Abstract
Modern humans arrived in Europe ~45,000 years ago, but little is known about their genetic composition before the start of farming ~8,500 years ago. We analyze genome-wide data from 51 Eurasians from ~45,000-7,000 years ago. Over this time, the proportion of Neanderthal DNA decreased from 3–6% to around 2%, consistent with natural selection against Neanderthal variants in modern humans. Whereas the earliest modern humans in Europe did not contribute substantially to present-day Europeans, all individuals between ~37,000 and ~14,000 years ago descended from a single founder population which forms part of the ancestry of present-day Europeans. A ~35,000 year old individual from northwest Europe represents an early branch of this founder population which was then displaced across a broad region, before reappearing in southwest Europe during the Ice Age ~19,000 years ago. During the major warming period after ~14,000 years ago, a new genetic component related to present-day Near Easterners appears in Europe. These results document how population turnover and migration have been recurring themes of European pre-history.
Modern humans arrived in Europe around 45,000 years ago and have lived there ever since, even during the Last Glacial Maximum 25,000-19,000 years ago when large parts of Europe were covered in ice1. A major question is how climatic fluctuations influenced the population history of Europe and to what extent changes in material cultures documented by archaeology and correlating to climatic events corresponded to movements of people. To date, it has been difficult to address this question because genome-wide ancient DNA has been retrieved from just five Upper Paleolithic individuals in Eurasia2–4. Here we assemble and analyze genome-wide data from 51 modern humans dating from 45,000 to 7,000 years ago (Table 1; Extended Data Table 1; Supplementary Information section 1).
Ancient DNA data
We extracted DNA from human remains in dedicated clean rooms5, and transformed the extracts into Illumina sequencing libraries6–8. A major challenge in ancient DNA research is that the vast majority of the DNA extracted from most specimens is of microbial origin, making random shotgun sequencing prohibitively expensive. We addressed this problem by enriching the libraries for between 390,000 and 3.7 million single nucleotide polymorphisms (SNPs) in the nuclear genome via hybridizing to pools of previously synthesized 52-base-pair oligonucleotide probes targeting these positions (this strategy makes it possible to generate genome-wide data from samples with high percentages of microbial DNA that are not practical to study by shotgun sequencing)3,9. We sequenced the isolated DNA fragments from both ends, and mapped the consensus sequences to the human genome (hg19), retaining fragments that overlapped the targeted SNPs. After removing fragments with identical start and end positions to eliminate duplicates produced during library amplification, we chose one fragment at random to represent each individual at each SNP.
Contamination from present-day human DNA is a danger in ancient DNA research. To address this we took advantage of three characteristic features of ancient DNA (Supplementary Information section 2). First, for an uncontaminated specimen, we expect only a single mitochondrial DNA sequence to be present, allowing us to detect contamination as a mixture of mitochondrial sequences. Second, because males carry a single X chromosome, we can detect contamination in male specimens as polymorphisms on chromosome X10. Third, cytosines at the ends of genuine ancient DNA molecules are often deaminated, resulting in apparent cytosine to thymine substitutions11. Thus, restricting analysis to molecules with evidence of such deamination filters out the great majority of contaminating molecules12. For libraries from males with evidence of mitochondrial DNA contamination or X chromosomal contamination estimates >2.5%—as well as for all libraries from females—we restricted the analyses to sequences with evidence of cytosine deamination (Supplementary Information section 2). After merging libraries from the same individual and limiting to individuals with >4,000 targeted SNPs covered at least once, 38 individuals remained, which we merged with newly generated shotgun sequencing data from the Karelia individual9 (2.0-fold coverage), and published data from ancient2–4,7,13–19 and present-day humans20. The final dataset includes 51 ancient modern humans, of which 16 had at least 790,000 SNPs covered (Figure 1; Table 1; Extended Data Table 1).
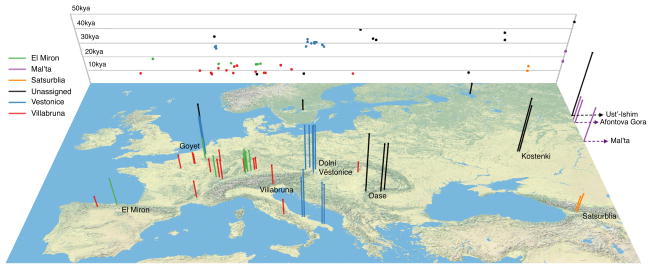
Figure 1. Location and age of 51 ancient samples.
Each bar corresponds to a sample, the color code designates the genetically defined sample cluster, and the height is proportional to sample age (the background grid shows a projection of longitude against sample age). To help in visualization, we add jitter for sites with multiple samples from nearby locations. Four samples that are from Siberia are plotted at the far eastern edge of the map.
Natural selection has reduced Neanderthal ancestry over the last 45,000 years
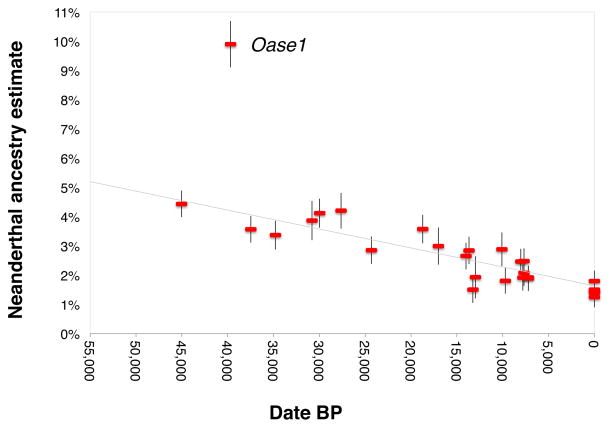
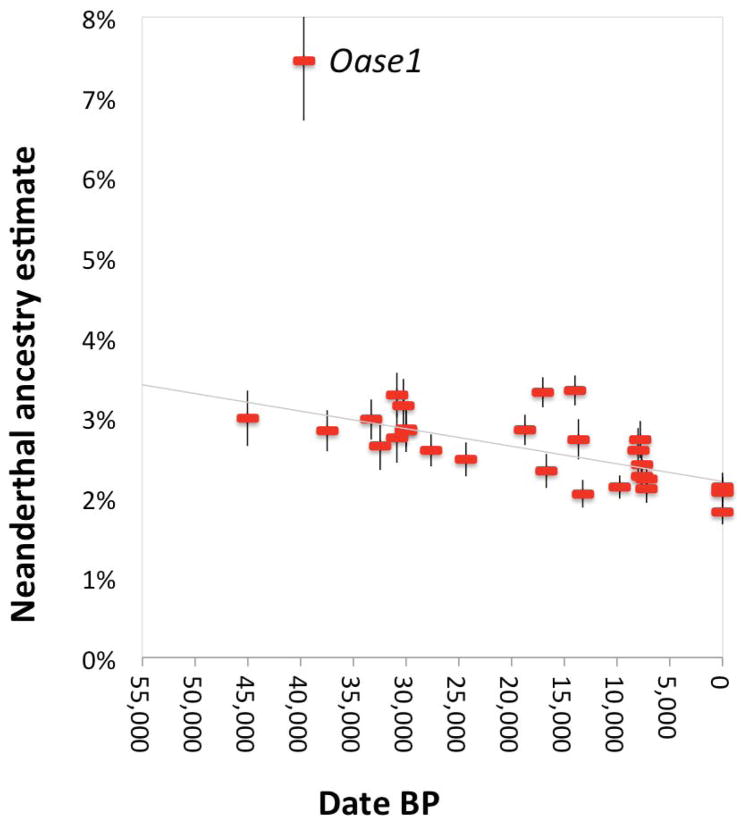
We used two previously published statistics3,7,21 to ask if the proportion of Neanderthal ancestry in Eurasians changed over the last 45,000 years. Whereas on the order of 2% of present-day Eurasian DNA is of Neanderthal origin (Extended Data Table 2), the ancient modern human genomes carry significantly more Neanderthal DNA (Figure 2) (P≪10−12). Using one statistic, we estimate a decline from 4.3–5.7% from a time shortly after introgression to 1.1–2.2% in Eurasians today (Figure 2). Using the other statistic, we estimate a decline from 3.2–4.2% to 1.8–2.3% (Extended Data Figure 1, Extended Data Table 3). Because all the European samples we analyzed dating to between 37,000 and 14,000 years ago are consistent with descent from a single founding population, admixture with populations with lower Neanderthal ancestry cannot explain the steady decrease in Neanderthal-derived DNA that we detect during this period, showing that natural selection against Neanderthal DNA must have driven this phenomenon (Figure 2). We also obtain an independent line of evidence for selection from our observation that the decrease in Neanderthal-derived alleles is more marked near genes than in less constrained regions of the genome (P=0.010) (Supplementary Information section 3; Extended Data Table 3)22–25.
Figure 2. Decrease of Neanderthal ancestry over time.
Plot of radiocarbon date against Neanderthal ancestry for samples with at least >200,000 SNPs covered, along with present-day Eurasians (standard errors are from a Block Jackknife). The least squares fit (gray) excludes the data from Oase1 (an outlier with recent Neanderthal ancestry) and three present-day European populations (known to have less Neanderthal ancestry than East Asians). The slope is significantly negative for all eleven subsets of samples we analyzed (10−29<P<10−11 based on a Block Jackknife) (Extended Data Table 3).
Y chromosomes, mitochondrial DNA and phenotypically important mutations
We used the proportion of sequences mapping to the Y chromosome to infer sex (Extended Data Table 4; Supplementary Information section 4), and determined Y chromosome haplogroups for the males. We were surprised to find haplogroup R1b in the ~14,000-year-old Villabruna individual from Italy. While the predominance of R1b in western Europe today is owes its origin to Bronze Age migrations from the eastern European steppe9, its presence in Villabruna and in a ~7,000-year-old farmer from Iberia9 document a deeper history of this haplotype in more western parts of Europe. Additional evidence of an early link between west and east comes from the HERC2 locus, where a derived allele that is the primary driver of light eye color in Europeans appears nearly simultaneously in specimens from Italy and the Caucasus ~14,000-13,000 years ago. Extended Data Table 5 presents results for additional alleles of known phenotypic importance. When analyzing the mitochondrial genomes we note the presence of haplogroup M in a ~27,000-year-old individual from southern Italy (Ostuni1) in agreement with the observation that this haplogroup, which today occurs in Asia and is absent in Europe, was present in pre-Last Glacial Maximum Europe and became lost during the Ice Age26. We also find that the ~33,000 year old Muierii2 from Romania carries a basal version of haplogroup U6, in agreement with the hypothesis that the presence of derived versions of this haplogroup in North Africans today is due to back-migration from western Eurasia27.
