Abstract
Tendon and ligament (T/L) pathologies account for a significant portion of musculoskeletal injuries and disorders. Tissue engineering has emerged as a promising solution in the regeneration of both tissues. Specifically, the use of multipotent human mesenchymal stromal cells (hMSC) has shown great promise to serve as both a suitable cell source for tenogenic regeneration and a source of trophic factors to induce tenogenesis. Using four donor sets, we investigated the bidirectional paracrine tenogenic response between human hamstring tenocytes (hHT) and bone marrow-derived hMSC. Cell metabolic assays showed that only one hHT donor experienced sustained notable increases in cell metabolic activity during co-culture. Histological staining confirmed that co-culture induced elevated collagen protein levels in both cell types at varying time-points in two of four donor sets assessed. Gene expression analysis using qPCR showed the varied up-regulation of anabolic and catabolic markers involved in extracellular matrix maintenance for hMSC and hHT. Furthermore, analysis of hMSC/hHT co-culture secretome using a reporter cell line for TGF-β, a potent inducer of tenogenesis, revealed a trend of higher TGF-β bioactivity in hMSC secretome compared to hHT. Finally, hHT cytoskeletal immunostaining confirmed that both cell types released soluble factors capable of inducing favorable tenogenic morphology, comparable to control levels of soluble TGF-β1. These results suggest a potential for TGF-β-mediated signaling mechanism that is involved during the paracrine interplay between the two cell types that is reminiscent of T/L matrix remodeling/ turnover. These findings have significant implications in the clinical use of hMSC for common T/L pathologies.
Keywords: MESENCHYMAL STROMAL CELLS, LIGAMENT, TENDON, TRANSFORMING GROWTH FACTOR BETA, PARACRINE SIGNALING, CO-CULTURE
Musculoskeletal injuries are a significant problem for the healthcare system. In the United States, there are approximately 32 million musculoskeletal injuries per year costing $950 billion in direct costs and lost wages with tendon and ligament (T/L) injuries accounting for about 45% of these injuries [Vunjak-Novakovic et al., 2004; Butler et al., 2008, Hast et al., 2014; Rothrauff and Tuan, 2014]. T/L are dense collagenous tissues involved in joint stability and locomotion. Ligaments are responsible for the structural support necessary to connect bones and stabilize joints, while tendons transfer the force generated from muscles into limb movement. Both tissues are composed of fibroblast cells embedded within an extracellular matrix (ECM) of collagens, elastin, and proteoglycans. They are hierarchical in architecture given that they operate primarily in tension. Injury due to trauma or genetic disorder often leads to varying degrees of change in the expression patterns of key structural proteins, tissue cellularity, disorganization of the collagen matrix, and inflammation [Sun et al., 2008; Fung et al., 2010]. Conventional methods of repair for the sub-failure (grades I, II) class of injuries include rest, ice, compression, and elevation or RICE [Lynch and Renstrom, 1999], which is contingent on the innate healing ability of the tissue. In severe cases such as ruptures and avulsions (grade III), surgical reconstruction may be required [Lynch and Renstrom, 1999; Vunjak-Novakovic et al., 2004; Sharma and Maffulli, 2005; James et al., 2008]. However due to the lack of sufficient vascularization and poor ECM remodeling, tissue healing is often lengthy and incomplete. As a result of the associated drawbacks with current treatment options, there is a need for functional tendon healing modalities.
Tissue engineering (TE) has emerged as a promising option for tendon repair. Biomaterials and cells, used individually or in combination, have shown the potential to repair numerous T/L dysfunctions in vitro and in vivo [Watanabe et al., 2002; Ge et al., 2005; Liu et al., 2008; Schneider et al., 2011; Canseco et al., 2012; Smith et al., 2013]. More specifically, the use of multipotent mesenchymal stem or stromal cells (MSC) as a cell source has proven to be a feasible modality for scaffold incorporation or direct injection. Recent in vitro and in vivo work has shown that localized MSC delivery may be beneficial for T/L repair by increasing cell number, enhancing ECM deposition and maturation, and increasing tissue biomechanical properties after injury [Awad et al., 1999; Watanabe et al., 2002; Luo et al., 2009; Lee et al., 2011; Lui et al., 2011]. This has led to work identifying MSC as both a suitable cell source for TE-inspired tenogenic regeneration and a potential source for the secretion of a plethora of soluble factors that mediate the healing response [Van Eijk et al., 2004; Caplan and Dennis, 2006; Shimode et al., 2007; Baraniak and McDevitt, 2010]. To this end, studies have identified several soluble factors as potential mediators in the enhancement of tenogenesis in both MSC and T/L fibroblasts [Schnabel et al., 2007; Schneider et al., 2011]. Amongst these factors, transforming growth factor-beta or TGF-β has been widely reported to be a potent inducer of tenogenic regeneration [Gafni et al., 2004; Lui et al., 2011].
Proteins of the TGF-β superfamily are considered pleiotropic cytokines that play a prominent role during wound healing and musculoskeletal tissue development [Leask and Abraham, 2004; Schiller et al., 2004]. More specifically, during T/L development, TGF-β has been reported to be a key mediator of a panel of genes that are responsible for the anabolic and catabolic maintenance of ECM in vitro and in vivo [Massague, 1998; Li et al., 2011]. Molecular changes evidenced in the altered expression of anabolic markers such as collagens and proteoglycans are known to accompany the healing of T/L [Kuo and Tuan, 2008]. Additionally, changes in the expression patterns of catabolic markers such as the collagen-degrading MMP family (matrix metalloproteinases) and proteoglycan-cleaving ADAMTS family (a disintegrin and metalloproteinase with thrombospondin motifs) have also been reported [Jones et al., 2006; Corps et al., 2008; Kuo and Tuan, 2008; Wylie et al., 2012; Maeda et al., 2013]. The balance between the regulation and production of these markers has significant implications in the extent of matrix remodeling during regeneration [Jones et al., 2006; Smith et al., 2008].
The objective of this study was to determine the effect of the paracrine signaling, or cross-talk, between primary human hamstring tenocytes (hHT) and hMSC on cell response and the expression of T/L markers in both cell types in vitro and screen the co-culture for TGF-β bioactivity. We hypothesize that the co-culture of hMSC with hHT will lead to enhanced tenogenic cell function when compared to populations cultured separately. We postulate that this exchange of soluble factors will facilitate the maintenance of ECM produced by both cell types, ultimately leading to enhanced tenogenic regeneration in vivo. To test this hypothesis, we employed an indirect cell co-culture model to investigate the effects of co-culture on cell metabolic activity, ECM production, and gene expression of anabolic and catabolic tenogenic markers. Additionally, we indirectly investigated TGF-β bioactivity in the secretome of each cell type and during co-culture via a TGF-β reporter bioassay. Lastly, we directly assayed for the effect of hMSC and hHT secretome on tenocyte morphology via immunostaining.
