboratorial criteria. The chest X-rays most frequently revealed interstitial infiltrate, 636 (55.26%) among the pregnant women confirmed, and 592 (53.48%) among those discarded, followed by a result considered normal in 284 (24.67%) of those confirmed and 295 (26.65%) of those discarded. Consolidation was presented in 189 (8.37%) of the cases notified, 93 of them confirmed and 96 discarded.
As to progression, 74.55% of the confirmed cases and 76.41% of those discarded were admitted to hospital.
In the analysis of outcomes, 2,730 of the pregnant women confirmed by a new viral subtype evolved to cure, 5 evolved to death due to other causes, and 307 did not have this information completed on the forms. Death by influenza occurred in 225 of the confirmed cases. The case fatality rate (225/3,267) was 6.88%.
DISCUSSION
Our results demonstrated that women, children and young adults were those most frequently reported and classified as confirmed cases of influenza A(H1N1) pdm09. These findings reproduce what was found in other studies and reflect the composition of the Brazilian population.(28)
In 2010, as is internationally known, the National Vaccination Campaign was conducted against influenza, in which 90 million people were vaccinated against virus A/H1N1pdm 2009. Although this is not the focal point of this study, we noted a significant decrease in the incidence of the disease after the said campaign, likely as an impact of this intervention.
The presence of fever, cough, and dyspnea, were the most frequent signs and symptoms of the cases classified as confirmed, discarded, and in pregnant women of our study. By and large, the clinical manifestations provoked by the pandemic influenza virus are slightly more severe than the infection caused by the seasonal virus. In updates of the definition of a case made by the Ministry of Health, the clinical picture of SARS, characterized by fever, cough, and dyspnea, was maintained.(1,7,27)
Therefore, in the cases classified as confirmed for pandemic influenza, we observed a discreet increase in the proportion of pregnant women (35.48%) who presented with comorbidities when compared to the general population (32.53%), and in fatality rate among pregnant women (6.88%) and general population (4.04%). This might be an important finding that demonstrates either greater severity in infections in pregnant women or a data capture bias of the institution, since they were already sensitive regarding vigilance and care to the population recognized as being at greater risk.
In reference to the presence of comorbidities, in the population of our study we found close proportions (32.53% of those confirmed, 38.29% of those discarded, 35.48% of the pregnant women confirmed, 39.01% of the pregnant women discarded, and 58.99% of deaths) with those of other studies.(9,14,16,18,22,24,29)
We were able to observe that our findings, both the signs and symptoms as to comorbidities, are similar to those found in literature. Patients with the characteristic SARS triad (fever, cough, and dyspnea), aged under 2 years or over 60 years, immunosuppressed, with chronic disease, and pregnant women are more vulnerable groups for worsening of the clinical condition of infection by the pandemic influenza H1N1 virus.(1,7,17,27)
As the pandemic went by, the number of cases classified as confirmed by means of the clinical-laboratorial criterion increased, which is expected (and what was determined) in a pandemic situation with a profile of respiratory disease transmission. Here we raise the hypothesis of an error in classification of the cases. We decided to not reclassify the cases and analyze a new scenario, since this situation reflects the reality of the information system and reinforces the discussion as to the quality of the data.
Clinical and radiological manifestations in the infection by the influenza virus are not specific.(8,15,18,23,29) As mentioned before, the clinical picture is similar to that of the other respiratory infections, and can be aggravated by characteristics of the infectious agent or by the condition of the host, which until then, were not associated with the viral load and severity of symptoms. The finding of interstitial infiltrate in X-ray testing may represent the clinical picture of various diseases, generally referring to inflammatory processes or a local exacerbated immune response.
The case fatality rate found in our study is consistent with the other rates available in the literature that were searched for this discussion.
Information of the cases notified represented only the cases captured by the surveillance system for mandatory notification diseases, and it is necessary to consider different sources of information for epidemiological monitoring.
Under-reporting of cases of SINAN should not occur, since human influenza by the new viral subtype is of mandatory notification for the purposes of health vigilance,(4) but it is known that this, along with quality of data, are the primary limitations found in studies with secondary data.
