Abstract
Antipsychotic sensitization and tolerance refer to the increased and decreased drug effects due to past drug use, respectively. Both effects reflect the long-term impacts of antipsychotic treatment on the brain and result from the brain’s adaptive response to the foreign property of the drug. In this review, clinical evidence of the behavioral aspect of antipsychotic sensitization and tolerance is selectively reviewed, followed by an overview of preclinical literature that examines these behavioral characteristics and the related pharmacological and nonpharmacological factors. Next, recent work on the developmental impacts of adolescent antipsychotic sensitization and tolerance is presented and recent research that delineates the neurobiological mechanisms of antipsychotic sensitization and tolerance is summarized. A theoretical framework based on “drug learning and memory” principles is proposed to account for the phenomena of antipsychotic sensitization and tolerance. It is maintained that antipsychotic sensitization and tolerance follow basic principles of learning or acquisition (“induction”) and memory (“expression”). The induction and expression of both effects reflect the consequences of associative and nonassociative processing and are strongly influenced by various pharmacological, environmental, and behavioral factors. Drug-induced neuroplasticity, such as functional changes of striatal dopamine D2 and prefrontal serotonin (5-HT)2A receptors and their mediated signaling pathways, in principle, is responsible for antipsychotic sensitization and tolerance. Understanding the behavioral characteristics and neurobiological underpinnings of antipsychotic sensitization and tolerance has greatly enhanced our understanding of mechanisms of antipsychotic action, and may have important implications for future drug discovery and clinical practice.
Keywords: Antipsychotic drugs, sensitization, tolerance, dopamine D2, serotonin 2A, GSK3 β, adolescent rats, psychosis
Introduction
Antipsychotic drugs are the primary medications for the treatment of schizophrenia and other neuropsychiatric disorders with a psychosis component (e.g. amphetamine psychosis, psychosis in Alzheimer’s disease, psychosis in Parkinson’s disease, etc.). Since the introduction of chlorpromazine in psychiatry in 1952, about 50 additional antipsychotic drugs have been developed for the treatment of schizophrenia. They are all significantly more effective than placebo and are often classified into two groups, typical (or first generation) and atypical (or second generation), with atypical drugs offering a reduced risk of extrapyramidal motor syndromes (EPS) (Kapur and Remington, 2001), although recent studies have questioned the validity of such a classification system (Leucht et al. 2013). The differences in efficacy among various commonly prescribed antipsychotic drugs are small yet robust, with clozapine being more efficacious than all the other drugs (e.g. amisulpride, olanzapine, risperidone, paliperidone, zotepine, haloperidol, quetiapine, aripiprazole, etc.).
Much research on antipsychotic drugs has four interconnected goals in mind. The first one is to understand the mechanisms of action of antipsychotic drugs at various levels (e.g. molecular, cellular, neural network, and behavioral) in an attempt to answer the basic question of how antipsychotic drugs work to achieve their therapeutic effects (Kapur, 2003, Kapur et al., 2005). There have been attempts to link actions of antipsychotic drugs at various receptor sites, notably dopamine D2, serotonin (5-HT)2A, and 5-HT1A receptors (Kapur et al., 2003; Meltzer et al., 1989; Richtand et al., 2007; Seeman, 2000) to their behavioral mechanisms of actions (Li et al., 2007). The second goal is to enhance our understanding of etiology and psychopathological mechanisms relevant for psychosis. The rationale is that dysfunction of the molecular targets of antipsychotic drugs such as D2 and 5-HT2A can be a possible cause of psychotic symptoms (Seeman, 2008). The third one is to provide better assays for new drug discovery. With the increase of our understanding of etiology of psychosis, molecular and behavioral mechanisms of antipsychotic action, behavioral and molecular assays with better predictive validity could be developed to identify new compounds useful for psychosis (Allen et al., 2011). The final one, which falls in the domain of behavioral neuroscience, is to use antipsychotic drugs as pharmacological tools to probe the neurochemical basis of behavior, as typical antipsychotic drugs such as haloperidol are potent D2 receptor antagonists and atypical drugs possess dual actions against serotonin 5-HT2A and dopamine D2 receptors. Research discussed in this review has been largely aimed at the first goal.
One important feature associated with repeated or chronic antipsychotic treatment is the alterations of drug sensitivity, a phenomenon largely ignored in the field of behavioral pharmacology in recent decades. In comparison to extensive research on changes of drug sensitivity induced by psychotomimetic drugs (e.g. amphetamine, cocaine and PCP etc.) (Pierce and Kalivas, 1997; Robinson and Becker, 1986), antipsychotic-induced alterations are not as well understood. This situation is peculiar given the fact that antipsychotics, like drugs of abuse, are often taken repeatedly by people for a prolonged period of time, and increase in antipsychotic response is thought to be an important mechanism supporting the maintenance of antipsychotic effect (Kapur et al., 2006). One of the major issues which may have contributed to this lack of attention is the difficulty in demonstrating its existence consistently. For example, in animal studies using the prepulse inhibition paradigm, alterations in antipsychotic drug sensitivity have never been consistently established among different antipsychotics (Geyer et al., 2001; Li et al., 2011). On the other hand, clinical studies often focus on the efficacy, tolerability, and side effect profiles of individual drugs, overlooking the temporal course of changes in drug sensitivity. Also, changes in antipsychotic efficacy in human studies typically do not become apparent within a limited trial period. It often requires years of medication in order to induce such a change (e.g. supersensitivity psychosis, tardive dyskinesia (TD)).
Several years ago, when we started looking into this issue, there were limited and scattered reports. There was also a lack of terminology used to describe drug-induced long-term changes in drug sensitivity and no standardized approach to study these changes. Current psychiatrists and psychopharmacologists do not talk about long-term antipsychotic effects in these terms, let alone discuss their clinical implications. We thus borrowed two terms from the literature of drugs of abuse and defined “antipsychotic sensitization” and “antipsychotic tolerance” as reflecting the increased and decreased drug effects due to past drug use, respectively. Antipsychotic sensitization and tolerance reflect the long-term consequences of chronic antipsychotic drug treatment on the brain and behavioral functions and are thought to be mediated by drug-induced changes in neuroplasticity and basic psychological processes. Therefore, understanding the behavioral characteristics and neurobiological underpinnings of antipsychotic sensitization and tolerance should greatly enhance our understanding of mechanisms of antipsychotic action, and may help future drug discovery and improve clinical treatment of schizophrenia. This understanding may also provide a different perspective of looking at some clinical effects. For example, antipsychotic sensitization and tolerance may explain why some recent clinical trials of promising novel therapeutics fail to demonstrate efficacy (Gill et al., 2014). The testing of novel compounds is often done in patients exposed to antipsychotic drugs (comparators) for years and briefly withdrawn. Due to the (cross)-tolerance effect, it is possible that prior antipsychotic exposure history and subsequent withdrawal affects the response of the brain to novel drugs to the extent that it effectively masks the true efficacy of novel compounds. On the other hand, as clinical responses of patients on novel compounds are often compared to those on treatment-as-usual (TAU) after a brief washout period. Re-exposure to the same drug may potentiate the TAU group’s clinical responses to the comparator drug, masking the true efficacy of novel compounds from another perspective. In addition to these implications for antipsychotic drug research, because antipsychotic sensitization and tolerance share many similarities with behavioral sensitization and tolerance induced by other psychoactive drugs such as psychostimulants (e.g. amphetamine, methamphetamine, nicotine, etc.), opioids, and dissociative anesthetics (e.g. phencyclidine, ketamine, MK-801) (Poulos et al., 1981; Robinson and Becker, 1986), studies of antipsychotic sensitization and tolerance could expand our understanding of sensitization and tolerance phenomena in general, and introduce new research ideas, tools, approaches, and knowledge.
This review will provide an overview of recent research in this area. We will focus on animal work that examines the behavioral characteristics of antipsychotic sensitization and tolerance, the possible underlying neurobiological mechanisms, their developmental impacts and clinical implications. To show the clinical relevance of these phenomena, human studies on antipsychotic sensitization and tolerance will be briefly reviewed at the beginning. It should be noted that sensitization and tolerance can develop in various domains involving different organ systems (e.g. cardiovascular, liver, blood, endocrine, brain, etc.) (Diamond and Borison, 1986; See and Kalivas, 1996). Because the behavior and associated brain functions are the focal targets of antipsychotics, we will restrict our use of antipsychotic sensitization and tolerance in the behavioral domain. Thus, we define antipsychotic sensitization as the consequence of repeated drug treatment that leads to increased behavioral effects of a drug, while antipsychotic tolerance as the decreased behavioral effects.
