Abstract
Background
Vitamin D is known to have diverse effects on various systems in the body. There is evidence to suggest that a link exists between the serum vitamin D status and tuberculosis. The present study was designed to assess the alterations in serum 25-hydroxyvitamin D levels in newly diagnosed sputum acid fast bacilli (AFB) positive pulmonary tuberculosis patients and to study the association, if any, between serum vitamin D levels and different levels of sputum smear positivity.
Methods
Serum 25-hydroxyvitamin D levels were estimated in 65 sputum AFB positive pulmonary tuberculosis patients and 65 age and gender-matched healthy controls.
Results
The levels of serum 25 hydroxy-vitamin D in tuberculosis patients were not statistically different from the levels of serum 25 hydroxy-vitamin D in healthy controls. However, among patients with pulmonary tuberculosis, there was a significant negative correlation between the levels of serum 25 hydroxy-vitamin D and levels of sputum positivity.
Conclusion
Serum vitamin D levels negatively correlates with bacterial load in patients with active pulmonary tuberculosis.
Keywords: Vitamin D; Tuberculosis, Pulmonary
Introduction
Tuberculosis caused by Mycobacterium tuberculosis, is considered as one of the leading causes of mortality and morbidity. According to World Health Organization (WHO), in 2011, 8.2 million cases were reported worldwide of which 2.0 million were from India, accounting for more than a fifth of the global burden1.
Vitamin D is known to be involved in both skeletal as well as extraskeletal functions like wound healing and regulation of immune response2. Vitamin D deficiency (VDD) is found to be very common worldwide with India, being a tropical country and majority of her people being exposed to sunlight throughout the year, there was a wrong notion that VDD is uncommon in India3. However, extensive data from the literature show that VDD is very common in India. Prevalence was found to be 74%–96% among the general healthy Indian population4. VDD has been linked to various diseases like diabetes mellitus, hypertension, myopathies, infections, inflammatory diseases, and autoimmune diseases5,6. VDD was also found to be one of the predisposing factors for acquiring tuberculosis because of its role in both innate and acquired immunity 7. This hypothesis was proposed by Davies et al.8 based on observations of high prevalence of tuberculosis among Asian immigrants in London who had low vitamin D levels.
While the association between 25-hydroxyvitamin D [25(OH)D] and tuberculosis has been studied in many Western countries, in India only limited and conflicting data were available regarding the same. Hence, the present study was designed to study serum 25(OH)D levels in newly diagnosed pulmonary tuberculosis patients and to assess its association with different sputum acid fast bacilli (AFB) positivity.
Materials and Methods
1. Study population and ethical approval
The present study is a cross-sectional study, conducted in Departments of Biochemistry, Pulmonary Medicine, and General Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India. This study was approved by the Institute Ethics Committee (IEC, Human Studies).
Sixty-five newly diagnosed sputum AFB positive pulmonary tuberculosis patients and 65 apparently healthy control subjects were recruited for the study. A new case was defined as a patient who has never received treatment for pulmonary tuberculosis or has taken anti-tubercular drugs for less than one month. Patients with extrapulmonary tuberculosis, history of alcohol intake, malabsorption, liver or renal disorders, intake of drugs which can alter vitamin D levels, human immunodeficiency virus infection, diabetes, immunosuppressive treatment, severe protein energy malnutrition are excluded from the study. The results of the patient group were compared with the apparently healthy subjects.
The sample size was estimated with an expected difference in VDD between the healthy population and tuberculosis patients as 20% (20% vs. 40%) at 5% level of significance and 90% power. Using the statistical formula for comparing two populations, the minimum sample size required for the study was estimated to be 659.
2. Assessment of sputum sample
Sputum AFB examination was done by fluorescent microscopy using Auramine-Rhodamine stain. The patients with confirmed tuberculosis were further classified into four groups Scanty, 1+, 2+, 3+ based on sputum AFB positivity as per Revised National Tuberculosis Control Program (RNTCP), Tuberculosis manual 200510. As there were only two patients in the scanty group, it was not considered for any further statistical analysis.