Genetic clustering of the ancient specimens
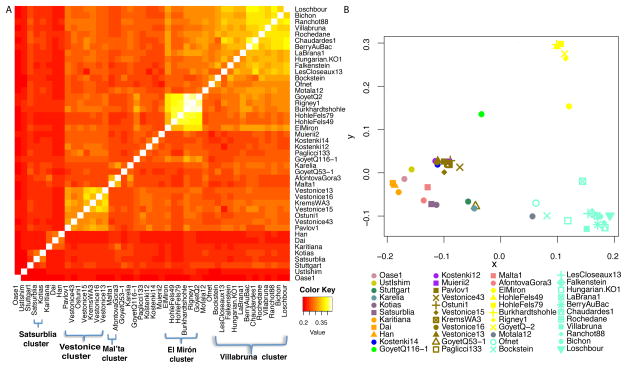
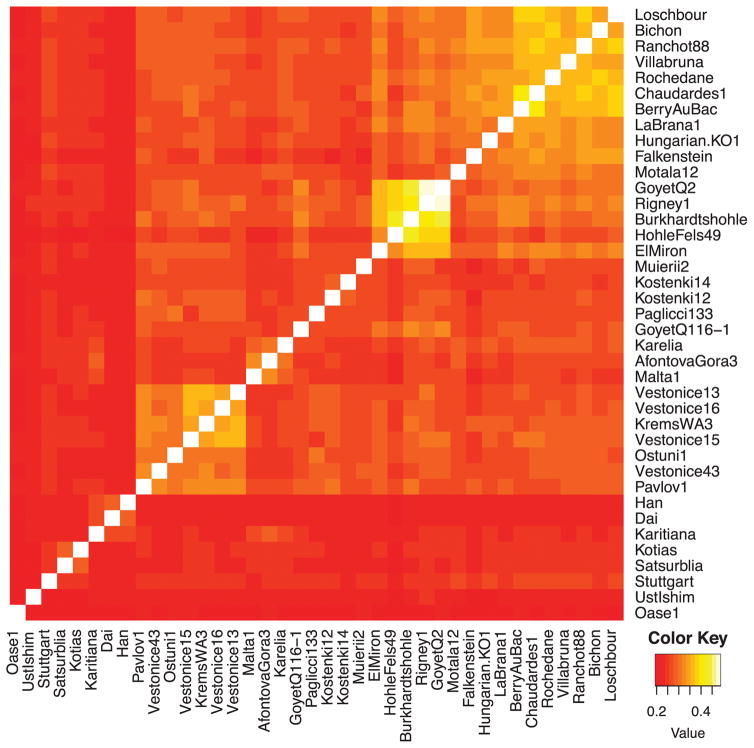
This dataset provides an unprecedented opportunity to study the population history of Upper Paleolithic Europe over more than 30,000 years. In order to not prejudice any association between genetic and archaeological groupings among the individuals studied, we first allowed the genetic data alone to drive the groupings of the specimens and only afterward examined their associations with archaeological cultural complexes. We began by computing f3-statistics14 of the form f3(X, Y; Mbuti), which measure shared genetic drift between a pair of ancient individuals after divergence from an outgroup (here Mbuti from sub-Saharan Africa), which allowed us to observe clear clusters of samples (Figure 3A; Extended Data Figure 2). Through Multi-Dimensional Scaling (MDS) analysis of this matrix (Figure 3B), as well as through D-statistic analyses28 (Supplementary Information section 5), we identified five clusters of individuals with substantial shared genetic drift, which we name after the oldest individual with >1.0-fold coverage in each cluster (Supplementary Information section 5; Table 1; Extended Data Table 1). In contrast, we were not able to identify clear structure among these samples based on model-based clustering29,30, which may reflect the fact that many of the samples are so ancient that present-day patterns of human variation are not very relevant to understanding their patterns of genetic differentiation4,13. The “Vestonice Cluster” is composed of 14 pre-Ice Age individuals from 34,000-26,000 years ago, who are all associated with the archaeologically defined Gravettian culture. The “Mal’ta Cluster” is composed of three individuals from the Glacial Maximum 24,000-17,000 years ago from the Lake Baikal region of Siberia. The “El Mirón Cluster” is composed of 6 Late Glacial individuals from 19,000-14,000 years ago, who are all associated with the Magdalenian culture. The “Villabruna Cluster” is composed of 13 post-Ice Age individuals from 14,000-7,000 years ago, associated with the Azilian, Epipaleolithic and Mesolithic cultures. The “Satsurblia Cluster” is composed of two individuals from 13,000-10,000 years ago from the northern Caucasus2. There were ten samples that we did not assign to any cluster, either because of evidence of representing distinct early lineages, (Ust’-Ishim, Oase1, Kostenki14, GoyetQ116-1, Muierii2, Cioclovina1, Kostenki12), or because they were admixed between major clusters (Karelia or Motala12), or of very different ancestry (Stuttgart). To classify the ancestry of additional low coverage samples, we built an admixture graph that fits the allele frequency correlation patterns among high coverage samples28 (Supplementary Information section 6; Figure 4a). We fit each low coverage sample into the graph in turn, including all fragments from every individual rather than just ones with evidence of cytosine deamination, accounting for contamination bias by modeling (Supplementary Information section 7).
Figure 3. Genetic clustering.
(A) Shared genetic drift measured by f3(X,Y; Mbuti) among samples with at least 30,000 SNPs covered (for AfontovaGora3, ElMiron, Falkenstein, GoyetQ-2, GoyetQ53-1, HohleFels49, HohleFels79, LesCloseaux13, Ofnet, Ranchot88 and Rigney1, we use all sequences for higher resolution). Lighter colors indicate more shared drift. (B) Multidimensional Dimensional Scaling (MDS) analysis, computed using the R software cmdscale package, highlights the main genetic groupings analyzed in this study: Vestonice Cluster (brown), Mal’ta Cluster (pink), El Mirón Cluster (yellow), Villabruna Cluster (light blue), and Satsurblia Cluster (dark purple). The affinity of GoyetQ116-1 (green) to the El Mirón Cluster is evident in both views of the data.
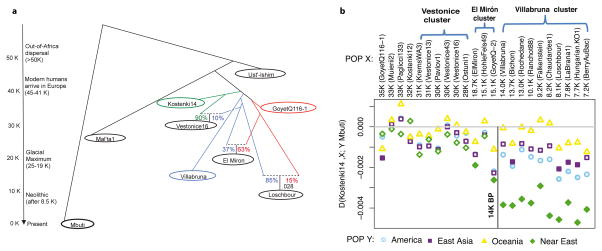
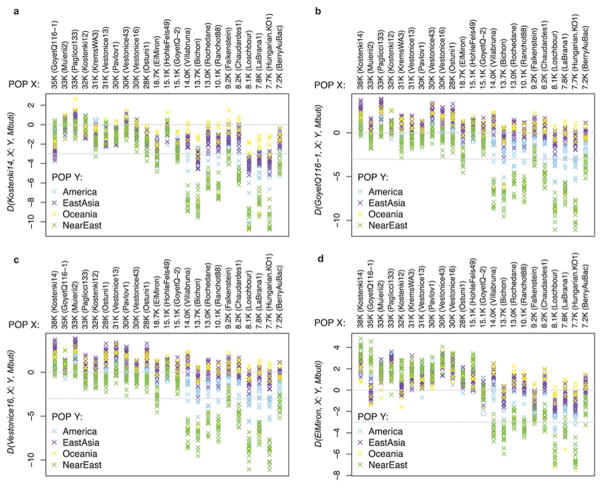
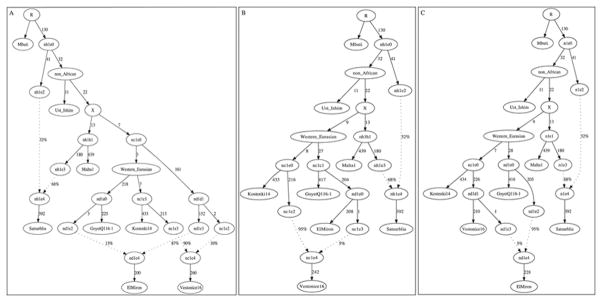
Figure 4. Population history inferences.
(A) Admixture Graph relating selected high coverage samples. Dashed lines show inferred admixture events; the estimated mixture proportions fitted using the ADMIXTUREGRAPH software are labeled28 (the estimated genetic drift on each branch is given in a version of this graph shown in Supplementary Information section 6). The samples are positioned vertically based on their radiocarbon date, but we caution that the population split times are not accurately known. We use color to highlight important early branches of the European founder population: the Kostenki14 lineage is modeled as the predominant contributor to the Vestonice Cluster (green); the GoyetQ116-1 lineage as the predominant contributor to the El Mirón Cluster (red); and the Villabruna lineage as broadly represented across many clusters. (B) Drawing together of European and Near Eastern populations ~14,000 years ago. Plot of affinity of each pre-Neolithic European population X to non-Africans outside Europe Y moving forward in time, comparing to Kostenki14 as a baseline; values Z<-3 standard errors below zero are indicated with filled symbols (we restricted to individuals with >50,000 SNPs). We observe an affinity to Near Easterners beginning with the Villabruna Cluster, and another to East Asians that affects a subset of the Villabruna Cluster.
A single founding population during most of the Upper Paleolithic period in Europe
Prior to this work, the most ambitious genetic analysis of early modern humans in Europe was based on the ~37,000-year-old Kostenki144. That analysis suggested that the population to which Kostenki14 belonged harbored within it the three major lineages that exist in mixed form in Europe today15: (1) a lineage related to all later pre-Neolithic Europeans, (2) a “Basal Eurasian” lineage that split from the ancestors of Europeans and East Asians before they separated from each other; and (3) a lineage related to the ~24,000-year-old Mal’ta1 from Siberia. With our more extensive sampling of Ice Age Europe, we find no support for this model. When we test whether the ~45,000-year-old Ust’-Ishim – an early Eurasian without any evidence of Basal Eurasian ancestry – shares more alleles with one test individual or another by computing statistics of the form D(Test1, Test2; Ust’-Ishim, Mbuti), we find that the statistic is consistent with zero when the Test populations are any pre-Neolithic Europeans or present-day East Asians3,13,31. This would not be expected if some of the pre-Neolithic Europeans, including Kostenki14, had Basal Eurasian ancestry (Supplementary Information section 8). We also find no evidence for the suggestion that the Mal’ta1 lineage contributed to Upper Paleolithic Europeans4, because when we compute the statistic D(Test1, Test2; Mal’ta1, Mbuti), we find that the statistic is consistent with zero when the Test populations are any pre-Neolithic Europeans beginning with Kostenki14, implying descent from a single founder population since separation from the lineage leading to Mal’ta1 (Supplementary Information section 9). A corollary of this finding is that the widespread presence of Mal’ta1-related ancestry in present-day Europeans15 is due to migrations from the Eurasian steppe in the Neolithic and Bronze Age periods9; it is not due to population structure within pre-Neolithic Europe as proposed in the initial analysis of the Kostenki14 genome4.