General issues
It is worth mentioning three general principles at the outset. Readers who are familiar with behavioral sensitization and tolerance associated with drugs of abuse can easily recognize them. First, sensitization and tolerance develop to the specific effects of a drug, not to a drug itself. Like many psychoactive drugs, antipsychotic drugs typically have multiple behavioral and physiological effects due to their complex pharmacodynamic receptor actions (Miyamoto et al., 2005). It is thus possible that antipsychotic sensitization and tolerance may develop to one effect of a drug, but not to another (Sun et al., 2009). It is also possible that sensitization is seen in one effect while at the same time tolerance is seen in others. Furthermore, the same drug may induce sensitization to a drug effect under some circumstances (e.g. dosage level, dosing regimen, and duration) but may induce tolerance to the same effect under other conditions (Stewart and Badiani, 1993). The second point is that multiple processes and mechanisms are involved in the development of antipsychotic sensitization and tolerance. At the behavioral level, antipsychotic sensitization and tolerance reflect a general nonassociative learning and memory process in which an organism modifies its responses to an exogenous stimulus (e.g. a drug) based on its past experience with this stimulus. The learning and memory processes involved in antipsychotic sensitization and tolerance are not dissimilar to those involved in the basic forms of habituation and sensitization. Because the induction and expression of both effects depend on the context in which drug treatment occurs and on the specific motoric response that the drug targets (Feng et al., 2013; Poulos and Hinson, 1982; Sun et al., 2014; Zhang and Li, 2012), other associative processes (e.g. conditioning, drug-setting, behavioral response) may also play a role in the development of antipsychotic sensitization and tolerance. At the brain level, drug-induced plastic changes on receptor density, intracellular signaling, electrophysiological property of neurons, and neuroanatomic volume are examples of many processesthat antipsychotic sensitization and tolerance exert on. Goudie (1993: 313) suggested that all these different processes and mechanisms associated with behavioral sensitization and tolerance can be classified into two general categories: “higher level mechanisms involving instrumental and classical conditioning processes, and more molecular mechanisms involving functional and dispositional adaptations.” He also pointed out that it would be easier to “derive general “laws” of sensitization and tolerance at the level of the first class rather than the second.” The third point is that many experimental and pharmacological factors influence the development of antipsychotic sensitization and tolerance. Notable factors include treatment schedule, drug dose, and behavioral testing conditions (Barnes et al., 1990; Remington and Kapur, 2010). Under some conditions, these factors could even determine whether a sensitization or tolerance will be developed (Klein and Schmidt, 2003; Poulos et al., 1981). With these points in mind, we will first review some human studies that examined antipsychotic sensitization and tolerance and their roles in explaining therapeutic and side effects of antipsychotic treatment.
Clinical phenomena associated with antipsychotic sensitization and tolerance
Like other psychoactive drugs, antipsychotic drugs are known to induce various clinically relevant sensitization and tolerance effects in many behavioral domains, including both therapeutic and side effects (Emmett-Oglesby and Goudie, 1989), resulting from the brain’s adaptive responses to the bombardment of long-term antipsychotic drug treatment (Konradi and Heckers, 2001; Schmitt et al., 2004). The observations that psychotic symptoms improve over time and extrapyramidal side effects get worst after years of medication could be considered examples of antipsychotic sensitization. On the other hand, chronic antipsychotic treatment can also induce tolerance in certain behavioral domains, as evidenced by the findings that in comparison to patients with chronic antipsychotic treatment, first-episode schizophrenia patients respond to lower doses of antipsychotics; are more sensitive to side effects; and have comparatively higher response rates than chronic schizophrenia patients (Lieberman et al., 1993; Kapur et al., 2000). These differences between drug naïve (first-episode) and drug experienced patients could be interpreted as a result of tolerance developed in the drug experienced group. The same drug treatment may induce sensitization in some patients, but tolerance in others (Sramek et al., 1990). Admittedly, the terms “antipsychotic sensitization” and “antipsychotic tolerance” have not been frequently used in describing many clinical phenomena. The above mentioned clinical phenomena could also be subject to other interpretations. However, as will become apparent, classifying antipsychotic phenomena in the framework of sensitization and tolerance would provide a unified theory (the brain’s adaptation responses to the bombardment of long-term antipsychotic drug treatment) to better understand their underlying mechanisms. In this section, the four best known phenomena consistent with the conceptualization of antipsychotic sensitization and tolerance will be selectively reviewed, including: exponential time course of symptom improvement, time-dependent sensitization (TDS), supersensitivity psychosis, and TD (Agid et al., 2003; Fallon and Dursun, 2011; Kapur et al., 2006).
Exponential time course of symptom improvement
When acute psychotic patients are treated with antipsychotic drugs, their symptoms improve gradually over time if they respond well to the chosen antipsychotic drugs. After 2–3 weeks of continuous treatment, a clear improvement can be noticed and patients report that they are less bothered by psychotic thoughts and bizarre perceptions (Kapur et al., 2006). Dopamine D2 receptor blockade is achieved within hours after drug administration (Nordstrom et al., 1992; Tauscher et al., 2002), however, it is not well understood and heatedly debated as to why it still takes 2–3 weeks in order to see clear therapeutic benefits. Traditionally, it is thought that the onset of antipsychotic response is delayed for 2–3 weeks, even though the receptor actions of antipsychotic drugs are well established within minutes (Gelder et al., 2000). However, recent re-examinations of the time course of antipsychotic effect cast doubt on this long-held idea of delayed onset (Agid et al., 2003; Kapur et al., 2005; Leucht et al., 2005). Agid et al. (2003) examined 42 double-blind, comparator-controlled studies (>7000 patients) using a meta-analysis technique, and found that psychotic symptoms improved within the first week of treatment and showed a progressive improvement over subsequent weeks, with the overall pattern of improvement approximating an exponential curve. In addition, Kapur et al. (2005) tested the hypothesis that psychosis improves within the first 24 h of antipsychotic treatment. They found that patients with schizophrenia receiving olanzapine (10 mg i.m.) or haloperidol (7.5 mg i.m.) treatment showed greater resolution of overall symptoms than those receiving placebo. An independent change in the psychotic symptoms, which included conceptual disorganization, hallucinatory behavior, or unusual thought content, was evident for both medications within the first 24 h of treatment. Leucht et al. (2005) analyzed a large homogeneous database of original patient data from seven randomized, double-blind studies of the efficacy of amisulpride in patients with schizophrenia spectrum disorders and found the same results. Therefore, the time course of the antipsychotic action reveals a progressively enhanced response to antipsychotic drugs, a sensitization-like pattern. It can be conceptualized that the reason that psychotic symptoms improve over time and follow an exponential curve is because antipsychotic effect intensifies with repeated drug administration.
TDS
TDS is a controversial concept that is not well understood. It refers to the observation that a brief exposure to a psychotherapeutic drug such as antipsychotic or antidepressant drugs induces a clinical effect that grows with the passage of time (Antelman et al., 2000), an effect indicative of antipsychotic sensitization. Antelman et al. (2000) have argued that TDS is a useful principle for the explanation of clinical improvement which grows with the passage of time, and a certain percentage of symptom improvement observed in patients is likely due to TDS. One direct implication is that “instead of managing disorders such as depression by multiple daily drug treatments, it may be possible to accomplish the same ends by treating once every few weeks.” (p. 354). As discussed above, psychotic symptoms do improve exponentially with the passage of time and with the increase of treatment duration (Agid et al., 2006; Kapur et al., 2006), but the relative contributions from each factor (i.e. time vs treatment duration) on symptom improvement has not been investigated. Currently, the most common practice in the clinic is to treat schizophrenic patients with antipsychotic drugs daily to achieve approximately 60%–80% of dopamine D2 receptor occupancy (Kapur, 1998). If we do not need to maintain a daily treatment schedule, it would avoid many side effects, including EPS and excess weight gain. Recent studies showing that dosing every 2–3 days is sufficient to maintain antipsychotic efficacy in schizophrenic patients is in support of this practice and the TDS principle (Remington et al., 2005, 2011). This finding suggests that upon initial exposure, physiological events initiated by a drug enhance the antipsychotic’s effects beyond its presence at the receptor, thereby inducing efficacy without requiring constant receptor binding. This idea is also supported by our recent preclinical findings that risperidone and asenapine sensitization persist and even increase to some degree with the passage of time (Gao and Li, 2013). More clinical and preclinical work is needed to determine how pharmacological factors and characteristics of patients influence TDS and identify relevant neurobiological mechanisms.
Supersensitivity psychosis
Supersensitivity psychosis refers to a drug-induced psychotic relapse following chronic neuroleptic treatment (Chouinard and Jones, 1980; Kirkpatrick et al., 1992). It has been reported that in some patients with schizophrenia, their psychotic symptoms return following withdrawal or decrease of doses of antipsychotic drugs. Some patients also report experiencing negative effects in the process of drug withdrawal, including difficulty falling or staying asleep, mood changes, increases in anxiety/agitation, difficulty concentrating/completing tasks, headaches, memory loss, nightmares, nausea, and vomiting etc. (Salomon et al., 2014). The underlying mechanism is suggested to be the drug-induced increase in the mesolimbic dopamine postsynaptic D2 receptors. It is well known, especially in preclinical studies, that chronic use of antipsychotic drugs often elicits dopamine supersensitivity (up-regulation of D2High receptors) (Seeman, 2011). The idea is that the cessation of chronic antipsychotic treatment induces a compensatory increase in the mesolimbic dopamine function, leading to psychotic relapse. Because supersensitivity psychosis is behaviorally (e.g. delusions, hallucinations, suspiciousness) and neurobiologically (e.g. increase in the mesolimbic dopamine function) similar to endogenous psychosis, reinstatement of antipsychotic treatment is efficacious to reduce this syndrome. In those patients, it is often observed that a gradual increase in the dosage is necessary to maintain a therapeutic effect, possibly due to the fact that antipsychotic treatment is needed to control both the endogenous psychosis and supersensitivity psychosis. This drug-induced increase in dosage increase indicates the development of tolerance to antipsychotic effect. In other words, the appearance of supersensitivity psychosis reflects the fact that chronic use of antipsychotic drugs causes a tolerance effect.
