Abstract
The voltage-gated sodium ion channel (VGSC) belongs to the largest superfamily of ion channels. Since VGSCs play key roles in physiological processes they are major targets for effective insecticides. RNA interference (RNAi) is widely used to analyse gene function, but recently, it has shown potential to contribute to novel strategies for selectively controlling agricultural insect pests. The current study evaluates the delivery of dsRNA targeted to the sodium ion channel paralytic A (TcNav) gene in Tribolium castaneum as a viable means of controlling this insect pest. Delivery of TcNav dsRNA caused severe developmental arrest with larval mortalities up to 73% post injection of dsRNA. Injected larvae showed significant (p < 0.05) knockdown in gene expression between 30–60%. Expression was also significantly (p < 0.05) reduced in pupae following injection causing 30% and 42% knockdown for early and late pupal stages, respectively. Oral delivery of dsRNA caused dose-dependant mortalities of between 19 and 51.34%; this was accompanied by significant (p < 0.05) knockdown in gene expression following 3 days of continuous feeding. The majority of larvae injected with, or fed, dsRNA died during the final larval stage prior to pupation. This work provides evidence of a viable RNAi-based strategy for insect control.
Insect pest control in agriculture is predominantly based on the use of synthetic chemical pesticides1,2,3. Despite their effectiveness at controlling pest insects, there is a real need to develop alternative approaches with lower environmental and non-target impacts4.
Current insecticides most commonly target components of the insect nervous system3, often targeting the ion channels responsible for perpetuating the action potential along neurons and the enzymes of the synaptic cleft responsible for the degradation of neurotransmitters. Of these, the voltage-gated sodium channel (VGSC) is the primary target of pyrethroids5,6,7. VGSCs are part of a super family of ion channels that includes the voltage-gated potassium channel, the voltage-gated calcium channel, TRP-related channels and cyclic nucleotide gated channels8. The correct functioning of these channels is essential for normal transmission of nerve impulse and any inhibition of the action potential as a result of pesticide binding will lead to paralysis and eventual death9. Insect VGSCs were first cloned in the late 1980s from Drosophila melanogaster10. Although initially it was believed that D. melanogaster possessed two distinct isoforms of sodium ion channels, the DSC-type and the para-type, Zhou et al.11 latterly showed DSC1 to be a Ca2+-selective cation channel. Thus, it is the para-type that are involved in the nervous system, acting as voltage-gated sodium transporters12,13,14,15,16. Whilst chemicals such as pyrethroids are very effective at killing insect pests they are neither targeted, nor impervious to loss of function from evolution of insect resistance17. The lack of specificity of pyrethroids is partly a consequence of the VGSCs being highly conserved, not only across different insect orders but across animal species18. Whilst this confers broad spectrum effects of insecticidal molecules targeting these particular ion channels, it results in limited specificity and, of more concern, non-target effects. Thus there is a need to develop novel insecticides which are of comparable efficacy but demonstrate higher specificity. The use of a molecular approach such as RNA interference provides a platform enabling a highly specific and targeted strategy to insect control.
RNA interference (RNAi) represents a unique form of post-transcriptional gene silencing (PTGS); it is a recognized cellular mechanism for defence against viral invasion and post-transcriptional regulation of mRNA19. Further, it is the specific downregulation of gene expression mediated by an artificial double-stranded RNA (dsRNA) molecule where one strand of the dsRNA corresponds to part or all of a specific gene transcript20. This sequence-based interaction confers an extremely high level of specificity. Although first described in plant and animal viruses, this evolutionarily conserved phenomenon of RNA silencing has now been observed in a range of organisms from Neurospora (an ascomycete fungus) to much more complex organisms including insects and mammals21,22,23,24,25,26,27,28.
RNAi-based gene silencing thus has the potential to represent a novel insecticide technology, since it is theoretically possible to protect plants against insects by down regulating the expression of essential genes in the pest20,29,30,31. Furthermore, this technology should also allow non-conserved sequences to be specifically targeted, thus conferring a high degree of specificity. The red flour beetle, Tribolium castaneum (Tc), is a major global storage pest of grain, legumes and cereal products both for human consumption and animal feed32. It has been demonstrated that T. castaneum is readily adaptable to all currently available classes of chemical insecticide. However, it is also particularly amenable to RNAi. In addition, there are many genetic and genomic tools available for this insect and it has become the genetic model for agriculturally important coleopteran species, representing an ideal system for the identification of novel pesticide targets33.
The present study demonstrates that RNA interference can be used to knockdown the expression of the D. melanogaster DmNav1 homologue in T. castaneum, resulting in high insect mortality. The results provide proof of concept for this approach to be used in the sustainable control of insect pests, since this strategy is both highly specific and effective towards the targeted species, and is therefore unlikely to effect beneficial insects such as pollinators and those involved in biological control, as there is no homology between the dsRNA fragment and off target sequences.
Materials and Methods
Insects
A culture of Tribolium castaneum was obtained from Blades Biological Ltd, Kent TN8 7DX and reared at 30 °C, 16:8 (L:D) on organic whole flour supplemented with 5% brewer’s yeast. Flour was replaced every 2–4 weeks.
Design of dsRNA
Selection of the target sequence used in the present study was made using the latest version of the E-RNAi web tool (http:// www.dkfz.de/signaling/e-rnai3//)34,35. Output from E-RNAi selected a region of TC004126 transcript that had no similarities with other transcripts or low-complexity regions in the T. castaneum genome. The same process was employed to select a region of the kanamycin resistance gene (nptII), JN638547 (synthetic construct) from the cloning vector pSC-A-amp/kan (Stratagene) to be used as a control to assess the effect of injecting and feeding target-less dsRNA.
Total RNA isolation and cDNA synthesis
Total RNA was isolated from 4th instar larvae using TRIzol® Plus RNA Purification Kit (Ambion, TRI reagent, #12183-555) following the manufacturer’s protocol. RNA integrity was evaluated on 1.5% agarose gels as described in Sambrook and Russell36, and quantified spectrophotometrically (NanoDrop, Labtech, ND-1000). cDNA synthesis was performed by reverse transcribing RNA using the i-Script™ reverse transcription supermix for RT-qPCR kit (BIO-RAD, 170-8841); 1000 ng of the extracted total RNA was used per each reaction.
