Abstract
Purpose
The incidence of gallstones after gastrectomy for gastric cancer is higher than in the general population. However, the causes and mechanisms of post-gastrectomy gallstones are unclear. The aim of this study was to evaluate the incidence of gallstone formation and the risk factors for their development after gastrectomy for gastric cancer.
Materials and Methods
Of 1,744 gastric cancer patients who underwent gastrectomy at Seoul National University Bundang Hospital between January 2010 and December 2012, 1,284 were included in this study and retrospectively reviewed. Patients' age, sex, body mass index (BMI), tumor location, stage, type of gastrectomy, type of reconstruction, and extent of node dissection were evaluated.
Results
The incidence of gallstones after gastrectomy for gastric cancer was significantly higher in men than in women (P=0.019). Exclusion of the duodenum during reconstruction was associated with a significantly higher incidence of gallstones (P=0.003). Overweight and obese patients with BMI ≥23 kg/m2 had significantly higher incidence of gallstones than those with a lower BMI (P=0.006). Multivariate analysis showed that obesity (hazard ratio, HR=1.614; 95% confidence interval, CI: 1.135~2.296; P=0.008), male sex (HR=1.515, 95% CI: 1.029~2.231, P=0.033), and exclusion of the duodenum (HR=1.648, 95% CI: 1.192~2.280, P=0.003) were significant, independent risk factors for gallstones after gastrectomy.
Conclusions
The cumulative incidence of gallstones for 5 years after gastrectomy was 15.3%. Male sex, obesity, and exclusion of the duodenum were risk factors for gallstone formation after gastrectomy. Careful surveillance will be required for these patient groups after gastrectomy.
Keywords: Gallstones, Stomach neoplasms, Gastrectomy, Incidence, Risk factors
Introduction
With the improvement of surgical techniques and postoperative comprehensive treatments, the survival time of patients with gastric cancer has extended after surgery; therefore, more attention has been paid to postoperative quality of life. The incidence of gallstones after gastrectomy for gastric cancer is higher than in the general population.1,2,3,4,5 Gallstones after gastrectomy may lead to cholecystitis, which requires further surgical treatment. Cholecystectomy after gastrectomy is a challenging procedure that can seriously affect quality of life; the incidence and risk factors of gallstone formation after gastric cancer surgery need to be reevaluated.
The causes and mechanisms underlying the development of post-gastrectomy gallstones are unclear. Pathophysiologic changes associated with gallstone formation after gastrectomy include alterations in gallbladder motility, release of cholecystokinin (CCK), and gallbladder responses.2,6,7,8 Gallstones may also result from surgical sequel such as resection of the vagal nerve and nonphysiological reconstruction of the gastrointestinal tract.2,6,7,8 The aim of this study was to evaluate the incidence of gallstone formation and the risk factors for their development after gastrectomy for gastric cancer.
Materials and Methods
1. Patients
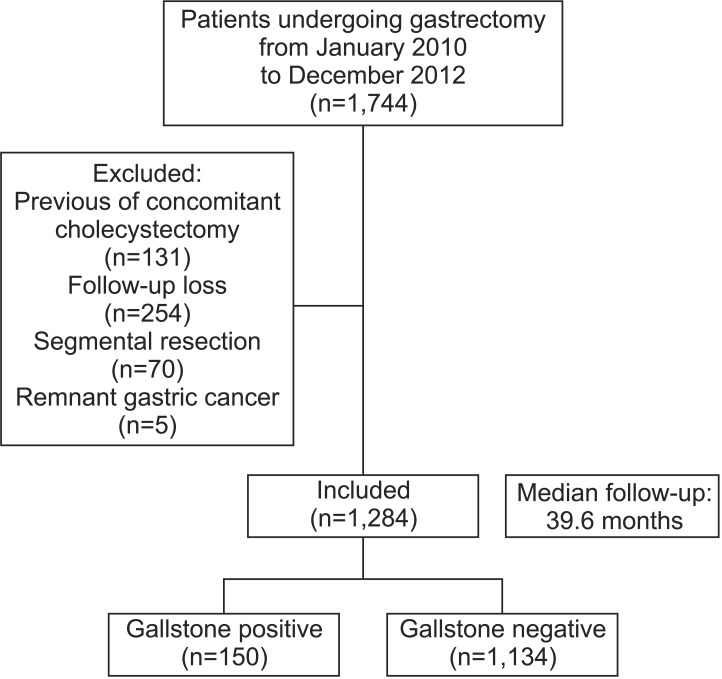
Between January 2010 and December 2012, 1,744 patients with gastric cancer underwent gastrectomy at the Seoul National University Bundang Hospital. Gallbladders were routinely evaluated by performing a computed tomography (CT) scan before each gastrectomy and cholecystectomy was performed if gallstones were detected. Of these 1,744 patients, 460 patients were excluded; 24 patients who had a history of cholecystectomy, 107 patients who underwent cholecystectomy at the time of their gastrectomy, 70 patients who had undergone segmental resection of the stomach, 5 patients who had remnant gastric cancer, and 254 patients who followed-up with other hospitals or failed to follow-up. The remaining 1,284 patients were analyzed for this study (Fig. 1). Patients' age, sex, preoperative body mass index (BMI), tumor location and stage, types of gastrectomy and reconstruction, and extent of node dissection were evaluated.
