Abstract
PAIRED (PRD)-like homeobox genes belong to a class of predicted transcription factor genes. Several of these PRD-like homeobox genes have been predicted in silico from genomic sequence but until recently had no evidence of transcript expression. We found recently that nine PRD-like homeobox genes, ARGFX, CPHX1, CPHX2, DPRX, DUXA, DUXB, NOBOX, TPRX1 and TPRX2, were expressed in human preimplantation embryos. In the current study we characterized these PRD-like homeobox genes in depth and studied their functions as transcription factors. We cloned multiple transcript variants from human embryos and showed that the expression of these genes is specific to embryos and pluripotent stem cells. Overexpression of the genes in human embryonic stem cells confirmed their roles as transcription factors as either activators (CPHX1, CPHX2, ARGFX) or repressors (DPRX, DUXA, TPRX2) with distinct targets that could be explained by the amino acid sequence in homeodomain. Some PRD-like homeodomain transcription factors had high concordance of target genes and showed enrichment for both developmentally important gene sets and a 36 bp DNA recognition motif implicated in Embryo Genome Activation (EGA). Our data implicate a role for these previously uncharacterized PRD-like homeodomain proteins in the regulation of human embryo genome activation and preimplantation embryo development.
Human preimplantation development, starting with the fusion of egg and sperm and continuing to blastocyst implantation1 is characterized by drastic changes in gene expression, especially a massive degradation of oocyte transcripts and a gradual, cascade-like initiation of transcription from the embryo genome2,3. The cellular factors responsible for the Embryo Genome Activation (EGA) have not been fully characterized. However, recently the PRD-like homeodomain transcription factors were implicated in human EGA and preimplantation development3.
The PRD-like homeobox genes form one of several classes of homeobox genes4. Homeobox genes are evolutionarily conserved and the majority of these genes are critical for the regulation of development5. Examples of homeobox genes include the HOX cluster genes6 that are known to play key roles in cell fate determination and cell differentiation. Homeobox genes are relatively well characterized in several model organisms, especially in Drosophila melanogaster, and several of the PRD-like homeobox genes have previously been suggested to be expressed in the human germline7. The PRD-like homeobox genes share a similar homeodomain, but they lack the actual PRD domain4. The homeodomain consists of approximately 60 amino acid (aa) residues and folds into a N-terminal arm and three alpha-helices8. The N-terminal arm and the third helix have been implicated in DNA-binding specificity and DNA-sequence recognition9.
Our recent study on human preimplantation development revealed the expression of 14 PRD-like homeodomain transcription factors during EGA, and we validated the presence of seven factors by cDNA cloning3. Most of these transcription factors had been predicted7,10, but only a few had been validated on transcript level11.
Here we describe the molecular cloning of several new splice variants for altogether nine human PRD-like homeobox genes, ARGFX (arginine-fifty homeobox; altogether six splice variants), CPHX1 (cytoplasmic polyadenylated homeobox 1), CPHX2 (cytoplasmic polyadenylated homeobox 2; three splice variants), DPRX (divergent paired-related homeobox; two splice variants), DUXA (double homeobox A), DUXB (double homeobox B; three splice variants), TPRX1 (tetra-peptide repeat homeobox 1), TPRX2 (tetra-peptide repeat homeobox 2; three splice variants) and NOBOX (NOBOX oogenesis homeobox). We show the first evidence of TPRX2P expression and propose that it should be renamed TPRX2 as previously suggested7. Many of these PRD-like homeobox genes appeared primate specific, expressed at very low levels in most tissues and restricted to preimplantation development. Experimental overexpression of the PRD-like homeodomain transcription factors in human embryonic stem cells (hESCs) reveals target genes that intersect with a significant number of the genes first activated in human embryos. The target gene promoters are enriched for the 36 bp motif previously predicted to play a major role in human EGA3.
Results
Cloning of PRD-like homeobox genes from human 8-cell stage embryos
Our previous transcription start site (TSS) enriched data on human preimplantation embryos suggested unannotated transcripts for ARGFX, CPHX1, CPHX2, DPRX, DUXA and DUXB3. Furthermore, two highly similar Tprx family genes TPRX1 and TPRX2 were predicted. We described previously the cDNA cloning of a major splice form for ARGFX, CPHX1, CPHX2, DPRX, DUXA and DUXB3. In the present study, we cloned novel splice variants of NOBOX, TPRX1 and TPRX2 together with additional splice variants for the ARGFX, CPHX2, DPRX and DUXB genes from human 8-cell embryos.
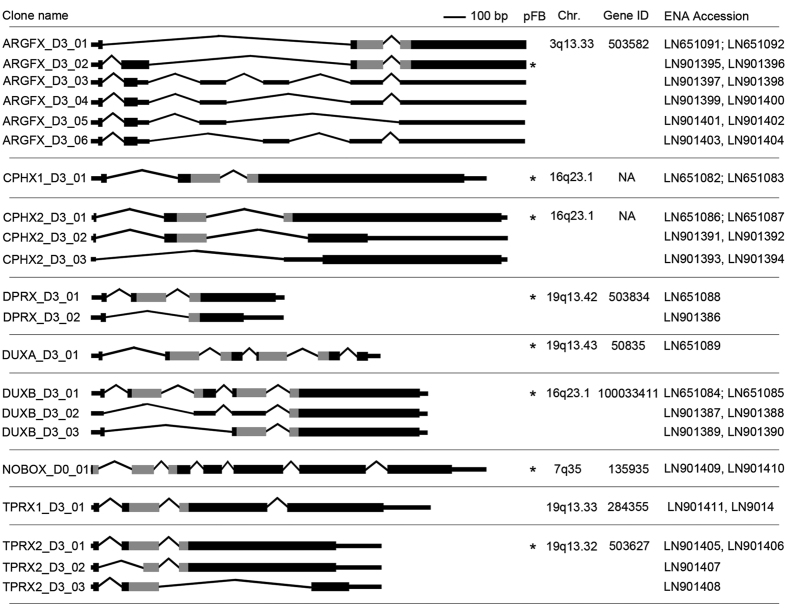
We amplified and cloned transcripts for each gene by PCR from non-sheared full-length cDNA libraries of three whole 8-cell embryos prepared according to the sequencing library preparation protocol by Tang et al.12. The validated transcripts are visualized in Fig. 1 and in UCSC Genome Browser views in Supplemental Figure S1 together with previously predicted gene sequences and the cloning primer locations. Forward primers were designed on the TSS sequences, and reverse primers by predicted gene structures and low level downstream reads (exon painting). The full gene names, references, identifiers, cloning primers and clone origins are shown in Table 1. The sequences for ARGFX, DPRX, DUXA and DUXB had been previously suggested7,10, but only ARGFX had been confirmed experimentally11. NOBOX was amplified from cDNA that was reverse transcribed from commercially available poly-A enriched human ovary RNA. The partial transcript sequence for NOBOX had been previously validated by cDNA cloning13, and two additional synthetic constructs are presented in GenBank (BC152834 and AB528776). Current RefSeq annotation follows the structure of the synthetic constructs for exons 1–8 (Supplementary Figure S2). However, our data suggested a novel intronic TSS resulting in a novel transcript with novel first exon, lacking exons number 1–4 and a shortened exon 5 compared to the RefSeq variant (Supplementary Figure S2). The longer exon 5 of the RefSeq variant disrupts the homeodomain, which is encoded by exons 1–3 of our new transcript variant. Our predicted homeodomain structure, however, corresponded to that previously presented13.
Figure 1. Exon-intron structure of PRD-like homeobox genes.
Exons are drawn to scale and represented by horizontal boxes. 5′ and 3′ UTRs are shown as thinner boxes, protein coding region as thicker boxes with homeobox sequence colored grey. Introns are represented by solid lines. Chromosomal locations and Gene IDs, if available, are given on the right. *Indicates the isoform used in the overexpression experiment. NA, not available.
