Abstract
Purpose
The aim of this study was to determine whether surgical resection of the primary tumor contributes to survival in patients with metastatic gastric cancer.
Materials and Methods
A total of 288 patients with metastatic gastric cancer from the Akdeniz University, Antalya Training and Research Hospital, and the Meram University of Konya database were retrospectively analyzed. The effect of primary tumor resection on survival of patients with metastatic gastric cancer was investigated using the log-rank test. Kaplan-Meier survival estimates were calculated. Multivariate analysis was performed using Cox proportional hazards regression modeling.
Results
The median overall survival was 12.0 months (95% confidence intewrval [CI], 10.4~13.6 months) and 7.8 months (95% CI, 5.5~10.0 months) for patients with and without primary tumor resection, respectively (P<0.001). The median progression-free survival was 8.3 months (95% CI, 7.1~9.5 months) and 6.2 months (95% CI, 5.8~6.7 months) for patients with and without primary tumor resection, respectively (P=0.002).
Conclusions
Non-curative gastrectomy in patients with metastatic gastric cancer might increase their survival rate regardless of the occurrence of life-threatening tumor-related complications.
Keywords: Stomach neoplasms, Surgery, Mortality
Introduction
Gastric cancer (GC) is one of the most common cancers in the word. Approximately 1 million new cases are diagnosed each year (7.8% of the total number of cancer cases) and half of these cases are diagnosed in East Asia. It is the second leading cause of cancer-related death in the world (738,000 deaths, 9.7% of the total).1 GC is usually diagnosed at locally advanced or metastatic stages. In untreated stage IV and stage III disease, the life expectancy is 3 and 6 months, respectively. In the presence of peritonitis carcinomatosa, life expectancy is only 2 months.2
The main curative treatment for GC is the combination of surgery and neoadjuvant chemotherapy, or adjuvant chemotherapy or radiotherapy alone. The higher the stage, the lower the contribution of surgery to survival; for example, the 5-year survival rate in stage I disease with R0 resection is 95% while in stage 2, it is 35% to 65%, depending on the number of lymph nodes involved and dissected.3 Most GC patients are at an advanced stage and therefore have no chance of curative treatment.4 The recommendation in the current National Comprehensive Cancer Network guidelines for stage IV GC patients is palliative care including best of care, a clinical study, or chemotherapy.5 Targeted molecular therapies (such as trastuzumab or bevacizumab) are available in addition to cytotoxic chemotherapy.6 Surgical resection in advanced stage disease is required for palliation of tumor-related symptoms such as hemorrhage, obstruction, or perforation.7
The contribution of palliative surgery to survival has been a question of debate for many years. In our study, we aimed to investigate the effect of non-curative primary tumor resectio n(PTR) on survival of patients with metastatic gastric cancer (mGC).
Materials and Methods
Between February 2008 and March 2015, a total of 288 patients with mGC from Akdeniz University, Meram University, and the Antalya Training and Research Hospital database were evaluated retrospectively. Median follow-up time was 11 months. Patients were evaluated in 3-month periods with radiological assessment. Among the 288, 184 patients were administered palliative chemotherapy and 104 patients underwent PTR. PTR was defined as a surgery for the removal of the primary tumor when the patient could not receive curative surgery due to non-resectable distant metastases or non-resectable peritoneal carcinomatosis. The patients were divided into two groups: PTR (+) and PTR (-).
Inclusion criteria for both groups were pathologically confirmed gastric adenocarcinoma, metastatic disease with radiological imaging with/without pathological confirmation, and metastatic disease inappropriate for surgery. The inclusion criterion for the surgery group was no prior treatment (chemotherapy or radiotherapy) before surgery. Resection was partial or total gastrectomy. Some of the patients with mGC did have resection of the primary tumor at the time of diagnosis for various reasons. However, non-curative tumor resection is not the standard of care for mGC in Turkey. Our criteria for choosing non-curative PTR included patients with critical symptoms such as bleeding, obstruction, or perforation (palliative aim), as well as patients diagnosed with metastatic disease intraoperatively or postoperatively (reductive aim).
