Abstract
Objective
Bevacizumab was recently approved by the US Food and Drug Administration for use in recurrent platinum resistant epithelial ovarian cancer (EOC), fallopian tube cancer (FTC), or primary peritoneal cancer (PPC) when no more than two prior cytotoxic regimens have been used; due to concerns for gastrointestinal perforation. We sought to determine bevacizumab-related toxicities in heavily pretreated recurrent EOC.
Methods
We performed a retrospective chart review of patients with recurrent EOC, FTC, and PPC from 2001 to 2011. Patients who received at least two prior chemotherapy regimens before bevacizumab were included. Medical records were reviewed for bevacizumab associated toxicities. The Wilcoxon-Mann-Whitney test was used to compare quantitative variables. Survival was estimated with the Kaplan-Meier method.
Results
Sixty patients met inclusion criteria. At the start of bevacizumab treatment, the median age was 60 years and the median body mass index was 26.5 kg/m2. More than 50% of patients received bevacizumab after three prior cytotoxic regimens. Grade 3 or higher bevacizumab associated toxicity events occurred in four patients, including one patient who developed a rectovaginal fistula. The median overall survival from the start of bevacizumab treatment was 21.05 months (95% CI, 18.23 to 32.67; range, 1.9 to 110 months). The number of cytotoxic regimens prior to bevacizumab treatment did not differ in those that experienced a toxicity versus those that did not (p=0.66).
Conclusion
The use of bevacizumab in heavily pretreated EOC, FTC, or PPC is worth consideration.
Keywords: Bevacizumab, Drug Resistance, Gastrointestinal Diseases, Ovarian Neoplasms, Recurrence, Survival Analysis
INTRODUCTION
Epithelial ovarian cancer (EOC) is the leading cause of death among women from a gynecological cancer [1]. While patients with EOC can be expected to respond to initial therapies, they often will become resistant to platinum-based cytotoxic regimens. Given that the response to cytotoxic chemotherapy can be diminished in this population, strategies for targeting vascular endothelial growth factor (VEGF) in EOC are being extensively evaluated. VEGF has been implicated in the proliferative vascularization that occurs in invasive tumors [2]. Bevacizumab is an anti-VEGF monoclonal antibody that blocks angiogenesis and has been shown to have efficacy in a variety of tumor types [2].
Bevacizumab has been shown in phase II and III trials to be active in the treatment of recurrent and/or platinum resistant EOC when used as a single agent or in combination with chemotherapy [3,4,5,6,7]. Specifically, the Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer (AURELIA) prospective phase III trial found a 3.3 months progression-free survival (PFS) benefit in women with recurrent platinum resistant EOC who were treated with chemotherapy in combination with bevacizumab compared to cytotoxic chemotherapy alone [6]. The findings of the AURELIA trial led the US Food and Drug Administration (FDA) to approve bevacizumab for use in platinum-resistant recurrent EOC.
Documented adverse events due to bevacizumab have included diarrhea, hypertension, vascular thrombosis, impaired wound healing, hemorrhage, and gastrointestinal perforation (GIP). Of these, GIP is the most concerning as there is up to a 50% incidence of mortality when GIP occurs in the setting of recurrent EOC [4]. Clinical findings associated with a higher risk of GIP in patients with EOC treated with bevacizumab include bowel obstruction, radiographic evidence of intestinal involvement by the tumor, and the number of prior cytotoxic regimens [4,8,9,10,11,12,13]. Most trials have limited the number of prior cytotoxic regimens that participants received prior to bevacizumab therapy to reduce the risk of bevacizumab-related GIP. Despite restrictive inclusion criteria, the rate of bevacizumab-related GIP reported in prior studies has varied, ranging from 0% to 11.4% [3,4,5,6,7,14,15,16,17]. Our objective was to determine the overall survival (OS) from the start of bevacizumab therapy and the rate of adverse events, in particular gastrointestinal toxicity, in heavily pretreated recurrent EOC.