Fig. 1. Flow chart showing patient characteristics following gastrectomy.
All of the 1,284 patients included patients underwent distal gastrectomy (DG), total gastrectomy (TG), proximal gastrectomy (PG), or pylorus-preserving gastrectomy (PPG), and systemic lymph node dissection. The reconstruction methods after gastrectomy included Billroth I or II anastomosis or Roux-en Y or uncut Roux-en Y reconstruction for DG, the Roux-en-Y method for TG, esophagogastrostomy or double tract reconstruction for PG, and gastrogastrostomy for PPG. The patients were followed for a median of 39.6 months, in accordance with institutional protocol. Ultrasonography (USG) was performed 3 months after surgery and an abdominal CT scan was performed 3 months after the USG. USG and CT scans were then performed alternatively every 3 months. After 3 years, an abdominal CT scan was generally performed every year until the end of the follow-up. Although the primary purpose of these examinations was to detect metastatic disease, gallbladder information was recorded in every examination. Patients were routinely followed-up for 5 years after their surgery. This study was approved by the ethics committee of the Seoul National University Bundang Hospital (No. B-1512-328-115). All patients provided informed consent for surgery.
2. Statistical analysis
Categorical data were compared by using the Pearson χ2 test or Fisher's exact test, as appropriate. The total incidence of gallstones after gastrectomy was evaluated by using the Kaplan-Meier method and differences between groups were evaluated by performing the log rank test. We analyzed patients who died during the follow-up as censored. Multivariate analysis with the Cox proportional hazards model was used to evaluate the risk factors for gallstones after gastric cancer surgery. P-values of less than 0.05 were considered to be statistically significant. All statistical analysis were performed with IBM SPSS ver. 21 for Windows (IBM Co., Armonk, NY, USA).