Patients who had stage IV kidney failure, New York Heart Association III or IV cardiac performance status, liver failure, or who could not receive chemotherapy because of poor performance (Eastern Cooperative Oncology Group Performance Status >2) were excluded from the study. Histological grades (G) of tumors were evaluated according to the American Joint Committee on Cancer Grading System: GX, cannot be assessed; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; and G4, undifferentiated.
Data on age, sex, comorbidities, smoking, alcohol consumption, tumor differentiation, CerbB2 status, metastatic area, first-line chemotherapy (first chemotherapy to be given for metastatic disease), second-line chemotherapy (second chemotherapy to be given after progression under first-line chemotherapy) (yes or no) were imported into the SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) from the medical records. In addition, the date of diagnosis, date of progression under first-line chemotherapy, and date of death of patients with mGC were also imported. Overall survival (OS) was defined as the time from the beginning of treatment to death.
Statistical analyses were performed using SPSS software ver. 16.0. To determine the properties and compare the patient and tumor characteristics of groups and to perform frequency analysis, chi-square tests and two independent sample t-tests were used. The effect of PTR on OS of patients with mGC was investigated using the log-rank test. Kaplan-Meier survival estimates were calculated. Multivariate analysis was performed using Cox proportional hazards regression modeling. The parameters with P-values <0.15 were included in multivariate analysis. A P-value of <0.05 was considered to be statistically significant.
Results
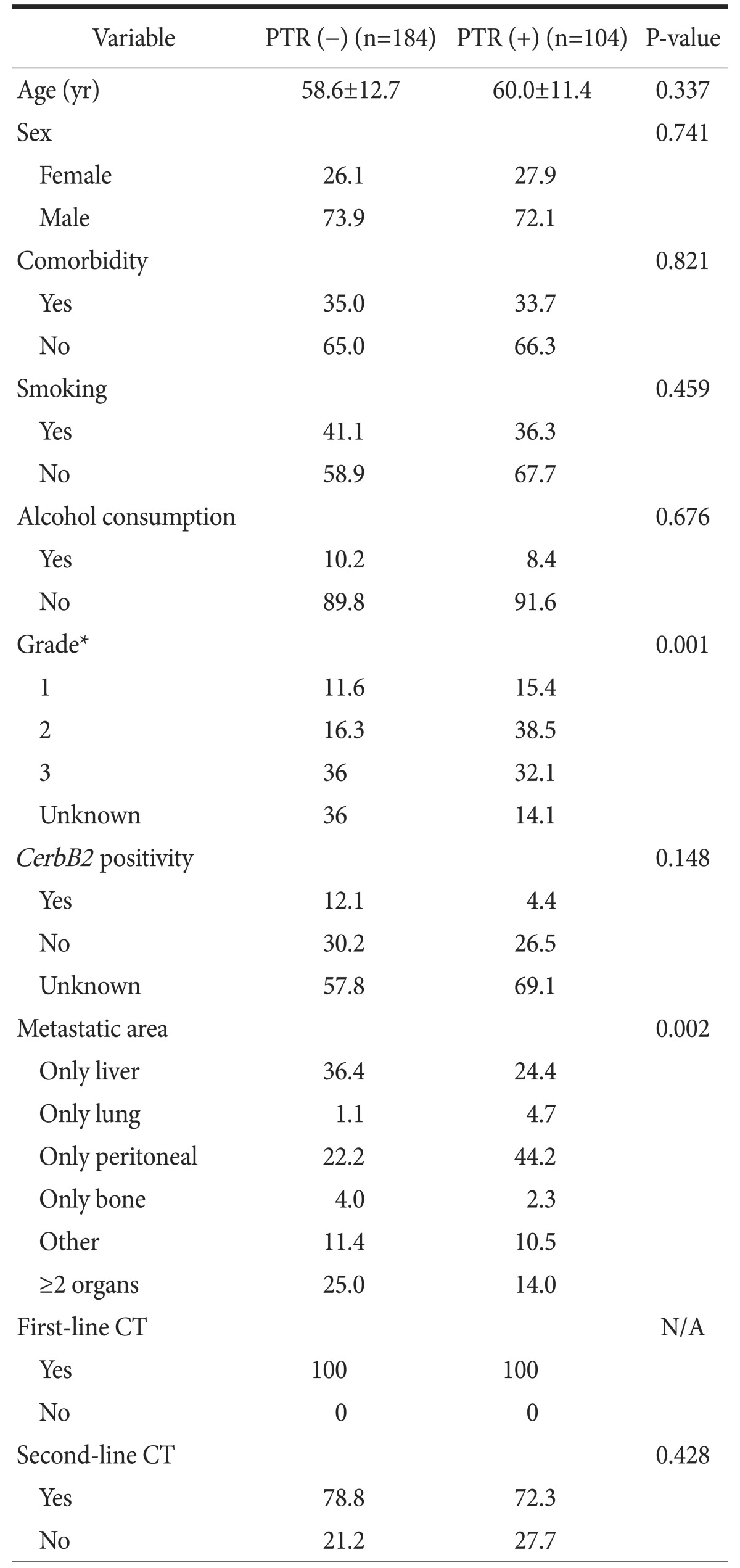
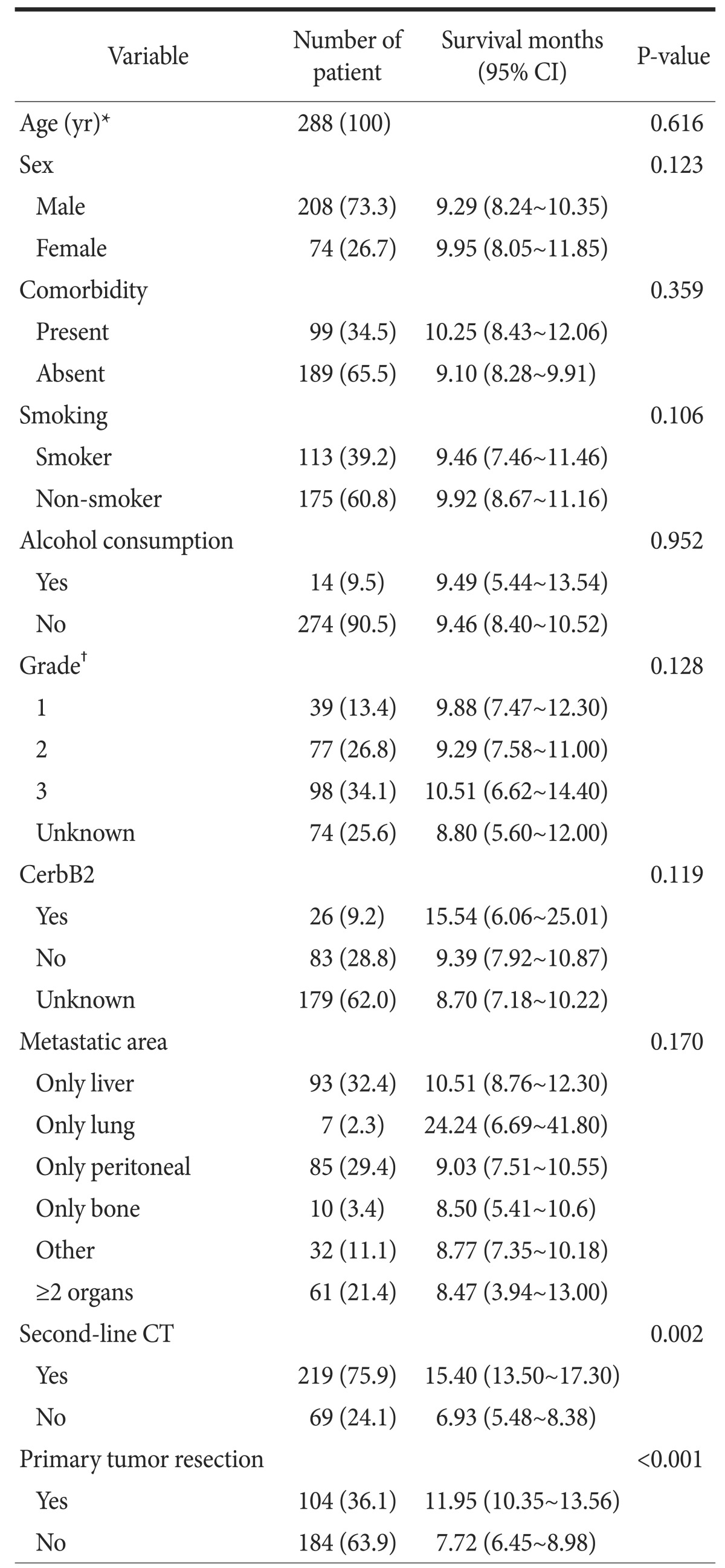
There was no significant difference between groups regarding age, sex, comorbidity, alcohol consumption, CerbB2 positivity, or second-line chemotherapy (P=0.337, P=0.741, P=0.821, P=0.676, P=0.148, and P=0.428, respectively). All patients received first-line chemotherapy. The characteristics of the groups are shown in Table 1. While the percentage of patients with a tumor grade 1 or 2 was higher in the PTR (+) group, the percentage of patients with grade 3 was higher in the PTR (-) group (P=0.001). In addition, the metastatic area was significantly different between groups (P=0.002). No significant pattern in tumor grade or metastatic area was seen in our results (P=0.350; Table 2).