Resurgence of an early branching European lineage during the Last Glacial Maximum
Among the newly reported individuals, GoyetQ116-1 from present-day Belgium is the oldest at ~35,000 years ago. It is similar to the ~37,000 year old Kostenki14 and all later samples in that it shares more alleles with present-day Europeans (e.g. French) than with East Asians (e.g. Han). In contrast, Ust’-Ishim and Oase1, which predate GoyetQ116-1 and Kostenki14, do not show any distinctive affinity to later Europeans (Extended Data Table 6). Thus, from at least about 37,000 years ago, populations in Europe shared at least some ancestry with present Europeans. However, GoyetQ116-1 differs from Kostenki14 and from all individuals of the succeeding Vestonice Cluster in that both f3-statistics (Figure 3; Extended Data Figure 2) and D-statistics show that it shares more alleles with members of the El Mirón Cluster who lived 19,000-14,000 years ago than with other pre-Neolithic Europeans (Supplementary Information section 10). Thus, GoyetQ116-1 has affinity to individuals who lived more than fifteen thousand years later. While at least half of the ancestry of all El Mirón Cluster individuals comes from the GoyetQ116-1 cluster, this proportion varies, with the largest amount in individuals outside Iberia (Z=−4.8) (Supplementary Information section 10).
A drawing together of the ancestry of Europe and the Near East after ~14,000 years ago
Beginning around 14,000 years ago with the Villabruna Cluster, the strong affinity to GoyetQ116-1 seen in El Mirón Cluster individuals who belong the Late Glacial Magdalenian Culture is greatly attenuated (Supplementary Information section 10). To test if this change might reflect gene flow from populations that did not descend from the >37,000 year old European founder population, we computed statistics of the form D(Early European, Later European; Y, Mbuti) where Y are various present-day non-Africans. If no gene flow from exogenous populations occurred, this statistic is expected to be zero. Figure 4b shows that it is consistent with zero (|Z|<3) for nearly all individuals dating to between about 37,000 and 14,000 years ago. However, beginning with the Villabruna Cluster, it becomes highly significantly negative in comparisons where the non-European population (Y) is Near Easterners (Figure 4b; Extended Data Figure 3; Supplementary Information section 11). This must reflect gene flow into the Villabruna Cluster from a population related to present-day Near Easterners rather than gene flow in the reverse direction, because we do not see similar patterns in earlier Europeans although they share substantial amounts of their ancestry with the Villabruna Cluster (Figure 4b). The “Satsurblia Cluster” individuals from the Caucasus dating to ~13,000-10,000 years ago2 share more alleles with the Villabruna Cluster individuals than they do with earlier Europeans, indicating that they are related to the population that contributed new alleles to people in the Villabruna Cluster, although they cannot be the direct source of the gene flow, among other reasons because they have large amounts of Basal Eurasian ancestry while Villabruna Cluster individuals do not2 (Supplementary Information section 12; Extended Data Figure 4). One possible explanation for the sudden drawing together of the ancestry of Europe and the Near East at this time is long-distance migrations from the Near East into Europe. However, a plausible alternative is population structure, whereby Upper Paleolithic Europe harbored multiple groups that differed in their relationship to the Near East, with the balance shifting among groups as a result of demographic changes after the Ice Age.
The Villabruna Cluster includes the largest group of samples in this study. This allows us to study heterogeneity within this cluster (Supplementary Information section 13). First, we detect differences in the degree of allele sharing with members of the El Mirón Cluster, as revealed by significant statistics of the form D(Test1, Test2; El Mirón Cluster, Mbuti). Second, we detect an excess of allele sharing with East Asians in a subset of Villabruna Cluster individuals - beginning with a ~13,000 year old sample from Switzerland - as revealed by significant statistics of the form D(Test1, Test2; Han, Mbuti) (Figure 4b and Extended Data Figure 3). For example, Han Chinese share more alleles with two Villabruna Cluster individuals (Loschbour and LaBrana1) than they do with Kostenki14, as reflected in significantly negative statistics of the form D(Kostenki14, Loschbour/LaBrana1; Han, Mbuti)4. This statistic was originally interpreted as evidence of Basal Eurasian ancestry in Kostenki14. However, because this statistic is consistent with zero when Han is replaced with Ust’-Ishim, these findings cannot be driven by Basal Eurasian ancestry (as we also discuss above), and must instead be driven by gene flow between populations related to East Asians and the ancestors of some Europeans (Supplementary Information section 8).
Conclusions
We have shown that the population history of pre-Neolithic Europe was complex in several respects. First, at least some of the initial modern humans to appear in Europe, exemplified by Ust’-Ishim and Oase1, failed to contribute appreciably to the current European gene pool. Only from around 37,000 years ago do all the European individuals analyzed share ancestry with present-day Europeans3. Second, from the time of Kostenki14 about 37,000 years ago until the time of the Villabruna Cluster about 14,000 years ago, all individuals seem to derive from a single ancestral population with no evidence of substantial genetic influx from elsewhere. It is interesting that during this time, the Mal’ta Cluster is not represented in any of the individuals we sampled from Europe. Thus, while individuals assigned to the Gravettian cultural complex in Europe are associated with the Vestonice Cluster, there is no genetic connection between them and the Mal’ta1 individual in Siberia despite the fact that Venus figurines are associated with both. This suggests that if this similarity is not a coincidence32, it reflects diffusion of ideas rather than movements of people. Third, we find that GoyetQ116-1 derives from a different deep branch of the European founder population than the Vestonice Cluster which became predominant in many places in Europe between 34,000 and 26,000 years ago including at Goyet Cave. GoyetQ116-1 is chronologically associated with the Aurignacian cultural complex. Thus, the subsequent spread of the Vestonice Cluster, which is associated with the Gravettian cultural complex, shows that the spread of the latter culture was mediated at least in part by population movements. Fourth, the population represented by GoyetQ116-1 did not disappear, as its descendants became widespread again after ~19,000 years ago in the El Mirón Cluster when we detect them in Iberia. The El Mirón Cluster is associated with the Magdalenian culture and may represent a post-ice age expansion from southwestern European refugia33. Fifth, beginning with the Villabruna Cluster at least ~14,000 years ago, all European individuals analyzed show an affinity to the Near East. This correlates in time to the Bølling-Allerød interstadial, the first significant warming period after the Ice Age34. Archaeologically, it correlates with cultural transitions within the Epigravettian in Southern Europe35 and the Magdalenian-to-Azilian transition in Western Europe36. Thus, the appearance of the Villabruna Cluster may reflect migrations or population shifts within Europe at the end of the Ice Age, an observation that is also consistent with the evidence of turnover of mitochondrial DNA sequences at this time26,37. One scenario that could explain these patterns is a population expansion from southeastern European or west Asian refugia after the Ice Age, drawing together the genetic ancestry of Europe and the Near East. Sixth, within the Villabruna Cluster, some, but not all, individuals have affinity to East Asians. An important direction for future work is to generate similar ancient DNA data from southeastern Europe and the Near East to arrive at a more complete picture of the Upper Paleolithic population history of western Eurasia38.
Extended Data
Extended Data Figure 1. A decrease in Neanderthal ancestry in the last 45,000 years.
This is similar to Figure 2, except we use ancestry estimates from rates of alleles matching to Neanderthal rather than f4-ratios, as described in Supplementary Information section 3). The least squares fit excludes Oase1 (as an outlier with recent Neanderthal ancestry) and Europeans (known to have reduce Neanderthal ancestry). The regression slope is significantly negative (P=0.00004, Extended Data Table 3).
Extended Data Fig. 2. Heat matrix of pairwise f3(X, Y; Mbuti) for selected ancient samples.
We analyze only samples with at least 30,000 SNPs covered at least once, which pass our quality control.
Extended Data Fig. 3. Studying how the relatedness of non-European populations to pairs of European hunter-gatherers changes over time.
We examine statistics of the form D(W, X; Y, Mbuti), with the Z-score given on the y-axis, where W is an early European hunter-gatherer, X is another European hunter-gatherer (in chronological order on the x-axis), and Y is a non-European population (see legend). A: W=Kostenki14. B: W=GoyetQ116-1. C: W=Vestonice16. D: W=ElMiron. |Z|>3 scores are considered statistically significant (horizontal line). The similar Figure 4b gives absolute D-statistic values rather than Z-scores (for W=Kostenki14) and uses pooled regions rather than individual populations Y.
Extended Data Figure 4. An Admixture Graph model that fits the data for Satsurblia, an Upper Paleolithic sample from the Caucasus.
This model uses 127,057 SNPs covered in all populations. Estimated genetic drifts are give along the solid lines in units of f2-distance (parts per thousand), and estimated mixture proportions are given along the dotted lines. All three models provide an fit to the allele frequency correlation data among Mbuti, UstIshim, Kostenki14, Vestonice16, Malta1, ElMiron and Satsurblia to within the limits of our resolution, in the sense that all empirical f2-, f3- and f4-statistics relating the samples are within three standard errors of the expectation of the model. Models in which Satsurblia is modeled as unadmixed cannot be fit.
Extended Data Table 1.
