Abstract
Recently, most anticancer drugs are derived from natural resources such as marine, microbial, and botanical sources, but the low success rates of chemotherapies and the development of multidrug resistance emphasize the importance of discovering new compounds that are both safe and effective against cancer. Ginseng types, including Asian ginseng, American ginseng, and notoginseng, have been used traditionally to treat various diseases, due to their immunomodulatory, neuroprotective, antioxidative, and antitumor activities. Accumulating reports have shown that ginsenosides, the major active component of ginseng, were helpful for tumor treatment. 20(S)-Protopanaxadiol (PDS) and 20(S)-protopanaxatriol saponins (PTS) are two characteristic types of triterpenoid saponins in ginsenosides. PTS holds capacity to interfere with crucial metabolism, while PDS could affect cell cycle distribution and prodeath signaling. This review aims at providing an overview of PTS and PDS, as well as their metabolites, regarding their different anticancer effects with the proposal that these compounds might be potent additions to the current chemotherapeutic strategy against cancer.
1. Introduction
Cancer is a group of diseases characterized by evading growth suppressors, activating invasion and metastasis, avoiding immune destruction, and deregulating cellular anabolism and metabolism. In 2015, a total of 1,658,370 new cancer cases and 589,430 cancer deaths were projected to occur in the United States [1]. In China, the estimates of new cancer incident cases and cancer deaths were 3,372,175 and 2,113,048, respectively [2]. However, chemotherapy always suffered from increasing multidrug resistance. Thus, identifying more chemicals extracted from herbal medicines is an essential step in advancing cancer treatment. Nowadays, many herbs, typically ginseng and notoginseng, have been used in clinical practice to treat advanced cancer in eastern countries, and researchers pay more and more attention to the potential therapeutic effects of those herbs. Therefore, it is very important to understand the bioactive effects and mechanism of the main ingredients and absorbed metabolites of these herbs.
Asian ginseng, American ginseng, and notoginseng, the roots and rhizome of Panax ginseng C. A. Meyer, Panax quinquefolius L., and Panax notoginseng (Burk.) F. H. Chen, belong to genus Panax of the family Araliaceae. These herbs have long been used for preventive and therapeutic purposes for thousands of years in Asian and North American countries. Ben Cao Gang Mu recorded that Asian ginseng was usually used as a tonic, sedative, life-prolonging, or gastrointestinal regulation drug to treat fatigue, blood deficiency, insomnia, and impotence [3]. American ginseng was first recorded in Ben Cao Cong Xin in 1757 and was used for relieving internal heat, cough, bloody phlegm, dysphoria and tiredness, and dry and thirsty mouth and throat. Notoginseng, another herb belonging to the genus Panax, is well known for treatment of blood disorders. Notoginseng is usually available in two different forms: the raw and steamed (processed) forms. The raw notoginseng has been traditionally used for its hemostatic and cardiovascular effects to arrest internal and external bleeding, reduce swelling and pain, and remove blood stasis and promote blood circulation [4]. Unlike raw notoginseng, the steamed form has been widely accepted to be a tonic to “nourish” the blood and to increase blood cells in anemic conditions [5].
Modern studies showed that Asian ginseng, American ginseng, and notoginseng exhibited various significant pharmacological effects, and the anticancer activities of ginseng types have been extensively investigated based on the functional capacity of inhibiting cancer cell growth, inducing angiogenesis, delaying invasion and metastasis, and regulating tumor-related immune suppression by their active ingredients, ginsenosides and their metabolites [6, 7]. PDS (20(S)-protopanaxadiol (PPD) saponins) and PTS (20(S)-protopanaxatriol (PPT) saponins) and their metabolites are the major anticancer active components of the most popular Panax herbs. Notably, ginsenoside Rg3 was produced as an antiangiogenic drug in China. In this review, we summarize and compare the regulatory effects of different ginsenosides and their metabolites on the development of cancer, and the corresponding mechanisms have also been discussed.
2. Chemical Structures and Metabolism of PDS and PTS
Saponins and sapogenins of ginseng types (also named ginsenosides) are the major bioactive constituents which were possibly responsible for the comparable and distinct pharmacological activities in the three Panax herbs [8]. All of the total ginsenosides extracts of these three herbs are chemical mixtures containing a group of triterpene glycosides with similar ingredients and structure, which have been shown to possess anticancer, anti-inflammatory, and neuroprotective activities and promote blood circulation to treat cardiocerebrovascular diseases [9]. Nowadays, more than sixty individual saponins were isolated from these three Panax herbs. They are classified into two main groups according to the different aglycone, namely, PDS, such as ginsenoside Rb1, and PTS, such as ginsenoside Rg1. The two types of triterpenoid saponins showed diverse or even antagonistic pharmacological activities [10]. Cumulated researches elucidated that the content of total saponins in notoginseng is higher than those in Asian ginseng [11], while ginsenosides Rb1, Re, and Rg1 are enriched in American ginseng, and ginsenosides Rf and Rb2 are enriched in Asian ginseng [12].
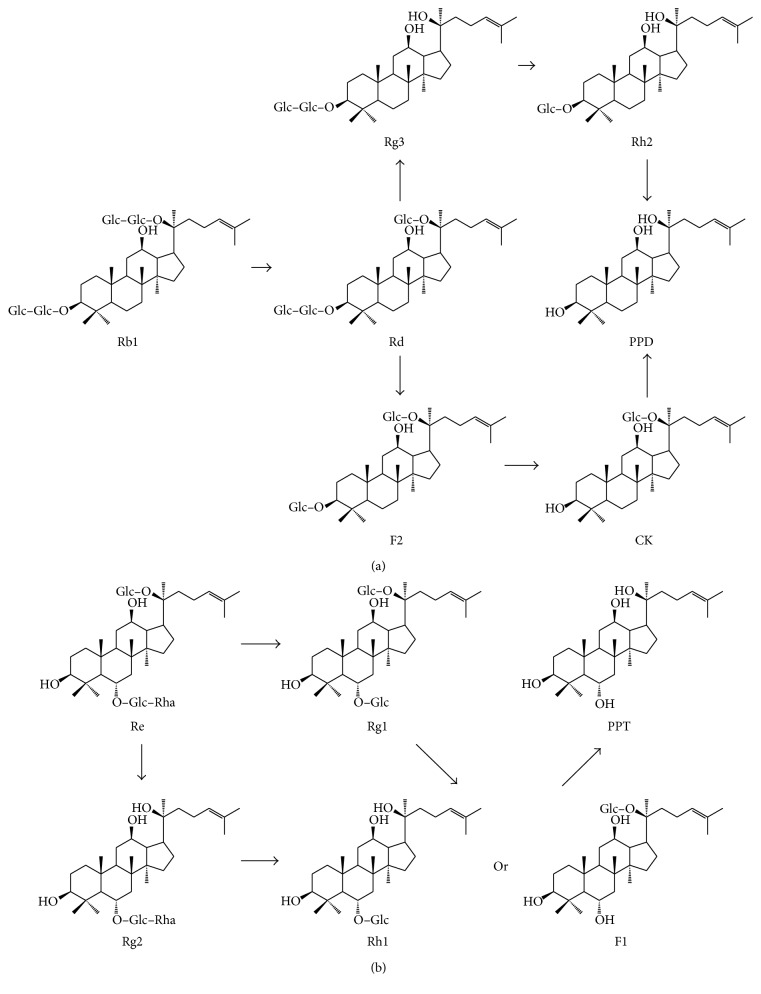
It is noteworthy that PDS and PTS are not easily absorbed by the body through the intestines due to their hydrophilicity [13]. Little amount of PDS could be absorbed in the gastrointestinal tract following oral intake. Therefore, these constituents inevitably come into contact with and are metabolized by microflora in the alimentary tract. As shown in Figure 1, upon oral consumption, ginsenosides are partly transformed into the PPD and PPT through a series of deglycosylation procedures by acid hydrolysis and intestinal bacterial actions [14]. All of the metabolites, such as compound K (CK), PPD, and PPT, are nonpolar compared to the parental components ginsenosides, which could be easily absorbed in the gastrointestinal tract and express biological actions [15]. The ability of PPD to be absorbed after oral administration had been demonstrated through pharmacokinetic studies. It was shown that PPD accumulated largely in the stomach (44%) and small in the intestine (32%) and was also present in the brain (0.01%) [16].
Figure 1.
Major metabolic processes of (a) 20(S)-protopanaxadiol- and (b) 20(S)-protopanaxatriol-type saponins. CK: compound K, PPD: 20(S)-protopanaxadiol, and PPT: 20(S)-protopanaxatriol.
Many reports reveal that the metabolites were assessed as more potent bioactive ingredients than the natural ones. It is validated that PPD, a stable deglycosylated PDS metabolic derivative that could be formulated for oral gavage, exerted antineoplastic actions, which were more effective than its prototype [17]. A good example is the improved anti-androgen-independent prostate cancer activity exerted by the intestinal bacterial metabolites compared to natural occurring ginsenosides, through decreasing survival rate, inhibiting proliferation, inducing apoptosis, and leading to cell cycle arrest in PC-3 cells [18]. The increase of lipotropy and decrease of C-6 steric hindrance, which were caused by deglycosylation by intestinal bacteria, might be the reason for the higher activity of metabolites.
3. Anticancer Effects of Ginsenosides
Preclinical and clinical researches demonstrated that ginsenosides have cancer preventing activities to various tumors, including gastric carcinoma, breast cancer, liver cancer, ovarian cancer, colon cancer, melanoma, and leukemia [19]. Extensive phytochemical and pharmacological studies on ginseng and notoginseng proved that the PDS and PTS are the main anticancer compositions and that the activities of PDS were more powerful than those of PTS [15, 20, 21]. In general, the anticancer effects of total ginsenoside from Asian ginseng and American ginseng, but not notoginseng, are widely reported; meanwhile, lots of publications indicated the anticancer effects of many pure ginsenosides, such as ginsenosides Rg3 and Rh2, isolated from all of the three ginseng types. In view of the results reported by Lee et al. [20] and Jin et al. [21], the relative nonpolar and PDS class ginsenosides exhibited more cytotoxicity on breast cancer cells and efficient cellular uptake on MCF-7 and MCF-7/MX cells compared with the relative polar and PTS class compounds. Shim et al. [15] suggested that the PDS and ginsenosides Rd, Rg3, Rk1, Rg5, and Rh2 showed potent or moderate inhibitory activities on inducing apoptosis of cancer cells through activating the caspase-3 pathway, whereas the PTS and ginsenosides Rf, Rg1, Re, Rh1, and Rg2 did not exhibit any inhibitory activity.
The structure-activity relationships indicated that both glycosylation at C-3-OH and nonoxidation at C-6 on ginsenosides might be important for the inhibition of the chymotrypsin-like activity of the 20S proteasome which plays an important role in selective protein degradation and regulates cellular events in anticancer process. On the other hand, several results indicate compound with less polar chemical structures possesses higher cytotoxic activity towards cancer cells. The ginsenosides with two molecules of glucose linked to C-3-OH have a less potent inhibitory activity than those with one molecule; for example, Rh2 (one glucose at C-3) showed more potent pharmacological activities than Rg3 (two types of glucose at C-3) [7]. From this perspective, cytotoxic potencies of the hydrolysates of PDS and PTS, especially PPD and CK (the hydrolysate of PPD-type ginsenoside fractions), are much stronger than the original ginsenosides.
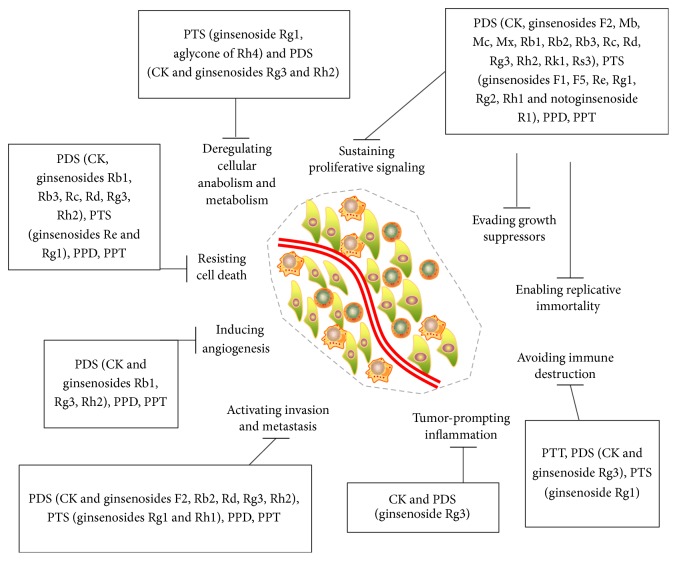
It appears that CK, Rh2, Rg3, PPD, and PPT are possibly responsible for the enhanced anticancer activity of ginseng. In fact, processing of herbs could change the chromatographic and pharmacological profiles of notoginseng and cause an increase of PDS and its hydrolysates, including ginsenosides Rh2, Rk1, Rk3, and Rg3, which might contribute to the greater antiproliferative effects against liver cancer cells, SNU449, SNU182, and HepG2 of steamed notoginseng than its raw form [22]. In another case, Lin et al. [23] attested that, after lactic acid bacteria fermentation, antihepatocarcinoma activity of notoginseng was improved, along with the fact that notoginsenoside R1 and ginsenosides Rg1, Rb1, Rd, and Rh4 were decreased, while ginsenosides Rh1 and Rg3 were increased during fermentation. Additionally, after it is orally taken, PTS would be inevitably deglycosylated by intestinal bacteria. For instance, biotransformation of ginsenoside Rg1 by the fungus Absidia coerulea AS3.2462 yielded five metabolites. Three of them exhibited moderate reversal activity towards A549/taxol MDR tumor cells in vitro [24]. The therapeutic targets of ginsenosides on cancer were summarized in Figure 2.
Figure 2.
Therapeutic targets of ginsenosides on cancer. PTS: 20(S)-protopanaxadiol saponins, PDS: 20(S)-protopanaxatriol saponins, PPT: protopanaxatriol, PPD: protopanaxadiol, and CK: compound K.
3.1. Inhibiting Cancer Cell Growth
Extensive experiment data indicates that ginsenosides could inhibit tumor growth by inhibiting cancer cell proliferation, which can be related with inducing apoptosis and autophage of cancer cells, inhibiting proliferative signaling pathways, or regulating the activity of tumor suppressors. For instance, ginsenoside Rh1 showed great estrogenic effect in human breast carcinoma MCF-7 cells [25]. The synthesized monoester of ginsenoside Rh1 showed moderate effects on murine H22 hepatoma cells [26]. Yang et al. [27] suggested that ginsenoside Rd could serve as a lead to develop novel chemotherapeutic or chemopreventive agents against human cervical cancer.