Synthesis of dsRNA molecules
PCR reactions were performed in a thermal cycler (Applied Biosystems, GeneAmp PCR system 2700), in 50 μl reaction volumes using (Thermo scientific PCR master mix (2x), #K0171). The final concentration of reagents used for the majority of PCRs was as follows: dNTP 0.4 mM, Magnesium Chloride 4 mM, primer concentrations 0.2 μM (Table 1), Taq DNA polymerase, 1.25 Units/reaction. The basic cycling parameters used were as follows: Denaturation step at 95 °C for 3 minutes, followed by a cycle of denaturation at 95 °C for 30 s, annealing at 57 °C for 30 s and elongation step at 72 °C for 15 s. This cycle was repeated 35 times followed by a final elongation cycle of 10 minutes. Following electrophoresis, amplified bands were gel purified using QIAquick MinElute Gel Extraction kit (Qiagen, #28604), cloned into StrataClone vector pSC-A-amp/kan (Stratagene, #240205) and then transformed into StrataClone SoloPack competent cells. Recombinant plasmids were sequenced to verify the cloned insert. The dsRNA was synthesized using replicator RNAi kit (Thermo Scientific, #F-610) and stored at −80 °C prior to injection.
Table 1. Primers used in PCR, synthesizing dsRNA, and performing qRT-PCR.
| Application Of Primers | Sequence (5′-3′) | Ampliconsize | ||
|---|---|---|---|---|
| PCR | F | AAGGCAAGGACATATTCCGA | 217 bp | |
| R | TCAAACGTGTAGATGCCAGTG | |||
| dsRNA synthesis | Ft7 | TAATACGACTCACTATAGGGAAGGCAAGGACATATTCCGA | 217 bp | |
| Rphi6 | GGAAAAAAATCAAACGTGTAGATGCCAGTG | |||
| qRT-PCR | Tc_paraA | F | TCATTCCGACTGTTGAGGGT | 100 bp |
| R | GATTCCCCAAAGCCCCCATC | |||
| Tc_RPS6 | F | GAAGCAGGGTGTTCTCACGA | 92 bp | |
| R | GTTTCCTTTCACCGTCACGC | |||
| Kanamycin resistance | F | TAATACGACTCACTATAGGGCATTCGCCGCCAAGTTCTTC | 468 bp | |
| R | TAATACGACTCACTATAGGGTGCTCGACGTTGTCACTGAA | |||
Delivery of the double-stranded RNA to larvae and pupae
Larvae and pupae (reared on organic whole wheat flour as above) were injected as described by Tomoyasu and Denell37, using a dissecting stereomicroscope. The dsRNA was injected into the dorsal side between the 1st and 2nd abdominal segments of 6th (final) instar larvae, and laterally between the 2nd and 3rd abdominal segments for pupal stages, using a NanojectII™ injector (Drummond Scientific Company). Three biological replicates (5 larvae or pupae/replicate) were injected from one dsRNA preparation (780 ng/μl in RNAs free water, equivalent to (53.6, 106.7, 160 ng/larva and 160 ng/pupa); 48 h post injection gene knockdown was measured by RT-qPCR using gene-specific primers. For the longer-term injection bioassays, 6th instar larvae (3 biological replicates, 15 larvae/rep) for each dose (53.6, 106.7, 160 ng/larva) were injected, returned to the diet and monitored on a daily basis until adult eclosion. As described previously35,38,39, all injected controls received RNAase free water at the same volumes as experimental conditions; a second control of dsRNA towards the microbial kanamycin resistance gene (nptII) was also included in addition to the non-injected insect group.
The dsRNA was also delivered orally via flour disks at a range of concentrations (0, 50, 100, 150 ng dsRNA/mg diet) prepared as described by Xie et al.40, in 96-wellflat-bottom well microtiter plates; one larva (6th instar) was added to each well. Three biological replicates, each of 15 larvae/rep were carried out for each dose; for all feeding assays controls were used as described above. Survival of larvae was monitored until adult eclosion. To monitor gene knockdown, three biological replicate (5 larvae/rep/dose) were allowed to feed for 72 h on flour disks as described above at the same doses, after which time larvae were snap frozen in liquid nitrogen prior to assessing gene knockdown by RT-qPCR.
Since preliminary studies with different doses showed no difference in either control mortality or expression of target gene30, subsequent controls were only carried out at a single (highest) dose for each treatment.
Gene expression studies with quantitative reverse transcription PCR (RT-qPCR)
cDNA, prepared as shown above, was used as the template; RT-qPCR was performed on the template in a final volume of 25 μl. Each reaction contained: 12.5 μl 2x Rotor-gene SYBR green PCR master mix solution (Qiagen Co.), forward and reverse primers (Table 1) were added in a final concentration of 0.5 mM, 9 μl nuclease free water and 1 μl of undiluted cDNA. To validate the primers, a standard curve based on a serial dilution of cDNA was carried out to determine the primer annealing efficiency, the presence of primer dimers and the production of a single PCR product. RT-qPCR conditions were as follows: 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 sec, 55 °C for 30 sec and 60 °C for 15 sec. Amplifications were carried out using three biological replicates of cDNA, each one from RNA obtained from five individuals, and the mean values of three technical replicates were analyzed. The efficiency of the primers were equivalent and the relative transcript quantity was calculated according to the delta-delta Ct method41, with Ct values of the respective target gene compared to those of the reference gene TcRPS6 (GenBank XP_968395.1); this gene is a reliable reference gene for RT-qPCR in T. castaneum42, and hence used to normalize gene expression.
Statistical analysis
Experimental data and qPCR results were analysed by ANOVA followed by Tukey Kramer Multiple Comparison; statistical differences are shown as different letters. Insect mortalities were analysed by Kaplan-Meyer survival analysis, SIGMAPLOT program, version 12.5, and larval mortality was corrected according to Abott’s formula43, with percent adult emergence calculated as previously described44,45.
Results
Bioinformatic analysis of targeted sequence
Homology of the TcNav dsRNA fragment designed by the E-RNAi web tool to the other 744 insect Nav sequences, including representatives from important pollinator species, held by NCBI was investigated using the MegaBLAST algorithm. No homology was reported under these highly stringent conditions to any other insect species. A more relaxed BLASTn search indicated similarity between the TcNav dsRNA fragment and the Nav coding sequence from a single coleopteran species, Brassicogethes aeneus (pollen beetle). Overall homology scores were 86% identity, total score 226 and E-value 1e-55 between the 217 base of the TcNav dsRNA fragment and the BaNav sequence.