The 51 ancient modern humans analyzed in this study
| Sample Code | Data source | Country | Lat. | Long. | Cal BP 95.4% | Date type (ref.) | Culture | Remain | SNP Panel | Sex | mtDNA haplogroup | Y chrom. haplogroup | Genetic Cluster | Damage restrict | Mean coverage+ | SNPs covered |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UstIshim | 1 | Russia | 57.43 | 71.10 | 47,480-42,560 | Direct-UF (1) | Unassigned | Femur | Shotgun | M | R | K (xLT) | Unassigned | No | 42 | 2,137,615 |
| Oase1 | 2 | Romania | 45.12 | 21.90 | 41,640-37,580 | Direct-UF (3) | Unassigned | Mandible | Shotgun | M | N | F | Unassigned | Yes | 0.156 | 285,076 |
| Kostenki14* | New | Russia | 51.23 | 39.30 | 38,680-36,260 | Direct-UF (4) | Unassigned | Tibia | 3.7M | M | U2 | C1b | Unassigned | No | 16.1 | 1,774,156 |
| GoyetQ116-1 | New | Belgium | 50.26 | 4.28 | 35,160-34,430 | Direct-NotUF (5) | Aurignacian | Humerus | 1240k | M | M | C1a | Unassigned | No | 1.046 | 846,983 |
| Muierii2 | New | Romania | 45.11 | 23.46 | 33,760-32,840 | Direct-UF (6) | Unassigned | Temporal | 3.7M | F | U6 | Unassigned | Yes | 0.049 | 98,618 | |
| Paglicci133 | New | Italy | 41.65 | 15.61 | 34,580-31,210 | Layer (7) | Gravettian | Tooth | 1240k | M | U8c | I | Vestonice | No | 0.041 | 82,330 |
| Cioclovina1 | New | Romania | 45.35 | 23.84 | 33,090-31,780 | Direct-UF (8) | Unassigned | Cranium | 1240k | M | U | CT | Unassigned | Yes | 0.006 | 12,784 |
| Kostenki12 | New | Russia | 51.23 | 39.30 | 32,990-31,840 | Layer (9) | Unassigned | Cranium | 3.7M | M | U2 | CT | Unassigned | No | 0.03 | 61,228 |
| KremsWA3 | New | Austria | 48.41 | 15.59 | 31,250-30,690 | Layer (10) | Gravettian | Cranium | 1240K | M | U5 | Vestonice | No | 0.11 | 203,986 | |
| Vestonice13 | New | Czech | 48.53 | 16.39 | 31,070-30,670 | Layer (9) | Gravettian | Femur | 3.7M | M | U8c | CT(notIJK) | Vestonice | Yes | 0.071 | 139,568 |
| Vestonice15 | New | Czech | 48.53 | 16.39 | 31,070-30,670 | Layer (9) | Gravettian | Femur | 3.7M | M | U5 | BT | Vestonice | Yes | 0.015 | 30,900 |
| Vestonice14 | New | Czech | 48.53 | 16.39 | 31,070-30,670 | Layer (9) | Gravettian | Femur | 390k | M | U | Vestonice | Yes | 0.003 | 5,677 | |
| Pavlov1 | New | Czech | 48.53 | 16.39 | 31,110-29,410 | Layer (9) | Gravettian | Femur | 3.7M | M | U5 | C1a2 | Vestonice | Yes | 0.028 | 57,005 |
| Vestonice43 | New | Czech | 48.53 | 16.39 | 30,710-29,310 | Layer (9) | Gravettian | Femur | 3.7M | M | U | F | Vestonice | Yes | 0.087 | 163,946 |
| Vestonice16 | New | Czech | 48.53 | 16.39 | 30,710-29,310 | Layer (9) | Gravettian | Femur | 3.7M | M | U5 | IJK | Vestonice | No | 1.31 | 945,292 |
| Ostuni2 | New | Italy | 40.73 | 17.57 | 29,310-28,640 | Direct-UF (New) | Gravettian | Femur | 3.7M | F | U2 | Vestonice | Yes | 0.008 | 17,017 | |
| GoyetQ53-1 | New | Belgium | 50.26 | 4.28 | 28,230-27,720 | Direct-NotUF (5) | Gravettian | Fibula | 1240k | F | U2 | Vestonice | Yes | 0.006 | 12,567 | |
| Paglicci108 | New | Italy | 41.65 | 15.61 | 28,430-27,070 | Layer (5) | Gravettian | Phalanx | 1240k | F | U2′3′4′7′8′9 | Vestonice | Yes | 0.002 | 4,330 | |
| Ostuni1 | New | Italy | 40.73 | 17.57 | 27,810-27,430 | Direct-UF (New) | Gravettian | Tibia | 3.7M | F | M | Vestonice | Yes | 0.245 | 369,313 | |
| GoyetQ376-19 | New | Belgium | 50.26 | 4.28 | 27,720-27,310 | Direct-NotUF (5) | Gravettian | Humerus | 1240k | F | U2 | Vestonice | Yes | 0.012 | 25,400 | |
| GoyetQ56-16 | New | Belgium | 50.26 | 4.28 | 26,600-26,040 | Direct-NotUF (5) | Gravettian | Fibula | 1240k | F | U2 | Vestonice | Yes | 0.005 | 9,988 | |
| Malta1 | 11 | Russia | 52.9 | 103.5 | 24,520-24,090 | Direct-UF (11) | Unassigned | Humerus | Shotgun | M | U | R | Mal’ta | No | 1.174 | 1439501 |
| ElMiron | New | Spain | 43.26 | −3.45 | 18,830-18,610 | Direct-UF (5) | Magdalenian | Toe | 3.7M | F | U5b | El Mirón | Yes | 1.012 | 797,714 | |
| AfontovaGora3 | New | Russia | 56.05 | 92.87 | 16,930-16,490 | Layer (5) | Unassigned | Tooth | 3.7M | F | R1b | Mal’ta | Yes | 0.17 | 286,355 | |
| AfontovaGora2 | 11 | Russia | 56.05 | 92.87 | 16,930-16,490 | Direct-UF (11) | Unassigned | Humerus | Shotgun | M | Mal’ta | No | 0.071 | 143,751 | ||
| Rigney1 | New | France | 47.23 | 6.10 | 15,690-15,240 | Direct-NotUF (12) | Magdalenian | Mandible | 1240k | F | U2′3′4′7′8′9 | El Mirón | Yes | 0.017 | 35,600 | |
| HohleFels49 | New | Germany | 48.22 | 9.45 | 16,000-14,260 | Layer (13) | Magdalenian | Femur | 390k | M | U8a | I | El Mirón | Yes | 0.033 | 63,151 |
| GoyetQ-2 | New | Belgium | 50.26 | 4.28 | 15,230-14,780 | Direct-NotUF (5) | Magdalenian | Humerus | 1240k | M | U8a | HIJK | El Mirón | Yes | 0.035 | 72,263 |
| Brillenhohle | New | Germany | 48.24 | 9.46 | 15,120-14,440 | Direct-UF (14) | Magdalenian | Cranium | 390k | M | U8a | El Mirón | Yes | 0.006 | 13,459 | |
| HohleFels79 | New | Germany | 48.22 | 9.45 | 15,070-14,270 | Direct-UF (5) | Magdalenian | Cranium | 390k | M | U8a | El Mirón | Yes | 0.005 | 11,211 | |
| Burkhardtshohle | New | Germany | 48.32 | 9.35 | 15,080-14,150 | Direct-UF (15) | Magdalenian | Cranium | 1240k | M | U8a | I | El Mirón | Yes | 0.018 | 38,376 |
| Villabruna | New | Italy | 46.15 | 12.21 | 14,180-13,780 | Direct-UF (16) | Epigravettian | Femur | 3.7M | M | U5b2b | R1b1 | Villabruna | No | 3.137 | 1,215,433 |
| Bichon | 17 | Switzerland | 47.01 | 6.79 | 13,770-13,560 | Direct-UF (17) | Azilian | Petrous | Shotgun | M | U5b1h | I2 | Villabruna | No | 8.119 | 2,116,782 |
| Satsurblia | 17 | Georgia | 42.24 | 42.92 | 13,380-13,130 | Direct-UF (17) | Epigravettian | Petrous | Shotgun | M | K3 | J2 | Satsurblia | No | 1.195 | 1,460,368 |
| Rochedane | New | France | 47.21 | 6.45 | 13,090-12,830 | Direct-NotUF (5) | Epipaleolithic | Mandible | 1240k | M | U5b2b | I | Villabruna | No | 0.131 | 237,390 |
| Iboussieres39 | New | France | 44.29 | 4.46 | 12,040-11,410 | Direct-NotUF (5) | Epipaleolithic | Femur | 390k | M | U5b2b | Villabruna | Yes | 0.005 | 9,659 | |
| Continenza | New | Italy | 41.96 | 13.54 | 11,200-10,510 | Layer (New) | Mesolithic | Cranium | 3.7M | F | U5b1 | Villabruna | Yes | 0.006 | 11,717 | |
| Ranchot88 | New | France | 47.91 | 5.43 | 10,240-9,930 | Direct-NotUF (5) | Mesolithic | Cranium | 1240k | F | U5b1 | Villabruna | Yes | 0.322 | 414,863 | |
| LesCloseaux13 | New | France | 48.52 | 2.11 | 10,240-9,560 | Direct-NotUF (18) | Mesolithic | Femur | 1240k | F | U5a2 | Villabruna | Yes | 0.004 | 8,635 | |
| Kotias | 17 | Georgia | 42.13 | 43.12 | 9,890-9,550 | Direct-UF (17) | Mesolithic | Tooth | Shotgun | M | H13c | J | Satsurblia | No | 12.157 | 2,133,968 |
| Falkenstein | New | Germany | 48.06 | 9.04 | 9,410-8,990 | Direct-UF (19) | Mesolithic | Fibula | 390k | M | U5a2c | F | Villabruna | Yes | 0.033 | 64,428 |
| Karelia | 20 | Russia | 61.65 | 35.65 | 8,800-7,950 | Layer (21) | Mesolithic | Tooth | Shotgun | M | C1g | R1a1 | Unassigned | No | 1.952 | 1,754,410 |
| Bockstein | New | Germany | 48.33 | 10.09 | 8,370-8,160 | Layer (22) | Mesolithic | Tooth | 390k | F | U5b1d1 | Villabruna | Yes | 0.011 | 21,977 | |
| Ofnet | New | Germany | 48.49 | 10.27 | 8,430-8,060 | Layer (23) | Mesolithic | Tooth | 390k | F | U5b1d1 | Villabruna | Yes | 0.003 | 6,263 | |
| Chaudardes1 | New | France | 49.24 | 3.46 | 8,360-8,050 | Direct-NotUF (5) | Mesolithic | Tibia | 1240k | M | U5b1b | I | Villabruna | Yes | 0.046 | 92,657 |
| Loschbour | 24 | Luxembourg | 49.70 | 6.24 | 8,160-7,940 | Direct-UF (24) | Mesolithic | Tooth | Shotgun | M | U5b1a | I2a1b | Villabruna | No | 20 | 2,091,584 |
| LaBrana1 | 25 | Spain | 42.93 | −5.35 | 7,940-7,690 | Direct-UF (26) | Mesolithic | Tooth | Shotgun | M | U5b2c1 | C1a2 | Villabruna | No | 3.338 | 1,884,745 |
| Hungarian.KO1 | 27 | Hungarian | 47.93 | 21.20 | 7,730-7,590 | Direct-UF (27) | Neolithic | Petrous | Shotgun | M | R3 | I2a | Villabruna | No | 1.1 | 1,410,303 |
| Motala12 | 24 | Sweden | 58.54 | 15.05 | 7,670-7,580 | Direct-UF (New) | Mesolithic | Tooth | Shotgun | M | U2e1 | I2a1b* | Unassigned | No | 2.185 | 1,874,519 |
| BerryAuBac | New | France | 49.24 | 3.54 | 7,320-7,170 | Direct-NotUF (5) | Mesolithic | Radius | 1240k | M | U5b1a | I | Villabruna | No | 0.027 | 54,690 |
| Stuttgart | 24 | Germany | 48.78 | 9.18 | 7,260-7,020 | Direct-UF (New) | Early Neolithic | Tooth | Shotgun | F | T2c1d1 | Unassigned | No | 19 | 2,078,724 |
Note: All dates are obtained as described in Supplementary Information section 1. When an individual has a direct date from an element from the same skeleton it is marked “Direct”, followed by a hyphen to indicate whether the date is obtained by ultrafiltration (“UF”) or without (“NotUF”). If the date is from the archaeological layers, we mark the date type as “Layer”. All the dates were calibrated using IntCal1328 and the OxCal4.2 program29.