MATERIALS AND METHODS
TISSUE HARVEST, CELL ISOLATION, AND hMSC CHARACTERIZATION
The experimental overview summarizing the experimental design and all cell and secretome analyses conducted is presented in Figure 1. All experiments were conducted in accordance with recommendations and approval from the Medical Ethical Research Committee at the Utrecht Medical Center and MST Twente. Following standard written informed consent, hamstring tendon (hHT) samples were harvested from four adult patients undergoing anterior cruciate ligament reconstruction. The tendons were isolated, rinsed with phosphate buffered saline (PBS), and excess muscle tissue was carefully removed prior to dissection and mincing into smaller pieces. Next, tendon pieces were cultured in growth medium of Dulbecco’s modified Eagle’s medium (PAA Laboratories, Australia) supplemented with 10% fetal bovine serum (FBS) (Lonza, Basel, Switzerland), 100 U/mL penicillin and 100 mg/mL streptomycin, and 0.2 mM ascorbic acid (Sigma– Aldrich, St. Louis, MO, USA) to allow the cells to migrate out from the tissue pieces.
Fig. 1.
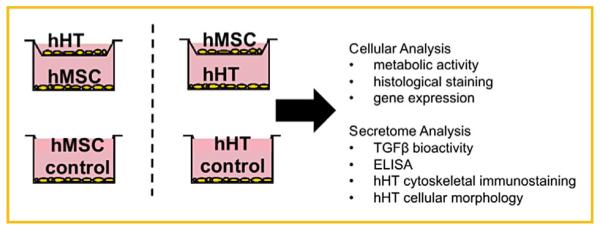
Experimental design showing co-culture configuration and non co-culture control groups. Experiments were performed in biological triplicate.
Bone marrow aspirates were obtained from four additional adult patients following written informed consent. Donor information for each cell type is presented in Table I. hMSC were isolated and cultured in hMSC basic medium consisting of alpha minimal essential medium (aMEM; Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Lonza), 100 U/mL penicillin and 100 mg/mL streptomycin (Life Technologies), 2 mM L-Glutamine (Life Technologies), and 0.2 mM ascorbic acid (Sigma–Aldrich), as previously described [Fernandes et al., 2010; Doorn et al., 2013]. Phenotypical characterization of hMSC was performed as previously described [Gothard et al., 2013]. Preliminary work with the hMSC used showed confirmation for self-renewal potential using a CFU assay and osteogenic differentiation potential using ALP mineralization staining (data not shown).
TABLE I.
Donor Set Patient Information
| Donor set | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Cell type | hMSC | hHT | hMSC | hHT | hMSC | hHT | hMSC | hHT |
| Gender | Female | Male | Female | Male | Male | Female | Male | Female |
| Age | 74 | 23 | 67 | 25 | 72 | 24 | 52 | 21 |
CO-CULTURE CONDITION
Cells between passage 2 and 5 were used for co-culture experiments and maintained for up to 14 days in a reduced factor medium containing 1% FBS and 1% P/S, without the addition of L-Glutamine or ascorbic acid. Indirect co-culture of hHT/hMSC was achieved using 1 μm pore sized permeable well plate inserts (Greiner Bio One, Kaysville, UT). In this system, 2.5 × 104 hHT (n ¼ 3) were seeded on the bottom of well-plates, hMSC were seeded on the permeable membrane at a 1:1 ratio and allowed to adhere overnight before placing the inserts in the well-plates. The reverse configuration was also studied to determine the effect of co-culture on hMSC. Assays were conducted on the cells seeded on the bottom well and control groups consisted of each cell type seeded separately with no co-culture inserts. After 3 days of co-culture, conditioned medium was collected for further analysis. For the first three donor sets, only half the cell culture medium was replaced 2–3 times per week to allow continuous cross-talk between cells and preserve the co-culture microenvironment. For the fourth donor set, culture medium was replaced with complete fresh medium after the first two time-points to further delineate the effects of preserving co-culture micro-environment on cell response. For the first two donor sets, assays were conducted at 1, 3, and 7-day time-points to assess the earlier cell response between the two cell types. For the last two donor sets, assays were conducted at 1, 3, 7, and 14-day time-points to also assess later cell responses.
CELLULAR METABOLIC ACTIVITY
Cell metabolic activity (n ¼ 3) was evaluated as previously described by Zilony et al. [2013] using the flurometric PrestoBlue assay according to manufacturer’s specifications. Briefly, at each time-point, the inserts were removed; medium aspirated and 10% (v/v) PrestoBlue solution in basic medium was added. After a 1 h incubation the fluorescence was measured in technical duplicate using a Victor3 1420 multilabel counter (Perkin Elmer, Waltham, MA, USA) at 560 nm excitation and 590 nm emission wavelengths. Fluorescent values were normalized for no-cell blank control wells as well as the first time-point non-co-culture control group to represent proliferation over time.
ECM DEPOSITION
To assess ECM deposition (n ¼ 3) at each time-point, we employed the semi-quantitative collagen/non-collagen staining assay as previously described [Tomikawa et al., 2012; Kwan et al., 2013]. In brief, medium was discarded, cells were washed with PBS and stained using Sirius Red/Fast Green Collagen Staining Kit (Chondrex Inc., Redmond, WA). After performing the assay according to manufacturer’s protocol, absorbance was read at 480 nm for Sirius Red and 605 nm for Fast Green with a spectrophotometer. Absorbance values for co-culture groups were normalized to control values.
REAL-TIME QUANTITATIVE REVERSE-TRANSCRIPTION POLYMERASE CHAIN REACTION (qPCR)
Expression of tenogenic markers in both cell types (n ¼ 3) was evaluated using qPCR as previously described [Kim et al., 2009; Canseco et al., 2012]. Total RNA was isolated and purified by spin protocol using Bioke RNA II Nucleospin RNA isolation kit (Machery Nagel, Düren, Germany) according to manufacturer’s protocol. Afterward, RNA concentrations were measured using a ND100 spectrophotometer (Thermo Fisher Scientific, Cambridge, MA, USA). Total RNA was normalized for all groups then reverse transcribed to obtain cDNA using iScript (BioRad, Hercules, CA, USA) according to the manufacturer’s directions. The cDNA was subjected to qPCR using iQ SYBR Green Supermix (Biorad) on a Real-time PCR Detection System (BioRad). Specific primer sequences are listed in Table II. Relative gene expression was calculated using the ΔΔCT method, normalized to beta-2-microglobulin (B2M) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an endogenous control.
TABLE II.