Results
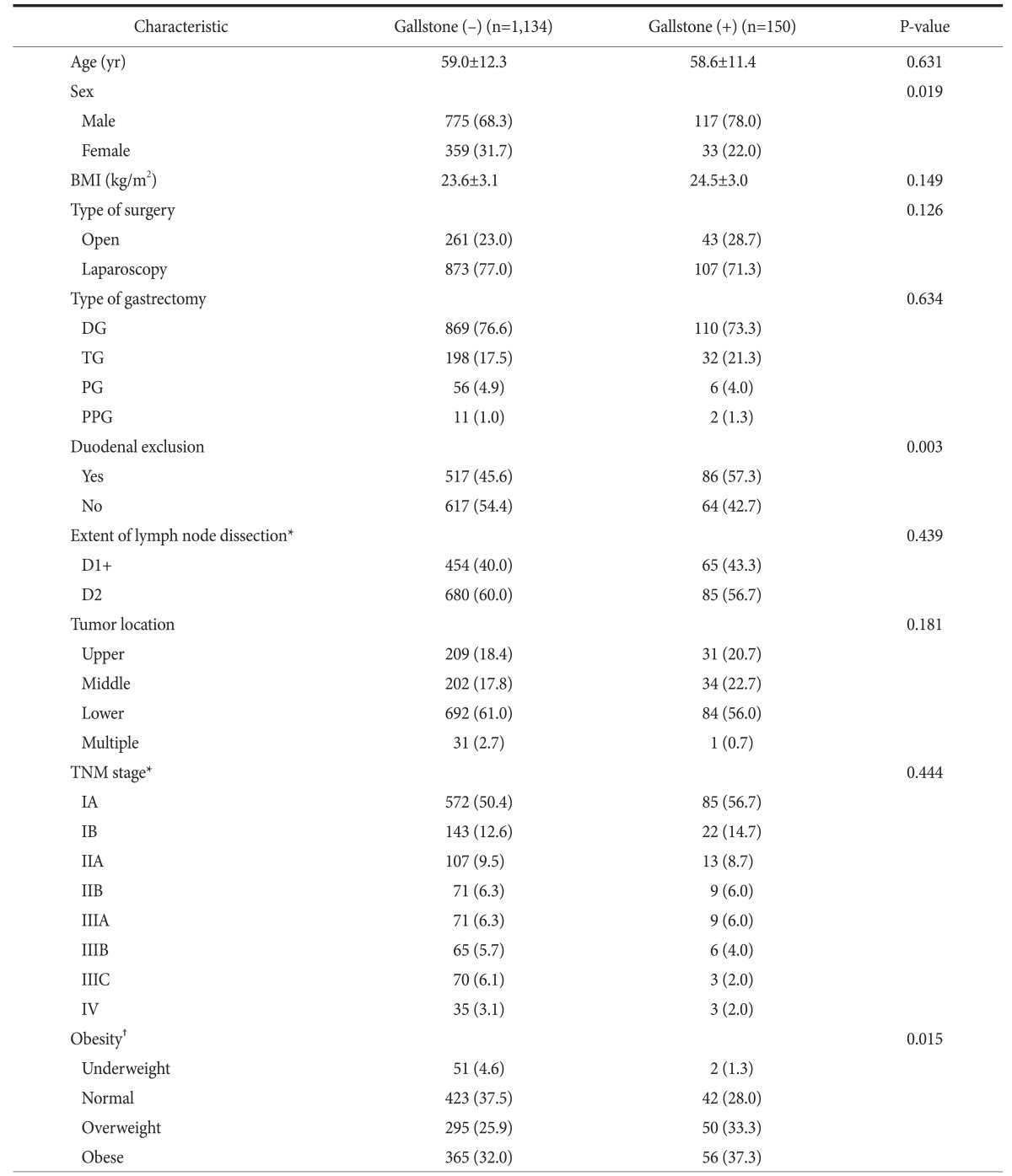
Of 1,284 patients included in this study, 892 were men (69.5% ) and the median age was 59.0 years. The median follow-up period was 39.6 months. The characteristics of these patients are shown in Table 1. Gallstone formation was observed in 150 of these patients. The incidence of gallstones 5 years after gastrectomy was 15.3% (Fig. 2A). The median interval between gastrectomy and the diagnosis of gallstones was 16.9 months. The incidence of gallstones at 5 years was significantly higher in men than in women (16.1% vs. 13.4%; P=0.019) (Fig. 2B).
Table 1. Characteristics of the 1,284 patients included in this study.
Values are presented as mean±standard deviation or number (%). BMI = body mass index; DG = distal gastrectomy; TG = total gastrectomy; PG = proximal gastrectomy; PPG = pylorus-preserving gastrectomy. *According to the 7th Union for International Cancer Control/American Joint Committee on Cancer TNM system. †Underweight, BMI<18.5 kg/m2; normal, BMI 18.5~23.0 kg/m2; overweight, BMI 23.0~25.0 kg/m2; obese, BMI≥25.0 kg/m2.
Fig. 2. (A) Cumulative incidence of gallstones after gastrectomy. (B) Incidence of post-gastrectomy gallstones in men and women (P<0.01, log rank test). (C) Incidence of gallstones after gastrectomy in patients who underwent reconstruction with or without duodenal exclusion (P<0.01, log rank test). (D) Incidence of post-gastrectomy gallstones in overweight and obese patients (≥23 kg/m2) vs. normal and underweight (<23 kg/m2) patients (P<0.001, log rank test). BMI = body mass index.