We represent Kostenki14 in most analyses by our newly reported 16.1x capture data, but repeat key analyses on the previously reported 2.8x shotgun data30.
Mean coverage is computed on the 3.7M SNP targets.
Extended Data Table 2.
Estimated proportion of Neanderthal ancestry
| f4-ratios | Archaic Ancestry Informative SNPs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample Code | Age BP | SNPs | Est. | 95% CI | SNPs | Est. | 95% CI | Increase in Neanderthal ancestry with B | S.E. |
| UstIshim | 45,020 | 2,137,615 | 4.4% | 3.6% – 5.3% | 778,774 | 3.0% | 2.3% – 3.7% | −0.9% | 1.3% |
| Oase1 | 39,610 | 285,076 | 9.9% | 8.4% – 11.4% | 59,854 | 7.5% | 6.0% – 8.9% | 2.5% | 1.8% |
| Kostenki14 | 37,470 | 1,774,156 | 3.6% | 2.7% – 4.4% | 632,748 | 2.8% | 2.3% – 3.3% | −1.0% | 1.0% |
| GoyetQ116-1 | 34,795 | 846,983 | 3.4% | 2.4% – 4.3% | |||||
| Muierii2 | 33,300 | 98,618 | 5.2% | 3.0% – 7.4% | 22,189 | 3.0% | 2.5% – 3.5% | 0.6% | 1.1% |
| Paglicci133 | 32,895 | 82,330 | 4.1% | 2.1% – 6.0% | |||||
| Cioclovina1 | 32,435 | 12,784 | 4.1% | −1.1% – 9.3% | |||||
| Kostenki12 | 32,415 | 61,228 | 1.9% | −0.7% – 4.4% | 13,385 | 2.6% | 2.1% – 3.2% | 1.7% | 1.5% |
| KremsWA3 | 30,970 | 203,986 | 3.9% | 2.6% – 5.2% | - | ||||
| Vestonice13 | 30,870 | 139,568 | 4.6% | 2.6% – 6.5% | 35,983 | 3.3% | 2.7% – 3.8% | 0.3% | 1.3% |
| Vestonice15 | 30,870 | 30,900 | 4.3% | 0.6% – 7.9% | 5,855 | 2.7% | 2.1% – 3.4% | −1.5% | 1.3% |
| Vestonice14 | 30,870 | 5,677 | 2.6% | −5.9% – 11.0% | |||||
| Pavlov1 | 30,260 | 57,005 | 4.4% | 1.6% – 7.1% | 9,327 | 3.1% | 2.5% – 3.8% | 0.7% | 1.2% |
| Vestonice43 | 30,010 | 163,946 | 6.9% | 5.2% – 8.5% | 38,749 | 2.9% | 2.4% – 3.3% | 0.9% | 0.9% |
| Vestonice16 | 30,010 | 945,292 | 4.1% | 3.1% – 5.1% | 268,157 | 2.8% | 2.3% – 3.3% | −0.1% | 1.0% |
| Ostuni2 | 28,975 | 17,017 | 1.6% | −3.2% – 6.3% | 2,746 | 2.3% | 1.4% – 3.1% | 1.3% | 1.6% |
| GoyetQ53-1 | 27,975 | 12,567 | 4.8% | −0.7% – 10.3% | |||||
| Paglicci108 | 27,750 | 4,330 | 3.4% | −6.0% – 12.7% | |||||
| Ostuni1 | 27,620 | 369,313 | 4.2% | 3.0% – 5.4% | 88,449 | 2.6% | 2.2% – 3.0% | 0.1% | 0.9% |
| GoyetQ376-19 | 27,515 | 25,400 | 6.5% | 2.7% – 10.2% | |||||
| GoyetQ56-16 | 26,320 | 9,988 | 3.6% | −1.9% – 9.1% | |||||
| Malta1 | 24,305 | 1,439,501 | 2.9% | 1.9% – 3.8% | 437,187 | 2.5% | 2.1% – 2.9% | 1.0% | 0.8% |
| ElMiron | 18,720 | 797,714 | 3.6% | 2.6% – 4.5% | 250,071 | 2.8% | 2.5% – 3.2% | 0.6% | 0.9% |
| AfontovaGora3 | 16,710 | 286,355 | 3.0% | 1.8% – 4.2% | 96,237 | 3.3% | 2.9% – 3.7% | −1.5% | 1.0% |
| AfontovaGora2 | 16,710 | 143,751 | 2.2% | 0.4% – 4.0% | 37,280 | 2.3% | 1.9% – 2.7% | −0.3% | 0.9% |
| Rigney1 | 15,465 | 35,600 | 0.8% | −2.6% – 4.2% | |||||
| HohleFels49 | 15,130 | 63,151 | 2.3% | −0.6% – 5.2% | |||||
| GoyetQ-2 | 15,005 | 72,263 | 1.7% | −0.6% – 4.0% | |||||
| Brillenhohle | 14780 | 13,459 | 2.5% | −3.0% – 8.1% | |||||
| HohleFels79 | 14,670 | 11,211 | 1.7% | −5.1% – 8.5% | |||||
| Burkhardtshohle | 14,615 | 38,376 | 1.7% | −1.6% – 5.0% | |||||
| Villabruna | 13,980 | 1,215,433 | 2.7% | 1.8% – 3.5% | 425,148 | 3.3% | 3.0% – 3.7% | 1.1% | 0.9% |
| Bichon | 13,665 | 2,116,782 | 2.9% | 1.9% – 3.8% | 769,422 | 2.7% | 2.2% – 3.2% | 0.7% | 1.3% |
| Satsurblia | 13,255 | 1,460,368 | 1.5% | 0.6% – 2.4% | 542,561 | 2.0% | 1.7% – 2.4% | 0.9% | 0.6% |
| Rochedane | 12,960 | 237,390 | 1.9% | 0.5% – 3.3% | |||||
| Iboussieres39 | 11,725 | 9,659 | 6.4% | −0.8% – 13.7% | |||||
| Continenza | 10,855 | 11,717 | 4.1% | −1.4% – 9.6% | 1,733 | 2.9% | 1.8% – 4.0% | −10.6% | 4.4% |
| Ranchot88 | 10,085 | 414,863 | 2.9% | 1.8% – 4.0% | |||||
| LesCloseaux13 | 9,900 | 8,635 | −3.0% | −9.7% – 3.8% | |||||
| Kotias | 9,720 | 2,133,968 | 1.8% | 1.0% – 2.7% | 779,146 | 2.1% | 1.8% – 2.4% | 0.7% | 0.5% |
| Falkenstein | 9,200 | 64,428 | 4.8% | 1.7% – 7.8% | |||||
| Karelia | 8,375 | 1,754,410 | 1.9% | 1.1% – 2.7% | 582,444 | 2.2% | 1.9% – 2.6% | −0.2% | 0.7% |
| Bockstein | 8,265 | 21,977 | 5.7% | 1.0% – 10.5% | |||||
| Ofnet | 8,245 | 6,263 | 9.8% | 1.4% – 18.1% | |||||
| Chaudardes1 | 8,205 | 92,657 | 1.9% | −0.2% – 3.9% | |||||
| Loschbour | 8,050 | 2,091,584 | 2.5% | 1.6% – 3.3% | 774,139 | 2.6% | 2.0% – 3.1% | 2.7% | 1.7% |
| LaBrana1 | 7,815 | 1,884,745 | 1.9% | 1.1% – 2.8% | 642,231 | 2.7% | 2.3% – 3.2% | 0.4% | 0.8% |
| Hungarian.KO1 | 7,660 | 1,410,303 | 2.1% | 1.2% – 3.0% | 439,408 | 2.4% | 2.0% – 2.8% | −0.1% | 1.2% |
| Motala12 | 7,625 | 1,874,519 | 2.5% | 1.6% – 3.3% | 655,685 | 2.3% | 1.9% – 2.7% | −0.1% | 0.7% |
| BerryAuBac | 7,245 | 54,690 | 2.5% | −0.2% – 5.1% | |||||
| Stuttgart | 7,140 | 2,078,724 | 1.9% | 1.1% – 2.7% | 767,813 | 2.1% | 1.8% – 2.5% | 0.0% | 0.7% |
| Dai | 0 | 2,144,502 | 1.4% | 0.7% – 2.1% | 782,066 | 1.8% | 1.5% – 2.1% | 1.4% | 0.4% |
| Han | 0 | 2,144,502 | 1.8% | 1.1% – 2.5% | 782,164 | 2.1% | 1.8% – 2.5% | 1.9% | 0.7% |
| English | 0 | 2,144,502 | 1.5% | 0.8% – 2.2% | |||||
| French | 0 | 2,144,502 | 1.5% | 0.9% – 2.1% | 782,386 | 1.7% | 1.4% – 1.9% | 1.4% | 0.6% |
| Sardinian | 0 | 2,144,502 | 1.2% | 0.6% – 1.9% | 782,351 | 1.7% | 1.4% – 2.0% | 0.7% | 0.5% |
| Karitiana | 0 | 782,037 | 2.1% | 1.7% – 2.4% | 1.5% | 1.0% | |||
Extended Data Table 3.