Temporal changes in TcNav gene expression
Relative abundance of TcNav mRNA at specific life stages was estimated using qRT-PCR to ensure that subsequent studies were carried out on appropriate stages. Transcripts were detected in all life stages investigated. Relative expression of TcNav was more abundant in the late pupal stage compared to either the adult or any of the larval stages, although these differences were not significant (p > 0.05; Fig. 1). Expression in the late pupal stages was 1.6 fold greater relative to the adult stage, but lower in the larval stages, with transcript levels in 6th, 4th and 2nd instar larvae being decreased (0.9, 0.6 and 0.4-fold, respectively). Based on these relative expression levels, both pupal stages (early and late) and 6th (final) instar larvae were selected as suitable stages for subsequent RNAi studies.
Figure 1. Stage-specific transcript levels in the whole body determined by qRT-PCR.
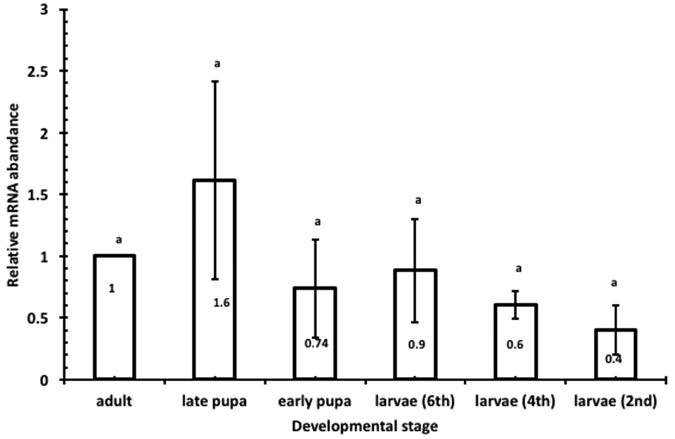
Mean ± SEM of three replications are shown. Means with the same letter are not significantly different (p = 0.05; ANOVA; with Tukey Kramer Multiple Comparison).
Physiological and molecular response of T. casteneum to TcNav dsRNA
Effects on changes in expression levels of TcNav transcripts and changes in phenotype (survival and adult emergence) were investigated using both oral delivery of dsRNA and via injection.
Delivery of dsRNA via injection
The dose response effects of dsRNA directed to TcNav on survival and subsequent development into adulthood of final (6th) instar larvae were investigated. Larvae were monitored daily post injection until pupation, and surviving insects recorded at eclosion. Injection of dsRNA caused a significant dose dependant decrease in survival (p < 0.05), (Fig. 2a). Six days post injection, larval mortality was 35.2% ± 1.7, 62.23% ± 2.4 and 73% ± 5.9 relative to the −ve control (injected with RNase free water), when injected with dsRNA at concentrations of 53.6, 106.7 and 160 ng/larva, respectively (Fig. 2a). Over this same time period, no mortality occurred in the non-injected control larvae; the 2 control groups (RNase free water and kanamycin dsRNA) each resulted in 17.8% mortality compared to the non-injected larvae. In addition to effects on survival, injection of the dsRNA inhibited adult emergence at eclosion in a similar dose-dependent manner, with reduction in adult emergence of 51.12% ± 2.21, 26.7% ± 0 and 17.8% ± 2.22 for the increasing doses, relative to the injected control with RNAase free water (Fig. 2b). However, these differences were only significant (p < 0.05) for the two higher concentrations of dsRNA. Probit analysis determined the delivery of dsRNA via injection to have a LC50 of 79.89 ng/larva.
Figure 2. Larval survival curves and adult eclosion bioassays following injected with dsRNA.
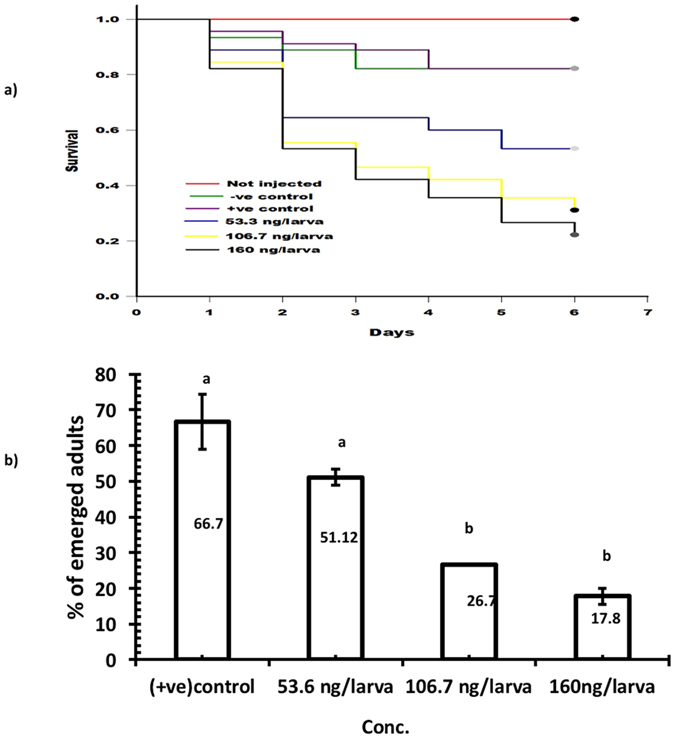
(a) Survival curves of larvae injected with dsRNA over 6 days, the different lines represent the survival curves for the different groups of injected larvae. (b) Effect on adult eclosion of dsRNA on different groups of injected larvae (p = 0.05; ANOVA; with Tukey Kramer Multiple Comparison), +ve control = larvae injected with Kanamycin resistance dsRNA, −ve control = larvae injected with RNAase free water.
Knockdown of TcNav expression following injection of dsRNA was demonstrated using RT-qPCR (Fig. 3). Analysis showed a direct correlation between the amounts of dsRNA injected with a subsequent decrease in the abundance of TcNav mRNA transcript. Gene knockdown at 48 h post injection of 6th instar larvae was shown to be significant (p < 0.05) between the different treatments, with expression of TcNav being reduced 1.43, 2.0 and 2.5-fold in response to increasing amounts of dsRNA corresponding to 53.6, 106.7 and 160 ng/larva, respectively, relative to larvae injected with RNase free water. Furthermore, qPCR analysis revealed that mRNA transcript levels in early stage and late stage pupae injected with 160 ng/pupae dsRNA were also significantly (p < 0.05) down regulated at 48 h post injection, with mRNA levels for TcNav being 1.43 and 1.73 fold lower, respectively (Fig. 4a,b) compared to their appropriate controls, which were injected with RNase free water. The differences between the two pupal stages were not significant (α = 0.05). The injection of naked off target dsRNA into either larvae or pupae did not affect expression of Nav in T. castaneum. This dsRNA molecule designed towards a 468 bp fragment of the microbial kanamycin resistance gene (nptII) was synthesised and injected at 160 ng/larva. RT-qPCR revealed that expression of TcNav in these individuals was 0.98-fold (p > 0.05) that of the control group that were injected solely with RNase free water.