3. Assessment of biochemical parameters
Five milliliters of venous blood sample collected from each of the subjects was centrifuged and sera used for the estimation of routine biochemical parameters immediately. Samples of sera for vitamin D analysis were stored in –80℃.
Serum glucose, calcium, phosphorus, total bilirubin, aspartate aminotransferase, alanine aminotransferase, creatinine, total protein, and albumin using reagent kits adapted to an automated clinical chemistry analyser (AU 680; Beckman Coulter, Brea, CA, USA). Serum 25(OH)D was estimated using enzyme-linked immunosorbent assay kits from DIAsource (S.A Rue du Bosquet, Belgium). Serum intact parathyroid hormone (iPTH) was assayed by two-site sandwich immunoassay direct chemiluminescence method using reagent kits adapted to ADVIA Centaur (Siemens, Berlin, Germany).
4. Statistical analysis
Normality of the data was analysed by Shapiro-Wilk test. Results were expressed as mean±standard deviation. Independent Student's t test was used to compare the results between cases and controls. Sputum grading and differences in vitamin D levels between and within groups of different ages and gender were analysed by one-way ANOVA. The association between vitamin D and sputum AFB positivity was analyzed using Pearson correlation test. Statistical analyses were performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). The analysis was carried out at 5% level of significance and the p-value of <0.05 was considered as statistically significant.
Results
1. Comparison of baseline characteristics
As compared to controls, serum iPTH levels were significantly elevated in tuberculosis patients. Though the serum 25(OH)D levels were found to be low in tuberculosis patients when compared with healthy controls, this difference was not statistically significant. Correspondingly we found the difference in total calcium, corrected calcium, phosphorus, and total protein levels. However, all parameters were in the reference range as shown in Table 1.
Table 1. Comparison of baseline characteristics between tuberculosis patients and controls.
| Parameter | Control (n=65) | Case (n=65) | p-value |
|---|---|---|---|
| Age, yr | 42.2±10.7 | 41.1±11.8 | NS |
| Gender | NS | ||
| Male | 25 | 31 | |
| Female | 40 | 34 | |
| 25-Hydroxyvitamin D, ng/mL | 17.5±5.7 | 15.4±6.8 | NS |
| iPTH, pmol/L | 3.13±0.77 | 3.48±1.11* | 0.046† |
| Total bilirubin, mmol/L | 11.4±2.90 | 11.1±2.56 | NS |
| AST, U/L | 27.5±6.1 | 25.7±5.4 | NS |
| ALT, U/L | 21.5±7.5 | 20.2±7.5 | NS |
| Creatinine, µmol/L | 72.4±10.6 | 74.2±14.1 | NS |
| Glucose, mmol/L | 4.4±0.47 | 4.79±0.73 | NS |
| Calcium, mmol/L | 2.3±0.18 | 2.18±0.19* | <0.001† |
| Corrected calcium, mmol/L | 2.3±0.18 | 2.2±0.22* | 0.021* |
| Phosphorus, mmol/L | 1.21±0.12 | 1.12±0.19* | <0.001† |
| Total protein, g/L | 78±6.2 | 69±9.2* | <0.001† |
| Albumin, g/L | 39.5±4.7 | 37.9±6.2 | NS |
Values are presented as mean±standard deviation.
Corrected calcium (mg/dL)=Total calcium+[(4–Albumin)×0.8].
*p<0.05. †p<0.001.