Significant correlation of Neanderthal ancestry estimate with specimen age
| Subset of samples | N | P-value for date correlation | Decrease in ancestry per 10,000 years | Estimate of Neanderthal ancestry at different time points | |||
|---|---|---|---|---|---|---|---|
| 0 years ago (present) | 50,000 years ago | 55,000 years ago | 60,000 years ago | ||||
| f4-ratio estimates | |||||||
| Core Set 1 (all ancient samples (except Oase1) + Han + Dai) | 57 | 5 × 10−22 | 0.48–0.73% | 1.1–2.2% | 4.0–5.4% | 4.3–5.7% | 4.5–6.0% |
| Subset of Core Set 1 (<32kya) | 50 | 2 × 10−15 | 0.59–0.98% | 0.9–2.1% | 4.5–6.4% | 4.8–6.9% | 5.1–7.4% |
| Subset of Core Set 1 (>32kya or <25kya) | 44 | 4 × 10−18 | 0.44–0.69% | 1.0–2.2% | 3.7–5.2% | 4.0–5.5% | 4.2–5.8% |
| Subset of Core Set 1 (>25kya or <14kya) | 47 | 5 × 10−21 | 0.48–0.73% | 1.0–2.2% | 3.9–5.3% | 4.2–5.7% | 4.5–6.0% |
| Subset of Core Set 1 (>14kya or present day) | 37 | 2 × 10−18 | 0.47–0.74% | 1.1–2.4% | 4.1–5.5% | 4.3–5.8% | 4.6–6.2% |
| Subset of Core Set 1 (only ancient samples) | 50 | 4 × 10−15 | 0.46–0.76% | 1.0–2.3% | 4.0–5.4% | 4.3–5.8% | 4.5–6.1% |
| Subset of Core Set 1 (individuals with >200,000 SNPs) | 28 | 4 × 10−19 | 0.46–0.71% | 1.1–2.3% | 3.9–5.3% | 4.2–5.7% | 4.4–6.0% |
| Modification of Core Set 1 (replace East Asians with Europeans) | 58 | 2 × 10−23 | 0.49–0.73% | 1.1–2.3% | 4.0–5.4% | 4.3–5.8% | 4.6–6.1% |
| All ancient samples including Oase1 + Han + Dai | 58 | 8 × 10−29 | 0.57–0.81% | 1.0–2.2% | 4.3–5.7% | 4.7–6.1% | 5.0–6.5% |
| All ancient samples | 51 | 1 × 10−20 | 0.57–0.86% | 0.9–2.2% | 4.4–5.8% | 4.7–6.2% | 5.0–6.6% |
| All ancient samples except Oase1 or UstIshim | 49 | 8 × 10−12 | 0.45–0.81% | 1.0–2.3% | 4.0–5.6% | 4.2–6.0% | 4.5–6.4% |
| Ancestry informative SNPs | |||||||
| Core Set 2 (all ancient samples (except Oase1) + Han + Dai + Karitiana) | 29 | 4 × 10−11 | 0.21–0.39% | 1.8–2.3% | 3.1–4.0% | 3.2–4.2% | 3.3–4.3% |
| Subset of Core Set 2 (no Han, Dai, Karitiana, Stuttgart) | 25 | 1 × 10−4 | 0.11–0.36% | 1.8–2.5% | 2.9–3.8% | 3.0–4.0% | 3.0–4.1% |
| Subset of Core Set 2 (no Han, Dai, Karitiana, Stuttgart, UstIshim) | 24 | 2 × 10−4 | 0.11–0.37% | 1.8–2.5% | 2.9–3.8% | 2.9–4.0% | 3.0–4.2% |
Note: The “Core Set 1,” used for the f4-ratio analyses, refers to 50 ancient samples (removing Oase1 as an outlier) along with 7 East Asians (Dai and Han). “Core Set 2,” used for the analyses of Neanderthal ancestry informative SNPs, refers to 26 ancient samples (removing Oase1) along with Han, Dai, and Karitiana
Extended Data Table 4.
Sex determination for newly reported samples.Y-rate is the ratio of NY/Nauto divided by the same quantity for the genome-wide target set. Female sex (F) is inferred as Y-rate<0.05 and male sex (M) as Y-rate>0.
| Sample | Target | Type | Nauto | NX | NY | NX/Nauto | NY/Nauto | X-rate | Y-rate | Sex |
|---|---|---|---|---|---|---|---|---|---|---|
| 1240k or 2.2M* | 1151240 | 49711 | 32681 | 0.0432 | 0.0284 | |||||
| 390k | 388745 | 1819 | 2242 | 0.0047 | 0.0058 | |||||
|
| ||||||||||
| Kostenki14 | 2.2M | all | 29633405 | 395534 | 262846 | 0.0133 | 0.0089 | 0.309 | 0.312 | M |
| GoyetQ116-1 | 1240k | all | 2122620 | 36391 | 22256 | 0.0171 | 0.0105 | 0.397 | 0.369 | M |
| Cioclovina1 | 1240k | Damage | 11521 | 184 | 125 | 0.0160 | 0.0108 | 0.370 | 0.382 | M |
| Kostenki12 | 2.2M | Subset | 63908 | 856 | 504 | 0.0134 | 0.0079 | 0.310 | 0.278 | M |
| Muierii2 | 2.2M | Damage | 81165 | 2177 | 8 | 0.0268 | 0.0001 | 0.621 | 0.003 | F |
| Vestonice13 | 2.2M | Damage | 119094 | 1578 | 1059 | 0.0133 | 0.0089 | 0.307 | 0.313 | M |
| Vestonice15 | 2.2M | Damage | 28762 | 338 | 227 | 0.0118 | 0.0079 | 0.272 | 0.278 | M |
| Vestonice14 | 390k | Damage | 4846 | 8 | 11 | 0.0017 | 0.0023 | 0.353 | 0.394 | M |
| Vestonice43 | 2.2M | Damage | 136933 | 1826 | 1204 | 0.0133 | 0.0088 | 0.309 | 0.310 | M |
| Pavlov1 | 2.2M | Damage | 54429 | 631 | 404 | 0.0116 | 0.0074 | 0.268 | 0.261 | M |
| Vestonice16 | 2.2M | Subset | 2433741 | 30463 | 20976 | 0.0125 | 0.0086 | 0.290 | 0.304 | M |
| KremsWA3 | 1240k | all | 235069 | 4119 | 2661 | 0.0175 | 0.0113 | 0.406 | 0.399 | M |
| Ostuni2 | 2.2M | Damage | 15749 | 138 | 1 | 0.0088 | 0.0001 | 0.203 | 0.002 | F |
| Ostuni1 | 2.2M | Damage | 427199 | 10868 | 47 | 0.0254 | 0.0001 | 0.589 | 0.004 | F |
| Paglicci108 | 1240k | Damage | 3883 | 124 | 2 | 0.0319 | 0.0005 | 0.740 | 0.018 | F |
| GoyetQ53-1 | 1240k | Damage | 10771 | 311 | 4 | 0.0289 | 0.0004 | 0.669 | 0.013 | F |
| GoyetQ376-19 | 1240k | Damage | 20052 | 680 | 10 | 0.0339 | 0.0005 | 0.785 | 0.018 | F |
| GoyetQ56-16 | 1240k | Damage | 8702 | 304 | 7 | 0.0349 | 0.0008 | 0.809 | 0.028 | F |
| Paglicci133 | 1240k | Subset | 81092 | 1641 | 983 | 0.0202 | 0.0121 | 0.469 | 0.427 | M |
| ElMiron | 2.2M | Damage | 1765696 | 40647 | 196 | 0.0230 | 0.0001 | 0.533 | 0.004 | F |
| HohleFels79 | 390k | Damage | 10188 | 28 | 22 | 0.0027 | 0.0022 | 0.587 | 0.374 | M |
| AfontovaGora3 | 2.2M | Damage | 291798 | 8705 | 37 | 0.0298 | 0.0001 | 0.691 | 0.004 | F |
| HohleFels49 | 390k | Damage | 61051 | 113 | 111 | 0.0019 | 0.0018 | 0.396 | 0.315 | M |
| Rigney1 | 1240k | Damage | 32797 | 1131 | 9 | 0.0345 | 0.0003 | 0.799 | 0.010 | F |
| GoyetQ-2 | 1240k | Damage | 65563 | 1123 | 706 | 0.0171 | 0.0108 | 0.397 | 0.379 | M |
| Brillenhohle | 390k | Damage | 12603 | 22 | 22 | 0.0017 | 0.0017 | 0.373 | 0.303 | M |
| Burkhardtshohle | 1240k | Damage | 34207 | 563 | 407 | 0.0165 | 0.0119 | 0.381 | 0.419 | M |
| Villabruna | 2.2M | Subset | 5505838 | 72055 | 52110 | 0.0131 | 0.0095 | 0.303 | 0.333 | M |
| Rochedane | 1240k | Subset | 256325 | 4780 | 2830 | 0.0186 | 0.0110 | 0.432 | 0.389 | M |
| Continenza | 2.2M | Damage | 10647 | 208 | 2 | 0.0195 | 0.0002 | 0.452 | 0.007 | F |
| Iboussieres39 | 390k | Damage | 8246 | 12 | 22 | 0.0015 | 0.0027 | 0.311 | 0.463 | M |
| Ranchot88 | 1240k | Damage | 594962 | 18520 | 119 | 0.0311 | 0.0002 | 0.721 | 0.007 | F |
| LesCloseaux13 | 1240k | Damage | 7326 | 275 | 2 | 0.0375 | 0.0003 | 0.869 | 0.010 | F |
| Falkenstein | 390k | Damage | 58970 | 113 | 102 | 0.0019 | 0.0017 | 0.410 | 0.300 | M |
| Bockstein | 390k | Damage | 20214 | 62 | 0 | 0.0031 | 0.0000 | 0.655 | 0.000 | F |
| Ofnet | 390k | Damage | 5294 | 13 | 1 | 0.0025 | 0.0002 | 0.525 | 0.033 | F |
| Chaudardes1 | 1240k | Damage | 84052 | 1429 | 865 | 0.0170 | 0.0103 | 0.394 | 0.363 | M |
| BerryAuBac | 1240k | All | 49670 | 902 | 554 | 0.0182 | 0.0112 | 0.421 | 0.393 | M |
We restrict analysis to the 1240k target set for study of the 2.2M capture datasets.
Extended Data Table 5.
Allele counts at SNPs thought to be affected by selection in samples that have at least 1.0-fold coverage.rs4988235 is responsible for lactase persistence in Europe59,60. The SNPs at SLC24A5 and SLC45A2 are responsible for light skin pigmentation. The SNP at EDAR61,62 affects tooth morphology and hair thickness. The SNP at HERC263,64 is the primary determinant of light eye color in present-day Europeans. We present the fraction of fragments overlapping each SNP that are derived; the observation of a low rate of derived alleles does not prove that the individual carried the allele, and instead may reflect sequencing error or ancient DNA damage. We highlight in light gray sites that we judge (based on the derived allele count) are likely to be heterozygous for the derived allele, and in dark gray sites that are likely to be homozygous.
