Abstract
Inter-arm blood pressure (BP) difference has been associated with ischemic stroke. Local atherosclerosis of stroke differ among vulnerable individuals, whereas intracranial arterial stenosis (ICAS) is more frequently affected Asians, and extracranial arterial stenosis (ECAS) is more prevalent among whites. We hereby sought to explore the association of inter-arm BP difference with ICAS and ECAS in stroke-free hypertensive patients in Chinese population. All the 885 subjects were evaluated of ICAS and ECAS through computerized tomographic angiography. Both arm BP was measured simultaneously by Vascular Profiler-1000 device. In the continuous study, ICAS was significantly associated with age, male, average brachial SBP, diabetes, anti-hypertensive treatment and inter-arm DBP difference. ECAS was associated with age, inter-arm SBP and LDL. In the categorical study, subjects with the top quartile of inter-arm DBP difference (≥4 mmHg) showed significantly higher risk of ICAS (OR = 2.109; 95% CI, 1.24–3.587). And the participants with the top quartile of inter-arm SBP difference (≥6 mmHg) showed significantly higher risk of ECAS (OR = 2.288; 95% CI, 1.309–3.998). In conclusion, we reported a diverse association of inter-arm SBP/DBP difference with the ICAS/ECAS. Inter-arm DBP difference might be the early symbol of ICAS in Chinese population, which need further verification in long-term cohort study.
Stroke is the second leading cause of death and the most common cause of adult disability worldwide1, with ischemic stroke as the predominant subtype in Chinese population. Atherosclerosis has been related to stroke for a long time, but the local expression and severity of atherosclerosis differ among vulnerable individuals, whereas intracranial arterial stenosis (ICAS) is more frequently affected Asians, blacks and Hispanics, extracranial arterial stenosis (ECAS) is more prevalent among whites2,3. The origin of the difference may be multiple, including genetic, environmental factors, and the different underlying disorders4. Hypertension is a worldwide health problem, which is especially common in China5, contributing to a high incidence of and mortality from stroke6,7, as well as for incidence of ICAS8. Therefore, high blood pressure (BP) may account for part of the distinctiveness of ICAS and ECAS in Chinese population.
A difference in BP readings between arms can be observed in various populations9, which is more prevalent in hypertension patients10. A great deal of evidence has indicated that inter-arm BP difference, especially SBP difference, is an independent risk factor of ischemic stroke11, left ventricular hypertrophy12, and other fatal and nonfatal cardiovascular events13. However, there is little study focusing on the relationship of inter-arm BP difference and asymptomatic ICAS or ECAS. Therefore, in the present study, we aim to investigate the association of both inter-arm SBP and DBP difference with ICAS and ECAS in stroke-free hypertensive patients in Chinese population.
Method
Study design
This study was undertaken within the framework of an ongoing cross-section and prospective study in China, which was a computerized tomographic angiography (CTA) based study of intra- and extracranial asymptomatic artery stenosis and stroke outcome in stroke-free hypertension patients. Participants of current study were recruited from hypertension outpatients who were identified in Xinzhuang Community hospital between May 2012 and December 2015, and then referred to Ruijin Hospital, a general hospital in Shanghai. Patients with hypertension were invited to participate in the study if they had ≥2 cardiovascular risk factors defined according to the Chinese hypertension guidelines14 and were willing to undergo an examination of brain using computerized tomographic angiography (CTA) and participate in long-term follow-up of their health. Hypertension was defined as blood pressure (BP) ≥140/90 mmHg, or taking antihypertensive medication. Those who had stroke, transient ischemic attack or atrial fibrillation identified from medical history was excluded. Those who were unfit for CTA examination because of iodine allergy were also excluded.
Ethics Statement
The study protocol was approved by the ethics committee of Ruijin Hospital and written informed consent was obtained from all participants. All experiments were performed in accordance with relevant guidelines and regulations of ethics committee of Ruijin Hospital.
Demographic and clinical measurements
Both arm BP was measured simultaneously by the use of the Vascular Profiler-1000 device (Omron, Kyoto, Japan), an oscillometric cuff technique. Trained technicians placed the pressure cuffs on both arms and performed the measurement after the subject had rested for ≈10 minutes in the supine position. The device simultaneously and automatically measures the both arm BPs twice, and then discards the first measurement and only stores the second one in the database.