Primer Sequences and Product Sizes for Quantitative Reverse Transcription-Polymerase Chain Reaction
| Gene | 5′ DNA sequence 3′ | Product size (bp) | |
|---|---|---|---|
| Collagen I | Forward | 5′ GTCACCCACCGACCAAGAAACC 3′ | 121 |
| Reverse | 5′ AAGTCCAGGCTGTCCAGGGATG 3′ | ||
| Collagen III | Forward | 5′ GCCAACGTCCACACCAAATT 3′ | 88 |
| Reverse | 5′ AACACGCAAGGCTGTGAGACT 3′ | ||
| Tenomodulin | Forward | 5′ TGTATTGGATCAATCCCACTCTAAT 3′ | 92 |
| Reverse | 5′ TTTTTCGTTGGCAGGAAAGT 3′ | ||
| Tenascin C | Forward | 5′ TGGGCAGATTTCACGGCTG 3′ | 207 |
| Reverse | 5′ TGCTCTGAGCCCGAATGTC 3′ | ||
| Aggrecan | Forward | 5′ AGGCAGCGTGATCCTTACC 3′ | 136 |
| Reverse | 5′ GGCCTCTCCAGTCTCATTCTC 3′ | ||
| TIMP-3 | Forward | 5′ CCAGGACGCCTTCTGCAAC 3′ | 71 |
| Reverse | 5′ CCTCCTTTACCAGCTTCTTCCC 3′ | ||
| MMP-1 | Forward | 5′ GGGAGATCATCGGGACAACTC 3′ | 72 |
| Reverse | 5′ GGGCCTGGTTGAAAAGCAT 3′ | ||
| MMP-3 | Forward | 5′ TGGCATTCAGTCCCTCTATGG 3′ | 116 |
| Reverse | 5′ AGGACAAAGCAGGATCACAGTT 3′ | ||
| MMP-13 | Forward | 5′ AAGGAGCATGGCGACTTCT 3′ | 72 |
| Reverse | 5′ TGGCCCAGGAGGAAAAGC 3′ | ||
| ADAMTS-4 | Forward | 5′ CAAGGTCCCATGTGCAACGT 3′ | 115 |
| Reverse | 5′ CATCTGCCACCACCAGTGTCT 3′ | ||
| ADAMTS-5 | Forward | 5′ TGGCTCACGAAATCGGACA 3′ | 74 |
| Reverse | 5′ GGAACCAAAGGTCTCTTCACAGA 3′ | ||
| B2M | Forward | 5′ GACTTGTCTTTCAGCAAGGA 3′ | 106 |
| Reverse | 5′ ACAAAGTCACATGGTTCACA 3′ | ||
| GAPDH | Forward | 5′ ACAACTTTGGTATCGTGGAA 3′ | 458 |
| Reverse | 5′ AAATTCGTTGTCATACCAGG 3′ |
SECRETED PROTEIN QUANTIFICATION
Conditioned medium collected from hHT and hMSC were analyzed for total secreted protein and total secreted TGF-β1. Total protein secreted was assayed using the Pierce BCA Total Protein Assay (Sigma). Total TGF-β1 was analyzed using ELISA (Biolegend, San Diego, CA, USA). Assays were performed in technical triplicate according to manufacturer’s protocol and absorbance was quantified using a microplate reader (Tecan, Medford, MA, USA).
TGF-β BIOASSAY
Quantification of soluble-TGF-β bioactivity was determined for co-culture conditions using transformed mink lung cells (TMLC) that have been genetically modified to produce luciferase under control of the TGF-β -responsive plasminogen activator inhibitor-1 (PAI-1) promoter [Abe et al., 1994; Wipff et al., 2007]. TMLC were a kind gift from Dr. Daniel Rifkin in the Department of Cell Biology at the New York University School of Medicine. TMLC (8 × 103/cm2;n ¼ 3) were grown overnight before being exposed to the conditioned medium from co-culture experiments for one day. TMLC cultured in 1% serum medium were utilized as basal controls. Afterwards, cells were assayed for metabolic activity using PrestoBlue then lysed and luciferase activity was assessed by light production from a luciferin substrate (Promega, Madison, WI, USA) using a luminometer (Perkin Elmer).
IMMUNOSTAINING
For cell morphology studies, all components utilized were from donor set 1. Here, hHT from the first donor set were seeded at 2.5 × 104 cells/cm2 (n ¼ 9) on tissue culture plates and allowed to attach overnight. The next day, conditioned medium samples collected from hMSC and hHT were analyzed for effect on hHT for 24 h. Control groups included basal conditions (1% FBS αMEM) and +10 ng/mL TGF-β1. Afterwards, cells were washed with PBS and fixed with 4% paraformaldehyde solution. After permeabilization with 0.1% Triton X-100/PBS and blocking with 2% BSA/PBS-Tween (0.1%), the cells were stained with Phalloidin fluorophore (1:40). Afterward, the cells were washed with PBS and counterstained for DAPI. Images were obtained using an EVOS FL Microscope (Life Technologies). Image analysis was performed using ImageJ Software (NIH).
STATISTICAL ANALYSIS
All numerical data reported as mean ± standard deviation. A oneway ANOVA with a Bonferroni’s multiple comparison post-hoc analysis was utilized for comparing groups in the secretome analysis studies. A two-way ANOVA with a Bonferroni’s multiple comparison post-hoc analysis was employed for comparing the various groups of cell populations for cell proliferation studies. A Student’s t-test was employed for comparing groups in the mRNA expression studies (GraphPad Prism Software 5.0, La Jolla, CA). Differences were considered statistically significant for P-values less than 0.05, unless otherwise stated (Table II).
RESULTS
EFFECT OF CROSS-TALK ON hMSC AND hHT METABOLIC ACTIVITY AND ECM DEPOSITION
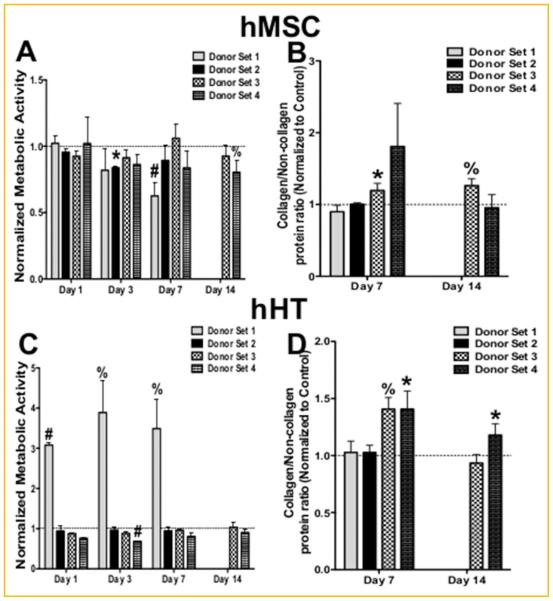
The goal of this study was to examine the effect of the paracrine interaction between hMSC and hHT on the tenogenic cell function of both cell types. Cell metabolic activity data obtained via the fluorometric PrestoBlue assay showed that for each hMSC donor, cell activity was never significantly increased, more so decreased, during co-culture with hHT (Fig. 2A). hMSC from donor set 3 were the only group to experience elevated proliferation during co-culture after 7 days, although not significant. Furthermore, Sirius Red/Fast Green staining showed a significant increase in collagen to non-collagen protein ratio after 7 and 14 days in donor set 3 (Fig. 2B).