Figure 3. Transcript levels of TcNav mRNA after normalization with the TcRPS6 as an endogenous control 48 h post larval injection.
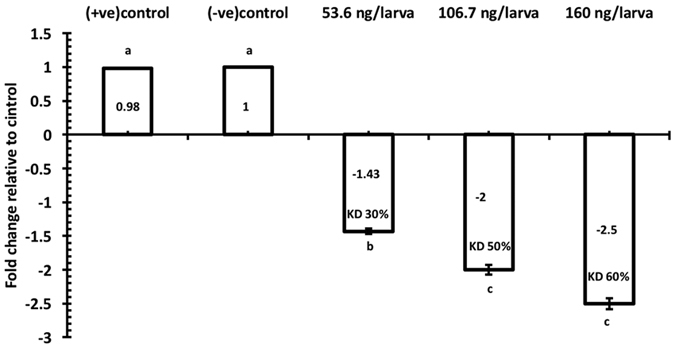
Mean ± SEM of three replications are shown. Means with the different letters are significantly different (p = 0.05; ANOVA; with Tukey Kramer Multiple Comparison). Knockdown of transcript level is indicated for each dose (KD%) with respect to the control group, +ve control = larvae injected with Kanamycin resistance dsRNA, −ve control = larvae injected with RNAase free water.
Figure 4. Transcript levels of TcNav mRNA after normalization with the TcRPS6 as an endogenous control 48 h post pupal injection.
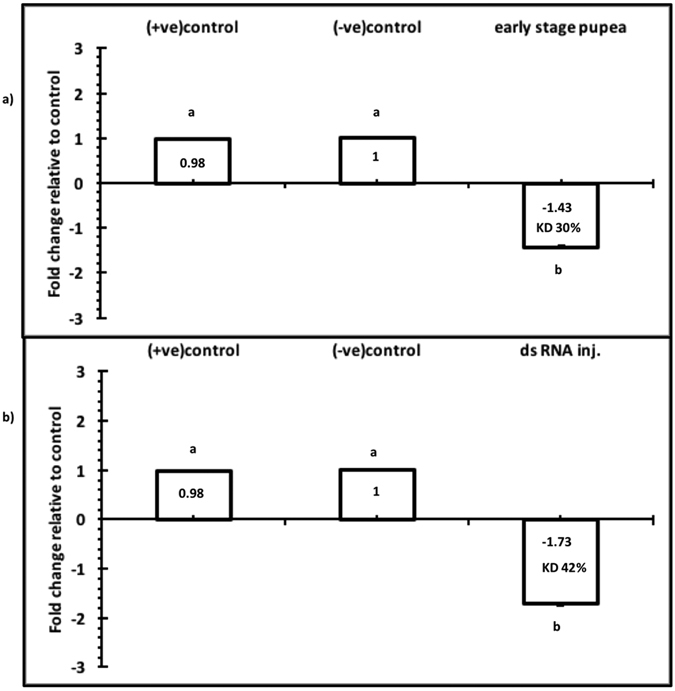
(a) Early stage pupae, (b) Late stage pupae. Mean ± SEM of three replications are shown. Means with the different letters are significantly different (p = 0.05; ANOVA; with Tukey Kramer Multiple Comparison). Knockdown of transcript level is indicated for each dose (KD%) with respect to the control group, +ve control = larvae injected with Kanamycin resistance dsRNA, −ve control = larvae injected with RNAase free water.
Oral delivery of dsRNA
Induction of RNA interference via oral delivery was carried out by restricted feeding of individual 6th instar larvae on flour disks containing the dsRNA at a range of different concentrations (0, 50, 100 and 150 ng/mg of diet). Relative to the control diet, which fed on flour disks containing kanamycin dsRNA, feeding of the dsRNA-containing diet for 6 days resulted in a significant (p < 0.05) impact on larval survival, with larval mortality of 19% ± 6.6, 37.83% ± 0.72 and 51.34% ± 6.8 respectively, for the 3 doses, 50, 100 and 150 ng/mg (Fig. 5a). Probit analysis showed this to be a linear dose response, with an LC50 of 150.23 ng dsRNA/mg of diet. As with the injection of dsRNA, oral delivery also resulted in a dose dependent response. At termination of the bioassay, reduction in adult emergence from insects reaching pupation correlated with the administered dose, with the higher doses causing greater reduction in development to adulthood, with reductions of 9.9% ± 13.2, 24.35% ± 6.2 and 51.26% ± 3.8 at 50, 100 and 150 ng/mg, respectively (Fig. 5b). Although the effects of the lowest dose were not significant (p > 0.05), the two higher doses did have significant (p < 0.05) effects on adult emergence.
Figure 5. Survival curves and bioassays of larvae fed on flour disks containing TcNav dsRNA over 6 days.
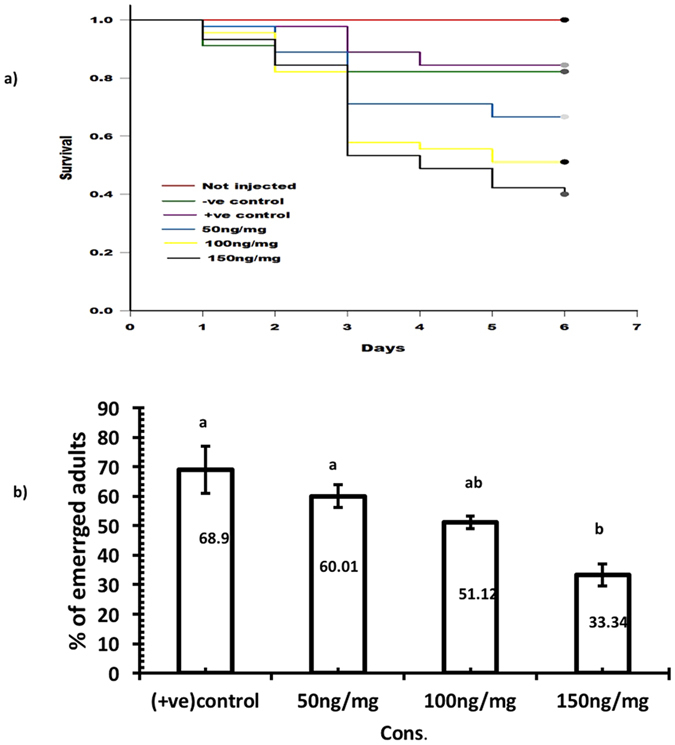
(a)Effect of dose on larval survival over time. (b) % of emerged adults from the different treatments above (p = 0.05; ANOVA; with Tukey Kramer Multiple Comparison), +ve control = larvae fed on Kanamycin resistance dsRNA flour disks, −ve control = larvae fed on RNAase free water flour disks.