Body weight and height were recorded with participants wearing light indoor clothing and no shoes. Clinical information was collected by interview, including smoking and drinking habits, current drug intake, personal history of diabetes, etc. Current smokers were defined as those who had smoked cigarettes on one or more days in the past 30 days. serum concentrations of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL), were performed in the Central Laboratory of Ruijin Hospital (Shanghai, China) using the standard protocols.
CTA protocol
CTA was performed with a 64-section helical CT scanner (GE FX/I, General Electric, Fairfield, CT) as previous described15. CTA acquisitions were obtained after a single bolus intravenous injection of 70 ml OptirayIoversol 320 into the antecubital vein at a rate of 3 ml/sec. Scanning covered the whole brain down to the level of aortic arch with 5-mm slice thickness. Images were reformatted in axial, sagittal, and coronal planes with 1.25-mm slice thickness. All images were read at a workstation with the software of AW4.4 vessel analysis independently by two experienced radiologist who were blinded to clinical data of the patients. Stenosis was defined as a lesion that decreased arterial internal diameter. The percentage of stenosis was calculated as the ratio of the diameter of the diseased artery at its most severe site divided by the diameter of a nearby normal segment. The intracranial arteries included intracranial segment of internal carotid artery and vertebral artery, basilar artery, anterior cerebral artery, middle cerebral artery and posterior cerebral artery. The extracranial arteries included extracranial segment of internal carotid artery and vertebral artery, external carotid artery, common carotid artery and subclavian artery. The greatest stenosis at an artery was chosen as being representative for each subject. The number of arteries with stenosis for each patient was also counted. The two radiologists had good agreement in the designation of stenosis (κ = 0.93, P < 0.001). All disagreements were reviewed and adjudicated by a senior radiologist to reach a consensus.
Statistic Analysis
For database management and statistical analysis, we used SPSS software (version 13.0; SPSS Inc., Chicago, Illinois, USA). Descriptive statistics for patients with or without ICAS were compared using a Pearson Chi-square test for categorical variables and Student t test for continuous variables. In continuous analyses, stepwise logistic regression in forward conditional method was performed to test the association of inter-arm SBP/DBP (1 mmHg) difference and other risk factors with ICAS/ECAS. The variables tested in the equation included age, sex, body mass index, current smoking and drinking status, diabetes, low-density lipoprotein, average brachial SBP, heart rate, anti-hypertensive treatment, statin use, inter-arm SBP/DBP difference. In categorical analyses, we tested the association of quartiles of inter-arm SBP/DBP difference (≥6 mm Hg or ≥4 mm Hg) and increased inter-arm SBP and DBP differences (≥10 mm Hg or ≥5 mm Hg) with ICAS/ECAS by the adjustment for age, sex, body mass index, current smoking and drinking status, diabetes, low-density lipoprotein, average brachial SBP, heart rate, anti-hypertensive treatment, statin use. All P values were 2-tailed, and a P value of <0.05 was considered statistically significant.
Results
Characteristics of the Study Participants
Of the 885 participants, 418 had no ICAS or ECAS, 191 had ICAS only, 129 had ECAS only, and 147 had concurrent extraintracranial artery stenosis (Table 1). Comparing with the stenosis absent group, the patients with ICAS were older and with higher frequency of male, higher both arm SBP, higher inter-arm DBP difference, and higher frequency of diabetes and anti-hypertensive treatment. The patients with ECAS were older and with higher frequency of male, higher inter-arm SBP difference and higher LDL.
Table 1. Clinical characteristics of hypertensive patients according to location of arterial stenosis.