Fig. 2.
Effect of co-culture on cell function. (A) Metabolic activity of hMSC during co-culture with hHT. Values have been normalized to non co-cultured control hMSC populations (dashed line at 1). (B) Sirius Red/Fast Green staining of hMSC during co-culture with hHT normalized non co-cultured control hMSC populations (dashed line at 1). (C) Metabolic activity of hHT during co-culture with hMSC normalized to non co-cultured control hHT populations (dashed line at 1). (D) Sirius Red/Fast Green staining of hHT during co-culture with hMSC normalized to non co-cultured control hHT populations (dashed line at 1) (* P < 0.05, %P < 0.01, #P < 0.001).
For hHT donors, cell activity data obtained via PrestoBlue assay only showed notable increases in metabolic activity in donor set 1. Cell populations from donor sets 2–4 had cell activity that ranged from staying close to control levels or falling slightly below during co-culture (Fig. 2C). Lastly, Sirius Red/Fast Green staining showed that hHT from donor sets 3 and 4 had elevated collagen to non-collagen protein levels during co-culture after 7 days. hHT from donor set 4 maintained higher collagen/non-collagen levels after 14 days of co-culture with hMSC (Fig. 2D).
mRNA EXPRESSION OF TENDON RELATED MARKERS
To further understand T/L matrix development during co-culture, we assessed a panel of anabolic markers that play critical roles in ECM development. Results of qPCR experiments are presented in Table III. Analysis of mRNA expression of anabolic genes shows that in the first donor set hMSC exhibited only a significant decrease in Tenascin C mRNA expression after 3 days. In this donor set, there was also a notable decrease in collagen I mRNA of hHT after 3 days. In the second donor set, hMSC experienced significantly higher levels of Tenomodulin expression during co-culture at 3 and7 days while hHT exhibited up-regulation of Tenascin C during co-culture after 7 days. In the third donor set, hHT experienced up-regulation of tissue inhibitor of metalloproteinase-3 (TIMP-3) during co-culture with hMSC at 7 and 14 days. There was also an up-regulation of collagen type I at 14 days. In the fourth donor set, hMSC experienced higher mRNA levels of Tenomodulin and Aggrecan during co-culture with hHT at 7 and 14 days. hHT exhibited a trend of up-regulation of Tenascin C and Tenomodulin after 7 and 14 days of co-culture with hMSC (Table III).
TABLE III.
Relative mRNA Expression of hMSC and hHT During Co-Culture
| A. hMSC |
||||||||
|---|---|---|---|---|---|---|---|---|
| Donor set 1 |
Donor set 2 |
Donor set 3 |
Donor set 4 |
|||||
| Gene | Day 3 | Day 7 | Day 3 | Day 7 | Day 7 | Day 14 | Day 7 | Day 14 |
| Collagen I | 1.01 ± 0.15 | 1.11 ± 0.13 | 1.1 ± 0.24 | 1.6 ± 0.27 | 1.1 ± 0.21 | 0.9 ± 0.48 | 1.0 ± 0.19 | 0.7 ± 0.16 |
| Collagen III | 1.05 ± 0.06 | 1.2 ± 0.30 | 1.7 ± 0.66 | 1.4 ± 0.49 | 1.0 ± 0.17 | 15 ± 032 | 0.9 ±0.12 | 1.0 ± 0.32 |
| Tenascin C | 0.49 ± 0.07% | 1.03 ± 0.30 | 10 ± 0.4 | 1.2 ± 0.21 | 0.90 ± 0.19 | 1.4 ± 039 | 0.5 ± 0.22 | 0.7 ± 0.40 |
| Tenomodulin | ND | ND | 5.2 ± 1.96* | 4.9 ± 20* | 1.0 ± 0.26 | 0.6 ± 020 | 4.2 ± 1.75* | 2.2 ± 0.20* |
| Aggrecan | 0.95 ± 1.13 | 0.80 ± 0.09 | 1.4 ± 0.25 | 2.5 ± 0.72* | 1.0 ± 0.39 | 0.7 ± 037 | 1.5 ± 0.15* | 3.8 ± 1.13* |
| TIMP-3 | 1.29 ± 0.87 | 0.77 ± 0.2I | 0.9 ± 0.21 | 1.5 ± 0.38 | 1.4 ± 0.22 | 0.8 ± 032 | 0.8 ± 0.05 | 0.9 ± 0.16 |
| MMP-1 | ND | ND | 1.4 ± 0.16 | 1.0 ± 0.45 | 2.6 ± 0.11* | 1.7 ± 0.47 | 1.7 ± 1.12 | 0.9 ± 0.15 |
| MMP-3 | ND | ND | 1.8 ± 0.70 | 0.8 ± 0.20 | 1.7 ± 0.42 | 12 ± 032 | 1.5 ± 0.55 | 0.8 ± 0.11 |
| MMP-13 | ND | 0.85 ± 0.28 | 1.0 ± 0.62 | 1.1 ± 0.47 | 2.0 ± 0.39* | 05 ± 034 | 0.8 ± 0.14 | 0.8 ± 0.14 |
| ADAMTS-4 | 1.20 ± 0.24 | 1.02 ± 0.03 | 1.0 ± 0.39 | 1.7 ± 0.58 | 1.7 ± 0.23 | 1.0 ± 039 | 1.0 ± 0.14 | 0.8 ± 0.25 |
| ADAMTS-5 | 1.07 ± 0.08 | 1.19 ± 0.52 | 1.7 ± 0.85 | 3.0 ± 1.21* | 1.8 ± 0.35* | 1.1 ± 039 | 1.6 ± 0.29* | 1.5 ± 0.30 |
|
| ||||||||
| B. hHT | ||||||||
|
| ||||||||
| Collagen I | 0.75 ± 0.11* | l.03 ± 0.28 | 1.19 ± 0.25 | 1.39 ± 0.52 | 1.33 ± 0.44 | 3.95 ± 1.49* | 1.50 ± 0.54 | 0.91 ± 0.27 |
| Collagen III | 0.67 ± 0.09 | 1.11 ± 0.30 | 1.75 ± 0.46 | 1.23 ± 0.32 | 1.41 ± 0.54 | 1.63 ± 0.64* | 1.51 ± 0.08 | 1.07 ± 0.64 |
