Philadelphia-chromosome negative myeloproliferative neoplasms (MPNs) including myelofibrosis (MF), polycythemia vera (PV), and essential thrombocythemia (ET), can transform into acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS),1,2 and have been associated with a greater incidence of lymphoid neoplasms.3 Epidemiologic data suggests increased incident non-hematologic malignancies among MPN patients,4–8 although the magnitude of risk is debated.5 One study of 7229 patients with ET, PV, or chronic myeloid leukemia (CML), with median follow-up of 4–5 years, found a modestly increased risk of developing a second non-hematologic malignancy (standardized incidence ratio 1.2–1.4) compared to the age-adjusted general population.6 Another study of 733 patients, with median follow-up of 6.45 years, found no increase in second non-hematologic malignancies, aside from melanoma.5 Therefore, the true second malignancy rate in this population remains unclear, which can impact optimal counseling to these patients. The Surveillance, Epidemiology, and End Results Program (SEER) Registry of the National Cancer Institute is a recognized standard for population-based cancer epidemiology in the United States, capturing approximately 28% of the population. Our objective was to determine the cumulative incidence of second malignancies among patients in the SEER database whose first malignancy is an MPN, and compare this rate to the age-adjusted US population cancer rate.
We used the SEER 18 registries database released in April 2014 (1973–2011; November 2013 submission). SEER included MPNs starting in 2001; we included patients diagnosed with an MPN as their first cancer, between 2001 and 2011, according to ICD-O-3 codes: PV (code 9950), chronic myeloproliferative disease, NOS (9960), MF (9961), and ET (9962). Diagnoses are confirmed pathologically according to WHO 2008 criteria and the International Classifications of Diseases for Oncology, Version 3.1,9 CML patients were excluded. Time to second malignancy was calculated by subtracting second malignancy survival/follow-up time from MPN survival/follow-up time.
We classified second malignancy according to SEER site-specific categories. The second malignancy incidence rate was the number of second cancers per person-years of follow-up. Site-specific malignancies could occur at any point in follow-up; these, therefore, may not be the immediate subsequent malignancy for a patient. Death was a competing risk. Age-adjusted malignancy incidence rates for the general population were calculated by weighting cancer incidence rates provided by the National Cancer Institute, SEER Cancer Statistic Review 2007–2011, according to the age distribution of the SEER MPN population.
Incident leukemia cases included AML, biphenotypic and undifferentiated acute leukemia, and MDS. Non-leukemic second malignancy rates were calculated by subtracting these cases, as well as cases of progression to another MPN. SEER documents radiation therapy but does not report chemotherapy data. Radiation given concurrently or following the original MPN diagnosis, and prior to AML/MDS diagnosis, was a variable in subgroup analysis.
Demographic characteristics were compared using chi-square and Fisher’s exact test. Cumulative incidences of second malignancies were calculated at 60 and 120 months using the %CIF macro package in SAS 9.4 (Cary, NC), using the method of Fine and Gray. Age-adjusted cancer incident rate ratios for MPN patients compared to the 2007–2011 population were calculated using STATA version 9.1 (College Station, TX). Overall survival (OS) was defined as time from MPN diagnosis to death, censored at last known alive, and estimated using the method of Kaplan and Meier. Cox regression was performed to calculate predictors of overall survival. Cumulative incidence of second malignancies was modeled using the subdistribution hazard, with death as a competing risk, by the %PSHREG macro package in SAS 9.4. All p-values are considered significant at the two-sided 0.05 level.
We identified 20,250 MPN patients diagnosed between January 2001 and December 2011, with 81,995.5 person-years follow-up; median follow-up was 42 months. Median age of diagnosis was 63 for PV, 66 for ET, 69 for MF, and 71 for MPN NOS. 38.8% of patients with ET were male, versus PV (58.6%), MF (58.2%), and MPN NOS (51.1%; p<0.0001). 0.57% were younger than 15 years at MPN diagnosis, and 1.84% were 90 years or older at MPN diagnosis.
At 5-years, survival was 75.3% for PV, 76.4% for ET, 50.2% for MPN NOS, and 38.9% for MF (p<0.0001). Compared to ET, there was an increase in the risk of death among patients with MF (HR 3.297), MPN NOS (HR 2.246), and PV (HR 1.141) (Figure 1). There was worse survival among patients with advancing age (HR 1.062/year), black race (HR 1.444), male sex (HR 1.317), and more than one lifetime malignancy (HR 1.123). Hispanic ethnicity was not associated with survival (p=0.0522).
