Abstract
Mipomersen is a 20mer antisense oligonucleotide (ASO) that inhibits apolipoprotein B (apoB) synthesis; its low-density lipoprotein (LDL)–lowering effects should therefore result from reduced secretion of very-low-density lipoprotein (VLDL). We enrolled 17 healthy volunteers who received placebo injections weekly for 3 weeks followed by mipomersen weekly for 7 to 9 weeks. Stable isotopes were used after each treatment to determine fractional catabolic rates and production rates of apoB in VLDL, IDL (intermediate-density lipoprotein), and LDL, and of triglycerides in VLDL. Mipomersen significantly reduced apoB in VLDL, IDL, and LDL, which was associated with increases in fractional catabolic rates of VLDL and LDL apoB and reductions in production rates of IDL and LDL apoB. Unexpectedly, the production rates of VLDL apoB and VLDL triglycerides were unaffected. Small interfering RNA–mediated knockdown of apoB expression in human liver cells demonstrated preservation of apoB secretion across a range of apoB synthesis. Titrated ASO knockdown of apoB mRNA in chow-fed mice preserved both apoB and triglyceride secretion. In contrast, titrated ASO knockdown of apoB mRNA in high-fat–fed mice resulted in stepwise reductions in both apoB and triglyceride secretion. Mipomersen lowered all apoB lipoproteins without reducing the production rate of either VLDL apoB or triglyceride. Our human data are consistent with longstanding models of posttranscriptional and posttranslational regulation of apoB secretion and are supported by in vitro and in vivo experiments. Targeting apoB synthesis may lower levels of apoB lipoproteins without necessarily reducing VLDL secretion, thereby lowering the risk of steatosis associated with this therapeutic strategy.
INTRODUCTION
Dyslipidemia, a major risk factor for cardiovascular disease (CVD), is characterized by elevated levels of apolipoprotein B100 (apoB) lipoproteins, including very-low-density lipoproteins (VLDL), carrying both triglycerides (TGs) and cholesterol, and low-density lipoproteins (LDL) carrying cholesterol (1). Although there is some heterogeneity in published results, increased secretion of apolipoprotein B (apoB) lipoproteins, particularly VLDL, is the characteristic abnormality observed in people with dyslipidemia (2, 3). On the basis of numerous clinical trials, however, lowering LDL cholesterol (LDL-C) remains the first-line therapy for reducing risk of CVD in such individuals (4). HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase inhibitors, better known as statins, are the most potent drugs available for reducing levels of apoB lipoproteins, mainly LDL, but also, to a lesser degree, VLDL. Although some studies have shown that statins can reduce production rates (PRs) of VLDL and LDL apoB, the central actions of statins result in an increase in the number of LDL receptors (LDLR) at the plasma membranes of cells, particularly the liver (5). More than 10% of individuals receiving statins are, however, clinically intolerant to these agents or can only take low doses of statin because of drug-specific side effects (6). Thus, about 50% of the patients on maximally tolerated statin therapy do not reach the recommended LDL-C levels established by National Cholesterol Education Program Adult Treatment Panel III guidelines, especially patients with genetic lipid disorders such as familial hypercholesterolemia (7). Interest remains high, therefore, in the development of other therapeutic approaches to reduce circulating levels of apoB lipoproteins.
Two such agents—one a small-molecule inhibitor of microsomal triglyceride transfer protein (MTP) (8) and the other a second-generation antisense oligonucleotide (ASO) to apoB (9)—were recently approved by the U.S. Food and Drug Administration (FDA) for patients with homozygous familial hypercholesterolemia. Despite the ability of both drugs to reduce apoB lipoproteins, there are concerns about the occurrence of hepatic steatosis. Preclinical studies in rodents with either an ASO against MTP or small-molecule MTP inhibitors resulted in significant increases in liver TG levels (10, 11). This adverse effect was confirmed in studies of homozygous familial hypercholesterolemia patients with the recently approved MTP inhibitor, lomitapide (JUXTAPID, Aegerion) (8, 12). In preclinical studies in mice treated with ASO to apoB, there was no hepatic steatosis (10, 13), although increased liver TG has been observed in clinical trials of patients receiving mipomersen (KYNAMRO, Sanofi-Genzyme)—a fully phosphorothioate 20mer oligonucleotide with 5 2′-methoxyethyl residues at the 5′ and 3′ poles and a 10 deoxynucleotide center—for as long as 26 weeks for the treatment of familial hypercholesterolemia (14, 15). A combined analysis of three randomized trials with mipomersen treatment of patients with familial hypercholesterolemia indicated stabilization of steatosis during long-term treatment of more than 2 years. Reversal to baseline levels of hepatic fat was demonstrated in a subset of about 25% of participants who had magnetic resonance imaging performed 24 weeks after cessation of treatment (16).
The unique mechanisms of lomitapide or mipomersen to inhibit apoB production could explain the marked differences in the accumulation of hepatic TG that have been observed in mice. When lomitapide inhibits MTP, the absence of transfer of TG, as well as phospholipids and cholesteryl esters, from the endoplasmic reticulum (ER) membrane to nascent apoB leads to degradation of apoB and the absence of lipid droplets in the ER lumen (17). In contrast, when apoB synthesis is sub-maximally inhibited, MTP is still able to transfer ER membrane lipids to apoB that escaped inhibition and was synthesized. This would be the case with the 200 mg/week dose of mipomersen that has been approved for patients with homozygous familial hypercholesterolemia as an adjunct to lipid-lowering medications and diet (18, 19). With the current dose of mipomersen used clinically, there could be increased “loading” of TG onto each apoB that is synthesized, resulting in the assembly and secretion of larger VLDL carrying more TG per particle.
Evidence supporting the ability of the liver to vary the amount of TG incorporated into nascent VLDL derives from studies of mice with increased de novo lipogenesis (20) and humans on high-carbohydrate diets (21) or with hepatic steatosis (22). This mechanism, which could compensate for reduced synthesis of apoB, would be unlikely to occur with inhibition of MTP, which is absolutely essential for the transfer of TG onto nascent apoB. An alternative or additional compensatory response to mipomersen-mediated decreases in the synthesis of apoB is suggested by many studies in hepatocytes demonstrating that only a portion of newly synthesized apoB is secreted, with the availability of hepatic lipids, particularly TG, being the major determinant of whether nascent apoB is secreted or degraded (17). In this model, submaximal inhibition of apoB synthesis might be compensated for by increasing the proportion of newly synthesized apoB that is secreted, thereby maintaining hepatic lipid homeostasis.
Here, we examined the effects of mipomersen treatment on fractional catabolic rates (FCRs) and PRs of apoB-containing lipoproteins and VLDL TG, as well as on hepatic de novo lipogenesis, in healthy volunteers. We also conducted studies of apoB and TG secretion during inhibition of apoB synthesis in a human hepatoma line and in mice. Our hypothesis was that mipomersen would decrease VLDL apoB PR, whereas VLDL TG PR would be unchanged, indicating the assembly and secretion of larger, TG-enriched VLDL particles. If our hypothesis is correct, mipomersen therapy could lower the levels of apoB-containing lipoproteins without necessarily causing significant hepatic steatosis.