Table 1. The properties of all patients.
Values are presented as mean±standard deviation or percent only. CT = computed tomography. *Classification according to the American Joint Committee on Cancer Grading System.
Table 2. The relationship between tumor grade and metastatic area (P=0.350).
Values are presented as percent only. *Classification according to the American Joint Committee on Cancer Grading System.
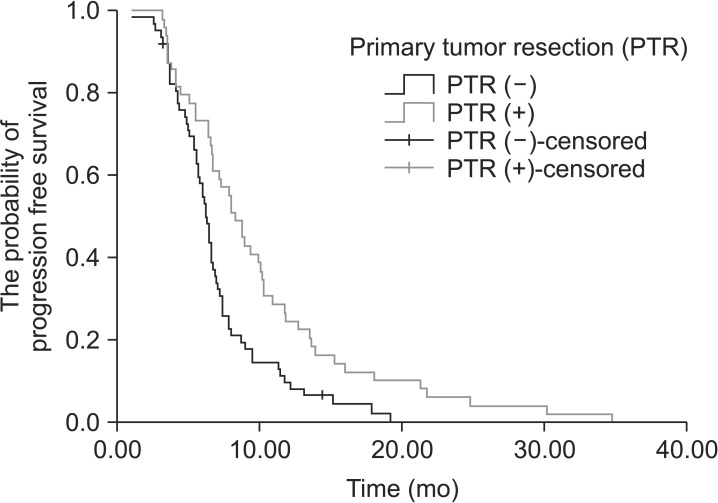
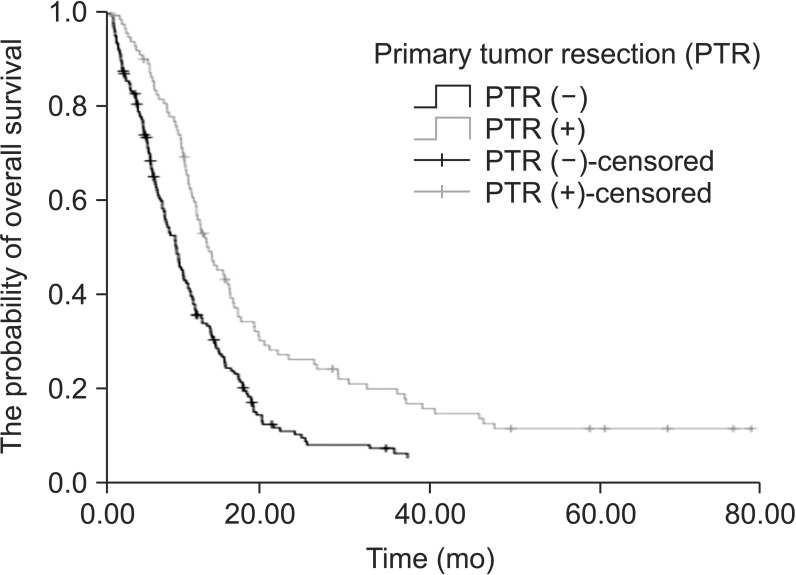
The median progression-free survival (PFS) was 8.3 months (95% confidence interval [CI], 7.1~9.5 months) in patients in the PTR (+) group and 6.2 months (95% CI, 5.8~6.7 months) in patients in the PTR (-) group. The median PFS was significantly higher in patients in the PTR (+) group (P=0.002) (Fig. 1). When we evaluated the groups in terms of OS, the median OS was significantly higher in patients in the PTR (+) group (P<0.001). The median OS was 12.0 months (95% CI, 10.4~13.6 months) in patients in the PTR (+) group and it was 7.8 months (95% CI, 5.5~10.0 months) in patients in the PTR (-) group (Fig. 2).