Figure 1.
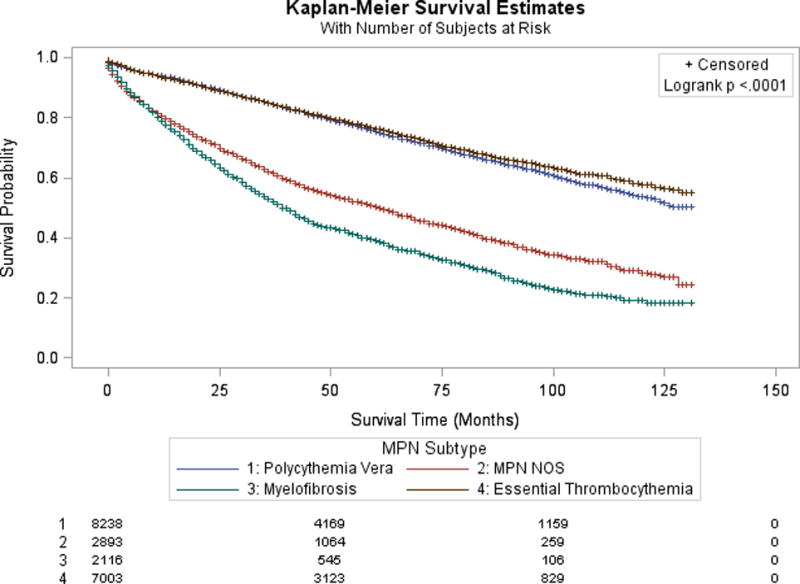
Kaplan-Meier survival estimate according to underlying MPN histology. The number of patients at risk at 0, 50, and 100 months, according to MPN histology, are shown at the bottom of the figure.
Of the 20,250 patients in the study, 1,866 developed at least one subsequent malignancy, including leukemic transformation, between 2001 and 2011, an incident rate of 2,386.4 per 100,000 person-years. The cumulative incidence of any second malignancy among MPN patients was estimated at 15.2% at 10 years (95% CI 14.4–16.0%; Table I). The risk of developing a second malignancy increased with advancing age at diagnosis (HR 1.017/year, p<0.0001) and male sex (HR 1.371, p<0.0001). In multivariable regression, the cumulative incidence of second malignancies in patients with MF and MPN NOS was lower than ET (HR 0.707, p=0.0001 and HR 0.742, p<0.0001, respectively).
Table I.
Incident second cancers among MPN patients compared to the US population. Sites with increased incidence relative to the US population are shaded light gray while those with decreased incidence are dark gray. Nominal p-value for significance < 0.009.
| Number of Patients with Second Cancer | Cumulative Incidence (%) | Incidence Rate Ratio Compared to General Population* (95% CI) | Nominal P-value | ||
|---|---|---|---|---|---|
| Site of second malignancy | (n) | 5 Year | 10 Year | ||
| All sites** | 1866 | 9.4 | 15.2 | 1.67 (1.59, 1.75) | <0.0001 |
| AML and MDS | 230 | 1.1 | 2.1 | 8.76 (7.36, 10.41) | <0.0001 |
| Second MPN Diagnosis | 74 | 0.4 | 0.6 | 10.77 (7.77, 14.90) | <0.0001 |
| Non-AML/MPN Second Malignancy*** | 1575 | 8.0 | 12.7 | 1.44 (1.37, 1.52) | <0.0001 |
| Hematologic Malignancies | |||||
| AML**** | 134 | 0.7 | 1.2 | 14.10 (10.91, 18.24) | <0.0001 |
| MDS | 80 | 0.4 | 0.7 | 4.77 (3.63, 6.20) | <0.0001 |
| ALL | 4 | 0 | 0.1 | 3.75 (0.89, 12.15) | 0.0424 |
| CLL | 33 | 0.2 | 0.3 | 2.46 (1.64, 3.59) | <0.0001 |
| CML | 16 | 0.1 | 0.1 | 4.15 (2.20, 7.46) | <0.0001 |
| CMML | 4 | 0.02 | 0.02 | 2.71 (0.67, 8.23) | 0.1026 |
| Lymphoma | |||||
| Hodgkin Lymphoma | 9 | 0.05 | 0.1 | 3.14 (1.33, 6.66) | 0.0065 |
| NHL | 115 | 0.6 | 0.9 | 2.27 (1.85, 2.77) | <0.0001 |
| Multiple Myeloma | 27 | 0.1 | 0.2 | 1.55 (1.00, 2.32) | 0.0414 |
| Non-Hematologic Malignancies | |||||
| Oral cavity/Pharynx | 37 | 0.2 | 0.3 | 1.47 (1.02, 2.07) | 0.0334 |
| Brain/nervous system | 74 | 0.4 | 0.5 | 2.15 (1.45, 3.22) | <0.0001 |