Table 1. Cloning of PRD-like homeobox genes.
| Gene name | Full name & first reference | Gene ID | TFE ID* | TFE Region | Strand | Forward/Reverse Primer | Clone origin |
|---|---|---|---|---|---|---|---|
| ARGFX | arginine-fifty homeobox7 | 503582 | FE367949 | chr3:121286750–121286860 | + | GAGAGACACACCACGTAGGAC/TCAGAGAAATCCCAAGTCTACC | 8-cell embryo |
| CPHX1 | cytoplasmic poyadenylated homeobox 114 | FE200101 | chr16:75760315–75760435 | − | TCTCAGTTGCTTGCTGGTCTC/CCTGACCTGCGACTGTGTTT | 8-cell embryo | |
| CPHX2 | cytoplasmic poyadenylated homeobox 214 | FE200054 | chr16:75710885–75711065 | − | GAGTTTCGACATGTCTTCCCAAG/TGACCTGTGGCTATGGTTCTGT | 8-cell embryo | |
| DPRX | divergent paired-related homeobox7 | 503834 | FE273734 | chr19:54135194–54135433 | + | TATCCCTGGACCTGAACCCA/ACACATCATTACAACATGTGACT | 8-cell embryo |
| DUXA | double homeobox A7 | 503835 | FE262918 | chr19:57678787–57678897 | − | GCTCAGCCTTCAGGACTCTCT/GTGAGACAGATTTGGGGTCCA | 8-cell embryo |
| DUXB | double homeobox B10 | 100033411 | FE200082 | chr16:75735289–75735420 | − | TTACTCGCTGATCTCCGTGG/TCAGCTGAGTGTGCCTACTG | 8-cell embryo |
| NOBOX | NOBOX oogenesis homeobox13,27 | 135935 | FE518599 | chr7:144100745–144100865 | − | ATGGAACCCACAGAGAATCC/ACAAGACGAGCCTACACAGG | Ovary |
| TPRX1 | Tetra-peptide repeat homeobox 17 | 284355 | chr19:48307780–48307850 | − | TCAGGACTCAGGATGCAAGAC/ATCCCAGCAGAGAAACGCTC | 8-cell embryo | |
| TPRX2 | tetra-peptide repeat homeobox 27 | 503627 | chr19:48362000–48363000 | + | TCAGGACTCAGGATGCAAGAC/ATCCCAGCAGAGAAACGCTC | 8-cell embryo |
*TFE, Transcript far 5′ end, TFE ID refers to TSS data by Töhönen&Katayama, et al.13.
Three genes, CPHX1, DUXB and CPHX2 form a gene cluster on 16q23.1 within a 68 kb segment on the same strand (chr16:75,693,001–75,761,000) (Supplemental Figure S3). These genes are currently lacking genome annotation, even though they have been predicted in human based on syntenic genomic location between human and mouse14. The alignment of amino acids between human CHPX1 and CPHX2 confirms high sequence conservation also outside the homeodomain (Supplemental Figure S4a).
CPHX2 amplification produced three different splice variants, two of which contained exons 1, 2, and variably exon 3, and one splice variant lacking exon 2. Different open reading frames (ORF) were predicted for each three splice variants, with only one having a complete homeodomain (Fig. 1).
The PCR amplification using the TPRX1 (Gene ID: 284355) TSS as a primer led to the discovery of TPRX1 itself from one 8-cell embryo, but in addition three additional splice variants of TPRX2P from three independent 8-cell embryos. TPRX2P is closely related to TPRX1 located 18.3 kb away at chr19:48,307,772–48,307,835. TPRX2P was previously annotated as a pseudogene but proposed to be named TPRX2 if shown to be expressed7. Therefore, we suggest to rename TPRX2P as TPRX2. All TPRX2 variants included three exons of variable lengths, but only the longest splice variant encoded a full homeodomain (Fig. 1). The amino acid sequence alignment between TPRX1 and TPRX2 showed high similarity of the first 140 amino acids including the homeodomain (Supplementary Figure S4b).
The PCR amplification revealed a single splice variant for CPHX1, DUXA and NOBOX, all encoding for at least one full homeodomain. Only one of the two validated DPRX transcripts encoded for full homeodomain. ARGFX amplification gave two splice variants with the predicted ORF consistent with gene model and four additional splice variants (Fig. 1). Amplification of DUXB resulted in three amplicons of which only the longest was predicted to contain both homeodomains.
The most likely functional splice variant containing the full-length homeodomain sequence and observed in all three independent 8-cell embryo libraries is marked in Fig. 1 with an asterisk. These isoforms were subsequently used in the hESC overexpression experiment. The genomic locations of the PRD-like homeobox genes are shown in Supplementary Figure S5.
Expression of PRD-like homeobox genes in early embryos and pluripotent stem cells
To investigate the developmental specificity of PRD-like homeobox gene expression, we used single cell RNA sequencing (RNAseq) on single blastomeres from two 8-cell stage embryos and two different hESC lines15 and compared the expression patterns (Fig. 2).
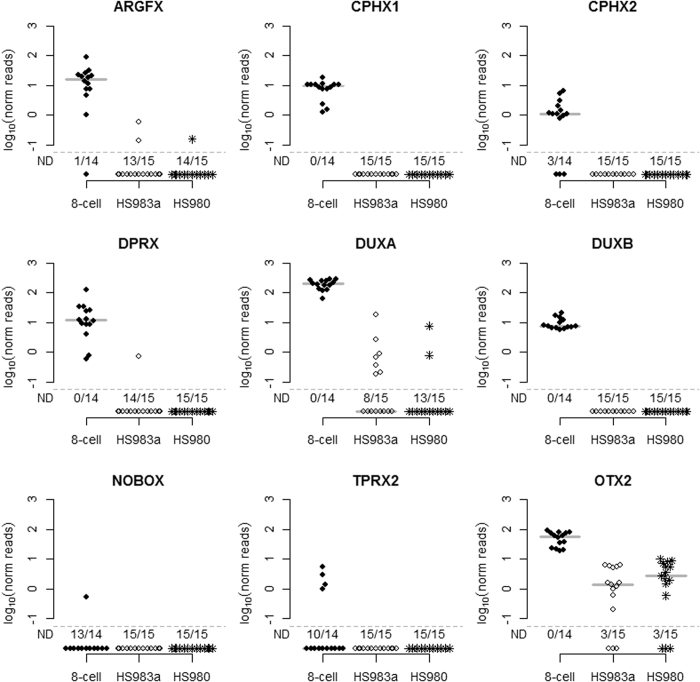
Figure 2. Expression of PRD-like homeobox genes in 8-cell blastomeres and hESCs.
STRT RNAseq expression values are shown for two 8-cell stage blastomeres (14 single cells in total) and for two different hESCs (HS980 and HS983a, 15 single cells each) at single cell resolution. Each dot indicates a single cell: 8-cell (filled circle), hES cell line HS983a (not filled circle) or hES cell HS980 (star). Y-axis shows log10 transformed spike-in normalized expression values. Vertical line indicates mean values. ND, not detected, indicates the number of cells in which the gene of interest was not detectable over the total number of cells analyzed with the threshold of one sequencing read per cell.
In concordance with our previous results3, we detected the expression of ARGFX, CPHX1, CPHX2, DPRX, DUXA, DUXB, NOBOX, TPRX2 and OTX2 in single blastomeres from 8-cell stage embryos. OTX2 was included as a positive control gene representing a previously characterized PRD-like homeobox gene. CPHX1, DPRX, DUXA, DUXB, and OTX2 expression was detected in all blastomeres with highest expression levels for DUXA and OTX2. ARGFX and CPHX2 expression was detected in the majority of blastomeres. NOBOX and TPRX2 expression was detected in one and four single blastomeres, respectively. The largest variation in expression levels among the different blastomeres was detected for ARGFX and DPRX, whereas DUXA and DUXB expression was at similar levels for all blastomeres.
In contrast to the expression of PRD-like homeobox genes in blastomeres, the expression in hESCs was much lower. Thus, CPHX1, CPHX2, DUXB, NOBOX and TPRX2 expression was not detected in any of the two different hESC lines (Fig. 2). Both ARGFX and DPRX expression was detected in only three or one single cell, respectively. Only DUXA and OTX2 were readily detectable in the hESCs, albeit only in a subset of the cells. DUXA was detected in seven out of 15 HS983a cells and in two out of 15 HS980 cells. The positive control OTX2 was detected in 12 out of 15 cells from both hESC lines. The expression levels detected in the hESCs were lower than that for human single blastomeres for all PRD-like homeobox genes detected.
