Summary
Mammalian colon harbors trillions of bacteria under physiologic conditions; this symbiosis is made possible because of a tolerized response from the mucosal immune system. The mechanisms underlying this tolerogenic phenomenon remain poorly understood. Here we show that Slc5a8, a Na+-coupled high-affinity transporter in colon for the bacterial fermentation product butyrate, plays a critical role in this process. Among various immune cells in colon, dendritic cells (DCs) are unique not only in their accessibility to luminal contents but also in their ability to induce tolerogenic phenotype in T cells. We found that DCs exposed to butyrate express the immunosuppressive enzymes indoleamine 2,3-dioxygenase 1 (IDO1) and aldehyde dehydrogenase 1A2 (Aldh1A2), promote conversion of naïve T cells into immunosuppressive FoxP3+ Tregs, and suppress conversion of naïve T cells into pro-inflammatory IFN-γ-producing cells. Slc5a8-null DCs do not induce IDO1 and Aldh1A2 and do not generate Tregs or suppress IFN-γ-producing T cells in response to butyrate. We also provide in vivo evidence for an obligatory role for Slc5a8 in suppression of IFN-γ-producing T cells. Furthermore, Slc5a8 protects against colitis and colon cancer under conditions of low-fiber intake but not when dietary fiber intake is optimal. This agrees with the high-affinity nature of the transporter to mediate butyrate entry into cells. We conclude that Slc5a8 is an obligatory link between dietary fiber and mucosal immune system via the bacterial metabolite butyrate, and that this transporter is a conditional tumor suppressor in colon linked to dietary fiber content.
Keywords: dietary fiber; bacterial metabolites; butyrate transporter; Slc5a8-null mouse; dendritic cell; histone deacetylase; indoleamine 2,3-dioxygenase 1; colitis; colon cancer
INTRODUCTION
Intestinal environment is a complex ecosystem that has been extensively studied; yet many fundamental aspects of this ecosystem remain poorly understood. The collective role of gut microbiome and its interaction with the intestinal immune system represent one such aspect. It is estimated that under healthy conditions, more than 100 trillion microorganisms colonize human intestinal lumen [1, 2]. With the advent of new high-throughput sequencing technologies, we are beginning to understand the dynamic nature and composition of gut microbiome. However, the physiologic impact of the gut microbiota on the host and the molecular aspects related to this phenomenon still remain largely unknown.
The presence of bacteria in a healthy gut lumen presents a unique challenge to the host immune system. In order to coexist with these bacteria, the immune system has adapted a ‘tolerogenic’ phenotype [1, 2]. Disruption of this delicate balance between bacteriome and immune cells results in inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis. Several immune cell types play crucial roles in maintaining this tolerogenic phenotype [3]. Dendritic cells (DCs) and regulatory T cells (Tregs) are two such critical immune cell types. DCs in the intestinal tract constantly sample the luminal contents for foreign antigens and, upon encountering such antigens, present them to naïve CD4+ and CD8+ T cells, thereby eliciting immune response. In the intestinal lamina propria, DCs possess capability to induce the conversion of naïve T cells into immunosuppressive FoxP3+ Tregs [4] and also to suppress the conversion of naïve T cells into IFN-γ-producing pro-inflammatory T cells [5]. Together, these processes lead to effective immune tolerance of luminal bacteria and enable coexistence of the gut bacteria with the host. Impairment of these processes underlies the pathogenesis, progression and prognosis of IBD. DCs and other antigen-presenting immune cell types express indoleamine 2,3-dioxygenase 1 (IDO1), a potent immunosuppressive enzyme [6]. IDO1 is a tryptophan-catabolizing enzyme, and IDO1-mediated depletion of tryptophan in the microenvironment induces arrest of proliferation of effector T cells, forcing them to anergy and apoptosis [6]. The expression of IDO1 in DCs is critical for their ability to induce the development of Tregs and suppress the development of IFN-γ+ T cells from naïve T cells. As such, IDO1 in DCs in the intestinal tract is critical for the maintenance of the tolerogenic phenotype of the mucosal immune system [7]. However, specific molecular processes that are responsible for the expression of high levels of IDO1 in intestinal DCs necessary for the maintenance of host-bacteria symbiosis remain poorly understood. Retinoic acid (RA), a vitamin A metabolite, is a key signaling molecule that induces immune suppression. Aldehyde dehydrogenases (ALDH1A) that generate RA are potent mediators of immune suppression [8]. Although the downstream signaling events of RA have been studied in great detail, molecular events involved upstream in the expression of ALDH1A enzymes remain largely unknown. In particular, very little is known on the role of gut bacteria themselves in inducing IDO1 and ALDH1A in DCs and hence in the maintenance of the tolerogenic phenotype of the mucosal immune system.
Dietary fiber plays an obligatory role in the survival and proliferation of normal gut bacteria by serving as the nutrient source. It consists of non-starch polysaccharides. Humans cannot digest dietary fiber due to lack of specific enzymes. Normal colonic bacteria ferment dietary fiber and generate short-chain fatty acids (SCFA) [9, 10]. Acetate, propionate and butyrate are the most abundant SCFA. Their collective concentration in colonic lumen in humans ranges 80–120 mM. Even though it is well recognized that SCFAs elicit a plethora of beneficial effects on colon, underlying molecular mechanisms remain poorly characterized. Among SCFAs, butyrate has been extensively studied, and the best understood mechanism of action of butyrate is its ability to inhibit histone deacetylases (HDACs) [11]. Butyrate as well as propionate, but not acetate, inhibit HDAC1 and HDAC3 [11–13]. Since HDACs are intracellular enzymes, the delivery of butyrate and propionate into cells to have access to HDACs is critical for this biological effect. A Na+-coupled high-affinity transporter for butyrate and other SCFAs, known as SLC5A8, is expressed in the colon [14, 15]. It is located in the lumen-facing apical membrane of colonic epithelial cells, thus having access to SCFAs in the lumen [15]. We have shown that this transporter is essential for the ability to butyrate to inhibit HDACs in colonic epithelial cells in vitro [13]. In addition, we have demonstrated that Slc5a8-dependent entry of butyrate and consequent inhibition of HDACs lead to specific blockade in development of DCs from pluripotent bone marrow precursor cells [16]. These studies have implicated Slc5a8 for the first time in mucosal immune cell function. In the present study, we show that the role of Slc5a8 goes even further with regard to immune cells.
Consumption of diets deficient in fiber increases the risk for intestinal inflammatory diseases and inflammation-associated colorectal cancer [17, 18]. Furthermore, inflammatory conditions of colon are associated with decreased abundance of SCFAs [17, 18]. Considering the fact that optimal fiber intake in the diet produces high concentrations of SCFAs in the lumen (~100 mM) and that SLC5A8 is a high-affinity transporter for these bacterial metabolites with Km values in the range of 50–100 μM, we hypothesized that the transporter may not be obligatory for the biological effects of SCFAs in vivo with optimal dietary fiber intake but would become essential only when dietary fiber intake is suboptimal, a condition associated with drastically reduced concentrations of SCFAs in the colonic lumen. In the present study we provide supporting evidence for this hypothesis.
MATERIALS AND METHODS
Animals
Wild type C57BL/6 mice and OT-II transgenic mice were purchased from the Jackson Laboratories (Bar Harbor, ME, USA). Generation of Slc5a8−/− mice has been described [19], and these mice have been used in our laboratory for several studies [16, 20–22]. Mice were maintained in the conventional animal housing with 12 h day-night cycles, with water and food provided ad libitum, and used between 8 – 12 weeks of age. Germ-free and age-matched conventional mice were obtained from Taconic Biosciences, Inc. (Hudson, NY, USA) and used as described previously [23]. Institutional Animal Care and Use Committee (IACUC) of the Georgia Regents University approved all animal procedures reported in this study.
Isolation of DCs and their culture
Mature DCs were isolated from mouse spleen using CD11c microbeads (Miltenyi Biotech, Auburn, CA, USA) followed by magnetic separation. Purity of isolated DCs was determined by flow cytometric analysis using CD11c antibody. DC purity for typical DC isolation was ~90%. DCs were then cultured in complete culture medium (RPMI 1640 medium, containing 10% fetal calf serum, 10 mM HEPES pH 7.4, 2 mM glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-mercaptoethanol) for 48 h.
Isolation and culture of CD4+ T cells
CD4+ CD25− CD44+ CD62LHi T cells were isolated from spleen and mesenteric lymph nodes of OT-II transgenic mice by FACS using fluorochrome-conjugated antibodies (both from eBioscience, San Diego, CA, USA). For in vitro FoxP3+ CD4+ Treg conversion assay, DCs pretreated with or without butyrate were recovered from culture at the end of 48 h and co-cultured for 4 days with these OT-II T cells at a ratio 1:2 in complete medium. The culture medium was supplemented with 0.5 µg/ml Ovalbumin peptide (ISQVHAAHAEINEA), 0.4 ng/ml TGFβ and 5 ng/ml IL-2. The cells were then fixed with FoxP3/Transcription Factor Fixation/Permeabilization kit (eBioscience, San Diego, CA, USA) and stained with antibodies against CD4 and FoxP3 for analysis on LSR II flow cytometer. For in vitro IFN-γ+ CD4+ T cell suppression assay, DCs pretreated with or without butyrate were recovered from culture at the end of 48 h and co-cultured for 4 days with the CD4+ CD25−CD44− CD62LHi T cells from OT-II transgenic mice at DC:T cell ratio 1:2 in the complete medium. The culture medium was supplemented with 0.5 μg/ml Ovalbumin peptide (ISQVHAAHAEINEA), 10 ng/ml IL-12, 10 μg/ml anti-IL4, and 5 ng/ml IL-2. On day 4 of co-culture, cells were recovered and cultured further in presence of 5 ng/ml IL-2 for 48 h, after which the cells were stimulated with phorbol myristate acetate plus ionomycin in the presence of GolgiStop and Golgiplug for 5 h. Finally, cells were fixed with FoxP3/Transcription Factor Fixation/Permeabilization kit (eBioscience, San Diego, CA, USA), stained with antibodies against CD4, CD25, and IFN-γ and analyzed on LSR II flow cytometer.