| Gastrointestinal System | |||||
| Esophagus | 21 | 0.1 | 0.2 | 1.70 (1.02, 2.69) | 0.032 |
| Stomach | 19 | 0.1 | 0.2 | 0.90 (0.53, 1.44) | 0.685 |
| Colorectal | 114 | 0.6 | 0.9 | 0.94 (0.77, 1.14) | 0.5061 |
| Liver and bile ducts | 13 | 0.1 | 0.1 | 0.66 (0.35, 1.15) | 0.129 |
| Pancreas | 43 | 0.2 | 0.4 | 1.16 (0.82, 1.58) | 0.364 |
| Larynx | 14 | 0.1 | 0.1 | 1.66 (0.88, 2.91) | 0.0909 |
| Respiratory System | |||||
| Lung and Bronchus | 279 | 1.3 | 2.4 | 1.55 (1.36, 1.75) | <0.0001 |
| Mesothelioma | 5 | 0 | 0.1 | 1.52 (0.47, 3.86) | 0.3758 |
| Breast (invasive) | 159 | 0.8 | 1.4 | 1.08 (0.91, 1.27) | 0.3605 |
| Melanoma | 104 | 0.5 | 1 | 2.18 (1.75, 2.68) | <0.0001 |
| Kaposi Sarcoma | 2 | 0 | 0 | 2.71 (0.28, 13.09) | 0.2459 |
| Female GU | |||||
| Cervix | 5 | 0.03 | 0.03 | 0.52 (0.17, 1.25) | 0.1343 |
| Corpus and Uterus | 25 | 0.1 | 0.2 | 0.46 (0.30, 0.69) | <0.0001 |
| Ovary | 14 | 0.1 | 0.1 | 0.51 (0.28, 0.87) | 0.0073 |
| Male GU | |||||
| Prostate | 228 | 1.1 | 1.9 | 0.56 (0.49, 0.64) | <0.0001 |
| Testis | 4 | 0.02 | 0.02 | 1.57 (0.40, 4.45) | 0.3943 |
| Kidney/renal pelvis | 75 | 0.4 | 0.5 | 2.01 (1.56, 2.58) | <0.0001 |
| Bladder | 72 | 0.4 | 0.5 | 1.11 (0.86, 1.42) | 0.3893 |
| Thyroid | 38 | 0.2 | 0.3 | 2.26 (1.56, 3.21) | <0.0001 |
General population incidence per SEER Cancer Statistics Review 1975–2011 (Age-adjusted incidence rate, 2007–2011)
The SEER incidence of all cancer sites was used as a comparator to the second cancer rate
Non-AML/MPN incidence rates in SEER were calculated by subtracting incident AML/MDS/MPN cases from the total
For this comparison, AML does not include acute leukemia NOS, undifferentiated or biphenotypic leukemia.
There was increased hazard of leukemic transformation with advancing age (HR 1.022/year, p<0.0001) and male compared to female sex (HR 1.364, p=0.0195). Accounting for these variables, PV was associated with a lower cumulative incidence of AML or MDS compared to other MPNs. However, looking specifically at AML transformation among MPN subtypes, there was a higher cumulative incidence of AML in MF (HR 2.223, p=0.0027) compared to ET. 520 patients received radiation either for MPN or a second non-leukemic malignancy, and prior to any leukemia diagnosis. There was no significant difference in the incidence of leukemia among those with radiation exposure (n=520) versus those without (n=19730) in multivariable analysis (p=0.1659).