TD
TD is a human choreic movement disorder associated with chronic exposure to antipsychotic drugs, especially to those with strong dopamine receptor blocking capacity (e.g. haloperidol, chlorpromazine). Clinically, TD includes a broad spectrum of symptoms that develop after chronic use of antipsychotic drugs, including involuntary movements of the tongue, jaw, trunk, or extremities. Abnormal movements could appear during treatment or withdrawal from the treatment, and typically persist for at least one month. The incidence of TD has not dramatically reduced with the widespread use of atypical antipsychotic drugs, suggesting that the common D2 blocking action of all antipsychotics is likely the main cause (Aquino and Lang, 2014).
TD is thought to reflect an antipsychotic sensitization effect in the side effect domain, as the syndromes emerge and deteriorate over time (however, see (Poulos et al., 1981)). The traditional view of the neurobiological mechanism of TD emphasizes the role of drug-induced upregulation of D2 function (D2 hypersensitisation) (Turrone et al., 2003), the same mechanism thought to be responsible for supersensitivity psychosis, although manifested in the motor function domain, not in the emotion and cognition domains. This distinction between TD and supersensitivity psychosis may be due to regional differences in D2 upregulation, with TD strongly associated with changes in the dorsal striatum, while supersensitivity psychosis with changes in the ventral striatum (including the nucleus accumbens) (Chouinard and Jones, 1980). Recently, the emphasis is shifted to the drug-induced synaptic plasticity in cortico-striatal transmission in the striatum. It is suggested that the synaptic plasticity is maladaptive, resulting in an imbalance between direct and indirect pathways in the striatum, and leads to perpetuating abnormal movements even after drug withdrawal (Loonen and Ivanova, 2013). Other ideas such as drug-induced disturbances of oxidative stress response systems and impacts on serotonin receptors and GABAergic medium spiny neurons have also been proposed (Aquino and Lang, 2014). Regardless of the precise mechanisms, TD is a cluster of persistent abnormal movement syndromes associated with long-term treatment with antipsychotic drugs. With its strong developmental feature, it likely reflects an increase of motor impairment effects of certain antipsychotic drugs, a type of antipsychotic sensitization.
Other phenomena
Other forms of antipsychotic sensitization and tolerance have been reported. For example, Williams et al. (1996) studied the time-based sensitization of cognitive impairment with haloperidol. They gave 24 healthy male subjects placebo on Day 1 and haloperidol (2 mg) on Days 2 and 25 and tested their cognitive function before dosing, and over a 24-hour period after dosing on Days 1, 2, and 25. They observed a clear impairment of cognitive function at 6–8 h after administration of haloperidol on Day 2. More importantly, when a single-dose of haloperidol was given again 25 days later, a greater level of impairment with earlier onset was noted in several tests in both treatment groups, indicating an antipsychotic sensitization effect. On the other hand, clozapine tolerance has been observed in some patients treated with clozapine. They show withdrawal symptoms (e.g. nausea, vomiting, insomnia, diarrhea, agitation, aggression, headache, etc.) (Touyz et al., 1978) and relapse to psychosis (Seppala et al., 2005), often seen with the discontinuation of clozapine use.
Overall, clinical studies have identified several clinical phenomena indicative of antipsychotic sensitization (e.g. exponential time course of symptom improvement, TDS, and TD) and antipsychotic tolerance (e.g. supersensitivity psychosis, clozapine withdrawal symptoms). However, most of them are descriptive and not mechanistic-oriented. After reviewing some recent clinical studies on antipsychotic tolerance, Goudie and Cole (2008: 815) concluded that “it seems highly likely that all antipsychotic treatments induce clinically important neuroadaptations during chronic drug administration, although the nature of such neuroadaptations remains unclear.” The possible distinctive neuroadaptations associated with sensitization and tolerance effect have not been explored. Sramek et al. (1990) conducted a retrospective review of neuroleptic dosages over a five-year period in 19 chronic schizophrenic patients. They found that some patients developed tolerance, while others developed sensitization, as indicated by their consistent yearly increases or decreases in dosage, suggesting that individual factors are also important in determining the direction of change in drug sensitivity. Unfortunately, it is not clear what the important pharmacological and dispositional factors are that influence these individual differences. Furthermore, the theoretical framework adequate to explain antipsychotic sensitization and tolerance is lacking. In the following, we will turn to preclinical animal work which in some way addressed these issues.
Preclinical evidence for antipsychotic-induced behavioral sensitization and tolerance
Sensitization and tolerance induced by antipsychotic drugs have a long research history. The first report of antipsychotic tolerance in English that can be found on the PubMed database is a study by Boyd (1960) who reported that Wistar rats developed tolerance to the motor suppressant and lethal effects of chlorpromazine over a period of 40 weeks when they were injected with increasing daily doses of chlorpromazine. Stille et al. (1971) also reported that tolerance occurred to repeated dosing with clozapine (2.5–20 mg/kg, p.o) and thioridazine (5–20 mg/kg, p.o), but not to haloperidol or perphenazine in locomotor activity in mice. At the end of the 19 days of drug administration, clozapine, and thioridazine even caused an increase in locomotor activity, a sign of behavioral supersensitivity (Seeman et al., 2005). On sensitization, Antelman et al. (1986: 58) reported that a single injection of low, clinically relevant doses of haloperidol and fluphenazine hydrochloride causes catalepsy in rats that grows over time “such that one re-exposure to the same compound up to 8 weeks later results in a marked enhancement (i.e. sensitization) of this response.”
Over the years, many preclinical studies provide strong support for chronic antipsychotic-induced sensitization and tolerance (Antelman et al., 1986). For example, intermittent haloperidol treatment via daily injection is shown to cause a progressively potentiated catalepsy (Amtage and Schmidt, 2003), enhanced vacuous chewing movements (VCMs, a proxy for tardive dyskinesia in humans) over time (Turrone et al., 2003), enhanced suppression of milk intake (Wolgin and Moore, 1992), enhanced disruption of conditioned avoidance responding (CAR) (Li et al., 2007), enhanced impairment of reward-based lever pressing rates (Trevitt et al., 1998; Varvel et al., 2002), enhanced disruption of maternal behavior (Zhao and Li, 2009b), and enhanced inhibition of phencyclidine (PCP)-induced hyperlocomotion (Sun et al., 2009). A similar effect on the PCP-induced hyperlocomotion has also been found with repeated clozapine and olanzapine treatment (Sun et al., 2009). In addition, repeated clozapine treatment is also shown to induce increasing numbers of myoclonic seizure-like jerks in rats (Stevens et al., 1997). Finally, Kaempf and Porter (1987) demonstrated sensitization for the rate-suppressing effects of the typical antipsychotic pimozide.
With regards to the antipsychotic-induced tolerance, continuous haloperidol treatment via osmotic mini-pump has been shown to cause a progressively decreased inhibition of spontaneous motor activity in rats (Carey and Deveaugh-Geiss, 1984), increased behavioral supersensitivity, as measured by increased amphetamine-induced locomotor activity following antipsychotic discontinuation (Samaha et al., 2007), and a progressively decreased disruption of avoidance responding over time (Samaha et al., 2008). Stanford and Fowler (1997) reported that clozapine-treated rats exhibited tolerance to the drug’s suppressive effect on the amount of time that rats were in contact with a force-sensing target disk. Trevitt et al. (1998) found that repeated injections of clozapine, but not haloperidol enhanced its suppression of lever pressing in a fixed ratio 5 (FR-5, 5 presses result in one reward). Porter and colleagues have conducted a series of experiments to identify differences between the acute and subchronic effects of antipsychotic drugs on operant responding in rats. In one earlier study, they demonstrated that acute treatment with clozapine significantly suppressed operant response rates on fixed-interval 60-second responding. With repeated drug administration and testing, the clozapine-treated rats gradually developed tolerance to the drug effects and recovered back to the vehicle control levels after seven days of drug treatment (Kaempf and Porter, 1987). Later, their group reported that although acute clozapine (10 mg/kg) significantly disrupted response rates and reinforcement rates and significantly increased response duration on a schedule of multiple random interval responding for food reinforcement, chronic administration of clozapine resulted in a development of tolerance (Villanueva and Porter, 1993). Varvel et al. (2002) also found that repeated clozapine produced a decrease in the rate of responding for food reward under a multiple FR 30/ fixed-interval (FI) 60-second schedule. More importantly, the degree to which clozapine tolerance develops appears to depend in part on the schedule of reinforcement, with more complete tolerance observed under a FI 60-second schedule (Kaempf et al., 1987), and only partial tolerance (approximately 50–75%) under a FR 30 schedule (Varvel et al., 2002; Villanueva et al., 1993). This differential tolerance was attributed to the different baseline levels of responding generated by these reinforcement schedules (Varvel et al., 2002). Clozapine-induced tolerance has also been observed in a drug discrimination task (Goudie et al., 2007) and rat maternal behavior (Zhao and Li, 2009b). Taken together, antipsychotic sensitization and tolerance appear to be inevitable features associated with repeated drug treatment. Therefore, understanding the neurobiological and behavioral factors that modulate the induction and expression of sensitization/tolerance is expected to greatly enhance our understanding of the effects of clinical treatment.
