Abstract
Objective
The aim of this study was to compare survival outcomes in two groups of patients with recurrent epithelial ovarian cancer (EOC) with initial recurrence detection by cancer antigen 125 (CA-125) elevation or imaging, and underwent secondary cytoreductive surgery (SCS).
Methods
A retrospective review of the medical records was performed on 99 recurrent EOC patients who underwent SCS at the Samsung Medical Center between January 2002 and December 2013. For follow-up after primary treatment, patients were routinely assessed by CA-125 levels every 3 months and computed tomography (CT) scan (or magnetic resonance imaging [MRI]) every 6 months for first 3 years, and by CA-125 every 6 months and CT scan (or MRI) every 12 months thereafter.
Results
The first recurrence was initially identified by either CA-125 elevation (n=41, 41.4%) or by imaging study (n=58, 58.6%). None of the patients showed the symptoms as initial sign of recurrence. There were higher percentages of extra-pelvic recurrence (87.8%) and multiple recurrences (78.0%) in the group diagnosed by CA-125 elevation. The proportion of no residual disease after SCS was comparably lower in the CA-125 group (22.0% vs. 72.4%). There were 19 cancer-associated deaths (19.2%) within a median follow-up period of 67 months. The group diagnosed by imaging had better overall survival from initial diagnosis (OS1), overall survival after SCS (OS2), progression-free survival after the initial treatment (PFS1) and progression-free survival after SCS compared to those of the CA-125 group (PFS2).
Conclusion
EOC patients with recurrence initially detected by imaging study showed better survival outcomes than patients diagnosed by CA-125 elevation.
Keywords: Diagnostic Techniques and Procedures, Ovarian Neoplasms, Recurrence
INTRODUCTION
Epithelial ovarian cancer (EOC) is known to be strongly associated with malignancy-related death in women [1,2]. In most cases, EOC is asymptomatic until late in the disease course. This delay in diagnosis is related to poor prognosis. Debulking surgery and chemotherapy are the primary therapies for EOC. Most patients respond to primary treatment; 75% of patients have a complete response. However, 40% to 60% of all EOC patients and 75% of patients with advanced stages eventually experience recurrence [3,4,5,6,7]. Diagnosing and effectively treating recurrent disease is considerably important in clinical management.
After primary treatment for EOC, the current National Comprehensive Cancer Network (NCCN) guideline recommends that patients followed up every 2 to 4 months for 2 years, then 3 to 6 months for 3 years, then annually after 5 years. During the follow-up period, patients are screened for recurrence based upon their clinical signs, serum tumor marker (cancer antigen 125 [CA-125]) levels, or radiologic studies [8,9]. Monitoring CA-125 alone is insufficient to detect recurrent disease [10,11,12,13]. Similarly, it is not currently advised to treat asymptomatic patients who have elevated tumor markers, but no imaging evidence of recurrence [10,11,12,13].
There is no defined standard treatment for recurrent EOC at this time. Currently, the NCCN guideline suggests that physicians consider secondary cytoreductive surgery (SCS) for platinum sensitive patients with radiologic and/or clinical relapse. Although the results of randomized trials addressing the role of SCS (GOG213, DESKTOP III, SOCceR) are not yet published, a Cochrane review based on the results of previous retrospective studies reported that complete reduction in SCS is associated with significant improvement in overall survival (OS) [14].
There has been number of criteria for selecting appropriate candidates for SCS [15,16]. However, negative predictive value of scores for surgical SCS continues to be poor [17] and additional information is needed for selecting candidates for SCS. In this study, we sought to understand which patients with recurrent EOC might be appropriate for SCS in addition to prior selection criteria. In order to do so, we compared survival outcomes in two groups of patients that differed by how their recurrence was initially diagnosed.
MATERIALS AND METHODS
We performed a retrospective review of all EOC patients who underwent surgery at Samsung Medical Center between January 2002 and December 2013. Data were obtained from the electric medical records. This study was approved by the Institutional Review Board.