Not only did oral delivery cause a reduction in larval survival, resulting in reduced adult emergence, there was also evidence of gene knockdown. Oral delivery of dsRNA caused significant reductions in transcript levels of TcNav. RT-qPCR analysis revealed that after 72 hours feeding by final (6th) instar larvae, transcript levels were significantly (p < 0.05) down regulated by 1.67 and 1.75-fold for the different treatments (100 and 150 ng dsRNA/mg diet) respectively, compared to the control group, which was not challenged with the TcNav dsRNA, but fed on kanamycin dsRNA flour disks (Fig. 6). However, oral delivery of dsRNA at 50 ng dsRNA/mg diet did not result in a significant decrease in gene TcNav gene expression (1.47-fold reduction, p > 0.05). Insects feeding on flour disks containing 150 ng/mg dsRNA towards the kanamycin gene did not show any significant reduction in expression of TcNav at any of the concentrations tested (0.83-fold of control, p > 0.05) compared to the group consuming control diet.
Figure 6. Transcript levels of TcNav mRNA after normalization with the TcRPS6 as an endogenous control 72 h post larval oral feeding.
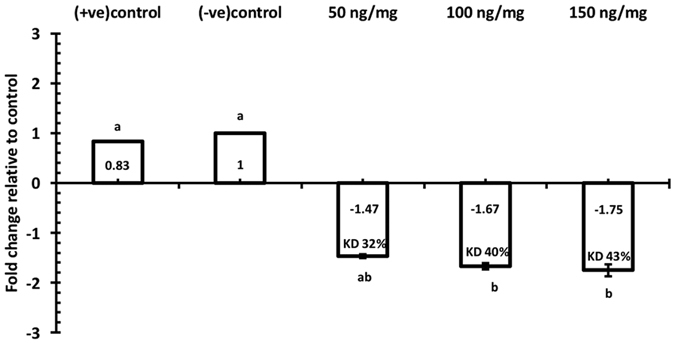
Mean ± SEM of three replications are shown. Means with the different letters are significantly different (p = 0.05; ANOVA; Tukey Kramer Multiple Comparison), +ve control = larvae fed on Kanamycin resistance dsRNA flour disks, −ve control= larvae fed on RNAase free water flour disks.
Discussion and Conclusions
The function of the voltage-gated sodium ion channels (VGSCs) is to initiate and then propagate action potentials in the nervous system and other excitable cells46. The transmembrane region S4 of each domain contains 3–5 positively charged residues that are essential for the channel to respond to changes in membrane potential. In response to membrane depolarization, electrostatic interactions between positively charged residues and the depolarized cytoplasm force the S4 region towards the extracellular surface causing a conformational change in S5-S6 that opens the channel pore and allows sodium influx47. These ion channels are the primary target for pyrethroid insecticides which act by preventing the closure of the VGSC. The pyrethroid group of insecticides has proven to be extremely successful in the broad spectrum control of common agricultural insect pest species. However target-site resistance to these insecticides has been demonstrated in several insect orders48 and is based on single point mutations within the DmNav orthologues7.
RNA interference for suppression of specific transcripts is proving to be a powerful tool in several insect species49, and, in contrast to many current chemical formulations, may provide a highly species specific control strategy. Hence, such an approach has the potential to significantly reduce non-target effects, including those on beneficial insects such as parasitoid wasps and pollinators50,51,52. T. castaneum has been shown to exhibit a robust systemic RNAi response in the postembryonic stages37,53,54, thus it has become a model insect in which RNAi proof of concept can be demonstrated. As far as the authors are aware, this is the first study to show RNAi-induced knockdown of VGSC gene expression in insects, resulting in a dose-dependent, fatal phenotype. In the present study RNAi was induced following administration of dsRNA corresponding to the DmNav orthologue from T. castaneum (TcNav, gene bank accession number NM_001165908.1 and TC004126 at Beetle-Base), with gene knockdown being observed following both injection into the insect haemocele and via oral uptake. Prior to delivery of the dsRNA it was first necessary to determine transcript abundances of this gene at different developmental stages to select for appropriate stages for study. Although not statistically significant, the results showed that transcript levels peaked during the late pupal stage followed by the adult and 6th instar larval stages. It is perhaps not surprising to find that TcNav transcripts were more abundant in late pupal stages, as it is during this time that axons and dendrites are remodelled to generate an adult-specific arbor during metamorphosis55 prior to adult eclosion. These results are consistent with those reported for two acetylcholinesterase genes (TcAce1 and TcAce2), where the transcript levels were more abundant in 6-day old pupae followed by 2-day old adult T. castaneum56. Furthermore, whilst transcript levels of three genes encoding diuretic hormones were present in all developmental stages of this insect, relative expression levels were similarly dependent upon the actual development stage57. In contrast, analysis of the developmental stage-specific expression pattern of a lethal giant larval gene (TcLg1) in T. castaneum showed constitutive expression30.
The injection of TcNav dsRNA into the haemocele of larval T. casteneum induced a quantifiable RNAi effect in a dose dependent manner resulting in reduction of gene expression after 2 days. Similar observations in T. castaneum have been reported by Lu et al.56 where transcript levels for two acetylcholinesterase genes (TcAce1 and TcAce2) were reduced by 92.3 and 95.2%, respectively, compared to the control 4 days post injection of corresponding dsRNA fragments. Similarly, studies with the crustacean cardioactive peptide (CCAP) showed that loss of CCAP activity can be induced through RNAi with dsRNA towards TcCcapr-2. Such gene knockdown resulted in no visible abnormal phenotype but demonstrated that TcCCAPR-2 is essential for cardioacceleratory activity58. Furthermore, Xiao et al.30 demonstrated that T. castaneum larvae injected with the double stranded RNA of TcLgl (lethal giant larvae) at each of the three doses (100, 200, and 400 ng/larva) resulted in significant and dose dependant suppression of TcLgl transcripts 2 days post injection.