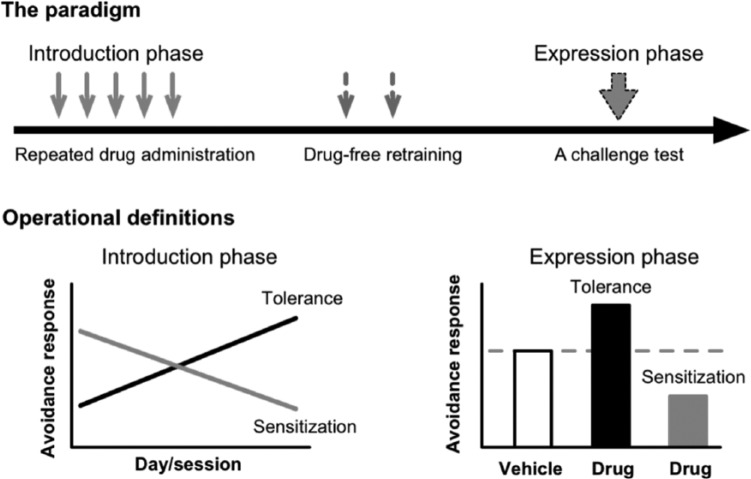
As mentioned above, antipsychotic sensitization refers to the increased behavioral responsiveness to an antipsychotic drug due to past drug treatment history, while tolerance refers to the opposite behavioral pattern (i.e. decreased responsiveness). In recent years, we developed a two-phase paradigm to study antipsychotic sensitization and tolerance. In the induction phase, different groups of animals are being repeatedly treated with various doses of an antipsychotic drug or vehicle for 3–7 days and tested in a behavioral model of antipsychotic activity (e.g. the conditioned avoidance response model) daily. In the expression phase, all animals are being challenged with a single dose of the drug and their performance in the test is compared. The magnitude of antipsychotic sensitization and tolerance can be measured in two ways in both phases (Qin et al., 2013; Swalve and Li, 2012), similar to the ones used in the behavioral sensitization induced by psychostimulants (Browman et al., 1998). The first index of antipsychotic sensitization or tolerance is revealed through a within-subjects comparison in the induction phase during which the behavioral effect of the drug is either stronger or weaker on the last day of drug treatment than that on the first day (e.g. a comparison between days 1 vs 5) (Zhang and Li, 2012). A second index is derived from a between-subjects comparison in the expression phase during which the behavioral responses of drug-pretreated and vehicle-pretreated animals are compared. With a between-groups analysis, antipsychotic sensitization or tolerance is indicated if drug-pretreated animals show a significantly greater or lower sensitivity to the drug challenge than vehicle-pretreated animals. Overall, it is believed that a between-subjects analysis provides a more “conservative” index of sensitization or tolerance (Browman et al., 1998), as this approach ensures that variables that could contribute to potential changes in behavior are present in both the drug and vehicle control groups. In some cases, the behavior affected by an antipsychotic drug (as an index of antipsychotic effect) is allowed to recover under the drug-free condition to the pre-drug and vehicle control level before the drug challenge, thus, any group difference found on the challenge test could only be attributed to past drug treatment history. This approach provides the strongest demonstration of antipsychotic sensitization and tolerance. Figure 1 illustrates such an approach in the conditioned avoidance response test of antipsychotic drugs.
Figure 1.
A schematic depiction of the two-phase paradigm used to study antipsychotic sensitization and tolerance. In the induction phase, different groups of animals are being repeatedly treated with various doses of an antipsychotic drug or vehicle for 3–7 days and tested in a behavioral model of antipsychotic activity daily. Antipsychotic sensitization or tolerance is revealed through a within-subjects comparison in this phase during which the behavioral effect of the drug is either stronger or weaker on the last day of drug treatment than that on the first day. In the expression phase, all animals are being challenged with a single dose of the drug and their performance in the test is compared. Antipsychotic sensitization or tolerance is indicated if drug-pretreated animals show a significantly greater or lower sensitivity to the drug challenge than vehicle-pretreated animals.
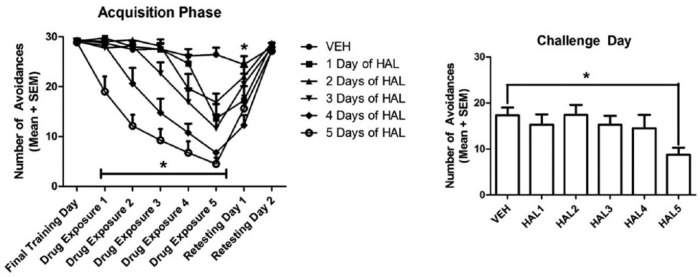
It is well established that at clinically relevant doses, all clinically approved antipsychotic drugs acutely suppress avoidance responding without altering unconditioned escape response in rats (Arnt, 1982; Wadenberg et al., 2001). Thus, the magnitude of avoidance suppression is frequently used as a validated behavioral index of antipsychotic activity (Arnt, 1982; Bignami, 1978; Shannon et al., 1999; Van Der Heyden and Bradford, 1988; Wadenberg and Hicks, 1999). As Figure 1 shows, in the induction phase, antipsychotic sensitization or tolerance in this test is observed when the avoidance-disruptive effect of the drug increases or decreases in magnitude throughout the treatment period. In the expression phase, sensitization or tolerance is shown when the drug-treated animals exhibit a lower or higher avoidance response than those treated with vehicle (Li et al., 2010). Several early studies have demonstrated both effects in the conditioned avoidance response test. For example, Fregnan and Chieli (1980) found that the anti-avoidance effect of haloperidol started on the first testing day and was progressively enhanced with each subsequent drug administration (across-session decline in avoidance responding). It reached a maximum level within 5–8 days. Kuribara and Tadokoro (1981) and Beninger et al. (1983) confirmed this finding and extended it to two other classes of antipsychotics, YM-08050, YM-08051 and pimizode respectively. Using a home-cage control group injected with drugs but not tested repeatedly for avoidance responding, they also showed that the across-session decline in avoidance responding was not due to the accumulation of the drugs with repeated dosing. Sanger (1985) showed that repeated administration of clozapine over four days induces a strong tolerance to the avoidance-disruptive effect of clozapine. It should be noted that many previous studies on antipsychotic sensitization and tolerance have not paid enough attention to the distinct processes of the induction and expression. Most of them only focused on the induction process. Figure 2 shows the results of haloperidol sensitization in the conditioned avoidance response model in adult rats (Swalve and Li, 2012). The sensitization pattern is clearly demonstrated in both phases.
Figure 2.
(a) Effect of repeated haloperidol (HAL) treatment (0.025 mg/kg, sc, -60 min) on conditioned avoidance responding across sessions. Number of avoidance responses made by the rats on the final training day (drug-free), five days of drug exposure and two drug-free retesting sessions are expressed as mean+standard error of the mean (SEM). Rats received either 0, 1, 2, 3, 4, or 5 days of HAL according to their group, *p<0.05. (b) Effect of number of drug exposure days on final challenge day. All groups were injected with HAL (0.025 mg/kg) and avoidance responses were measured, *p<0.05. Adapted from Swalve N and Li M (2012) Parametric studies of antipsychotic-induced sensitization in the conditioned avoidance response model: Roles of number of drug exposure, drug dose, and test-retest interval. Behav Pharmacol 23: 380–391 with permission from Wolters Kluwer Health, Inc.
VEH: vehicle; SC: subcutaneously; -60 min: 60 min before test.