RESULTS
Effects of mipomersen on lipid and apoB levels
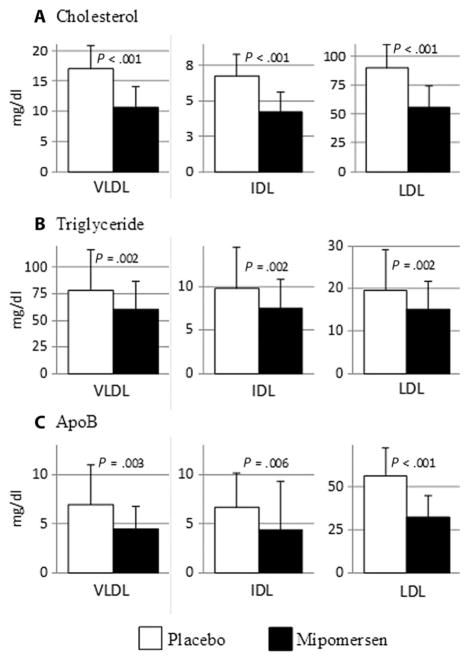
Thirty-one subjects were screened, 20 enrolled, and 8 healthy men and 9 healthy women completed the study (table S1). Baseline characteristics are in Table 1. Participants were not taking medications known to affect lipid metabolism and had normal plasma glucose levels and normal hepatic and renal function. They received three weekly doses of placebo followed by seven weekly doses of mipomersen treatment (fig. S1). Mipomersen treatment resulted in significant reductions in plasma levels of total cholesterol, TG, LDL-C, and apoB, but not high-density lipoprotein cholesterol (HDL-C) (Table 2). The cholesterol and TG concentrations in VLDL, IDL, and LDL were decreased significantly by mipomersen treatment (Fig. 1, A and B), and there were marked reductions in the levels of VLDL apoB (−29%), IDL apoB (−25%), and LDL apoB (−42%) (Fig. 1C). We observed reductions in VLDL, IDL, and LDL particle numbers as determined by ion mobility (23) (table S2).
Table 1. Subject baseline characteristics.
Twenty individuals were enrolled and 17 completed the study (table S1).
| Mean age, years (±SD) | 43.5 (14.2) |
|---|---|
| Gender, n (%) | |
| Male | 8 (47.1) |
| Female | 9 (52.9) |
| Race (%) | |
| White | 29.4 |
| Black | 47.1 |
| Asian | 5.9 |
| Unknown/mixed race | 17.6 |
| Mean BMI (±SD) | 27.6 (3.2) |
Table 2. Plasma lipid levels after 3 weeks of placebo and 7 weeks of mipomersen treatment.
Data are means (±SD) of one sample obtained from each subject (n = 17) just before the kinetic study in each period. Percent change is based on the difference between levels during mipomersen treatment and during placebo treatment for each subject. The significance of percent change for each endpoint was examined by paired t test after determining that the data were normally distributed using the Kolmogorov-Smirnov normality test.
| Parameter | Placebo (mg/dl) | Mipomersen (mg/dl) | Change (%) | P value |
|---|---|---|---|---|
| Totalcholesterol | 187.2 (25.7) | 132.4 (29.7) | −28.6 (16.1) | <0.001 |
| TG | 86.9 (49.3) | 57.1 (30.4) | −28.0 (28.0) | <0.001 |
| LDL-C | 114.7 (22.3) | 61.9 (24) | −45.5 (18.9) | <0.001 |
| ApoB | 69.6 (18.9) | 40.8 (14.1) | −40.3 (17.5) | <0.001 |
| HDL-C | 55.2 (14.3) | 59.1 (17.9) | 6.8 (19.3) | 0.17 |
Fig. 1. Effects of mipomersen treatment on human lipoprotein lipids and apoB levels.
Blood samples were obtained from subjects during their kinetic studies performed after 3 weeks of treatment with placebo and after 7 weeks of treatment with mipomersen. Plasma was separated from those samples, and VLDL, IDL, and LDL were isolated by sequential ultracentrifugation. (A and B) Cholesterol and TG were measured by enzymatic methods. (C) ApoB was determined by commercial enzyme-linked immunosorbent assay. Data are means ± SD (n = 5 samples obtained from each of the 17 subjects during their two kinetic studies). P values determined by paired t tests after determining that the data were normally distributed using the Kolmogorov-Smirnov normality test.
Effects of mipomersen on apoB metabolism in VLDL, IDL, and LDL
The reduction in VLDL apoB concentration during mipomersen treatment compared to placebo (Fig. 1C) was due to a 23% increase in the median FCR of VLDL apoB (Table 3). There was no significant effect of mipomersen treatment on VLDL apoB PR. The percent of VLDL apoB converted to IDL declined from 70 to 58%. This change, which reflects direct removal of VLDL apoB from plasma during mipomersen treatment, was associated with a significant 33% reduction in IDL apoB PR (Table 3) associated with the significant reduction in IDL apoB level (Fig. 1C) on mipomersen.
Table 3. ApoB kinetics.
The effects of mipomersen on apoB metabolism were determined from 17 kinetic studies at the end of the placebo and mipomersen treatment periods. Absolute values for the placebo and mipomersen periods are shown. If the data were found to be normally distributed by the Kolmogorov-Smirnov test, they are presented as means (±SD), and statistical analyses were performed on percent change for each endpoint on mipomersen from placebo using paired t tests. If the data were not normally distributed, the median and interquartile values (in parentheses) are shown for percent change, and the Wilcoxon rank-sum test was performed to test for significance.
| Parameter | Placebo | Mipomersen | Change (%) | P value |
|---|---|---|---|---|
| VLDL apoB FCR (pools/day) | 7.6 (3.6) | 9.9 (4.6) | 23.0 (−7.4, 55.6) | 0.023 |
| VLDL apoB PR (mg kg−1 day−1) | 21.7 (11.3) | 19.0 (10.9) | −17.6 (−32.7, −0.9) | 0.15 |
| VLDL to IDL (% conversion)* | 70.2 (24.2) | 58.2 (24.5) | −12.1 | 0.09 |
| IDL apoB FCR (pools/day) | 5.5 (3.5) | 5.8 (3.6) | 11.1 | 0.30 |
| IDL apoB PR (mg kg−1 day−1) | 15.0 (8.1) | 10.2 (6.9) | −32.7 (−53.5, −5.7) | 0.05 |
| IDL to LDL (% conversion)* | 76.2 (23) | 74.4 (26) | −1.7 | 0.84 |
| LDL apoB FCR (pools/day) | 0.52 (0.14) | 0.65 (0.19) | 29.7 | <0.001 |
| LDL apoB PR (mg kg−1 day−1) | 12.7 (4.6) | 8.8 (3.1) | −26.5 | 0.001 |
| Direct LDL PR (mg kg−1 day−1)† | 2.6 (3.5) | 2.2 (1.5) | 0.0 (−0.8, 1.5) | 0.89 |
| LDL apoB PR from direct LDL secretion (%)* | 21.3 (22) | 26.8 (18) | 5.5 | 0.38 |
| Total apoB PR (sum VLDL PR and direct LDL PR) (mg kg−1 day−1) | 24.3 (11.5) | 21.3 (10.7) | −15.1 (−34.5, −7.4) | 0.26 |
| VLDL TG FCR (pools/hour) | 10.5 (7.9) | 13.2 (8.4) | 46.3 | 0.03 |
| VLDL TG PR (mg kg−1 hour−1) | 13.1 (6.4) | 13.3 (7.0) | 10.0 | 0.40 |
| VLDL TG PR/VLDL apoB PR ratio | 22.9 (27.0) | 26.2 (37.0) | 16.5 (−7.8, 93.2) | 0.05 |
| Plasma VLDL TG/VLDL apoB ratio | 14.5 (13.7) | 15.3 (9.0) | 24.6 | 0.03 |
For the percent conversions (VLDL to IDL; IDL to LDL), absolute differences between placebo and mipomersen results were used to test for significance.