Fig. 1. Progression-free survival curve of patients who had primary tumor resection vs. patients who did not (P=0.002).
Fig. 2. Overall survival curve of patients who had primary tumor resection vs. patients who did not (P<0.001).
Data on age, sex, comorbidity, smoking, alcohol consumption, grade, CerbB2 status, metastatic area, second-line chemotherapy, and PTR were included in a univariate analysis. Metastatic areas were different between patients who did and did not have PTR (P=0.002). However, all metastatic areas in both patient groups were unfit for surgery. The P-values for these factors are shown in Table 3 and the univariate analysis results are presented in Table 4. The factors (sex, smoking, grade, CerbB2 status, second-line chemotherapy, and PTR) that had P-values <0.15 were included in the multivariate analysis (Table 5). In the final multivariate analysis, second-line chemotherapy and PTR were found to be significant (P=0.002 and P=0.005, respective).
Table 3. The properties of patients according to primary tumor resection (PTR).
Values are presented as mean±standard deviation or percent only. CT = computed tomography; N/A = non-applicable. *Classification according to the American Joint Committee on Cancer Grading System.
Table 4. Variables affecting survival of patients with metastatic gastric cancer: univariate analysis.
Values are presented as number (%) or median (range). CT = computed tomography; CI= confidence interval. *Value of mean±standard deviation: 58.6±12.7 years. †Classification according to the American Joint Committee on Cancer Grading System.
Table 5. Multivariate analysis of factors affecting survival of patients with metastatic gastric cancer.
Values are presented as number (%). CT = computed tomography; CI= confidence interval. *Classification according to the American Joint Committee on Cancer Grading System.
Discussion
In Stage IV GC, one or more areas can be involved, and patients can present with peritoneal metastases, peritoneal malignant fluid cytology, non-regional lymph node metastases, liver metastases, and other organ metastases. In this patient group, the OS rate is 3~5 months with best supportive care and 9~11 months with chemotherapy.8 If there is human epidermal growth factor receptor-2 overexpression, trastuzumab can be added to the therapy, increasing the survival rate to 11~14 months.9
The benefit of PTR for survival when the mGC patient does not have distressing symptoms such as tumor-related hemorrhage, obstruction, or perforation has been a question of debate for a long time. Some studies investigated the potential benefit of palliative gastric resection on survival.10,11,12,13,14,15,16,17 The Dutch Gastric Cancer Trial found that palliative resection (total or partial gastrectomy) may be more beneficial and increase the survival rate to 8.1 vs. 5.4 months, a statistically significant difference in the patient group aged less than 70 years with a tumor burden limited to one metastatic site.10 In a retrospective study that compared the data from 677 patients who underwent palliative resection and 532 patients who received non-surgical palliation in 21 French surgical centers, the median survival was 11.9 months versus 8.5 months in the surgery and non-surgery groups, respectively (P<0.001). In this study, subgroup analyses demonstrated that this benefit was further increased in patients selected by tumor and patient related factors. The investigators recommended palliative resection based on their results.15