To examine the influence that food passage through the duodenum has on the incidence of gallstones, patients who had reconstructions that excluded the duodenum (Billroth II, Roux-en Y, and uncut Roux-en Y) were compared to patients in whom passage through the duodenum was maintained (Billroth I, PG, and PPG). The incidence of gallstones was significantly higher in the group with duodenal exclusion (P=0.003) (Fig. 2C).
To evaluate the influence of degree of obesity on gallstone formation, patients were divided into four groups based on BMI: underweight (BMI<18.5 kg/m2), normal (18.5 kg/m2≤BMI <23.0 kg/m2), overweight (23.0 kg/m2≤BMI<25.0 kg/m2), and obese (25.0 kg/m2≤BMI). The prevalence of gallstones tended to be higher in the obese group than in the non-obese groups; however, the difference was not statistically significant. The incidence of gallstones was significantly higher in overweight and obese patients (P=0.006) compared to the incidence in underweight and normal patients (Fig. 2D).
Gallstones developed in 43 patients (14.1%) of the 304 patients who underwent open surgery and in 107 patients (10.9%) of the 980 patients who underwent laparoscopic surgery. While the incidence was higher in the open group, there was no significant difference between the two groups (P=0.126). Of the 765 patients who underwent gastrectomy with standard D2 or D2+ lymph node dissection, 85 patients (11.1%) developed gallstones, compared to development of gallstones in 65 patients (12.5%) of 519 patients who underwent gastrectomy with D1+ or D1 lymph node dissection (P=0.439).
The incidence of gallstones for each type of gastrectomy was evaluated. Gallstones developed in 110 patients (11.2%) of the 979 patients who underwent DG, 32 patients (13.9%) of the 230 patients who underwent TG, 6 patients (9.7%) of the 62 patients who underwent PG, and 2 patients (15.4%) of the 13 patients who underwent PPG. There were no significant differences between the incidence of gallstones and the type of gastrectomy (P=0.634). Combining patients who underwent DG, PG, and PPG into a partial gastrectomy group, the incidences of gallstones in patients who underwent partial gastrectomy and TG were 11.2% and 13.9%, respectively. Although the incidence was higher in the TG group, the difference was not statistically significant (P=0.245).
The incidence of gallstones was also evaluated according to the type of reconstruction after DG. Gallstones developed in 56 patients (9.2%) of the 606 patients reconstructed by performing Billroth I anastomosis, 11 patients (13.4%) of 82 patients who underwent Billroth II reconstruction, 5 patients (22.7%) of 22 patients were reconstructed by the Roux-en-Y procedure, and 38 patients (14.1%) of 269 patients who underwent the uncut Roux-en-Y procedure. The proportion of patients who developed gallstones was significantly higher after a Roux-en-Y procedure than after a Billroth I anastomosis in the DG group (P=0.036).