Some direct LDL secretion rates were zero, so no percent change was calculated for this parameter; instead, values are absolute differences.
The FCR of IDL apoB did not change, and there was no effect of mipomersen on the conversion of IDL apoB to LDL (76 and 74%, respectively) (Table 3). However, the reduced IDL apoB PR, together with a modest fall in the secretion of LDL-like particles directly from the liver, resulted in a significant fall in LDL apoB concentration (Fig. 1C) owing to a 27% reduction in mean LDL apoB PR on mipomersen (Table 3). There was no significant change in total apoB PR (sum of VLDL apoB PR and direct LDL apoB PR) on mipomersen (Table 3).
The marked fall in LDL apoB levels during mipomersen therapy (Fig. 1C) was due not only to decreased PR but unexpectedly to a 30% increase in the FCR of LDL apoB (Table 3). Because circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) is an important regulator of hepatic LDLR (24), we quantified this protein in plasma. There was no significant effect of mipomersen on circulating concentrations of PCSK9 (261.7 ± 63.6 ng/ml on placebo versus 241.2 ± 58.2 ng/ml on mipomersen) (P = 0.57; paired t test). Alterations in LDL particle size, particularly leading to a greater proportion of medium-size particles, could increase affinity for the LDL receptor (25). However, mipomersen treatment was associated with a shift in the distribution of LDL toward small particles (table S3). Conversely, reductions in the numbers of all larger-size LDL subfractions (table S4) suggested increased numbers of LDL receptors during administration of mipomersen, with preferential removal of larger particles. Changes in hepatic cholesterol balances could alter LDL receptor levels: mipomersen had no effects on plasma levels of lathosterol, a marker of cholesterol synthesis, or either campesterol or β-sitosterol, both markers of cholesterol absorption (table S5).
Changes in FCRs of VLDL and LDL could have been affected by mipomersen-mediated changes in apoC-III or apoE concentrations. There were no changes in plasma apoE levels from placebo (4.9 ± 1.5 mg/dl) to mipomersen treatment (4.5 ± 1.3 mg/dl) (P = 0.2; paired t test). In contrast, there was a fall in plasma apoC-III of 20% from placebo (9.3 ± 2.7) to mipomersen (7.3 ± 3.2) (P = 0.02; paired t test).
Effects of mipomersen on VLDL TG metabolism
The concentration of VLDL TG fell by 20% during mipomersen treatment (Fig. 1B). This was accompanied by a 46% increase in the mean FCR of VLDL TG during mipomersen treatment, with no change seen in the PR of VLDL TG (Table 3). The percentage of VLDL TG produced from hepatic de novo lipogenesis did not change (11.2 ± 6.3% and 10.5 ± 5.6% during placebo and mipomersen, respectively) (P = 0.50; paired t test). To gain insight into the increase in VLDL TG FCR, we measured post-heparin hepatic lipase (HL) and lipoprotein lipase (LpL) activities in plasma. Neither of these enzymes was affected by mipomersen treatment (table S6). Fasting plasma levels of fatty acid (FA) and β-hydroxybutyrate also did not change between periods (table S6). The individual FCRs and PRs for VLDL, IDL, and LDL apoB and VLDL TG, for the 17 subjects, are in table S7.
We hypothesized that mipomersen treatment might be associated with a shift in the size distribution of secreted VLDL, with a larger TG-rich VLDL entering the circulation. The ratio of PRs of VLDL TG to VLDL apoB, which should reflect the size of newly secreted VLDL particles, increased by a median value of 17%, indicating assembly and secretion of larger VLDL (Table 3). These data were supported by a significant increase of 25% in the ratio of TG/apoB in circulating VLDL during mipomersen treatment (Table 3).
Effects of siRNA knockdown of APOB in HepG2 cells on apoB secretion
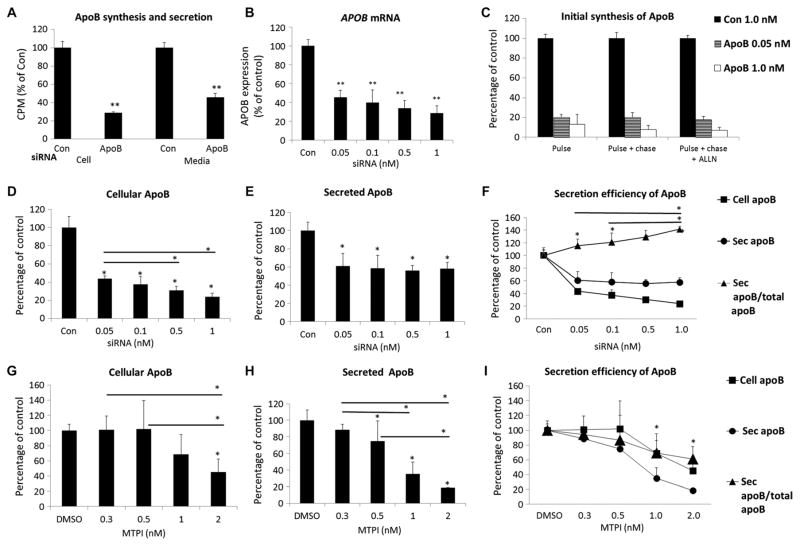
To better understand the lack of effect of mipomersen on the secretion of VLDL apoB in vivo, we examined the effects of the knockdown of APOB on the secretion of apoB in the human hepatoma cell line HepG2 in vitro. For these studies, we used a small interfering RNA (siRNA) that has been extensively validated against human APOB in vitro in HepG2 cells (26) and in vivo in nonhuman primates (27) to titrate the knockdown of APOB mRNA. We determined that maximal siRNA-mediated knockdown of APOB mRNA was attained at a concentration of 10 nM, with about 80% inhibition of apoB synthesis (cell) and 60% inhibition of apoB secretion (media) during a 2-hour steady-state labeling study compared to a scrambled siRNA control (Fig. 2A). Titration of the siRNA between 0.05 and 1.0 nM resulted in dose-related reductions of APOB mRNA (Fig. 2B) and synthesis of the protein in short-pulse and pulse-chase experiments with or without inhibition of the proteasome (Fig. 2C). There was an obvious relationship between increasing siRNA-mediated knockdown of APOB expression and cellular accumulation of newly synthesized apoB in 2-hour labeling studies (Fig. 2D). However, secretion of apoB remained constant across the doses of siRNA (Fig. 2E).
Fig. 2. Effects of siRNA-mediated knockdown of APOB on apoB secretion in human HepG2 cells.