MATERIALS AND METHODS
The Institutional Review Board (IRB) granted permission for this retrospective cohort study (IRB Protocol # 48078). A departmental database was used to identify patients who had received bevacizumab either alone or in combination with cytotoxic chemotherapy between 2001 and 2011. Subjects were eligible for inclusion if they had a histopathological confirmed diagnosis of EOC, fallopian tube cancer (FTC) or primary peritoneal cancer (PPC) after their initial cytoreductive surgery and if they had received bevacizumab either alone or in combination with cytotoxic therapy at our institution after two or more prior cytotoxic regimens. Individuals who had neoadjuvant chemotherapy were eligible for inclusion in the study. Subjects were excluded if they received chemotherapy at another institution and their chemotherapy records were not available for review or if they previously had bevacizumab during front line or second line therapy. The clinical practice at our institution is to not administer bevacizumab to patients with significant gastrointestinal symptoms or extensive tumor involvement of the bowel by radiographic studies, exploratory surgery, or clinical exam based on the findings of prior studies utilizing bevacizumab [4,10,11,12,13]. This clinical practice was confirmed during the review of medical records.
The medical records were systematically reviewed for the following variables: stage and histological grade at diagnosis, demographic information, surgical history, chemotherapy regimens, chemotherapy-related adverse events, date of death, and date of last follow-up. The number of cytotoxic regimens before bevacizumab were determined from the adjuvant chemotherapy regimens. The regimen that contained bevacizumab was the last chemotherapy regimen considered for the purpose of this study. The National Cancer Institute's Common Toxicity Criteria for Adverse Events (CTCAE) ver. 4.0 was used to grade any bevacizumab related toxicities [18]. OS was determined in months from the first date that bevacizumab was given until death occurred or last documented follow-up. The Kaplan-Meier method with right censoring at the time of last documented follow-up was used to estimate survival. The log-rank test was used to compare survival curves. The central tendency of variables were described with the median. Additionally, the chi-square test was used to compare categorical variables and the Wilcoxon-Mann-Whitney test was used to compare quantitative variables.
RESULTS
One hundred sixty-three medical charts of patients who received bevacizumab were evaluated. Sixty subjects met our inclusion criteria. Subjects were excluded from analysis because they received chemotherapy at another institution (9.2%), because they had received bevacizumab in frontline or second line therapy (49.1%), or because they did not have a histopathological confirmed diagnosis of EOC, FTC or PPC (4.9%). Demographic and treatment information are presented in Table 1. At the start of bevacizumab treatment, the median age in the cohort was 60 years and the median body mass index was 26.5 kg/m2. Sixty-three percent had serous histology and 52% of patients were platinum resistant. Six patients had a history of radiation to the abdominopelvic area. Neoadjuvant chemotherapy was administered to four patients who received bevacizumab after less than three prior cytotoxic regimens and to three patients who received bevacizumab after three or more cytotoxic regimens. Thirty-one patients (51.7%) had a history of bowel surgery prior to bevacizumab use. The median time from the date of bowel surgery to the start of bevacizumab was 23.1 months in those who had less than three prior regimens before bevacizumab and 45.9 months in those who had three or more prior regimens before bevacizumab. Thirty-five percent of patients received bevacizumab alone and 65% received it in combination with cytotoxic therapy. The bevacizumab containing regimens used can be seen in Table 2. The median number of prior cytotoxic regimens before bevacizumab was 3 (range, 2 to 9); 48.3% of patients had received 2 prior cytotoxic regimens, 40% had been treated with 3 prior cytotoxic regimens, and 11.7% of patients had received 4 or more prior cytotoxic regimens. Fifteen mg/kg every 3 weeks was the bevacizumab dosing most commonly used and 59 subjects (98.3%) received this dose. One patient required a dose reduction to 7.5 mg/kg due to refractory hypertension. Patients received a median of 10 doses of bevacizumab (range, 2 to 45) with median cumulative dose of 9,675 mg (range, 1,440 mg to 81,525 mg; 95% CI, 5,995.05 to 13,354.95). The majority of subjects eventually had progressive disease while on the bevacizumab containing regimen (76.67%). The remaining subjects either had stable disease (15.00%), complete response (6.67%), or a partial response (1.67%). The bevacizumab treatment characteristics by platinum resistance status can be seen in Table 3. Those who were platinum sensitive received a higher number of bevacizumab doses compared to those who were platinum resistant (p=0.03).