In order to independently confirm the developmental pattern of PRD-like homeobox gene expression, we applied PCR on an additional set of cDNA samples, including HS980 and whole 8-cell stage embryo libraries. In this manner, we detected only low levels of expression of ARGFX, CPHX1, DPRX, DUXA and DUXB in three hESC lines, including HS980 (Supplementary Figure S6).
The majority of cell types and tissues lack PRD-like homeobox gene expression
To investigate mRNA expression of the PRD-like homeobox genes in different cell types and tissues, we used the FANTOM5 database16,17. Only OTX2 was shown to be expressed in the FANTOM5 database, whereas all the new genes were barely detectable in very few of the 1829 samples (Supplemental Table S1). Specifically, none of the new transcripts had an annotated transcriptional start site (TSS) in FANTOM5 data. Only one unannotated promoter corresponded to one of our genes, namely the DUXB TSS, but it lacked correct annotation due to the fact that there was no annotation for DUXB itself in FANTOM5.
The lack of expression in FANTOM5 data suggested possible silencing of the genes in later stages of development. To investigate methylation as a possible silencing mechanism of the PRD-like homeobox genes, we considered the DNA methylation status of their promoters in human sperm, preimplantation embryos and embryonic tissues18. Percentage of methylated CpG-s from around 1000 bp from the homeobox genes TSS was plotted for all the samples and replicas with sequencing coverage of at least 5x (Supplementary Figure S7). The results show hypomethylation for all the detected homeobox genes in preimplantation embryos compared to various ESC-s and embryonic tissues. This suggests a rapid epigenetic silencing mechanism after the preimplantation development. The more widely expressed control gene OTX2 however was not similarly methylated in course of development. Additionally we assessed the methylation status of these genes in blood cells19. The results show that in blood cells these genes were highly methylated, supporting the mechanism of epigenetic silencing in adult tissues (Supplementary Figure S8).
Overexpression of the PRD-like homeobox genes in hESC identifies target genes
We overexpressed the PRD-like homeobox genes in hESC to identify their target genes. The pFastBac vector used contained both the gene of interest and an eGFP fluorescent marker, enabling separation of transfected cells from non-transfected cells by Fluorescence Activated Cell Sorting (FACS). We could then verify the co-expression of the two proteins in the same cells by the simultaneous expression of eGFP and the red fluorescence gene mCherry control both by microscopy (Supplementary Figure S9a) and by FACS analysis (Supplementary Figure S9b). Successful sorting of the transfected versus non-transfected cells was further confirmed by mapping the sequencing reads to the original pFastBac vector backbone. The vast majority of reads from the GFP positive samples originated from the overexpression vector, while there were very few reads detected in the GFP negative samples (Supplementary Figure S9c).
After quality control and the exclusion of one disqualified GFP negative control sample, we performed the differential expression analysis as previously described20. We listed the differentially expressed TSSs from each comparison at p < 0.05, and for robustness, we considered for further analyses the intersection of lists against three control conditions. The analysis yielded 18 gene sets in total: upregulated and downregulated gene sets for all the PRD-like homeobox genes and the OTX2 control (Fig. 3a).
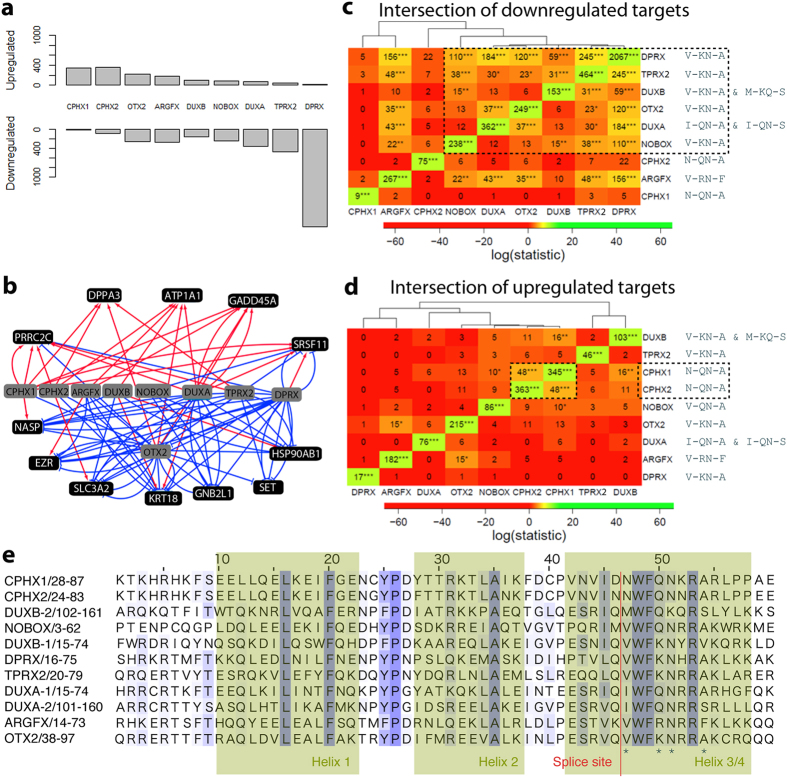
Figure 3. Target genes of the PRD-like homeobox genes in hESCs.
The homeobox genes were overexpressed in hESCs followed by transcriptional profiling by RNAseq. (a) The number of up- and down-regulated target genes following transfection for 9–11 hours. (b) The most commonly occurring up- and down-regulated target genes are shown as a gene network. (c) Similarity of the down-regulated target gene lists as observed by performing chi-squared test to the pairwise overlap of all the gene sets. (d) Similarity of the up-regulated target genes lists as observed by performing chi-squared test to the pairwise overlap of all the gene sets. The color indicates logarithmic value of the chi-squared test statistics, and the significance of higher than expected overlap is shown by asterisk according to the multiple-testing corrected Fisher’s exact test value (p < 10−2*, p < 5 × 10−5**, p < 5 × 10−8***). (e) Alignment of the amino acid sequences for all homeodomains in the CPHX1, CPHX2, ARGFX, DUXB, NOBOX, DUXA, TPRX2 and DPRX genes. Conserved residues are marked in blue, homeodomain helices have a green background. A conserved splice-site is marked by a red line. *Mark the position of variable amino acids in the DNA-binding domain that are indicated in (c,d). Dotted line around the amino acid stretch shows grouping of similar genes (c,d). The red line marks the splice site characteristic for PRD-like homeobox genes4,36.
Among the regulated target genes we identified 12 common target genes that were either upregulated by at least four or downregulated by at least five of the different PRD-like homeodomain transcription factors. These 12 common target genes included, among others, the cell-cycle gene NASP, the pluripotency associated gene DPPA3, the trophoblast determining gene KRT18, the pre-mRNA processing genes PRRC2C and SRSF11 and the homeodomain gene OTX2 itself (Fig. 3b).
Furthermore, there were some targets that were specific for a subset of the homeodomain genes. For example, three out of the four Yamanaka factors (KLF4, MYC, POU5F1, SOX2) were differentially regulated by TPRX2, OTX2, DUXA and DPRX: SOX2 was downregulated by TPRX2 and OTX2, MYC was downregulated by OTX2, DUXA and DPRX, and POU5F1 was upregulated by DPRX and downregulated by OTX2. Interestingly, CPHX1 upregulated BCAP31 and USP9X, two genes important for gamete generation21.
The PRD-like homeodomain transcription factors may act both as activators and repressors
The number of differentially expressed genes varied between the different PRD-like homeodomain transcription factors, with DPRX regulating the largest number of genes. The majority of DPRX target genes were downregulated and showed overlap with TPRX2 and ARGFX downregulated target genes (Fig. 3c). The smallest number of significantly regulated genes was found for DUXB. We found distinct roles for the PRD-like homeodomain genes as either mainly activators, such as ARGFX, CPHX1 and CPHX2 (Fig. 3a,c), or as mainly repressors, such as DPRX, DUXA and TPRX2 (Fig. 3a,c). For testing the application of our results to the context of human embryos, we looked at the expression of up-regulated genes by the three activators (CPHX1, CPHX2 and ARGFX) in human embryos2. An independent RNA sequencing dataset from human embryos and ESC-s showed up-regulation of the target genes in the course of embryo development for all three activators (Supplementary Figure S10). Full lists of the target genes, their corresponding TSSs and genomic annotations are given in Supplementary Table S2.