3.1.1. Inducing Apoptosis and Autophage of Cancer Cells
(1) PPD. Nowadays, PPD has been well characterized to possess the pleiotropic anticarcinogenesis capabilities in many cancer cell lines, including A172, A549, B16, Caco-2, Ehrlich ascites cells L1210, H1299, H358, H838, HCT-116, HCT-8, HeLa, HepG2, HPAC, Int-407, Jurkat, LNCaP, MCF-7, MDA-MB-468, Me180, Molt-4, Panc-1, P388, PC3, Raji, SK-HEP-1, SW-480, T98G, and THP-1. PPD could induce different forms of programmed cell death, including typical apoptosis and autophagy through both caspase-dependent and caspase-independent mechanisms, which could be testified in models of two human glioma cell lines, SF188 and U87MG. For the SF188 cells, PPD activated caspase-3, caspase-7, caspase-8, and caspase-9 and induced rapid apoptosis. Interestingly, in U87MG cells PPD induces cell death without activating any caspases, but with promoting the dramatic autophagy of cells [28]. Additionally, PPD induced the intrinsic and extrinsic apoptotic pathways, activated death receptor 5 and caspase-3, caspase-8, and caspase-9, and upregulated the mRNA and/or protein levels of endoplasmic reticulum stress-associated molecules in HepG2 cells [29]. The research reported by Popovich and Kitts [30] showed that PPD possessed characteristic effects on the proliferation of human leukemia cells THP-1 and that the presence of sugars in PPD aglycone structures reduced the potency to induce apoptosis. It could also inhibit the growth of acute lymphoblastic leukemia (ALL) cell lines Reh and RS411 cells by stimulating differentiation and inhibiting growth and cell cycle progression of ALL cells without changing cell apoptosis [31].
The analogue of PPD is now also known to be helpful for tumor treatment. 20(S)-25-Methoxyl-dammarane-3β,12β,20-triol (25-OCH3-PPD) is a dammarane-type triterpene sapogenin isolated from P. notoginseng, which has been regarded as the principal medicinal component of the plant and has shown anticancer effects in vitro and in vivo with a low toxicity to noncancer cells [32]. Bi et al. [33] added it to LS174, SW620, SW480, and A549 cells and demonstrated that it significantly inhibited cell proliferation and induced apoptosis by modulation on β-catenin, a key mediator in the Wnt pathway. Meanwhile, other researchers found that 25-OCH3-PPD exhibited activity against human H358 and H838 lung cancer cells and androgen-dependent prostate cancer cells, LNCaP and PC3, through decreasing survival, inhibiting proliferation, and inducing apoptosis and G1 cell cycle arrest. This compound also decreased the levels of cell proliferation-associated proteins (MDM2, E2F1, cyclin D1, and cyclin-dependent kinase 2 (CDK2) and CDK4) and increased the activation of proapoptotic proteins (cleaved PARP, cleaved caspase-3, caspase-8, and caspase-9) [32]. Wu et al. [34] detected that 25-OCH3-PPD produced a significant inhibitory effect on activated t-HSC/Cl-6 cells based on its regulatory function to increase the level of cellular GSH and cleaved caspase-3, while PPT, Rg3, Rb1, Rb3, Rg1, Rg2, and Re showed little or no cytotoxic effects. Aside from 25-OCH3-PPD, 20(R)-dammarane-3β,12β,20,25-tetrol (25-OH-PPD) has abilities to inhibit proliferation, leading to cycle arrest, inducing apoptosis on cancer cells, and inhibiting the growth of xenograft tumors without any host toxicity [35].
(2) CK. 20-O-D-Glucopyranosyl-20(S)-protopanaxadiol (CK, IH-901, or M1), one of the most important metabolites of PDS by intestinal microorganisms, is drawing increasing attention recently due to its potent inhibitory benefits on cancer, including hepatocarcinoma cells, breast cancer cells, lymphoma cells, and melanoma cells, in vitro and in vivo (shown in Table 1) [36]. The IC50 of CK to inhibit the proliferation was 12.7, 11.4, 8.5, and 9.7 μM for B16-BL6, HepG2, K562, and 95-D cell lines, respectively [37]. Oral administration of CK significantly inhibited the tumor proliferation at the implantation site after intrapulmonary implantation of Lewis lung carcinoma and colon 26-L5 tumor cells in concentration- and time-dependent manners and suppressed the metastasis to meditational lymph nodes, which was primarily due to induce caspase-3-dependent apoptosis [38]. Moreover, the analogue of CK, 3-O-oleoyl CK (OCK), caused 2.6-fold suppression of tumor growth compared with CK on the growth and metastasis of murine B16-F10 melanoma cells in C57BL/6 mice [39].
Table 1.
Summary of the anticancer activities of PPD and PDS.
| Compounds | Activities | Mechanisms |
|---|---|---|
| Protopanaxadiol (PPD) | Antiproliferation | G1 phase arrest; promotes melanin production and increases DNA binding sites on the cell surface and cell adhesiveness [69]; stimulates differentiation [31]; induces caspase-dependent apoptosis [70]; activates NF-κB signaling [71]; downregulates PI3K/Akt signaling pathway [50] |
| Inhibit tumor growth | Suppresses NF-κB, JNK, and MAPK/ERK signaling pathways [48]; downregulates full-length AR expression and activity and its constitutively active splice variants [6] | |
| Antimetastasis | Downregulates MMP-9 [72] and MMP-2 [73] | |
| Antiangiogenesis | Inhibits the proliferation HUVECs | |
| Synergy and attenuation | Synergies with cyclophosphamide, mitoxantrone, 5-FU, docetaxel, epicatechin, paclitaxel or vinblastine, irinotecan, tamoxifen, or doxorubicin [74–76]. Reverses MDR and inhibits P-gp [77] | |
|
| ||
| Ginsenoside Rg3 | Antiproliferation | Induces calcium-dependent apoptosis and autophagy [78]; induces DNA double-strand breaks [79]; downregulates HIF-1α expression under hypoxia conditions [80]; downregulates PI3K/Akt [81] and three modules of MAP kinases [82]; inhibits COX-2, NF-κB, phosphorylation of STAT3, ERK1/2, and JNK [83] |
| Active tumor suppressors | Induces senescence-like growth arrest by regulating Akt and p53/p21-dependent signaling pathways [61] | |
| Inhibit cellular metabolism | Increases the cellular GSH/GSSG ratio, enhances the γ-GCS activity, and suppresses ROS generation [84] | |
| Antiangiogenesis | Degrades serum IGF-1 level [85]; downregulates KDR and VEGF expressions [86]; decreases intratumoral microvessel density [87]; inhibits VEGF dependent p38/ERK signaling in vitro and inhibits the mobilization of endothelial progenitor cells from the bone marrow microenvironment to the peripheral circulation [88] | |
| Inhibit tumor growth | Downregulates Wnt/β-catenin signaling [54]; decreases HDAC3 and increases acetylation of p53 [63]; decreases FUT4/LeY expression and inhibits the activation of EGFR/MAPK pathway [89] | |
| Antimetastasis | Suppresses invasion and MMP-9 expression level [90]; inhibits micro-lymphatic metastasis of colorectal neoplasms [91]; blocks hypoxia-induced EMT, activates the ubiquitin-proteasome pathway to promote HIF-1α degradation, upregulates E-cadherin via transcriptional suppression of Snail, and downregulates vimentin under hypoxic conditions [92] | |
| Synergy and attenuation | Reverses P-gp-mediated MDR [93]; increases radiosensitivity [94]; synergies with 5-FU, As2O3 (arsenic trioxide), capecitabine, cisplatin, CTX, docetaxel, doxorubicin, gemcitabine, gemcitabine plus cisplatin, mitomycin C and tegafur, paclitaxel, ribonuclease inhibitor, suramin, tamoxifen, TRAIL, verapamil, and vinorelbine+cisplatin [95–100] | |
| Immunomodulation | Improves cellular immunity and stimulates ConA-induced lymphocyte proliferation and augmentation of Th1-type cytokines IL-2 and IFN-γ levels in mice [101]; improves the immune function [102] | |
| Prevent tumorigenesis | Reduces tumor incidence in N:GP(S) newborn mice injected with benzo(a)pyrene [103]; downregulates NF-κB and AP-1 [104] | |
|
| ||
| Ginsenoside Rh2 | Antiproliferation | G1 phase arrest [105]; induces cell differentiation and reduces telomerase activity [106]; induces Ca2+-dependent mitochondrial apoptosis pathway [107]; induces autophagy [78]; activates NF-κB signaling pathway and upregulates TNF-α [108]; reduces Akt/mTOR signaling [109] |
| Active tumor suppressors | Increases the expression level of DR4 death receptor [65]; upregulates miRNA-128 expression [110]; activates p53 [64] | |
| Inhibit cellular metabolism | Induces AMPK and p38 MAPK activation. AMPK determines apoptotic sensitivity of cancer cells to Rh2 [111] | |
| Inhibit tumor growth | Inhibits EGFR signaling through PI3K/Akt/mTOR signaling pathways [112] and upregulation of miR-491 [113]; augment of TGF-β receptor signaling [114] | |
| Antiangiogenesis | Inhibits angiogenesis and lymphangiogenesis and downregulates JAM expression [115] | |
| Synergy and attenuation | Synergies with cisplatin, betulinic acid, CTX, daunomycin, vinblastine, docetaxel, paclitaxel, and mitoxantrone [116, 117]; reverses P-gp-mediated MDR [118] | |
| Prevent tumorigenesis | Decreases the tumor incidence in N:GP(S) newborn mice injected with benzo(a)pyrene model [103, 119] | |
|
| ||
| CK/IH-901/M1 | Antiproliferation | G1 phase arrest [120]. Inhibits telomerase activity and downregulates the human TERT gene [121]; induces mitochondria-dependent apoptotic pathway [122]; inhibits FGFR3 expression and signaling [52]; induces autophagy [123] |
| Active tumor suppressors | Inhibits DNA methyltransferase 1 and reactivates epigenetically silenced genes. IC50: 20 ± 1.0 μg/mL [124]; upregulates cytochrome C, p53, and Bax expression [125] | |
| Inhibit cellular metabolism | CAMK-IV/AMPK pathways [126]; inhibits histone deacetylase activity [127]; modulates AMPK-COX-2 signaling [40] | |
| Anti-inflammation | Inhibits colonic inflammation and tumorigenesis promoted by Western diet. Inhibits tumor xenograft growth. Reduces EGFR and ErbB2 activation and Cox-2 expression [128] | |
| Inhibit tumor growth | Bid-mediated mitochondrial pathway [42]; increases Ca2+ influx, mainly through TRPC channels and by targeting AMPK [129] | |
| Antimetastasis | Inhibits adhesion, invasion, and spontaneous metastatic growth. Inhibition of AP-1 and MAPK pathways [130]; suppresses the activation of the NF-κB pathway and inhibition of MMP-2/MMP-9 expression [131] | |
| Antiangiogenesis | Regulates MMP expression, as well as the activity of sphingosine kinase-1 and its related sphingolipid metabolites [132]; blockades of type IV collagenase secretion [133] | |
| Synergy and attenuation | Synergies with cisplatin, CTX [134]; increases radiosensitivity [135]; decreases the toxicity of irinotecan [136] | |
| Prevent tumorigenesis | Prevents tumorigenesis of aberrant crypts in C57BL:6 mice colonized with ginseng-hydrolyzing bacteria | |
|
| ||
| Ginsenoside Rb1 | Antiproliferation | Increases the expression levels of caspase-3 and caspase-8 [137] |
| Antiangiogenesis | Inhibits the HGF/SF-induced chemoinvasion. Inhibits tyrosine kinase [10]; regulates pigment epithelium-derived factor through the estrogen β receptor [138] | |
| Attenuation | Reduces CTX-induced DNA damage and apoptosis effects [139] | |
| Chemoprevention | Induces cytochrome P450 1A1 expression. Aryl hydrocarbon receptor [140] | |
|
| ||
| Ginsenoside Rb2 | Antiproliferation | Increases the expression levels of caspase-3 and caspase-8 [137] |
| Antimetastasis | Inhibits the adhesion and invasion and suppression of MMP-2 [141] | |
| Prevent tumorigenesis | Prevents the downregulation of gap junctional intercellular communication by TPA and hydrogen peroxide [142] | |
|
| ||
| Ginsenoside Rb3 | Antiproliferation | Inhibits DNA transferring and duplication [143]; inhibits RNF-α-induced NF-κB activity and inhibits COX-2 and iNOS mRNA levels [144] |
| Synergy and attenuation | Increases cisplatin's antiproliferative activity in MCF-7 cells [145] | |
|
| ||
| Ginsenoside Rc | Antiproliferation | Antiproliferation of HCT-116 and HT-29 cells [146] |
| Synergy and attenuation | Reverses MDR, reduces the activity of the efflux pump, enhances T cell proliferation, and increases the NK cell activity [147] | |
|
| ||
| Ginsenoside Rd | Antiproliferation | Inhibits the chymotrypsin-like activity of 26S proteasome [148]; induces apoptosis and reduces oxidative stress and associates with DNA replication and repair, protein synthesis and degradation, mutagenesis, and metastasis [149] |
| Antimetastasis | Blocks MMP activation and MAPK signaling pathways [55] | |
| Synergy and attenuation | Reverses MDR, reduces the activity of the efflux pump, enhances T cell proliferation, and increases the NK cell activity [147] | |
|
| ||
| Ginsenoside Rk1 | Antiproliferation | Induces apoptosis, upregulation of Fas, FasL, and Bax, and downregulation of procaspase-8 and procaspase-3, mutant p53, and Bcl-2 [59] |
|
| ||
| Ginsenoside Rs3 | Antiproliferation | G1/S phase arrest. Elevates protein levels of p53 and p21WAF1 and downregulates the activities of the cyclin-dependent kinases [60] |
|
| ||
| Ginsenoside F2 | Antiproliferation | Induces apoptosis accompanied by protective autophagy. Activates intrinsic apoptotic pathway and mitochondrial dysfunction [150] |
| Inhibit tumor growth | IC50: 49.9 ± 4.2 μM. Accumulation of ROS and activating the ASK-1/JNK signaling pathway [19] | |
| Antimetastasis | IC50: 50 μg/mL. Activation of caspase-3 and caspase-8 and inhibition of MMP-9 [151] | |
|
| ||
| Ginsenosides Mb, Mc, and Mx | Antiproliferation | Antiproliferation of HCT-116 and HT-29 cells [146], as well as HL-60, HGC-27, Colon205, and Du145 cells [152] |
CK exhibits cytotoxicity largely by inducing apoptosis via generation of reactive oxygen species (ROS), regulating on cell cycle arrest at the G1 phase, upregulating Bax, disrupting the mitochondrial membrane potential, activating caspase-3, caspase-8, and caspase-9, inhibiting of telomerase activity, and decreasing cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) levels via an AMP-activated protein kinase- (AMPK-) dependent pathway [40]. The treatment of MDA-MB-231 with CK upregulated COX-2 mRNA and protein and enhanced the production of PGE2 [41]. Hu et al. [42] and Cho et al. [43] reported that CK significantly inhibits the viabilities of BGC823, SGC7901, and HL-60 cells in dose- and time-dependent manners mainly through mitochondria-mediated internal pathway. In the HL-60 treatment, this compound still induced a series of intracellular events associated with death receptor-dependent apoptotic pathway [43].