1. Patients
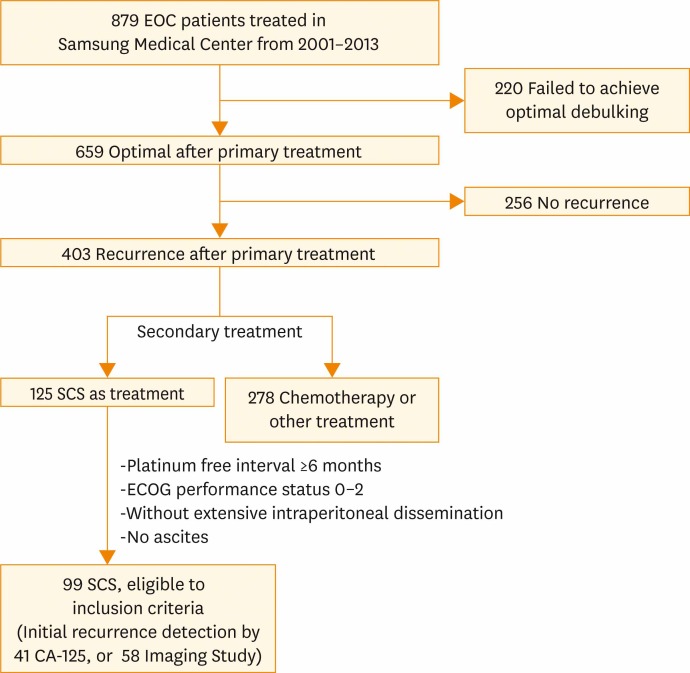
Among patients who had primary treatment for EOC in Samsung Medical Center, 99 EOC patients were identified for having SCS after recurrence. Patients who had undergone SCS were included according to the following inclusion criteria: (1) optimal debulking after primary treatment, (2) platinum-free interval (PFI) ≥6 months, (3) good performance status before surgery as defined by Eastern Cooperative Oncology Group (performance status 0 to 2), (4) single or multiple recurrence sites without extensive intraperitoneal dissemination or ascites on imaging. These inclusion criteria were based on those used in other major retrospective studies [16,18,19]. None of the candidates had received chemotherapy prior to SCS. Flowchart of included patients is shown in Fig. 1.
Fig. 1.
Flowchart of included patients. CA-125, cancer antigen 125; ECOG, Eastern Cooperative Oncology Group; EOC, epithelial ovarian cancer; SCS, secondary cytoreductive surgery.
Patients who underwent SCS were divided into two groups according to the initial diagnostic methods: CA-125 elevation or positive imaging findings. None of the patients showed the symptoms as initial sign of recurrence. The diagnostic criteria for recurrence or metastasis during the follow-up period included rising CA-125 levels or clinical signs of recurrence, which include positive conventional imaging findings.
2. Treatment and follow-up
The standard primary surgical treatment consisted of hysterectomy, bilateral salpingo-oophorectomy, omentectomy, retroperitoneal (pelvic and paraaortic) lymphadenectomy, and tumorectomy of any metastatic lesions. Peritoneal washing was routinely performed. Peritoneal biopsies were performed if any abnormalities were identified. After primary debulking surgery (PDS), either abdominopelvic computed tomography (CT) or pelvic magnetic resonance imaging (MRI) was performed to confirm the disease status.
Within two weeks of surgery, patients started their first cycle of platinum based combination chemotherapy. Cycles were repeated every 3 weeks until 4 to 6 cycles were completed. The CA-125 level was routinely checked to determine whether CA-125 was in the normal range. After primary treatment, patients were assessed by physical examination, complete blood count and chemistries, including CA-125 levels, every 3 months for the first 3 years, and every 6 months thereafter. Chest radiography and abdominopelvic CT scan (or alternatively abdominopelvic MRI) were performed every 6 months for the first 3 years, and every 12 months thereafter (although this may not be standard follow-up schedule, it had been proceeding in our institution as routine protocol). Additional diagnostic procedures were performed according to specific clinical suspicions. If recurrence was suspected with symptoms or CA-125 elevation, additional imaging studies were performed. Recurrence could have been detected by imaging studies with or without CA-125 elevation. After detection of recurrence in EOC patients, time and method of secondary treatment were decided by physicians, usually SCS or chemotherapy. After secondary treatment was performed, the same follow up schedules and treatment were conducted as were those after PDS.