To further investigate the target gene profiles, we performed cross-comparisons between all upregulated or downregulated genes. The significance of the overlap was evaluated by Chi-squared test statistic or Fisher’s exact test. A major overlap was found between the upregulated targets of CPHX1 and CPHX2 (p < 2 × 10−14) (Fig. 3d), and genes upregulated by DUXB and CPHX1 (p < 2 × 10−8), ARGFX and OTX2 (p < 2 × 10−5), and CPHX1 and NOBOX (p < 2 × 10−5).
In the same manner, the comparison between downregulated target genes yielded a number of significant overlaps; the target genes downregulated by DPRX, TPRX2, DUXB and NOBOX all have significant overlap between each other (Fig. 3c). Furthermore, DPRX and ARGFX share a significant number of downregulated target genes with all other PRD-like homeodomain genes except for CPHX1, CPHX2 (both) or DUXB (only ARGFX). We also tested the overlaps of the target genes upregulated by one factor and downregulated by another (Supplementary Figure S11). A significant overlap was observed between the downregulated targets of DPRX and upregulated targets of both CPHX1 (p < 2 × 10−8) and CPHX2 (p < 2 × 10−14). These data suggest tentative regulatory networks controlling human EGA.
Target gene profiles can be influenced by the amino acid sequence of the homeodomain
Since the members of the PRD-like homeobox gene family share similar amino acid sequences in the homeodomains (Fig. 3e), we hypothesized that they might target the same genes through similar DNA-binding properties. Thus, we correlated the observed overlaps of the target genes with sequence similarities of the homeobox domains (Fig. 3c,d).
CPHX1 and CPHX2 shared a large number of upregulated target genes (Fig. 3c), and they had an almost identical homeodomain, with only 4 aa difference (Fig. 3e). DUXA and DUXB differed from the others in having two homeodomains. Only DUXB had the residues V-KN-A, but both DUXA and DUXB shared a number of target genes with the others (Fig. 3d). ARGFX stood out with a different homeodomain sequence and significant overlap between its downregulated target genes and the target genes of DPRX, TPRX2, OTX2, DUXA and NOBOX. Comparing the overall homeodomain sequence, ARGFX shared 35% and 33% aa identity with DUXA, whereas the homeodomains in DUXB shared 33% and 28% aa identity with ARGFX. The same degree of aa identity in the homeodomain (35%) was seen between ARGFX and TPRX2. This shows that although the similarities in the DNA binding domain indicate similar target gene profiles, the homeodomain similarity was not alone determining the target gene expression profiles.
Target genes of PRD-like transcription factors are activated during human preimplantation development
We suggested earlier that the new PRD-like homeobox genes might act as key regulators of human early development3. In order to further test this hypothesis, we analyzed the overlap between the differentially expressed target genes in our overexpression experiments and the genes upregulated at various stages of preimplantation development: EGA2,3, trophectoderm (TE), epiblast (EPI), primitive endoderm (PE) and inner cell mass (ICM)22 (Fig. 4). The similarity of targets was tested by Chi-squared test after matching the dataset-specific TSSs for EGA genes from Töhönen, et al. The lists of upregulated genes from Yan et al. and Blakeley et al. were calculated as described in materials and methods, and the Chi-squared test was performed by matching the gene names. The number of observed intersecting genes between the experimentally regulated gene sets and the preimplantation gene sets was calculated and compared with the expected number of intersecting genes occurring by chance. We report multiple testing corrected p-values.
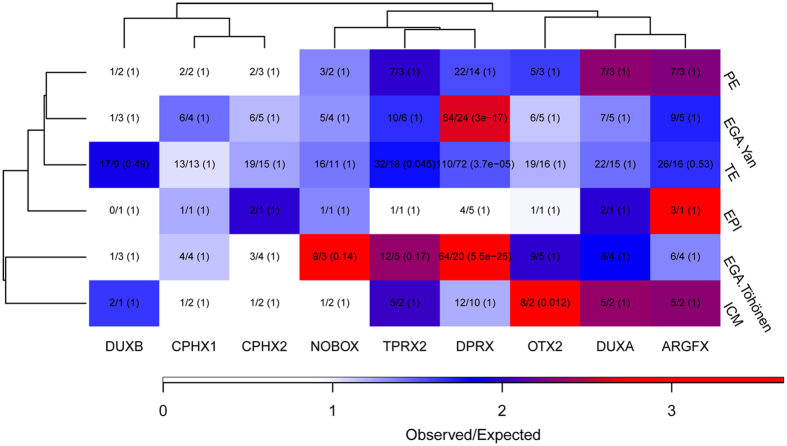
Figure 4. Overlap of target genes with developmentally important gene sets.
Chi-squared test was performed for identifying significant number of intersecting genes. Target genes from our overexpression experiment were compared to the developmentally important datasets of genes up-regulated in embryonic genome activation (EGA) (two independent datasets for EGA.Yan2 and EGA.Töhönen3), epiblast (Epi), Trophectoderm (TE) or inner cell mass (ICM)22. The number of observed divided by expected number of intersecting genes is indicated by color scale, and written on the plot followed by multiple-testing corrected p-value in brackets.
Most of the comparisons with PE, ICM and EPI gene sets yielded no statistically significant overlaps with the experimental gene sets, except for OTX2. Eight target genes of OTX2 overlapped with ICM genes, while overlap of only 2 genes was expected (p = 0.012). Larger overlap than expected was noted for DPRX (110/72, p = 3.7 × 10−5) and TPRX2 (32/18, p = 0.045) with the TE genes. The most significant overlaps were observed for DPRX with both EGA datasets (Töhönen et al.3; 64/20, p = 5.5 × 10−25 and Yan et al.2; 64/24, p = 3 × 10−17).
Activity of the homeobox genes via DNA motif implicated in preimplantation development
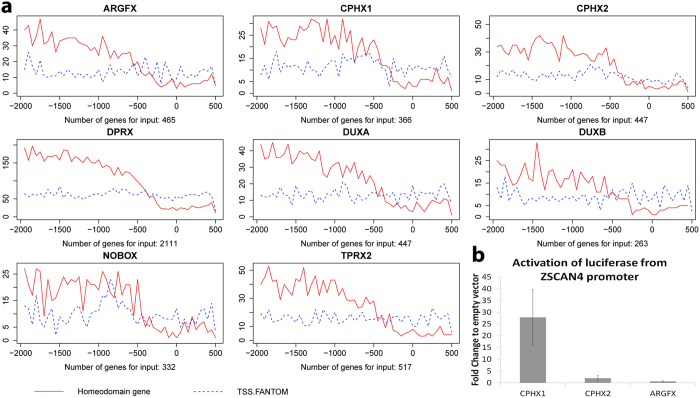
The PRD-like homeobox genes were suggested to act on a motif found enriched in the promoters of EGA genes3. In order to test further the hypothesis, we studied the enrichment of the 36 bp embryo motif in the promoters of the experimental target genes (2000 bp upstream and 500 bp downstream from TSS) by the MAST software23. The results confirmed the enrichment of the 36 bp motif upstream of the TSS for genes regulated by most of the PRD-like homeobox genes (Fig. 5a). This result further strengthened the notion of the functionality of the predicted motif for PRD-like genes, and supported the 36 bp motif as a key regulatory element in human preimplantation development.
Figure 5. Promoter properties of the homeodomain genes.