NS: not significant; iPTH: intact parathyroid hormone; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
2. Comparison of biochemical parameters in the study patients
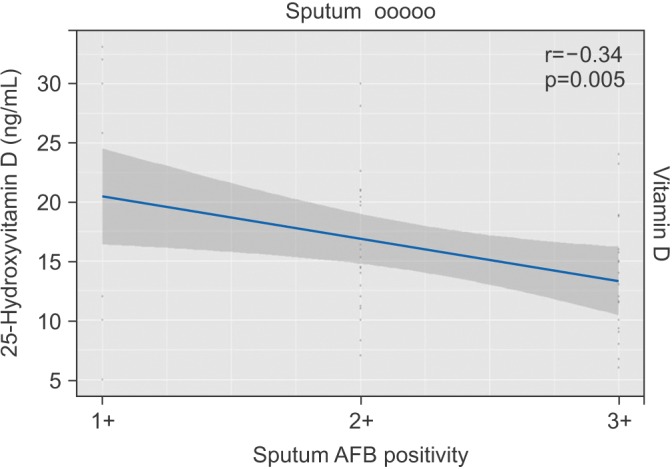
To assess the association of serum 25(OH)D with the severity of tuberculosis, we studied serum 25(OH)D with respect to different levels of sputum AFB positivity, which indirectly reflects the severity of the disease. In the present study, 53% (n=35) had 3+, 35% (n=22) had 2+, and 12% (n=8) had 1+ sputum AFB positivity, respectively. The mean vitamin D levels amongst different grades of sputum smear positivity were found to be statistically significant. No statistically significant difference was found in iPTH, calcium, phosphorus, and total protein levels as shown in Table 2. A strong negative correlation was found between serum vitamin D levels and different levels of sputum AFB positivity (r=–0.34, p=0.005) (Figure 1).
Table 2. Comparison of age and biochemical parameters with respect to sputum AFB positivity.
| Parameter | Sputum microscopy | p-value | ||
|---|---|---|---|---|
| Sputum 1+ (n=8) | Sputum 2+ (n=22) | Sputum 3+ (n=35) | ||
| Age, yr | 39.8±10.3 | 41.27±10.4 | 41.46±13.1 | NS |
| 25-Hydroxyvitamin D, ng/mL | 22.2±11.3 | 16.7±5.9 | 13.1±4.5 | 0.001* |
| iPTH, pmol/L | 3.39±1.01 | 3.50±1.17 | 3.82±1.33 | NS |
| Total bilirubin, mmol/L | 11.11±2.56 | 11.79±2.56 | 10.77±2.73 | NS |
| AST, U/L | 27. 2±4.7 | 23.3±5.1 | 26.9±5.7 | NS |
| ALT, U/L | 21.1±6.3 | 21.3±8.2 | 19.3±7.3 | NS |
| Creatinine, µmol/L | 72.9±12.3 | 74.6±15.9 | 76±13.2 | NS |
| Glucose, mmol/L | 4.83±0.6 | 5.04±0.7 | 4.62±0.7 | NS |
| Calcium, mmol/L | 2.2±0.19 | 2.19±0.21 | 2.27±0.19 | NS |
| Phosphorus, mmol/L | 1.1±0.17 | 1.2±0.16 | 1.0±0.15 | NS |
| Total protein, g/L | 68±7.9 | 69±10.4 | 69±10 | NS |
Values are presented as mean±standard deviation.
*p<0.001.
AFB: acid fast bacilli; NS: not significant; iPTH: intact parathyroid hormone; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
Figure 1. Correlation of serum vitamin D levels with sputum acid fast bacilli (AFB) positivity.
Discussion
Vitamin D is known to have an immunomodulating effect. However, earlier studies have reported conflicting data regarding vitamin D levels in tuberculosis patients. So, we investigated 25(OH)D levels in newly diagnosed sputum AFB positive pulmonary tuberculosis patients and its association with bacterial load.
The present study showed a decreasing trend in vitamin D levels in tuberculosis patients, but this finding was not statistically significant when compared with healthy controls. In general, Indians even if apparently normal are known to have lower serum vitamin D levels11 and hence, we did not find significant difference among cases and controls.
Even though previous studies have demonstrated decreased vitamin D levels in tuberculosis patients, their selection criteria for cases and controls were different, like the inclusion of tuberculosis patients on treatment, extrapulmonary tuberculosis, multidrug-resistant tuberculosis which might have influenced vitamin D levels. Few studies have also included patients with liver disease, renal disease, diabetes, and alcoholics in whom there might have been an altered vitamin D metabolism9,12,13,14. The strength of our study is that we excluded most of the possible causes that would interfere with vitamin D metabolism.
Significantly elevated levels of iPTH was found in patients with tuberculosis when compared with controls. This is considered as a normal regulatory response to decreased calcium and phosphorus levels.