For each patient, the method of recurrence detection was determined from a review of the electric medical record. Detection was attributed to an office visit if the patient presented with symptoms of recurrence or clinical signs on exam. In contrast, detection was attributed to imaging if this was the first or only evidence of disease recurrence. Lastly, detection was attributed to CA-125 level if elevations in this marker were the initial abnormal finding leading to the diagnosis. Elevated CA-125 levels were defined as >35.0 U/mL by the laboratory at our institution. Forty-one of the 99 patients who underwent SCS were initially diagnosed with recurrence based on a CA-125 elevation. In contrast, 58 patients were diagnosed by imaging findings, and did not have elevated CA-125 levels. Patients with both CA-125 elevation and imaging findings were categorized as CA-125 elevation group, and no patients were diagnosed by symptoms alone.
3. Statistical analysis
Summary statistics were used to describe the data. Continuous variables are presented as medians (range). Categorical variables are presented as frequencies (percentages). The baseline clinical characteristics and study outcomes were compared between the two groups using the Mann-Whitney test for continuous variables, and the chi-square test or Fisher exact test for categorical variables, as appropriate. In order to define the recurrence pattern, the location of the recurrence site was divided into two categories. Recurrences in the pelvis occurring below the pelvic brim were considered pelvic recurrences. Extra-pelvic recurrences were those outside of this defined area. The spread patterns were classified as peritoneal, lymphatic, or hematogenous metastases. Peritoneal recurrences were defined as cases with a recurrence of metastatic lesions in the peritoneum of the pelvis and abdominal area. Hematogenous recurrences were defined as visceral metastases, such as in the liver and lung parenchyma. Lymphatic metastases were defined as metastases to lymph nodes, mostly pelvic and paraaortic, as well as distant lymph nodes. The Kaplan-Meier method was used for survival analysis. Multivariate analyses were performed with the Cox proportional hazards model to evaluate the importance of the recurrence type and other clinicopathological features as prognostic factors. Multivariate p-values were used to characterize the independence of these factors. A 95% CI was used to quantify the relationship between survival time and each independent factor. Statistical analyses were performed using SPSS ver. 21.0 (IBM Co., Armonk, NY, USA). A two-sided p≤0.05 was considered statistically significant. The PFI was defined as the time from the completion of primary treatment to recurrence, death or loss to follow-up. The overall survival from initial diagnosis (OS1) was described as the time between diagnosis and the patient's death or loss to follow-up. The overall survival after SCS (OS2) was described as the time from SCS to the date of the patient's death or loss to follow-up. We defined the progression-free survival from the initial treatment (PFS1) as the time between the diagnosis and first relapse, or the final follow-up visit. We defined progression-free survival from SCS (PFS2) as the period between the time of SCS to relapse, or the final follow-up visit.
RESULTS
The baseline patient characteristics are shown in Table 1. The largest proportion of patients had stage 3 (n=71, 71.7%), grade 3 (n=71, 71.7%), and serous type (n=70, 70.7%) disease. There were no statistically significant differences between the two groups with regard to age, BMI, stage, grade, histology or residual disease after PDS. In contrast, there were statistically significant differences in the median CA-125 levels at primary treatment as well as at recurrence between the two groups.