Meanwhile, CK led to G1 cell cycle arrest in tumor cells. Exposure to CK for 48 hours led to G1 arrest in Hep3B, U937 cells, MDA-MB-231, Hs578T, and MKN28 cells [40, 41, 44]. The CK-mediated G1 arrest resulted from an increase in p27Kip1 mRNA and protein expression followed by a decrease in CDK2 kinase activity. Saiki [45] proposed that the induction of apoptosis of tumor cells by CK involved the upregulation of the CDK inhibitor p27Kip1 as well as the downregulation of c-Myc and cyclin D1 in a time-dependent manner.
In addition to PPD and CK, Rh2 and Rg3 may be also effective preclinical candidate compounds for liver cancer, breast cancer, prostatic cancer, pediatric acute myeloid leukemia and glioblastoma, and so forth (shown in Table 1). X. Wang and Y. Wang indicated that Rh2 significantly prolonged the survival of mice with pediatric leukemia and induced apoptosis of leukemia cells through miR-21-modulated suppression of Bcl-2 [46]. Liu et al. found that Rh2 dose-dependently reduced SCC viability and increased autophagy and reduced β-catenin signaling in SCC cells [47].
3.1.2. Inhibiting Proliferative Signaling Pathways
Besides the activities mentioned above, PPD and CK can exert their anticancer effect through direct antiproliferation activation. In fact, our results suggest that the anticancer activity of PPD in colon cancer cells may be mediated through targeting multiple cancer signaling pathways, namely, nuclear factor-kappa B (NF-κB), JNK, and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathways [48]. Hwang et al. [49] also demonstrated the antiproliferative and proapoptotic effects of an enzymatically fortified ginseng extract on KATO3 cells appear to be associated with the upregulation of Bax, the activation of NF-κB, and the inhibition of mTOR and PKB signals. PPD also downregulates the PI3K/Akt signaling pathway in A549 cells [50]. Yu et al. [51] found that PPD inhibited estrogen-stimulated gene expression and cell proliferation both in vitro and in vivo through blocking [3H]-17-β-estradiol- (E2-) induced transcriptional activation and inhibiting colony formation of endometrial cancer cells. Kang et al. described the fact that CK induced the activation of c-Jun N-terminal kinase (JNK) and the transcription factor AP-1, a downstream target of JNK [44]. Furthermore, CK induced apoptosis and inhibits fibroblast growth factor receptor 3 (FGFR3) expression and signaling in myeloma cell line KMS-11, suggesting candidacy for the chemoprevention and the treatment of myeloma [52].
CK also depresses several cell proliferation signaling pathways, for example, ERK, Janus activated kinase (JAK) 1/signal transducer and activator of transcription 3 (STAT3), AMPK pathway, and FGFR3 signaling. The treatment of MDA-MB-231 with CK upregulated COX-2 mRNA and protein, enhanced the production of PGE2, and induced the sustained activation of ERK [41]. Furthermore, CK inhibited phosphorylation of STAT3 and its upstream activator, JAK1, but not JAK2, downregulated STAT3 target genes bcl-xL, bcl-2, survivin, cyclin E, and D1, and enhanced the expression of protein tyrosine phosphatase SHP-1, but not phosphatase and tensin homolog (PTEN) in this treatment [53].
Similar to PPD and CK, other PDS also target in the cancer cell proliferation related signaling pathways. For example, our results strongly suggest that ginsenoside Rg3 diminishes nuclear staining intensity of β-catenin; the anticancer activity of Rg3 may be in part caused by blocking the nuclear translocation of β-catenin in colon cancer cells [54]. Besides, treatment with ginsenoside Rd dose- and time-dependently inhibited the migration and invasion of human hepatocellular carcinoma HepG2, Hep3B, SNU-398, and SNU-878 cells via blocking MAPK signaling, inhibiting the phosphorylation of ERK and p38 MAPK, inhibiting AP-1 activation [55].
3.1.3. Regulating the Activity of Tumor Suppressors
Tumor suppressor, such as p53, VHL, ING4, Rb, PTEN, p16, p21, APC, DCC, NF1, NF2, WT1, and DR4 death receptor, is a type of genes that protects a cell from one step on the path to cancer [56]. Defects in the tumor suppressor pathway make tumors vulnerable to varieties of stresses, which can be exploited therapeutically. CK inhibits HCT-116, SW-480, and HT-29 cancer cell growth by upregulation of p53/p21, FoxO3a-p27/p15, and Smad3 and downregulation of cdc25A, CDK4/CDK6, and cyclin D1/3 [57]. Similarly, PPD could activate p53 and p21 and downregulate the levels of CDK2, cyclin E and cyclin D1, and PCNA in NIH3T3 cells [58].
As shown in Table 1, several other PDS, such as ginsenosides Rg3, Rh2, Rk1, and Rs3, also demonstrated activation of the activity of p53 and p21 [59]. Rs3 could elevate the protein levels of p53 and p21WAF1 and then downregulate the activities of the cyclin-dependent kinases in SK-HEP-1 cells [60]. Sin et al. indicated that Rg3 could induce senescence-like growth arrest by regulating Akt and p53/p21-dependent signaling pathways in human glioma cells [61]. Rg3-mediated phosphorylation of p53 resulted in inhibition of Akt phosphorylation, which in turn reduced MDM2-mediated p53 degradation [62]. Rg3 also has antiproliferative activity against melanoma by decreasing HDAC3 and increasing acetylation of p53 on lysine (k373/k382) both in vitro and in vivo [63]. Moreover, Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53 [64] and increases the expression level of DR4 death receptor [65]. Guo et al. [66] found that significant increases in Fas expression and caspase-8 activity temporally coincided with an increase in p53 expression in p53-nonmutated HeLa and SK-HEP-1 cells upon Rh2 treatment.
3.2. Antiangiogenesis
Tumor-induced angiogenesis (neovascularization) is one of the most important events concerning tumor growth and metastasis [45]. As shown in Tables 1 and 2, PPT, PPD, and several PDS, namely, CK, Rg3, Rh2, Rb1, and F2, presented significantly antiangiogenic effect. Through investigating their antiangiogenic effects in an angiogenesis model of human umbilical vein endothelial cells (HUVECs), Usami et al. [67] found that PPD displayed inhibition on proliferative activity of HUVECs in a dose-dependent manner and had potential as anticancer drug candidates.
Table 2.
Summary of the anticancer activities of PPT and PTS.
| Effects | Activities | Mechanisms |
|---|---|---|
| Ginsenosides F1 and F5 | Antiproliferation | Induces chromatin condensation and increases sub-G1hypodiploid cells. IC50: 23.2 μM and 62.4 μM [153] |
|
| ||
| Ginsenoside Re | Antiproliferation | Increases GSH/GSSG ratio, enhances the γ-GCS activity, and suppresses ROS generation [84]; inhibits the transferring and duplication of DNA [143] |
| Synergy and attenuation | Synergies with cisplatin. Increases cisplatin's antiproliferative activity [145] | |
|
| ||
| Ginsenoside Rf | Antiproliferation | G2/M phase arrest. IC50: 11.36 μM. Upregulates Bax and downregulates Bcl-2, CDK1, and cyclin B1, activates caspase-3 and caspase-9, and releases cytochrome C [154] |
|
| ||
| Ginsenoside Rg1 | Antiproliferation | Inhibits ubiquitin activating enzyme (E1) activity [155]; S phase arrest and induces cell senescence [156]; increases the expression levels of caspase-3 and caspase-8; induces apoptosis [137]; inhibits EpoR-mediated JAK2/STAT5 signal pathway [157] |
| Immunomodulation | Activates tumor killer cells and enhances the production of NO from IFN-γ activated-macrophages [158] | |
| Inhibit tumor growth | Inhibits colon cancer growth. Downregulates the expression of cyclin D1, PCNA, and VEGF [159] | |
| Antimetastasis | Suppresses TPA-induced tumor cell invasion and migration by inhibition of NF-κB-dependent MMP-9 expression [160]; inhibits transforming growth factor-β1-induced epithelial-to-mesenchymal transition [161] | |
| Synergy and attenuation | Synergist with IL-2. Activates lymphokine activated killer cells as a synergistic of IL-2 [162] | |
| Chemoprevention | Induces cytochrome P450 1A1 expression. Aryl hydrocarbon receptor [140] | |
|
| ||
| Ginsenoside Rg2 | Antiproliferation | IC50: 9.0 μM [153] |
|
| ||
| Ginsenoside Rh1 | Antiproliferation | Induces differentiation. Stimulates the nuclear translocation of glucocorticoid receptor [163]; induces apoptosis [30] |
| Antimetastasis | Inhibits the invasion and migration. Suppresses MMP-1 expression through inhibition of AP-1 and MAPK signaling pathway [164]; inhibits MMPs gene expression by suppressing MAPKs, PI3K/Akt, and downstream NF-κB and AP-1 [165] | |
|
| ||
| Notoginsenoside R1 | Antiproliferation | Induces differentiation; affects synthesis of DNA and RNA [166]; upregulates p53 gene and downregulates Bcl-2 gene [167] |
|
| ||
| Protopanaxatriol (PPT) | Antiproliferation | Increases sub-G1 cells [168]; induces apoptosis. Activation of p53 and p21 and downregulation of CDK2, cyclin E and cyclin D1, and PCNA [58] |
| Antiangiogenesis | Inhibits the proliferative activity of HUVECs in a dose-dependent manner. EC50: 6.64 μg/mL [67] | |
| Antimetastasis | Enhances natural-killer cytotoxicity by esterified protopanaxatriol [169]; inhibits invasion and downregulation of MMP-9 [72] | |
| Synergy and attenuation | Reverses daunomycin and vinblastine resistance [170]; synergies with mitoxantrone; inhibits BCRP-associated vanadate sensitive ATPase activity [21] | |
Jeong et al. [68] investigated the antiangiogenic activity and relative mechanisms of CK in HUVECs. The outcomes revealed that CK significantly inhibited the proliferation and downregulated phosphorylation of p38 MAPK and AKT in bFGF treated HUVECs. Besides, it inhibited the migration and tube formation, reduced secreted level of vascular endothelial growth factor (VEGF), and increased the secreted level of pigment epithelium-derived factor (PEDF) at noncytotoxic concentrations. In vivo experimental results revealed that CK effectively disrupted bFGF-induced neovascularization in the Matrigel plugs excised from mice and inhibited the tumor formation of SGC7901 cells in nude mice [42, 68].
Recently, 20(S)-ginsenoside Rg3 was produced as a new anticancer drug in China due to its antiangiogenic effect. Clinical studies show that Rg3, especially in combination with chemotherapy, can reduce chemotherapy side effect and improve life quality and survival rates of patients with non-small cell lung cancer [171], gastric cancer [172], esophageal cancer [173], and so forth. The mechanism might be correlated with antitumor angiogenesis and improving the immune function. The results were confirmed in many animal models, such as C57BL/6 mice bearing Lewis lung tumor model and rabbits inoculated with liver VX2 tumor model. Yu et al. found that Rg3 could suppress the tumor growth and angiogenesis in VX2 transplanted hepatic tumor model in experimental rabbits. The tumor microvessel density (MVD) and the expression of VEGF were significantly lower than those of the control group [174]. Rg3 also enhanced the antiangiogenic of capecitabine in a model of BALB/c mice inoculated with 4T1 breast cancer [175] and inhibited neovascularization and growth of mouse Lewis lung carcinoma with gemcitabine in C57BL/6 mice inoculated with Lewis lung carcinoma [176].
3.3. Delaying Invasion and Metastasis
Besides the activities mentioned above, some compounds belong to PDS and PTS also exert other pharmacological effects about anticancer. PDS and its metabolites, including Rb2, Rd, F2, Rh2, Rg3, CK, and PPD, could inhibit the tumor invasion and metastasis. Moreover, PTS (shown in Table 2) and its metabolites, including Rg1, Rh1, and PPT, also affect the tumor invasion and metastasis process. Inhibiting epithelial-mesenchymal transition (EMT) and regulating the expression and activity of cellular adhesive molecules, matrix metalloproteinases (MMPs), and collagenases are involved in the anti-invasion effect of ginsenosides.
The results obtained by Li et al. [73] indicated that PPD significantly inhibited the invasiveness of HT1080 cells in vitro, and this action is primarily due to downregulating the expression of MMP-2. Cathcart et al. found that ginsenoside Rd dose- and time-dependently inhibited the migration and invasion of human hepatocellular carcinoma HepG2, Hep3B, SNU-398, and SNU-878 cells via reducing the expression of MMP-1, MMP-2, and MMP-7 [177] and inducing focal adhesion formation and modulating vinculin localization and expression [55].
Other reports indicated that both Rg1 and Rg3 suppress liver cancer cell HepG2 or lung cancer cell A549 migration and invasion in vitro by inhibiting the transforming growth factor- (TGF-) β1-induced EMT [161, 178]. The anti-invasion effects of Rg3 and Rh2 were proved related with the expression of MMP-13 both in B16F10 mouse melanoma cancer cells and in glioblastoma multiforme cells [179, 180]. 20(S)-Rg3 also effectively inhibits EMT in nude mouse xenograft models of ovarian cancer by blocking hypoxia-induced epithelial-mesenchymal transition [92] and limited metastasis and promoted survival by downregulating VEGF overexpression in HCC tumor [181].