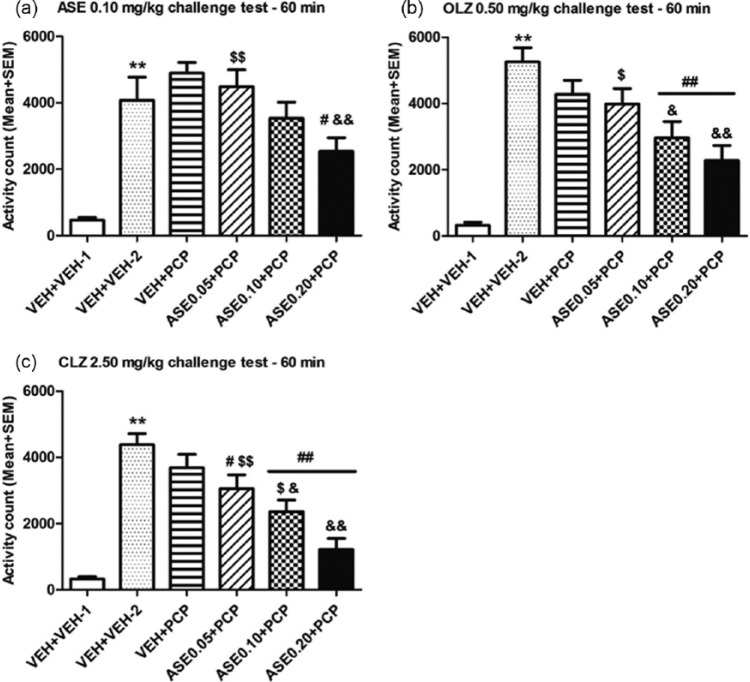
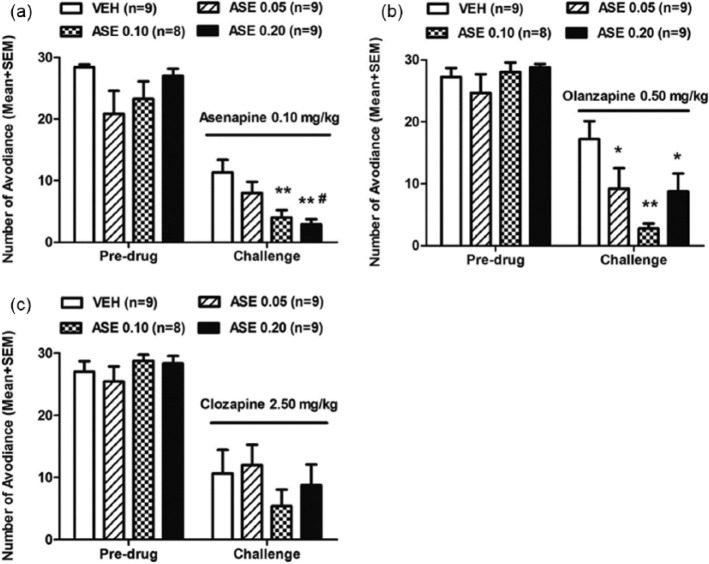
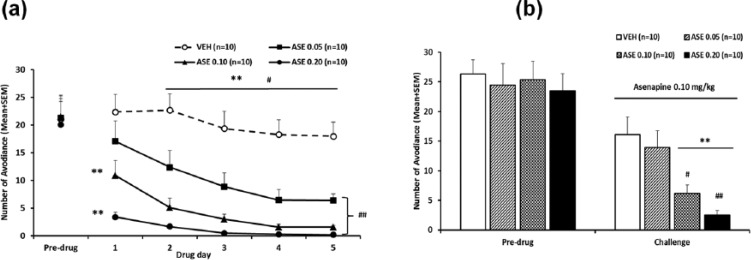
Similarly, it is possible to apply this basic paradigm to other behavioral tests of antipsychotic drugs. For example, the same paradigm has been used to demonstrate that repeated administration of olanzapine (also, risperidone, asenapine) or clozapine induces a potentiated (sensitization) or a decreased (tolerance) inhibition of the PCP-induced hyperlocomotion, respectively (Feng et al., 2013; Qin et al., 2013; Sun et al., 2009; Zhang and Li, 2012; Zhao et al., 2012), another preclinical test for antipsychotic activity (Gleason and Shannon, 1997) (see Figure 4(a)). Furthermore, this paradigm could also be used to conduct cross-sensitization or cross-tolerance studies by challenging animals with a different antipsychotic drug during the expression phase. As an example, Zhang et al. (2011) shows that rats previously treated with risperidone in the induction phase showed stronger reactivity to the avoidance-disruptive effect of olanzapine administered in the expression phase. Recently, a cross-sensitization from asenapine to olanzapine in both the conditioned avoidance response model (Figure 3) and the PCP-induced hyperlocomotion model (Figure 4) has also been observed (Qin et al., 2013).
Figure 4.
Prior asenapine (ASE) treatment increased the inhibition of PCP-induced hyperlocomotion upon asenapine re-exposure and on olanzapine (OLZ) and clozapine (CLZ) treatment in adult rats. (a) Locomotor activity was measured for 60 min after vehicle (for the vehicle (VEH)+VEH-1 group) or PCP (3.20 mg/kg, sc, for the other five groups) injection and expressed as mean+standard error of the mean (SEM) for each group. ASE (0.10 mg/kg, sc) was injected 30 min before the vehicle or PCP injection. (b) Locomotor activity was measured for 60 min after vehicle or PCP injection and expressed as mean+SEM for each group. OLZ (0.50 mg/kg, sc) was injected 30 min before the vehicle or PCP injection. (c) Locomotor activity was measured for 60 min after vehicle or PCP injection and expressed as mean+SEM for each group. CLZ (2.50 mg/kg, sc) was injected 30 min before the vehicle or PCP injection. (n=8/group). Both the VEH+VEH-1 and VEH+VEH-2 groups were repeatedly injected with 0.9% saline for five consecutive days in the induction phase. The only difference was that on the challenge test days, the VEH+VEH-1 group was injected with ASE/OLZ/CLZ followed by another saline injection, whereas the VEH+VEH-2 group was injected with ASE/OLZ/CLZ followed by a PCP injection. **p⩽0.001 relative to VEH+VEH-1 group; #p<0.05, ##p≤0.001 relative to VEH+VEH-2; &p<0.05, &&p⩽0.004 relative to VEH+PCP; $p<0.05, $$p⩽0.009 relative to ASE 0.20+PCP group. Adapted from Qin R, Chen Y and Li M (2013) Repeated asenapine treatment produces a sensitization effect in two preclinical tests of antipsychotic activity. Neuropharmacology 75C: 356–364 with permission from Elsevier.
PCP: phencyclidine.
Figure 3.
Prior asenapine (ASE) treatment increased sensitivity to ASE re-exposure and olanzapine (OLZ) exposure in the avoidance response. (as) Number of avoidance responses in the ASE (0.10 mg/kg) challenge test; (b) OLZ (0.50 mg/kg) challenge test and (c) clozapine (CLZ, 2.50 mg/kg) challenge test is expressed as mean+standard error of the mean (SEM), *p<0.05, **p<0.01 relative to the vehicle (VEH) group; #p<0.05 relative to the ASE 0.05 group. Adapted from Qin R, Chen Y and Li M (2013) Repeated asenapine treatment produces a sensitization effect in two preclinical tests of antipsychotic activity. Neuropharmacology 75C: 356–364 with permission from Elsevier.
Factors that influence antipsychotic sensitization and tolerance
It is common knowledge that both pharmacological factors (e.g. dose, schedule, and route of drug administration, presence of other drugs, etc.) and nonpharmacological factors (e.g. environmental stimuli, selected behavioral responses, passage of time, etc.) affect the development of behavioral sensitization and tolerance induced by psychoactive drugs (Emmett-Oglesby and Goudi, 1989). Behavioral sensitization and tolerance induced by antipsychotic drugs are no exceptions. This section selectively reviews relevant reports, illustrating the principles of how these two classes of factors exert their impacts on the induction and expression of antipsychotic sensitization and tolerance.
Pharmacological factors
Drug dose
Antipsychotic sensitization and tolerance are dependent upon a number of factors including dose and number of exposures. In fact, it is well known that drug doses can even determine whether a sensitization effect or tolerance effect will be observed. The general observation is that a sensitization is likely to occur if a low dose is being used, whereas a tolerance often results from a treatment with a higher dose. For instance, haloperidol at low and medium doses in rats (e.g. 0.25 mg/kg) cause a sensitization in a catalepsy test (Klein and Schmidt, 2003), at high doses (e.g. 1.0 mg/kg) it tends to cause a tolerance (Ezrin-Waters and Seeman, 1977; Poulos and Hinson, 1982). Similarly, clozapine at the high and medium doses (e.g. 5.0 to 10.0 mg/kg) causes a tolerance but at low doses (e.g. 1.0 mg/kg) cause a sensitization (Stevens et al., 1997). Thus, sensitization or tolerance may not be an intrinsic feature of any particular drug or its particular behavioral effect, but is modulated by drug dose.
Within a dose range that typically induces either a sensitization or tolerance, the higher the dose, the stronger the sensitization or tolerance effect. This conclusion was recently demonstrated in the conditioned avoidance response test. Swalve and Li (2012) tested three doses of haloperidol (0.025, 0.05 and 0.10 mg/kg) and three doses of olanzapine (0.5, 1.0 and 2.0 mg/kg) using the two-phase paradigm. Rats were first repeatedly treated with haloperidol or olanzapine for three consecutive days and tested for avoidance response. Three days later, all of them were challenged with haloperidol or olanzapine. Haloperidol or olanzapine at the low dose was unable to induce a long-term sensitization as assessed in the expression phase. In contrast, the medium or high doses induced robust sensitization with just three days of drug treatment. Similarly, Feng et al. (2013) showed that clozapine tolerance is dose-dependent, as a higher dose (e.g. 10 mg/kg) induces a stronger tolerance than that induced by a lower one (2.5 or 5.0 mg/kg).
Number of drug administrations
Antipsychotic-induced sensitization and tolerance have drug memory-like property. From the learning and memory perspective, the induction and expression phases can be characterized as the training (i.e. acquisition) and memory testing phases. The number of drug injections can thus be conceptualized as the number of learning trials (sessions). Therefore, it is expected that the strength of antipsychotic sensitization and tolerance will be more prominent following a greater number of drug injections. In one study (Li et al., 2010), rats that were treated with olanzapine (1.0 mg/kg) for 3 days displayed a relatively less robust sensitization effect than those who were treated with the drug for 5-7 days in other studies (Li et al., 2007; (Li et al., 2009a; Mead and Li, 2010). Swalve and Li (2012) compared 5 groups of rats that received 1 to 5 days of drug administration and found that sensitization induced by haloperidol (0.025 mg/kg) or olanzapine (0.5 mg/kg) was only apparent in rats that received injections for 5 days. The groups that had 1 to 4 days of injections did not even have slightly lower avoidance levels on the challenge day; instead, their levels were no different from that of the vehicle control group. These results suggest that antipsychotic sensitization is dependent on the number of drug exposures, with more exposures leading to a stronger sensitization effect.