| ICAS/ ECAS absent | ICAS present | ECAS present | COMB present | P | |
|---|---|---|---|---|---|
| N | 418 | 191 | 129 | 147 | |
| Age (years) | 62.9 ± 5.7 | 65.5 ± 5.9† | 65 ± 5.6† | 67.1 ± 5† | <0.001 |
| Male (N,%) | 150 (35.9) | 101 (52.9)† | 60 (46.5)* | 90 (61.2)† | <0.001 |
| Smoking (N,%) | 52 (12.4) | 34 (17.8) | 21 (16.3) | 26 (17.7) | 0.224 |
| Dringking (N,%) | 57 (13.6) | 34 (17.8) | 15 (11.6) | 21 (14.3) | 0.420 |
| Body mass index (kg/m2) | 25 ± 3.1 | 25.3 ± 2.9 | 25 ± 3.2 | 25.4 ± 2.9 | 0.403 |
| Left arm SBP (mmHg) | 133.9 ± 15.4 | 138.8 ± 15.6† | 134 ± 15.6 | 140 ± 16.9† | <0.001 |
| Left arm DBP (mmHg) | 79.1 ± 9.7 | 79.5 ± 9.3 | 77.3 ± 10.2 | 78.7 ± 8.9 | 0.218 |
| Right arm SBP (mmHg) | 134.9 ± 15.5 | 140.1 ± 15.6† | 135.8 ± 16.1 | 141.3 ± 16.9† | <0.001 |
| Right arm DBP (mmHg) | 79.3 ± 9.5 | 79.7 ± 9.2 | 77.9 ± 10.3 | 79.2 ± 8.8 | 0.355 |
| Inter arm SBP difference (mmHg) | 3.2 ± 2.6 | 3.2 ± 2.6 | 4.2 ± 4.1† | 3.7 ± 3.4* | 0.002 |
| Inter arm DBP difference (mmHg) | 2.5 ± 2 | 3 ± 2.3† | 2.8 ± 2.3 | 2.8 ± 2.4 | 0.021 |
| Heart rate (beats/min) | 71.9 ± 11.4 | 69.9 ± 10.5* | 71.8 ± 11.7 | 71.6 ± 11.4 | 0.219 |
| Plasma glucose (mmol/L) | 5.1 ± 1.1 | 5.3 ± 1.6 | 5.2 ± 1.1 | 5.4 ± 1.4* | 0.056 |
| Total cholesterol (mmol/L) | 4.9 ± 0.8 | 4.8 ± 0.9 | 5 ± 0.9 | 4.9 ± 0.9 | 0.154 |
| Low-density lipoprotein (mmol/L) | 2.8 ± 0.8 | 2.9 ± 0.8 | 3 ± 0.7* | 3.1 ± 0.8† | 0.016 |
| Diabetes mellitus (N,%) | 74 (17.7) | 48 (25.1)* | 24 (18.6) | 39 (26.5)* | 0.047 |
| Anti-hypertensive treatment (N,%) | 354 (84.7) | 176 (92.1)* | 117 (90.7) | 135 (91.8)* | 0.014 |
| Statin use (N,%) | 27 (6.4) | 12 (6.3) | 10 (7.8) | 19 (12.9)* | 0.068 |
Data are expressed as mean ± SD, or percentage (%). ECAS, extracranial arterial stenosis; ICAS, intracranial arterial stenosis; COMB, combined extra- and intracranial arterial stenosis. SBP, systolic blood pressure; DBP, diastolic blood pressure. P indicates the comparison among four groups. *p < 0.05 and †p < 0.01 in comparison with ICAS/ ECAS absent group.
Continuous Analyses
In order to detected all the independent risk factors associated with ICAS/ECAS, we performed a stepwise logistic regression in forward conditional method, including the variables of age, sex, body mass index, current smoking and drinking status, diabetes, low-density lipoprotein, average brachial SBP, heart rate, anti-hypertensive treatment, statin use, and inter-arm SBP/DBP difference (1 mmHg). Variables potentially associated with ICAS/ECAS were shown in Table 2. ICAS was significantly associated with age, male, average brachial SBP, diabetes, anti-hypertensive treatment and inter-arm DBP difference. While ECAS was significantly associated with age, inter- arm SBP difference and LDL.
Table 2. The risk factors of intracranial and extracranial arterial stenosis.