| LCT | SLC45A2 | SLC24A5 | EDAR | HERC2 | ||
|---|---|---|---|---|---|---|
| SNP | rs4988235 | rs16891982 | rs1426654 | rs3827760 | rs12913832 | |
| Ancestral | G | C | G | A | A | |
| Derived | A | G | A | G | G | |
| UstIshim | Coverage | 31 | 46 | 52 | 42 | 50 |
| Derived allele frequency | 0% | 0% | 2% | 0% | 0% | |
|
| ||||||
| Kostenki14 | Coverage | 140 | 113 | 6 | 45 | 52 |
| Derived allele frequency | 0% | 2% | 17% | 0% | 0% | |
|
| ||||||
| GoyetQ116-1 | Coverage | 8 | 6 | 0 | 9 | 1 |
| Derived allele frequency | 0% | 0% | n/a | 0% | 0% | |
|
| ||||||
| Vestonice16 | Coverage | 13 | 18 | 0 | 4 | 5 |
| Derived allele frequency | 0% | 6% | 0% | 0% | ||
|
| ||||||
| Malta1 | Coverage | 1 | 0 | 2 | 2 | 2 |
| Derived allele frequency | 0% | 0% | 0% | 0% | ||
|
| ||||||
| ElMiron | Coverage | 2 | 10 | 0 | 7 | 5 |
| Derived allele frequency | 0% | 0% | 0% | 0% | ||
|
| ||||||
| Villabruna | Coverage | 17 | 52 | 5 | 19 | 10 |
| Derived allele frequency | 0% | 0% | 0% | 0% | 100% | |
|
| ||||||
| Bichon | Coverage | 11 | 4 | 25 | 16 | 9 |
| Derived allele frequency | 0% | 0% | 0% | 0% | 33% | |
|
| ||||||
| Satsurblia | Coverage | 1 | 2 | 4 | 1 | 4 |
| Derived allele frequency | 0% | 0% | 100% | 0% | 50% | |
|
| ||||||
| Kotias | Coverage | 16 | 22 | 13 | 20 | 15 |
| Derived allele frequency | 0% | 0% | 100% | 0% | 0% | |
|
| ||||||
| Loschbour | Coverage | 19 | 18 | 20 | 17 | 21 |
| Derived allele frequency | 0% | 0% | 0% | 0% | 100% | |
|
| ||||||
| LaBrana1 | Coverage | 8 | 6 | 2 | 11 | 3 |
| Derived allele frequency | 12% | 0% | 0% | 0% | 100% | |
|
| ||||||
| Hungarian.KO1 | Coverage | 1 | 2 | 2 | 1 | 2 |
| Derived allele frequency | 0% | 0% | 50% | 0% | 100% | |
|
| ||||||
| Motala12 | Coverage | 2 | 0 | 3 | 3 | 1 |
| Derived allele frequency | 0% | 0% | 33% | 100% | ||
|
| ||||||
| Karelia | Coverage | 1 | 9 | 4 | 0 | 1 |
| Derived allele frequency | 0% | 67% | 0% | 0% | ||
|
| ||||||
| Stuttgart | Coverage | 25 | 21 | 15 | 29 | 21 |
| Derived allele frequency | 0% | 0% | 100% | 0% | 0% | |
Extended Data Table 6.
All European hunter-gatherers after Kostenki14 share genetic drift with present-day Europeans.We compute the statistic D(Han, Test; French, Mbuti). Measuring whether present-day French share more alleles with Han or with a Test population (restricting to ancient samples with at least 30,000 SNPs covered at least once). Present-day Europeans share significantly more genetic drift with European hunter-gatherers from Kostenki14 onward than they do with Han. Thus, by the date of Kostenki14, there was already West Eurasian-specific genetic drift.
| Test | SNPs used | D-value | Z score |
|---|---|---|---|
| Ust’-Ishim | 2,050,358 | 0.003 | 6.6 |
| Oase1 | 278,785 | 0.005 | 10.6 |
| Kostenki14 | 1,676,253 | −0.002 | −5.5 |
| Muierii2 | 95,787 | −0.004 | −6.3 |
| GoyetQ116-1 | 811,756 | −0.004 | −8.0 |
| Kostenki12 | 59,850 | −0.004 | −5.1 |
| Paglicci133 | 79,624 | −0.004 | −5.5 |
| Vestonice13 | 136,598 | −0.004 | −7.1 |
| Vestonice15 | 30,252 | −0.006 | −6.4 |
| Vestonice16 | 914,141 | −0.004 | −9.1 |
| Pavlov1 | 55,835 | −0.005 | −6.3 |
| Vestonice43 | 160,463 | −0.004 | −6.9 |
| KremsWA3 | 229,187 | −0.005 | −10.2 |
| Ostuni1 | 360,347 | −0.004 | −8.6 |
| Malta1 | 1,401,718 | −0.005 | −11.3 |
| ElMiron | 777,654 | −0.007 | −14.7 |
| AfontovaGora2 | 141,073 | −0.007 | −13.6 |
| AfontovaGora3 | 707,617 | −0.006 | −13.6 |
| HohleFels49 | 62,816 | −0.004 | −5.2 |
| Rigney1 | 34,445 | −0.006 | −6.1 |
| GoyetQ-2 | 70,210 | −0.006 | −8.8 |
| Burkhardtshohle | 37,234 | −0.006 | −6.2 |
| Villabruna | 1,170,777 | −0.010 | −24.7 |
| Bichon | 2,034,069 | −0.009 | −23.6 |
| Satsurblia | 1,419,824 | −0.005 | −13.1 |
| Rochedane | 229,806 | −0.011 | −20.8 |
| Ranchot88 | 402,274 | −0.010 | −21.8 |
| Kotias | 2,047,856 | −0.006 | −15.8 |
| Falkenstein | 64,043 | −0.008 | −11.6 |
| Chaudardes1 | 90,047 | −0.011 | −16.0 |
| Loschbour | 2,037,082 | −0.011 | −25.4 |
| LaBrana1 | 1,824,307 | −0.009 | −23.0 |
| Motala12 | 1,816,201 | −0.009 | −23.8 |
| Hungarian.KO1 | 1,372,801 | −0.010 | −26.5 |
| Karelia | 1,701,664 | −0.009 | −21.9 |
| Stuttgart | 2,023,939 | −0.009 | −23.9 |
| BerryAuBac | 53,028 | −0.011 | −14.0 |
Supplementary Material
Acknowledgments
We thank Bridget Alex, David Meltzer, Priya Moorjani, Iñigo Olalde, Sriram Sankararaman and Bence Viola for critical comments, Kristin Stewardson and Eadaoin Harney for sample screening, and Fredrik Hallgren for sharing a radiocarbon date for Motala12. The Figure 1 map is plotted using data available under the Open Database License © OpenStreetMap (www.openstreetmap.org/copyright). The Goyet project led by HR was funded by the Wenner-Gren Foundation (Gr. 7837), the College of Social and Behavioral Sciences of CSUN, and the RBINS. The excavation of the El Mirón Cave burial, led by LGS and MRGM, was supported by the Gobierno de Cantabria, the L.S.B. Leakey Foundation, the University of New Mexico, the Stone Age Research Fund (J. and R. Auel, principal donors), the town of Ramales de la Victoria and the Universidad de Cantabria. Excavations at Grotta Paglicci were performed by Professor A. Palma di Cesnola in collaboration with the Soprintendenza Archeologia della Puglia (founded by MIUR and local Institutions). Research at Riparo Villabruna was supported by MIBACT and the Veneto Region. QF was funded by the Special Foundation of the President of the Chinese Academy of Sciences (2015–2016), the Bureau of International Cooperation of Chinese Academy of Sciences, Chinese Academy of Sciences (XDA05130202), the National Natural Science Foundation of China (L1524016) and the Chinese Academy of Sciences Discipline Development Strategy Project (2015-DX-C-03). DFe was supported by an Irish Research Council grant (GOIPG/2013/36). IM was supported by a long-term fellowship from the Human Frontier Science Program LT001095/2014-L. PSk was supported by the Swedish Research Council (VR 2014-453). ST, MPR and SP were funded by the Max Planck Society and the Krekeler Foundation. CN-M was funded by FWF P-17258, P-19347, P-21660 and P-23612. SC and OTM were funded by a “Karsthives” Grant PCCE 31/2010 (CNCS-UEFISCDI, Romania). APD, ND, VSla and ND were funded by the Russian Science Foundation (Project No.14-50-00036). MAM was funded by a Marie Curie Intra-European Fellowship within the 7th European Community Framework Programme (grant number PIEF-GA-2008-219965). MLa and DC were funded by grants PRIN 2010-11 and 2010EL8TXP_003. CC and the research about the French Jura sites of Rochedane, Rigney and Ranchot was funded by the Collective Research Program (PCR) (2005–2008). KH was supported by the European Research Council (ERC StG 283503) and the Deutsche Forschungsgemeinschaft (DFG INST37/706-1FUGG, DFG FOR2237). DGD was funded by the European Social Fund and Ministry of Science, Research and Arts of Baden-Württemberg. RP was funded by ERC starting grant ADNABIOARC (263441). JKr was funded by DFG grant KR 4015/1-1, the Baden Württemberg Foundation, and the Max Planck Society. JKe was funded by a grant from the Deutsche Forschungsgemeinschaft (SFB1052, project A02). DR was funded by NSF HOMINID grant BCS-1032255, NIH (NIGMS) grant GM100233, and the Howard Hughes Medical Institute.
Footnotes
Author Contributions
JKr, SP and DR conceived the idea for the study. QF, CP, MH, WH, MMe, VSlo, RGC, APD, ND, VSla, AT, FM, BG, EV, MRG, LGS, CN-M, MT-N, SC, OTM, SB, MPer, DCo, MLa, SR, AR, FV, CT, KW, DG, HR, IC, DFl, PSe, MAM, CC, HB, NJC, KH, VM, DGD, JS, DCa, RP, JKr, SP and DR assembled archaeological material. QF, CP, MH, DFe, AF, WH, MMe, AM, BN, NR, VSlo, ST, HB, DGD, MPR, RP, JKr, SP and DR performed or supervised wet laboratory work. QF, CP, MH, MPet, SM, AP, IL, MLi, IM, SS, PSk, JKe, NP and DR analyzed data. QF, CP, MH, MPet, JKe, SP and DR wrote the manuscript and supplements.
The aligned sequences are available through the European Nucleotide Archive under accession number PRJEB13123.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of the paper.