Table 1. Baseline patient characteristics.
| Characteristic | All patients (n=99) | Initial recurrence diagnosed by imaging (n=58) | Initial recurrence diagnosed by CA-125 elevation (n=41) | p-value | |
|---|---|---|---|---|---|
| Age (yr) | 49 (24–77) | 49.5 (24–77) | 49 (31–72) | 0.773* | |
| Body mass index (kg/m2) | 22.8 (16.3–31.2) | 22.7 (16.3–30.4) | 23.2 (18.0–31.2) | 0.137* | |
| Stage | 0.258† | ||||
| I | 9 (9.1) | 5 (8.6) | 4 (9.8) | ||
| II | 14 (14.1) | 11 (19.0) | 3 (7.3) | ||
| III | 71 (71.7) | 38 (65.5) | 33 (80.5) | ||
| IV | 5 (5.1) | 4 (6.9) | 1 (2.4) | ||
| Grade | 0.896† | ||||
| 1 | 4 (4.0) | 2 (3.4) | 2 (4.9) | ||
| 2 | 20 (20.3) | 12 (20.7) | 8 (19.5) | ||
| 3 | 71 (71.7) | 41 (70.7) | 30 (73.2) | ||
| Unknown | 4 (4.0) | 3 (5.2) | 1 (2.4) | ||
| Histology subtype | 0.496† | ||||
| Serous | 70 (70.7) | 41 (70.7) | 29 (70.7) | ||
| Endometrioid | 6 (6.0) | 5 (8.6) | 1 (2.4) | ||
| Clear cell | 3 (3.0) | 2 (3.4) | 1 (2.4) | ||
| Transitional | 2 (2.0) | 1 (1.7) | 1 (2.4) | ||
| Mixed | 7 (7.1) | 5 (8.6) | 2 (4.9) | ||
| Other | 11 (11.1) | 4 (6.9) | 7 (17.1) | ||
| CA-125 level at primary treatment (U/mL) | 527.0 (5.0–17,593.0) | 398.8 (5.0–11,800.0) | 783.0 (21.1–17,593.0) | 0.023* | |
| CA-125 level at recurrence (U/mL) | 24.8 (1.6–2,446.1) | 10.0 (1.6–34.0) | 79.5 (42.2–2,446.1) | <0.001* | |
| Residual after PDS | 0.371† | ||||
| No residual | 34 (34.3) | 22 (37.9) | 12 (29.3) | ||
| <1 cm | 65 (65.7) | 36 (62.1) | 29 (70.7) | ||
Values are presented as median (range) or number (%).
CA-125, cancer antigen 125; PDS, primary debulking surgery.
*Mann-Whitney test. †Fisher exact test.
Table 2 shows the recurrence patterns of the first relapse and the surgical characteristics of SCS. There were statistically significant differences in the locations and number of recurrence lesions. In the group with CA-125 elevation, 87.8% of patients had extra-pelvic recurrence, compared to 58.6% of the group diagnosed by imaging. A higher percentage of multiple recurrence lesions were found in the CA-125 elevation group (78%) than in the imaging diagnosis group (39.7%). Differences in recurrence sites and spread patterns (peritoneal/lymphatic/hematogenous) between two groups were not statistically significant.
Table 2. Recurrence patterns and surgical characteristics by group.