Table 1. Patient demographic and tumor characteristics per chemotherapy regimens prior to bevacizumab.
| Variable | <3 Prior regimens (n=29) | ≥3 Prior regimens (n=31) | p-value | |
|---|---|---|---|---|
| Race | 0.63 | |||
| White | 22 (75.86) | 26 (83.87) | ||
| Black | 3 (10.34) | 1 (3.23) | ||
| Hispanic | 2 (6.9) | 2 (6.45) | ||
| American-Indian | 0 | 1 (3.23) | ||
| Unknown | 2 (6.9) | 1 (3.23) | ||
| Age at start of bevacizumab (yr) | 57 (37–83) | 60 (35–79) | 0.98 | |
| BMI at bevacizumab start (kg/m2) | 26.56 (16.65–39.39) | 24.67 (18.61–42.58) | 0.85 | |
| Stage | 0.36 | |||
| IA–IIIC | 1 (3.44) | 2 (6.5) | ||
| IIA–IV | 28 (96.6) | 29 (93.5) | ||
| Histology | 0.50 | |||
| Serous | 20 (68.97) | 18 (58.1) | ||
| Adenocarcinoma | 8 (27.59) | 10 (32.26) | ||
| Clear cell, mixed | 1 (3.44) | 3 (9.68) | ||
| Debulking | 0.51 | |||
| Optimal | 18 (62.07) | 22 (70.97) | ||
| Platinum resistant | 0.99 | |||
| Yes | 15 (51.72) | 16 (51.61) | ||
| Prior radiation to abdominopelvic area | 0.93 | |||
| Yes | 3 (10.34) | 3 (9.68) | ||
| Prior bowel surgery | 0.61 | |||
| Yes | 14 (48.28) | 17 (54.84) | ||
| Time from bowel surgery to start of bevacizumab (mo) | 23.11 (8.17–41.6) | 45.93 (21.2–103.07) | <0.001 | |
| Bevacizumab combined with cytotoxic chemotherapy | 0.24 | |||
| Yes | 21 (72.41) | 18 (58.06) | ||
| Number of prior cytotoxic regimens | 2 (2) | 3 (3-9) | <0.001 | |
| Number of bevacizumab doses | 9 (3–45) | 10 (2–33) | 0.97 | |
| Cumulative bevacizumab received (mg) | 8,800 (1,440–81,525) | 10,000 (1,710–36,300) | 0.63 | |
| Follow-up after last dose of bevacizumab (mo) | 11.3 (1.47–60.4) | 10.73 (0.27–51.3) | 0.60 | |
Values are presented as number (%) or median (range).
BMI, body mass index.
Table 2. Lists of Bevacizumab containing regimens.
| Regimen | No. (%) |
|---|---|
| Bevacizumab | 21 (35.00) |
| Bevacizumab/paclitaxel | 2 (3.33) |
| Bevacizumab/carboplatin | 4 (6.67) |
| Bevacizumab/topotecan | 24 (40.00) |
| Bevacizumab/gemcitabine | 4 (6.67) |
| Bevacizumab/paclitaxel/carboplatin | 2 (3.33) |
| Bevacizumab/carboplatin/gemcitabine | 2 (3.33) |
| Bevacizumab/doxil | 1 (1.67) |
Table 3. Bevacizumab treatment characteristics by platinum resistance status.
| Characteristic | Platinum sensitive (n=29) | Platinum resistant (n=31) | p-value | |
|---|---|---|---|---|
| Bevacizumab combined with cytotoxic chemotherapy (yes) | 16 (55.17) | 23 (74.19) | 0.12 | |
| Number of prior cytotoxic regimens | 3 (2–4) | 3 (2–9) | 0.55 | |
| of bevacizumab doses | 13 (4–45) | 8 (2–33) | 0.03 | |
| Cumulative bevacizumab received (mg) | 11,700 (2,400–81,525) | 6,500 (1,440–36,300) | 0.03 | |
| Follow-up after last dose of bevacizumab (mo) | 13.1 (1.1–51.3) | 8.67 (0.27–60.4) | 0.17 | |
Values are presented as number (%) or median (range).