In vivo IFN-γ+ CD4+ T cell suppression assay
OVA-specific CD4+ CD25−CD44− CD62LHiCD90.1+ (Thy1.1) T cells from OT-II donor mice were injected (i.v.) into recipient WT or Slc5a8−/− (Thy1.2) mice 1 day before immunization. Mice were then immunized with a mixture of ovalbumin dissolved in PBS and complete Freud’s adjuvant at 1:1 ratio (s.c.). Two weeks later, animals were sacrificed to obtain cells from spleen. These cells were then stimulated with phorbol myristate acetate plus ionomycin in the presence of GolgiStop and Golgiplug for 5 h. Finally, cells were fixed with FoxP3/Transcription Factor Fixation/Permeabilization kit (eBioscience, San Diego, CA, USA), stained with antibodies against CD4, CD25, and IFN-γ and analyzed on LSR II flow cytometer.
RNA isolation and real-time PCR
Total RNA was isolated from cells using RNeasy Plus Micro kit (Qiagen). RNA was quantified, and reverse-transcribed using Superscript III Reverse transcriptase kit (Invitrogen). Real-time PCR was performed using mouse IDO1 primers (forward: 5′-TGG CAA ACT GGA AGA AAA AG-3′; reverse: 5′-AAT GCT TTC AGG TCT TGA CG-3′) and mouse Aldh1A2 primers (forward: 5′-TGG GTG AGT TTG GCT TAC GG-3′; reverse: 5′-AGA AAC GTG GCA GTC TTG GC-3′). All PCR data were normalized to the data for mouse HPRT1 (primers: 5′-GCG TCG TGA TTA GCG ATG ATG AAC-3′ and 5′-CCT CCC ATC TCC TTC ATG ACA TCT-3′). Relative gene expression in treated cells was assessed using the ΔΔCt method with expression levels in respective control (untreated) cells taken as 1.
Analysis of STAT3 acetylation and its role in butyrate-induced IDO1 expression
DC2.4 cells were cultured in presence or absence of 0.5 mM butyrate in complete medium for 16 h. Cells were then lysed in RIPA buffer supplemented with protease and phosphatase inhibitor cocktail (Cell Signaling, Danvers, MA, USA) and 10 mM nicotinamide. Total STAT3 was immunoprecipitated from cell lysates using an antibody specific for STAT3. The immune-precipitates were then used for western blotting and probed with an antibody specific for acetyllysine and also with the antibody specific for STAT3. To determine the role of STAT3 in butyrate-induced IDO1 expression, DCs isolated from wild type mouse spleen were cultured in the absence or presence of 0.5 mM butyrate with and without JSI-124 (1 μM), an inhibitor of STAT3 signaling. After 48 h treatment, RNA was isolated from the cells and used for analysis of IDO1 expression by real-time PCR. Data were normalized to the expression of HPRT1 as an internal control.
Analysis of HDAC activity
HDAC enzymatic activity in whole colonic lysates was measured using a commercially available kit (BioVision, Inc. Milpitas, CA, USA).
Histology
Mouse tissues were washed with PBS and fixed in 10% formaldehyde in PBS (v/v) for 24 h before incubation with 70% ethanol in water (v/v). Tissues were then embedded into paraffin and sectioned into 8-μm thick slices on clear glass slides and stained with H&E at GRU histology core facility. Slides were washed and counterstained with DAPI, and then examined under a microscope.
Animal diets
Mice were fed a diet containing dietary fibers (Fiber-containing diet; FC diet) or a diet without fibers (Fiber-free diet; FF diet). Both diets contained Lactalbumin 20.5%, DL-Methionine 0.22%, Dextrose monohydrate 52.839%, Maltodextrin 15%, Soyabeal Oil 5%, Mineral Mix AIN-93G 3.5%, Vitamin Mix AIN-93 1.5%, Potassium phosphate monobasic 0.84%, Calcium carbonate 0.3%, Choline bitartarate 0.3% and TBHQ antioxidant 0.001%. The only difference between the two diets was the presence (FC diet) or absence (FF diet) of 5% cellulose as the fiber source. These diets were custom-manufactured by Harlan Laboratories (Indianapolis, IN, USA). The diets were autoclaved and vacuum-packed by the manufacturer and kept at 4 °C until used to feed the animals. The diets were provided to the animals ad libitum. We observed no significant difference between consumption of FC or FF diets by the animals.
Lamina propria immune cell isolation
To isolate immune cells from colonic lamina propria, we followed a method described earlier in one of our previous studies [24]. Briefly, intestinal tissues harvested from mice were cleaned by opening longitudinally and then by gentle shaking in HBSS. Tissues were then cut into approximately 0.5-cm long pieces and immersed in HBSS containing 5% fetal bovine serum (v/v) and 5 mM EDTA. Tissues were incubated at 37 °C with continuous agitation for 40 min. The epithelial cells thus separated were removed by filtering and the tissues pieces were further incubated with 1 mg/ml Collagenase D and 1 mg/ml DNase I (Roche, Nutley, NJ, USA) in RPMI1640 supplemented with 5% fetal bovine serum (v/v), with continuous agitation for 20 min. Resultant supernatant was filtered through 70-μm nylon mesh, and washed with complete medium to dilute collagenase and DNase. After preparation of single cell suspensions from lamina propria, cells were cultured in presence of phorbol myristate acetate plus ionomycin in the presence of GolgiStop and Golgiplug for 5 h. Cells were fixed with FoxP3/Transcription Factor Fixation/Permeabilization kit (eBioscience, San Diego, CA, USA), stained with antibodies against CD45, CD90.2, CD4, CD25, and IFN-γ and analyzed on LSR II flow cytometer.
Development of acute and chronic intestinal inflammation and colon cancer in mice
This was done as described previously from our laboratory [24]. For development of acute inflammation, wild type and Slc5a8−/− mice were kept on FC diet or FF diet beginning 4 weeks prior to the onset of experiment until the end. Acute intestinal inflammation was induced by feeding animals 2% (w/v) dextran sulfate sodium, mw 36kDa – 50kDa, (DSS, MP Biomedicals, Santa Ana, CA, USA) in drinking water ad libitum. Acute intestinal colitis developed 4 to 6 days following the initiation of DSS administration. Animals were monitored for morbidity and disease progression. For histological analyses, animals were sacrificed on day 5 to obtain tissues. Chronic intestinal inflammation and inflammation-associated colon cancer were induced by AOM-DSS model. Briefly, wild type and Slc5a8−/− mice were kept on FC diet or FF diet beginning 4 weeks prior to the onset of experiment until the end. On day 0, animals were injected intraperitonially with 10 mg/kg Azoxymethane (AOM; Sigma Aldrich, St. Louis, MO, USA), followed by cyclic administration of 1% (w/v) DSS. Two cycles of 5-day treatment with DSS with a 24-day interval were used. The experiment was terminated on day 70 following the day of AOM injection. The progression of chronic intestinal colitis was monitored and scored daily as follows: weight loss was measured by weighing animals and recording percent weight relative to weight of animals on day 0; rectal bleeding was scored as 0 - no bleeding, 2 – occult bleeding marked by reddish pink spots in stool, 4 – obvious fresh blood observed around anus; diarrhea was scored as 0 - no diarrhea, 1 – soft, but well formed stool pellet, 2 – soft stool that sticks to surface of cage or bedding, 3 – diarrhea with stool sticking to the fur or tail, 4 – continuous streak of watery diarrhea mixed with blood. To monitor inflammation-associated intestinal polyps, animals were sacrificed on day 70 and colons isolated for assessment of the numbers of polyps by visual inspection under a microscope.
Measurement of intestinal permeability
Mice were given fluorescein isothiocyanate (FITC)-dextran by oral gavage at a dose of 0.5 mg/g of body weight. Four hours later, mice were bled and FITC-dextran was quantified in the serum using a fluorescence spectrophotometer.
Statistical analysis
Experiments were repeated 3–5 times, and the data are presented as means ± S. E. Statistical significance was determined by Student’s t test. A p <0.05 was considered significant.
RESULTS
IDO1 expression in colon correlates with bacterial colonization
To determine if colonic bacteria impact on the expression of the immunosuppressive enzyme IDO1 in colon, we monitored its expression in colon in conventional mice with normal colonic bacteria and in germ-free (GF) mice with no bacteria anywhere in the body including the intestinal tract. We found that, compared to GF animals, colons of conventionally raised animals had higher expression of IDO1 (Fig. 1A). Since retinoic acid (RA) signaling is an important determinant of IDO1 expression in colon, we assessed the colonic expression of Aldh1A2, an enzyme that generates RA, in conventional and GF mice. We found the expression of this enzyme higher in conventional mouse colon than in GF mouse colon (Fig. 1B), suggesting that RA signaling might underlie the increased expression of IDO1 in colon in association with the presence of bacteria. To confirm that RA is indeed an inducer of IDO1, we cultured neonatal colon tissues in vitro in the presence of RA for 16 h and then monitored the expression of IDO1. Treatment with RA led to a marked induction of IDO1 in colon (Fig. 1C).