Excluding progression to, or diagnosis of, a different MPN, or leukemic transformation, MPN patients had a HR of 1.44 for developing a second cancer versus the age-adjusted general population (IR 2004.6/100,000 person-years). The risk of second non-AML/MPN cancers was higher with age (HR 1.016/year, p<0.0001) and male sex (HR 1.431, p<0.0001) and lower for Hispanic ethnicity (HR 0.798, p=0.0394). Such cancers were seen in 708 PV patients (IR 2,008.9/100,000 person-years, IRR vs. general population 1.44 [95%CI 1.34, 1.56], p<0.0001), 531 ET patients (IR 1922.8/100,000 person-years, IRR 1.39 [1.27, 1.51], p<0.0001), 123 MF patients (IR 2097.3/100,000 person-years, IRR 1.51 [1.25, 1.80], p<0.0001) and 213 MPN NOS patients (IR 2,163.3/100,000 person-years, IRR 1.56 [1.35, 1.78], p<0.0001). A subgroup analysis of MPN patients under age 40 (n=1617) or under age 50 (n=3860) also revealed an increase in second non-AML/MPN cancers versus the age-adjusted US population under 40 (27 second cancers, IRR 4.24, [2.64, 6.62], p<0.0001) and 50 (133 second cancers, IRR 3.51, [2.80, 4.38], p<0.0001), respectively.
Leukemic transformation to AML or MDS occurred in 230 patients, an incidence rate ratio of 8.76 compared to the incidence of AML or MDS in the age-adjusted general population (p<0.0001). MPN patients had significantly increased incident rates of CLL, CML, multiple myeloma, Hodgkin and Non-Hodgkin lymphoma, and second MPNs (Table I), as well as brain and nervous system cancers, melanoma, and lung, renal, and thyroid cancers. There was a decreased incidence of uterine, ovarian, and prostate cancer among MPN patients compared to the age-adjusted general population.
Leukemic transformation of MPNs is well-established, but published data regarding other second cancers in these patients is more inconsistent. To our knowledge, this study encompasses the largest population of MPN patients to undergo such an analysis. Excluding progression to or diagnosis of another MPN, or leukemic transformation, the cumulative incidence of second cancers among patients with MPNs was estimated at 8.0% at 5 years and 12.7% at 10 years. Our findings verify and expand upon smaller studies of second malignancy in MPN patients.3–5 A Danish population study found a standardized incidence ratio of second non-hematologic cancers at 1.2 to 1.4,6 but, did not include MF. Similarly, we found an IRR of 1.44 for second cancers compared to the age-adjusted US population, including MF patients. This increased risk appeared more pronounced among those younger than age 50, although further interpretation for specific malignancies is limited by the relatively few patients and events.
The estimated cumulative incidence of leukemic transformation was 2% at 10 years, lower than prior estimates10,11 and approximating rates reported in ET or PV.12,13 This likely reflects the study’s 42-month median follow-up, and predominance of patients with ET or PV at later time points due to longer survival. An explanation for increased incidence of second malignancies among MPN patients is elusive, but may relate to exposure to alkylating or radioactive therapies,10 chronic inflammation,5,7,14,15 and acquired somatic mutations.2 Unfortunately, SEER does not report chemotherapy administration, limiting our ability to infer causes. Further follow-up of this cohort will be valuable to assess longer-term outcomes.
The current analysis is the largest such study of patients with MPN to assess second cancer rates, and establishes a modest but significant risk of second malignancy, in addition to leukemic transformation. It is important to recognize these risks when caring for and counseling patients with MPNs, particularly in relation to cancer screening and patient survivorship.
Acknowledgments
Role of the Funding Source: AMB is supported in part by the T32 CA 71345-18 training grant. MMJ is supported by the Betty Lea Stone Fellowship from the American Cancer Society. This project is facilitated through the assistance of the Dana-Farber Cancer Center Core Grant 5P30 CA 006516.