The mean serum 25(OH)D levels amongst different levels of sputum smear positivity revealed a significant reduction in vitamin D levels with increase in sputum smear positivity and also there was a strong negative correlation between AFB sputum positivity and serum 25(OH)D levels (r=–0.34, p=0.005) indicating that low vitamin D is associated with increased bacterial load. It has been found that vitamin D inhibit the multiplication of Mycobacterium tuberculosis in the macrophages by induction of anti-microbial peptide cathelicidin15. So, we speculate that with a decrease in vitamin D level in high sputum positivity (3+) cases reduces the anti-microbial activity and increases the multiplication of tubercle bacilli.
Kim et al.16 shown that vitamin D inhibits collagen degradation by regulating interleukin 1–mediated matrix metalloproteinase synthesis, which helps in lung tissue repair and therefore better prognosis in tuberculosis patients.
In summary, the findings from the present study suggest that lowered levels of vitamin D were associated with an increase sputum AFB load in patients with tuberculosis. Therefore, the effect of vitamin D supplementation on sputum AFB load in tuberculosis patients needs to be explored.
Acknowledgements
This work was supported by a grant from JIPMER intramural grant to the corresponding author.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.World Health Organization. Global tuberculosis control: WHO report 2011 [Internet] Geneva: World Health Organization; 2014. [cited 2014 Jul 16]. Available from: http://www.who.int/tb/publications/global_report/2011/en/ [Google Scholar]
- 2.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Londhey V. Vitamin D deficiency: Indian scenario. J Assoc Physicians India. 2011;59:695–696. [PubMed] [Google Scholar]
- 4.Harinarayan CV, Joshi SR. Vitamin D status in India: its implications and remedial measures. J Assoc Physicians India. 2009;57:40–48. [PubMed] [Google Scholar]
- 5.Zasloff M. Fighting infections with vitamin D. Nat Med. 2006;12:388–390. doi: 10.1038/nm0406-388. [DOI] [PubMed] [Google Scholar]
- 6.Jhun BW, Kim SJ, Kim K, Lee JE, Hong DJ. Vitamin D status in South Korean military personnel with acute eosinophilic pneumonia: a pilot study. Tuberc Respir Dis. 2015;78:232–238. doi: 10.4046/trd.2015.78.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies PD, Church HA, Brown RC, Woodhead JS. Raised serum calcium in tuberculosis patients in Africa. Eur J Respir Dis. 1987;71:341–344. [PubMed] [Google Scholar]
- 9.Sasidharan PK, Rajeev E, Vijayakumari V. Tuberculosis and vitamin D deficiency. J Assoc Physicians India. 2002;50:554–558. [PubMed] [Google Scholar]
- 10.RNTCP. Tuberculosis manual [Internet] New Delhi: Government of India, Central Tuberculosis; 2005. [cited 2016 Mar 1]. Available from: http://www.tbcindia.nic.in/pdfs/RNTCP%20Lab%20Network%20Guidelines.pdf. [Google Scholar]
- 11.G R, Gupta A. Vitamin D deficiency in India: prevalence, causalities and interventions. Nutrients. 2014;6:729–775. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 13.Wejse C, Olesen R, Rabna P, Kaestel P, Gustafson P, Aaby P, et al. Serum 25-hydroxyvitamin D in a West African population of tuberculosis patients and unmatched healthy controls. Am J Clin Nutr. 2007;86:1376–1383. doi: 10.1093/ajcn/86.5.1376. [DOI] [PubMed] [Google Scholar]
- 14.Tostmann A, Wielders JP, Kibiki GS, Verhoef H, Boeree MJ, van der Ven AJ. Serum 25-hydroxy-vitamin D3 concentrations increase during tuberculosis treatment in Tanzania. Int J Tuberc Lung Dis. 2010;14:1147–1152. [PubMed] [Google Scholar]
- 15.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Baek MS, Yoon DS, Park JS, Yoon BW, Oh BS, et al. Vitamin D inhibits expression and activity of matrix metalloproteinase in human lung fibroblasts (HFL-1) cells. Tuberc Respir Dis. 2014;77:73–80. doi: 10.4046/trd.2014.77.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]