Figure 1. Induction of IDO1 and Aldh1A2 by colonic bacteria and the relevance of retinoic acid to the process.
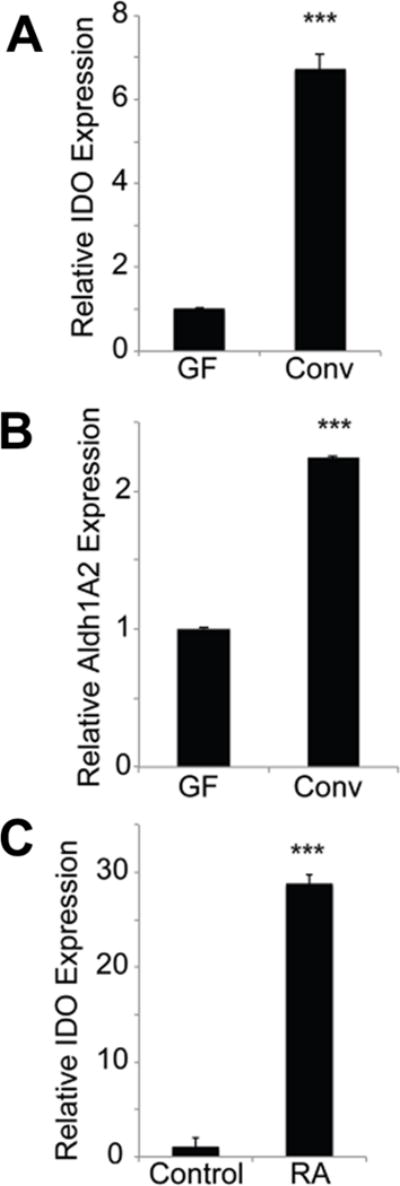
(A, B) Total RNA isolated from colon tissues from conventional or germ-free mice was used for real-time PCR to monitor the expression of IDO1 and Aldh1A2. (C) Colon tissues from 4-day-old mouse pups were cut into pieces and cultured in vitro in the presence or absence of all-trans retinoic acid (RA; 1 μM) for 16 h. RNA was then isolated from these tissues and used for real-time PCR. The experiment was repeated independently with five different mice and the data (means ± S. E.) are from these biological replicates. ***, p < 0.001 compared to the respective controls.
Impact of SCFAs on IDO1 and Aldh1A2 expression in mature DCs
SCFAs (acetate, propionate, and butyrate), which are generated by bacterial fermentation of dietary fiber, have been implicated in the host-bacteria communication. Therefore, we asked whether SCFAs have any effect on the expression of IDO1 and Aldh1A2 in dendritic cells (DCs) as a potential molecular mechanism for the induction of these two enzymes in association with colonic bacteria. We found that mature DCs isolated from murine spleen expressed higher levels of IDO1 and Aldh1A2 when treated with butyrate (Fig. 2A, C). The maximal induction was seen with 0.5 mM butyrate. Propionate also induced the expression of both enzymes to a significant extent but acetate did not (Fig. 2B, D). Butyrate and propionate are HDAC inhibitors; in contrast, acetate has no effect on HDACs. As such, these findings implicate inhibition of histone deacetylases as a potential mechanism by which the bacterial metabolites butyrate and propionate induce IDO1 and Aldh1A2 in colon.
Figure 2. Induction of IDO1 and Aldh1A2 in CD11c+ DCs by colonic bacterial fermentation products.
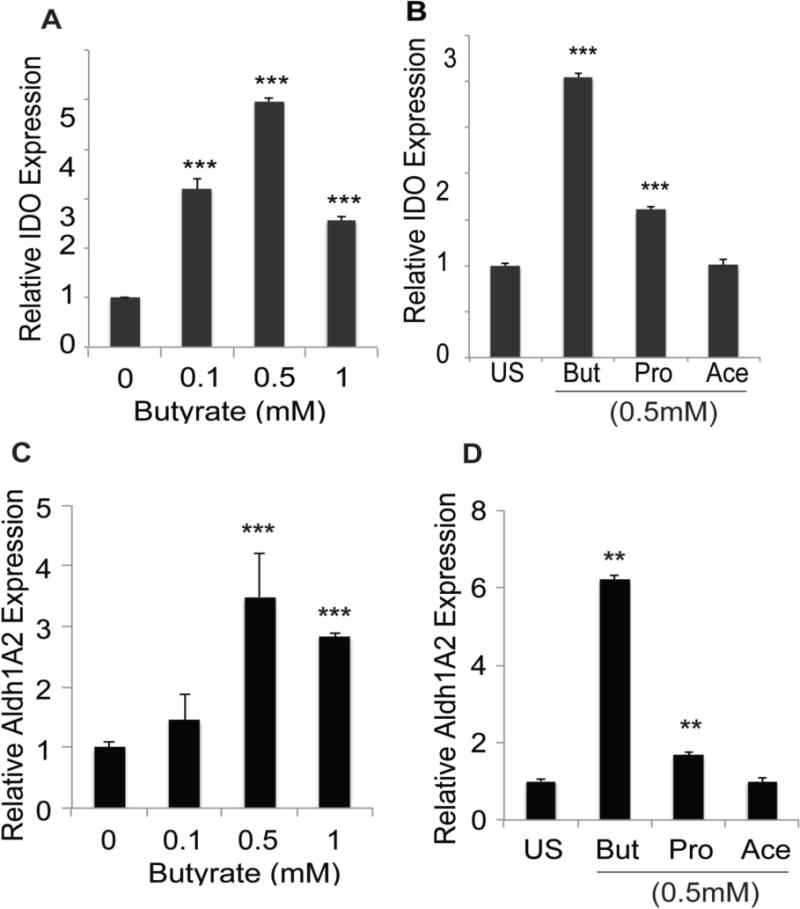
(A, C) CD11c+ DCs obtained from the spleens of conventional mice were cultured in the presence of absence of butyrate at indicated concentrations for 48 h. RNA was then isolated from the cells and used for real-time PCR. (B, D) CD11c+ DCs isolated from the spleens of conventional mice were cultured in the presence or absence of 0.5 mM butyrate (But), propionate (Pro) or acetate (Ace) for 48 h. RNA was then isolated from the cells and used for real-time PCR. In each case, the experiment was repeated three times and the data (means ± S. E.) are from these biological replicates. **, p < 0.01; ***, p < 0.001 compared to untreated controls.
Relevance of the SCFA transporter Slc5a8 in butyrate-induced expression of IDO1 and Aldh1A2 in DCs
For butyrate and propionate to cause inhibition of HDACs in DCs, they have to first enter the cells to have access to the intracellular HDACs. In vivo, DCs do not encounter SCFAs at high levels even though the luminal concentrations of these bacterial metabolites are present at high millimolar concentrations. Colonic epithelial cells metabolize SCFAs robustly, and as a result SCFAs enter the lamina propria only at submillimolar concentrations. Therefore, we hypothesized that the entry of butyrate into DCs might require the high-affinity transporter Slc5a8. To test this hypothesis, we compared the ability of extracellular butyrate and propionate to induce IDO1 and Aldh1A2 in DCs prepared from the spleens of wild type mice and Slc5a8−/− mice. Both of these HDAC inhibitors were able to induce IDO1 and Aldh1A2 in wild type DCs but had no effect in Slc5a8−/− DCs (Fig. 3A, B). Acetate, a SCFA with no effect on HDACs, did not induce IDO1 or Aldh1A2 in DCs irrespective of whether the cells were prepared from wild type mice or Slc5a8−/− mice (Fig. 3A, B). These data strongly suggest a role of HDAC inhibition in butyrate/propionate-induced expression of IDO1 and Aldh1A2 in DCs.
Figure 3. IDO1 and Aldh1A2 expression in DCs in response to butyrate and propionate is dependent on Slc5a8.
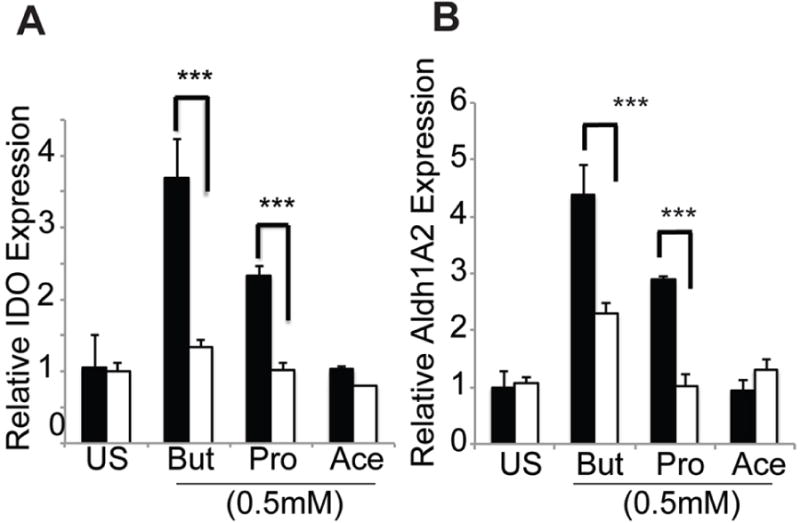
CD11c+ DCs from the spleens of wild type mice (closed bars) or Slc5a8−/− mice (open bars) were cultured in the presence or absence of 0.5 mM butyrate (But), propionate (Pro) or acetate (Ace) for 48 h. RNA was then isolated from the cells and used for real-time PCR to monitor the expression of IDO1 (A) and Aldh1A2 (B). ***, p < 0.001 compared to Scl5a8−/− DCs.