(A) APOB siRNA (10 nM) inhibits the synthesis and secretion of apoB in HepG2 cells. HepG2 cells were transfected with 10 nM APOB or 10 nM irrelevant control (Con) siRNA for 2 days, radiolabeled continuously with [35S]methionine/cysteine for 2 hours, and radioactivity [counts per minute (CPM)] in cellular or media apoB was determined. (B) APOB siRNA knocks down APOB mRNA in a dose-dependent manner. HepG2 cells were transfected at varying doses of APOB siRNA for 2 days. Total RNA was extracted and APOB mRNA levels were analyzed by quantitative polymerase chain reaction (qPCR). (C) APOB siRNA reduces initial synthesis rates of apoB in a dose-dependent manner. Cells were labeled with [35S]methionine/cysteine for 10 min and chased for 10 min with or without proteasome inhibitor ALLN pretreatment for 1 hour. (D and E) The effect of APOB siRNA doses on cellular and secreted apoB in HepG2 cells transfected as in (B), and apoB synthesis and secretion analyzed as in (A). (F) Efficiency of apoB secretion as a function of APOB siRNA knockdown. Efficiency was defined as the percentage of apoB secreted into the medium compared with the amount of total newly synthesized apoB (cellular and secreted apoB over 2 hours). (G and H) Cellular and secreted apoB in HepG2 cell culture after treatment with an MTP inhibitor (MTPI) for 1 hour (before steady-state labeling for 2 hours). (I) Efficiency of apoB secretion as a function of MTP inhibition. Data were plotted using the cell and secreted apoB data, as described in (F). All data are mean percentage of control [siRNA (1 nM) in (B) to (F); dimethyl sulfoxide (DMSO) in (G) to (I)] ± SD (n = 3). *P < 0.05; **P < 0.01 versus respective control, unless otherwise noted, one-way analysis of variance (ANOVA) followed by post hoc comparisons using the Fisher LSD (least significant difference) method.
When we depicted secretion of apoB as the percent of the total newly synthesized apoB in cells plus the media after 2 hours of radio-labeling, there was increased efficiency of secretion (Fig. 2F). In contrast, a small-molecule inhibitor of MTP caused parallel decreases in cellular (Fig. 2G) and secreted (Fig. 2H) apoB after 2 hours of radio-labeling. Thus, with MTP inhibition, there was no change in the efficiency of secretion of apoB with increasing inhibition of MTP (Fig. 2I). siRNA knockdown of APOB did not affect expression of LDL receptor (LDLR), inducible degrader of the LDLR (IDOL), or PCSK9 in HepG2 cells (fig. S2).
These results demonstrate that HepG2 cells, under culture conditions where there is very little intracellular TG, are able to accommodate submaximal reductions in apoB synthesis by increasing the proportion of newly synthesized apoB that is secreted.
Effect of ASO knockdown of ApoB in mice on secretion of apoB and TG
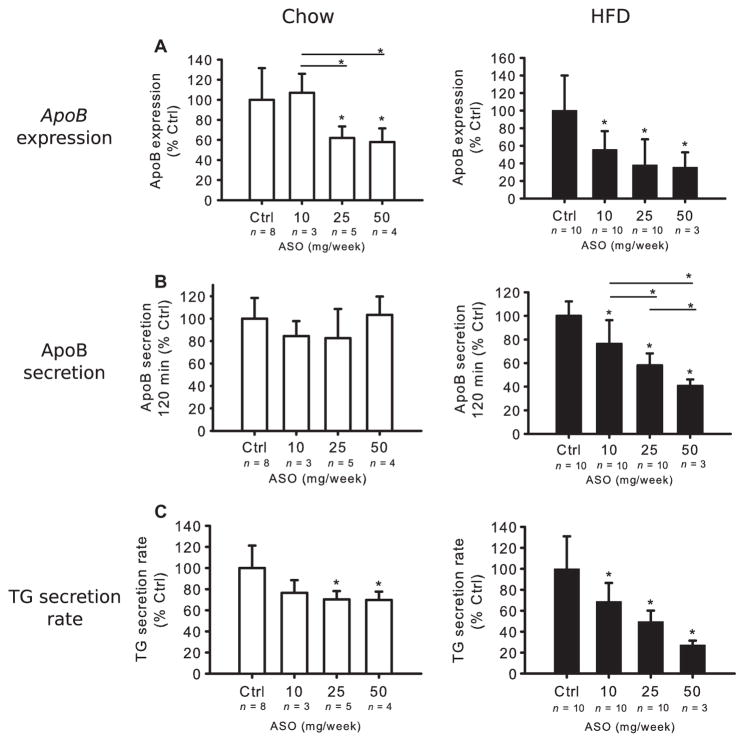
To further support the validity of our observation of a lack of effect of mipomersen on VLDL apoB secretion in normal humans, we performed similar experiments in vivo in Apobec-1 knockout mice, using a murine-specific ApoB ASO. Apobec-1 knockout mice were used because they synthesize and secrete only apoB from their livers, making them more like humans. Mice on either chow or a high-fat diet (HFD) for 6 weeks were treated with ApoB ASO in doses ranging from 10 to 50 mg/week for an additional 3 weeks, while continuing their diets, at which time apoB and TG secretion were measured. The doses were chosen from preliminary studies where 200 or 100 mg/week of ApoB ASO significantly reduced both TG and apoB secretion in chow-fed mice (fig. S3).
In the titration experiment, ApoB ASO reduced hepatic ApoB mRNA levels in both groups of mice in a dose-dependent manner, with the response more pronounced in the HFD-fed mice (Fig. 3A), similar to the results of Lee et al., who conducted experiments with the same ASO in mice on chow or HFD (10). Relevant to this experiment is the evidence that ApoB ASO does not significantly alter ApoB mRNA levels after 2 weeks of treatment (13) in the small intestine, and although it does lower ApoB mRNA after 6 to 8 weeks of treatment, APOB protein is not altered at those times (10, 13). The effects of ASO treatment on apoB and TG secretion demonstrated different patterns in chow-versus HFD-fed mice. Whereas apoB secretion was unaffected by apoB ASO at the doses administered in the chow-fed mice, it was inhibited in a stepwise, dose-dependent manner in the HFD mice (Fig. 3B). Furthermore, apoB ASO had modest, non–dose-related effects on TG secretion in the chow-fed mice, but caused greater reductions of TG secretion in the HFD mice with a clear trend for dose responsiveness (Fig. 3C). The hepatic TG concentration in chow-fed mice receiving control ASO was 159 ± 33 μg/mg protein and, as expected, much higher (625 ± 207 μg/mg protein) in the HFD group on control ASO (P < 0.001, by unpaired t test).
Fig. 3. Effects of ApoB ASO treatment on apoB and TG secretion in Apobec-1 knockout mice.
Chow-and HFD-fed mice were treated for 3 weeks with ApoB ASO. (A) ApoB mRNA expression was determined using qPCR of livers from both chow and HFD mice and normalized to liver β-actin mRNA. In each diet group, irrelevant, control (Ctrl) ASO-treated mRNA levels were set as 100%. (B) Mice were fasted 4 hours and injected with Tyloxapol and [35S]methionine intravenously. Plasma 35S-labeled apoB levels in CPM were determined in samples obtained at 120 min after the start of the study. ApoB radioactivity in mice receiving the irrelevant control ASO was set as 100%. (C) Blood samples were collected at 0, 30, 60, 90, and 120 min for measurement of TG levels. TG secretion rate was determined by the change in plasma TG concentration between 30 and 120 min after Tyloxapol injection and divided by 1.5. The secretion rate of the mice receiving irrelevant control ASO was set at 100%. Data in (A) to (C) are means ± SD (n noted on figure). *P < 0.05 versus control, unless otherwise noted, one-way ANOVA and Tukey’s post hoc test.
Hepatic steatosis in the HFD group was associated with greater absolute rates of secretion of newly synthesized apoB in the control ASO-treated mice compared to chow-fed control ASO-treated mice (fig. S4). ApoB ASO treatment did not affect expression of the genes for Ldlr, Idol, or Pcsk9 (fig. S5). Furthermore, ApoB ASO treatment did not alter the level of LDLR protein in the livers of mice on either diet (fig. S6).
These in vivo data are consistent with greater need to use a larger proportion of newly synthesized apoB for assembly with TG to secrete VLDL when there is steatosis. In this situation, therefore, any reduction of apoB synthesis will result in decreased apoB secretion. Indeed, an exploratory analysis of the relationship between baseline VLDL apoB production and the decrease in VLDL apoB on mipomersen in the human study indicated just such a relationship, although the correlation did not achieve significance (fig. S7).