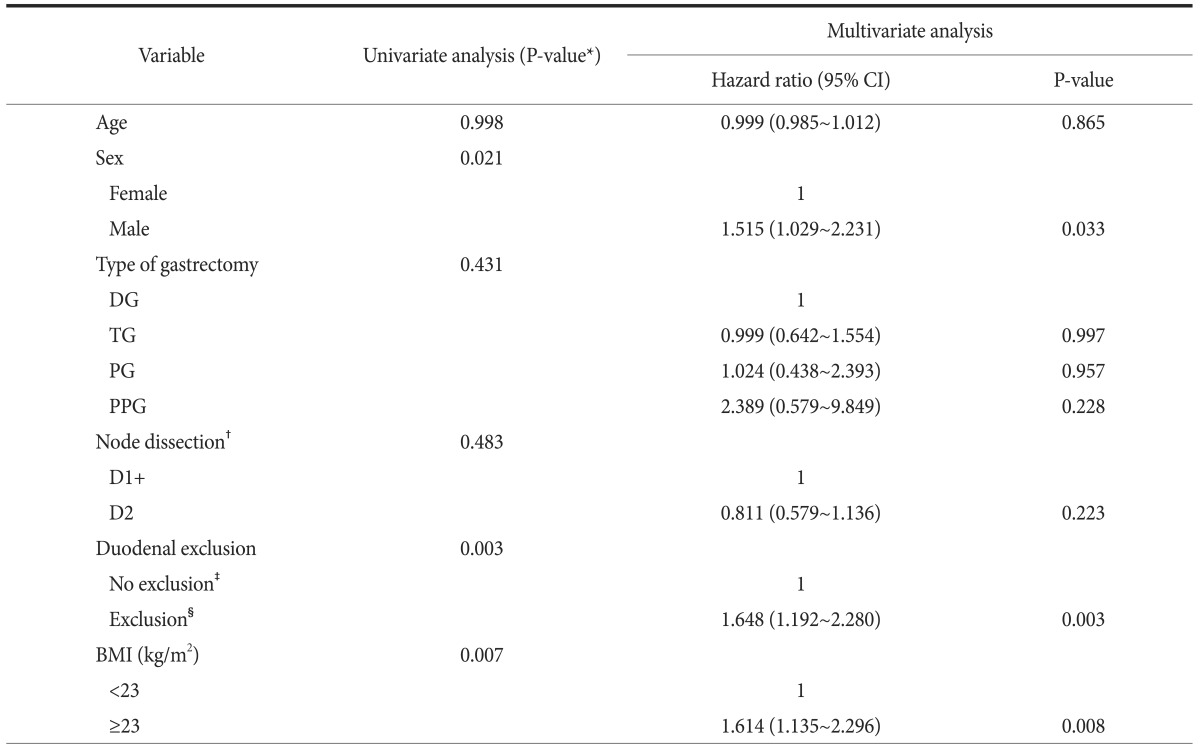
Variables that showed significant difference on using univariate analysis and were known risk factors for gallstone development after gastrectomy were selected for multivariate analysis. Multivariate analysis performed by using the Cox proportional hazard model showed that obesity, male sex, and exclusion of the duodenum were significant independent risk factors for gallstone development after gastrectomy (Table 2).
Table 2. Multivariate analysis of risk factors for gallstones after gastrectomy.
DG = distal gastrectomy; TG = total gastrectomy; PG = proximal gastrectomy; PPG = pylorus-preserving gastrectomy; BMI = body mass index; CI = confidence interval. *Cox proportional hazards model. †According to the 7th Union for International Cancer Control/American Joint Committee on Cancer TNM system. ‡Billroth I; proximal gastrectomy = pylorus preserving gastrectomy. §Billroth II; Roux-en-Y = uncut Roux-en-Y.
Discussion
In Asian countries, the prevalence of gallstone disease in the general population ranges from 3% to 10%.9 Thus, the rate of gallstone formation is higher after gastrectomy than in the general population. In this study, the cumulative incidence of gallstones 5 years after gastrectomy was 15.2%, which is within the previously reported range of 15% to 25%. 1,2,3,5,10,11,12
The incidence of gallstone formation differed significantly in patients who underwent Billroth I and Roux-en-Y reconstruction for DG. The reason for the higher incidence of gallstone formation in the Roux-en-Y group was evaluated further by analyzing the influence that excluding the duodenum from reconstruction had on the development of gallstones. Exclusion of the duodenum during reconstruction has been associated with gallstone development after gastrectomy.2,4,5 Food passage through the duodenum stimulates the secretion of CCK, which induces contractions of the gallbladder. If the duodenum is excluded, food would directly enter the jejunum, reducing CCK secretion and gallbladder contractions, thus increasing the risk of gallstones.
We found that the incidence of gallstones after gastrectomy was higher in overweight and obese patients than in those who were underweight and normal weight, which supports previous findings showing an association between high BMI and gallstone disease.13,14,15,16 BMI is the method most frequently used to assess adiposity. Obesity may contribute to the increased hepatic secertion of cholesterol, a key event in the development of cholesterol gallstones.14,15 Weight cycling (i.e., weight loss and regain) and larger weight fluctuation are risk factors for gallstone disease.17 In general, a larger number of obese patients lost a great amount of weight after gastrectomy, and showed a larger weight fluctuation, suggesting a link between higher BMI and a higher incidence of gallstone formation after gastrectomy. Although gallstone incidence is reported to be higher in women than in men,13,18,19,20 this study found that the incidence of gallstones after gastrectomy was higher in men. The mechanism underlying this phenomenon has not yet been clearly revealed; it remains uncertain and more studies are needed.