| Characteristic | All patients (n=99) | Initial recurrence diagnosed by imaging (n=58) | Initial recurrence diagnosed by CA-125 elevation (n=41) | p-value | |
|---|---|---|---|---|---|
| Recurrence site | 0.653* | ||||
| Peritoneal surface | 37 (37.4) | 25 (43.1) | 12 (29.3) | ||
| Mesentery and omentum | 15 (15.2) | 7 (12.1) | 8 (19.5) | ||
| Lymph nodes | 17 (17.2) | 10 (17.2) | 7 (17.1) | ||
| Spleen | 13 (13.1) | 8 (13.8) | 5 (12.2) | ||
| Liver | 12 (12.1) | 6 (10.3) | 6 (14.6) | ||
| Diaphragm | 5 (5.1) | 2 (3.4) | 3 (7.3) | ||
| Location of recurrence | 0.002* | ||||
| Pelvic | 29 (29.3) | 24 (41.4) | 5 (12.2) | ||
| Extra-pelvic | 70 (70.7) | 34 (58.6) | 36 (87.8) | ||
| No. of recurrent lesions | <0.001* | ||||
| Single | 44 (44.4) | 35 (60.3) | 9 (22.0) | ||
| Multiple | 55 (55.6) | 23 (39.7) | 32 (78.0) | ||
| Spread pattern | 0.852* | ||||
| Peritoneal | 78 (78.8) | 45 (77.6) | 33 (80.5) | ||
| Lymphatic | 17 (17.2) | 11 (19.0) | 6 (14.6) | ||
| Hematogenous | 4 (4.0) | 2 (3.4) | 2 (4.9) | ||
| Size of largest recurrent lesion (mm) | 30 (5–130) | 30 (5–120) | 30 (10–130) | 0.235† | |
| Operation type | 0.817* | ||||
| Laparotomy | 74 (74.7) | 44 (75.9) | 30 (73.2) | ||
| Laparoscopy | 25 (25.3) | 14 (24.1) | 11 (26.8) | ||
| Residual after SCS | <0.001* | ||||
| No residual | 51 (51.5) | 42 (72.4) | 9 (22.0) | ||
| Residual present | 48 (48.5) | 16 (27.6) | 32 (78.0) | ||
| PFI (mo) | 22 (6–151) | 26 (9–151) | 19 (6–83) | 0.003† | |
| Interval from initial recurrence detection to SCS (day) | 32 (7–216) | 32.5 (9–203) | 24 (7–216) | 0.324† | |
Values are presented as number (%) or median (range).
CA-125, cancer antigen 125; PFI, platinum-free interval; SCS, secondary cytoreductive surgery.
*Fisher exact test. †Mann-Whitney test.
With regard to surgical SCS characteristics, 72.4% of patients diagnosed with imaging had no residual disease after SCS; in contrast, only 22.0% of those diagnosed by CA-125 elevation had no residual disease (p<0.001). Also, the median PFI was significantly longer in patients with imaging diagnoses compared to that of the CA-125 group (26 months vs. 19 months, p=0.003). There were no significant differences for interval from initial recurrence detection to SCS between two groups (p=0.324).
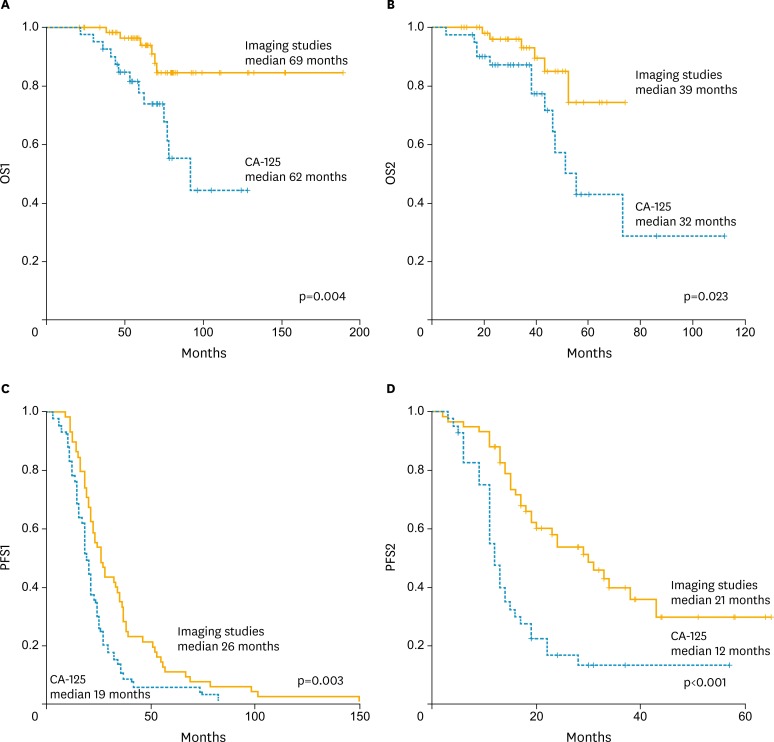
The survival analyses are shown in Fig. 2. The Kaplan-Meier curves for OS1, OS2, PFS1, and PFS2, revealed that patients in the imaging group had significantly better survival than did those with elevated CA-125. The median OS1, OS2, PFS1, and PFS2 were comparably longer in the imaging group than they were in the CA-125 group. In multivariate analysis with clinicopathological variables and recurrent patterns, CA-125 elevation at recurrence diagnosis was a significant factor for poorer survival in this patients group (Table 3).