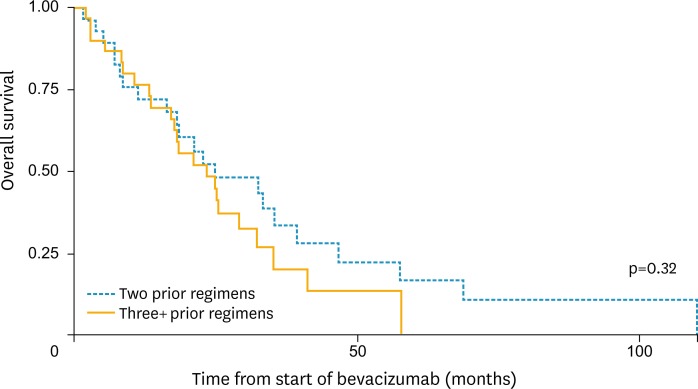
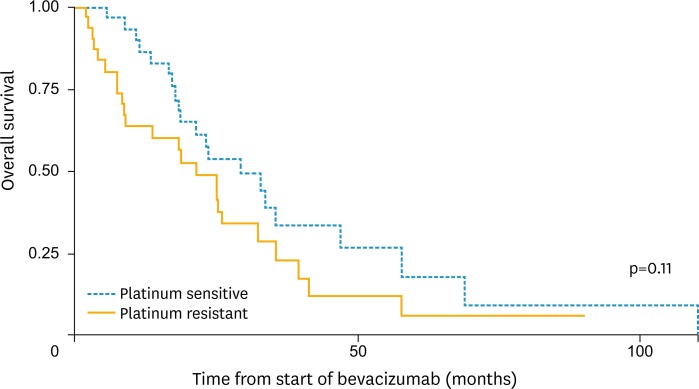
There were 4 bevacizumab associated grade 3 or higher adverse events. The most common bevacizumab-related adverse events were hypertension and hemorrhage (Table 4). One grade 3 or higher adverse event occurred in a platinum resistant patient while the other 3 were in platinum sensitive patients. There was one case of a rectovaginal fistula which occurred in a platinum sensitive patient who had received 3 cytotoxic regimens prior to receiving bevacizumab alone. The rectovaginal fistula occurred 18 months after the bevacizumab dose was given. At the time the rectovaginal fistula was discovered the patient was noted to have progressive intra-abdominal tumor burden as well as diverticulosis on imaging. One 74-year-old patient with platinum sensitive recurrent ovarian cancer, a history of migraine headaches, and Raynaud's disease experienced cerebrovascular ischemia while on bevacizumab. There was no difference in the number of cytotoxic regimens previously received in those patients who experienced a toxicity compared to those who did not (p=0.66). The median OS from the start of bevacizumab treatment was 21.05 months (95% CI, 18.23 to 32.67). There was no significant difference in the median OS for those who had 2 prior cytotoxic regimens (21.17 months, 95% CI, 16.60 to 39.53) and ≥3 (20.93 months; 95% CI, 13.67 to 32.47) prior cytotoxic regimens before bevacizumab (p=0.32) (Fig. 1). The median OS for those who were platinum sensitive at the start of bevacizumab was 23.33 months (95% CI, 18.23 to 46.93) versus 18.10 months (95% CI, 8.77 to 32.47) for those who were platinum resistant at the start of bevacizumab (p=0.11) (Fig. 2). The median follow-up from the last date of bevacizumab therapy until death or the last known time in the cohort was 10.9 months.
Table 4. Non-hematologic adverse events*.
| Variable | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| No. (%) | 11 (68.8) | 1 (6.3) | 3 (18.8) | 1 (6.3) | |
| Cardiovascular | |||||
| Hypertension | 4 | - | 1 | - | |
| Venous thrombosis | - | - | 1 | - | |
| Gastrointestinal | |||||
| Diarrhea | - | 1 | - | - | |
| Hemorrhage | |||||
| Gastrointestinal bleeding | 1 | - | - | - | |
| Epistaxis | 2 | - | - | - | |
| Bleeding gum | 1 | - | - | - | |
| Other | |||||
| Proteinuria | 2 | - | - | - | |
| Cerebrovascular ischemia | - | - | 1 | - | |
| Headache | 1 | - | - | - | |
| Rectovaginal fistula | - | - | - | 1 | |
*Per Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0.