Drug dosing regimen
Previous work suggests that drug dosing regimens determine many features of long-term treatment outcomes, with an intermittent and transient treatment (e.g. daily injection) tending to cause a sensitization effect while a continuous treatment (e.g. osmotic minipump) causes a tolerance (Remington and Kapur, 2010). Indeed, it has been shown that continuous haloperidol or olanzapine exposure to rats via osmotic minipump caused a greater increase in VCMs (a proxy for tardive dyskinesia in humans) than transient subcutaneous injections (Turrone et al., 2005). Similarly, continuous haloperidol treatment caused an attenuated disruption (tolerance) of avoidance responding (a measure of antipsychotic activity), while intermittent haloperidol treatment potentiated avoidance disruption (sensitization) (Samaha et al., 2007, 2008). Recently, we also demonstrated that haloperidol sensitization induced throughout adolescence in the conditioned avoidance response test persisted into adulthood only when haloperidol was administered via daily injection. If haloperidol was administered via osmotic minipump, the sensitization effect was not apparent (Gao and Li, 2013). This differential response to intermittent versus continuous treatment probably reflects differential effects of antipsychotic drugs on dopamine systems, especially on postsynaptic D2 receptors (Samaha et al., 2007).
Drug-drug interactions
Most patients with schizophrenia are being treated with multiple psychotherapeutic drugs, such as antipsychotics, SSRIs and benzodiazepines in order to control their diverse symptoms and co-morbid anxiety and depression (Zumbrunnen and Jann, 1998). This practice of psychotropic polypharmacy has raised some concerns regarding the efficacy, costs and possible adverse effects of drug-drug interactions (DDIs) (Alfaro, 2001; Rupnow et al., 2007; Sandson et al., 2005). However, because current clinical data come mostly from case reports and limited uncontrolled studies, it is difficult to assess the extent and nature of DDIs and determine how antipsychotic sensitization or tolerance might be altered by DDIs. A few years ago, a drug-drug conditioning paradigm was developed to examine how concurrent use of chlordiazepoxide with haloperidol or olanzapine might affect the induction and expression of antipsychotic sensitization in the conditioned avoidance response test (Li et al., 2009b). It was observed that pairing of chlordiazepoxide with haloperidol during the repeated drug test phase for seven days attenuated the anti-avoidance effect of haloperidol, indicating an attenuation of the development of haloperidol sensitization. However, such pairing did not have a lasting effect on the expression of haloperidol sensitization, as there was no group difference between the group that received the chlordiazepoxide+haloperidol pairing and those that received no such pairing in the haloperidol challenge test. In contrast, pairing of chlordiazepoxide with olanzapine had little effect on the induction of olanzapine sensitization, but did reduce its expression. This effect of chlordiazepoxide is due to drug-drug conditioning, as the control groups that received the same treatment (i.e. chlordiazepoxide with haloperidol or olanzapine) but separated by 24 h did not show such an effect. These findings suggest that concurrent use of chlordiazepoxide with antipsychotics, especially with olanzapine, may cause a long-term attenuation of olanzapine sensitization through a drug-drug interaction mechanism.
Following a similar approach, a recent study examined how the antidepressant citalopram pairing with haloperidol or olanzapine during the induction phase affects antipsychotic sensitization in the conditioned avoidance response model (Sparkman and Li, 2012). It was reported that concurrent use of citalopram with both antipsychotic drugs potentiated the anti-avoidance effect of olanzapine or haloperidol (to a lesser extent) during the seven drug test sessions, indicating that citalopram enhanced the development of antipsychotic sensitization. However, in the subsequent challenge test, no group difference was found, suggesting that repeated pairing of citalopram with haloperidol or olanzapine did not affect the expression of antipsychotic sensitization. These findings suggest that the presence of an antidepressant could potentially change the strength of antipsychotic sensitization, and possibly the antipsychotic efficacy of haloperidol and olanzapine in the treatment of schizophrenia. Recently, we observed that concurrent nicotine treatment attenuated haloperidol’s sensitized effect on avoidance response (unpublished observation). This finding also suggests that haloperidol sensitization might involve drug-induced changes in nicotinic receptor. It has been reported that haloperidol non-competitively inhibits the function of mammalian neuronal nicotinic α4β2 and α7 receptors with potencies comparable to that of mecamylamine (a classical nicotinic receptor antagonist) (Grinevich et al., 2009).
Nonpharmacological factors
Like behavioral sensitization and tolerance induced by other psychoactive drugs such as amphetamine (Browman et al., 1998) and morphine (Siegel, 1978), antipsychotic sensitization and tolerance are also greatly affected by nonpharmacological factors such as environmental stimuli, selected behavioral responses, behavioral testing contingencies, and passage of time, etc.. In the following, I will summarize some relevant work on this topic.
Environmental cues and selected behavioral responses
It is well established that the manifestations of behavioral sensitization and tolerance induced by many psychoactive drugs are not mere consequences of the pharmacological actions of the drugs, but are the result of interactions amongst the pharmacological effects of drugs and the environmental cues during drug administration. The importance of environmental factors in modulating antipsychotic sensitization and tolerance has been demonstrated by many investigators. The typical approach is to compare a “paired” group (a group that receives drug injection in the test environment) with an “unpaired” group (a group that receives vehicle injection in the test environment, and drug in the home cage) (Amtage and Schmidt, 2003; Poulos and Hinson, 1982). The influence of environment is assessed on a test day, when all animals receive a challenge injection of the drug in the test environment. If a stronger or weaker drug effect is detected in the “paired” group, it would suggest that environmental stimuli have an influence on the drug effect (Robinson et al., 1998). Using such an approach, Poulos and Hinson (1982) demonstrated that Pavlovian conditioning factors determine the expression of tolerance to haloperidol catalepsy. They found that rats exhibited tolerance only in the environment previously associated with haloperidol injections, but not in the environment previously associated with saline injections. In addition, a drug-induced increase in the number of brain dopamine receptors, by itself, cannot account for the conditional occurrence of such tolerance. Schmidt’s group reported that intermittent haloperidol treatment and repeated catalepsy testing caused a sensitized cataleptic response over time and this sensitization was completely context specific, since context changes abolished catalepsy sensitization (Amtage and Schmidt, 2003; Klein and Schmidt, 2003). They reported that rats treated with haloperidol (0.25 mg/kg, i.p.) and tested over a nine-day period showed intensification of catalepsy. However, when the rats were tested in another environment, this change of the environmental context abolished the catalepsy sensitization. In addition, they found that rats that were treated with haloperidol in the home cages but not repeatedly tested for catalepsy also did not show catalepsy sensitization; often they developed tolerance towards the cataleptogenic effects of haloperidol (Schmidt et al., 1999). Similarly, sensitization induced by haloperidol and olanzapine in the conditioned avoidance response test was also context dependent, as only the rats treated with both drugs in the avoidance test apparatus and tested for avoidance responding exhibited such a sensitization; those that received the identical treatments in the home cages did not (Li et al., 2009b; Sparkman and Li, 2012).
Recently, a different approach was employed to examine the context-dependent sensitization and tolerance. It takes advantage of the fact that repeated antipsychotic treatment induces sensitization or tolerance in both the conditioned avoidance response and PCP-induced hyperlocomotion models, and sensitization or tolerance induced in these two models presumably reflects the same antipsychotic activity over time. If antipsychotic sensitization or tolerance results from inevitable neurobiological adaptations produced by the direct pharmacological actions of the drug (Tarsy and Baldessarini, 1974), it should be transferrable across models and suggests that contextual and behavioral variables have little influence on the development of antipsychotic sensitization or tolerance. On the other hand, if context and behaviors associated with drug administration have a powerful control on the expression of antipsychotic sensitization or tolerance, it should not be transferrable between models. In the first study (Zhang and Li, 2012), we tested haloperidol and olanzapine sensitizations and examined their bi-directional transfer between the conditioned avoidance response model and PCP model. Results showed that haloperidol and olanzapine sensitization induced in both models only manifested itself when the induction model was the same as the expression model. There was no expression of such a sensitization effect when the tested environment and required behavioral response were different from the original ones. These findings suggest the expression of haloperidol and olanzapine sensitization in the conditioned avoidance response model and PCP model is strongly influenced by test environment and/or selected behavioral response (Zhang and Li, 2012).
Feng et al. (2013) used a similar approach and examined how the environmental cues and behavioral responses affect the expression of clozapine tolerance. They found that when tested in the PCP model, rats previously treated with clozapine in the avoidance model did not show an immediate weaker inhibition of PCP-induced hyperlocomotion than those treated with clozapine for the first time, but showed a significantly weaker inhibition over time, suggesting that switching the environments diminished the initial expression of clozapine tolerance. In contrast, when tested in the avoidance response model, rats previously treated with clozapine in the PCP model showed an immediate weaker disruption of avoidance response than those treated with clozapine for the first time, but this weaker effect reduced over time. Therefore, similar to antipsychotic sensitization, the expression of clozapine tolerance is also strongly modulated by the test environment and/or selected behavioral response.