As with larvae, suppression of TcNav expression levels were also observed both in the early and late pupal stages, again showing consistency with those reported by30 where injection of 1-day old pupae with dsTcLgl, caused significant suppression of the corresponding transcript on days 2, 4, 6 and 8. Furthermore, studies demonstrated that freshly moulted pupae of T. castaneum injected with dsRNA led to a specific knockdown of the respective neuropeptides genes and that this dsRNA-mediated silencing caused adult loss-of-function phenotypes59.
The present study demonstrates, irrespective of the method of delivery, significant and severe developmental arrest (in terms of larval mortalities and reduced pupation) following knock-down of the VGSC, the severity being dependant on the dose administered. Injection of dsRNA into Locusta migratoria showed that locusts also have a sensitive and systemic dose-dependent RNAi response60. Similar responses to oral delivery of dsRNA have also been reported in T. castaneum30,42,56,58.
Data presented by Clemens and Elia, Oates et al. and Geiss et al.61,62,63 confirmed that the interferon-regulated innate immunity pathway that protects vertebrates from invasion of long dsRNA is absent in insects. It is thus feasible and preferable to deliver long dsRNAs to insects to ensure a maximal RNAi effect. Furthermore, Whyard et al.50 observed that it is possible to deliver relatively short (29–40 nt) dsRNAs designed towards insect vATPase transcripts whilst maintaining levels of induced mortality. This observation will prove advantageous if RNAi is to be developed as a viable tool in crop protection. Several T. castaneum genes such as heat shock protein 90, chitin synthase, the segmentation gene hairy, and a matrix metalloprotease have been showed to be down regulated by RNAi, and therefore may represent good candidate genes to target for future RNAi-based crop protection strategies. The high degree of specificity of the dsRNA molecules used in the present study towards their individual targets, as shown by the MegaBLAST homology searches, lend support for the use of this technology in crop protection. Use of this novel technology is further supported by the lack of any appreciable dsRNA uptake from the diets of higher animals. Therefore, dietary dsRNA is not anticipated to represent any hazard or risk to humans, mammals, or other vertebrates64,65. For these reasons transgenic crops expressing dsRNA are currently under investigation as a new tool for targeted and specific protection of crops from insect pests28,66,67,68,69,70.
The findings of the present study provide the first demonstration of the silencing of a voltage-gated sodium ion channel gene (TcNav) via RNAi in T. Castaneun. It provides evidence to suggest that this gene is a feasible candidate to target for the control of this insect pest using RNAi. Furthermore, the findings presented here support the temporal expression of the genes during development, which will help in targeting the key developmental stage if this technology is to be used in crop protection.
Additional Information
How to cite this article: Abd El Halim, H. M. et al. RNAi-mediated knockdown of the voltage gated sodium ion channel TcNav causes mortality in Tribolium castaneum. Sci. Rep. 6, 29301; doi: 10.1038/srep29301 (2016).
Acknowledgments
The authors are grateful to Gillian Davison, Aishah Alatawi and Francesca Laudani for their help in the lab. This research was funded by the Cultural Affairs & Mission Sector, Ministry of Higher Education, Egypt through a scholarship awarded to Hesham Mohamed Abd El Halim. Munawar S. Ahmad was supported through a Commonwealth Fellowship.
Footnotes
Author Contributions H.M.A.E.H., M.G.E., E.Y.T.N. and A.M.R.G. designed the overall experiment. H.M.A.E.H., M.G.E. and M.S.A. designed the dsRNA fragment and performed the molecular cloning from T. castaneum. H.M.A.E.H., M.G.E., E.Y.T.N. and M.S.A. performed the insect injections and the RT-qPCR. B.M.H.A. performed the additional bioassays and expression analysis requested after initial review. H.M.A.E.H., M.G.E. and A.M.R.G. the interpreted of results. H.M.A.E.H., M.H.A. and E.M.S. wrote the manuscript. A.M.R.G. and M.G.E. corrected the manuscript for final submission. All of the authors reviewed and contributed to the manuscript.
References
- Nauen R., Stumpf N. & Elbert A. Toxicological and mechanistic studies on neonicotinoid cross resistance in Q-type Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 58, 868–875, doi: 10.1002/ps.557 (2002). [DOI] [PubMed] [Google Scholar]
- Aktar W., Sengupta D. & Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdisciplinary toxicology 2, 1–12 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware G. W. W. D. M. The pesticide book. (Meister Media Worldwide, 2004). [Google Scholar]
- Defra. Food 2030. Department for Environment, Food and Rural Affairs. P84 (2010).