Fig. 2.
Kaplan-Meier curves for (A) overall survival from initial diagnosis (OS1), (B) overall survival after secondary cytoreductive surgery (SCS; OS2), (C) progression-free survival after the initial treatment (PFS1), and (D) progression-free survival from SCS (PFS2) in patients with recurrent ovarian cancer according to the initial diagnostic method of recurrence. CA-125, cancer antigen 125.
Table 3. Multivariate analysis for PFS2, OS1, OS2 according to clinicopathological features and recurrence patterns in epithelial ovarian cancer patients with SCS (n=99).
| Variable | No. | PFS2 | OS1 | OS2 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| Stage | ||||||||
| I, II | 23 | 1 | 1 | 1 | ||||
| III, IV | 76 | 0.870 (0.468–1.618) | 0.661 | 1.921 (0.483–7.638) | 0.354 | 1.426 (0.377–5.392) | 0.601 | |
| Histology | ||||||||
| Serous | 80 | 1 | 1 | 1 | ||||
| Non-serous | 19 | 1.010 (0.507–2.013) | 0.978 | 3.549 (0.891–14.138) | 0.072 | 3.058 (0.805–11.616) | 0.101 | |
| Location of recurrence | ||||||||
| Pelvic | 29 | 1 | 1 | 1 | ||||
| Extra-pelvic | 70 | 0.651 (0.344–1.231) | 0.187 | 0.882 (0.240–3.240) | 0.849 | 0.939 (0.270–3.269) | 0.922 | |
| No. of recurrent lesions | ||||||||
| Single | 44 | 1 | 1 | 1 | ||||
| Multiple | 55 | 1.580 (0.877–2.849) | 0.128 | 1.112 (0.329–3.752) | 0.865 | 1.481 (0.451–4.863) | 0.517 | |
| Residual after SCS | ||||||||
| No residual | 51 | 1 | 1 | 1 | ||||
| Residual present | 48 | 1.330 (0.727–2.433) | 0.354 | 1.275 (0.340–4.779) | 0.718 | 1.191 (0.375–3.783) | 0.767 | |
| Initial sign of recurrence | ||||||||
| Imaging study | 58 | 1 | 1 | 1 | ||||
| CA-125 elevation | 41 | 2.791 (1.701–4.579) | <0.001 | 3.779 (1.433–9.969) | 0.007 | 2.935 (1.107–7.783) | 0.030 | |
The Cox's proportional hazard regression test was used for multivariate analysis.
CA-125, cancer antigen 125; HR, hazard ratio; OS1, overall survival from initial diagnosis; OS2, overall survival after SCS; PFS2, progression-free survival after SCS; SCS, secondary cytoreductive surgery.
DISCUSSION
There were differences in recurrence patterns and survival outcomes between two groups of patients who had secondary cytoreduction with distinct initial methods of recurrence diagnosis. It is difficult to directly derive the role of SCS in this study, because patients included in this study were limited to patients who underwent SCS for treatment, not all patients with recurrence. We compared the characteristics and survival of patients with different initial diagnostic methods of EOC recurrence to determine which group is appropriate for SCS.