| OR | 95% CI | P | |
|---|---|---|---|
| Isolated ICAS | |||
| Age | 1.063 | 1.028–1.098 | <0.001 |
| Male | 1.872 | 1.296–2.704 | 0.001 |
| Average brachial SBP | 1.002 | 1.001–1.003 | 0.005 |
| Inter-arm DBP difference | 1.012 | 1.004–1.021 | 0.004 |
| Diabetes | 1.511 | 1.001–2.300 | 0.049 |
| Anti-hypertensive treatment | 0.539 | 0.291–0.998 | 0.048 |
| Isolated ECAS | |||
| Age | 1.064 | 1.026–1.104 | 0.001 |
| Inter-arm SBP difference | 1.01 | 1.004–1.017 | 0.001 |
| Low-density lipoprotein | 1.417 | 1.078–1.862 | 0.012 |
| COMB | |||
| Age | 1.129 | 1.083–1.178 | <0.001 |
| Male | 2.757 | 1.780–4.270 | <0.001 |
| Average brachial SBP | 1.002 | 1.001–1.003 | 0.003 |
| Low-density lipoprotein | 1.756 | 1.316–2.343 | <0.001 |
| Diabetes | 1.712 | 1.042–2.812 | 0.034 |
| Statin use | 0.314 | 0.154–0.641 | 0.001 |
Stepwise logistic regression in forward conditional method was performed to test the risk factors of stenosis. The variables tested in the equation included age, sex, body mass index, current smoking and drinking status, diabetes, logw-density lipoprotein, average brachial SBP, heart rate, anti-hypertensive treatment, statin use, inter-arm SBP difference and inter-arm DBP difference. The odds ratio expressed the risk in the ICAS and ECAS group compared with the non-stenosis group. Isolated ECAS, extracranial arterial stenosis only; Isolated ICAS, intracranial arterial stenosis only; COMB, combined extra- and intracranial arterial stenosis; OR, odds ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Categorical Analyses
The categorical analyses of inter-arm SBP/DBP and ICAS/ECAS were in Table 3. After adjustment for the abovementioned covariates, the risk of ICAS was independently associated with the inter-arm DBP difference (P = 0.048). Comparing with the lowest quartile, the subjects with the top quartile of inter-arm DBP difference (≥4 mmHg) showed significantly higher risk of ICAS (P = 0.006) with the corresponding OR = 2.109 (95% CI, 1.24–3.587). The risk of ICAS in patients with inter-arm DBP difference ≥5 mmHg was 92% higher (P = 0.014). On the contrary, there was no significant association was detected between ECAS and inter-arm DBP difference. But the participants with the top quartile of inter-arm SBP difference (≥6 mmHg) showed significantly higher risk of ECAS (P = 0.004) with the corresponding OR = 2.288 (95% CI, 1.309–3.998). The risk of ECAS in patients with inter-arm SBP difference ≥10 mmHg was 254% higher. Neither inter-arm SBP difference nor DBP difference was associated with presence of concurrent extraintracranial artery stenosis.
Table 3. Associations of inter arm blood pressure difference with intracranial and extracranial arterial stenosis OR, odds ratio.
| Isolated ICAS |
Isolated ECAS |
COMB |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Inter arm SBP quartiles | 0.900 | 0.003 | 0.435 | ||||||
| Q1 (<1 mmHg) | 1 | 1–1 | 1 | 1–1 | 1 | 1−1 | |||
| Q2 (1–3 mmHg) | 1.166 | 0.734–1.852 | 0.515 | 0.871 | 0.501–1.516 | 0.625 | 1.446 | 0.823–2.541 | 0.2 |
| Q3 (3–6 mmHg) | 1.034 | 0.609–1.758 | 0.900 | 0.938 | 0.496–1.772 | 0.843 | 1.517 | 0.819–2.809 | 0.185 |
| Q4 (≥6 mmHg) | 0.976 | 0.557–1.713 | 0.933 | 2.288 | 1.309–3.998 | 0.004 | 1.584 | 0.827–3.033 | 0.165 |
| Inter arm SBP> = 10 mmHg | 0.585 | 0.171–2.002 | 0.393 | 3.544 | 1.482–8.48 | 0.004 | 1.12 | 0.366–3.425 | 0.842 |
| Inter arm DBP quartiles | 0.048 | 0.495 | 0.128 | ||||||
| Q1 (<1 mmHg) | 1 | 1–1 | 1 | 1–1 | 1 | 1–1 | |||
| Q2 (1–2.2 mmHg) | 1.290 | 0.752–2.213 | 0.355 | 1.11 | 0.619–1.992 | 0.726 | 1.013 | 0.556–1.847 | 0.966 |
| Q3 (2.2–4 mmHg) | 1.447 | 0.859–2.435 | 0.165 | 1.091 | 0.618–1.926 | 0.763 | 0.775 | 0.423–1.419 | 0.409 |
| Q4 (≥4 mmHg) | 2.109 | 1.24–3.587 | 0.006 | 1.539 | 0.858–2.763 | 0.148 | 1.614 | 0.89–2.924 | 0.115 |
| Inter arm DBP> = 5 mmHg | 1.917 | 1.141–3.219 | 0.014 | 1.464 | 0.798–2.686 | 0.218 | 1.433 | 0.732–2.808 | 0.294 |
Aadjusted for age, sex, BMI, current smoking and drinking status, diabetes, low density lipoprotein, average brachial SBP, heart rate, anti-hypertensive treatment, and statin use. The odds ratio expressed the risk in the ICAS and ECAS group compared with the non-stenosis group. Isolated ECAS, extracranial arterial stenosis only; Isolated ICAS, intracranial arterial stenosis only; COMB, combined extra- and intracranial arterial stenosis.