This study also assessed the incidence of post-gastrectomy gallstones in subgroups of patients classified by types of gastrectomy. Patients undergoing TG were reported to have a higher incidence of gallstones than patients undergoing other types of gastrectomy.2,4 Although this study observed a higher incidence in patients who underwent TG, these differences were not statistically significant, the rate of gallstone development did not differ by type of gastrectomy.
Although a higher degree of lymph node dissection has been associated with a higher incidence of gallstones after gastrectomy,3,8,11,21,22 we observed no significant difference in the rate of gallstone formation between the D1 plus D1+ groups and the D2 plus D2+ groups, although the incidence was higher in the D1 plus D1+ groups. The incidence of gallstones is reported to be significantly higher in patients who underwent lymph node dissection in the hepatoduodenal ligament than in those who did not.2 Our facility started performing laparoscopic gastrectomy in May 2003; 75% of these operations between 2010 and 2012 were performed laparoscopically. Laparoscopic surgery has several advantages over traditional open surgery, including a magnified view that allows for more precise surgery; efforts were always made to identify and preserve the vagus nerve during dissection around the hepatoduodenal ligament. We found that the incidence of gallstones was not different between these two groups.
This study had several limitations, including its retrospective design. In addition, the preoperative work-up for gallstones were performed with CT scan, which is recognized as being less accurate than ultrasound in detecting gallstones. Therefore, gallstones present before gastrectomy may have been missed and we may have overestimated the incidence of post-gastrectomy gallstones. Prospective, multicenter, well-designed clinical studies in larger patient cohorts are needed to evaluate the incidence of and factors relating to post-gastrectomy gallstones.
In conclusion, multivariate analysis in this study showed that obesity, male sex, and exclusion of the duodenum increased the incidence of post-gastrectomy gallstones. Obese male patients should be carefully followed-up due to their increased potential to develop gallstones after gastrectomy. In addition, reconstruction that maintains the duodenum may better prevent gallstones after gastrectomy.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Hwang JY, Lee JH, Chi KC, Park SI. Clinical review of cholelithiasis after gastric resection in gastric cancer patients. J Korean Surg Soc. 2004;67:198–203. [Google Scholar]
- 2.Kobayashi T, Hisanaga M, Kanehiro H, Yamada Y, Ko S, Nakajima Y. Analysis of risk factors for the development of gallstones after gastrectomy. Br J Surg. 2005;92:1399–1403. doi: 10.1002/bjs.5117. [DOI] [PubMed] [Google Scholar]
- 3.Fukagawa T, Katai H, Saka M, Morita S, Sano T, Sasako M. Gallstone formation after gastric cancer surgery. J Gastrointest Surg. 2009;13:886–889. doi: 10.1007/s11605-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 4.Chen XJ, Li N, Huang YD, Ren S, Liu F, Chen L, et al. Factors for postoperative gallstone occurrence in patients with gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:877–881. doi: 10.7314/apjcp.2014.15.2.877. [DOI] [PubMed] [Google Scholar]
- 5.Jun KH, Kim JH, Kim JJ, Chin HM, Park SM. Retrospective analysis on the gallstone disease after gastrectomy for gastric cancer. Gastroenterol Res Pract. 2015;2015:827864. doi: 10.1155/2015/827864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue K, Fuchigami A, Hosotani R, Kogire M, Huang YS, Miyashita T, et al. Release of cholecystokinin and gallbladder contraction before and after gastrectomy. Ann Surg. 1987;205:27–32. [PMC free article] [PubMed] [Google Scholar]