DISCUSSION
Mipomersen is a second-generation ApoB ASO that inhibits the synthesis of apoB (13, 28). In a phase 1 study, treatment with 50 to 400 mg of mipomersen injected subcutaneously weekly produced dose-dependent reductions in plasma LDL-C and apoB concentrations (29). On the basis of those results and phase 2 studies (18, 19), a dose of 200 mg per week, by subcutaneous injection, was chosen for further development and was the dose approved by the FDA for treatment of patients with homozygous familial hypercholesterolemia (9). The phase 1 and 2 data indicated that the 300- and 400-mg weekly doses had greater LDL-C and apoB-lowering efficacy than the 200-mg weekly dose, which was the dose used here. Thus, it is reasonable to conclude that the 200-mg weekly dose results in submaximal reductions in apoB synthesis; this, we believe, is critical to the interpretation of our results.
As expected, mipomersen significantly reduced plasma levels of LDL-C and apoB. VLDL, IDL, and LDL apoB concentrations were also reduced, as were levels of VLDL TG. The reductions in LDL apoB levels resulted from decreased LDL PRs, but unexpectedly, the fall in LDL PR did not result from decreased VLDL apoB secretion into plasma, which was our primary hypothesis. Instead, decreased production of LDL resulted from increased direct clearance of VLDL apoB with less conversion to IDL, the immediate precursor of LDL. A modest reduction in the direct secretion of LDL into plasma during mipomersen treatment also contributed to the fall in LDL PR. Additionally, and also unexpectedly, an increase in FCR contributed to the reduced LDL apoB levels present during mipomersen treatment.
Why would treatment with a drug that reduces APOB mRNA, thereby reducing apoB synthesis, not lower VLDL apoB PR? In vitro studies in a variety of primary hepatocytes and hepatic cell lines have demonstrated repeatedly that apoB is typically synthesized constitutively and that its assembly into a VLDL and secretion involves the co-translational lipidation of the nascent polypeptide as it traverses the ER (17). The latter step plays a crucial role in determining how much VLDL is secreted; in the absence of adequate lipids or MTP activity, co-translational and co-translocational degradation of apoB results (17). Additional sites of intracellular degradation of apoB can also occur during or after the assembly of apoB with lipids (30), adding further complexity to the secretory process. The extensive post-transcriptional and posttranslational control of the assembly and secretion of VLDL results in a range of secretion from 20 to 70% of newly synthesized apoB from cultured hepatocytes. The relevance of these cell-based studies to human physiology is supported by the wide range of secretory rates of VLDL in normal and hyperlipidemic individuals, and the ability to alter VLDL PR in individuals with different diets and drugs (31, 32).
With these data in mind, we carried out a series of studies in HepG2 cells in which we reduced APOB mRNA in a stepwise fashion with siRNA (26, 27). Our findings of increased efficiency of apoB secretion across a range of inhibition of apoB synthesis strongly support our in vivo results. For example, if someone with normal liver TG levels makes 100 apoB molecules per minute and only needs to secrete 50 VLDL particles (with one apoB per particle) to maintain hepatic lipid homeostasis, inhibition of synthesis by 40%, to 60 molecules of apoB produced per minute, should allow the liver to maintain hepatic lipids at pretreatment levels by increasing the proportion of nascent apoB that is secreted. In contrast, if hepatic lipids are elevated and a greater percentage of newly synthesized apoB is constantly being secreted as the liver attempts to reduce hepatic TG, inhibiting synthesis of apoB would likely lead to decreased secretion of VLDL.
This is what we observed in HFD-fed mice compared to chow-fed mice. The HFD mice had steatosis and much higher rates of apoB secretion when treated with control ASO and had dose-dependent reductions in apoB secretion when treated with apoB ASO; this was in marked contrast to what was observed in chow-fed mice. Results of an exploratory analysis in our subjects provided support for our model of the role of apoB in maintaining hepatic lipid homeostasis. In our normolipidemic participants, higher VLDL apoB PRs on placebo were associated with greater reductions in VLDL apoB secretion on mipomersen. Further studies of the effects of mipomersen on apoB metabolism in people with hepatic steatosis and a dyslipidemia characterized by increased VLDL apoB and VLDL TG secretion will be required to definitively address this important question.
Our second hypothesis, that VLDL TG PR would not be affected by mipomersen owing to assembly and secretion of larger VLDL, was supported by our findings of increased ratios of TG to apoB in circulating VLDL and an increased ratio of VLDL TG PR to VLDL apoB PR. A rigorous examination of this hypothesis, which is supported by in vitro studies in cultured liver cells that were treated with FAs (33) and in vivo studies in mice and humans in which hepatic de novo lipogenesis was increased (20, 34), must await further studies of mipomersen treatment of hypertriglyceridemic subjects. Plasma and VLDL TG levels fell despite any change in VLDL TG PR. Our finding of increased FCRs for VLDL TG in the absence of increases in post-heparin LpL and HL activities points to previous studies showing increased efficiency of LpL-mediated lipolysis of larger and more TG-enriched particles (35). Additionally, we observed an increase in both the FCR of VLDL apoB and the direct removal of VLDL apoB without conversion to IDL or LDL. The increased FCR of VLDL apoB could simply have been the result of a more efficient LpL-mediated lipolysis of larger VLDL TG or a result of reduced levels of apoC-III we observed during mipomersen treatment. However, that would have resulted in a more rapid and efficient conversion of VLDL to IDL and LDL, rather than the decreased conversion we observed.
Both apoE and apoC-III affect hepatic removal of VLDL; apoE levels in plasma were not altered by mipomersen treatment, but the reductions in apoC-III levels we observed could have resulted in greater removal of VLDL or their remnants by the liver with less conversion to IDL. The latter would be more likely if the reduced apoC-III levels resulted from mipomersen-mediated decreased secretion of the protein by the liver rather than those lower concentrations being the result of lower levels of VLDL, IDL, and LDL, which all carry apoC-III. Further studies will be required to determine if decreased apoB synthesis affects apoC-III synthesis or secretion by the liver.
The reductions in LDL we observed resulted from both decreased PR due to less conversion of VLDL to IDL and LDL, and a significant and unexpected increase in LDL FCR. We were unable to discern why inhibition of hepatic apoB synthesis would lead to an increase in LDL FCR, which is typically associated with increased levels of hepatic LDL receptors. However, we did measure plasma PCSK9 concentrations as a surrogate, because lower PCSK9 levels would mean increased hepatic LDL receptors available to bind LDL (24). Mipomersen had no effect on plasma PCSK9 levels in humans. Reduced rates of endogenous cholesterol synthesis or intestinal absorption of cholesterol could also lead downstream to increased LDLR, but we saw no effects of mipomersen treatment on the three markers of the whole-body cholesterol metabolism: lathosterol, campesterol, or β-sistosterol. LDLR, PCSK9, and IDOL mRNA were unchanged in human HepG2 cells treated with maximally suppressive doses of siRNA. Furthermore, mipomersen did not affect mRNA levels of the Ldlr, Pcsk9, and Idol, or the level of LDLR protein in mice. An explanation that is not dependent on the number of LDL receptors in the liver would be greater affinity of LDL for the same number of receptors; we did see a change in the distribution of LDL size, in the direction of smaller LDL particles, but small LDLs have lower affinity for the LDLR. The reductions that occurred in the numbers of particles in all the large LDL subfractions are consistent, however, with increased LDLR-mediated clearance. Additionally, the cholesterol-to-apoB ratio in LDL increased; larger, cholesterol-enriched LDL is believed to have greater affinity for the LDLR. As noted above, we observed reductions in apoC-III, which can inhibit the uptake of LDL by its receptor. However, apoC-III is only carried on a small fraction of LDL, and the changes we observed in apoC-III are unlikely to explain the significant increase in LDL FCR with mipomersen treatment. Overall, we do not have a clear explanation for the increased LDL apoB FCR during mipomersen treatment.