Inter-arm BP and number/severity of ICAS/ECAS
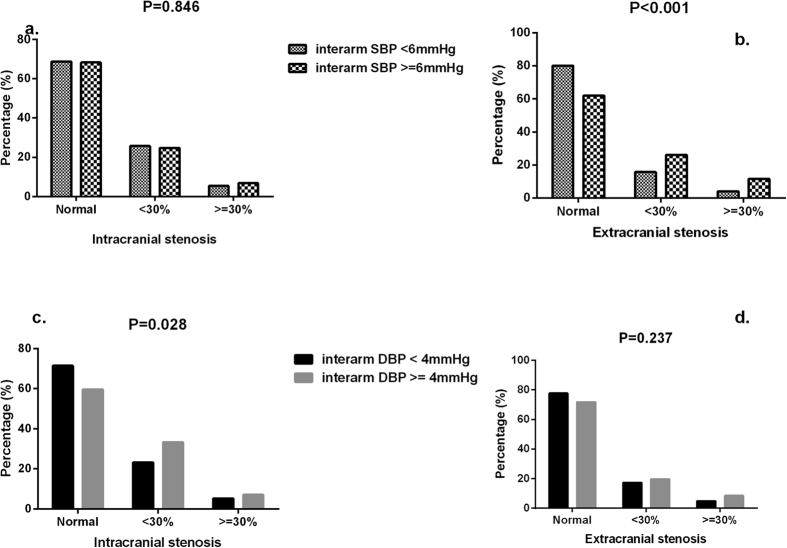
Then, the severity of ICAS/ECAS was compared between patients with the top quartile of inter-arm SBP/DBP and the others. Comparing with the subjects with the inter-arm DBP <4 mmHg (5.3%), the subjects in the top quartile of inter-arm DBP difference (≥4 mmHg) had more moderate to severe ICAS (7.1%) (P = 0.028) (Fig. 1c). In contrast, comparing with the subjects with the inter-arm SBP difference <6 mmHg (4.1%), the subjects in the top quartile of inter-arm SBP difference (≥6 mmHg) had more moderate to severe ECAS (11.7%) (P < 0.001) (Fig. 1b).
Figure 1.
Prevalence of severity (%) of ECAS and ICAS stenosis according to top quartile of inter-arm SBP (a,b) and DBP (c,d). ECAS, extracranial arterial stenosis; ICAS, intracranial arterial stenosis.
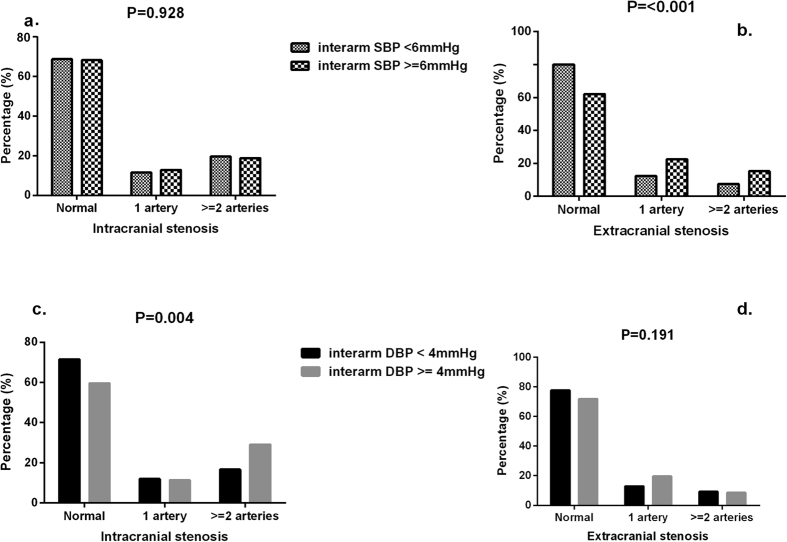
Moreover, patients in the highest quartile of inter-arm DBP difference showed higher frequency of multiple ICAS (29.1%) than the rest of participants (16.6%) (P = 0.004) (Fig. 2c). The patients in the highest quartile of inter-arm SBP difference showed higher frequency of multiple ECAS (15.3%) than the rest of participants (7.6%) (P < 0.001) (Fig. 2b).