| Tenascin C | l.32 ± 0.48 | 1.14 ± 0.33 | 2.19 ± 0.96 | 1.87 ± 0.35* | 0.95 ± 0.41 | 1.89 ± 1.18 | 2.09 ± 0.84 | 2.05 ± 0.35 |
| Tenomodulin | ND | ND | ND | ND | ND | ND | 1.39 ± 0.90 | 2.80 ± 1.52 |
| Aggrecan | 2.33 ± 1.90 | 0.84 ± 0.08 | 1.24 ± 0.40 | 1.04 ± 0.42 | 0.77 ± 039 | 0.62 ± 0.23 | 1.47 ± 0.43 | 0.74 ± 0.46 |
| TIMP-3 | 2.12 ± 1.93 | 0.94 ± 0.33 | 1.85 ± 1.12 | 0.95 ± 0.20 | 2.06 ± 0.43* | 2.66 ± 0.64* | 2.27 ± 0.82 | 0.74 ± 0.30 |
| MMP-1 | 4.32 ± 0.39# | 10.01 ± 4.73* | 0.65 ± 0.62 | 1.11 ± 0.08 | 1.16 ± 020 | 3.72 ± 0.77% | 4.14 ± 0.94* | 0.61 ± 0.30 |
| MMP-3 | ND | 3.51 ± 1.34* | 1.37 ± 0.92 | 1.14 ± 0.14 | 1.90 ± 022* | 0.90 ± 0.23 | 2.06 ± 0.67 | 0.94 ± 0.45 |
| MMP-13 | 8.07 ± 0.71# | 16.43 ± 5.15% | 1.0 ± 0.62 | 1.33 ± 0.26 | 1.05 ± 0.14 | 2.12 ± 0.29% | 3.16 ± 0.98* | 1.61 ± 0.11 |
| ADAMTS-4 | 1.29 ± 0.45 | 3.08 ± 1.04* | 2.53 ± 0.94 | 1.43 ± 0.21 | 1.61 ± 0.19* | 2.03 ± 0.41* | 2.47 ± 1.01 | 0.98 ± 0.42 |
| ADAMTS-5 | 0.44 ± 0.15 | 0.53 ± 0.25 | 0.54 ± 0.44 | 0.90 ± 0.24 | 0.64 ± 0.17 | 120 ± 0.37 | 1.18 ± 0.46 | 0.73 ± 0.29 |
Transcript levels expressed as relative compared to non co-cultured control cell populations. Normalized to B2M or GAPDH.
P < 0.05,
P < 0.01,
P < 0.001.
Next, we screened a panel of catabolic markers characteristic of T/L ECM development. Expression analysis of catabolic markers in the first donor set revealed that hHT experienced a marked increase in the mRNA expression of MMP-1, MMP-3, MMP-13, and ADAMTS-4 during the 7 days of co-culture with hMSC. Results from the second donor set showed that hMSC exhibited up-regulation of ADAMTS-5 at 7 days during co-culture with hHT. In the third donor set, hMSC showed up-regulation of MMP-1, MMP- 13, and ADAMTS-5 after 7 days of co-culture while the hHT showed elevated mRNA levels of ADAMTS-4 and MMP-3 at 7 days and ADAMTS-4, MMP-1, and MMP-13 at 14 days after co-culture with hMSC. The fourth donor set showed that hMSC had higher mRNA expression of ADAMTS-5 at 7 days of co-culture while hHT experienced up-regulation of MMP-1 and MMP-13 after 7 days of co-culture (Table III). Overall, our data indicates that in addition to affecting the expression patterns of anabolic markers, cross-talk between hHT and hMSC may also activate variable changes in the expression patterns of catabolic markers.
CONDITIONED MEDIUM TGF-β BIOASSAY
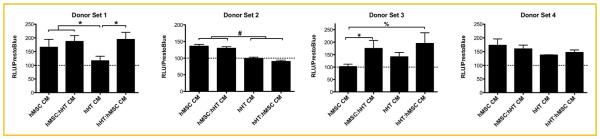
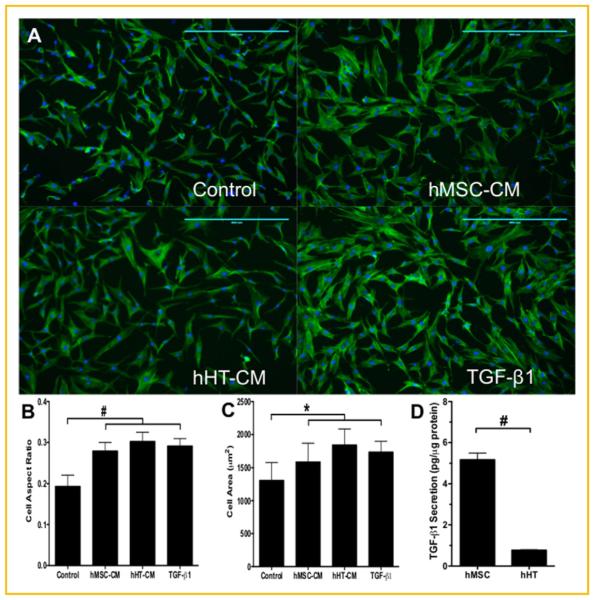
Assuming that the cross-talk between the two cell types was predominantly responsible for the differences in cell response, we conducted a preliminary screen of the culture medium for soluble TGF-β bioactivity. After three days of co-culture, the conditioned medium (CM) was collected and analyzed for cell secretome effect on TGF-β bioactivity using TMLC, a luciferase-producing reporter cell line. After 1 day of culture with the CM, the first donor set showed that TGF-β bioactivity was lower in hHT-CM (n ¼ 3) when compared to the other groups. The second donor set showed that hMSC-CM had higher TGF-β bioactivity than hHT-CM (n ¼ 3). The third donor set showed higher TGF-β bioactivity in the co-culture media for both cell types when compared to hMSC-conditioned medium (n ¼ 3). Lastly, the fourth donor set showed no significant difference in TGF-β bioactivity between treatment groups (Fig. 3). Next, we used the conditioned medium from the first donor set to culture hHT and analyzed the effects on cell morphology. Results showed that hHT cultured in both hHT and hMSC-CM appeared to be more elongated and have a larger and fiatter cytoskeleton similar to cells cultured in 10 ng/mL TGF-β1 according to the fluorescent staining (Fig. 4A). Image analysis results also confirmed this, showing that the treatment groups (n ¼ 540) had significantly higher cell aspect ratios and cell areas (Fig. 4B, C). Lastly, further secretome analysis of CM using ELISA showed that the hMSC secreted approximately 5 times as much TGF-β1 per total protein as hHT (Fig. 4D).
Fig. 3.
Relative TGF-β bioactivity of TMLC after culture with co-culture derived conditioned medium (CM) normalized to TMLC cultured in basal conditions (dashed line) (* P < 0.05, %P < 0.01, #P < 0.001).
Fig. 4.