In a study that investigated survival after surgery of 82 patients diagnosed with preoperative metastatic gastric and gastroesophageal cancer at the MD Anderson Cancer Center, patients that underwent explorative surgery, PTR with or without resection of the metastatic site, and resection of the metastatic site only, median survival for all patients was 1.5 years (range, 0.1~14 years). Five year OS for patients with peritoneal metastases, positive cytology only, distant lymph nodes, and distant organ involvement was 13%, 42%, 20%, and 34%, respectively. This study demonstrated that surgery increases the survival rate in stage IV patients.16
Several Japanese studies conducted in East Asia, where half of the annual cases of the disease occur, investigated the efcfte of tumor burden on palliative surgery.18,19,20 One study demonstrated that peritoneal involvement impairs quality of life but does not affect survival, and that palliative resection shows benefits for survival.18 Another study with similar results demonstrated that peritoneal metastasis does not have prognostic relevance, and that palliative surgery shows benefits for survival among patients without liver metastases.19 In another study, the patients were divided into four groups: unresectable tumor, hepatic metastasis, peritoneal metastasis, and distant lymph node metastasis; the impact of palliative surgery on survival was then examined. The 5-year survival rate was 14% in the patients with one factor and 6% in those with two factors. The authors concluded that if the prognostic factors are well-defined preoperatively, surgery will be beneficial in the presence of one factor but not in the presence of multiple factors.20
Numerous studies, some of which are limited to a selected patient population, have demonstrated no benefits of PTR on survival of mGC. In a retrospective study of 289 patients, 10 patients had undergone emergency surgery on diagnosis, 110 patients had undergone elective surgery (46 patients with palliative resection [group A], and 64 patients with surgery without resection, such as laparoscopy, laparotomy, or G/J tube [group B]) and 169 patients had not undergone surgery. When the elective surgery patient group (group A and B) was compared with the patient group without surgery, the median OS did not reach statistical significance (8.6 months [group A] vs. 9.2 months [group B] vs. 7.7 months; P>0.05). Based on these results, the authors stated that non-curative resection (excluding emergency situations) significantly increased perioperative mortality and morbidity and was also associated with limited survival, making it unsuitable for locally advanced and metastatic disease because it delayed chemotherapy. Therefore, it should be limited only to a selected patient group.21
We examined our data in the context of the ongoing debates and of studies with opposing views on PTR for mGC. In our study, both OS and PFS were longer for patients who had PTR for mGC. However, the most important limitation to our study is that it is of a retrospective design and no data were available on details about the surgery (total or subtotal), surgery related mortality and morbidity, hospital stay, regimens of first and second lines of chemotherapy, or the quality of life of the patients. In addition, patients with grade 1 or 2 tumors had PTR more frequently than patients with grade 3 tumors did. Since patients who did not have surgery were diagnosed through biopsies, some patients may have had insufficient pathological samples for tumor grading. This may have caused a difference in the ratios of grades in both groups, which may be a statistical limitation. To the author's knowledge, this is the first study to demonstrate a possible relationship between grade and PTR in patients with mGC. We also demonstrated that patients who had PTR for mGC received significantly higher rates of second line chemotherapy. We hypothesize that tumor grade could be an important marker for operability in patients with mGC. PTR may be a treatment option to improve PFS and OS results, especially for patients with low-grade tumors.