Figure 2.
Prevalence of involved ECAS and ICAS arteries according to top quartile of inter-arm SBP (a,b) and DBP (c,d). ECAS, extracranial arterial stenosis; ICAS, intracranial arterial stenosis.
Furthermore, the patients with inter-arm SBP difference ≥10 mmHg suffered more severe ECAS and involved ECAS vessels. While the patients with inter-arm DBP difference ≥5 mmHg suffered more severe ICAS and involved ICAS vessels (Supplementary Figs 1 and 2).
Discussion
In this stroke-free Chinese hypertension population who underwent both intra- and extracranial CTA, a diverse association of inter-arm SBP/DBP difference with the ICAS/ECAS was detected. The inter-arm SBP difference was only significantly associated with the presence of ECAS and the severity of ECAS. On the contrary, the inter-arm DBP difference was only associated with the presence of ICAS and the severity of ICAS.
It was a very interesting thing that the different component of BP might relate to the stenosis in different part of the arterial tree separately. Although both ICAS and ECAS were process of atherogenesis and had the similar vascular risk factors, the distribution of atherogenesis varied among different populations, whereas patients of Asian possessed more ICAS16, while whites more frequently suffered from extracranial carotid lesions17. The genetic background heterogeneity might influence the atherogenesis at the different location of arteries. Uehara et al. had explored the risk factors of ICAS and ECAS in 425 stroke-free Japanese patients, they found that the independent predictors of ECAS were age, hyperlipidemia and ischemic heart disease (IHD), while those for ICAS were age, hypertension, diabetes mellitus and IHD18. Such results were in accordance with our findings, whereas we found that ECAS was associated with age and the concentration of LDL, and ICAS was related to age, sex, SBP, diabetes and the use of anti-hypertensive treatment. Although simultaneous hypercholesterolemia and hypertension management was suggested by several guidelines19, comparing with the ECAS which was more likely to be found by carotid ultrasonography and therefore drew more attention for anti-hypertensive and statin treatment, the patients with ICAS in the present study suffered higher BP and lower frequency of lipid lowering therapy. The uncontrolled BP and consequent augmented inter-arm DBP difference might cause the site specific atherogenesis, which was probably induced by various shear stress dependent endothelial gene expressions20, and diverse shear stress dependant accumulation of inflammatory cells in specific vascular regions21.
BP difference between arms was a common phenomenon which could be observed in various populations22, but the underlying mechanism of such difference between arms was still unclear23. There was an assumption that the asymmetrical arms arterial pressure was due to the atherosclerotic stenotic lesion24. And the relationship between inter-arm SBP and subclavian stenosis and cardiovascular mortality had been reported by a great deal of studies25. Exaggerated absolute SBP difference >10 mmHg or >15 mmHg has been associated with peripheral vascular disease, pre-existing cerebrovascular disease. In accordance with the former studies, we confirmed the association of inter-arm SBP difference with the ECAS, including the subclavian stenosis. Although the difference of DBP in two arms was also universal, there were limited studies analyzing the relation between inter-arm DBP differences and cardiocerebrovascular disorders. A study performed in general population found that exaggerated absolute inter-arm DBP (≥5 mmHg) but not inter-arm SBP was associated with left ventricular mass index26, Johansson et al. explained that the DBP might play more important role in the early phase of cardiovascular disease. In addition, Hu et al. had also found that inter-arm DBP difference but not SBP difference was associated with the flow-mediated dilatation of arm, which was an early index of arterial endothelium lesion27. The ICAS is the early lesion of cerebral vessels, and most of subjects in the present study suffered mild ICAS, so that the difference of inter-arm DBP might respond for early ICAS through disturbing the blood flow and subsequently influence the atherogenesis.