3.4. Regulating of Tumor-Related Immune Suppression
The evidences support the effect of ginsenosides on overcoming tumor to evade the immune system. Wang et al. [182] reported that CK could inhibit tumor growth by decreasing the expressions of immunosuppression-related gene and suppressing the production of proinflammatory cytokines. Hao et al. found that total ginsenosides from Asian ginseng can promote the growth inhibition and apoptosis of human T lymphocyte Jurkat cells induced by PG human lung carcinoma cells, which may be related to the upregulation of cytokine TGF-β1 secretion in PG cells [183]. Zhou et al. [184] have compared the anticancer activity of CK plus cyclophosphamide (CTX) with that of CTX alone. The result exhibited that the combination effect was significantly superior and synergistic, which might due to immunoregulation activity of CK by improving the WBC, interleukin- (IL-) 2, and interferon- (IFN-) γ degraded of CTX. Further studies implied that OCK did not directly affect tumor growth in vitro, whereas it promoted tumor cell lysis by lymphocytes, particularly nonadherent splenocytes [185].
Dendritic cell (DC) plays a pivotal role in the initiation of T cell-mediated immune responses through influencing T cell differentiation towards the Th1, Th2, or Th17 type and regulating factors related to the direction of the T cell polarization [186]. PPT exerts anticancer bioactivity mainly through its ability to improve immunity on DC-based vaccines [187], and the activity of PPT is stronger than its original ginsenosides form, PTS. Stimulation with 20 μM of PPT increased expression of CD80, CD83, and CD86 and decreased endocytic activity in DCs [188]. As the most important anticancer compound in ginseng, Rg3 also presented inhibition of tumor growth and immunomodulation activities in H22-tumor bearing mice attributed to the improvement of cellular immunity. It could stimulate ConA-induced lymphocyte proliferation and augmentation of Th1-type cytokines IL-2 and INF-γ levels in mice [101].
In addition to the above, ginsenosides also improve the immune destruction of organism. Jang et al. [189] indicated that methanol extract of cultured wild ginseng cambial meristematic cells (CMCs) is effective for potentiation of NK cell and anticancer activity. PPT-primed mature DCs displayed enhanced T cells stimulatory capacity in an allogeneic mixed lymphocyte reaction. Mature DCs differentiated with PPT induced the differentiation of naive T cells towards a Th1 response. The production of IFN-γ and 51Cr release on PPT-primed mature DCs was augmented, while small amounts of IL-4 depending on IL-12 secretion were investigated [188].
3.5. Deregulating Cellular Anabolism and Metabolism
More and more evidence indicated that the anticancer effect of ginsenosides is also related with its abilities on regulating abnormal tumor anabolism, metabolism, and glycolysis. Li et al. [190] showed that 20(S)-Rg3 could inhibit Warburg effect through STAT3/HK2 pathways, and 20(S)-Rg3 decreased metabolic enzymes in glycolysis including PKM2, HK2, GLUT1, and LDH, but the mechanism still needed further study. Aglycone of Rh4 inhibits melanin synthesis in B16 melanoma cells via the protein kinase A pathway [191]. Investigations indicated that Rh4 significantly reduced the cyclic AMP (cAMP) level and downregulated microphthalmia-associated transcription factor and tyrosinase in B16 melanoma cells. Otherwise, Rg1 has been shown to bind to the glucocorticoid receptor (GR), leading to the downregulation of GR expression and the induction of GR-mediated transcription synergistically with cAMP in FTO2B rat hepatoma cells [192].
As a kind of aldose reductase inhibitor, Rh2 induced AMPK and p38 MAPK activation and thus determined the apoptotic sensitivity of cancer cells [111]. Rg3 and its metabolite CK also impact on the cancer-related metabolism pathways like AMPK. Yuan et al. found that Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway [193]. CK-mediated cell death of HT-29 colon cancer cells is regulated by calcium/calmodulin-dependent protein kinase- (CAMK-) IV/AMPK pathways [126] and CK induced apoptosis by modulating AMPK-COX-2 signaling in MCF-7 human breast cancer cells [40].
3.6. Inhibiting Tumor-Prompting Inflammation
Tumor promotion often accompanies an elevated ornithine decarboxylase (ODC) activity, acute inflammation, and induction of COX-2 activity, and the eukaryotic transcription factor NF-κB has been involved in intracellular signaling pathways associated with inflammation and carcinogenesis [194]. CK has been reported to possess anti-inflammatory effects by inhibiting 12-O-tetradecanoylphorbol-13-acetate- (TPA-) induced COX-2 expression. Lee et al. [195] showed that topical application of CK onto shaven backs of female ICR mice led to the inhibition of TPA-induced expression of COX-2 and production of PGE2. CK pretreatment inhibited TPA-induced epidermal NF-κB DNA binding in mouse skin, which appeared to be mediated by blocking phosphorylation and subsequent degradation of IκBα. The regulatory effect on COX-2 and NF-κB has also been found in Rg3-pretreated mouse skin stimulated by TPA [104].
3.7. Depressing Carcinogenesis
Eliminating and reducing risk factors of carcinogenesis are considered a critical step to tumor prevention and control. Korean investigators carried out extensive long-term anticarcinogenicity experiments with 2000 newborn mice stimulated by several chemical carcinogens and suggested that traditional Chinese medicine ginseng holds a potential anticancer effect [196]. There was a 22% decrease (p < 0.05) in the incidence of urethane induced lung adenoma by the use of red ginseng extract. Yun and colleagues indicated that red ginseng extract showed inhibition of lung tumor incidence, while fresh ginseng did not [196]. Another research from Yun's group also demonstrated that the anticarcinogenicity of ginseng was more prominent in aged or heat treated extracts of ginseng and red ginseng made by steaming. Moreover, ginsenosides Rg3, Rg5, and Rh2 were found to be active anticarcinogenic compounds [103].
The report by Keum and colleagues suggested that Rg3 also inhibits the tumor promotion. Pretreatment of dorsal skins of female ICR mice with Rg3 significantly inhibited TPA-induced ornithine decarboxylase activity and 7,12-dimethylbenz[a]anthracene-initiated papilloma formation. Rg3 pretreatment also abrogated the expression of COX-2 in TPA-stimulated mouse skin possibly through downregulation of NF-κB and AP-1 transcription factors [104]. Furthermore, Rb2 prevents human cancers by downregulation of gap junctional intercellular communication by TPA and hydrogen peroxide in WB-F344 rat liver epithelial cells [142]. CK could prevent tumorigenesis of aberrant crypts in C57BL:6 mice colonized with ginseng-hydrolyzing bacteria [197].
Phase 2 detoxification enzymes protect against carcinogenesis and oxidative stress. Lee et al. [198] illustrated that PPD induced the activity of phase 2 detoxification enzymes. Ginseng extracts and components (such as PPD and PPT) were assayed for inducer activity of NQO1 (quinone reductase), a phase 2 enzyme, in Hepa1c1c7 cells. Wang et al. [140] suggested that the chemopreventive effect of Panax ginseng may be also due, in part, to ginsenosides Rg1 and Rb1's ability to compete with aryl hydrocarbons for both the aryl hydrocarbon receptor and CYP1A1 in HepG2 cell.
3.8. Synergy with Chemotherapy
It is suggested that the combination of ginsenoside or notoginsenoside with chemotherapy drugs acts synergistically to produce therapeutic effects greater than those that can be achieved with single use. With the aim of increasing the activities and decreasing the side effects, the adjuvant potentials of saponins had been screened. Combining 25-OCH3-PPD with conventional chemotherapeutic agents or radiation led to potent anticancer effects. The tumor regression was almost complete following administration of 25-OCH3-PPD and taxotere/gemcitabine [35]. Researchers had also hypothesized that the potential therapeutic efficacy of PTS and PDS possibly could be enhanced when they are cotreated with various kinds of known tumor necrosis factor- (TNF-) α antagonists [119].
As is mentioned above, PPD could also be an adjuvant agent to achieve more effective anticancer activities. It has been assessed by calcein AM efflux assay that PPD was able to inhibit P-glycoprotein (P-gp) activity as potently as verapamil on MDR cells, while it did not affect ATPase activity of P-gp [77]. Combinations of PPD and docetaxel yield more additive or synergistic activity on established PC-3 tumors compared to animals treated with docetaxel alone [117]. Besides, PPD synergistically enhances cytotoxicity of tamoxifen and mitoxantrone in an estrogen receptor-independent fashion, probably by downregulating Akt activity [21, 51].
As shown in Table 1, PDS including Rc, Rd, Rb1, Rh2, and Rb3 show synergistic activity with chemotherapeutic drugs. Choi et al. [199] demonstrated that PTS isolated from ginseng also has a chemosensitizing effect on P-gp-mediated multidrug resistance (MDR) cells by increasing the intracellular accumulation of drugs through direct interaction with P-gp at the azidopine site. Kitagawa et al. [200] examined that PPT increased the accumulation of P-gp substrate daunorubicin 3.6-fold, more potent than that of CK. Collectively, ginseng types or ginsenosides administration might render an improved efficiency and an ameliorated toxicity of chemotherapy during cancer treatment.
4. Conclusion
Observations published in the last years suggest that ginsenoside could be an anticancer agent for various cancers, and the anticancer property of ginsenoside is associated with the induction of apoptosis or autophagy and inhibition of cell proliferation, metastasis, and angiogenesis, as well as modulating the immune system. As the major active components of ginseng types, PTS and PDS ginsenosides have shown wide anticancer properties with respective characteristics. Compared with PTS, PDS ginsenosides (e.g., Rg3) and its metabolites or derivates have stronger therapeutic potential for inhibiting the growth, angiogenesis, metastasis, inflammation, and immune evasion of cancer. On the other hand, PTS and PPT regulate abnormal tumor anabolism, metabolism, and glycolysis in cancer. PTS and its derivatives also depress carcinogenesis and improve the antitumor activity of chemotherapeutic drugs.
As a result of the multiple targets and signaling pathways of ginsenosides, we still could not get a clear understanding of the anticancer effect of ginseng types. But the current research has confirmed the anticancer effect of ginseng types in the aspects mentioned above. Although the research progress on ginseng has greatly promoted its application, how PTS and PDS target cancer-related signaling pathways remains unclear, and the further details and mechanism are still unknown. Thus, it is of importance to understand the characteristics and possible mechanisms associated with the anticancer effects of ginseng derivatives.
Acknowledgments
This work was supported by the funds from the National Natural Science Foundation of China (NSFC) (81473575 to Jian-Li Gao and 81503288 to Jian-Bo Wan).
Abbreviations
- 25-OCH3-PPD:
20(S)-25-Methoxyl-dammarane-3β,12β,20-triol
- 25-OH-PPD:
20(R)-Dammarane-3β,12β,20,25-tetrol
- Akt:
Protein kinase B
- ALL:
Acute lymphoblastic leukemia
- AMPK:
AMP-activated protein kinase
- cAMP:
Cyclic AMP
- AP-1:
Activator protein-1
- AR:
Androgen receptor
- ASK-1:
Apoptosis signal regulating kinase-1
- ATP:
Adenosine triphosphate
- Bcl-2:
B-cell lymphoma-2
- BCRP:
Breast cancer resistance protein
- CDK:
Cyclin-dependent kinase
- CK:
Compound K
- COX-2:
Cyclooxygenase-2
- CTX:
Cyclophosphamide
- DC:
Dendritic cell
- EGFR:
Epidermal growth factor receptor
- EMT:
Epithelial-mesenchymal transition
- ERK:
Extracellular signal-regulated kinase
- bFGF:
Basic fibroblast growth factor
- FGFR3:
Fibroblast growth factor receptor 3
- FUT4:
Fucosyltransferase 4
- GCS:
Glasgow Coma Scale
- GR:
Glucocorticoid receptor
- GSH:
Glutathione
- GSSG:
Oxidized glutathione
- HDAC3:
Histone deacetylase 3
- HGF/SF:
Hepatocyte growth factor/scatter factor
- HIF-1:
Hypoxia inducible factor-1
- HUVEC:
Human umbilical vein endothelial cell
- IFN:
Interferon
- IL:
Interleukin
- JAK:
Janus activated kinase
- JAM:
Junctional adhesion molecule
- JNK:
c-Jun N-terminal kinase
- KDR:
Kinase insert domain receptor
- MAPK:
Mitogen-activated protein kinase
- MDR:
Multidrug resistance
- MMP:
Matrix metalloproteinase
- MVD:
Mevalonate (diphosphate) decarboxylase
- NADPH:
Nicotinamide adenine dinucleotide phosphate
- NF-κB:
Nuclear factor-kappa B
- NO:
Nitric oxide
- NQO1:
NADPH quinone oxidoreductase 1
- OCK:
3-O-Oleoyl compound K
- PARP:
Poly(ADP-ribose) polymerase
- PCNA:
Proliferating cell nuclear antigen
- PDS:
20(S)-Protopanaxadiol saponin
- PGE2:
Prostaglandin E2
- PI3K:
Phosphatidylinositol 3-kinase
- PPD:
20(S)-Protopanaxadiol
- PPT:
20(S)-Protopanaxatriol
- PTEN:
Phosphatase and tensin homolog
- PTS:
20(S)-Protopanaxatriol saponin
- RNF-α:
RING finger protein-alpha
- ROS:
Reactive oxygen species
- TERT:
Telomerase reverse transcriptase
- TGF:
Transforming growth factor
- TPA:
12-O-Tetradecanoylphorbol-13-acetate
- TRAIL:
Tumor necrosis factor-related apoptosis-inducing ligand
- TRPC:
Transient receptor potential channel
- VEGF:
Vascular endothelial growth factor.