Immunofluorescent imaging of hHT from donor set 1 fixed and stained for actin (green) and nuclei (blue). (A) Cells were cultured for 24 h under basal conditions, with hMSC-conditioned medium, hHT-conditioned medium, and 10 ng/mL TGF-β1 (scale bar ¼ 400 mm). (B) Cell aspect ratios and (C) cell area measurements obtained from image analysis (n ¼ 540). (D) Total TGF-β1 secreted by hMSC and hHT after 3 days of culture (* P < 0.05, #P < 0.001).
DISCUSSION
We investigated the effects of cross-talk between hHT and hMSC on the cell response of both cell types. While the majority of the data sets between donors appeared consistent, these studies revealed some unexpected inter-donor variability. Previous work investigating this response between T/L cells and MSC has reported a noticeable enhancement of cell response evidenced in increased cellular proliferation, ECM deposition, and up-regulation of several tenogenic markers [Shimode et al., 2007; Luo et al., 2009; Schneider et al., 2011]. Thus, we hypothesized that the indirect co-culture of the two cell types would lead to an enhancement of tenogenic function in both cell types. To the best of our knowledge, this is the first study on the paracrine interaction between these two cell types across four unique sets of adult human donors. Initially, we observed differences amongst the donor sets in proliferation, ECM production, and mRNA expression. With this in mind, we aimed to consider trends common amongst donor sets.
Our results showed that there was no notable response in metabolic activity amongst all donor sets except for the cells in the first donor set (Fig. 2A, and C). In this donor set, the hMSC appeared to exhibit decreases in metabolic activity during co-culture with hHT, while hHT experienced strong increases in metabolic activity during co-culture with hMSC. In some aspects, this observation both disagreed with and supported some of the responses seen in similar studies conducted with rat tissue. Conversely, Luo et al. [2009] showed that after 3 days of co-culturing with tenocytes, rat MSC experienced elevated metabolic activity. Similarly, Shimode et al. [2007] observed significant increases in cell number of Achilles tendon tenocytes after co-culture with bone marrow-derived MSC. To our knowledge, our present study with a human-derived cell model, is the first to report on the cell response of these co-cultured cell types. One explanation for the differences in response observed could be the species difference, or more specific, could be that there was a higher total number of cells/mL of culture medium in the co-culture groups compared to the control groups (as performed by Luo et al. [2009]). This difference in cell number could lead to a faster consumption of serum components, hindering cell proliferation.
We then analyzed the matrix produced by the cells during co-culture (Fig. 2B and D). According to our data, in two donor sets (n ¼ 6), there was an increase in the collagen/non-collagen ratio of the ECM deposited by each cell type. The increase in collagen is a notable result because it is the most abundant protein in T/L [Ekwueme et al., 2011; Voleti et al., 2012]. Increases in the amount of collagen deposited may accelerate the functional recovery upon cell transplantation. Although we saw no enhancement in cell metabolic activity, the cross-talk between hHT and hMSC may have instead induced more matrix production in both cell types. It is also likely that there are factors, such as TGF-β, being released that may be inhibiting cell proliferation while concurrently inducing higher ECM production [Leask and Abraham, 2004].
The mRNA expression analysis showed, for both cell types, an up-regulation of several anabolic and catabolic markers prevalent in T/L cells (Table III). For the anabolic markers assessed, Tenomodulin was up-regulated in hMSC in the presence of hHT for two donors that mRNA for Tenomodulin was detected for. Tenomodulin has been described as a late stage tenogenic marker during tendon development and is often used as a marker to indicate tenogenesis [Docheva et al., 2005, 2010; Shukunami et al., 2006]. This portion of the data supports the notion that factors released from hHT have the potential to induce tenogenesis in undifferentiated hMSC [Lee et al., 2007; Luo et al., 2009]. For hHT, there was an observed up-regulation of markers including collagen type I and TIMP-3 in one donor set and Tenomodulin in another set. Also, Tenascin-C for one donor after 7 days. All of these markers have been well documented as tenogenic markers involved with matrix development and assembly. Markers including collagen type I, Tenomodulin, and Tenascin C are known to be directly involved in the anabolic maintenance of T/L ECM. TIMP-3 belongs to a family of proteinases that have specificity for members of the MMP family and inhibit MMP activity [Lee et al., 2007; Liu et al., 2008].
Analysis of catabolic markers showed that hMSC co-cultured with hHT experienced significant up-regulation of ADAMTS-5, MMP-1, and MMP-13. Also, hHT co-cultured with hMSC experienced significant up-regulation of MMP-1, MMP-3, MMP-13, and ADAMTS-4. Previous in vitro and in vivo work on the ADAMTS and MMP family showed that tenocyte mechanotransduction [Kuo and Tuan, 2008; Maeda et al., 2013] and growth factors such as TGF-β, TNF-α, IL-1a, and IL-1b had significant effects on catabolic activity [Corps et al., 2008; Sun et al., 2008; Wylie et al., 2012]. In addition to the up-regulation of anabolic markers, the co-culture induced up-regulation of several catabolic markers thus signifying increased matrix turnover and remodeling. The balance or imbalance between MMPs and TIMPs strongly infiuences matrix remodeling [Attia et al., 2013]. Therefore, this data indicates that co-culture may activate changes in the mRNA expression patterns of markers involved with the anabolic maintenance of T/L ECM. It is possible that the matrix remodeling induced during co-culture could lead to a more mature collagen matrix and ultimately the enhancement of overall tissue biomechanics in vivo.
The mRNA expression data showed significant differences between control and treatment groups for several markers described above that are often referred to as TGF-β-target genes. Due to these findings, we performed a preliminary screen for TGF-β bioactivity in the secretome of the co-culture conditions using a TGF-β reporter cell line (Fig. 3). Results showed that in direct comparison, TGF-β bioactivity was higher in the hMSC groups in three out of four of the donor sets examined (n ¼ 9). Two donor sets showed TGF-β bioactivity to be higher in the co-culture groups of both cell types. These results suggest that hMSC may be able to secrete higher amounts of TGF-β or lower amounts of inhibitors of TGF-β activity. Next, we aimed to explore the effect of hMSC secretome directly on hHT cell morphology. Since the crosstalk in the first donor set appeared to have the most pronounced effect on the cells analyzed, we performed further studies with the CM and hHT from this donor set. After 24 h of culture with the CM, hHT appeared to have a more elongated morphology when compared to controls medium and very similar to groups cultured with soluble TGF-β1 (Fig. 4A–C), a known potentiator of tenogenic differentiation. Fibroblast elongation and alignment are hallmarks of tenogenic differentiation. Parameters such as morphology and actin cytoskeletal organization are all implicated during this process [Erisken et al., 2013].