We understand that the results of mipomersen treatment of humans with homozygous familial hypercholesterolemia suggest that the LDL receptor is not needed for efficacy of this agent (9). Studies of apoB metabolism in homozygous familial hypercholesterolemia with null mutations have demonstrated increased secretion of apoB lipoproteins (36, 37); in our model, mipomersen inhibition of apoB synthesis would decrease apoB secretion in those individuals. In summary, our study of the effects of mipomersen in healthy subjects, supported by our studies in HepG2 cells and mice, provides new and unique insights into mechanisms for the maintenance of hepatic lipid homeostasis when apoB synthesis is reduced. Our results indicate that inhibition of apoB synthesis should remain a target for therapies to reduce levels of all atherogenic lipoproteins.
MATERIALS AND METHODS
Study design
This was a single blind, two-period, linear, placebo-controlled, phase 1 study in healthy volunteers. The first primary hypothesis was that mipomersen, administered at a dose of 200 mg weekly, would significantly reduce the PR of VLDL apoB. The second hypothesis was that there would be an increase in the efficiency of VLDL TG fractional clearance from the circulation during mipomersen treatment. Exploratory hypotheses included that there would not be a change in VLDL TG production; that there would not be a change in direct removal of VLDL apoB from the circulation; that LDL apoB production would be reduced, but fractional removal would not be affected; that hepatic DNL would not be affected; and that FA oxidation in the liver, as estimated by measurement of circulating β-hydroxybutyrate, would not change during mipomersen treatment. The primary endpoint was the percent change in VLDL apoB PR from the end of placebo to the end of mipomersen treatment. The sample size calculation indicated that 16 subjects would provide 80% power to detect a 20% reduction in VLDL apoB PR, on the basis of a paired t test with a 5% type I error rate, assuming an SD of about 6 mg kg−1 day−1 as observed in previous research (38, 39) and an expected VLDL apoB PR of about 25 mg kg−1 day−1 at the end of the placebo period. Additional details of the study protocol are in the Supplementary Materials and fig. S1.
Human volunteers
All subjects signed informed consent. For screening, see the Supplementary Materials. Subjects were nonsmokers, were of ages 18 to 75 years, had a body mass index (BMI) of less than 38 kg/m2, and were not on any lipid-altering medications. All participants had blood glucose <115 mg/dl, TG <170 mg/dl, and LDL-C >100 mg/dl and <160 mg/dl if they had more than two cardiovascular risk factor. Subjects who met the eligibility criteria were instructed to follow an American Heart Association–recommended diet. After an additional week, subjects returned to the Irving Institute for Clinical and Translational Research (IICTR) for study randomization and fasting measurements of plasma lipids, a chemistry panel, hematology studies, and vital signs.
Mathematical modeling of the apoB and TG enrichment data
ApoB kinetics in VLDL, IDL, and LDL were estimated using the leucine and phenylalanine enrichment data from the constant infusion and the bolus injection, respectively (38, 39). The kinetics of VLDL TG were analyzed using the enrichment data generated by the injection of D5-glycerol. Mathematical modeling is described in the Supplementary Methods.
In vitro studies of APOB knockdown and inhibition of MTP activity in HepG2 cells
HepG2 cells were obtained from American Type Culture Collection and cultured in a growth medium of Dulbecco’s modified Eagle’s medium (high glucose, 5% antibiotics, and 10% fetal bovine serum). Transfection of HepG2 cells and metabolic labeling to measure the secretion and synthesis of apoB were performed with both steady-state and pulse-chase labeling protocols as described previously in our laboratory; details are provided in the Supplementary Materials (40, 41).
In vivo studies of ApoB knockdown in mice
All animal experiments were approved by the Columbia University Institutional Animal Care and Use Committee. Details regarding the mice are in the Supplementary Materials. After 6 weeks on HFD, Apobec-1 knockout mice were started on treatment with mouse control ASO (ISIS #141923) or a second-generation mouse apoB ASO (Ionis Pharmaceuticals #147764) for 3 weeks; these reagents were provided by Ionis Pharmaceuticals. Mice remaining on chow diet were started on ASO treatment at the same age as the mice on HFD. The ApoB ASO was administered at different doses (10, 25, or 50 mg/week) mixed with control ASO to give equal amounts of ASO in each injection. At the end of 3 weeks of ASO treatment, mice were fasted for 4 hours, and TG and apoB secretion was measured by intravenously injecting Tyloxapol (500 mg/kg) (T8761-50G, Sigma-Aldrich) and 200 μCi per mouse [35S]methionine (1175 Ci/mmol; PerkinElmer Life Sciences) as described previously (42).
Statistical analysis
The Wilcoxon signed-rank test and the paired t test were used to test the hypothesis that there was no change between the first and second turnover studies. The Kolmogorov-Smirnov statistic tested the hypothesis that the percent/nominal change was normally distributed. For the in vitro cell and in vivo mouse studies, all data are presented as means ± SD. Differences in the means among multiple groups were compared for significance using a one-way ANOVA followed by individual comparisons when appropriate. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We acknowledge laboratory assistance from M. Pavlyha and K. Poulos, The Research Pharmacy at Columbia University Medical Center, and the inpatient and outpatient nursing staff and Bionutrition unit of the IICTR at Columbia University Medical Center. We are grateful to N.R. Matthan and A.H. Lichtenstein (Tufts University) for providing the plasma sterol data. M. Graham (Ionis Pharmaceuticals) provided the apoB and MTP ASOs. N. Davidson (Washington University School of Medicine) provided the Apobec-1 knockout mice. I. Goldberg (New York University School of Medicine) provided advice for the measurement of HL and LpL. R. M. Krauss (Children’s Hospital Oakland Research Institute) provided assistance with the analysis of lipoprotein size/particle number and data interpretation.
Funding: This study was funded by Sanofi-Genzyme. Additional support for this work was obtained from the National Center for Advancing Translational Sciences, NIH (through UL1 TR000040), and from the National Heart, Lung, and Blood Institute (through R01 HL55638). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Author contributions: G.R.-S. designed and conducted all the human studies and participated in all aspects of writing the manuscript; B.M. designed and conducted all the cell experiments and wrote part of the manuscript; A.H.-O. designed and conducted all the mouse experiments and wrote part of the manuscript; M.D.-D. conducted the human studies; J.J. conducted the human studies; J.O. designed and conducted some of the human experiments; T.T. designed and conducted some of the human studies and contributed to the manuscript; C.N. conducted the human studies; N.F. conducted the human experiments; D.S.D. conducted the human studies; W.K. designed and conducted some of the human studies and wrote parts of the manuscript; S.H. designed and conducted the human studies; R.R. designed and conducted the human studies and wrote part of the manuscript; R.S.M. designed the human studies and wrote part of the manuscript; H.N.G. designed the human, cell, and mouse studies, conducted the human studies, and participated in all aspects of writing the manuscript.