Disclosure
Xiao-Jia Chen and Xiao-Jing Zhang contributed equally to this work and should be considered co-first authors.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2015. CA Cancer Journal for Clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Zeng H., Zhang S., He J. Annual report on status of cancer in China, 2011. Chinese Journal of Cancer Research. 2015;27(1):2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen L. S. Bencao Gangmu. 1st. Beijing, China: Huaxia; 2005. [Google Scholar]
- 4.The State Pharmacopoeia Commission of People’s Republic of China. Pharmacopoeia of the People's Republic of China. Beijing, China: Chemical Industry Press; 2000. [Google Scholar]
- 5.State Administration of Traditional Chinese Medicine. ZhongHua Ben Cao JinXuan Ben. Shanghai, China: Shanghai Science and Technology; 1996. [Google Scholar]
- 6.Cao B., Qi Y., Yang Y., et al. 20(S)-protopanaxadiol inhibition of progression and growth of castration-resistant prostate cancer. PLoS ONE. 2014;9(11, article e111201) doi: 10.1371/journal.pone.0111201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong H., Bai L.-P., Wong V. K. W., et al. The in vitro structure-related anti-cancer activity of ginsenosides and their derivatives. Molecules. 2011;16(12):10619–10630. doi: 10.3390/molecules161210619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang K. S., Yokozawa T., Yamabe N. Y., Kim H. Y., Park J. H. ESR study on the structure and hydroxyl radical-scavenging activity relationships of ginsenosides isolated from Panax ginseng C. A. Meyer. Biological and Pharmaceutical Bulletin. 2007;30(5):917–921. doi: 10.1248/bpb.30.917. [DOI] [PubMed] [Google Scholar]
- 9.Wan J.-B., Lee S. M.-Y., Wang J.-D., et al. Panax notoginseng reduces atherosclerotic lesions in ApoE-deficient mice and inhibits TNF-α-induced endothelial adhesion molecule expression and monocyte adhesion. Journal of Agricultural and Food Chemistry. 2009;57(15):6692–6697. doi: 10.1021/jf900529w. [DOI] [PubMed] [Google Scholar]
- 10.Sengupta S., Toh S.-A., Sellers L. A., et al. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;110(10):1219–1225. doi: 10.1161/01.cir.0000140676.88412.cf. [DOI] [PubMed] [Google Scholar]
- 11.Rhule A., Navarro S., Smith J. R., Shepherd D. M. Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells. Journal of Ethnopharmacology. 2006;106(1):121–128. doi: 10.1016/j.jep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. Journal of Korean Medical Science. 2001;16:S28–S37. doi: 10.3346/jkms.2001.16.s.s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W., Wang G.-J., Xie H.-T., et al. Determination of ginsenoside Rd in dog plasma by liquid chromatography-mass spectrometry after solid-phase extraction and its application in dog pharmacokinetics studies. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2007;852(1-2):8–14. doi: 10.1016/j.jchromb.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 14.Yue P. Y. K., Mak N. K., Cheng Y. K., et al. Pharmacogenomics and the Yin/Yang actions of ginseng: anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chinese Medicine. 2007;2, article 6 doi: 10.1186/1749-8546-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shim S. H., Baek K.-H., Kim Y. S. Inhibition of human 20S proteasome by ginsenosides from Panax ginseng. Bulletin of the Korean Chemical Society. 2009;30(6):1385–1387. doi: 10.5012/bkcs.2009.30.6.1385. [DOI] [Google Scholar]
- 16.Musende A. G., Eberding A., Wood C. A., et al. A novel oral dosage formulation of the ginsenoside aglycone protopanaxadiol exhibits therapeutic activity against a hormone-insensitive model of prostate cancer. Anti-Cancer Drugs. 2012;23(5):543–552. doi: 10.1097/CAD.0b013e32835006f5. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. Journal of Pharmacological Sciences. 2004;95(2):153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- 18.Li W., Liu Y., Zhang J. W., et al. Anti-androgen-independent prostate cancer effects of ginsenoside metabolites In vitro: mechanism and possible structure-activity relationship investigation. Archives of Pharmacal Research. 2009;1:49–57. doi: 10.1007/s12272-009-1117-1. [DOI] [PubMed] [Google Scholar]
- 19.Mao Q., Zhang P.-H., Wang Q., Li S.-L. Ginsenoside F2 induces apoptosis in humor gastric carcinoma cells through reactive oxygen species-mitochondria pathway and modulation of ASK-1/JNK signaling cascade in vitro and in vivo. Phytomedicine. 2014;21(4):515–522. doi: 10.1016/j.phymed.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Lee J. I., Ha Y. W., Choi T. W., et al. Cellular uptake of ginsenosides in Korean white ginseng and red ginseng and their apoptotic activities in human breast cancer cells. Planta Medica. 2011;77(2):133–140. doi: 10.1055/s-0030-1250160. [DOI] [PubMed] [Google Scholar]
- 21.Jin J., Shahi S., Kang H. K., van Veen H. W., Fan T.-P. Metabolites of ginsenosides as novel BCRP inhibitors. Biochemical and Biophysical Research Communications. 2006;345(4):1308–1314. doi: 10.1016/j.bbrc.2006.04.152. [DOI] [PubMed] [Google Scholar]
- 22.Toh D.-F., Patel D. N., Chan E. C. Y., Teo A., Neo S.-Y., Koh H.-L. Anti-proliferative effects of raw and steamed extracts of Panax notoginseng and its ginsenoside constituents on human liver cancer cells. Chinese Medicine. 2011;6, article 4 doi: 10.1186/1749-8546-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y.-W., Mou Y.-C., Su C.-C., Chiang B.-H. Antihepatocarcinoma activity of lactic acid bacteria fermented Panax notoginseng . Journal of Agricultural and Food Chemistry. 2010;58(15):8528–8534. doi: 10.1021/jf101543k. [DOI] [PubMed] [Google Scholar]
- 24.Liu X., Qiao L., Xie D., et al. Microbial transformation of ginsenoside-Rg 1 by Absidia coerulea and the reversal activity of the metabolites towards multi-drug resistant tumor cells. Fitoterapia. 2011;82(8):1313–1317. doi: 10.1016/j.fitote.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Bae E.-A., Shin J.-E., Kim D.-H. Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Biological and Pharmaceutical Bulletin. 2005;28(10):1903–1908. doi: 10.1248/bpb.28.1903. [DOI] [PubMed] [Google Scholar]
- 26.Han M., Hou J.-G., Dong C.-M., et al. Isolation, synthesis and structures of ginsenoside derivatives and their anti-tumor bioactivity. Molecules. 2010;15(1):399–406. doi: 10.3390/molecules15010399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Z.-G., Sun H.-X., Ye Y.-P. Ginsenoside Rd from Panax notoginseng is cytotoxic towards HeLa cancer cells and induces apoptosis. Chemistry and Biodiversity. 2006;3(2):187–197. doi: 10.1002/cbdv.200690022. [DOI] [PubMed] [Google Scholar]
- 28.Liu G.-Y., Bu X., Yan H., Jia W. W.-G. 20S-Protopanaxadiol-induced programmed cell death in glioma cells through caspase-dependent and -independent pathways. Journal of Natural Products. 2007;70(2):259–264. doi: 10.1021/np060313t. [DOI] [PubMed] [Google Scholar]
- 29.Zhu G.-Y., Li Y.-W., Tse A. K.-W., et al. 20(S)-Protopanaxadiol, a metabolite of ginsenosides, induced cell apoptosis through endoplasmic reticulum stress in human hepatocarcinoma HepG2 cells. European Journal of Pharmacology. 2011;668(1-2):88–98. doi: 10.1016/j.ejphar.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Popovich D. G., Kitts D. D. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Archives of Biochemistry and Biophysics. 2002;406(1):1–8. doi: 10.1016/s0003-9861(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 31.Sun L. H., Wang Q., Liu X. M., et al. Anti-cancer effects of 20(S)-protopanoxadiol on human acute lymphoblastic leukemia cell lines Reh and RS4;11. Medical Oncology. 2011;28(3):813–821. doi: 10.1007/s12032-010-9508-1. [DOI] [PubMed] [Google Scholar]
- 32.Wang W., Rayburn E. R., Hang J., Zhao Y. Q., Wang H., Zhang R. W. Anti-lung cancer effects of novel ginsenoside 25-OCH3-PPD. Lung Cancer. 2009;65(3):306–311. doi: 10.1016/j.lungcan.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bi X., Zhao Y., Fang W., Yang W. Anticancer activity of Panax notoginseng extract 20(S)-25-OCH3-PPD: Targetting β-catenin signalling. Clinical and Experimental Pharmacology and Physiology. 2009;36(11):1074–1078. doi: 10.1111/j.1440-1681.2009.05203.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y.-L., Wan Y., Jin X.-J., et al. 25-OCH3-PPD induces the apoptosis of activated t-HSC/Cl-6 cells via c-FLIP-mediated NF-κB activation. Chemico-Biological Interactions. 2011;194(2-3):106–112. doi: 10.1016/j.cbi.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Wang W., Wang H., Rayburn E. R., Zhao Y., Hill D. L., Zhang R. 20(S)-25-methoxyl-dammarane-3β, 12β, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action. British Journal of Cancer. 2008;98(4):792–802. doi: 10.1038/sj.bjc.6604227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akao T., Kanaoka M., Kobashi K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration: measurement of compound K by enzyme immunoassay. Biological & Pharmaceutical Bulletin. 1998;21(3):245–249. doi: 10.1248/bpb.21.245. [DOI] [PubMed] [Google Scholar]
- 37.Zhou W., Feng M.-Q., Li J.-Y., Zhou P. Studies on the preparation, crystal structure and bioactivity of ginsenoside compound K. Journal of Asian Natural Products Research. 2006;8(6):519–527. doi: 10.1080/10286020500208600. [DOI] [PubMed] [Google Scholar]
- 38.Suda K., Murakami K., Murata J., Hasegawa H., Saiki I. An intestinal bacterial metabolite (M1) of ginseng protopanaxadiol saponins inhibits tumor-induced neovascularization. Wakan Iyakugaku Zasshi. 2000;17(4):144–150. [Google Scholar]
- 39.Hasegawa H. Metabolic activation of ginsenoside against cancer: intestinal bacterial deglycosylation and hepatic fatty-acid esterification. Wakan Iyakugaku Zasshi. 1997;18(6):217–228. [Google Scholar]
- 40.Kim A. D., Kang K. A., Zhang R., et al. Ginseng saponin metabolite induces apoptosis in MCF-7 breast cancer cells through the modulation of AMP-activated protein kinase. Environmental Toxicology and Pharmacology. 2010;30(2):134–140. doi: 10.1016/j.etap.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Yim H. W., Jong H.-S., Tai Y. K., et al. Cyclooxygenase-2 inhibits novel ginseng metabolite-mediated apoptosis. Cancer Research. 2005;65(5):1952–1960. doi: 10.1158/0008-5472.can-04-1740. [DOI] [PubMed] [Google Scholar]
- 42.Hu C., Song G., Zhang B., et al. Intestinal metabolite compound K of panaxoside inhibits the growth of gastric carcinoma by augmenting apoptosisviaBid-mediated mitochondrial pathway. Journal of Cellular and Molecular Medicine. 2012;16(1):96–106. doi: 10.1111/j.1582-4934.2011.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho S.-H., Chung K.-S., Choi J.-H., Kim D.-H., Lee K.-T. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer. 2009;9, article 149:449–461. doi: 10.1186/1471-2407-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang K. A., Kim Y. W., Kim S. U. G1 phase arrest of the cell cycle by a ginseng metabolite, compound K, in U937 human monocytic leukamia cells. Archives of Pharmacal Research. 2005;28(6):685–690. doi: 10.1007/bf02969359. [DOI] [PubMed] [Google Scholar]
- 45.Saiki I. In vivo anti-metastatic action in ginseng saponins is based on their intestinal bacterial metabolites after oral administration. Journal of Ginseng Research. 2007;31(1):1–13. [PubMed] [Google Scholar]
- 46.Wang X., Wang Y. Ginsenoside Rh2 mitigates pediatric leukemia through suppression of Bcl-2 in leukemia cells. Cellular Physiology and Biochemistry. 2015;37(2):641–650. doi: 10.1159/000430383. [DOI] [PubMed] [Google Scholar]
- 47.Liu S., Chen M., Li P., et al. Ginsenoside Rh2 inhibits cancer stem-like cells in skin squamous cell carcinoma. Cellular Physiology and Biochemistry. 2015;36(2):499–508. doi: 10.1159/000430115. [DOI] [PubMed] [Google Scholar]
- 48.Gao J.-L., Lv G.-Y., He B.-C., et al. Ginseng saponin metabolite 20(S)-protopanaxadiol inhibits tumor growth by targeting multiple cancer signaling pathways. Oncology Reports. 2013;30(1):292–298. doi: 10.3892/or.2013.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang J.-W., Baek Y.-M., Jang I.-S., et al. An enzymatically fortified ginseng extract inhibits proliferation and induces apoptosis of KATO3 human gastric cancer cells via modulation of Bax, mTOR, PKB and IκBα . Molecular Medicine Reports. 2015;11(1):670–676. doi: 10.3892/mmr.2014.2704. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y.-L., Zhang R., Xu H.-L., Yu X.-F., Qu S.-C., Sui D.-Y. 20(S)-protopanaxadiol triggers mitochondrial-mediated apoptosis in human lung adenocarcinoma A549 cells via inhibiting the PI3K/Akt signaling pathway. The American Journal of Chinese Medicine. 2013;41(5):1137–1152. doi: 10.1142/S0192415X13500778. [DOI] [PubMed] [Google Scholar]
- 51.Yu Y., Zhou Q., Hang Y., Bu X., Jia W. Antiestrogenic effect of 20S-protopanaxadiol and its synergy with tamoxifen on breast cancer cells. Cancer. 2007;109(11):2374–2382. doi: 10.1002/cncr.22659. [DOI] [PubMed] [Google Scholar]