The group diagnosed by imaging demonstrated a high percentage of pelvic recurrence and single site recurrence. This may be attributed to residual disease after SCS. The residual disease after SCS was relatively more frequent in the elevated CA-125 group than in the imaging group. Although it was not clearly demonstrated in our data, these differences may be related to differences in survival outcomes between the two groups; residual tumor after cytoreduction is widely known as a powerful prognostic factor in EOC patients [20,21,22].
Previous studies have addressed differences in outcomes according to the time of relapse and method of diagnosing recurrence. The CA-125 levels in EOC patients might have been elevated several months before symptoms arise [23]. In contrast, imaging studies in EOC patients are known to be useful in the detection of late recurrence, especially in patients who may benefit from SCS [24]. In one study from the United Kingdom, treating recurrent EOC characterized by CA-125 elevation alone did not increase OS. Imaging studies, such as CT scan, are capable of diagnosing asymptomatic recurrence; however, previous studies have not clarified the presence of survival differences [25,26]. Although the objective and patients group of this study were different from that of other previous studies, our data showed significant differences in the survival outcomes of patients with recurrence diagnosed by CA-125 elevation and imaging studies.
Our study is limited by its retrospective nature and single institution data. We are planning the validation with multicenter data sets for more definite results. In our study, patients had initial signs of recurrence recognized by either CA-125 elevation or imaging study. We could not compare the patients with symptoms as initial sign because all patients were asymptomatic at the time of recurrence. In addition, the results of this study can only be applied to restrictive criteria of EOC patients because patients included in this study were limited to patients who underwent SCS for treatment, not all patients with recurrence. Patients without definitive recurrence lesions on imaging or those with diffuse, extensive recurrence lesions who were unlikely to have SCS as a secondary treatment were not included. Patients selected for SCS may have more limited recurrence lesions and better performance status than other patients.
Despite some limitations, we concluded that, in platinum sensitive patients who had undergone SCS, patients with recurrence diagnosed on imaging had better survival outcomes than did those diagnosed by CA-125 elevation. Patients whose recurrence was diagnosed by imaging also had more pelvic recurrence, single lesion recurrences and no residual after SCS compared to those whose recurrence was diagnosed by CA-125 elevation. In this context, patients with radiologic evidence of recurrence (but without CA-125 elevation) are likely to be more appropriate for SCS than are patients with CA-125 elevation as initial sign of recurrence.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999-2010. J Gynecol Oncol. 2013;24:298–302. doi: 10.3802/jgo.2013.24.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Vaidya AP, Curtin JP. The follow-up of ovarian cancer. Semin Oncol. 2003;30:401–412. doi: 10.1016/s0093-7754(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 5.Rubin SC, Randall TC, Armstrong KA, Chi DS, Hoskins WJ. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol. 1999;93:21–24. doi: 10.1016/s0029-7844(98)00334-2. [DOI] [PubMed] [Google Scholar]
- 6.Cotte E, Glehen O, Mohamed F, Lamy F, Falandry C, Golfier F, et al. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg. 2007;31:1813–1820. doi: 10.1007/s00268-007-9146-8. [DOI] [PubMed] [Google Scholar]