Data and materials availability: Data are stored at Columbia and Sanofi-Genzyme. Data requests should be addressed to C. Sensinger of Genzyme (Charotte.Sensinger@genzyme.com) or the study authors. Ionis Pharmaceuticals holds the patent on human apoB ASO (mipomersen), but mipomersen is now FDA-approved for the treatment of homozygous FH. Mouse apoB ASO was provided by Ionis Pharmaceuticals via a material transfer agreement (MTA) with Columbia University. The human siRNA for APOB was provided by Merck Pharmaceutical via an MTA with Columbia University. As of December 2, 2015, Isis Pharmaceuticals Inc. has changed its name to Ionis Pharmaceuticals Inc.
www.sciencetranslationalmedicine.org/cgi/content/full/8/323/323ra12/DC1
Methods
Fig. S1. Study flow diagram.
Fig. S2. No changes in LDLR, IDOL, or PCSK9 mRNA levels in HepG2 cells after knockdown of APOB.
Fig. S3. ApoB ASO inhibits hepatic secretion of TG and apoB from mice on either chow or HFD diet.
Fig. S4. ApoB secretion is increased in Apobec-1 knockout mice on HFD.
Fig. S5. ApoB siRNA treatment does not affect expression of Ldlr, Idol, or Pcsk9 in mouse livers.
Fig. S6. Hepatic LDL receptor was not affected by ApoB ASO.
Fig. S7. Relationship between mipomersen-induced reductions in VLDL apoB PR and baseline VLDL PR.
Table S1. Participant information.
Table S2. Effect of mipomersen on apoB lipoprotein particle number by ion mobility analysis.
Table S3. Effect of mipomersen on apoB lipoprotein size distribution by ion mobility analysis.
Table S4. Effect of mipomersen on LDL size subfraction particle numbers by ion mobility analysis.
Table S5. Markers of cholesterol synthesis and absorption.
Table S6. Hepatic lipase, lipoprotein lipase, FFA, and β-hydroxybutyrate levels.
Table S7. Individual participant apoB kinetics of VLDL, IDL, LDL, and VLDL TG FCR.
Competing interests: R.S.M. was an employee of Sanofi-Genzyme during the active study phase. G.R.-S. reports grants and nonfinancial support from Merck during the conduct of the study. D.S.D. reports grants from Merck during the conduct of the study. H.N.G. reports grants from Merck during the conduct of the study and personal consulting fees from Merck outside the submitted work. H.N.G. was also on the Sanofi advisory board during the time that this work was done. All other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Nordestgaard BG, Ray KK, Reiner Z, Taskinen M-R, Tokgözoglu L, Tybjærg-Hansen A, Watts GF European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur Heart J. 2011;32:1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low–density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Artheroscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 3.Chan DC, Barrett PHR, Watts GF. Lipoprotein transport in the metabolic syndrome: Pathophysiological and interventional studies employing stable isotopy and modelling methods. Clin Sci. 2004;107:233–249. doi: 10.1042/CS20040109. [DOI] [PubMed] [Google Scholar]
- 4.Cholesterol Treatment Trialists’ (CTT) Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 6.Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, März W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: Impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gitt AK, Drexel H, Feely J, Ferrières J, Gonzalez-Juanatey JR, Thomsen KK, Leiter LA, Lundman P, da Silva PM, Pedersen T, Wood D, Jünger C, Dellea PS, Sazonov V, Chazelle F, Kastelein JJP DYSIS Investigators. Persistent lipid abnormalities in statin-treated patients and predictors of LDL-cholesterol goal achievement in clinical practice in Europe and Canada. Eur J Prev Cardiol. 2012;19:221–230. doi: 10.1177/1741826711400545. [DOI] [PubMed] [Google Scholar]
- 8.Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Du Plessis AME, Propert KJ, Sasiela WJ, Bloedon LT, Rader DJ Phase 3 HoFH Lomitapide Study investigators. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: A single-arm, open-label, phase 3 study. Lancet. 2013;381:40–46. doi: 10.1016/S0140-6736(12)61731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: A randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 10.Lee RG, Fu W, Graham MJ, Mullick AE, Sipe D, Gattis D, Bell TA, Booten S, Crooke RM. Comparison of the pharmacological profiles of murine antisense oligonucleotides targeting apolipoprotein B and microsomal triglyceride transfer protein. J Lipid Res. 2013;54:602–614. doi: 10.1194/jlr.M029215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wierzbicki AS, Hardman T, Prince WT. Future challenges for microsomal transport protein inhibitors. Curr Vasc Pharmacol. 2009;7:277–286. doi: 10.2174/157016109788340703. [DOI] [PubMed] [Google Scholar]
- 12.Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 13.Crooke RM, Graham MJ, Lemonidis KM, Whipple CP, Koo S, Perera RJ. An apolipo-protein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. J Lipid Res. 2005;46:872–884. doi: 10.1194/jlr.M400492-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Visser ME, Akdim F, Tribble DL, Nederveen AJ, Kwoh TJ, Kastelein JJP, Trip MD, Stroes ESG. Effect of apolipoprotein-B synthesis inhibition on liver triglyceride content in patients with familial hypercholesterolemia. J Lipid Res. 2010;51:1057–1062. doi: 10.1194/jlr.M002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein EA, Dufour R, Gagne C, Gaudet D, East C, Donovan JM, Chin W, Tribble DL, McGowan M. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: Results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation. 2012;126:2283–2292. doi: 10.1161/CIRCULATIONAHA.112.104125. [DOI] [PubMed] [Google Scholar]
- 16.Santos RD, Duell PB, East C, Guyton JR, Moriarty PM, Chin W, Mittleman RS. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension. Eur Heart J. 2015;36:566–575. doi: 10.1093/eurheartj/eht549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J Lipid Res. 2009;50:S162–S166. doi: 10.1194/jlr.R800090-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akdim F, Stroes ESG, Sijbrands EJG, Tribble DL, Trip MD, Jukema JW, Flaim JD, Su J, Yu R, Baker BF, Wedel MK, Kastelein JJP. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J Am Coll Cardiol. 2010;55:1611–1618. doi: 10.1016/j.jacc.2009.11.069. [DOI] [PubMed] [Google Scholar]
- 19.Akdim F, Tribble DL, Flaim JD, Yu R, Su J, Geary RS, Baker BF, Fuhr R, Wedel MK, Kastelein JJP. Efficacy of apolipoprotein B synthesis inhibition in subjects with mild-to-moderate hyperlipidaemia. Eur Heart J. 2011;32:2650–2659. doi: 10.1093/eurheartj/ehr148. [DOI] [PubMed] [Google Scholar]
- 20.Horton JD, Shimano H, Hamilton RL, Brown MS, Goldstein JL. Disruption of LDL receptor gene in transgenic SREBP-1a mice unmasks hyperlipidemia resulting from production of lipid-rich VLDL. J Clin Invest. 1999;103:1067–1076. doi: 10.1172/JCI6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melish J, Le NA, Ginsberg H, Steinberg D, Brown WV. Dissociation of apoprotein B and triglyceride production in very-low-density lipoproteins. Am J Physiol. 1980;239:E354–E362. doi: 10.1152/ajpendo.1980.239.5.E354. [DOI] [PubMed] [Google Scholar]