Involvement of acetyl-STAT3 in butyrate-induced expression of IDO1
STAT3 (signal transducer and activator of transcription 3) plays a key role in generation of tolerogenic DCs, and acetylated STAT3 (acetylation site, Lys685) moves to the nucleus and acts as a transcription factor on IDO1 promoter to induce expression [25, 26]. This transcriptional activity can be abrogated with JSI124, an inhibitor of the biological activity of acetyl-STAT3. Since butyrate is an HDAC inhibitor, we tested whether butyrate-induced HDAC inhibition underlies IDO1 expression in DCs. We found that butyrate treatment increased the acetylation status of STAT3 in DC2.4 cells, suggesting butyrate-mediated HDAC inhibition (Fig. 4A). Furthermore, pharmacological inhibition of STAT3 signaling using JSI-124 completely abolished butyrate-mediated IDO1 induction in splenic DCs (Fig. 4B). These studies indicate a direct role for HDAC inhibition and acetyl-STAT3 in butyrate-mediated IDO1 induction in DCs.
Figure 4. Butyrate-mediated induction of IDO1 expression involves acetyl-STAT3.
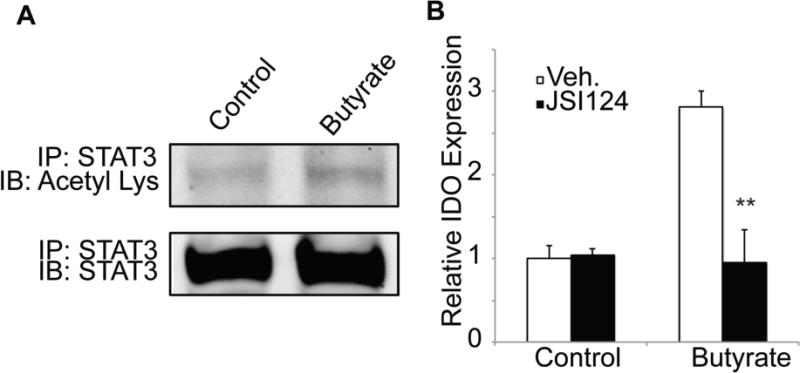
(A) Mouse DC cell line DC2.4 was cultured in the presence or absence of 0.5 mM butyrate for 16 h. Total STAT3 was immunoprecipitated from the cell lysates using an antibody specific for STAT3 and then used for immunoblotting with an antibody specific for acetyl-lysine. The same blot was then probed with the STAT3-specific antibody to determine the levels of total STAT3. (B) DCs isolated from WT mouse spleen were cultured in the presence or absence of 0.5 mM butyrate with and without the STAT3 signaling inhibitor JSI-124 (1 μM). After 48 h treatment, total RNA was isolated from the cells and used to analyze IDO expression by real-time PCR. Data were normalized to expression of HPRT1 as an internal control. **, p < 0.01 compared to butyrate treatment in the absence of JSI-124.
Role of butyrate and Slc5a8 in the induction of tolerogenic phenotype in DCs
DCs act as master regulators of tolerogenic immune response by their ability to promote the conversion of naïve T cells into immunosuppressive Tregs and also to suppress the conversion of naïve T cells into pro-inflammatory IFN-γ+ T cells. We asked whether the butyrate-induced expression of IDO1 and Aldh1A2 had any relationship to the ability of DCs to control the generation of Tregs and IFN-γ+ T cells. To address this question, we assessed the ability of butyrate to induce tolerogenic phenotype in DCs by monitoring their ability to generate Foxp3+ Treg cells in vitro. To assess the specific role of IDO1 in this process, we used 1-MT, an inhibitor of IDO1. We observed that DCs cultured in the presence of butyrate induced Tregs much more robustly than the DCs cultured in the absence of butyrate (Fig. 5A); the IDO1 inhibitor 1-MT almost completely abolished this effect. To access the role of Aldh1A2 (i.e., RA signaling) in the phenomenon, we used an inhibitor of RA signaling (LE135). Again, butyrate-treated DCs were able to generate Tregs but lost this ability in the presence of LE135 (Fig. 5B). The role of Slc5a8 in the process was examined by comparing the effect of butyrate on wild type DCs and Slc5a8−/− DCs with regard to their ability to convert naïve T cells into Tregs. Butyrate treatment potentiated the ability of wild type DCs to generate Tregs, but the effect was significantly reduced with Slc5a8−/− DCs (Fig. 5C). The ability to convert naïve T cells into Tregs in the absence of butyrate was not different between wild type and Slc5a8−/− DCs in this in vitro assay.
Figure 5. Role of IDO1 and retinoic acid signaling in the tolerogenic phenotype of CD11c+ DCs and the relevance of Slc5a8 to the process in vitro and in vivo.
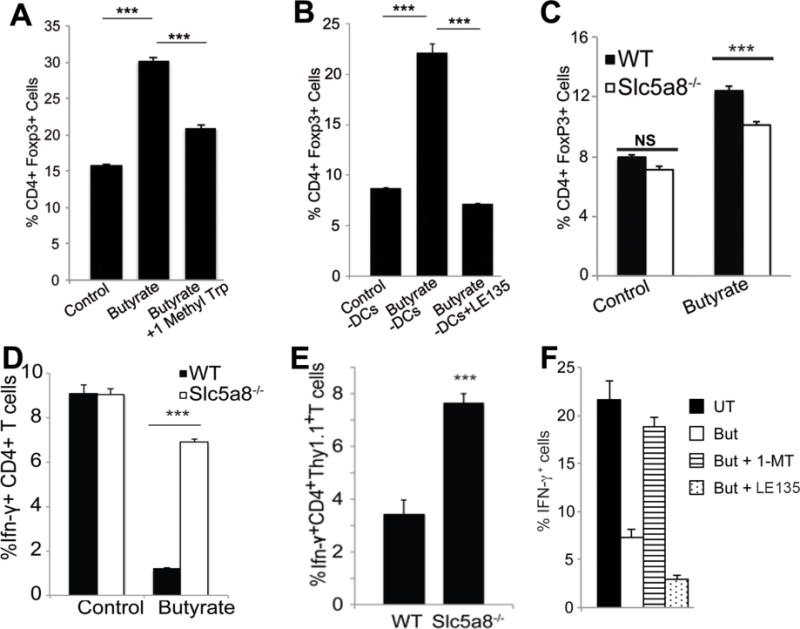
(A) CD11c+ DCs were isolated from wild type mice and treated with or without butyrate (0.5 mM) in the presence or absence of the IDO1 inhibitor 1-MT (1 mM) for 48 h. DCs were then recovered and co-cultured for 5 days with CD4+ CD25− T cells (naïve T cells) from OT-II transgenic mice. T cells were then stained with anti-CD4 and anti-FoxP3 antibodies and analyzed on a flow cytometer. Data (means ± S. E.) are from three independent experiments. (B) The experimental procedure was the same as above except that the DCs were treated with or without butyrate (0.5 mM) in the presence or absence of LE135 (1 μM), an inhibitor of RA signaling. Data (means ± S. E.) are from three independent experiments. (C) CD11c+ DCs from wild type mice and Slc5a8−/− mice were treated with or without butyrate (0.5 mM) for 48 h. The cells were then used for conversion of naïve T cells into FoxP3+ Tregs as described above. Data (means ± S. E.) are from three independent experiments with three different mice for each genotype. NS, not significant; ***, p < 0.001. (D) CD11c+ DCs from wild type mice and Slc5a8−/− mice were cultured in the presence or absence of butyrate (0.5 mM) for 48 h. The cells were then recovered and cultured in the presence of CD4+ CD25− CD44− CD62LHi T cells from OT-II transgenic mice. T cells were stimulated with phorbol myristate acetate and ionomycin in the presence of Golgiplug and GolgiStop for 5 h and stained with anti-CD4 and anti-IFN-γ antibodies and analyzed on a flow cytometer. Data (means ± S. E.) are from biological triplicates. ***, p < 0.001 compared to wild type control DCs. (E) CD90.1+ CD4+ CD25− CD44− CD62LHi T cells from OT-II transgenic mice (Thy1.1) were injected (2 × 106 cells/mouse) intravenously into either wild type mice or Slc5a8−/− mice (Thy1.2). After 2 days, mice received subcutaneous immunization with ovalbumin. After 2 weeks, splenic T cells were isolated and stimulated with phorbol myristate acetate and ionomycin in the presence of GolgiPlug and GolgiStop for 5 h and then stained with anti-CD4, anti-IFN-γ and anti-CD90.1 antibodies. The cells were then analyzed on a flow cytometer. Data (means ± S. E.) are from three independent experiments. ***, p < 0.001 compared to wild type mice. (F) CD11c+ DCs from wild type mice were cultured with or without butyrate (But, 0.5 mM) in the absence or presence of the IDO1 inhibitor 1-MT (1 mM) or the retinoic acid signaling inhibitor LE135 (1 μM) for 48 h. The cells were then recovered and cultured in the presence of CD4+ CD25− CD44− CD62LHi T cells from OT-II transgenic mice. T cells were stimulated with phorbol myristate acetate and ionomycin in the presence of Golgiplug and GolgiStop for 5 h and stained with anti-CD4 and anti-IFN-γ antibodies and analyzed on a flow cytometer. Data (means ± S. E.) are from biological triplicates.