- 7.Chiang YC, Chen CA, Chiang CJ, Hsu TH, Lin MC, You SL, et al. Trends in incidence and survival outcome of epithelial ovarian cancer: 30-year national population-based registry in Taiwan. J Gynecol Oncol. 2013;24:342–351. doi: 10.3802/jgo.2013.24.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skates SJ, Xu FJ, Yu YH, Sjövall K, Einhorn N, Chang Y, et al. Toward an optimal algorithm for ovarian cancer screening with longitudinal tumor markers. Cancer. 1995;76(Suppl):2004–2010. doi: 10.1002/1097-0142(19951115)76:10+<2004::aid-cncr2820761317>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Högberg T, Kågedal B. Long-term follow-up of ovarian cancer with monthly determinations of serum CA 125. Gynecol Oncol. 1992;46:191–198. doi: 10.1016/0090-8258(92)90254-g. [DOI] [PubMed] [Google Scholar]
- 10.Gadducci A, Conte P, Cianci C, Negri S, Genazzani AR. Treatment options in patients with recurrent ovarian cancer. Anticancer Res. 2001;21:3557–3564. [PubMed] [Google Scholar]
- 11.Sharma A, Bernacki RJ. Ovarian cancer patients with high CA-125 but no symptoms--should antiangiogenic treatments be considered? Oncol Res. 1997;9:53–54. [PubMed] [Google Scholar]
- 12.Fleming LW. Playing the waiting game ... the asymptomatic patient with recurrent ovarian cancer detected only by rising Ca125 levels. Scott Med J. 2001;46:81–83. doi: 10.1177/003693300104600306. [DOI] [PubMed] [Google Scholar]
- 13.Goonewardene TI, Hall MR, Rustin GJ. Management of asymptomatic patients on follow-up for ovarian cancer with rising CA-125 concentrations. Lancet Oncol. 2007;8:813–821. doi: 10.1016/S1470-2045(07)70273-5. [DOI] [PubMed] [Google Scholar]
- 14.Al Rawahi T, Lopes AD, Bristow RE, Bryant A, Elattar A, Chattopadhyay S, et al. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev. 2013;2:CD008765. doi: 10.1002/14651858.CD008765.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian WJ, Chi DS, Sehouli J, Tropé CG, Jiang R, Ayhan A, et al. A risk model for secondary cytoreductive surgery in recurrent ovarian cancer: an evidence-based proposal for patient selection. Ann Surg Oncol. 2012;19:597–604. doi: 10.1245/s10434-011-1873-2. [DOI] [PubMed] [Google Scholar]
- 16.Harter P, du Bois A, Hahmann M, Hasenburg A, Burges A, Loibl S, et al. AGO Ovarian Cancer Study Group Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702–1710. doi: 10.1245/s10434-006-9058-0. [DOI] [PubMed] [Google Scholar]
- 17.Janco JM, Kumar A, Weaver AL, McGree ME, Cliby WA. Performance of AGO score for secondary cytoreduction in a high-volume U.S. center. Gynecol Oncol. 2016;141:140–147. doi: 10.1016/j.ygyno.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Chi DS, McCaughty K, Diaz JP, Huh J, Schwabenbauer S, Hummer AJ, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933–1939. doi: 10.1002/cncr.21845. [DOI] [PubMed] [Google Scholar]
- 19.Onda T, Yoshikawa H, Yasugi T, Yamada M, Matsumoto K, Taketani Y. Secondary cytoreductive surgery for recurrent epithelial ovarian carcinoma: proposal for patients selection. Br J Cancer. 2005;92:1026–1032. doi: 10.1038/sj.bjc.6602466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90:390–396. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 21.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecol Oncol. 2006;100:283–287. doi: 10.1016/j.ygyno.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 23.von Georgi R, Schubert K, Grant P, Münstedt K. Post-therapy surveillance and after-care in ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2004;114:228–233. doi: 10.1016/j.ejogrb.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Gadducci A, Cosio S, Zola P, Landoni F, Maggino T, Sartori E. Surveillance procedures for patients treated for epithelial ovarian cancer: a review of the literature. Int J Gynecol Cancer. 2007;17:21–31. doi: 10.1111/j.1525-1438.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 25.Tanner EJ, Chi DS, Eisenhauer EL, Diaz-Montes TP, Santillan A, Bristow RE. Surveillance for the detection of recurrent ovarian cancer: survival impact or lead-time bias? Gynecol Oncol. 2010;117:336–340. doi: 10.1016/j.ygyno.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Gadducci A, Fuso L, Cosio S, Landoni F, Maggino T, Perotto S, et al. Are surveillance procedures of clinical benefit for patients treated for ovarian cancer?: A retrospective Italian multicentric study. Int J Gynecol Cancer. 2009;19:367–374. doi: 10.1111/IGC.0b013e3181a1cc02. [DOI] [PubMed] [Google Scholar]