ECAS was the main lesion in the arterial tree in whites and was considered as an important risk factor for cardiovascular diseases. Inter-arm SBP difference had long been associated with ECAS, so that it was undoubtedly that the increased SBP difference between arms might be an indicator for fatal and nonfatal events in European population. However, ICAS accounted for 30–50% of ischemic stroke and >50% of transient ischemic attack in Asians28. Leng et al. had found that ICAS was not independently correlated with carotid intima-media thickness and conferred that atherosclerosis might start earlier in ICAS than in ECAS in Chinese population29. Therefore, Inter-arm DBP difference, as a possible indicator for ICAS in Chinese and other Asian population, should be paid more attention and deserved further researches.
We acknowledge that there were several limitations of the present study. First, as a cross-sectional study, we could not infer any causal relationship between inter-arm BP and ICAS/ECAS. Second, the majority of subjects were under antihypertensive treatment, which induced that the BP differences between arms were lower than the previous findings in hypertension population. But the prevalence of BP difference was similar with the results in general population30, and we had detected the association of inter-arm BP with ICAS/ECAS in both binary and continuous analysis. Third, nearly half of the participants had ICAS or ECAS, which seemed higher than other reports. The hypertension patients enrolled had at least two other risk factor, which might have higher prevalence of ICAS and ECAS than the common population. Moreover, most the involved vessels were with mild stenosis (<30%), and the prevalence of moderate to severe stenosis of intracranial arteries (15.2%) was similar to previous survey in high risk patients31. Forth, the ICAS was more common in Asians than the whites, so that our finding might not be directly generalized to other populations.
In conclusion, we found a diverse association of inter-arm SBP/DBP difference with the ICAS/ECAS. Inter-arm DBP difference might be the early symbol of ICAS in Chinese population. In addition, the further long-term cohort study was warranted to elucidate the concrete effect of inter-arm BP difference on the asymptomatic ICAS/ECAS.
Additional Information
How to cite this article: Wang, Y. et al. Association of Inter-arm Blood Pressure Difference with Asymptomatic Intracranial and Extracranial Arterial Stenosis in Hypertension Patients. Sci. Rep. 6, 29894; doi: 10.1038/srep29894 (2016).
Supplementary Material
Acknowledgments
This project was supported by grants from the National Key Program for Basic Research (2009CB521905), National Natural Science Foundation of China (81000037 and 81370205). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author Contributions Conceived and designed the experiments: D.Z., Y.L. and P.G. Performed the experiments: Y.W., J.Z., X.T., Y.Q., H.L. and K.C., Analyzed the data: Y.W. Contributed analysis tools: H.L. and K.C. Wrote the paper: Y.W. and D.Z.
References
- Bonita R. et al. The global stroke initiative. Lancet Neurol 3, 391–3 (2004). [DOI] [PubMed] [Google Scholar]
- Caplan L. R., Gorelick P. B. & Hier D. B. Race, sex and occlusive cerebrovascular disease: a review. Stroke 17, 648–55 (1986). [DOI] [PubMed] [Google Scholar]
- White H. et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 111, 1327–31 (2005). [DOI] [PubMed] [Google Scholar]
- Lopez-Cancio E. et al. Biological signatures of asymptomatic extra- and intracranial atherosclerosis: the Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) study. Stroke 43, 2712–9 (2012). [DOI] [PubMed] [Google Scholar]
- Gu D. et al. Prevalence, awareness, treatment, and control of hypertension in china. Hypertension 40, 920–7 (2002). [DOI] [PubMed] [Google Scholar]
- He J. et al. Premature deaths attributable to blood pressure in China: a prospective cohort study. Lancet 374, 1765–72 (2009). [DOI] [PubMed] [Google Scholar]
- Banach M. et al. Association of systolic blood pressure levels with cardiovascular events and all-cause mortality among older adults taking antihypertensive medication. Int J Cardiol 176, 219–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. T. et al. Association between ambulatory systolic blood pressure during the day and asymptomatic intracranial arterial stenosis. Hypertension 63, 61–7 (2014). [DOI] [PubMed] [Google Scholar]