CONCLUSION
The epidemiological profile of the cases classified as confirmed for pandemic influenza found in our group does not include specific groups in the profile of the epidemic. Although the age range of those affected (children and young adults) differs partially from the profile expected for the cases of seasonal influenza (younger than 2 years of age and older than 60 years), no differences were found in the descriptions between the cases classified as confirmed and discarded.
In reference to the epidemiological profile of the pregnant women, the findings of our study suggested a greater morbidity-mortality for this group. The severity of the infection by influenza virus in pregnant women may be related to the very alterations that the gestational process triggers in the female organism, with an overload of the circulatory, respiratory, and immune systems. This population is delivered greater care, since they routinely present with better compliance to the services, as they are systematically included in antenatal care. In this way, more attention given to care of the pregnant women may have contributed towards greater detection of suspects and diagnostic confirmation, and consequently, with lower rates of complications, hospitalizations, and mortality in this group.
In our study, the profile observed relative to the pandemic influenza clinical picture, as well as with the more vulnerable groups and with a worse prognosis, was similar to what was described in other studies. The combination young adults-obesity seems to require more attention in order to avoid progression towards conditions of greater severity of the infection.
The greater proportion of comorbidities recorded in the cases classified as discarded suggested that the population was informed as to the characteristics of the symptoms of pandemic virus infection, alert to the conditions of risk, and instructed to seek healthcare services. Consequently, it suggested concern of the healthcare system with these groups of risk, and that the quality of care may have favored a lower mortality rate.
The investigation of the chest X-ray on the SINAN form showed a result for clinical management and not for epidemiological vigilance.
One of the highlights of this study is the quality of the SINAN data, which made clear the need for system consistency analysis for this disease. We recommend to healthcare professionals that, when facing cases of pandemic influenza with severe clinical condition, comorbidities, or in pregnant women, they should consider the need for hospital care, since these factors compose a worse prognosis of the infectious status by the pandemic influenza virus.
REFERENCES
- 1.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica . Guia de Vigilância Epidemiológica. 7a ed. Brasília, (DF): Ministério da Saúde; 2009. Influenza. Influenza. Caderno 1; pp. 1–23. série A normais e manuais técnicos. [Google Scholar]
- 2.Freitas MP. Estudo temporal da mortalidade de idosos por doenças respiratórias associadas à influenza no Brasil, 1996-2001. Belo Horizonte: Faculdade de Medicina, Universidade Federal de Minas Gerais; 2004. dissertação. [Google Scholar]
- 3.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde O desafio da influenza: epidemiologia e organização da vigilância no Brasil [Internet] [[citado 2015 Mar 4]];Bol Eletr Epid. 2004 Disponível em: http://bvsms.saude.gov.br/bvs/periodicos/boletim_eletronico_epi_ano04_n01.pdf. [Google Scholar]
- 4.Brasil. Ministério da Saúde Define as terminologias adotadas em legislação nacional, conforme o disposto no Regulamento Sanitário Internacional 2005 (RSI 2005), a relação de doenças, agravos e eventos em saúde pública de notificação comp. Portaria GM n° 104, de 25 de janeiro de 2011. 2011 Jan 26;:37–37. Diário Oficial da União. Brasília (DF) Seção 1.