We then interrogated the role of butyrate and Slc5a8 in the ability of DCs to generate IFN-γ+ T cells from naïve T cells. Treatment of wild type DCs with butyrate markedly suppressed their ability to convert naïve T cells into IFN-γ+ T cells, but this action of butyrate was almost completely abrogated in Slc5a8−/− DCs (Fig. 5D). To further corroborate our findings, we performed an in vivo T cell conversion assay, in which we introduced CD90.1+ CD4+ CD25− CD44− CD62LHi T cells from OT-II transgenic mice (Thy1.1) into either wild type mice or Slc5a8−/− mice (Thy1.2) intravenously. Mice were then immunized with ovalbumin. Two weeks later, antigen-specific conversion of naïve T cells into IFN-γ+ CD4+ OT-II T cells (Thy1.1) was quantified in spleen by flow cytometry. Consistent with our in vitro findings, Slc5a8−/− mice had significantly higher levels of IFN-γ+ T cells than wild type mice (Fig. 5E). We then investigated the involvement of IDO1 and Aldh1A2 in butyrate-mediated suppression of the conversion of naïve T cells into IFN-γ+ cells by DCs by using 1-MT as an inhibitor of IDO1 and LE135 as an inhibitor of retinoic acid signaling. Treatment of splenic DCs from wild type mice with 1-MT almost completely reversed the ability of butyrate to suppress the development of IFN-γ+ T cells but LE135 failed to do so (Fig. 5F). These data indicate the involvement of IDO1 but not retinoic acid signaling in butyrate-mediated suppression of the pro-inflammatory IFN-γ+ T cells.
Impact on Slc5a8 on T cell repertoire in vivo
Intestinal lamina propria immune cells are important orchestrators of intestinal immune response. Similar to immune cells elsewhere, lamina propria immune cells are also in a dynamic equilibrium with the systemic immune cell repertoire. To understand the impact of Slc5a8 on immune cells repertoire in vivo, we analyzed the steady-state levels of Tregs and IFN-γ+ T cells in colonic lamina propria in wild type mice and Slc5a8−/− mice. We observed no significant difference in CD4+ FoxP3+ T cells (Tregs) between wild type mice and Slc5a8−/− mice in colon (data not shown). Since in vitro experiments clearly showed that Slc5a8 was required for the ability of DCs to generate Tregs, we expected the levels of Tregs to be reduced in colon in Slc5a8−/− mice. There was however a significant difference in the steady-state levels of IFN-γ+ T cells in colon between wild type mice and Slc5a8−/− mice. The knockout mice had higher levels of these pro-inflammatory T cells (9.2 ± 1.9% of CD4+ T cells) than the wild type mice (3.9 ± 1.1% of CD4+ T cells) as expected based on our in vitro findings in which butyrate and Slc5a8 were essential for the ability of DCs to suppress IFN-γ+ T cells. The difference was significant (p < 0.01). The observed differences between in vitro and in vivo data in terms of steady-state levels of Tregs and IFN-γ+ T cells between wild type mice and Slc5a8−/− mice might suggest that the impact of butyrate/Slc5a8 on DCs is more robust in the suppression of the conversion of naïve T cells into IFN-γ+ T cells than in the promotion of the conversion of naïve T cells into Tregs.
Relevance of dietary fiber content to the in vivo role of Slc5a8 in the mucosal immune system
With intake of optimal dietary fiber, SCFAs are present in colonic lumen at high millimolar concentrations. Under these conditions, these monocarboxylates can be absorbed in the intestinal tract by diffusion and/or low-affinity/high-capacity monocarboxylate transporters such as SLC16A1 (also known as MCT1). SLC5A8 is a high-affinity/low-capacity transporter for SCFAs; its affinity for butyrate and propionate is in the high micromolar range [14], thus contributing only minimally to the overall entry process. But this is true only for the colonic epithelial cells that are exposed to luminal SCFAs. The presence or absence of this transporter is not likely to impact on the entry of SCFAs into colonic epithelial cells when dietary fiber intake is optimal. In contrast, when dietary fiber intake is low, the luminal concentrations of SCFAs decrease proportionately. Under these conditions, the kinetic features of Slc5a8 make it essential for the entry of SCFAs into colonic epithelial cells. The situation is different for the immune cells that reside in the lamina propria. Irrespective of the concentrations of SCFAs in the lumen, only a small proportion of these bacterial metabolites ever reach the lamina propria because of their metabolism in the epithelial cells. Therefore, under physiologic conditions in vivo, the lamina propria immune cells encounter 10 or 20 times less concentrations of SCFAs than in the lumen. Accordingly, the high-affinity transporter Slc5a8 might be obligatory for immune cells independent of the dietary fiber content. To ascertain the role of Slc5a8 in the delivery of SCFAs in the colon for subsequent inhibition of HDACs and its relevance to dietary fiber content, we measured HDAC activity in colon. We found no significant difference in HDAC activity in colon tissue between wild type mice and Slc5a8−/− mice when the mice were fed optimal dietary fiber (FC diet) (Fig. 6A). However, the findings were different when the mice were fed a fiber-free diet (FF diet). HDAC activity in colon was significantly higher in Slc5a8−/− mice than in wild type mice (Fig. 6A). This is expected because the predominant source for HDAC activity in the whole colon tissue is the epithelial cells rather than immune cells. The high-affinity transporter Slc5a8 is obligatory for the entry of butyrate and propionate into colonic epithelial cells only when the dietary fiber intake is low. The epithelial cell function and integrity were almost intact in Slc5a8−/− mice when fed a diet with optimal fiber content. This is evident from the findings that the epithelial barrier function, as monitored by the leakage of orally administered FITC-dextran into the blood, remained intact in Slc5a8−/− mice with FC diet (Fig. 6B). In contrast, the barrier function was compromised in Slc5a8−/− mice when fed FF diet (Fig. 6B). Thus, the low-fiber diet does not compromise the epithelial barrier function in the intestinal tract as long as the high-affinity SCFA transporter is functional. The transporter is not essential when dietary fiber intake is optimal but becomes essential under conditions of low dietary fiber intake.
Figure 6. Impact of dietary fiber content on colonic HDAC activity, intestinal barrier function, and frequency of IFN-γ+ T cells in colonic lamina propria in wild type mice and Slc5a8−/− mice.
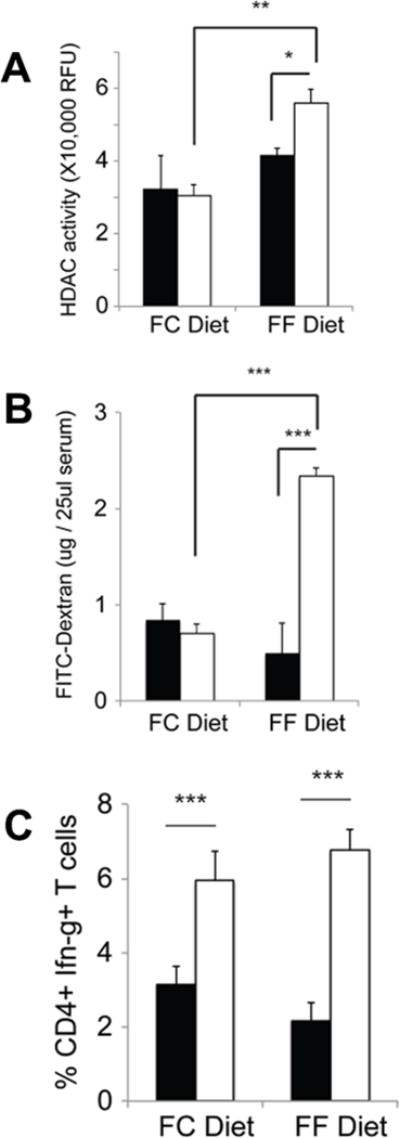
Wild type mice (closed bars) and Slc5a8−/− mice (open bars) were kept on either a fiber-containing diet (FC diet) or a fiber-free diet (FF diet) for 4 weeks. (A) HDAC activity was measured in colonic tissue lysates using a commercially available kit. (B) Intestinal barrier function was monitored by assessing the appearance of orally administered FITC-dextran in blood. (C) Colon tissues were used to determine the frequency of IFN-γ+ T cells in the lamina propria. Data (means ± S. E.) were from three independent animals for each genotype.
We then quantified IFN-γ+ T cells in the lamina propria of the colon in wild type mice and Slc5a8−/− mice with or without dietary fiber (FC diet or FF diet). We observed no difference in the frequency of IFN-γ+ T cells in wild type mice with intact functional Slc5a8 whether or not the mice were fed FC diet or FF diet (Fig. 6C). However, the frequency of these pro-inflammatory T cells increased significantly in Slc5a8−/− mice, and the increase was seen irrespective of the dietary fiber content (Fig. 6C). These findings are congruent with our rationale that the mucosal immune cells encounter only submillimolar concentrations of SCFAs irrespective of dietary fiber content and that the high-affinity SCFA transporter Slc5a8 is obligatory for the entry of these SCFAs into immune cells under these conditions.