Fig. 1.
Overall survival (OS) curve by 3 or more cytotoxic regimens versus less than 3 prior cytotoxic regimens.
Fig. 2.
Overall survival (OS) curve by platinum sensitivity.
DISCUSSION
Bevacizumab has been proven to be effective in recurrent and platinum resistant EOC in both retrospective [19,20,21] and prospective studies [3,4,6]. The impact on survival is even greater when bevacizumab is combined with cytotoxic therapy [6]. In a prospective trial of bevacizumab in persistent or recurrent EOC, the Gynecologic Oncology Group reported a response rate of 17.7% with a median response duration of 10.3 months [3]. Cannistra et al. [4] in their prospective trial of bevacizumab in platinum resistant recurrent EOC reported a similar response of 15.9%. However, 11.4% of the patients in this trial experienced a GIP. Cannistra et al. [4] noted that the trial included "higher risk patients" in that 47.7% had received 3 prior cytotoxic regimens and 90% had tumor involving the bowel at the time of enrollment. The AURELIA trial, a prospective phase III trial of 360 patients with recurrent EOC and no more than 2 prior cytotoxic regimens, randomized patients to single agent chemotherapy alone or bevacizumab plus chemotherapy. The study found that the median response rate was 27.3% when bevacizumab was combined with cytotoxic chemotherapy, compared with only 11.8% for cytotoxic therapy alone, and that median PFS was improved as well (6.7 months vs. 3.4 months) [6]. Overall, bevacizumab in combination with cytotoxic therapy was well tolerated with four patients (2.2%) experiencing GIP [6]. Moreover, quality of life outcomes were assessed in the AURELIA trial [22]. A greater proportion of women treated with chemotherapy and bevacizumab compared to chemotherapy alone had a significant improvement in abdominal and gastrointestinal symptoms (21.9% vs. 9.3%) [22]. The efficacy and tolerability of bevacizumab in combination with cytotoxic chemotherapy compared to bevacizumab alone in heavily pretreated EOC was examined in a retrospective cohort study [23]. The investigators found that bevacizumab in combination with cytotoxic chemotherapy was associated with improved PFS and OS compared to bevacizumab alone. Additionally, there was no difference in the rate of adverse events including gastrointestinal events between the two groups [23].
The current study demonstrates our institutional experience with recurrent EOC in patients who received a median of 3 prior cytotoxic regimens before receiving bevacizumab. We found that bevacizumab alone and in combination with cytotoxic therapy is efficacious and more importantly, well-tolerated. This cohort of patients received considerably higher amounts of bevacizumab compared to previous trials. The median number of bevacizumab doses received was 10. In comparison, the median number of doses of bevacizumab in prior trials was 5 in the Cannistra et al. [4] trial, 7 in the GOG phase II trial [3], and 8.9 in the German cohort [9]. The majority of the patients in our cohort discontinued bevacizumab due to eventual progression of disease. However, given the median of 10 doses per patient we can extrapolate a median duration of response including stable disease of 7.5 months and a median OS from the start of bevacizumab treatment of 18.2 months.
In this heavily pretreated patient population with recurrent EOC, FTC or PPC only 6.6% experienced a grade 3 or 4 non-hematologic bevacizumab-associated toxicity. In additional phase II trials of either bevacizumab alone or with concurrent cytotoxic chemotherapy, the GIP rate with bevacizumab use has ranged from 0% to 5.7% [3,5,7,14,15,16]. In these phase II trials, the eligibility criteria for inclusion limited the number of prior chemotherapy regimens to 1 or 2 [3,5,7,14,15,16]. Few studies have reported on the outcome of bevacizumab-related GIP in an unselected heavily pretreated recurrent EOC population (Table 5). In 2011, a retrospective cohort study from Germany evaluated 15 patients with platinum resistant recurrent EOC and a median of 5.4 prior chemotherapy regimens. Thirteen patients received bevacizumab dosed at 7.5 mg/m2 either alone (46.7%) or combination with cytotoxic therapy (53.3%) for a median of 8.9 cycles per patient [9]. They reported a partial response of 13.3% [9]. They noted that none of the patients experienced a GIP but did note that three patients (20%) experienced a gastrointestinal fistula [9].