Because the context-dependent feature of antipsychotic sensitization and tolerance resembles the one found in psychomotor sensitization (Anagnostaras and Robinson, 1996; Anagnostaras et al., 2002; Browman et al., 1998; Robinson et al., 1998; Stewart and Vezina, 1991, Vezina et al., 1989), and tolerance (Poulos et al., 1981; Siegel, 1978; Siegel et al., 2000), the theoretical conceptualization of antipsychotic sensitization and its situational specificity can gain insights from the theoretical accounts of behavioral sensitization and tolerance. Based on the present study, our previous work (Li et al., 2004, Li et al., 2007, 2009, 2009b, 2010, Mead and Li, 2010) and the work of others (Anagnostaras et al., 2002; Stewart and Vezina, 1991), we propose that three psychological processes may govern the effect of antipsychotic sensitization or tolerance and its situational specificity (Zhang and Li, 2012): (a) repeated antipsychotic treatment induces an unconditioned and nonassociative increase or decrease of behavioral effects (i.e. sensitization); an effect attributable to the direct pharmacological action of a drug, likely mediated by drug-induced time-dependent brain changes involving various receptors or other molecules that antipsychotic drugs target; (b) distinct contextual cues (e.g. environmental stimuli, interoceptive drug cue, etc.) develop an association with unconditional drug effects via a Pavlovian conditioning process and thus become excitatory conditioned stimuli. These cues acquire the ability to elicit an antipsychotic-like effect by themselves, and may potentiate the sensitized or diminished response in an expected situation; (c) situational cues, including the contextual stimuli, interoceptive drug state, as well as topographic difference in motor responses, serve as occasion-setters to modulate the manifestation of altered responses. Occasion-setters are a class of conditional stimuli that do not themselves elicit an antipsychotic-like effect, but modulate the ability of other stimuli to elicit responses (Holland, 1989). According to this hypothesis, the same situational cue could function as both a drug-like CS and an occasion-setter.
At last, we would to emphasize that there are not two forms of antipsychotic sensitization or tolerance: “context-specific” and “context-independent”, just as there are not two forms of behavioral sensitization induced by psychostimulants (Anagnostaras et al., 2002). There is just one non-associative form of neuroplasticity manifesting behaviorally as an alteration in antipsychotic responses. This manifestation and its modulation by environmental cues and behaviors is dependent on specific experimental and drug treatment factors. Only under certain circumstances do environmental cues or behavioral responses modulate the development and expression of antipsychotic sensitization or tolerance. Therefore, the environmental cues and behavioral responses of animals may not fundamentally alter drug-induced neurobiological changes, say, in D2 or 5-HT2A receptors. They only impact the functional manifestations of drug-induced brain changes. One recent study clearly illustrates this point because it demonstrates both the “context-dependent” and “context-independent” antipsychotic sensitization and tolerance for some drugs but not others and under one condition but not others (Sun et al., 2014). In the first experiment, which examined the extent to which prior antipsychotic treatment in the home cages affected a drug’s ability to inhibit PCP-induced hyperlocomotion in a novel motor activity test apparatus, it was shown that five days of repeated haloperidol and olanzapine treatment in the home cages still potentiated their inhibition of PCP-induced hyperlocomotion (i.e. the expression of antipsychotic sensitization) assessed in a new environment, whereas the clozapine treatment enhanced the development of clozapine tolerance. These findings indicate a lack of environmental modulation of antipsychotic efficacy, a finding different from Zhang and Li (2012) and Feng et al. (2013). The second experiment examined the impact of different numbers of antipsychotic administrations in either the home environment or test environment (e.g. 4, 2, or 0) on a drug’s ability to inhibit PCP-induced hyperlocomotion. No environmental modulation was found for clozapine and olanzapine but a strong modulation was found for haloperidol, as evidenced by the finding that four-day haloperidol treatment in the test apparatus had a significantly higher inhibition than four-day home cage treatment. These findings collectively suggest that prior antipsychotic treatment in one environment could alter later antipsychotic-like response assessed in a different environment but only under certain test conditions. Therefore, whether the circumstances surrounding antipsychotic drug administration exert a powerful control of the expression of antipsychotic-like efficacy is dependent on many factors, including the degree of similarity between different test environments, drug doses, and number of drug treatments, etc. The environmental modulation on antipsychotic sensitization and tolerance may have a significant clinical implication. For one thing, it suggests that the environment where the drug is being administered could potentially change how a patient responds to the drug.
Passage of time (i.e. test interval between the induction and expression phase)
Antipsychotic sensitization and tolerance likely reflect a composite impact from two sources. One is the relatively specific pharmacological actions of a given antipsychotic drug. As mentioned before, this is likely mediated by a drug’s actions on its immediate neuroreceptor targets (e.g. D2 and 5-HT2A receptors) (Li et al., 2010) and should follow the basic principles of learning and memory, as antipsychotic sensitization and tolerance represent a non-associative form of learning and memory. Under this principle, the magnitude of sensitization and tolerance should decrease with the passage of time due to a memory trace decay process (similar to forgetting). Another source is the ubiquitous adaptive response to the foreign aspect of the drug (any drug is an exogenous agent to an organism), which tends to follow the TDS principle (Antelman et al., 1986, 2000) and this response should increase with the passage of time upon acute exposure to the drug. Therefore, under one circumstance, we may see an increase of antipsychotic sensitization and tolerance when the experimental condition favors the TDS principle, whereas under other circumstances, the sensitization and tolerance effect may decrease when the forgetting force dominates. The ultimate intensity of antipsychotic sensitization or tolerance at any given time point likely reflects the consequence of a joint action from these two forces. An earlier study did not find that the magnitude of haloperidol and olanzapine sensitization in the conditioned avoidance response test changed across the three time intervals between the induction and expression phases (i.e. 4, 10, or 17 days after the last drug treatment) (Swalve and Li, 2012). Recently, this issue was re-examined using 3 longer intervals (10, 20 and 40 days between the last drug treatment and challenge test) (Gao and Li, 2013). Once again, no increase or decrease in sensitization magnitude was observed at these test points. Thus, although theoretically, antipsychotic sensitization and tolerance could be a function of time, empirical evidence is lacking. Future research needs to examine the importance of different challenge doses and numbers of drug administration to determine the experimental conditions that favor TDS as it relates to antipsychotic sensitization and tolerance. In this regard, it appears that one single injection of haloperidol is able to induce a sensitization effect in the PCP-induced hyperlocomotion test and this effect is larger when assessed at the three-week post-injection point than at one-week post-injection (unpublished observation). We are actively pursuing this line of research to verify its robustness and its generality. It is also important to keep in mind that because environmental stimuli and behavioral response have a profound impact on the induction and expression of antipsychotic sensitization and tolerance (see the above discussion), in searching for the optimal condition that is conducive to TDS, we should pay more attention to the environmental cues and behavioral responses that are associated with drug administration.
Developmental impacts: Altered drug sensitivity due to adolescent drug exposure
Antipsychotic treatment in children and adolescents has increased dramatically in recent decades (Kalverdijk et al., 2008; Olfson et al., 2006; Rani et al., 2008). Epidemiological surveys conducted in many countries (e.g. UK, US, Germany, Netherlands) indicate a two- to six-fold increase in the number of prescribed antipsychotics for young patients (⩽20 years) between the 1990s and the mid-2000s (Kalverdijk et al., 2008; Olfson et al., 2006; Rani et al., 2008). More than 90% of the children and adolescents who are treated with antipsychotic medications are on atypical drugs (e.g. risperidone, olanzapine, and aripiprazole) for the management of disruptive behavior disorders (37.8%), mood disorders (31.8%), pervasive developmental disorders, or mental retardation (17.3%) and psychotic disorders (14.2%) (Olfson et al., 2006). Clinical research on antipsychotic treatment in children and adolescents primarily focuses on the efficacy, tolerability, and side effect profiles of individual drugs. There is a general lack of research on the long-term consequences of adolescent antipsychotic treatment on the brain and the behavioral development of patients.
Preclinical studies strongly suggest that antipsychotic exposure in adolescence could alter brain and behavioral functions. For example, animal receptor binding studies show that antipsychotic exposure during adolescence increases or decreases various neuroreceptors, including dopamine D1, D2, and D4 receptors (Moran-Gates et al., 2006; Vinish et al., 2013), serotonin 5-HT1A and 5-HT2A receptors (Choi et al., 2010), and ionotropic NMDA and AMPA glutamatergic receptors (Choi et al., 2009). Behavioral studies also suggest that early adolescent antipsychotic exposure enhances animals’ sensitivity to reward stimuli (Vinish et al., 2013), impairs their working memory, delays the extinction process of fear memory in adulthood (Milstein et al., 2013), and prevents the development of various psychosis-like behaviors (e.g. prepulse inhibition (PPI) deficit, latent inhibition deficit, etc.) induced by maternal immune activation (PolyI:C injection during pregnancy), while impairing certain behavioral functions of normal animals (Meyer et al., 2010; Piontkewitz et al., 2009, 2011, 2012).