Footnotes
Declaration of Interests: The authors report no conflicts of interest related to this work.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition. 4th. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Abdel-Wahab O, Manshouri T, Patel J, Harris K, Yao J, Hedvat C, Heguy A, Bueso-Ramos C, Kantarjian H, Levine RL, et al. Genetic analysis of transforming events that convert chronic myeloproliferative neoplasms to leukemias. Cancer research. 2010;70(2):447–452. doi: 10.1158/0008-5472.CAN-09-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rumi E, Passamonti F, Elena C, Pietra D, Arcaini L, Astori C, Zibellini S, Boveri E, Pascutto C, Lazzarino M. Increased risk of lymphoid neoplasm in patients with myeloproliferative neoplasm: a study of 1,915 patients. Haematologica. 2011;96(3):454–458. doi: 10.3324/haematol.2010.033779. [accessed 2014 Sep 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kissova J, Ovesna P, Penka M, Bulikova A, Kiss I. Second Malignancies in Philadelphia-negative Myeloproliferative Neoplasms–Single-center Experience. Anticancer Research. 2014;34(5):2489–2496. [accessed 2014 Oct 5] [PubMed] [Google Scholar]
- 5.Susini MC, Masala G, Antonioli E, Pieri L, Guglielmelli P, Palli D, Bosi A, Vannucchi AM. Risk of second cancers in chronic myeloproliferative neoplasms. Blood. 2012;119(16):3861–3862. doi: 10.1182/blood-2011-12-401455. [accessed 2014 Oct 5] [DOI] [PubMed] [Google Scholar]
- 6.Frederiksen H, Farkas DK, Christiansen CF, Hasselbalch HC, Sørensen HT. Chronic myeloproliferative neoplasms and subsequent cancer risk: a Danish population-based cohort study. Blood. 2011;118(25):6515–6520. doi: 10.1182/blood-2011-04-348755. [accessed 2014 Oct 5] [DOI] [PubMed] [Google Scholar]
- 7.Fallah M, Kharazmi E, Sundquist J, Hemminki K. Higher risk of primary cancers after polycythaemia vera and vice versa. British Journal of Haematology. 2011;153(2):283–285. doi: 10.1111/j.1365-2141.2010.08538.x. [accessed 2014 Oct 5] [DOI] [PubMed] [Google Scholar]
- 8.Radaelli F, Onida F, Rossi FG, Zilioli VR, Colombi M, Usardi P, Calori R, Zanella A. Second malignancies in essential thrombocythemia (ET): a retrospective analysis of 331 patients with long-term follow-up from a single institution. Hematology (Amsterdam, Netherlands) 2008;13(4):195–202. doi: 10.1179/102453308X316022. [DOI] [PubMed] [Google Scholar]
- 9.Percy C, Fritz A, Jack A, Shanmugarathan S, Sobin L, Parkin DM, Whelan S. International classification of diseases for oncology (ICD-O) World Health Organization; Geneva: 2000. [Google Scholar]
- 10.Bjorkholm M, Derolf AR, Hultcrantz M, Kristinsson SY, Ekstrand C, Goldin LR, Andreasson B, Birgegard G, Linder O, Malm C, et al. Treatment-Related Risk Factors for Transformation to Acute Myeloid Leukemia and Myelodysplastic Syndromes in Myeloproliferative Neoplasms. Journal of Clinical Oncology. 2011;29(17):2410–2415. doi: 10.1200/JCO.2011.34.7542. [accessed 2014 Aug 15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finazzi G, Caruso V, Marchioli R, Capnist G, Chisesi T, Finelli C, Gugliotta L, Landolfi R, Kutti J, Gisslinger H, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005;105(7):2664–2670. doi: 10.1182/blood-2004-09-3426. [accessed 2014 Dec 3] [DOI] [PubMed] [Google Scholar]
- 12.Palandri F, Catani L, Testoni N, Ottaviani E, Polverelli N, Fiacchini M, De Vivo A, Salmi F, Lucchesi A, Baccarani M, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: Safety of cytoreductive therapy. American Journal of Hematology. 2009;84(4):215–220. doi: 10.1002/ajh.21360. [accessed 2014 Dec 3] [DOI] [PubMed] [Google Scholar]
- 13.Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, Randi ML, Vaidya R, Cazzola M, Rambaldi A, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874–1881. doi: 10.1038/leu.2013.163. [accessed 2014 Dec 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating Interleukin (IL)-8, IL-2R, IL-12, and IL-15 Levels Are Independently Prognostic in Primary Myelofibrosis: A Comprehensive Cytokine Profiling Study. Journal of Clinical Oncology. 2011;29(10):1356–1363. doi: 10.1200/JCO.2010.32.9490. [accessed 2014 Oct 20] [DOI] [PubMed] [Google Scholar]
- 15.Kristinsson SY, Björkholm M, Hultcrantz M, Derolf ÅR, Landgren O, Goldin LR. Chronic Immune Stimulation Might Act As a Trigger for the Development of Acute Myeloid Leukemia or Myelodysplastic Syndromes. Journal of Clinical Oncology. 2011;29(21):2897–2903. doi: 10.1200/JCO.2011.34.8540. [accessed 2012 Apr 16] [DOI] [PMC free article] [PubMed] [Google Scholar]