Table 5. Study characteristics for bevacizumab-related gastrointestinal perforation.
| Study | Design | No. | Platinum resistance | Number of prior regimens | Bevacizumab with cytotoxic chemo | Gastrointestinal perforation or fistula rate |
|---|---|---|---|---|---|---|
| Diaz et al. (2010) [24] | Retrospective cohort | 160 | 44 (27.5) | 4 (2–8) | 127 (79) | 6 (4) |
| Emile et al. (2013) [8] | Retrospective cohort | 37 | 33 (89.2) | 4 (NR) | 0 | 0 |
| Pietzner et al. (2011) [9] | Retrospective cohort | 15 | 15 (100) | 5.4 (1–7) | 6 (40) | 3 (20) |
| Richardson et al. (2010) [11] | Retrospective cohort | 112 | 37 (33.0) | 4 (1–10) | 108 (96.4) | 10 (9) |
| Simpkins et al. (2007) [12] | Retrospective cohort | 25 | 25 (100) | 3 (1–6) | 15 (60) | 0 |
| Sfakianos et al. (2009) [25] | Retrospective cohort | 68 | NR | 5* | 45 (67) | 5 (7.2) |
| Tanyi et al. (2011) [13] | Retrospective cohort | 82 | NR | 3 (1–9) | NR | 8 (9.76) |
Values are presented as number (%) or median (range).
NR, not reported
*Mean.
Predictors for bevacizumab-related GIP in the treatment of recurrent EOC have been previously considered [11,13]. Richardson et al. [11] examined several characteristics of patients with recurrent EOC treated with bevacizumab. Subjects included in the cohort were treated with a median of 4 prior regimens and 9% experienced GIP [11]. They found that those with rectovaginal nodularity was associated with a higher risk of bevacizumab-related GIP [11]. Tanyi et al. [13], in their retrospective review, did not assess for rectovaginal nodularity in patients with recurrent EOC treated with bevacizumab. They found that patients with a history of bowel surgery or bowel obstruction had a higher incidence of bevacizumab-related GIP [13]. Simpkins et al. [12] in their retrospective cohort study described that limiting bevacizumab treatment in recurrent EOC to patients without clinical signs of bowel obstruction, tumor involvement of the rectosigmoid, or bowel involvement on computed tomography scan may limit the risk of GIP. Using this criteria, there were no cases of GIP in their heavily pretreated cohort of recurrent EOC [12].
In our cohort, 31 patients (51.7%) had prior bowel surgery and 27 patients (45%) had tumor involving the bowel at the start of bevacizumab treatment as determined by imaging or exploratory surgery. This rate is much lower than the 90% of tumor burden on bowel noted in the Cannistra et al. [4] trial. In our retrospective cohort, clinicians carefully selected which heavily pretreated patients received bevacizumab; however the tumor burden on the bowel was likely higher than 45% because not all patients were imaged prior to starting bevacizumab. Cannistra et al. [4] concluded that the GIP rate seen in their trial was not associated with the tumor burden on the bowel but instead was due to those patients being heavily pretreated with 3 cytotoxic regimens prior to bevacizumab. This led to the recommendation that women with EOC have no more than 2 prior cytotoxic regimens. Diaz et al. [24] in a retrospective cohort study examined the incidence of GIP with bevacizumab use. They reported a GIP rate of 4% with no difference in the median number of prior regimens in those who experienced GIP versus those who did not, 4 and 5, respectively [24]. In our study, in which 52% of patients had received 3 or more cytotoxic regimens prior to bevacizumab, we found very minimal toxicity with only 1.7% experiencing rectovaginal fistula, without the GIP experience seen in the Cannistra et al. [4] cohort.