Relevance of dietary fiber content to the role of Slc5a8 in protection against colonic inflammation and inflammation-associated colon cancer
We then wanted to determine how the dietary fiber impacts on the essential role of Slc5a8 in protection against colonic inflammation and inflammation-associated colon cancer. First, we assessed the progression of acute colitis induced by DSS (2% in drinking water for 6 days) in wild type mice and Slc5a8−/− mice under conditions of optimal fiber intake (FC diet) or a fiber-free diet (FF diet). We observed no significant difference between wild type mice and Slc5a8−/− mice in terms of survival, rectal bleeding, and diarrhea when the mice were fed the FC diet. In contrast, when the mice were fed the FF diet, Slc5a8−/− mice, but not wild type mice, demonstrated acute morbidity at around day 5 of DSS administration (Fig. 7A). There was also intense intestinal (small intestine and colon) bleeding and, as a result, anemia in Slc5a8−/− mice (Fig. 7B–D). The bleeding was minimal or non-existent in wild type mice under identical dietary conditions. Microscopically, we observed mild inflammation with infiltration of immune cells in colonic lamina propria in wild type mice as well as in Slc5a8−/− mice when fed the FC diet (Fig. 7E), and the extent of inflammation was similar between the two genotypes. When fed the FF diet, the inflammation was slightly higher in wild type mice compared to that with the FC diet, indicating that the fiber-free diet does accelerate DSS-induced colonic inflammation to some extent in wild type mice. But the impact of the fiber-free diet on colonic inflammation was much more pronounced in Slc5a8−/− mice (Fig. 7E). There was severe tissue destruction with heavy infiltration of immune cells throughout the colonic mucosal layer in Slc5a8−/− mice on the FF diet. Comparatively, the colonic inflammation was milder in wild type mice under identical dietary conditions.
Figure 7. Protective role of Slc5a8 against acute DSS-induced colitis and the relevance of dietary fiber to the process.
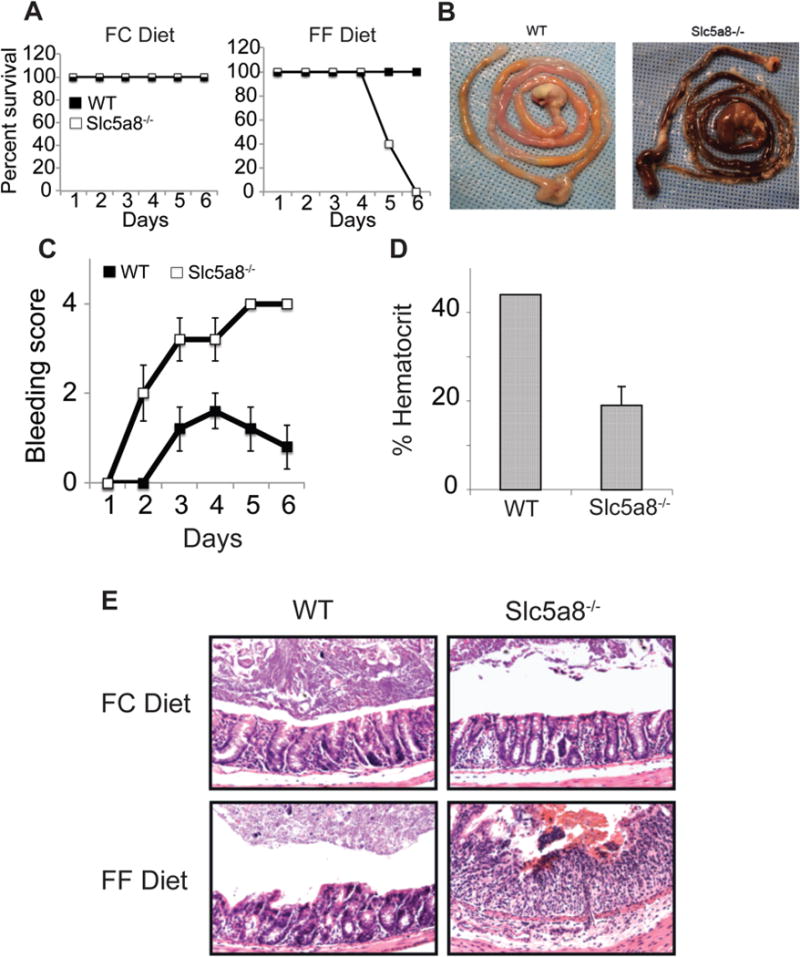
Wild type mice and Slc5a8−/− mice were fed either a fiber-containing diet (FC diet) or a fiber-free diet (FF diet) for 4 weeks prior to the initiation of colitis with administration of 2% DSS in drinking water. The DSS administration was continued for 6 days and the mice were kept on the respective diets all through the experiment. Animal survival and rectal bleeding were recorded each day of the experiment. Some mice were killed on the 5th of DSS administration for the analysis of the entire intestinal tract and for the histological evaluation of colonic sections. Blood was collected by retro-orbital bleeding and used for determination of hematocrit. (A) Mouse survival. (B) Gross appearance of the entire intestinal tract. (C) Rectal bleeding score. (D) Hematocrit. (E) Histological evaluation of colonic sections. Data (means ± S.E.) are from two independent experiments with n = 5 in each group.
We then assessed the progression of chronic inflammation in colon and the associated colon cancer in wild type mice and Slc5a8−/− mice under the two dietary conditions using the DSS/AOM (Fig. 8A). Again, we observed no significant difference between wild type mice and Slc5a8−/− mice in loss of body weight, diarrhea, and rectal bleeding when the mice were fed the FC diet (Fig. 8B–D). In contrast, when the mice were fed the FF diet, there was a marked difference between the two genotypes. Slc5a8−/− mice developed a more severe colitis than their wild type counterparts as evident from an exaggerated weight loss, diarrhea, and rectal bleeding in Slc5a8−/− mice than in wild type mice (Fig. 8B–D). Furthermore, wild type mice on FF diet showed improvement in all three disease-score indices upon termination of DSS treatment; in contrast, Slc5a8−/− mice demonstrated a significant delay in the improvement of the disease under identical conditions. Thus, the essential role of Slc5a8 in protection against chronic colonic inflammation became clearly evident only when the mice were on the FF diet.
Figure 8. Protective role of Slc5a8 against chronic DSS-induced colitis and the relevance of dietary fiber to the process.
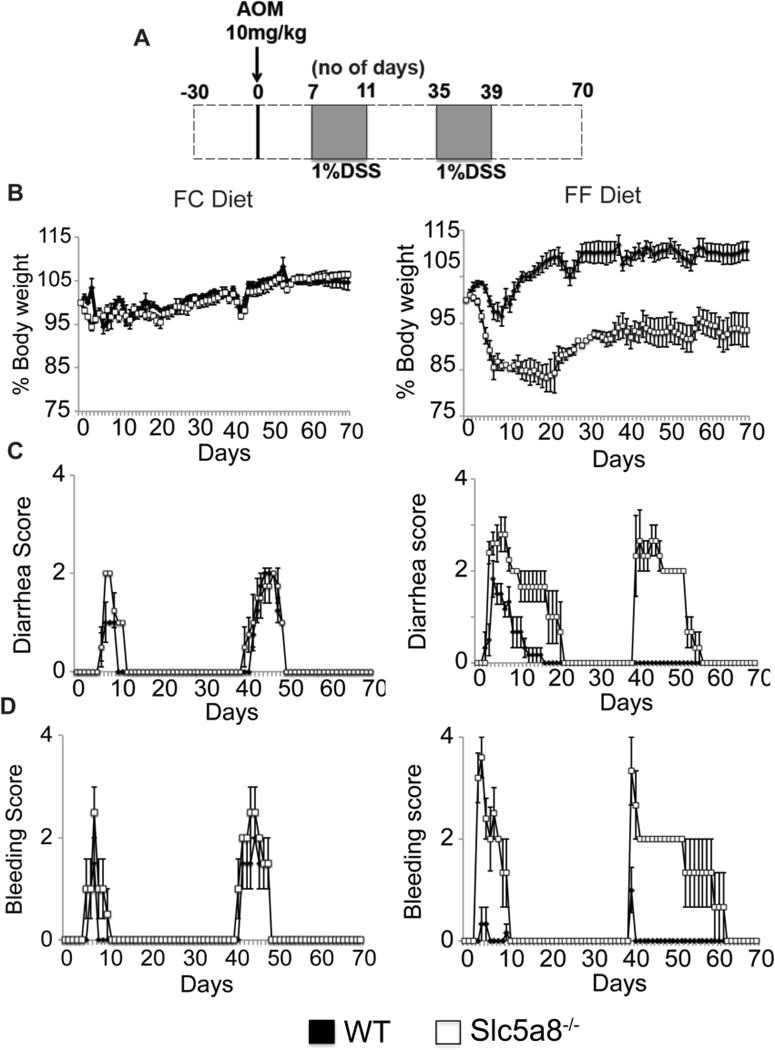
Wild type mice and Slc5a8−/− mice were fed either a fiber-containing diet (FC diet) or a fiber-free diet (FF diet) for 4 weeks prior to the injection of AOM. The experimental paradigm for the administration of DSS following AOM injection is given in (A). The mice were killed on day 70 following AOM injection. The animals were kept on the respective diets all through the experimental period. Body weight (B), diarrhea (C) and rectal bleeding (D) were monitored every day. Data (means ± S. E.) are from two independent experiments with n = 5 in each group.
We then studied the incidence and progression of colon cancer in the same cohort at the end of the experimental paradigm (Fig. 9). With FC diet, there was a trend of an increase in the number of polyps in colon in Slc5a8−/− mice compared to wild type mice but the difference was not statistically significant. In contrast, the absence of fiber in the diet increased the number of polyps in colon in wild type mice as well as in Slc5a8−/− mice. There was also a significant difference between wild type mice and Slc5a8−/− mice in the number of polyps. Thus, similar to our observations in the case of colitis, the essential nature of Slc5a8 as a protector of inflammation-associated colon cancer was evident only with the fiber-free diet.