(a) Enrichment of a 36 bp DNA motif in the promotor region (−2,000 ~ + 500 bp distance around the center of the TFE) of the PRD-like homeodomain transcription factor target genes. The motif was enriched upstream of the promoters of genes during human pre-implantation development (red line). The motif is over-represented upstream of the up- and down-regulated genes by homeobox genes and under-represented from about 0 to 500 bp downstream from the TFE position. The motif is not enriched in any specific regions among random start-sites from the FANTOM database (blue dotted line). (b) Luciferase expression Fold Change between ZSCAN4 promoter-containing vector and corresponding empty vector (pGL4.25) with transfection by activators CPHX1, CPHX2 and ARGFX in HEK293 cell lines. The average values from three biological replicas are shown, error bars represent standard deviation. The values are normalized to corresponding vector without transcription factor overexpression.
To further investigate the suggested main activators CPHX1, CPHX2 and ARGFX as transcription activators, we performed luciferase reporter assays after co-transfecting human embryonic kidney cells (HEK-293) with a transcription factor at a time and a reporter construct with ZSCAN4 promoter upstream of the luciferase gene. The ZSCAN4 promoter contained multiple repeats of the 36 bp motif, and ZSCAN4 was upregulated in EGA3. The ZSCAN4 promoter yielded up to 27 fold increase in luciferase expression when co-transfected with CPHX1, but not with CPHX2 or ARGFX, suggesting further specificity of function (Fig. 5b).
Discussion
The homeodomain proteins make up approximately 15–30% of all animal transcription factors24. A number of homeodomain proteins have been shown to be important for early development in vertebrate species25,26, and some homeodomain proteins are restricted in expression to specific cells at specific developmental stage. In our previous study we detected the expression of PRD-like homeobox genes expressed in oocytes, zygotes or single blastomeres from 4- and 8- cell embryos3. The transcript variants of the PRD-like homeobox genes ARGFX, CPHX1, CPHX2, DPRX, DUXA, DUXB, TPRX1 and TPRX2 cloned in this study may be expressed only during a restricted time period, explaining their recent discovery. A thorough investigation of human homeobox loci revealed that ARGFX, DPRX, TPRX1 and DUXA were initially predicted and annotated based on a few existing EST, retrotransposed pseudogene and genomic sequences7,10. Of the genes presented here only NOBOX was previously well characterized and found expressed at high levels in the ovary, specifically in primordial and growing oocytes27. Interestingly, our sequencing data indicated a new TSS yielding a novel splice variant of NOBOX that we confirmed by cDNA cloning. This novel NOBOX TSS had barely detectable expression in 4- or 8-cell embryos and embryonic stem cells.
In our database searches the PRD-like homeobox genes were undetectable in most cell types and tissues, including the comprehensive FANTOM5 database. In agreement with their absent expression, these genes were rapidly methylated in directly after preimplantation stage in human embryos18 , suggesting an epigenetic silencing mechanism (Supplementary Figure S7). This observation was also consistent with the commonly observed silencing of Alu elements enriched in the promoters of the new genes3,28. During human preimplantation development the DNA methylation levels are reset, which is critical for the normal development29,30.
Due to ethical reasons, functional studies in human embryos were not possible for these genes. However, we chose the closest and biologically most relevant cell line hESC-s for overexpression experiment. The PRD-like homeobox genes could induce both up- and downregulation of target genes based on the hESC overexpression experiment (Fig. 3a), and the target genes showed significant overlaps both between the different factors themselves (Fig. 3c,d) and between previously published human preimplantation data sets (Fig. 4). While the target genes show expected overlaps with published datasets from embryos, we have to acknowledge that some of the identified target genes might not be the same in human embryos due to the usage of cell model. Since the expression of PRD-like homeodomain transcription factors was very lowly or not at all detected in hESC-s (Supplementary Figure 6), some of the target genes in human embryos might not be overexpressed due to the cellular context such as due to methylation of the gene region.
In the hESCs, DPRX was found to downregulate the largest number of target genes (2067), followed by TPRX2 (464) and DUXA (362). We propose that DPRX might be needed to restrict the expression of a number of activated genes after the EGA, allowing later lineage specification.
The luciferase reporter assay using a putative ZSCAN4 promoter placed upstream of the luciferase gene further confirmed that CPHX1, but not the other activators CPHX2 and ARGFX acts on ZSCAN4 promoter (Fig. 5b). Thus the activity of these factors was not determined only by their homeodomain, but rather a combination of homeodomain specificity, possible transactivating domains in the protein or their cellular context and interaction with other proteins. For example, OTX2 has been shown to interact on protein level with LHX1 and FOXA231. Thus, the cellular context may affect the transcriptional activity of the PRD-like homeodomain proteins.
We studied the possibility that the overlap of target genes for the PRD-like homeobox genes might result from the similarities in the DNA-binding regions of their homeodomains. We observed a large number of genes upregulated by CPHX1 and CPHX2, and they differed in their homeodomain aa sequence at six positions only with a high overall protein sequence similarity (Supplementary Figure S4 a). ARGFX, on the other hand, only shared 17 aa identity with the homeodomains of CPHX1 and CPHX2, possibly explaining the low overlap of target genes for these transcription factors even though all three seemed to function mainly as activators (Fig. 3c,d). As an example, ZSCAN4, a known inducer of iPS cells32 was significantly upregulated by CPHX1 only. Further experimental evidence is needed in order to define the specificity of the target gene profiles of the homeodomain proteins.
It is known that an aa change at position 52 in the DNA-recognition part of the homeodomain in NOBOX may cause premature ovarian failure due to disruption of the transcription factor binding to the NOBOX-binding consensus sequence33. When aligning the different homeodomains for our 9 transcription factors we see that at position 52 the aa varies between the different factors. For example, ARGFX, OTX2, TPRX2 and DUXA have an arginine residue at position 52, just as NOBOX. The overall highly similar CPXH1 and CPXH2 differ at this position (lysine or asparagine).
PRD-like transcription factors have been implicated in early development in other organisms such as Drosophila melanogaster. For example, the Drosophila prd-like homeobox gene Odysseus is involved in germ cell formation34. Interestingly, we found that CPHX1 upregulates the expression of BCAP31 and USP9X, whereas CPHX2 upregulates SPIN3, all three genes involved in human gamete generation according to DAVID annotation. In addition, BCAP31 is downregulated by DUXB and TPRX2. Also other germ cell related genes, such as ZFP42, TEX14 and TFAP2C were regulated in our overexpression experiment. Targets of the PRD-like homeodomain transcription factors were enriched in genes upregulated during early development (Fig. 4). OTX2 targets were enriched in ICM, TPRX2 targets in TE and DPRX targets in TE and EGA.
In conclusion, this study presents further evidence of expression for the nine PRD-like genes that we recently identified from human preimplantation embryos3. Our data support the hypothesis that the previously uncharacterized PRD-like homeobox genes are specifically expressed during early development, have a key role in the transcriptional dynamics during EGA, and are subsequently silenced as the development progresses from a pluripotent stage towards more highly differentiated stage.
Materials and Methods
Human embryos
Human embryos used in this study were collected in Sweden and donated by informed consent by couples who underwent infertility treatment by in vitro fertilization (IVF). Cryopreserved cells that were not needed for IVF treatment were donated as an alternative to being destroyed as they had exceeded the maximum legal storage time. Cleavage stage embryos were frozen at the 4-cell stage on day 2 after fertilization. After thawing, embryos were allowed to develop until the 8-cell stage in a sequential culture system (G1/CCM medium, Vitrolife at 37 °C and 5% CO2 and 5% O2). This study was reviewed and approved by the ethics review boards according to Swedish law (Dnr 2010/937-31/4 of the Regional Ethics Board in Stockholm). All the methods were carried out in accordance with the approved guidelines.
Library preparation
Human embryo libraries were prepared after thawing the embryos at the 4-cell stage and incubation allowing them to develop until the 8-cell stage. Each single embryo was put into a 0.5 mL PCR tube containing 4.45 μl of freshly prepared cell lysis buffer and the libraries were processed according to the protocol by Tang et al.12. In total, three 8-cell libraries were prepared.
Human ovary poly-A + RNA was obtained from Clontech (cat# 636152). Human ovary cDNA was prepared using a first strand cDNA synthesis kit (Invitrogen) according to the manufacturer’s instructions.