Another retrospective cohort study compared GIP and/or fistula rates in those with heavily pretreated recurrent EOC who received bevacizumab plus chemotherapy as opposed to chemotherapy alone [25]. Patients were not selected for therapy based on risk factors for bowel perforation and those in the bevacizumab containing group had received a median of 5 prior cytotoxic regimens prior to bevacizumab. The proportion of patients who experienced GIP/fistula was 7.2% of the bevacizumab and chemotherapy group versus 6.5% in the chemotherapy alone group [25]. They concluded that the GIP was not likely related to bevacizumab but to carcinomatosis [25]. Indeed, in our cohort the proportion of patients who experienced a GIP/fistula was much less than that seen in the Sfakianos group, likely due to our careful selection of patients without significant tumor burden on the bowel.
The strengths of this study are the size of the cohort and the larger number of doses of bevacizumab that patients received compared to prior studies with no patients lost to follow-up. The limitations include the retrospective nature of the data. Because we relied on medical records, there may have been under reporting of adverse events. Furthermore, there was likely an inherent selection bias as bevacizumab treatment was given based on clinician preference, which may have caused an underestimation of toxicities. Additionally, 52% of the cohort received 3 or more cytotoxic regimens prior to bevacizumab with the remainder of the cohort receiving less than 3 cytotoxic regimens prior to bevacizumab. While the group of most interest are those who received 3 or more cytotoxic regimens we were able to demonstrate that there was no appreciable difference in toxicity by the number of prior cytotoxic regimens. Furthermore, given that surgical exploration was never undertaken for the rectovaginal fistula case in our study it is not clear if the fistula was due to a bevacizumab-related spontaneous perforation, inflammatory process from a diverticular abscess, or due to tumor burden in the area. Lastly, bevacizumab was given in combination with cytotoxic therapy 65% of the time, limiting our ability to determine grade 3 or 4 hematological toxicities due to bevacizumab alone.
We have shown that the use of bevacizumab in heavily pretreated EOC appears to be tolerable with a GIP/fistula rate of 1.7% which is lower than demonstrated in prior studies. The risk for GIP in this patient population appears to be multifactorial and not just related to the number of prior cytotoxic regimens or the use of bevacizumab. While currently, bevacizumab is approved for use in women with platinum resistant EOC, FDA recommendations limit the number or prior cytotoxic regimens to two. Our study suggests that women who have received three or more prior cytotoxic regimens who do not have can be safely treated with bevacizumab.
Footnotes
Previously presented as a poster presentation at the Western Association of Gynecologic Oncologists Annual Meeting, June 2015, Santa Rosa, CA, USA.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 3.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 4.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 5.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 6.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 7.Tillmanns TD, Lowe MP, Walker MS, Stepanski EJ, Schwartzberg LS. Phase II clinical trial of bevacizumab with albumin-bound paclitaxel in patients with recurrent, platinum-resistant primary epithelial ovarian or primary peritoneal carcinoma. Gynecol Oncol. 2013;128:221–228. doi: 10.1016/j.ygyno.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 8.Emile G, Chauvenet L, Tigaud JM, Chidiac J, Pujade Lauraine E, Alexandre J. A clinical experience of single agent bevacizumab in relapsing ovarian cancer. Gynecol Oncol. 2013;129:459–462. doi: 10.1016/j.ygyno.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Pietzner K, Richter R, Chekerov R, Erol E, Oskay-Özcelik G, Lichtenegger W, et al. Bevacizumab in heavily pre-treated and platinum resistant ovarian cancer: a retrospective study of the North-Eastern German Society of Gynaecologic Oncology (NOGGO) Ovarian Cancer Study Group. Anticancer Res. 2011;31:2679–2682. [PubMed] [Google Scholar]