References
- 1.Gamble C, Davies W, Pettitt P, Richards M. Climate change and evolving human diversity in Europe during the last glacial. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2004;359:24–53. doi: 10.1098/rstb.2003.1396. discussion 253–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones ER, et al. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nature communications. 2015;6:8912. doi: 10.1038/ncomms9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Q, et al. An early modern human from Romania with a recent Neanderthal ancestor. Nature. 2015 doi: 10.1038/nature14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seguin-Orlando A, et al. Paleogenomics. Genomic structure in Europeans dating back at least 36,200 years. Science. 2014;346:1113–1118. doi: 10.1126/science.aaa0114. [DOI] [PubMed] [Google Scholar]
- 5.Dabney J, et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc Natl Acad Sci U S A. 2013;110:15758–15763. doi: 10.1073/pnas.1314445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer M, Kircher M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harbor protocols. 2010;2010 doi: 10.1101/pdb.prot5448. pdb prot5448. [DOI] [PubMed] [Google Scholar]
- 7.Meyer M, et al. A High-Coverage Genome Sequence from an Archaic Denisovan Individual. Science. 2012 doi: 10.1126/science.1224344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohland N, Harney E, Mallick S, Nordenfelt S, Reich D. Partial uracil-DNA-glycosylase treatment for screening of ancient DNA. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2015;370:20130624. doi: 10.1098/rstb.2013.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haak W, et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature. 2015 doi: 10.1038/nature14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korneliussen TS, Albrechtsen A, Nielsen R. ANGSD: Analysis of Next Generation Sequencing Data. BMC bioinformatics. 2014;15:356. doi: 10.1186/s12859-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krause J, et al. A complete mtDNA genome of an early modern human from Kostenki, Russia. Current biology: CB. 2010;20:231–236. doi: 10.1016/j.cub.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 12.Skoglund P, et al. Origins and genetic legacy of Neolithic farmers and hunter-gatherers in Europe. Science. 2012;336:466–469. doi: 10.1126/science.1216304. [DOI] [PubMed] [Google Scholar]
- 13.Fu Q, et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514:445–449. doi: 10.1038/nature13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghavan M, et al. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature. 2014;505:87–91. doi: 10.1038/nature12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazaridis I, et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature. 2014;513:409–413. doi: 10.1038/nature13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olalde I, et al. Derived immune and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature. 2014;507:225–228. doi: 10.1038/nature12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamba C, et al. Genome flux and stasis in a five millennium transect of European prehistory. Nature communications. 2014;5:5257. doi: 10.1038/ncomms6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green RE, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. 328/5979/710 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prufer K, et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature. 2014;505:43–49. doi: 10.1038/nature12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallick S. The landscape of human genome diversity. In submission. 2015 [Google Scholar]
- 21.Reich D, et al. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature. 2010;468:1053–1060. doi: 10.1038/nature09710. nature09710 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sankararaman S, et al. The genomic landscape of Neanderthal ancestry in present-day humans. Nature. 2014;507:354–357. doi: 10.1038/nature12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernot B, Akey JM. Resurrecting surviving Neandertal lineages from modern human genomes. Science. 2014;343:1017–1021. doi: 10.1126/science.1245938. [DOI] [PubMed] [Google Scholar]
- 24.Harris K, Nielsen R. The Genetic Cost of Neanderthal Introgression. biorxiv.org. 2015 doi: 10.1534/genetics.116.186890. http://dx.doi.org/10.1101/030387. [DOI] [PMC free article] [PubMed]
- 25.Juric I, Aeschbacher S, Coop C. The Strength of Selection Against Neanderthal Introgression. biorxiv.org. 2015 doi: 10.1371/journal.pgen.1006340. http://dx.doi.org/10.1101/030148. [DOI] [PMC free article] [PubMed]
- 26.Posth C, et al. Pleistocene Mitochondrial Genomes Suggest a Single Major Dispersal of Non-Africans and a Late Glacial Population Turnover in Europe. Current Biology. doi: 10.1016/j.cub.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 27.Olivieri A, et al. The mtDNA legacy of the Levantine early Upper Palaeolithic in Africa. Science. 2006;314:1767–1770. doi: 10.1126/science.1135566. [DOI] [PubMed] [Google Scholar]
- 28.Patterson NJ, et al. Ancient Admixture in Human History. Genetics. 2012 doi: 10.1534/genetics.112.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome research. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skotte L, Korneliussen TS, Albrechtsen A. Estimating individual admixture proportions from next generation sequencing data. Genetics. 2013;195:693–702. doi: 10.1534/genetics.113.154138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu Q, et al. A revised timescale for human evolution based on ancient mitochondrial genomes. Curr Biol. 2013;23:553–559. doi: 10.1016/j.cub.2013.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conard NJ. A female figurine from the basal Aurignacian of Hohle Fels Cave in southwestern Germany. Nature. 2009;459:248–252. doi: 10.1038/nature07995. [DOI] [PubMed] [Google Scholar]
- 33.Straus LG. After the Deep Freeze: Confronting “Magdalenian” Realities in Cantabrian Spain And Beyond. Journal of Archaeological Method and Theory. 2013;20:236–255. doi: 10.1007/s10816-012-9152-5. [DOI] [Google Scholar]
- 34.Weaver AJ, Saenko OA, Clark PU, Mitrovica JX. Meltwater pulse 1A from Antarctica as a trigger of the Bolling-Allerod warm interval. Science. 2003;299:1709–1713. doi: 10.1126/science.1081002. [DOI] [PubMed] [Google Scholar]
- 35.Montoya C, Peresani M. Premiers éléments de diachronie dans l’Epigravettien récent des Préalpes de la Vénétie. Bracco JP, Montoya C, editors. D’un monde à l’autre. Les systèmes lithiques pendant le Tardiglaciaire autour de la Méditerranée nord-occidentale. Mémoire Societé Préhistorique Française. 2005:123–138. [Google Scholar]
- 36.Valentin B. Paris, Publications de la Sorbonne, Cahiers archéologiques de Paris 1, 1. 2008. Jalons pour une Paléohistoire des derniers chasseurs (XIVe-VIe millénaire avant J.-C.) [Google Scholar]
- 37.Pala M, et al. Mitochondrial DNA signals of late glacial recolonization of Europe from near eastern refugia. American journal of human genetics. 2012;90:915–924. doi: 10.1016/j.ajhg.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McVean G, Awadalla P, Fearnhead P. A coalescent-based method for detecting and estimating recombination from gene sequences. Genetics. 2002;160:1231–1241. doi: 10.1093/genetics/160.3.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rougier H, et al. Pestera cu Oase 2 and the cranial morphology of early modern Europeans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1165–1170. doi: 10.1073/pnas.0610538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marom A, McCullagh JSO, Higham TFG, Sinitsyn AA, Hedges REM. Single amino acid radiocarbon dating of Upper Paleolithic modern humans. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6878–6881. doi: 10.1073/pnas.1116328109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soficaru A, Dobos A, Trinkaus E. Early modern humans from the Pestera Muierii, Baia de Fier, Romania. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17196–17201. doi: 10.1073/pnas.0608443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palma di Cesnola A. L’Aurignaziano e il Gravettiano antico. Claudio Grenzi Ed; 2004. Paglicci. [Google Scholar]
- 43.Soficaru A, Petrea C, Dobos A, Trinkaus E. The human cranium from the Pestera Cioclovina Uscata, Romania - Context, age, taphonorny, morphology, and paleopathology. Curr Anthropol. 2007;48:611–619. doi: 10.1086/519915. [DOI] [Google Scholar]
- 44.Trinkaus E, Svoboda J. Early Modern Human Evolution in Central Europe: The People of Dolní Věstonice and Pavlov. Vol. 12. Oxford University Press; 2006. [Google Scholar]
- 45.Simon U, Haendel M, Einwoegerer T, Neugebauer-Maresch C. The archaeological record of the Gravettian open air site Krems-Wachtberg. Quaternary International. 2014;351:5–13. doi: 10.1016/j.quaint.2013.08.009. [DOI] [Google Scholar]
- 46.Cupillard C, et al. Changes in ecosystems, climate and societies in the Jura Mountains between 40 and 8 ka cal BP. Quaternary International. 2015;378:40–72. doi: 10.1016/j.quaint.2014.05.032. [DOI] [Google Scholar]
- 47.Housley RA, Gamble CS, Street M, Pettitt PB. Radiocarbon evidence for the Lateglacial human recolonisation of Norther Europe. Proc Prehist Soc. 1997;63:25–54. [Google Scholar]
- 48.Benazzi S, et al. Early dispersal of modern humans in Europe and implications for Neanderthal behaviour. Nature. 2011;479:525–528. doi: 10.1038/nature10617. [DOI] [PubMed] [Google Scholar]
- 49.Simon U. Die Burkhardtshöhle - eine Magdalénienstation am Nordrand der Schwäbischen Alb. Magisterarbeit. 1993 [Google Scholar]
- 50.Vercellotti G, Alciati G, Richards MP, Formicola V. The Late Upper Paleolithic skeleton Villabruna 1 (Italy): a source of data on biology and behavior of a 14.000 year-old hunter. Journal of Anthropological Sciences. 2008;86:143–163. [PubMed] [Google Scholar]
- 51.Valentin F, et al. Découvertes récentes d‘inhumations et d’incinération datées du Mésolithique en Ile de France. Revue Archéologique d’Ile-de-France. 2008:21–42. [Google Scholar]
- 52.Bramanti B, et al. Genetic discontinuity between local hunter-gatherers and central Europe’s first farmers. Science. 2009;326:137–140. doi: 10.1126/science.1176869. [DOI] [PubMed] [Google Scholar]
- 53.Price TD, Jacobs K. Olenii Ostrov - 1st radiocarbon dates from a major mesolithic cemetery in Karelia, USSR. Antiquity. 1990;64:849–853. [Google Scholar]
- 54.Wehrberger K. Der Streit ward definitiv beendet...” Eine mesolithische Bestattung aus der Bocksteinhöhle im Lonetal, Alb-Donau-Kreis. Zur Erinnerung an Ludwig Bürger (1844–1898) Archäologisches Korrespondenzblatt. 2000;30:15–31. [Google Scholar]
- 55.Orschiedt J. Dissertation. Vol. 13. Urgeschichtliche Materialhefte; 1999. Manipulationen an menschlichen Skelettresten. Taphonomische Prozesse, Sekundärbestattungen oder Kannibalismus. [Google Scholar]
- 56.Sanchez-Quinto F, et al. Genomic affinities of two 7,000-year-old Iberian hunter-gatherers. Curr Biol. 2012;22:1494–1499. doi: 10.1016/j.cub.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Reimer PJ, et al. Intcal13 and Marine13 Radiocarbon Age Calibration Curves 0–50,000 Years cal BP. Radiocarbon. 2013;55:1869–1887. [Google Scholar]
- 58.Ramsey CB, Lee S. Recent and planned developments of the program OxCal. Radiocarbon. 2013;55:720–730. [Google Scholar]
- 59.Enattah NS, et al. Identification of a variant associated with adult-type hypolactasia. Nature genetics. 2002;30:233–237. doi: 10.1038/ng826. [DOI] [PubMed] [Google Scholar]
- 60.Bersaglieri T, et al. Genetic signatures of strong recent positive selection at the lactase gene. American journal of human genetics. 2004;74:1111–1120. doi: 10.1086/421051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujimoto A, et al. A scan for genetic determinants of human hair morphology: EDAR is associated with Asian hair thickness. Human molecular genetics. 2008;17:835–843. doi: 10.1093/hmg/ddm355. [DOI] [PubMed] [Google Scholar]
- 62.Kimura R, et al. A common variation in EDAR is a genetic determinant of shovel-shaped incisors. American journal of human genetics. 2009;85:528–535. doi: 10.1016/j.ajhg.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sturm RA, et al. A single SNP in an evolutionary conserved region within intron 86 of the HERC2 gene determines human blue-brown eye color. American journal of human genetics. 2008;82:424–431. doi: 10.1016/j.ajhg.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eiberg H, et al. Blue eye color in humans may be caused by a perfectly associated founder mutation in a regulatory element located within the HERC2 gene inhibiting OCA2 expression. Human genetics. 2008;123:177–187. doi: 10.1007/s00439-007-0460-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.