- Yu F. H. & Catterall W. A. Overview of the voltage-gated sodium channel family. Genome biology 4, 207 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich F. D., Du Y. & Dong K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pesticide Biochemistry and Physiology 106, 93–100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K. et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol 50, 1–17, doi: 10.1016/j.ibmb.2014.03.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. et al. Voltage-gated ion channels and gating modifier toxins. Toxicon 49, 124–141, doi: 10.1016/j.toxicon.2006.09.022 (2007). [DOI] [PubMed] [Google Scholar]
- Davies T. G. E., Field L. M., Usherwood P. N. R. & Williamson M. S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 59, 151–162, doi: 10.1080/15216540701352042 (2007). [DOI] [PubMed] [Google Scholar]
- Loughney K., Kreber R. & Ganetzky B. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 58, 1143–1154 (1989). [DOI] [PubMed] [Google Scholar]
- Zhou W., Chung I. B., Liu Z. Q., Goldin A. L. & Dong K. A voltage-gated calcium-selective channel encoded by a sodium channel-like gene. Neuron 42, 101–112, doi: 10.1016/s0896-6273(04)00148-5 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M. & Tanouye M. A. Two sodium-channel genes in Drosophila: implications for channel diversity. Proceedings of the National Academy of Sciences of the United States of America 86, 2079–2082, doi: 10.1073/pnas.86.6.2079 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackeray J. R. & Ganetzky B. Conserved alternative splicing patterns and splicing signals in the Drosophila sodium channel gene para. Genetics 141, 203–214 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan C. J., Palladino M. J., Ganetzky B. & Reenan R. A. RNA editing of the Drosophila para Na(+) channel transcript. Evolutionary conservation and developmental regulation. Genetics 155, 1149–1160 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K. Insect sodium channels and insecticide resistance. Invertebrate neuroscience: IN 7, 17–30, doi: 10.1007/s10158-006-0036-9 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur E. et al. NMR analysis of interaction of LqhalphaIT scorpion toxin with a peptide corresponding to the D4/S3-S4 loop of insect para voltage-gated sodium channel. Biochemistry 47, 911–921, doi: 10.1021/bi701323k (2008). [DOI] [PubMed] [Google Scholar]
- Devine G. & Furlong M. Insecticide use: Contexts and ecological consequences. Agric Hum Values 24, 281–306, doi: 10.1007/s10460-007-9067-z (2007). [DOI] [Google Scholar]
- Zlotkin E. The insect voltage-gated sodium channel as target of insecticides. Annual review of entomology 44, 429–455, doi: 10.1146/annurev.ento.44.1.429 (1999). [DOI] [PubMed] [Google Scholar]
- Miller S. C., Miyata K., Brown S. J. & Tomoyasu Y. Dissecting Systemic RNA Interference in the Red Flour Beetle Tribolium castaneum: Parameters Affecting the Efficiency of RNAi. PLoS ONE 7, e47431, doi: 10.1371/journal.pone.0047431 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. R. G. & Gatehouse J. A. RNAi-mediated crop protection against insects. Trends in Biotechnology 26, 393–400, doi: 10.1016/j.tibtech.2008.04.004 (2008). [DOI] [PubMed] [Google Scholar]
- Fire A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811, doi: 10.1038/35888 (1998). [DOI] [PubMed] [Google Scholar]
- Cogoni C. et al. Transgene silencing of the al-1 gene in vegetative cells of Neurospora is mediated by a cytoplasmic effector and does not depend on DNA-DNA interactions or DNA methylation. The EMBO journal 15, 3153–3163 (1996). [PMC free article] [PubMed] [Google Scholar]
- Kennerdell J. R. & Carthew R. W. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 95, 1017–1026 (1998). [DOI] [PubMed] [Google Scholar]
- Kennerdell J. R. & Carthew R. W. Heritable gene silencing in Drosophila using double-stranded RNA. Nat Biotechnol 18, 896–898, doi: 10.1038/78531 (2000). [DOI] [PubMed] [Google Scholar]
- Svoboda P., Stein P., Hayashi H. & Schultz R. M. Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development (Cambridge, England) 127, 4147–4156 (2000). [DOI] [PubMed] [Google Scholar]
- Wianny F. & Zernicka-Goetz M. Specific interference with gene function by double-stranded RNA in early mouse development. Nature cell biology 2, 70–75, doi: 10.1038/35000016 (2000). [DOI] [PubMed] [Google Scholar]
- Billy E., Brondani V., Zhang H., Muller U. & Filipowicz W. Specific interference with gene expression induced by long, double-stranded RNA in mouse embryonal teratocarcinoma cell lines. Proc Natl Acad Sci USA 98, 14428–14433, doi: 10.1073/pnas.261562698 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Li H. C. & Miao X. X. Feasibility, limitation and possible solutions of RNAi-based technology for insect pest control. Insect science 20, 15–30, doi: 10.1111/j.1744-7917.2012.01513.x (2013). [DOI] [PubMed] [Google Scholar]
- Wuriyanghan H., Rosa C. & Falk B. W. Oral Delivery of Double-Stranded RNAs and siRNAs Induces RNAi Effects in the Potato/Tomato Psyllid, Bactericerca cockerelli. PLoS ONE 6, e27736, doi: 10.1371/journal.pone.0027736 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D., Liang X., Gao X., Yao J. & Zhu K. The lethal giant larvae Gene in Tribolium castaneum: Molecular Properties and Roles in Larval and Pupal Development as Revealed by RNA Interference. International Journal of Molecular Sciences 15, 6880–6896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich J. Application of RNA Interference for the Study of Lethal Genes and Dynamic Processes. (2016).
- Phillips T. W. & Throne J. E. Biorational approaches to managing stored-product insects. Annual review of entomology 55, 375–397, doi: 10.1146/annurev.ento.54.110807.090451 (2010). [DOI] [PubMed] [Google Scholar]
- Morris K. et al. Tribolium castaneum larval gut transcriptome and proteome: A resource for the study of the coleopteran gut. J Proteome Res 8, 3889–3898, doi: 10.1021/pr900168z (2009). [DOI] [PubMed] [Google Scholar]
- Horn T. & Boutros M. E-RNAi: a web application for the multi-species design of RNAi reagents–2010 update. Nucleic Acids Res 38, W332–W339, doi: 10.1093/nar/gkq317 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapountzis P. et al. New insight into the RNA interference response against cathepsin-L gene in the pea aphid, Acyrthosiphon pisum: Molting or gut phenotypes specifically induced by injection or feeding treatments. Insect Biochemistry and Molecular Biology 51, 20–32, doi: 10.1016/j.ibmb.2014.05.005 (2014). [DOI] [PubMed] [Google Scholar]
- Sambrook J. & Russel D. W. Molecular cloning : a laboratory manual. 3rd ed. edn, (Cold Spring Harbor Laboratory Press, 2001). [Google Scholar]
- Tomoyasu Y. & Denell R. E. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol 214, 575–578, doi: 10.1007/s00427-004-0434-0 (2004). [DOI] [PubMed] [Google Scholar]