- 10.Randall LM, Monk BJ. Bevacizumab toxicities and their management in ovarian cancer. Gynecol Oncol. 2010;117:497–504. doi: 10.1016/j.ygyno.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson DL, Backes FJ, Hurt JD, Seamon LG, Copeland LJ, Fowler JM, et al. Which factors predict bowel complications in patients with recurrent epithelial ovarian cancer being treated with bevacizumab? Gynecol Oncol. 2010;118:47–51. doi: 10.1016/j.ygyno.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Simpkins F, Belinson JL, Rose PG. Avoiding bevacizumab related gastrointestinal toxicity for recurrent ovarian cancer by careful patient screening. Gynecol Oncol. 2007;107:118–123. doi: 10.1016/j.ygyno.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Tanyi JL, McCann G, Hagemann AR, Coukos G, Rubin SC, Liao JB, et al. Clinical predictors of bevacizumab-associated gastrointestinal perforation. Gynecol Oncol. 2011;120:464–469. doi: 10.1016/j.ygyno.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 14.del Carmen MG, Micha J, Small L, Street DG, Londhe A, McGowan T. A phase II clinical trial of pegylated liposomal doxorubicin and carboplatin plus bevacizumab in patients with platinum-sensitive recurrent ovarian, fallopian tube, or primary peritoneal cancer. Gynecol Oncol. 2012;126:369–374. doi: 10.1016/j.ygyno.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EL, Zanagnolo V, Cohn DE, Salani R, O'Malley DM, Sutton G, et al. A phase II study of gemcitabine, carboplatin and bevacizumab for the treatment of platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2014;134:262–266. doi: 10.1016/j.ygyno.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Hagemann AR, Novetsky AP, Zighelboim I, Gao F, Massad LS, Thaker PH, et al. Phase II study of bevacizumab and pemetrexed for recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer. Gynecol Oncol. 2013;131:535–540. doi: 10.1016/j.ygyno.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenham RM, Lapolla J, Lin HY, Apte SM, Lancaster JM, Judson PL, et al. A phase II trial of docetaxel and bevacizumab in recurrent ovarian cancer within 12 months of prior platinum-based chemotherapy. Gynecol Oncol. 2013;130:19–24. doi: 10.1016/j.ygyno.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 4.0 [Internet] Bethesda, MD: National Institutes of Health, National Cancer Institute; 2016. [cited 2016 May 4]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [Google Scholar]
- 19.Cohn DE, Valmadre S, Resnick KE, Eaton LA, Copeland LJ, Fowler JM. Bevacizumab and weekly taxane chemotherapy demonstrates activity in refractory ovarian cancer. Gynecol Oncol. 2006;102:134–139. doi: 10.1016/j.ygyno.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Monk BJ, Han E, Josephs-Cowan CA, Pugmire G, Burger RA. Salvage bevacizumab (rhuMAB VEGF)-based therapy after multiple prior cytotoxic regimens in advanced refractory epithelial ovarian cancer. Gynecol Oncol. 2006;102:140–144. doi: 10.1016/j.ygyno.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Numnum TM, Rocconi RP, Whitworth J, Barnes MN. The use of bevacizumab to palliate symptomatic ascites in patients with refractory ovarian carcinoma. Gynecol Oncol. 2006;102:425–428. doi: 10.1016/j.ygyno.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Stockler MR, Hilpert F, Friedlander M, King MT, Wenzel L, Lee CK, et al. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol. 2014;32:1309–1316. doi: 10.1200/JCO.2013.51.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuh KC, Secord AA, Bevis KS, Huh W, ElNaggar A, Blansit K, et al. Comparison of bevacizumab alone or with chemotherapy in recurrent ovarian cancer patients. Gynecol Oncol. 2015;139:413–418. doi: 10.1016/j.ygyno.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 24.Diaz JP, Tew WP, Zivanovic O, Konner J, Sabbatini PJ, dos Santos LA, et al. Incidence and management of bevacizumab-associated gastrointestinal perforations in patients with recurrent ovarian carcinoma. Gynecol Oncol. 2010;116:335–339. doi: 10.1016/j.ygyno.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Sfakianos GP, Numnum TM, Halverson CB, Panjeti D, Kendrick JE, 4th, Straughn JM., Jr The risk of gastrointestinal perforation and/or fistula in patients with recurrent ovarian cancer receiving bevacizumab compared to standard chemotherapy: a retrospective cohort study. Gynecol Oncol. 2009;114:424–426. doi: 10.1016/j.ygyno.2009.05.031. [DOI] [PubMed] [Google Scholar]