Finally, using ELISA and a BCA protein assay we see that hMSC secreted up to five times as much total TGF-β as hHT (Fig. 4D). This is notable because hMSC have recently been shown to have secretomes that are rich in both immunomodulatory and trophic factors [Caplan and Dennis, 2006; Baraniak and McDevitt, 2010; Barminko et al., 2011; Faulknor et al., 2015]. The soluble factors released from the hMSC may be responsible for the favorable response in cell morphology evidenced by the hHT. These data suggest that cross-talk between the two cell types may synergistically potentiate an increase in TGF-β signaling for both cell types and that hMSC secretome may have favorable effects on tenocyte cellular morphology. On-going studies are further examining this trend and identifying other active components involved during cross-talk between the two cell types.
The “training” or pre-differentiation of MSC before trans-plantation has been discussed as a method of increasing their therapeutic efficacy and perhaps further control their in vivo response [Barminko et al., 2011; Doorn et al., 2013]. The results obtained in our studies suggest that the secretome of hMSC in their undifferentiated state can also be harnessed to potentiate ECM turnover in the target tissue. Also, given that a cell’s micro-environment can modulate its secretome, further investigation into the effect of pre-differentiated MSC secretome on the tissue healing response is warranted. On-going studies are exploring these effects in vitro. Furthermore, the in vivo response of the paracrine interaction between these two cell types will be investigated using a small animal model. The effect of the therapy will be evaluated using biomechanical tissue testing and imaging techniques such as immunohistochemistry and magnetic reso-nance imaging.
In conclusion, the results from these studies suggest that the paracrine interaction between adult human tenocytes and hMSC induces changes in the cellular response of both cell types. Clearly, there may be major differences between rat and human systems, considering human variation and age of injury/surgical repair. Here we show most notably that soluble factors were exchanged and were shown to stimulate higher collagen/non-collagen production by both cell types in two of four donor sets. Additionally, mRNA expression analysis showed variable changes in the expression patterns of prominent anabolic and catabolic markers of T/L ECM for all donor sets. Lastly, a preliminary screen of the individual and co-culture secretomes of both cell types showed that the hMSC secretome possessed higher TGF-β bioactivity. This work provides insight into the paracrine effect of the relationship between tenocytes and MSC on T/L ECM maintenance and explores TGF-β as a potential mediator of this response. We aim to use this cell model to begin to explore possible therapeutic interventions as a method to enhance tenogenic regeneration in vivo.
ACKNOWLEDGMENTS
The authors gratefully acknowledge financial support from NSF CBET 1243144 and NSF DGE 0801620, Integrative Graduate Education and Research Traineeship (IGERT) on the Integrated Science and Engineering of Stem Cells.
Grant sponsor: National Science Foundation; Grant numbers: CBET 1243144, DGE 0801620.
Footnotes
Conflicts of interest: No competing financial interests exist.
Portions of this work presented at the Netherlands Society for Biomaterials and Tissue Engineering (NBTE) 2013 Annual Meeting in Lunteren, The Netherlands.
REFERENCES
- Abe M, Harpel J, Metz C, Nunes I, Loskutoff D, Rifkin D. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Attia M, Huet E, Gossard C, Menashi S, Tassoni MC, Martelly I. Early events of overused supraspinatus tendons involve matrix metalloproteinases and emmprin/cd147 in the absence of infiammation. Am J Sports Med. 2013;41:908–917. doi: 10.1177/0363546512473817. [DOI] [PubMed] [Google Scholar]
- Awad H, Butler D, Boivin G, Smith F, Malaviya P, Huibregtse B, Caplan A. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267–277. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- Baraniak P, McDevitt T. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barminko J, Kim JH, Otsuka S, Gray A, Schloss R, Grumet M, Yarmush ML. Encapsulated mesenchymal stromal cells for in vivo transplantation. Biotechnol Bioeng. 2011;108:2747–2758. doi: 10.1002/bit.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, Awad H. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- Canseco J, Kojima K, Penvose A, Ross J, Obokata H, Gomoll A, Vacanti C. Effect on ligament marker expression by direct-contact co-culture of mesenchymal stem cells and anterior cruciate ligament cells. Tissue Eng: Part A. 2012;18:2549–2558. doi: 10.1089/ten.tea.2012.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Corps AN, Jones GC, Harrall RL, Curry VA, Hazleman BL, Riley GP. The regulation of aggrecanase adamts-4 expression in human achilles tendon and tendon-derived cells. Matrix Biol. 2008;27:393–401. doi: 10.1016/j.matbio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docheva D, Padula D, Popov C, Weishaupt P, Pragert M, Miosge N, Hickel R, Bocker W, Clausen-Schaumann H, Schieker M. Establishment of immortalized periodontal ligament progenitor cell line and its behavioural analysis on smooth and rough titanium surfaces. Eur Cells Mater. 2010;19:228–241. doi: 10.22203/ecm.v019a22. [DOI] [PubMed] [Google Scholar]
- Doorn J, Fernandes HA, Le BQ, van de Peppel J, van Leeuwen JP, De Vries MR, Aref Z, Quax PH, Myklebost O, Saris DB, van Blitterswijk CA, de Boer J. A small molecule approach to engineering vascularized tissue. Biomaterials. 2013;34:3053–3063. doi: 10.1016/j.biomaterials.2012.12.037. [DOI] [PubMed] [Google Scholar]
- Ekwueme E, Kwansa A, Sharif K, El-Amin S, Freeman J. Recent advancements in ligament replacement. Recent Pat Biomed Eng. 2011;4:196–204. [Google Scholar]
- Erisken C, Zhang X, Moffat KL, Levine WN, Lu HH. Scaffold fiber diameter regulates human tendon fibroblast growth and differentiation. Tissue Eng Part A. 2013;19:519–528. doi: 10.1089/ten.tea.2012.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulknor RA, Olekson MA, Nativ NI, Ghodbane M, Gray AJ, Berthiaume F. Mesenchymal stromal cells reverse hypoxia-mediated suppression of alpha-smooth muscle actin expression in human dermal fibroblasts. Biochem Biophys Res Commun. 2015;458:8–13. doi: 10.1016/j.bbrc.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Fernandes H, Mentink A, Bank R, Stroop R, van Blitterswijk C, de Boer J. Endogenous collagen influences differentiation of human multipotent mesenchymal stromal cells. Tissue Eng Part A. 2010;16:1693–1702. doi: 10.1089/ten.TEA.2009.0341. [DOI] [PubMed] [Google Scholar]
- Fung DT, Wang VM, Andarawis-Puri N, Basta-Pljakic J, Li Y, Laudier DM, Sun HB, Jepsen KJ, Schaffier MB, Flatow EL. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech. 2010;43:274–279. doi: 10.1016/j.jbiomech.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni Y, Turgeman G, Liebergal M, Pelled G, Gazit Z, Gazit D. Stem cells as vehicles for orthopedic gene therapy. Gene Ther. 2004;11:417–426. doi: 10.1038/sj.gt.3302197. [DOI] [PubMed] [Google Scholar]