- 5.Brasil. Congresso Nacional . Aprova o texto revisado do Regulamento Sanitário Internacional, acordado na 58ª Assembléia Geral da Organização Mundial de Saúde, em 23 de maio de 2005. 2 ed. Brasília, DF: Congresso Nacional; 2009. Decreto Legislativo no 395, de 2009. [Google Scholar]
- 6.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde . Notificação de Influenza no SINAN. Brasília, (DF): Ministério da Saúde; 2009. Nota técnica n°3/2009 - GT-SINAN/CIEVS e COVER/CGDT/DEVEP. [Google Scholar]
- 7.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde . Protocolo de manejo clínico epidemiológica da Influenza. Versão II. Brasília, (DF): Ministério da Saúde; 2009. Gabinete Permanente de Emergências em Saúde Pública. [Google Scholar]
- 8.Lenzi L, Mello ÂM, Silva LR, Grochocki MH, Pantarolo R. Pandemic influenza A (H1N1) 2009: risk factors for hospitalization. J Bras Pneumol. 2012;38(1):57–65. doi: 10.1590/s1806-37132012000100009. [DOI] [PubMed] [Google Scholar]
- 9.Lenzi L, Mello ÂM, Silva LR, Grochocki MH, Pontarolo R. Manifestações clínicas, desfechos e fatores prognósticos da influenza pandêmica A (H1N1) de 2009 em crianças. Rev Paul Pediatr. 2012;30(3):346–352. [Google Scholar]
- 10.Melchior TB, Guatura SB, Camargo CN, Watanabe AS, Granato C, Bellei N. Casos confirmados de influenza em pacientes hospitalizados com suspeita de infecção por influenza A (H1N1) em 2010 em um hospital sentinela na cidade de São Paulo, Brasil. J Bras Pneumol. 2011;37(5):655–658. doi: 10.1590/s1806-37132011000500013. [DOI] [PubMed] [Google Scholar]
- 11.Nassar AP, Junior, Mocelin AO, Nunes AL, Brauer L. Apresentação clínica e evolução de pacientes com infecção por Influenza A (H1N1) que necessitaram de terapia intensiva durante a pandemia de 2009. Rev Bras Ter Intensiva. 2010;22(4):333–338. [PubMed] [Google Scholar]
- 12.Divisão de Doenças de Transmissão Respiratória, Centro de Vigilância Epidemiológica Prof. Alexandre Vranjac, Coordenadoria de Controle de Doenças, Secretaria de Estado da Saúde de São Paulo Characteristics of Influenza A (H1N1) notified cases. Rev Saude Publica. 2009;43(5):900–904. doi: 10.1590/s0034-89102009000500024. Portuguese. [DOI] [PubMed] [Google Scholar]
- 13.Duarte PA, Venazzi A, Youssef NC, Oliveira MC, Tannous LA, Duarte CB, et al. Pacientes com infecção por vírus A (H1N1) admitidos em unidades de terapia intensiva do Estado do Paraná, Brasil. Rev Bras Ter Intensiva. 2009;21(3):231–236. [PubMed] [Google Scholar]
- 14.Oliveira W, Carmo E, Penna G, Kuchenbecker R, Santos H, Araujo W, Malaguti R, Duncan B, Schmidt M. Surveillance Team for the pandemic influenza A(H1N1) 2009 in the Ministry of Health. Pandemic H1N1 influenza in Brazil: analysis of the first 34,506 notified cases of influenza-like illness with severe acute respiratory infection (SARI) Euro Surveill. 2009;14(42) doi: 10.2807/ese.14.42.19362-en. pii: 19362. Erratum in: Euro Surveill. 2009;14(43) pii: 19382. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro SA, Brasileiro GS, Soleiman LN, Silva CC, Kavaguti CS. Síndrome respiratória aguda grave causada por influenza A (subtipo H1N1) J Bras Pneumol. 2010;36(3):386–389. doi: 10.1590/s1806-37132010000300017. [DOI] [PubMed] [Google Scholar]
- 16.Schuelter-Trevisol F, Dutra MC, Uliano EJ, Zandomênico J, Trevisol DJ. Perfil epidemiológico dos casos de gripe A na região sul de Santa Catarina, Brasil, na epidemia de 2009. Rev Panam Salud Publica. 2012;32(1):82–86. doi: 10.1590/s1020-49892012000700013. [DOI] [PubMed] [Google Scholar]