- 7.Hahm J, Park J, Cho Y, Eun C, Lee Y, Choi H, et al. Changes in gallbladder motility in gastrectomized patients. Korean J Intern Med. 2000;15:19–24. doi: 10.3904/kjim.2000.15.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi SQ, Ohta T, Tsuchida A, Terayama H, Naito M, Li J, et al. Surgical anatomy of innervation of the gallbladder in humans and Suncus murinus with special reference to morphological understanding of gallstone formation after gastrectomy. World J Gastroenterol. 2007;13:2066–2071. doi: 10.3748/wjg.v13.i14.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang YR, Jang JY, Kwon W, Park JW, Kang MJ, Ryu JK, et al. Changes in demographic features of gallstone disease: 30 years of surgically treated patients. Gut Liver. 2013;7:719–724. doi: 10.5009/gnl.2013.7.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura K, Ogoshi K, Makuuchi H. Clinicopathological study of cholelithiasis following gastric cancer surgery. Eur Surg Res. 2005;37:29–35. doi: 10.1159/000083145. [DOI] [PubMed] [Google Scholar]
- 11.Akatsu T, Yoshida M, Kubota T, Shimazu M, Ueda M, Otani Y, et al. Gallstone disease after extended (D2) lymph node dissection for gastric cancer. World J Surg. 2005;29:182–186. doi: 10.1007/s00268-004-7482-5. [DOI] [PubMed] [Google Scholar]
- 12.Sugita H, Kojima K, Inokuchi M, Kato K. Long-term outcomes of laparoscopic gastrectomy for gastric cancer. J Surg Res. 2015;193:190–195. doi: 10.1016/j.jss.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 13.Unisa S, Jagannath P, Dhir V, Khandelwal C, Sarangi L, Roy TK. Population-based study to estimate prevalence and determine risk factors of gallbladder diseases in the rural Gangetic basin of North India. HPB (Oxford) 2011;13:117–125. doi: 10.1111/j.1477-2574.2010.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stender S, Nordestgaard BG, Tybjaerg-Hansen A. Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a Mendelian randomization study. Hepatology. 2013;58:2133–2141. doi: 10.1002/hep.26563. [DOI] [PubMed] [Google Scholar]
- 15.Radmard AR, Merat S, Kooraki S, Ashraf M, Keshtkar A, Sharafkhah M, et al. Gallstone disease and obesity: a population-based study on abdominal fat distribution and gender differences. Ann Hepatol. 2015;14:702–709. [PubMed] [Google Scholar]
- 16.Aune D, Norat T, Vatten LJ. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur J Epidemiol. 2015;30:1009–1019. doi: 10.1007/s10654-015-0081-y. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Weight cycling and risk of gallstone disease in men. Arch Intern Med. 2006;166:2369–2374. doi: 10.1001/archinte.166.21.2369. [DOI] [PubMed] [Google Scholar]
- 18.Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Ikematsu H, Noguchi A, et al. Prevalence of gallstone disease in a general population of Okinawa, Japan. Am J Epidemiol. 1988;128:598–605. doi: 10.1093/oxfordjournals.aje.a115007. [DOI] [PubMed] [Google Scholar]
- 19.Heaton KW, Braddon FE, Mountford RA, Hughes AO, Emmett PM. Symptomatic and silent gall stones in the community. Gut. 1991;32:316–320. doi: 10.1136/gut.32.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun H, Tang H, Jiang S, Zeng L, Chen EQ, Zhou TY, et al. Gender and metabolic differences of gallstone diseases. World J Gastroenterol. 2009;15:1886–1891. doi: 10.3748/wjg.15.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando S, Tsuji H. Surgical technique of vagus nerve-preserving gastrectomy with D2 lymphadenectomy for gastric cancer. ANZ J Surg. 2008;78:172–176. doi: 10.1111/j.1445-2197.2007.04396.x. [DOI] [PubMed] [Google Scholar]
- 22.Inokuchi M, Sugita H, Otsuki S, Sato Y, Nakagawa M, Kojima K. Long-term effectiveness of preserved celiac branch of vagal nerve after Roux-en-Y reconstruction in laparoscopy-assisted distal gastrectomy. Dig Surg. 2014;31:341–346. doi: 10.1159/000368703. [DOI] [PubMed] [Google Scholar]