Figure 9. Protective role of Slc5a8 against AOM/DSS-induced inflammation-associated colon cancer and the relevance of dietary fiber to the process.
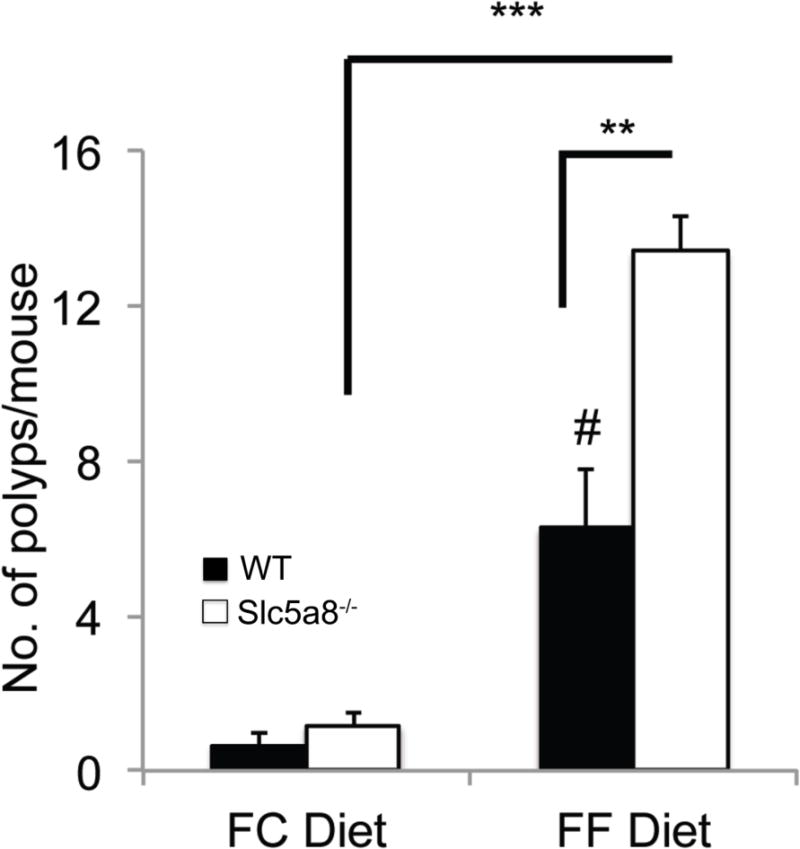
Wild type mice and Slc5a8−/− mice were fed either a fiber-containing diet (FC diet) or a fiber-free diet (FF diet) for 4 weeks prior to the injection of AOM. The experimental paradigm for the administration of DSS following AOM injection is given in Fig. 10A. The animals were kept on the respective diets all through the experimental period. The mice were killed on day 70 following AOM injection. Colons were removed and cut open longitudinally. The mucosal surface was examined under a microscope for polyps. Data (means ± S. E.) are from two independent experiments with n = 5 in each group. #, p < 0.05 compared to wild type mice on FC diet; **, p < 0.01; ***, p < 0.001.
DISCUSSION
The human gut harbors a large range of microorganisms, playing a critical role in the maintenance of colonic health. In our body, the number of bacterial cells outnumber human cells by 10 to 1, and 99% of functional genes in our body are microbial. The term “commensal bacteria” is often used to describe the colonic bacteria, meaning that only the bacteria benefit from the coexistence while the host is neither harmed nor receives any benefit from the relationship. But from what we know now on the biologic and metabolic crosstalk between the colonic bacteria and the host, the term “commensal” is misleading. Normal bacteria in colon elicit a plethora of beneficial effects on the host, including protection against enteropathogenic bacteria, supply of many water-soluble and fat-soluble vitamins, supply of energy substrates for colonic epithelial cells, intestinal maturation, and modulation of the mucosal immune system and the epigenetic landscape in the gut [27, 28]. In return, the host provides the home and the nourishment to the bacteria. Thus, the term “mutualism” is more appropriate than “commensalism” to describe the relationship between gut bacteria and the host. There is a general consensus that the bacterial fermentation products, namely short-chain fatty acids (SCFAs), play a significant role as the mediators of the beneficial effects of colonic bacteria on the host [9, 10]. It has been recognized for several decades that dietary fiber protects against colonic inflammation and colon cancer [17, 18]. The use of dietary fiber by colonic bacteria as substrates for fermentation resulting in the generation of SCFAs provides a molecular link between dietary fiber/colonic bacteria and colonic health. However, the molecular mechanisms by which the SCFAs contribute to the maintenance of colonic health in the host remain poorly understood.
In recent years, significant progress has been made in understanding the molecular targets for SCFAs in the host intestinal tract [29, 30]. Cell-surface receptors for SCFAs have been identified in colonic epithelial cells that impact on intracellular signaling in response to SCFAs in the lumen; these receptors, GPR109A and GPR43, are expressed on the lumen-facing apical membrane of these cells where they have direct contact with the bacterial metabolites present in the lumen. It has been shown convincingly that these two receptors protect against colonic inflammation and colon carcinogenesis [24, 31, 32]. In addition to these extracellular actions of SCFAs, intracellular actions also play an essential role in the biological effects of these bacterial metabolites. This primarily involves their functions as important energy source for the colonocytes and also as potent epigenetic modifiers. The latter function is related to the ability of butyrate and propionate to inhibit HDACs. For this intracellular function, SCFAs have to enter the cells to have access to HDACs inside the cells. Colonic epithelial cells express both low-affinity and high-affinity transporters for SCFAs. The low-affinity entry mechanism involves SLC16A1 and the high-affinity entry mechanism involves SLC5A8 [33, 34]. Since the luminal concentrations of SCFAs can vary widely depending on the content of fiber in the diet, the relative contribution of these two entry mechanisms might depend on dietary fiber content.
Until now, research on the biological functions of SCFAs in the colon focused mostly on the colonic epithelial cells as the target. Relatively less attention was given to the potential role of these bacterial metabolites on the mucosal immune system. The immune cells in the lamina propria are exposed to SCFAs at least at micromolar concentrations, though these concentrations represent only a fraction of their concentrations in the lumen. The high-affinity SCFA transporter SLC5A8 is capable of working efficiently even at these low concentrations. As such, potential actions of these bacterial metabolites on immune cells in the lamina propria cannot be discounted.
In the present study, we focused on the role of the high-affinity transporter Slc5a8 on the biology of dendritic cells (DCs). The findings of this study can be summarized as follows: (a) normal bacteria in the colon induce the immunosuppressive enzymes IDO1 and Aldh1A2 in the colon and in antigen-presenting immune cells via SCFAs, (b) among the three major SCFAs, only butyrate and propionate are responsible for this effect through their ability to inhibit HDACs, (c) Slc5a8, the high-affinity transporter for SCFAs, is obligatory for the ability of butyrate and propionate to induce IDO1 and Aldh1A2 in DCs, (d) butyrate/propionate-dependent induction of IDO1 and Aldh1A2 in DCs potentiates the ability of DCs to convert naïve T cells into FoxP3+ Tregs and also potentiate their ability to suppress naïve T cells into IFN-γ+ T cells, and (e) the high-affinity/low-capacity nature of Slc5a8 makes this transporter dispensable in vivo under conditions of optimal dietary fiber intake but makes it obligatory under conditions of low dietary fiber intake to mediate the protective effects of SCFAs against colonic inflammation and colon cancer. These studies unravel an important function for SLC5A8 in the mucosal immune system and establish this transporter as a conditional tumor suppressor linked to dietary fiber content.
Exposure of DCs to butyrate imparts a tolerogenic phenotype on these cells. Butyrate potentiates the ability of DCs to convert naïve T cells into immunorsuppressive Tregs and also augments the ability of DCs to suppress the conversion of naïve T cells into pro-inflammatory IFN-γ+ T cells. This provides an important molecular mechanism by which colonic bacteria impose a tolerogenic phenotype on the mucosal immune system to enable their coexistence with the host. For both of these functions, the butyrate transporter Slc5a8 seems obligatory.
In addition to the establishment of the obligatory nature of Slc5a8 for the tolerogenic phenotype of DCs, the present study also demonstrates an important role for this transporter in protection against colonic inflammation and colon cancer. Interestingly, this function depends on the dietary fiber content. The present study constitutes the first in vivo evidence in support of the anti-inflammatory and tumor-suppressive function of this transporter. SLC5A8 was first identified as a candidate tumor suppressor in colon [35]. We showed that it is a Na+-coupled transporter for SCFAs [14] and that its ability to energize the entry of the SCFA butyrate, a bacterial fermentation product of dietary fiber and an inhibitor of HDACs, underlies its tumor-suppressive function in colon in vitro [29, 30]. Nonetheless, in vivo studies by Frank et al [19] with Slc5a8−/− mice failed to support such a function. Interestingly, our recent studies using the same Slc5a8−/− mice generated by Frank et al [19] have shown that absence of Slc5a8 promotes breast cancer [22]. The difference between colon and mammary gland was puzzling. We hypothesized that the vastly varying levels of butyrate in colonic lumen (~15 mM) vs blood (~10 μM) might provide a logical solution to the puzzle. SLC5A8 has high-affinity for butyrate (Km, ~100 μM), and at high concentrations butyrate diffuses or is taken up into colon cells by the low-affinity transporters (MCTs). As such, SLC5A8-mediated butyrate entry into these cells constitutes only a small fraction of the total entry process; hence SLC5A8 is dispensable for butyrate to inhibit HDACs in these cells when dietary fiber intake is high. SLC5A8 is obligatory for HDAC inhibition in colon cells only when the luminal butyrate levels are low (e.g., low-fiber diet). The present study provides strong evidence in support of our hypothesis. Slc5a8−/− mice do show increased risk for colonic inflammation and colon cancer but only on a fiber-free diet, thus establishing the transporter as a conditional tumor suppressor.