PCR primer design
The putative novel transcripts were predicted based on our previous RNAseq data3 and these predictions were used for designing PCR cloning primers.
NOBOX primers were designed based on an RNAseq peak at FE518599 (chr7:144100745-144100865,-) located in an intron of the human RefSeq sequence NM_001080413.3 (GeneID135935), that was connected to the 3′ UTR of the predicted sequence. The sequences containing ORFs for CPHX1, CPHX2 and DUXB were predicted based on FE200101 (chr16:75760315-75760435,-), FE200082 (chr16:75710885-75711065,-) and FE200054 (chr16:75735289-75735420,-), respectively and used for primer design. Predicted sequences used for primer design are provided as Supplemental Text S1. TPRX1/TPRX2 primer design was based on prediction for TPRX1 with an undefined TSS or TFE at chr19:48,307,772-48,307,835 -, and human RefSeq sequence NM_198479.2 leading to a design of not perfectly matching primers for TPRX2. DUXA, DPRX and ARGFX cloning primers were designed based on the human RefSeq NM_001012729.1 (NCBI GeneID 503835), NM_001012728.1 (GeneID 503834) and NM_001012659.1 (GeneID 503582) respectively (Table 1).
Cloning of ARGFX, CPHX1, CPHX2, DPRX, DUXA, DUXB and TPRX2
cDNA libraries from three single 8-cell embryos were used for cloning of the putative transcripts. The transcripts were amplified from the cDNA libraries using Phusion High-Fidelity DNA polymerase (New England Biolabs) according to the manufacturer’s instructions. For amplification of DUXA, DPRX, DUXB, TPRX1 and TPRX2, the following PCR program was used: 98 °C for 30 s; 40 cycles of 98 °C for 10 s, 65.9 °C (DUXB)/67.9 °C (DPRX, TPRX1,TPRX2)/71.5 °C (DUXA) for 30 s, 72 °C for 1 min; final extension 72 °C for 10 min. ARGFX, CPHX1 and CPHX2 were amplified using Touchdown PCR: 98 °C for 30 s; 24 cycles of 98 °C for 10 s, annealing for 30 s, temperature decreasing from 63 °C to 56 °C, 1 °C/3 cycles, 72 °C for 30 s; 16 cycles of 98 °C for 10 s, 55 °C for 30 s, 72 °C for 30 s; final extension 72 °C for 10 min. In order to clone the three differently sized TPRX2 isoforms, the PCR products were purified from agarose gel using the QIAquick Gel Extraction Kit (Qiagen), and the obtained amplicons were then reamplificated in order to obtain sufficient amount of amplicons for subsequent cloning. PCR products were cloned into the pCR4Blunt-TOPO vector using the Zero Blunt TOPO PCR Cloning kit (Invitrogen) and verified by Sanger sequencing (Eurofins Genomics).
Cloning of NOBOX
Human ovary cDNA was used for cloning putative NOBOX transcript. The transcript was amplified from cDNA using HotStarPlus Taq DNA polymerase (Qiagen) according to manufacturer’s instructions. For PCR amplification following steps were applied: 95 °C for 5 min; 40 cycles of 95 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min; final extension 72 °C for 10 min. PCR product was cloned into pCRII-dual promoter TOPO vector using TOPO TA cloning kit (Invitrogen), and sequence was verified by Sanger sequencing (Eurofins Genomics).
Prediction of ORFs, functional protein domains, and alignment on UCSC genome browser
For open reading frame (ORF) prediction, forward and reverse sequences from each clone were first trimmed for vector sequence using Pregap4 version 1.6-r and then the contig sequences were formed using Gap4 v4.11.2-r (both from the Staden package, http://staden.sourceforge.net/). Mismatching bases were manually checked for quality and edited accordingly. ORFs were predicted and translated using ApE plasmid editor (http://biologylabs.utah.edu/jorgensen/wayned/ape/). Conserved protein domains were predicted applying an NCBI Blastx (http://blast.ncbi.nlm.nih.gov/Blast.cgi) translated nucleotide query on each complete clone sequence against non-redundant protein sequences (nr) and EMBL-EBI InterPro (http://www.ebi.ac.uk/interpro/) on translated sequences. The consensus cDNA and amino acid sequences were aligned by Blat with “psl” as an output (UCSC Genome Browser) and the information was combined to generate a BED file for visualization of custom tracks on a browser (Supplemental Text S2). The alignments were edited manually in order to include short exons.
Gene expression profiling of hESC lines and single 8-cell blastomeres
A total of 48 single cells were manually picked directly to STRT lysis buffer. The cells included 16 human regular ES cells (HS980), 16 human single-8-cell blastomere derived ES cells (HS983a) and 16 human single blastomeres from 8-cell embryos. The embryos were thawed at 4-cells stage (ThawKit™ Cleave, VitroLife) and cultured in G-1™ Plus media (VitroLife) overnight under standard conditions as performed in the IVF Clinic (5% CO2/5%O2). The zona pellucidae were removed with Tyrode’s solution (Sigma-Aldrich) and embryos were dissociated into single cells using enzymatic (TrypLE, Life technologies) and mechanical separation. The STRT library was prepared according to the modified STRT protocol (Krjutškov, et al., accepted) and sequenced on four lanes of Illumina HiSeq 2000 instrument. Pre-processing of STRT reads, quality control and alignments were performed as previously described (Krjutškov, et al., accepted). After quality control, 4 outliers (one regular ES cell, one blastomere-derived ES cell and two 8-cell blastomeres) were removed from further analysis due to low RNA content. The gene expression was quantified per transcript far 5′ end (TFE) units3. The TFE coordinates for the PRD-like homeobox genes are given in Table 1. The reference file for counting the reads per TFE was defined for PRD-like homeobox genes and spike-in RNAs only. The reads per TFE were counted using SAMtools version 1.1 and htseq-count version 0.6.1, and the data was normalized using external spike-in RNAs as described previously20. The raw read counts and normalized expression values for PRD-like homeobox genes and spike-in RNAs used for creating beeswarm plots are given in Supplemental Table S3. The original sequence files as well as aligned BAM-files are accessible from European Nucleotide Archive (ENA) under the accession number PRJEB12467.
Analysis of methylation pattern for homeobox genes
Publicly available dataset for human sperm, preimplantation embryo, ESC and embryonic tissues was downloaded from GEO (accession number GSE51239, file hDev100bpMeFinal.txt.gz)18. 100 bp methylation value percentages were used for genomic positions in the range of 500 bp up- and downstream from the middle of TFE-s for the homeobox genes.
Publicly available dataset for methylation in human blood cells was downloaded19. The M-values within the entire homeobox genes’ region was used.
PCR detection of PRD-like homoebox gene expression
Total RNA extracted from three hESC lines (H9, HS401 and HS980) in three biological replicates were used for cDNA synthesis using SuperScript III First-Strand Synthesis Supermix for qRT-PCR (Invitrogen) according to the manufacturer’s instructions. For PCR we used an input of 22 ng of cDNA and the reactions were done in three biological replicates. As a control, we used 10 ng cDNA from a human 8-cell cDNA library. The PCR reactions were carried out using an ABI PRISM 7500 Fast Real-Time PCR System with FastStart Universal SYBR Green Master mix (Roche) according to the manufacturer’s instructions. The primer sequences are shown in Supplemental Table S4. For validation of the amplicons, the PCR products were cloned into a pCRII-dual promoter TOPO vector using the TOPO TA cloning kit (Invitrogen), and verified by Sanger sequencing (Eurofins Genomics).
hESC expression vector construction
In order to overexpress ARGFX, CPHX1, CPHX2, DPRX, DUXA, DUXB, NOBOX, OTX2 and TPRX2 in mammalian cells, the sequences were cloned into a bicistronic pFastBac expression vector. The pFastBac vector was modified as described previously3. Briefly, the transcript sequences were amplified from the TOPO vectors using primers containing AscI and PacI restriction sites, digested using AscI and PacI (New England Biolabs) and ligated into pFastBac vector. The primer sequences for cloning the genes in this study are shown in Supplemental Table S5. mCherry fluorescent protein was used as a control, and was amplified from the standard injection marker construct elt-2::GFP for C. elegans (kindly provided by Gert Jansen, The Erasmus University Medical Center, Holland). The final structure of the modified expression pFastBac vector was as follows: CMV enhancer – EF1a promoter – AscI – Gene Of Interest – PacI – IRES – eGFP – WPRE. The IRES (internal ribosomal entry site) element allows for simultaneous expression of two proteins: the inserted gene of interest, and a green fluorescent protein (GFP) marker.