- 22.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Häkkinen A, Olofsson SO, Yki-Järvinen H, Borén J. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 23.Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, Reitz RE, Krauss RM. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54:1307–1316. doi: 10.1373/clinchem.2007.100586. [DOI] [PubMed] [Google Scholar]
- 24.Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 25.Campos H, Arnold KS, Balestra ME, Innerarity TL, Krauss RM. Differences in receptor binding of LDL subfractions. Arterioscler Thromb Vasc Biol. 1996;16:794–801. doi: 10.1161/01.atv.16.6.794. [DOI] [PubMed] [Google Scholar]
- 26.Tadin-Strapps M, Peterson LB, Cumiskey AM, Rosa RL, Mendoza VH, Castro-Perez J, Puig O, Zhang L, Strapps WR, Yendluri S, Andrews L, Pickering V, Rice J, Luo L, Chen Z, Tep S, Ason B, Somers EP, Sachs AB, Bartz SR, Tian J, Chin J, Hubbard BK, Wong KK, Mitnaul LJ. siRNA-induced liver ApoB knockdown lowers serum LDL-cholesterol in a mouse model with human-like serum lipids. J Lipid Res. 2011;52:1084–1097. doi: 10.1194/jlr.M012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamza MS, Kumar C, Chia SM, Anandalakshmi V, Boo N, Strapps W, Robinson M, Caguyong M, Bartz S, Tadin-Strapps M, van Gool A, Shih SJ. Alterations in the hepatic transcriptional landscape after RNAi mediated ApoB silencing in cynomolgus monkeys. Atherosclerosis. 2015;242:383–395. doi: 10.1016/j.atherosclerosis.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Lee RG, Crosby J, Baker BF, Graham MJ, Crooke RM. Antisense technology: An emerging platform for cardiovascular disease therapeutics. J Cardiovasc Transl Res. 2013;6:969–980. doi: 10.1007/s12265-013-9495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kastelein JJP, Wedel MK, Baker BF, Su J, Bradley JD, Yu RZ, Chuang E, Graham MJ, Crooke RM. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114:1729–1735. doi: 10.1161/CIRCULATIONAHA.105.606442. [DOI] [PubMed] [Google Scholar]
- 30.Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J Biol Chem. 2001;276:27855–27863. doi: 10.1074/jbc.M008885200. [DOI] [PubMed] [Google Scholar]
- 31.Ginsberg HN, Le NA, Gibson JC. Regulation of the production and catabolism of plasma low density lipoproteins in hypertriglyceridemic subjects. Effect of weight loss. J Clin Invest. 1985;75:614–623. doi: 10.1172/JCI111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arad Y, Ramakrishnan R, Ginsberg HN. Lovastatin therapy reduces low density lipoprotein apoB levels in subjects with combined hyperlipidemia by reducing the production of apoB-containing lipoproteins: Implications for the pathophysiology of apoB production. J Lipid Res. 1990;31:567–582. [PubMed] [Google Scholar]
- 33.Dixon JL, Ginsberg HN. Regulation of hepatic secretion of apolipoprotein B-containing lipoproteins: Information obtained from cultured liver cells. J Lipid Res. 1993;34:167–179. [PubMed] [Google Scholar]
- 34.Grefhorst A, Elzinga BM, Voshol PJ, Plösch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Kuipers F. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 35.Brunzell JD, Hazzard wR, Porte D, Jr, Bierman EL. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoprotein in man. J Clin Invest. 1973;52:1578–1585. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bilheimer DW, Goldstein JL, Grundy SM, Brown MS. Reduction in cholesterol and low density lipoprotein synthesis after portacaval shunt surgery in a patient with homozygous familial hypercholesterolemia. J Clin Invest. 1975;56:1420–1430. doi: 10.1172/JCI108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millar JS, Maugeais C, Ikewaki K, Kolansky DM, Barrett PHR, Budreck EC, Boston RC, Tada N, Mochizuki S, Defesche JC, Wilson JM, Rader DJ. Complete deficiency of the low-density lipoprotein receptor is associated with increased apolipoprotein B-100 production. Arterioscler Thromb Vasc Biol. 2005;25:560–565. doi: 10.1161/01.ATV.0000155323.18856.a2. [DOI] [PubMed] [Google Scholar]
- 38.Nagashima K, Lopez C, Donovan D, Ngai C, Fontanez N, Bensadoun A, Fruchart-Najib J, Holleran S, Cohn JS, Ramakrishnan R, Ginsberg HN. Effects of the PPARγ agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. J Clin Invest. 2005;115:1323–1332. doi: 10.1172/JCI23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millar JS, Reyes-Soffer G, Jumes P, Dunbar RL, deGoma EM, Baer AL, Karmally W, Donovan DS, Rafeek H, Pollan L, Tohyama J, Johnson-Levonas AO, Wagner JA, Holleran S, Obunike J, Liu Y, Ramakrishnan R, Lassman ME, Gutstein DE, Ginsberg HN, Rader DJ. Anacetrapib lowers LDL by increasing ApoB clearance in mildly hypercholesterolemic subjects. J Clin Invest. 2015;125:2510–2522. doi: 10.1172/JCI80025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon JL, Furukawa S, Ginsberg HN. Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J Biol Chem. 1991;266:5080–5086. [PubMed] [Google Scholar]
- 41.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siri P, Candela N, Zhang YL, Ko C, Eusufzai S, Ginsberg HN, Huang LS. Post-transcriptional stimulation of the assembly and secretion of triglyceride-rich apolipoprotein B lipoproteins in a mouse with selective deficiency of brown adipose tissue, obesity, and insulin resistance. J Biol Chem. 2001;276:46064–46072. doi: 10.1074/jbc.M108909200. [DOI] [PubMed] [Google Scholar]
- 43.Magkos F, Fabbrini E, Korenblat K, Okunade AL, Patterson BW, Klein S. Reproducibility of glucose, fatty acid and VLDL kinetics and multi-organ insulin sensitivity in obese subjects with non-alcoholic fatty liver disease. Int J Obes. 2011;35:1233–1240. doi: 10.1038/ijo.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: Theoretical, analytic, and experimental considerations. Am J Physiol. 1999;276:E1146–E1170. doi: 10.1152/ajpendo.1999.276.6.E1146. [DOI] [PubMed] [Google Scholar]
- 45.Ramakrishnan R, Ramakrishnan JD. Using mass measurements in tracer studies—a systematic approach to efficient modeling. Metabolism. 2008;57:1078–1087. doi: 10.1016/j.metabol.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthan NR, Raeini-Sarjaz M, Lichtenstein AH, Ausman LM, Jones PJH. Deuterium uptake and plasma cholesterol precursor levels correspond as methods for measurement of endogenous cholesterol synthesis in hypercholesterolemic women. Lipids. 2000;35:1037–1044. doi: 10.1007/s11745-000-0616-9. [DOI] [PubMed] [Google Scholar]
- 47.Lutz EP, Merkel M, Kako Y, Melford K, Radner H, Breslow JL, Bensadoun A, Goldberg IJ. Heparin-binding defective lipoprotein lipase is unstable and causes abnormalities in lipid delivery to tissues. J Clin Invest. 2001;107:1183–1192. doi: 10.1172/JCI11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hocquette JF, Graulet B, Olivecrona T. Lipoprotein lipase activity and mRNA levels in bovine tissues. Comp Biochem Physiol B Biochem Mol Biol. 1998;121:201–212. doi: 10.1016/s0305-0491(98)10090-1. [DOI] [PubMed] [Google Scholar]
- 49.Hirano K-I, Young SG, Farese RV, Jr, Ng J, Sande E, Warburton C, Powell-Braxton LM, Davidson NO. Targeted disruption of the mouse apobec-1 gene abolishes apolipoprotein B mRNA editing and eliminates apolipoprotein B48. J Biol Chem. 1996;271:9887–9890. doi: 10.1074/jbc.271.17.9887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.