- Philip B. & Tomoyasu Y. In Molecular Methods for Evolutionary Genetics Vol. 772 Methods in Molecular Biology (eds Orgogozo Virginie & Rockman Matthew V.) Ch. 28, 471–497 (Humana Press, 2011). [DOI] [PubMed] [Google Scholar]
- Rinkevich F. D. & Scott J. G. Limitations of RNAi of α6 nicotinic acetylcholine receptor subunits for assessing the in vivo sensitivity to spinosad. Insect science 20, 101–108, doi: 10.1111/j.1744-7917.2012.01523.x (2013). [DOI] [PubMed] [Google Scholar]
- Xie Y. S., Bodnaryk R. P. & Fields P. G. A rapid and simple flour-disk bioassay for testing substances active against stored-product insects. The Canadian Entomologist 128, 865–875, doi: 10.4039/Ent128865-5 (1996). [DOI] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 25, 402–408, doi: 10.1006/meth.2001.1262 (2001). [DOI] [PubMed] [Google Scholar]
- Broehan G., Kroeger T., Lorenzen M. & Merzendorfer H. Functional analysis of the ATP-binding cassette (ABC) transporter gene family of Tribolium castaneum. BMC Genomics 14, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott W. S. A method of computing the effectiveness of an insecticide. 1925. Journal of the American Mosquito Control Association 3, 302–303 (1987). [PubMed] [Google Scholar]
- Khazanie R. Elementary statistics. 488 pp (Good year publishing, 1979). [Google Scholar]
- Jahan N., Razaq J. & Jan A. Laboratory evaluation of chitin synthesis inhibitors (Diflubenzuron and Buprofezin) against Aedes aegypti larvae from Lahore, Pakistan. Pakistan J. Zool 43, 1079–1084 (2011). [Google Scholar]
- Du Y. et al. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proceedings of the National Academy of Sciences 110, 11785–11790, doi: 10.1073/pnas.1305118110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarov-Yarovoy V. et al. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proceedings of the National Academy of Sciences 109, E93–E102, doi: 10.1073/pnas.1118434109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund D. M. In Comprehensive Molecular Insect Science (ed Gilbert Lawrence I.) 1–24 (Elsevier, 2005). [Google Scholar]
- Park Y. Endocrine regulation of insect diuresis in the early postgenomic era1. Canadian Journal of Zoology 90, 507–520, doi: 10.1139/z2012-013 (2012). [DOI] [Google Scholar]
- Whyard S., Singh A. D. & Wong S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochemistry and Molecular Biology 39, 824–832, doi: : 10.1016/j.ibmb.2009.09.007 (2009). [DOI] [PubMed] [Google Scholar]
- Porta H. et al. Tobacco plants expressing the Cry1AbMod toxin suppress tolerance to Cry1Ab toxin of Manduca sexta cadherin-silenced larvae. Insect Biochem Mol Biol 41, 513–519, doi: 10.1016/j.ibmb.2011.04.013 (2011). [DOI] [PubMed] [Google Scholar]
- Gong L., Yang X., Zhang B., Zhong G. & Hu M. Silencing of Rieske iron-sulfur protein using chemically synthesised siRNA as a potential biopesticide against Plutella xylostella. Pest Manag Sci 67, 514–520, doi: 10.1002/ps.2086 (2011). [DOI] [PubMed] [Google Scholar]
- Park Y. & Beeman R. W. Postgenomics of Tribolium: Targeting the endocrine regulation of diuresis. Entomological Research 38, 93–100, doi: 10.1111/j.1748-5967.2008.00143.x (2008). [DOI] [Google Scholar]
- Tomoyasu Y. et al. Exploring systemic RNA interference in insects: a genome-wide survey for RNAi genes in Tribolium. Genome biology 9, R10, doi: 10.1186/gb-2008-9-1-r10 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger A. & Dura J.-M. Nuclear receptors and Drosophila neuronal remodeling. Biochimica Et Biophysica Acta-Gene Regulatory Mechanisms 1849, 187–195, doi: 10.1016/j.bbagrm.2014.05.024 (2015). [DOI] [PubMed] [Google Scholar]
- Lu Y. et al. Cholinergic and non-cholinergic functions of two acetylcholinesterase genes revealed by gene-silencing in Tribolium castaneum. Sci. Rep. 2, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme L. V. Diuretic hormones of Tribolium castaneum (Herbst)(Coleoptera: tenebrionidae), Kansas State University, (2009).
- Li B., Beeman R. W. & Park Y. Functions of duplicated genes encoding CCAP receptors in the red flour beetle, Tribolium castaneum. Journal of Insect Physiology 57, 1190–1197, doi: 10.1016/j.jinsphys.2011.05.011 (2011). [DOI] [PubMed] [Google Scholar]
- Arakane Y. et al. Functional analysis of four neuropeptides, EH, ETH, CCAP and bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum. Mechanisms of Development 125, 984–995 (2008). [DOI] [PubMed] [Google Scholar]
- Park Y. Endocrine regulation of insect diuresis in the early postgenomic era1This review is part of a virtual symposium on recent advances in understanding a variety of complex regulatory processes in insect physiology and endocrinology, including development, metabolism, cold hardiness, food intake and digestion, and diuresis, through the use of omics technologies in the postgenomic era. Canadian Journal of Zoology 90, 507–520, doi: 10.1139/z2012-013 (2012). [DOI] [Google Scholar]
- Clemens M. J. & Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 17, 503–524 (1997). [DOI] [PubMed] [Google Scholar]
- Oates A. C., Bruce A. E. & Ho R. K. Too much interference: injection of double-stranded RNA has nonspecific effects in the zebrafish embryo. Developmental biology 224, 20–28, doi: 10.1006/dbio.2000.9761 (2000). [DOI] [PubMed] [Google Scholar]
- Geiss G. et al. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J Biol Chem 276, 30178–30182 (2001). [DOI] [PubMed] [Google Scholar]
- Baum J. A. & Roberts J. K. Progress Towards RNAi-Mediated Insect Pest Management. Insect Midgut and Insecticidal Proteins 47, 249–295 (2014). [Google Scholar]
- Petrick J. S. et al. A 28-day oral toxicity evaluation of small interfering RNAs and a long double-stranded RNA targeting vacuolar ATPase in mice. Regulatory Toxicology and Pharmacology 71, 8–23, doi: 10.1016/j.yrtph.2014.10.016 (2015). [DOI] [PubMed] [Google Scholar]
- Gordon K. H. & Waterhouse P. M. RNAi for insect-proof plants. Nat Biotechnol 25, 1231–1232, doi: 10.1038/nbt1107-1231 (2007). [DOI] [PubMed] [Google Scholar]
- Baum J. A. et al. Control of coleopteran insect pests through RNA interference. Nat Biotech 25, 1322–1326, doi: 10.1038/nbt1359 (2007). [DOI] [PubMed] [Google Scholar]
- Gatehouse A. M. R., Ferry N., Edwards M. G. & Bell H. A. Insect-resistant biotech crops and their impacts on beneficial arthropods. Philosophical Transactions of the Royal Society B: Biological Sciences 366, 1438–1452, doi: 10.1098/rstb.2010.0330 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abhinav K. RNAi: A Promising Approach to Develop Transgenic Plants Against Geminiviruses and Insects. Journal of Plant Physiology & Pathology (2013). [Google Scholar]
- Zhang J. et al. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. science 347, 991–994 (2015). [DOI] [PubMed] [Google Scholar]