SLC5A8 is silenced in cancers of every tissue that has been examined so far, and in each of these tissues the transporter has been proposed to function as a tumor suppressor (reviewed in Ref. 36, 37). While the connection of SLC5A8 to the bacterial metabolite butyrate, which functions as an HDAC inhibitor, provides a logical explanation for the tumor-suppressive function of the transporter in colon, why would the transporter be silenced in cancers of so many non-colonic tissues which never encounter butyrate at relevant concentrations? We believe that our recent findings that pyruvate is almost as potent as butyrate as an HDAC inhibitor and that this ubiquitous metabolite is a high-affinity substrate for SLC5A8 [38] provide a sound rationale for the tumor-suppressive function of this transporter in non-colonic tissues. While SLC5A8/butyrate-induced HDAC inhibition is critical for the transporter’s tumor-suppressive function in the colon, SLC5A8/pyruvate-induced HDAC inhibition is critical for the transporter’s tumor-suppressive function in non-colonic tissues. Pyruvate is present in systemic blood at ~100 μM. Tumor cells are programmed to keep their intracellular levels of pyruvate very low by converting it into lactate, most likely to prevent pyruvate-induced HDAC inhibition [12]. These cells do not want SLC5A8 on their membrane; lest it would mediate Na+-coupled active entry of extracellular pyruvate into cells causing cell death. This provides a molecular basis as to why all tumor types examined to date silence SLC5A8. The present study establishing the tumor-suppressive and immunomodulatory function of this transporter in colon for the first time in vivo highlights the importance of this transporter not only in colon cancer but also in cancers of various other organs.
Abbreviations
- Slc5a8
solute carrier gene family 5a, member 8
- IBD
inflammatory bowel disease
- IDO1
indoleamine 2,3-dioxygenase 1
- SCFA
short-chain fatty acid
- RA
all-trans retinoic acid
- Aldh1A2
aldehyde dehydrogenase 1A2
- DC
dendritic cells
- 1-MT
1-methyl-D, L-tryptophan
- DSS
dextran sulfate sodium
- GF
germ-free
- IFN-γ
interferon-γ
- IL
interleukin
- HDAC
histone deacetylase
- FC diet
fiber-containing diet
- FF diet
fiber-free diet
References
- 1.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 2.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden EK, Snapper SB. Regulatory T cells in inflammatory bowel disease. Curr Opin Gastroenterol. 2008;24:733–741. doi: 10.1097/mog.0b013e328311f26e. [DOI] [PubMed] [Google Scholar]
- 4.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 6.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 7.Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 8.Feng T, Cong Y, Qin H, Benveniste EN, Elson CO. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. J Immunol. 2010;185:5915–5925. doi: 10.4049/jimmunol.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short-chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 11.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 12.Thangaraju M, Carswell KN, Prasad PD, Ganapathy V. Colon cancer cells maintain low levels of pyruvate to avoid cell death caused by inhibition of HDAC1/HDAC3. Biochem J. 2009;417:379–389. doi: 10.1042/BJ20081132. [DOI] [PubMed] [Google Scholar]
- 13.Thangaraju M, Cresci G, Itagaki S, Mellinger J, Browning DD, Berger FG, Prasad PD, Ganapathy V. Sodium-coupled transport of the short-chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J Gastrointest Surg. 2008;12:1773–1782. doi: 10.1007/s11605-008-0573-0. [DOI] [PubMed] [Google Scholar]
- 14.Miyauchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J Biol Chem. 2004;279:13293–13296. doi: 10.1074/jbc.C400059200. [DOI] [PubMed] [Google Scholar]
- 15.Gopal E, Miyauchi S, Martin PM, Ananth S, Roon P, Smith SB, Ganapathy V. Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharm Res. 2007;24:575–584. doi: 10.1007/s11095-006-9176-1. [DOI] [PubMed] [Google Scholar]
- 16.Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285:27601–27608. doi: 10.1074/jbc.M110.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning TS, Gibson GR. Microbial-gut interactions in health and disease. Prebiotics. Best Pract Res Clin Gastroenterol. 2004;18:287–298. doi: 10.1016/j.bpg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 18.World Cancer Research Fund and American Institute for Cancer Research. Patterns of diet and cancer. In: Potter JD, editor. Food, Nutrition and Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 1997. pp. 20–52. [DOI] [PubMed] [Google Scholar]
- 19.Frank H, Groger N, Diener M, Becker C, Braun T, Boettger T. Lactaturia and loss of sodium-dependent lactate uptake in the colon of SLC5A8-deficient mice. J Biol Chem. 2008;283:24729–24737. doi: 10.1074/jbc.M802681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babu E, Ananth S, Veeranan-Karmegam R, Coothankandaswamy V, Smith SB, Boettger T, Ganapathy V, Martin PM. Transport via SLC5A8 (SMCT1) is obligatory for 2-oxothiazolidine-4-carboxylate to enhance glutathione production in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:5749–5757. doi: 10.1167/iovs.10-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ananth S, Babu E, Veeranan-Karmegam R, Bozard Baldowski BR, Boettger T, Martin PM. Induction of the cystine/glutamate exchanger SLC7A11 in retinal pigment epithelial cells by the antipsoriatic drug monomethylfumarate. Invest Opthalmol Vis Sci. 2013;54:1592–1602. doi: 10.1167/iovs.12-11289. [DOI] [PubMed] [Google Scholar]
- 22.Elangovan S, Pathania R, Ramachandran S, Ananth S, Padia RN, Srinivas SR, Babu E, Howthorn L, Schoenlein PV, Boettger T, Smith SB, Prasad PD, Ganapathy V, Thangaraju M. Molecular mechanism of SLC5A8 inactivation in breast cancer. Mol Cell Biol. 2013;33:3920–3935. doi: 10.1128/MCB.01702-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cresci GA, Thangaraju M, Mellinger JD, Liu K, Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg. 2010;14:449–461. doi: 10.1007/s11605-009-1045-x. [DOI] [PubMed] [Google Scholar]
- 24.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia RN, Shi H, Thangaraju M, Prasad PD, Maniccasamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang GM, He YW, Fang R, Zhang G, Zeng J, Yi YM, Zhang S, Bu XZ, Cai SH, Du J. Sodium butyrate down-regulation of indoleamine 2,3-dioxygenase at the transcriptional level and post-transcriptional levels. Int J Biochem Cell Biol. 2010;42:1840–1846. doi: 10.1016/j.biocel.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P, Jiang G, Gao J, Li L, Du J, Jiao X. SAHA down-regulates the expression of indoleamine 2,3-dioxygenase via inhibition of the JAK/STAT1 signaling pathway in gallbladder carcinoma cells. Oncol Rep. 2013;29:269–275. doi: 10.3892/or.2012.2073. [DOI] [PubMed] [Google Scholar]
- 27.Tsai F, Coyle WJ. The microbiome and obesity: is obesity linked to our gut flora? Curr Gastroenterol Rep. 2009;11:307–313. doi: 10.1007/s11894-009-0045-z. [DOI] [PubMed] [Google Scholar]
- 28.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganapathy V, Prasad PD, Thangaraju M, Martin PM, Singh N. Butyrate-mediated protection against colonic inflammation and colon carcinogenesis: Role of butyrate transporters and butyrate receptors. In: Li C, editor. Butyrate: Food Sources, Functions and Health Benefits. Nova Science Publishers, Inc; Hauppauge, New York: 2014. pp. 157–176. [Google Scholar]
- 30.Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Transporters and receptors for short-chain fatty acids as a link between colonic bacteria and the host. Curr Opin Pharmacol. 2013;13:869–874. doi: 10.1016/j.coph.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, Prasad PD, Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Y, Chen Y, Jiang H, Robbins GT, Nie D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int J Cancer. 2011;128:847–856. doi: 10.1002/ijc.25638. [DOI] [PubMed] [Google Scholar]
- 33.Goncalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab. 2013;14:994–1008. doi: 10.2174/1389200211314090006. [DOI] [PubMed] [Google Scholar]
- 34.Ganapathy V, Gopal E, Miyauchi S, Prasad PD. Biological functions of SLC5A8, a candidate tumor suppressor. Biochem Soc Trans. 2005;33:237–240. doi: 10.1042/BST0330237. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, Kasturi L, Lutterbaugh J, Rerko RM, Casey G, Issa JP, Willis J, Willson JK, Plass C, Markowitz SD. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci USA. 2003;100:8412–8417. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganapathy V, Thangaraju M, Gopal E, Itagaki S, Miyauchi S, Prasad PD. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10:193–199. doi: 10.1208/s12248-008-9022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Thangaraju M, Gopal E, Martin PM, Ananth S, Smith SB, Prasad PD, Sterneck E, Ganapathy V. SLC5A8 triggers tumor cell apoptosis through pyruvate-dependent inhibition of histone deacetylases. Cancer Res. 2006;66:11560–115. doi: 10.1158/0008-5472.CAN-06-1950. [DOI] [PubMed] [Google Scholar]