- Clark C. E., Campbell J. L., Evans P. H. & Millward A. Prevalence and clinical implications of the inter-arm blood pressure difference: A systematic review. J Hum Hypertens 20, 923–31 (2006). [DOI] [PubMed] [Google Scholar]
- Pesola G. R., Pesola H. R., Lin M., Nelson M. J. & Westfal R. E. The normal difference in bilateral indirect blood pressure recordings in hypertensive individuals. Acad Emerg Med 9, 342–5 (2002). [DOI] [PubMed] [Google Scholar]
- Kim J. et al. Interarm blood pressure difference and mortality in patients with acute ischemic stroke. Neurology 80, 1457–64 (2013). [DOI] [PubMed] [Google Scholar]
- Su H. M. et al. Association of interarm systolic blood pressure difference with atherosclerosis and left ventricular hypertrophy. PLoS One 7, e41173 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. E., Campbell J. L. & Powell R. J. The interarm blood pressure difference as predictor of cardiovascular events in patients with hypertension in primary care: cohort study. J Hum Hypertens 21, 633–8 (2007). [DOI] [PubMed] [Google Scholar]
- Liu L. S. [Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi 39, 579–615 (2010). [PubMed] [Google Scholar]
- Wang Y. et al. Association of Lp-PLA2 Mass and Aysmptomatic Intracranial and Extracranial Arterial Stenosis in Hypertension Patients. PLoS One 10, e0130473 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick P. B., Wong K. S., Bae H. J. & Pandey D. K. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 39, 2396–9 (2008). [DOI] [PubMed] [Google Scholar]
- Hsia D. C., Moscoe L. M. & Krushat W. M. Epidemiology of carotid endarterectomy among Medicare beneficiaries: 1985–1996 update. Stroke 29, 346–50 (1998). [DOI] [PubMed] [Google Scholar]
- Uehara T., Tabuchi M. & Mori E. Risk factors for occlusive lesions of intracranial arteries in stroke-free Japanese. Eur J Neurol 12, 218–22 (2005). [DOI] [PubMed] [Google Scholar]
- Banach M. et al. Lipids, blood pressure and kidney update 2014. Pharmacol Res 95-96, 111–125 (2015). [DOI] [PubMed] [Google Scholar]
- Resnick N. et al. Endothelial gene regulation by laminar shear stress. Adv Exp Med Biol 430, 155–64 (1997). [DOI] [PubMed] [Google Scholar]
- Honda H. M. et al. A complex flow pattern of low shear stress and flow reversal promotes monocyte binding to endothelial cells. Atherosclerosis 158, 385–90 (2001). [DOI] [PubMed] [Google Scholar]
- Verberk W. J., Kessels A. G. & Thien T. Blood pressure measurement method and inter-arm differences: a meta-analysis. Am J Hypertens 24, 1201–8 (2011). [DOI] [PubMed] [Google Scholar]
- Clark C. E. & Aboyans V. Interarm blood pressure difference: more than an epiphenomenon. Nephrol Dial Transplant 30, 695–7 (2015). [DOI] [PubMed] [Google Scholar]
- Aboyans V. Asymmetrical limbs arterial pressures: a new marker of atherosclerosis. Hypertens Res 36, 394–5 (2013). [DOI] [PubMed] [Google Scholar]
- Clark C. E., Taylor R. S., Shore A. C., Ukoumunne O. C. & Campbell J. L. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet 379, 905–14 (2012). [DOI] [PubMed] [Google Scholar]
- Johansson J. K., Puukka P. J. & Jula A. M. Interarm blood pressure difference and target organ damage in the general population. J Hypertens 32, 260–6 (2014). [DOI] [PubMed] [Google Scholar]
- Hu W. et al. The inter-arm diastolic blood pressure difference induced by one arm ischemia: a new approach to assess vascular endothelia function. PLoS One 9, e84765 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. S. et al. Intracranial stenosis in Chinese patients with acute stroke. Neurology 50, 812–3 (1998). [DOI] [PubMed] [Google Scholar]
- Leng X. Y. et al. Correlation of large artery intracranial occlusive disease with carotid intima-media thickness and presence of carotid plaque. Stroke 44, 68–72 (2013). [DOI] [PubMed] [Google Scholar]
- Kimura A. et al. Patient characteristics and factors associated with inter-arm difference of blood pressure measurements in a general population in Ohasama, Japan. J Hypertens 22, 2277–83 (2004). [DOI] [PubMed] [Google Scholar]
- Wong K. S. et al. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology 68, 2035–8 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.