Overexpression experiments and cell sorting for expression profiling
The hESC line HS401 was cultured on Laminin-521 (Biolamina) in mTeSR™1 media (Stemcell Technologies). One ug of expression vector containing the gene of interest was incubated with 3 μl of Lipofectamine2000 in 50 μl of DMEM. Confluent cells (95–100%) were trypsinized, gently washed with DPBS, and re-suspended in 50 μl of transfection solution. The cell suspension was transferred to a new Laminin-521 coated plate with 50 μl of DMEM and the cells were allowed to settle in the transfection solution for 15 minutes. The media was subsequently changed to mTeSR™1. After 9–11 h of transfection, the cells were washed with DPBS and treated with trypsin to get single-cell solution, washed again with DPBS and suspended in ice-cold DPBS until the analysis. The overexpression time point was chosen by time-course analysis using pFastBac-mCherry vector and further estimation when the first wave of target genes would be expressed. Cell viability was assessed by 1:1000 propidium iodide (Molecular Probes) and cell clusters were removed by FSC and SSC. 75 cells per sample from GFP positive and GFP negative cells were sorted in three replicates into 5 μl of library lysis buffer. The sorting was performed in three replicas from the same transfection well for 75 transfected and 75 non-transfected cells in each replica (altogether 6 samples per gene) for each gene and mCherry control (n = 9), providing two libraries for pooled sequencing of 54 samples by modified STRT protocol (Krjutškov, et al. accepted).
The homeobox genes were distributed between two libraries that were processed simultaneously. The first of these, Library 3, included DUXA, DPRX, ARGFX, OTX2 and mCherry. The second library, Library 4, included CPHX1, CPHX2, DUXB, TPRX1, NOBOX and mCherry.
STRT RNAseq analysis for target gene detection
The STRT data was analyzed as described previously3. Briefly, the reads were filtered, de-multiplexed by barcodes, joined for the same unique molecular identifiers (UMI), trimmed for the barcodes and UMI-s, and mapped to the human UCSC genome hg19 by TopHat. Outliers were identified by analysis of distance correlation dendrograms, which indicated two outliers for one library (Library 3, Supplementary Figure S12a) and no outliers for the second library (Library 4, Supplementary Figure S12b). The outlier E8 in Library 3 (mCherry, GFP negative) was removed, resulting in one library having one less GFP negative control. The principal components one and two separated two different DPRX wells from the controls, and the components 3 and 4 separated none, therefore DPRX was not removed.
The signal of STRT sequencing forms clusters that were identified as Transcript Far 5′ End (TFE) as described previously (Krjutškov, et al. accepted). Briefly, the aligned BAM files were assembled into putative transcripts by sample types using Cufflinks, followed by extraction of only the 5′-end exons by UCSC tools and followed by merging reads from various sample types by BEDTools. The reads were counted on the identified TFE-s by htseq.
The raw reads were aligned by TopHat to the modified pFastBac backbone vector, and reads counted by htseq, in order to confirm overexpression in the GFP positive versus GFP negative cells.
Three sets of controls were used for performing differential analysis: only mCherry control GFP positive wells (n = 3), only GFP-negative wells from the whole library (n = 22 or n = 23), and the combination of both mCherry control and homeobox specific GFP-negative wells (n = 3 + 3). Estimates of differential expression for the TFE-s were obtained using the R package SAMstrt with positive samples compared to each of the three control (FDR < 0.05 unless indicated otherwise)20. Only intersection of the differentially expressed TFE-s from three analysis was used in the study. The identifier TFE-s were mapped to the genome and annotated for genes and genomic regions as described previously3. Only the reads mapping to 5′ UTR of coding genes were used in the study.
The original sequence files as well as aligned BAM-files are accessible from ENA by the accession number PRJEB12453.
Gene expression analysis for public preimplantation datasets
Three publicly available datasets were used for gaining differentially expressed genes lists. Two independent studies were used for gaining the EGA genes lists2,3. The TFE identifiers up-regulated by either 4- or 8-cell stage were gained from Töhönen et al. and the identifiers were matched with at least 1 bp overlap by BEDTools.
The RPKM values were downloaded from Yan et al. and up-regulated genes by either 4- or 8-cell stage were calculated by students t-test (FDR < 0.05). Only gene names that had a match in our dataset were used for the analysis. Average RPKM values in Oocytes, Zygotes, 2-, 4-, 8-cell embryos, Morluae, Epiblast and passage 0 ESC-s were used for calculating the gene expression levels of up-regulated target genes.
Raw count values were downloaded from a study that performed RNA sequencing on different lineages of human blastocysts22. Students t-test was done for four comparisons in order to obtain genes specifically expressed in TE (TE vs EPI), ICM (EPI vs TE), PE (PE vs EPI) and EPI (EPI vs PE). Ensembl ID-s for the differentially expressed genes (p < 0.05) were converted to the associated gene names by Ensembl Biomart tool35 and intersecting gene names with our dataset were used.
The number of intersecting identifiers present in both datasets, not present in either one or both of the datasets were calculated for the Chi-squared test.
Motif analysis
The MAST tool23 was used to search for significant sequence patterns to all given motifs in a gene set. Sequences −2000 and +500 bp from the center of each TFE for differentially regulated genes (FDR < 0.1) were analyzed. A list of all transcription start sites was gained from FANTOM database, and used as a control set to the THE-s.
Luciferase reporter assay
HEK-293 cells (ATCC) were seeded on 48-well plates in DMEM containing 1 g/l glucose, L-glutamine, pyruvate and supplemented with 10% FBS and 2 mM L-glutamine (all from Gibco). Cells were grown overnight at 37 °C in 5% CO2 and transfected with the ZSCAN4 promoter in pGL4.25 reporter vector described previously3 or pGL4.25 luciferase vector (Promega) in combination with CPHX1, CPHX2 or ARGFX, all cloned into pFastBac vector as described above. Renilla luciferase vector pGL4.74 [hRluc/TK] (Promega) was co-transfected with other constructs to enable normalization. The concentrations for single constructs were as follows: Luciferase vector 100 ng/well, pFastBac vector 100 ng/well and Renilla luciferase vector 10 ng/well. The transfections were performed using Lipofectamine 2000 (Invitrogen) 0.5 μL/well according to manufacturer’s instructions. Cells were incubated at 37 °C in 5% CO2, harvested 24 h after transfection and subjected to Dual luciferase assay (Promega) in three biological replicates with two technical replicates each, according to manufacturer’s protocol. Luciferase signals were measured using a TECAN infinite M200 (Tecan, Männedorf, Switzerland).
Data availability statement
All the novel homeobox genes clone sequences were submitted to EMBL, and their ENA accession numbers are given in Fig. 1. The short read sequences and aligned BAM-files from the RNA-seq experiments for the overexpression in ES-cells are uploaded in ENA with the accession number PRJEB12453. The RNA-seq dataset from single-cell hESC-s and 8-cell human embryo have the accession number PRJEB12467.
Additional Information
How to cite this article: Madissoon, E. et al. Characterization and target genes of nine human PRD-like homeobox domain genes expressed exclusively in early embryos. Sci. Rep. 6, 28995; doi: 10.1038/srep28995 (2016).