- 52.Choi H. H., Jong H.-S., Park J.-H., et al. A novel ginseng saponin metabolite induces apoptosis and down-regulates fibroblast growth factor receptor 3 in myeloma cells. International Journal of Oncology. 2003;23(4):1087–1093. [PubMed] [Google Scholar]
- 53.Park S., Lee H.-J., Jeong S.-J., et al. Inhibition of JAK1/STAT3 signaling mediates compound K-induced apoptosis in human multiple myeloma U266 cells. Food and Chemical Toxicology. 2011;49(6):1367–1372. doi: 10.1016/j.fct.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 54.He B.-C., Gao J.-L., Luo X., et al. Ginsenoside Rg3 inhibits colorectal tumor growth through the down-regulation of Wnt/β-catenin signaling. International Journal of Oncology. 2011;38(2):437–445. doi: 10.3892/ijo.2010.858. [DOI] [PubMed] [Google Scholar]
- 55.Yoon J.-H., Choi Y.-J., Cha S.-W., Lee S.-G. Anti-metastatic effects of ginsenoside Rd via inactivation of MAPK signaling and induction of focal adhesion formation. Phytomedicine. 2012;19(3-4):284–292. doi: 10.1016/j.phymed.2011.08.069. [DOI] [PubMed] [Google Scholar]
- 56.Riaz N., Morris L. G., Lee W., Chan T. A. Unraveling the molecular genetics of head and neck cancer through genome-wide approaches. Genes and Diseases. 2014;1(1):75–86. doi: 10.1016/j.gendis.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z., Du G.-J., Wang C.-Z., et al. Compound K, a ginsenoside metabolite, inhibits colon cancer growth via multiple pathways including p53-p21 interactions. International Journal of Molecular Sciences. 2013;14(2):2980–2995. doi: 10.3390/ijms14022980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang S. J., Cha J. Y., Park S. G., et al. Diol- and triol-type ginseng saponins potentiate the apoptosis of NIH3T3 cells exposed to methyl methanesulfonate. Toxicology and Applied Pharmacology. 2002;181(3):192–202. doi: 10.1006/taap.2002.9413. [DOI] [PubMed] [Google Scholar]
- 59.Kim J. S., Joo E. J., Chun J., et al. Induction of apoptosis by ginsenoside Rk1 in SK-MEL-2-human melanoma. Archives of Pharmacal Research. 2012;35(4):717–722. doi: 10.1007/s12272-012-0416-0. [DOI] [PubMed] [Google Scholar]
- 60.Kim S.-E., Lee Y. H., Park J. H., Lee S. K. Ginsenoside-Rs3, a new diol-type ginseng saponin, selectively elevates protein levels of p53 and p21(WAF1) leading to induction of apoptosis in SK-HEP-1 cells. Anticancer Research. 1999;19(1):487–491. [PubMed] [Google Scholar]
- 61.Sin S., Kim S. Y., Kim S. S. Chronic treatment with ginsenoside Rg3 induces Akt-dependent senescence in human glioma cells. International Journal of Oncology. 2012;41(5):1669–1674. doi: 10.3892/ijo.2012.1604. [DOI] [PubMed] [Google Scholar]
- 62.Choi Y.-J., Kang L.-J., Lee S.-G. Stimulation of DDX3 expression by ginsenoside Rg3 through the Akt/p53 pathway activates the innate immune response via TBK1/IKKε/IRF3 signalling. Current Medicinal Chemistry. 2014;21(8):1050–1060. doi: 10.2174/09298673113206660306. [DOI] [PubMed] [Google Scholar]
- 63.Shan X., Fu Y.-S., Aziz F., Wang X.-Q., Yan Q., Liu J.-W. Ginsenoside Rg3 inhibits melanoma cell proliferation through down-regulation of histone deacetylase 3 (HDAC3) and increase of p53 acetylation. PLoS ONE. 2014;9(12, article e115401) doi: 10.1371/journal.pone.0115401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li B., Zhao J., Wang C.-Z., et al. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Letters. 2011;301(2):185–192. doi: 10.1016/j.canlet.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng C.-C., Yang S.-M., Huang C.-Y., Chen J.-C., Chang W.-M., Hsu S.-L. Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemotherapy and Pharmacology. 2005;55(6):531–540. doi: 10.1007/s00280-004-0919-6. [DOI] [PubMed] [Google Scholar]
- 66.Guo X.-X., Li Y., Sun C., et al. p53-dependent Fas expression is critical for Ginsenoside Rh2 triggered caspase-8 activation in HeLa cells. Protein and Cell. 2014;5(3):224–234. doi: 10.1007/s13238-014-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Usami Y., Liu Y.-N., Lin A.-S., et al. Antitumor agents. 261. 20(S)-protopanaxadiol and 20(S)-protopanaxatriol as antiangiogenic agents and total assignment of 1H NMR spectra. Journal of Natural Products. 2008;71(3):478–481. doi: 10.1021/np070613q. [DOI] [PubMed] [Google Scholar]
- 68.Jeong A., Lee H.-J., Jeong S.-J., et al. Compound K inhibits basic fibroblast growth factor-induced angiogenesis via regulation of p38 mitogen activated protein kinase and AKT in human umbilical vein endothelial cells. Biological & Pharmaceutical Bulletin. 2010;33(6):945–950. doi: 10.1248/bpb.33.945. [DOI] [PubMed] [Google Scholar]
- 69.Ota T., Maeda M., Odashima S. Mechanism of action of ginsenoside Rh2: uptake and metabolism of ginsenoside Rh2 by cultured B16 melanoma cells. Journal of Pharmaceutical Sciences. 1991;80(12):1141–1146. doi: 10.1002/jps.2600801210. [DOI] [PubMed] [Google Scholar]
- 70.Jin Y. H., Yim H., Park J. H., Lee S. K. Cdk2 activity is associated with depolarization of mitochondrial membrane potential during apoptosis. Biochemical and Biophysical Research Communications. 2003;305(4):974–980. doi: 10.1016/S0006-291X(03)00870-2. [DOI] [PubMed] [Google Scholar]
- 71.Wang C.-Z., Li B., Wen X.-D., et al. Paraptosis and NF-κB activation are associated with protopanaxadiol-induced cancer chemoprevention. BMC Complementary and Alternative Medicine. 2013;13, article 2 doi: 10.1186/1472-6882-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park M.-T., Cha H.-J., Jeong J.-W., et al. Glucocorticoid receptor-induced down-regulation of MMP-9 by ginseng components, PD and PT contributes to inhibition of the invasive capacity of HT1080 human fibrosarcoma cells. Molecules and Cells. 1999;9(5):476–483. [PubMed] [Google Scholar]
- 73.Li G., Wang Z. H., Sun Y. X., Liu K., Wang Z. R. Ginsenoside 20(S)-protopanaxadiol inhibits the proliferation and invasion of human fibrosarcoma HT1080 cells. Basic and Clinical Pharmacology and Toxicology. 2006;98(6):588–592. doi: 10.1111/j.1742-7843.2006.pto_415.x. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y., Yang G., Bu X., et al. Cell-type-specific regulation of raft-associated Akt signaling. Cell Death and Disease. 2011;2(4, article e145) doi: 10.1038/cddis.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du G.-J., Wang C.-Z., Zhang Z.-Y., et al. Caspase-mediated pro-apoptotic interaction of panaxadiol and irinotecan in human colorectal cancer cells. Journal of Pharmacy and Pharmacology. 2012;64(5):727–734. doi: 10.1111/j.2042-7158.2012.01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park B., Lee Y.-M., Kim J.-S., et al. Neutral sphingomyelinase 2 modulates cytotoxic effects of protopanaxadiol on different human cancer cells. BMC Complementary and Alternative Medicine. 2013;13, article 194 doi: 10.1186/1472-6882-13-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y., Bu L., Yan H., Jia W. 20S-protopanaxadiol inhibits P-glycoprotein in multidrug resistant cancer cells. Planta Medica. 2009;75(10):1124–1128. doi: 10.1055/s-0029-1185477. [DOI] [PubMed] [Google Scholar]
- 78.Cheong J. H., Kim H., Hong M. J., et al. Stereoisomer-specific anticancer activities of ginsenoside Rg3 and Rh2 in HepG2 cells: disparity in cytotoxicity and autophagy-inducing effects due to 20(S)-epimers. Biological and Pharmaceutical Bulletin. 2015;38(1):102–108. doi: 10.1248/bpb.b14-00603. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y.-H., Li H.-D., Li B., Jiang S.-D., Jiang L.-S. Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells and reduces MNNG-induced DNA damage and apoptosis in normal human cells. Oncology Reports. 2014;31(2):919–925. doi: 10.3892/or.2013.2914. [DOI] [PubMed] [Google Scholar]
- 80.Wang B.-S., Zhang L.-S., Song D.-M., Zhang J.-H., Liu Y.-M. Effect of gensenoside Rg3 on apoptosis of Hep-2 and expression of HIF-1alha in human laryngeal cancer cell line under anoxic conditions. Journal of Chinese Medicinal Materials. 2009;32(1):102–106. [PubMed] [Google Scholar]
- 81.Wang J.-H., Nao J.-F., Zhang M., He P. 20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer HO-8910 cells through PI3K/Akt and XIAP pathways. Tumor Biology. 2014;35(12):11985–11994. doi: 10.1007/s13277-014-2497-5. [DOI] [PubMed] [Google Scholar]
- 82.Kim H.-S., Lee E.-H., Ko S.-R., Choi K.-J., Park J.-H., Im D.-S. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Archives of Pharmacal Research. 2004;27(4):429–435. doi: 10.1007/bf02980085. [DOI] [PubMed] [Google Scholar]
- 83.Chen Q.-J., Zhang M.-Z., Wang L.-X. Gensenoside Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells. Cellular Physiology and Biochemistry. 2010;26(6):849–858. doi: 10.1159/000323994. [DOI] [PubMed] [Google Scholar]
- 84.Ng W. Y., Yang M. S. Effects of ginsenosides Re and Rg3 on intracellular redox state and cell proliferation in C6 glioma cells. Chinese Medicine. 2008;3, article 8 doi: 10.1186/1749-8546-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang H.-B., Ren Y.-P., Zhang J., Ma S.-H., Gao F., Wu Y.-P. Correlation of insulin-like growth factor-1 (IGF-1) to angiogenesis of breast cancer in IGF-1-deficient mice. Chinese Journal of Cancer. 2007;26(11):1215–1220. [PubMed] [Google Scholar]
- 86.Wang X., Zheng Y.-L., Li K., Lin N., Fan Q.-X. The effects of ginsenosides Rg3 on the expressions of VEGF and KDR in human lung squamous cancer cells. Journal of Chinese medicinal materials. 2009;32(11):1708–1710. [PubMed] [Google Scholar]
- 87.Tao H., Yao M., Zou S., Zhao D., Qiu H. Effect of angiogenesis inhibitor Rg3 on the growth and metastasis of gastric cancer in SCID mice. Zhonghua Wai Ke Za Zhi. 2002;40(8):606–608. [PubMed] [Google Scholar]
- 88.Kim J.-W., Jung S.-Y., Kwon Y.-H., et al. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biology and Therapy. 2012;13(7):504–515. doi: 10.4161/cbt.19599. [DOI] [PubMed] [Google Scholar]
- 89.Shan X., Aziz F., Tian L. L., Wang X. Q., Yan Q., Liu J. W. Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation inhibits melanoma cell proliferation by decreasing FUT4/LeY expression. International Journal of Oncology. 2015;46(4):1667–1676. doi: 10.3892/ijo.2015.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang X., Xin Y., Xu T.-M., Qu Y.-Q. Effects of ginsenoside Rg3 on the invasion and metastasis of mouse melanoma cell line B16 as well as the expression of MMP-9. Tumor. 2011;31(2):117–121. [Google Scholar]
- 91.Yu Y., Zhang G.-M., Su J., Shang L.-H., Chen G. Effects of ginsenoside Rg3 on the micro-lymphatic metastasis of colorectal neoplasms. Chinese Journal of Oncology. 2005;27(12):p. 742. [PubMed] [Google Scholar]
- 92.Liu T., Zhao L., Zhang Y., et al. Ginsenoside 20(S)-Rg3 targets HIF-1α to Block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0103887.e103887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He J.-B., Liao H.-Z., Zhang Y.-Q. Ginsenoside Rg3 reverses multidrug resistance of lung adenocarcinoma in mice. Journal of Practical Oncology. 2012;27(4):373–378. [Google Scholar]
- 94.Sun B.-S., Liu X.-L., Liu L.-L., Fu S.-B., Wu Z.-F., Gong S.-L. Suppressive effects of ginsenoside Rg3 combined with X-ray irradiation on growth of melanoma cells. Journal of Jilin University Medicine Edition. 2006;32(6):1016–1018. [Google Scholar]
- 95.Nie N., Wu X.-L., Luo H.-C., Liu B., Liu Q. Inhibitory effect of 5-fluorouracil combined with Rg3 on proliferation of gastric cancer cells. Journal of Shanghai Jiaotong University (Medical Science) 2014;34(5):645–649. [Google Scholar]
- 96.Lee C.-K., Park K.-K., Chung A.-S., Chung W.-Y. Ginsenoside Rg3 enhances the chemosensitivity of tumors to cisplatin by reducing the basal level of nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone oxidoreductase-1 and prevents normal tissue damage by scavenging cisplatin-induced intracellular reactive oxygen species. Food and Chemical Toxicology. 2012;50(7):2565–2574. doi: 10.1016/j.fct.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 97.Kim S.-S., Seong S., Kim S.-Y. Synergistic effect of ginsenoside Rg3 with verapamil on the modulation of multidrug resistance in human acute myeloid leukemia cells. Oncology Letters. 2014;7(4):1265–1269. doi: 10.3892/ol.2014.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang L.-Q., Wang B., Gan H., et al. Enhanced oral bioavailability and anti-tumour effect of paclitaxel by 20(s)-ginsenoside Rg3 in vivo . Biopharmaceutics & Drug Disposition. 2012;33(8):425–436. doi: 10.1002/bdd.1806. [DOI] [PubMed] [Google Scholar]
- 99.Xu X.-H., He J.-B., Zhang P. Effects of Ginsenoside Rg3 in combination with Suramin on growth of lung cancer in mice. Chinese Journal of Cancer Prevention and Treatment. 2013;20(2):97–101. [Google Scholar]
- 100.Che J.-B., Liu Z.-H., Ma H.-B., et al. Influence of As2O3 combined with ginsenosides Rg3 on inhibition of lung cancer NCI-H1299 cells and on subsistence of nude mice bearing hepatoma. Asian Pacific Journal of Tropical Medicine. 2014;7(10):772–775. doi: 10.1016/s1995-7645(14)60134-6. [DOI] [PubMed] [Google Scholar]
- 101.Wu R., Ru Q., Chen L., Ma B., Li C. Stereospecificity of ginsenoside Rg3 in the promotion of cellular immunity in hepatoma H22-bearing mice. Journal of Food Science. 2014;79(7):H1430–H1435. doi: 10.1111/1750-3841.12518. [DOI] [PubMed] [Google Scholar]