- 17.Yokota RT, Skalinski LM, Igansi CN, de Souza LR, Iser BP, Reis PO, et al. Risk factors for death from pandemic (H1N1) 2009, southern Brazil. Emerg Infect Dis. 2011;17(8):1467–1471. doi: 10.3201/eid1708.101233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camargo LF, de Sandes-Freitas TV, Silva CD, Bittante CD, Ono G, Corrêa L, et al. Morbimortality of pandemic influenza A H1N1 infection in kidney transplant recipients requiring hospitalization: a comparative analysis with nonimmunocompromised patients. Transplantation. 2012;93(1):69–72. doi: 10.1097/TP.0b013e31823aa528. [DOI] [PubMed] [Google Scholar]
- 19.Lenzi L, Pontarolo R. Evaluation of pregnancy as a risk factor in the outcomes of influenza A (H1N1)/2009 in women of childbearing age. Cad Saude Publica. 2012;28(2):395–399. doi: 10.1590/s0102-311x2012000200018. [DOI] [PubMed] [Google Scholar]
- 20.Abdulkader RC, Ho YL, Santos SS, Caires R, Arantes MF, Andrade L. Characteristics of acute kidney injury in patients infected with the 2009 influenza A (H1N1) virus. Clin J Am Soc Nephrol. 2010;5(11):1916–1921. doi: 10.2215/CJN.00840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenzi L, Wiens A, Grochocki MH, Pontarolo R. Study of the relationship between socio-demographic characteristics and new influenza A (H1N1) Braz J Infect Dis. 2011;15(5):457–461. doi: 10.1016/s1413-8670(11)70227-6. [DOI] [PubMed] [Google Scholar]
- 22.Souza TM, Salluh JI, Bozza FA, Mesquita M, Soares M, Motta FC, et al. H1N1pdm influenza infection in hospitalized cancer patients: clinical evolution and viral analysis. PLoS One. 2010;5(11):e14158–e14158. doi: 10.1371/journal.pone.0014158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenzi L, Wiens A, Pontarolo R. The characteristics, clinical manifestations and outcomes of pandemic influenza A (H1N1) 2009 in the elderly. Rev Soc Bras Med Trop. 2013;46(2):135–140. doi: 10.1590/0037-8682-0024-2012. [DOI] [PubMed] [Google Scholar]
- 24.Pires Rda J, Neto, Lemos DR, Cavalcanti LP, Ramos AN, Junior, Alencar CH, Façanha MC, et al. Pandemic influenza A (H1N1) 2009: epidemiological analysis of cases in a tropical/semi-arid region of Brazil. Rev Soc Bras Med Trop. 2013;46(2):141–146. doi: 10.1590/0037-8682-0016-2012. [DOI] [PubMed] [Google Scholar]
- 25.Raboni SM, Stella V, Cruz CR, França JB, Moreira S, Gonçalves L, et al. Laboratory diagnosis, epidemiology, and clinical outcomes of pandemic influenza A and community respiratory viral infections in southern Brazil. J Clin Microbiol. 2011;49(4):1287–1293. doi: 10.1128/JCM.02205-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schout D, Hajjar LA, Galas FR, Uip DE, Levin AS, Caiaffa HH, Filho, et al. Epidemiology of human infection with the novel virus influenza A (H1N1) in the Hospital das Clínicas, São Paulo, Brazil-June-September 2009. Clinics (São Paulo) 2009;64(10):1025–1030. doi: 10.1590/S1807-59322009001000014. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica . Protocolo de vigilância epidemiológica da influenza pandêmica (H1N1) 2009 - Notificação, Investigação e Monitoramento. Brasília, DF: Ministério da Saúde; 2010. pp. 1–32. [Google Scholar]
- 28.Brasil. Ministério do Orçamento Planejamento e Gestão. Instituto Brasileiro de Geografia e Estatística . Censo demográfico 2010. Características gerais da população, religião e pessoas com deficiência. Brasília, DF: Ministério do Orçamento Planejamento e Gestão; 2010. [Google Scholar]
- 29.Del Bianco R, Santos MS, Ribeiro MC, Viso AT, Carvalho V. Clinical aspects of influenza A (H1N1) in HIV-infected individuals in São Paulo during the pandemic of 2009. Braz J Infect Dis. 2011;15(2):170–173. doi: 10.1016/S1413-8670(11)70166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]