Supplementary Material
Acknowledgments
We thank the anonymous donors of cells for this study. The pFastBac expression vector was a kind gift from Professor Shu Wang. We thank Lovisa Reinius for the help in interpreting the methylation data. This work was supported by the Karolinska Institutet Distinguished Professor Award, the EU MAARS project and the Strategic Research program for Diabetes funding at Karolinska institutet to J.K.; the Swedish Research Council to F.L., O.H. and J.K., the ALF Project Funding (Stockholm County and Karolinska Institutet) to O.H.; the Jane & Aatos Erkko Foundation, the Instrumentarium Science Foundation and the Orion Research Foundation to E.-M.J.; the EU FP7-PEOPLE-2012-IAPP grant SARM (324509) to K.K. and J.K.; the Ragnar Söderberg Foundation (M67/13), the Swedish Foundation for Strategic Research (ICA12-0001) and Åke Wibergs Foundtaion (3772745) to F.L.; the Mats Sundin Fellowship in Developmental Health to S.P. and Åke Wiberg and Magnus Bergvall foundation to V.T. Part of this work was assisted by Karolinska High Throughput Center, a core facility at Karolinska Institutet with affiliation to SciLifeLab (https://www.scilifelab.se/facilities/khtc/). The computations were performed on resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Project b2014069.
Footnotes
Author Contributions E.M., E.M.J., L.V., R.M., S.P. and K.K. performed the experiments; E.M., E.M.J., L.V. and J.K. wrote the manuscript; E.M., E.M.J., E.E., T.B. and S.K. analyzed the data; V.T., S.L., F.L. and O.H. provided the materials and methods to perform the study; all authors contributed to the planning of the experiments, interpretation of the data, critical review to the manuscript text, and approved the final version of the manuscript.
References
- Niakan K. K., Han J., Pedersen R. A., Simon C. & Pera R. A. Human pre-implantation embryo development. Development 139, 829–841, doi: 10.1242/dev.060426 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L. et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol, doi: 10.1038/nsmb.2660 (2013). [DOI] [PubMed] [Google Scholar]
- Tohonen V. et al. Novel PRD-like homeodomain transcription factors and retrotransposon elements in early human development. Nat Commun 6, 8207, doi: 10.1038/ncomms9207 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burglin T. R. & Affolter M. Homeodomain proteins: an update. Chromosoma, doi: 10.1007/s00412-015-0543-8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. W. Evolution of homeobox genes. Wiley Interdiscip Rev Dev Biol 2, 31–45, doi: 10.1002/wdev.78 (2013). [DOI] [PubMed] [Google Scholar]
- Bürglin T. R. A Handbook of Transcription Factors, Chapter 5: Homeodomain Subtypes and Functional Diversity. Vol. 52 (Springer Netherlands, 2011). [DOI] [PubMed] [Google Scholar]
- Booth H. A. & Holland P. W. Annotation, nomenclature and evolution of four novel homeobox genes expressed in the human germ line. Gene 387, 7–14, doi: 10.1016/j.gene.2006.07.034 (2007). [DOI] [PubMed] [Google Scholar]
- D'Elia A. V. et al. Missense mutations of human homeoboxes: A review. Hum Mutat 18, 361–374, doi: 10.1002/humu.1207 (2001). [DOI] [PubMed] [Google Scholar]
- Qian Y. Q. et al. Nuclear magnetic resonance spectroscopy of a DNA complex with the uniformly 13C-labeled Antennapedia homeodomain and structure determination of the DNA-bound homeodomain. J Mol Biol 234, 1070–1083, doi: 10.1006/jmbi.1993.1660 (1993). [DOI] [PubMed] [Google Scholar]
- Holland P. W., Booth H. A. & Bruford E. A. Classification and nomenclature of all human homeobox genes. BMC Biol 5, 47, doi: 10.1186/1741-7007-5-47 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. & Holland P. W. The origin and evolution of ARGFX homeobox loci in mammalian radiation. BMC Evol Biol 10, 182, doi: 10.1186/1471-2148-10-182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F. et al. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat Protoc 5, 516–535, doi: 10.1038/nprot.2009.236 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntriss J., Hinkins M. & Picton H. M. cDNA cloning and expression of the human NOBOX gene in oocytes and ovarian follicles. Mol Hum Reprod 12, 283–289, doi: 10.1093/molehr/gal035 (2006). [DOI] [PubMed] [Google Scholar]
- Zhong Y. F. & Holland P. W. The dynamics of vertebrate homeobox gene evolution: gain and loss of genes in mouse and human lineages. BMC Evol Biol 11, 169, doi: 10.1186/1471-2148-11-169 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin S. et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun 5, 3195, doi: 10.1038/ncomms4195 (2014). [DOI] [PubMed] [Google Scholar]
- Forrest A. R. et al. A promoter-level mammalian expression atlas. Nature 507, 462–470, doi: 10.1038/nature13182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizio M. et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol 16, 22, doi: 10.1186/s13059-014-0560-6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Z. D. et al. DNA methylation dynamics of the human preimplantation embryo. Nature 511, 611–615, doi: 10.1038/nature13581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinius L. E. et al. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One 7, e41361, doi: 10.1371/journal.pone.0041361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama S., Tohonen V., Linnarsson S. & Kere J. SAMstrt: statistical test for differential expression in single-cell transcriptome with spike-in normalization. Bioinformatics 29, 2943–2945, doi: 10.1093/bioinformatics/btt511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25, 25–29, doi: 10.1038/75556 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley P. et al. Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development 142, 3151–3165, doi: 10.1242/dev.123547 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T. L. & Gribskov M. Combining evidence using p-values: application to sequence homology searches. Bioinformatics 14, 48–54 (1998). [DOI] [PubMed] [Google Scholar]
- de Mendoza A. et al. Transcription factor evolution in eukaryotes and the assembly of the regulatory toolkit in multicellular lineages. Proceedings of the National Academy of Sciences of the United States of America 110, E4858–4866, doi: 10.1073/pnas.1311818110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W. & Krumlauf R. Homeobox genes and axial patterning. Cell 68, 283–302 (1992). [DOI] [PubMed] [Google Scholar]
- Parker H. J., Bronner M. E. & Krumlauf R. A Hox regulatory network of hindbrain segmentation is conserved to the base of vertebrates. Nature 514, 490–493, doi: 10.1038/nature13723 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumori N., Yan C., Matzuk M. M. & Rajkovic A. Nobox is a homeobox-encoding gene preferentially expressed in primordial and growing oocytes. Mech Dev 111, 137–141 (2002). [DOI] [PubMed] [Google Scholar]
- Luo Y., Lu X. & Xie H. Dynamic Alu methylation during normal development, aging, and tumorigenesis. Biomed Res Int 2014, 784706, doi: 10.1155/2014/784706 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H. et al. The DNA methylation landscape of human early embryos. Nature 511, 606–610, doi: 10.1038/nature13544 (2014). [DOI] [PubMed] [Google Scholar]
- Okae H. et al. Genome-wide analysis of DNA methylation dynamics during early human development. PLoS Genet 10, e1004868, doi: 10.1371/journal.pgen.1004868 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Murata T., Matsuo I. & Aizawa S. OTX2 directly interacts with LIM1 and HNF-3beta. Biochem Biophys Res Commun 267, 64–70, doi: 10.1006/bbrc.1999.1872 (2000). [DOI] [PubMed] [Google Scholar]
- Jiang J. et al. Zscan4 promotes genomic stability during reprogramming and dramatically improves the quality of iPS cells as demonstrated by tetraploid complementation. Cell Res 23, 92–106, doi: 10.1038/cr.2012.157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y. et al. NOBOX homeobox mutation causes premature ovarian failure. Am J Hum Genet 81, 576–581, doi: 10.1086/519496 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting C. T., Tsaur S. C., Wu M. L. & Wu C. I. A rapidly evolving homeobox at the site of a hybrid sterility gene. Science 282, 1501–1504 (1998). [DOI] [PubMed] [Google Scholar]
- Cunningham F. et al. Ensembl. Nucleic Acids Res 43, D662–669, doi: 10.1093/nar/gku1010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W. J., Affolter M. & Burglin T. Homeodomain proteins. Annu Rev Biochem 63, 487–526, doi: 10.1146/annurev.bi.63.070194.002415 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the novel homeobox genes clone sequences were submitted to EMBL, and their ENA accession numbers are given in Fig. 1. The short read sequences and aligned BAM-files from the RNA-seq experiments for the overexpression in ES-cells are uploaded in ENA with the accession number PRJEB12453. The RNA-seq dataset from single-cell hESC-s and 8-cell human embryo have the accession number PRJEB12467.