- Ge Z, Goh J, Lee E. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005;14:573–583. doi: 10.3727/000000005783982819. [DOI] [PubMed] [Google Scholar]
- Gothard D, Dawson JI, Oreffo RO. Assessing the potential of colony morphology for dissecting the cfu-f population from human bone marrow stromal cells. Cell Tissue Res. 2013;352:237–247. doi: 10.1007/s00441-013-1564-3. [DOI] [PubMed] [Google Scholar]
- Hast M, Zuskov A, Soslowsky L. The role of animal models in tendon research. Bone Joint Res. 2014;3:193–202. doi: 10.1302/2046-3758.36.2000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R, Kesturu G, Balian G, Chhabra A. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Jones GC, Corps AN, Pennington CJ, Clark IM, Edwards DR, Bradley MM, Hazleman BL, Riley GP. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006;54:832–842. doi: 10.1002/art.21672. [DOI] [PubMed] [Google Scholar]
- Kim K, Dean D, Mikos AG, Fisher JP. The effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on crosslinked poly(propylene fumarate) disks. Biomacromo- lecules. 2009;10:1810–1817. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- Kwan KH, Yeung KW, Liu X, Wong KK, Shum HC, Lam YW, Cheng SH, Cheung KM, To MK. Silver nanoparticles alter proteoglycan expression in the promotion of tendon repair. Nanomedicine. 2013;10:1375–1383. doi: 10.1016/j.nano.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. TGF-βeta signaling and the fibrotic response. Faseb J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Lee IC, Wang JH, Lee YT, Young TH. The differentiation of mesenchymal stem cells by mechanical stress or/and co-culture system. Biochem Biophys Res Commun. 2007;352:147–152. doi: 10.1016/j.bbrc.2006.10.170. [DOI] [PubMed] [Google Scholar]
- Lee J, Zhou Z, Taub P, Ramcharan M, Li Y, Akinbiyi T, Maharam E, Leong D, Laudier D, Ruike T, Torina P, Zaidi M, Majeska R, Schaffier M, Flatow E, Sun H. Bmp-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS ONE. 2011;6:e17531. doi: 10.1371/journal.pone.0017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Klim JR, Derda R, Courtney AH, Kiessling LL. Spatial control of cell fate using synthetic surfaces to potentiate TGF-βeta signaling. Proc Natl Acad Sci USA. 2011;108:11745–11750. doi: 10.1073/pnas.1101454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fan H, Toh SL, Goh JC. A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials. 2008;29:1443–1453. doi: 10.1016/j.biomaterials.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Lui P, Rui Y, Ni M, Chan K. Tenogenic differentiation of stem cells for tendon repair-what is the current evidence? J Tissue Eng Regen Med. 2011:1–20. doi: 10.1002/term.424. [DOI] [PubMed] [Google Scholar]
- Luo Q, Song G, Song Y, Xu B, Qin J, Shi Y. Indirect co-culture with tenocytes promotes proliferation and mrna expression of tendon/ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology. 2009;61:1–10. doi: 10.1007/s10616-009-9233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S, Renstrom P. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27:61–71. doi: 10.2165/00007256-199927010-00005. [DOI] [PubMed] [Google Scholar]
- Maeda E, Sugimoto M, Ohashi T. Cytoskeletal tension modulates mmp-1 gene expression from tenocytes on micropillar substrates. J Biomech. 2013;46:991–997. doi: 10.1016/j.jbiomech.2012.11.056. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-βeta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Rothrauff BB, Tuan RS. Cellular therapy in bone-tendon interface regeneration. Organogenesis. 2014;10:13–28. doi: 10.4161/org.27404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M, Javelaud D, Mauviel A. TGF-βeta-induced smad signaling and gene regulation: Consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Schnabel LV, Mohammed HO, Miller BJ, McDermott WG, Jacobson MS, Santangelo KS, Fortier LA. Platelet rich plasma (prp) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230–240. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- Schneider PR, Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Three- dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J Orthop Res. 2011;29:1351–1360. doi: 10.1002/jor.21400. [DOI] [PubMed] [Google Scholar]
- Sharma P, Maffulli N. Tendon injury and tendinopathy: Healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- Shimode K, Iwasaki N, Majima T, Funakoshi T, Sawaguchi N, Onodera T, Minami A. Bone marrow stromal cells act as feeder cells for tendon fibroblasts through soluble factors. Tissue Eng. 2007;13:333–341. doi: 10.1089/ten.2006.0079. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Smith MM, Sakurai G, Smith SM, Young AA, Melrose J, Stewart CM, Appleyard RC, Peterson JL, Gillies RM, Dart AJ, Sonnabend DH, Little CB. Modulation of aggrecan and adamts expression in ovine tendinopathy induced by altered strain. Arthritis Rheum. 2008;58:1055–1066. doi: 10.1002/art.23388. [DOI] [PubMed] [Google Scholar]
- Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS ONE. 2013;8:e75697. doi: 10.1371/journal.pone.0075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HB, Li Y, Fung DT, Majeska RJ, Schaffier MB, Flatow EL. Coordinate regulation of il-1beta and mmp-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res. 2008;466:1555–1561. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomikawa K, Yamamoto T, Shiomi N, Shimoe M, Hongo S, Yamashiro K, Yamaguchi T, Maeda H, Takashiba S. Smad2 decelerates re- epithelialization during gingival wound healing. J Dental Res. 2012;91:764–770. doi: 10.1177/0022034512451449. [DOI] [PubMed] [Google Scholar]
- Van Eijk F, Saris D, Riesle J, Willems W, Van Blitterswijk C, Verbout A, Dhert W. Tissue engineering of ligaments: A comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue Eng. 2004;10:893–903. doi: 10.1089/1076327041348428. [DOI] [PubMed] [Google Scholar]
- Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: Repair and regeneration. Annu Rev Biomed Eng. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Altman G, Horan R, Kaplan D. Tissue engineering of ligaments. Annu Rev Biomed Eng. 2004;6:131–156. doi: 10.1146/annurev.bioeng.6.040803.140037. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Woo S, Papageorgiou C, Celechovsky C, Takai S. Fate of donor bone marrow cells in medial collateral ligament after simulated autologous transplantation. Microsc Res Tech. 2002;58:39–44. doi: 10.1002/jemt.10115. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-βeta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie JD, Ho JC, Singh S, McCulloch DR, Apte SS. Adamts5 (aggrecanase-2) is widely expressed in the mouse musculoskeletal system and is induced in specific regions of knee joint explants by inflammatory cytokines. J Orthop Res. 2012;30:226–233. doi: 10.1002/jor.21508. [DOI] [PubMed] [Google Scholar]
- Zilony N, Tzur-Balter A, Segal E, She? O. Bombarding cancer: Biolistic delivery of therapeutics using porous si carriers. Sci Rep. 2013;3:2499. doi: 10.1038/srep02499. [DOI] [PMC free article] [PubMed] [Google Scholar]