Acknowledgement
We would like to thank Dr. Gokhan Tazegul for his efforts in his help with the manuscript.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364–367. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Feng XY, Chen YB, Chen S, Li YF, Zhou ZW, Li W, et al. Relationship between the number of lymph node detection and prognosis in stage II gastric cancer after D(2) dissection. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13:346–349. [PubMed] [Google Scholar]
- 4.Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw. 2010;8:437–447. doi: 10.6004/jnccn.2010.0033. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN) NCCN Guidelines; Gastric Cancer [Internet] Fort Washington (PA): NCCN; [cited 2015 Dec 13]. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 6.Meza-Junco J, Au HJ, Sawyer MB. Trastuzumab for gastric cancer. Expert Opin Biol Ther. 2009;9:1543–1551. doi: 10.1517/14712590903439702. [DOI] [PubMed] [Google Scholar]
- 7.Kasakura Y, Ajani JA, Mochizuki F, Morishita Y, Fujii M, Takayama T. Outcomes after emergency surgery for gastric perforation or severe bleeding in patients with gastric cancer. J Surg Oncol. 2002;80:181–185. doi: 10.1002/jso.10127. [DOI] [PubMed] [Google Scholar]
- 8.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 9.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 10.Hartgrink HH, Putter H, Klein Kranenbarg E, Bonenkamp JJ, van de Velde CJ Dutch Gastric Cancer Group. Value of palliative resection in gastric cancer. Br J Surg. 2002;89:1438–1443. doi: 10.1046/j.1365-2168.2002.02220.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JZ, Lu HS, Huang CM, Wu XY, Wang C, Guan GX, et al. Outcome of palliative total gastrectomy for stage IV proximal gastric cancer. Am J Surg. 2011;202:91–96. doi: 10.1016/j.amjsurg.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Bozzetti F, Bonfanti G, Audisio RA, Doci R, Dossena G, Gennari L, et al. Prognosis of patients after palliative surgical procedures for carcinoma of the stomach. Surg Gynecol Obstet. 1987;164:151–154. [PubMed] [Google Scholar]
- 13.Huang KH, Wu CW, Fang WL, Chen JH, Lo SS, Wang RF, et al. Palliative resection in noncurative gastric cancer patients. World J Surg. 2010;34:1015–1021. doi: 10.1007/s00268-010-0467-7. [DOI] [PubMed] [Google Scholar]
- 14.Kunisaki C, Makino H, Takagawa R, Oshima T, Nagano Y, Fujii S, et al. Impact of palliative gastrectomy in patients with incurable advanced gastric cancer. Anticancer Res. 2008;28:1309–1315. [PubMed] [Google Scholar]
- 15.Mariette C, Bruyère E, Messager M, Pichot-Delahaye V, Paye F, Dumont F, et al. Palliative resection for advanced gastric and junctional adenocarcinoma: which patients will benefit from surgery? Ann Surg Oncol. 2013;20:1240–1249. doi: 10.1245/s10434-012-2687-6. [DOI] [PubMed] [Google Scholar]
- 16.Badgwell B, Roy-Chowdhuri S, Chiang YJ, Matamoros A, Blum M, Fournier K, et al. Long-term survival in patients with metastatic gastric and gastroesophageal cancer treated with surgery. J Surg Oncol. 2015;111:875–881. doi: 10.1002/jso.23907. [DOI] [PubMed] [Google Scholar]
- 17.Dittmar Y, Rauchfuss F, Goetz M, Jandt K, Scheuerlein H, Heise M, et al. Non-curative gastric resection for patients with stage 4 gastric cancer: a single center experience and current review of literature. Langenbecks Arch Surg. 2012;397:745–753. doi: 10.1007/s00423-012-0902-3. [DOI] [PubMed] [Google Scholar]
- 18.Ouchi K, Sugawara T, Ono H, Fujiya T, Kamiyama Y, Kakugawa Y, et al. Therapeutic significance of palliative operations for gastric cancer for survival and quality of life. J Surg Oncol. 1998;69:41–44. doi: 10.1002/(sici)1096-9098(199809)69:1<41::aid-jso8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi S, Arai Y, Morise M, Kobayashi N, Tsukamoto H, Shimao H, et al. Gastric cancer with metastases to the distant peritoneum: a 20-year surgical experience. Hepatogastroenterology. 1998;45:1183–1188. [PubMed] [Google Scholar]
- 20.Maekawa S, Saku M, Maehara Y, Sadanaga N, Ikejiri K, Anai H, et al. Surgical treatment for advanced gastric cancer. Hepatogastroenterology. 1996;43:178–186. [PubMed] [Google Scholar]
- 21.Schmidt B, Look-Hong N, Maduekwe UN, Chang K, Hong TS, Kwak EL, et al. Noncurative gastrectomy for gastric adenocarcinoma should only be performed in highly selected patients. Ann Surg Oncol. 2013;20:3512–3518. doi: 10.1245/s10434-013-3024-4. [DOI] [PubMed] [Google Scholar]