- 102.Lu P., Su W., Miao Z.-H., Niu H.-R., Hua Q.-L. Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with non-small cell lung cancer. Chinese Journal of Integrative Medicine. 2008;14(1):33–36. doi: 10.1007/s11655-007-9002-6. [DOI] [PubMed] [Google Scholar]
- 103.Yun T.-K. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutation Research—Fundamental and Molecular Mechanisms of Mutagenesis. 2003;523-524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 104.Keum Y.-S., Han S.-S., Chun K.-S., et al. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-κB activation and tumor promotion. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 2003;523-524:75–85. doi: 10.1016/S0027-5107(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 105.Ota T., Maeda M., Odashima S., Ninomiya-Tsuji J., Tatsuka M. G1 phase-specific suppression of the Cdk2 activity by ginsenoside Rh2 in cultured murine cells. Life Sciences. 1996;60(2):PL39–PL44. doi: 10.1016/S0024-3205(96)00608-X. [DOI] [PubMed] [Google Scholar]
- 106.Zeng X.-L., Tu Z.-G. Effects of ginsenoside Rh2 on signal transduction in hepatocarcinoma SMMC-7721 cells. Chinese Journal of Hepatology. 2004;12(9):565–566. [PubMed] [Google Scholar]
- 107.Ge W., Yang S.-J. Molecular mechanism of G-Rh2-induced apoptosis in gastric carcinoma cells. World Chinese Journal of Digestology. 2007;15(28):2972–2976. [Google Scholar]
- 108.Yu H., Hou J., Qi X., Shi Y., Li S., Zhang C. 20 (S)-ginsenoside-Rh2 induces apoptosis in human lung adenocarcinoma A549 cells by activating NF-κB signaling pathway. Journal of Pure and Applied Microbiology. 2013;7(1):287–294. [Google Scholar]
- 109.You Z.-M., Zhao L., Xia J., et al. Down-regulation of phosphoglucose isomerase/autocrine motility factor enhances gensenoside Rh2 pharmacological action on leukemia KG1α cells. Asian Pacific Journal of Cancer Prevention. 2014;15(3):1099–1104. doi: 10.7314/apjcp.2014.15.3.1099. [DOI] [PubMed] [Google Scholar]
- 110.Wu N., Wu G.-C., Hu R., Li M., Feng H. Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128. Acta Pharmacologica Sinica. 2011;32(3):345–353. doi: 10.1038/aps.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim M.-J., Yun H., Kim D.-H., et al. Amp-activated protein kinase determines apoptotic sensitivity of cancer cells to ginsenoside-Rh2. Journal of Ginseng Research. 2014;38(1):16–21. doi: 10.1016/j.jgr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li S., Gao Y., Ma W., et al. EGFR signaling-dependent inhibition of glioblastoma growth by ginsenoside Rh2. Tumor Biology. 2014;35(6):5593–5598. doi: 10.1007/s13277-014-1739-x. [DOI] [PubMed] [Google Scholar]
- 113.Chen W., Qiu Y. Ginsenoside Rh2 targets EGFR by up-regulation of miR-491 to enhance anti-tumor activity in hepatitis B virus-related hepatocellular carcinoma. Cell Biochemistry and Biophysics. 2015;72(2):325–331. doi: 10.1007/s12013-014-0456-9. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Q., Hong B., Wu S., Niu T. Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumor Biology. 2015;36(4):2377–2381. doi: 10.1007/s13277-014-2845-5. [DOI] [PubMed] [Google Scholar]
- 115.Yang H.-K., Lü Y.-H., Liu H.-D., et al. Effect of ginsenoside Rh2 on VEGF-C and lymphangiogenesis in the mouse-transplanted tumor. Acta Anatomica Sinica. 2009;40(2):265–268. doi: 10.3969/j.issn.0529-1356.2009.02.019. [DOI] [Google Scholar]
- 116.Li Q., Wang X.-Y., Li Y., Jin Y.-H. Mechanism of ginsenoside Rh2 in enhancing sensitivity of human liver cancer HepG2 cells to betulinic acid. Chinese Journal of Biologicals. 2011;24(5):531–542. [Google Scholar]
- 117.Musende A. G., Eberding A., Jia W., Ramsay E., Bally M. B., Guns E. T. Rh2 or its aglycone aPPD in combination with docetaxel for treatment of prostate cancer. Prostate. 2010;70(13):1437–1447. doi: 10.1002/pros.21179. [DOI] [PubMed] [Google Scholar]
- 118.Zhang H., Wang H.-Q., Zhang H.-L., Kong D., Wu X.-Z. Ginsenoside Rh2 reverses P-glycoprotein-mediated multidrug resistance of MCF-7/ADM cells. Tumor. 2007;27(5):365–369. [Google Scholar]
- 119.Cho J. Y., Yoo E. S., Baik K. U., Park M. H., Han B. H. In vitro inhibitory effect of protopanaxadiol ginsenosides on tumor necrosis factor (TNF)-α production and its modulation by known TNF-α antagonists. Planta Medica. 2001;67(3):213–218. doi: 10.1055/s-2001-12005. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y., Xu Y., Zhu Y., Li X. Anti-cancer effects of ginsenoside compound k on pediatric acute myeloid leukemia cells. Cancer Cell International. 2013;13, article 24 doi: 10.1186/1475-2867-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kang K. A., Lee K. H., Chae S., et al. Inhibition of telomerase activity in U937 human monocytic leukemia cells by Compound K, a ginseng saponin metabolite. Biotechnology and Bioprocess Engineering. 2006;11(1):7–12. doi: 10.1007/BF02931861. [DOI] [Google Scholar]
- 122.Lee I. K., Kang K. A., Lim C. M., et al. Compound K, a metabolite of ginseng saponin, induces mitochondria-dependent and caspase-dependent apoptosis via the generation of reactive oxygen species in human colon cancer cells. International Journal of Molecular Sciences. 2010;11(12):4916–4931. doi: 10.3390/ijms11124916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim A. D., Kang K. A., Kim H. S., et al. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death and Disease. 2013;4(8, article e750) doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kang K. A., Kim H. S., Kim D. H., Hyun J. W. The role of a ginseng saponin metabolite as a DNA methyltransferase inhibitor in colorectal cancer cells. International Journal of Oncology. 2013;43(1):228–236. doi: 10.3892/ijo.2013.1931. [DOI] [PubMed] [Google Scholar]
- 125.Ming Y.-L., Song G., Chen L.-H., et al. Anti-proliferation and apoptosis induced by a novel intestinal metabolite of ginseng saponin in human hepatocellular carcinoma cells. Cell Biology International. 2007;31(10):1265–1273. doi: 10.1016/j.cellbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 126.Kim D. Y., Park M. W., Yuan H. D., Lee H. J., Kim S. H., Chung S. H. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. Journal of Agricultural and Food Chemistry. 2009;57(22):10573–10578. doi: 10.1021/jf902700h. [DOI] [PubMed] [Google Scholar]
- 127.Kang K. A., Piao M. J., Kim K. C., et al. Compound K, a metabolite of ginseng saponin, inhibits colorectal cancer cell growth and induces apoptosis through inhibition of histone deacetylase activity. International Journal of Oncology. 2013;43(6):1907–1914. doi: 10.3892/ijo.2013.2129. [DOI] [PubMed] [Google Scholar]
- 128.Dougherty U., Mustafi R., Wang Y., et al. American ginseng suppresses Western diet-promoted tumorigenesis in model of inflammation-associated colon cancer: role of EGFR. BMC Complementary and Alternative Medicine. 2011;11, article 111 doi: 10.1186/1472-6882-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hwang J. A., Hwang M. K., Jang Y., et al. 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol, a metabolite of ginseng, inhibits colon cancer growth by targeting TRPC channel-mediated calcium influx. Journal of Nutritional Biochemistry. 2013;24(6):1096–1104. doi: 10.1016/j.jnutbio.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 130.Jung S.-H., Woo M.-S., Kim S.-Y., et al. Ginseng saponin metabolite suppresses phorbol ester-induced matrix metalloproteinase-9 expression through inhibition of activator protein-1 and mitogen-activated protein kinase signaling pathways in human astroglioma cells. International Journal of Cancer. 2006;118(2):490–497. doi: 10.1002/ijc.21356. [DOI] [PubMed] [Google Scholar]
- 131.Ming Y., Chen Z., Chen L., et al. Ginsenoside compound K attenuates metastatic growth of hepatocellular carcinoma, which is associated with the translocation of nuclear factor-κB p65 and reduction of matrix metalloproteinase-2/9. Planta Medica. 2011;77(5):428–433. doi: 10.1055/s-0030-1250454. [DOI] [PubMed] [Google Scholar]
- 132.Shin K.-O., Seo C.-H., Cho H.-H., et al. Ginsenoside compound K inhibits angiogenesis via regulation of sphingosine kinase-1 in human umbilical vein endothelial cells. Archives of Pharmacal Research. 2014;37(9):1183–1192. doi: 10.1007/s12272-014-0340-6. [DOI] [PubMed] [Google Scholar]
- 133.Hasegawa H., Uchiyama M. Antimetastatic efficacy of orally administered ginsenoside Rb1 in dependence on intestinal bacterial hydrolyzing potential and significance of treatment with an active bacterial metabolite. Planta Medica. 1998;64(8):696–700. doi: 10.1055/s-2006-957560. [DOI] [PubMed] [Google Scholar]
- 134.Lee S.-J., Sung J.-H., Lee S.-J., Moon C.-K., Lee B.-H. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Letters. 1999;144(1):39–43. doi: 10.1016/S0304-3835(99)00188-3. [DOI] [PubMed] [Google Scholar]
- 135.Chae S., Kang K. A., Chang W. Y., et al. Effect of compound K, a metabolite of ginseng saponin, combined with γ-ray radiation in human lung cancer cells in vitro and in vivo. Journal of Agricultural and Food Chemistry. 2009;57(13):5777–5782. doi: 10.1021/jf900331g. [DOI] [PubMed] [Google Scholar]
- 136.Chen Y., Wang P., Liang H., Liu S., Mu L., Zhao Y. Therapeutic window alteration of irinotecan by ginsenosides. Latin American Journal of Pharmacy. 2014;33(10):1731–1734. [Google Scholar]
- 137.Lee D. G., Jang S.-I., Kim Y.-R., et al. Anti-proliferative effects of ginsenosides extracted from mountain ginseng on lung cancer. Chinese Journal of Integrative Medicine. 2014 doi: 10.1007/s11655-014-1789-8. [DOI] [PubMed] [Google Scholar]
- 138.Leung K. W., Cheung L. W. T., Pon Y. L., et al. Ginsenoside Rb1 inhibits tube-like structure formation of endothelial cells by regulating pigment epithelium-derived factor through the oestrogen β receptor. British Journal of Pharmacology. 2007;152(2):207–215. doi: 10.1038/sj.bjp.0707359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Q. H., Wu C. F., Yang J. Y., Mu Y. H., Chen X. X., Zhao Y. Q. Reduction of cyclophosphamide-induced DNA damage and apoptosis effects of ginsenoside Rb1 on mouse bone marrow cells and peripheral blood leukocytes. Environmental Toxicology and Pharmacology. 2009;27(3):384–389. doi: 10.1016/j.etap.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 140.Wang Y., Ye X., Ma Z., et al. Induction of cytochrome P450 1A1 expression by ginsenoside Rg1 and Rb1 in HepG2 cells. European Journal of Pharmacology. 2008;601(1–3):73–78. doi: 10.1016/j.ejphar.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 141.Fujimoto J., Sakaguchi H., Aoki I., Toyoki H., Khatun S., Tamaya T. Inhibitory effect of ginsenoside-Rb2 on invasiveness of uterine endometrial cancer cells to the basement membrane. European Journal of Gynaecological Oncology. 2001;22(5):339–341. [PubMed] [Google Scholar]
- 142.Kang K.-S., Kang B.-C., Lee B.-J., et al. Preventive effect of epicatechin and ginsenoside Rb2 on the inhibition of gap junctional intercellular communication by TPA and H2O2 . Cancer Letters. 2000;152(1):97–106. doi: 10.1016/s0304-3835(99)00438-3. [DOI] [PubMed] [Google Scholar]
- 143.Xie J.-T., Du G.-J., McEntee E., et al. Effects of triterpenoid glycosides from fresh ginseng berry on SW480 human colorectal cancer cell line. Cancer Research and Treatment. 2011;43(1):49–55. doi: 10.4143/crt.2011.43.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.He F., Ding Y., Liang C., et al. Antitumor effects of dammarane-type saponins from steamed notoginseng. Pharmacognosy Magazine. 2014;10(39):314–317. doi: 10.4103/0973-1296.137372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aung H.-H., Mehendale S.-R., Wang C.-Z., Xie J.-T., McEntee E., Yuan C.-S. Cisplatin's tumoricidal effect on human breast carcinoma MCF-7 cells was not attenuated by American ginseng. Cancer Chemotherapy and Pharmacology. 2007;59(3):369–374. doi: 10.1007/s00280-006-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hao M., Zhang L., Zheng Y., Song C., Zhou Y., Cong X. Anti-proliferative effects of protopanaxadiol-type ginsenosides on human colon epithelial cancer cells. Journal of Chemical and Pharmaceutical Research. 2013;5(12):141–144. [Google Scholar]
- 147.Berek L., Szabó D., Petri I. B., Shoyama Y., Lin Y.-H., Molnár J. Effects of naturally occurring glucosides, solasodine glucosides, ginsenosides and parishin derivatives on multidrug resistance of lymphoma cells and leukocyte functions. In Vivo. 2001;15(2):151–156. [PubMed] [Google Scholar]
- 148.Chang T.-L., Ding H.-Y., Kao Y.-W. Role of ginsenoside Rd in inhibiting 26S proteasome activity. Journal of Agricultural and Food Chemistry. 2008;56(24):12011–12015. doi: 10.1021/jf801427e. [DOI] [PubMed] [Google Scholar]
- 149.Lee S. Y., Kim G. T., Roh S. H., et al. Proteome changes related to the anti-cancer activity of HT29 cells by the treatment of ginsenoside Rd. Pharmazie. 2009;64(4):242–247. doi: 10.1691/ph.2009.8734. [DOI] [PubMed] [Google Scholar]
- 150.Mai T. T., Moon J., Song Y., et al. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Letters. 2012;321(2):144–153. doi: 10.1016/j.canlet.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 151.Shin J. Y., Lee J. M., Shin H. S., et al. Anti-cancer effect of ginsenoside F2 against glioblastoma multiforme in xenograft model in SD rats. Journal of Ginseng Research. 2012;36(1):86–92. doi: 10.5142/jgr.2012.36.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Han Y., Sun B., Hu X., et al. Transformation of bioactive compounds by Fusarium sacchari fungus isolated from the soil-cultivated ginseng. Journal of Agricultural and Food Chemistry. 2007;55(23):9373–9379. doi: 10.1021/jf070354a. [DOI] [PubMed] [Google Scholar]
- 153.Tung N. H., Song G. Y., Van Minh C., et al. Steamed ginseng-leaf components enhance cytotoxic effects on human leukemia HL-60 cells. Chemical and Pharmaceutical Bulletin. 2010;58(8):1111–1115. doi: 10.1248/cpb.58.1111. [DOI] [PubMed] [Google Scholar]
- 154.Shangguan W.-J., Li H., Zhang Y.-H. Induction of G2/M phase cell cycle arrest and apoptosis by ginsenoside Rf in human osteosarcoma MG-63 cells through the mitochondrial pathway. Oncology Reports. 2014;31(1):305–313. doi: 10.3892/or.2013.2815. [DOI] [PubMed] [Google Scholar]
- 155.Chang T.-L., Huang Y.-H., Ou Y.-D. The role of ginsenosides in inhibiting ubiquitin activating enzyme (E1) activity. Journal of Functional Foods. 2014;7(1):462–470. doi: 10.1016/j.jff.2014.01.010. [DOI] [Google Scholar]
- 156.Wang H.-N., Zuo G.-W., Li C.-L., et al. Effects of ginsenoside Rb1, Rg1 on proliferation of leukemia K562 cells. Journal of Clinical Rehabilitative Tissue Engineering Research. 2009;13(40):7829–7832. doi: 10.3969/j.issn.1673-8225.2009.40.004. [DOI] [Google Scholar]
- 157.Xia J., Li J., Zuo G.-W., et al. The effect of ginsenoside Rg1 on EPOR pathway in leukemia cell line TF-1. Tumor. 2014;34(2):113–120. [Google Scholar]
- 158.Fan Z.-H., Isobe K., Kiuchi K., Nakashima I. Enhancement of nitric oxide production from activated macrophages by a purified form of ginsenoside (Rg1) The American Journal of Chinese Medicine. 1995;23(3-4):279–287. doi: 10.1142/S0192415X9500033X. [DOI] [PubMed] [Google Scholar]
- 159.Zhang L.-J., Zhou E.-F. In vitro and in vivo inhibitory effects of ginsenoside Rg1 on proliferation of colon cancer cells. World Chinese Journal of Digestology. 2014;22(30):4599–4603. doi: 10.11569/wcjd.v22.i30.4599. [DOI] [Google Scholar]
- 160.Li L., Wang Y., Qi B., et al. Suppression of PMA-induced tumor cell invasion and migration by ginsenoside Rg1 via the inhibition of NF-κB-dependent MMP-9 expression. Oncology Reports. 2014;32(5):1779–1786. doi: 10.3892/or.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yu M., Yu X., Guo D., et al. Ginsenoside Rg1 attenuates invasion and migration by inhibiting transforming growth factor-beta1-induced epithelial to mesenchymal transition in HepG2 cells. Molecular Medicine Reports. 2015;11(4):3167–3173. doi: 10.3892/mmr.2014.3098. [DOI] [PubMed] [Google Scholar]
- 162.Zhao T.-H. Enhancing effect of ginsenoside Rg1 on LAK cells antitumor activity in vitro. Chinese Journal of Clinical Oncology. 1994;21(1):48–49. [Google Scholar]
- 163.Lee Y.-N., Lee H.-Y., Lee Y. M., et al. Involvement of glucocorticoid receptor in the induction of differentiation by ginsenosides in F9 teratocarcinoma cells. The Journal of Steroid Biochemistry and Molecular Biology. 1998;67(2):105–111. doi: 10.1016/S0960-0760(98)00080-6. [DOI] [PubMed] [Google Scholar]
- 164.Yoon J.-H., Choi Y.-J., Lee S.-G. Ginsenoside Rh1 suppresses matrix metalloproteinase-1 expression through inhibition of activator protein-1 and mitogen-activated protein kinase signaling pathway in human hepatocellular carcinoma cells. European Journal of Pharmacology. 2012;679(1–3):24–33. doi: 10.1016/j.ejphar.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 165.Jung J.-S., Ahn J.-H., Le T.-K., Kim D.-H., Kim H.-S. Protopanaxatriol ginsenoside Rh1 inhibits the expression of matrix metalloproteinases and the in vitro invasion/migration of human astroglioma cells. Neurochemistry International. 2013;63(2):80–86. doi: 10.1016/j.neuint.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 166.Xu L., Wang B., Gao J. Preliminary studies of notoginsenoside R1-induced differentiation of HL-60 cell lines in vitro. Journal of West China University of Medical Sciences. 1991;22(2):124–127. [PubMed] [Google Scholar]
- 167.Wang C.-Z., Xie J.-T., Zhang B., et al. Chemopreventive effects of Panax notoginseng and its major constituents on SW480 human colorectal cancer cells. International Journal of Oncology. 2007;31(5):1149–1156. doi: 10.3892/ijo.31.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Popovich D. G., Kitts D. D. Ginsenosides 20(S)-protopanaxadiol and Rh2 reduce cell proliferation and increase sub-G1 cells in two cultured intestinal cell lines, Int-407 and Caco-2. Canadian Journal of Physiology and Pharmacology. 2004;82(3):183–190. doi: 10.1139/y04-001. [DOI] [PubMed] [Google Scholar]
- 169.Hasegawa H., Suzuki R., Nagaoka T., Tezuka Y., Kadota S., Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biological and Pharmaceutical Bulletin. 2002;25(7):861–866. doi: 10.1248/bpb.25.861. [DOI] [PubMed] [Google Scholar]
- 170.Hasegawa H., Sung J.-H., Matsumiya S., et al. Reversal of daunomycin and vinblastine resistance in multidrug-resistant P388 leukemia in vitro through enhanced cytotoxicity by triterpenoids. Planta Medica. 1995;61(5):409–413. doi: 10.1055/s-2006-958126. [DOI] [PubMed] [Google Scholar]
- 171.Sun Y., Lin H., Zhu Y., et al. A randomized, prospective, multi-centre clinical trial of NP regimen (vinorelbine+cisplatin) plus Gensing Rg3 in the treatment of advanced non-small cell lung cancer patients. Chinese Journal of Lung Cancer. 2006;9(3):254–258. doi: 10.3779/j.issn.1009-3419.2006.03.09. [DOI] [PubMed] [Google Scholar]
- 172.Chen Z.-J., Cheng J., Huang Y.-P., et al. Effect of adjuvant chemotherapy of ginsenoside Rg3 combined with mitomycin C and tegafur in advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2007;10(1):64–66. [PubMed] [Google Scholar]
- 173.Huang J.-Y., Sun Y., Fan Q.-X., Zhang Y.-Q. Efficacy of Shenyi Capsule combined with gemcitabine plus cisplatin in treatment of advanced esophageal cancer: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2009;7(11):1047–1051. doi: 10.3736/jcim20091105. [DOI] [PubMed] [Google Scholar]
- 174.Yu Y., Zhang C., Liu L., Li X. Hepatic arterial administration of ginsenoside Rg3 and transcatheter arterial embolization for the treatment of VX2 liver carcinomas. Experimental and Therapeutic Medicine. 2013;5(3):761–766. doi: 10.3892/etm.2012.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Q., Kang X., Yang B., Wang J., Yang F. Antiangiogenic effect of capecitabine combined with ginsenoside Rg3 on breast cancer in mice. Cancer Biotherapy and Radiopharmaceuticals. 2008;23(5):647–653. doi: 10.1089/cbr.2008.0532. [DOI] [PubMed] [Google Scholar]
- 176.Huang X., Hou M., Yi C., Song H. Anti-angiogenic effects of low-dose gemcitabine combined with ginsenoside Rg3 on mouse Lewis lung carcinoma. Chinese Journal of Lung Cancer. 2006;9(2):132–136. doi: 10.3779/j.issn.1009-3419.2006.02.07. [DOI] [PubMed] [Google Scholar]
- 177.Cathcart J., Pulkoski-Gross A., Cao J. Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes & Diseases. 2015;2(1):26–34. doi: 10.1016/j.gendis.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim Y.-J., Choi W.-I., Jeon B.-N., et al. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-beta1-induced epithelial-mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology. 2014;322:23–33. doi: 10.1016/j.tox.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 179.Lee S. G., Kang Y. J., Nam J.-O. Anti-metastasis effects of ginsenoside Rg3 in B16F10 cells. Journal of Microbiology and Biotechnology. 2015;25(12):1997–2006. doi: 10.4014/jmb.1506.06002. [DOI] [PubMed] [Google Scholar]
- 180.Guan N., Huo X., Zhang Z., Zhang S., Luo J., Guo W. Ginsenoside Rh2 inhibits metastasis of glioblastoma multiforme through Akt-regulated MMP13. Tumor Biology. 2015;36(9):6789–6795. doi: 10.1007/s13277-015-3387-1. [DOI] [PubMed] [Google Scholar]
- 181.Zhou B., Wang J., Yan Z. Ginsenoside Rg3 attenuates hepatoma VEGF overexpression after hepatic artery embolization in an orthotopic transplantation hepatocellular carcinoma rat model. OncoTargets and Therapy. 2014;7:1945–1954. doi: 10.2147/OTT.S69830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang R., Li Y., Wang W., Zhou M., Cao Z. Compound K suppresses myeloid-derived suppressor cells in a mouse model bearing CT26 colorectal cancer xenograft. Journal of Southern Medical University. 2015;35(5):748–752. [PubMed] [Google Scholar]
- 183.Hao Y., Wang P., Wu J., Qiu Q.-Y. Effects of ginsenoside and berberine on secretion of immunosuppressive cytokines in lung carcinoma cell line PG. Zhong Xi Yi Jie He Xue Bao. 2008;6(3):278–282. doi: 10.3736/jcim20080312. [DOI] [PubMed] [Google Scholar]
- 184.Zhou W., Feng M.-Q., Li X.-W., et al. X-ray structure investigation of (20S)-20-O-β-D-glucopyranosyl-protopanaxadiol and antitumor effect on Lewis lung carcinoma in vivo. Chemistry & Biodiversity. 2009;6:380–388. doi: 10.1002/cbdv.200700444. [DOI] [PubMed] [Google Scholar]
- 185.Hasegawa H., Saiki I. Oleoyl triterpene glycoside biotransformed from ginseng suppresses growth and metastasis of murine B16-F10 melanoma via immunostimulation. Wakan Iyakugaku Zasshi. 2000;17(5):186–193. [Google Scholar]
- 186.Mellman I., Steinman R.-M. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106(3):255–258. doi: 10.1016/S0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 187.Takei M., Tachikawa E., Umeyama A. Dendritic cells promoted by ginseng saponins drive a potent Th1 polarization. Biomarker Insights. 2008;3:269–286. doi: 10.4137/bmi.s585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Takei M., Tachikawa E., Hasegawa H., Lee J.-J. Dendritic cells maturation promoted by M1 and M4, end products of steroidal ginseng saponins metabolized in digestive tracts, drive a potent Th1 polarization. Biochemical Pharmacology. 2004;68(3):441–452. doi: 10.1016/j.bcp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 189.Jang A. Y., Song E. J., Shin S. H., et al. Potentiation of natural killer (NK) cell activity by methanol extract of cultured cambial meristematic cells of wild ginseng and its mechanism. Life Sciences. 2015;135:138–146. doi: 10.1016/j.lfs.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 190.Li J., Liu T., Zhao L., et al. Ginsenoside 20(S)Rg3 inhibits the Warburg effect through STAT3 pathways in ovarian cancer cells. International Journal of Oncology. 2015;46(2):775–781. doi: 10.3892/ijo.2014.2767. [DOI] [PubMed] [Google Scholar]
- 191.Jeong Y.-M., Oh W.-K., Tran T.-L., et al. Aglycone of Rh4 inhibits melanin synthesis in B16 melanoma cells: possible involvement of the protein kinase A pathway. Bioscience, Biotechnology, and Biochemistry. 2013;77(1):119–125. doi: 10.1271/bbb.120602. [DOI] [PubMed] [Google Scholar]
- 192.Chung E., Lee K.-Y., Lee Y.-J., Lee Y.-H., Lee S.-K. Ginsenoside-Rg1 down-regulates glucocorticoid receptor and displays synergistic effects with cAMP. Steroids. 1998;63(7-8):421–424. doi: 10.1016/S0039-128X(98)00043-9. [DOI] [PubMed] [Google Scholar]
- 193.Yuan H.-D., Quan H.-Y., Zhang Y., Kim S.-H., Chung S.-H. 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway. Molecular Medicine Reports. 2010;3(5):825–831. doi: 10.3892/mmr.2010.328. [DOI] [PubMed] [Google Scholar]
- 194.Dolcet X., Llobet D., Pallares J., Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Archiv. 2005;446(5):475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 195.Lee J.-Y., Shin J.-W., Chun K.-S., et al. Antitumor promotional effects of a novel intestinal bacterial metabolite (IH-901) derived from the protopanaxadiol-type ginsenosides in mouse skin. Carcinogenesis. 2005;26(2):359–367. doi: 10.1093/carcin/bgh313. [DOI] [PubMed] [Google Scholar]
- 196.Yun T.-K., Kim S.-H., Lee Y.-S. Trial of a new medium-term model using benzo(a)pyrene induced lung tumor in newborn mice. Anticancer Research. 1995;15(3):839–845. [PubMed] [Google Scholar]
- 197.Hasegawa H., Benno Y. Anticarcinogenesis in mice by ginseng-hydrolyzing colonic bacteria. Microbial Ecology in Health and Disease. 2000;12(2):85–91. doi: 10.1080/089106000435473. [DOI] [Google Scholar]
- 198.Lee L.-S., Stephenson K.-K., Fahey J.-W., et al. Induction of chemoprotective phase 2 enzymes by ginseng and its components. Planta Medica. 2009;75:1129–1133. doi: 10.1055/s-0029-1185508. [DOI] [PubMed] [Google Scholar]
- 199.Choi C.-H., Kang G., Min Y.-D. Reversal of P-glycoprotein-mediated multidrug resistance by protopanaxatriol ginsenosides from korean red ginseng. Planta Medica. 2003;69(3):235–240. doi: 10.1055/s-2003-38483. [DOI] [PubMed] [Google Scholar]
- 200.Kitagawa S., Takahashi T., Nabekura T., Tachikawa E., Hasegawa H. Inhibitory effects of ginsenosides and their hydrolyzed metabolites on daunorubicin transport in KB-C2 cells. Biological & Pharmaceutical Bulletin. 2007;30(10):1979–1981. doi: 10.1248/bpb.30.1979. [DOI] [PubMed] [Google Scholar]