In addition to the effects on basic brain and behavioral functions, adolescent antipsychotic exposure can also alter later antipsychotic responses in adulthood. Since 2012, we have conducted a series of experiments and delineated the extent to which antipsychotic exposure during adolescence affects ‘exposure-dependent’ alterations. Similar to what has been reported in adult animal studies, two patterns of alterations: sensitization and tolerance are also identified. The first study used the conditioned avoidance response model and addressed two important issues: first, whether olanzapine sensitization and clozapine tolerance can be induced in adolescent rats; second, the extent to which olanzapine sensitization and clozapine tolerance induced in adolescence persist into adulthood (Qiao et al., 2013). The basic paradigm is similar to that depicted in Figure 1. Male adolescent rats (~postnatal days (P) 43–47) were first treated with olanzapine or clozapine daily for five consecutive days and then challenged either in adolescence (~P 50) or after they matured into adults (~P 76 and 92). Olanzapine sensitization and clozapine tolerance were found in the behavioral measures of antipsychotic activity (e.g. avoidance response and intertrial crossing), but not in the measure of fear (e.g. CS-induced 22 kHz ultrasonic vocalizations (USVs)) (Mead et al., 2008; Sun et al., 2010). These findings suggest that antipsychotic treatment during adolescence can induce a long-term specific alteration in antipsychotic effect that persists into adulthood despite the brain maturation. Both olanzapine sensitization and clozapine tolerance effects are dose-dependent, specific to the antipsychotic effect (e.g. anti-avoidance), but not to the anxiolytic effect (e.g. a decreasing effect on 22-kHz USVs). These results also support the idea that the different behavioral effects of an antipsychotic drug undergo different time courses of change after repeated administration (Stewart and Badiani, 1993).
Following this initial study, a series of studies have been conducted on other antipsychotic drugs. Risperidone, asenapine, and haloperidol are all found to cause a sensitization effect that persists into adulthood in a similar fashion as olanzapine in the conditioned avoidance response model (Gao and Li, 2014; Qiao et al., 2014b; Shu et al., 2014a) (see Figure 5). In addition, adolescent risperidone treatment could even alter adulthood responsiveness to olanzapine (a cross-sensitization effect) and clozapine (Qiao et al., 2014b). Specifically, evidence indicates that adolescent risperidone treatment essentially enhances olanzapine sensitization and clozapine tolerance. These long-lasting changes are likely mediated by drug-induced neuroplastic changes and could have significant clinical implications because risperidone has been one of the most prescribed antipsychotic agents for children and adolescents (Patel et al., 2005) and drug switching is quite common in people with schizophrenia during the course of optimizing therapeutic regimens for individual patients (Rosenheck et al., 2009). These findings suggest that the past history of a patient’s experience with a given drug may impact his/her later response to a new drug. Thus, clinicians working with adult patients who have been treated with one drug (e.g. risperidone) but wish to switch to another drug (e.g. olanzapine or clozapine) may need to consider possible changes in antipsychotic efficacy and monitor patients’ symptom response to the new drug during this switching process.
Figure 5.
Repeated asenapine (ASE) treatment increased the suppression of avoidance response in adolescent rats (postnatal days, P 43–48) (a) and increased sensitivity to ASE re-exposure in the challenge test in adulthood (P ~76) (b). Number of avoidance responses made by the rats from the ASE (0.05 mg/kg), ASE (0.10 mg/kg), ASE (0.20 mg/kg) and vehicle groups on the last training (pre-drug) day, during the five drug test days and on the challenge test day are expressed as mean+standard error of the mean (SEM). **p<0.004, three ASE groups relative to the VEH group; #p<0.05, ASE 0.10 and ASE 0.20 groups relative to the ASE 0.05 group, respectively. Adapted from Shu Q, Qin R, Chen Y, et al. (2014b) Asenapine sensitization from adolescence to adulthood and its potential molecular basis. Behav Brain Res 273: 166–176 with permission from Elsevier.
VEH: vehicle.
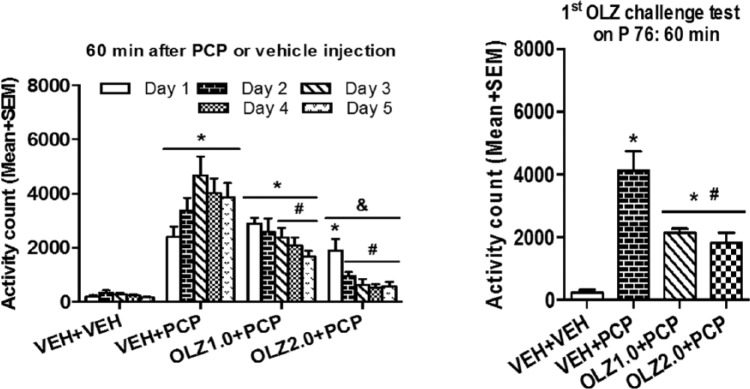
In order to validate the generality of adolescent antipsychotic sensitization and clozapine tolerance effects, it is necessary to employ a similar test paradigm used in one test (e.g., the conditioned avoidance response model) and apply it in another (e.g. PCP-induced hyperlocomotion model). Shu et al. (2014a) did just that (see Figure 6). This study showed that during adolescence, repeated olanzapine or clozapine treatment produced a persistent inhibition of PCP-induced hyperlocomotion across the five test days. In the challenge test during adolescence, rats previously treated with olanzapine did not show a significantly stronger inhibition of PCP-induced hyperlocomotion than those previously treated with vehicle. In contrast, those previously treated with clozapine showed a weaker inhibition than the vehicle controls. When assessed in adulthood, the enhanced sensitivity to olanzapine and the decreased sensitivity to clozapine were detected on ~P 76, even on ~P 91 in the case of olanzapine. These findings suggest that adolescent olanzapine or clozapine exposure can induce long-term alterations in antipsychotic response that persist into adulthood. A subsequent study demonstrated that repeated risperidone treatment in adolescence could also cause a sensitization effect in this model of antipsychotic activity (Qiao et al., 2014a).
Figure 6.
Olanzapine (OLZ) sensitization from adolescence to adulthood. Locomotor activity was measured during the 60-minute test period after daily PCP injection throughout the five test days (left) and during the OLZ challenge test on postnatal day (P) 76 (right). OLZ at 1.0 and 2.0 mg/kg induced a sensitization effect in adulthood. Adapted from Shu Q, Hu G and Li M (2014a) Adult response to olanzapine or clozapine treatment is altered by adolescent antipsychotic exposure: A preclinical test in the phencyclidine hyperlocomotion model. J Psychopharmacol 28: 363–375.
VEH: vehicle; PCP: phencyclidine.
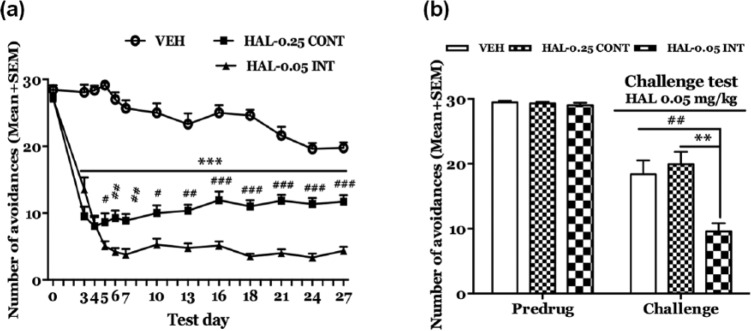
Much of our adolescent antipsychotic sensitization and tolerance work has relied on a daily intermittent drug injection schedule for a short period of time (e.g. five days). How these long-term effects are modulated by treatment schedule has never been examined. In a recent study (Gao and Li, 2014), we explored how haloperidol sensitization induced throughout adolescence and tested in adulthood was differentially impacted by these two dosing regimens in the conditioned avoidance response test (Figure 7). Adolescent rats were treated with haloperidol continuously (via osmotic minipump) or intermittently (via daily injection) from P 44 to 71. Haloperidol sensitization was assessed in a challenge test in adulthood (>P 80) in which all rats were injected with haloperidol. Interestingly, only the intermittent dosing group showed a robust sensitization effect. This finding suggests that adolescent haloperidol sensitization is a schedule-specific phenomenon, much like what we observe in other behavioral effects of antipsychotic drugs (Samaha et al., 2008; Turrone et al., 2005). It is more likely to be seen under an intermittent dosing regimen than under a continuous dosing one.
Figure 7.
Effects of chronic continuous versus intermittent haloperidol (HAL) treatment on conditioned avoidance responding over time. (a) Number of avoidance responses made by the rats treated with HAL-0.25 CONT (0.25 mg/kg/day via minipump, n=14), HAL-0.05 INT (0.05 mg/kg/injection/day sc, n=14) or vehicle (VEH, n=13) on the predrug (0) day, and drug test days. ***p<0.001 for comparisons between HAL-(0.05 INT and 0.25 CONT) and vehicle (VEH); ###p<0.001, ##p<0.01, #p<0.05 for comparisons between HAL-0.05 INT and HAL-0.25 CONT on each test day. (b) Number of avoidance responses made by the three groups of rats in the HAL 0.05 mg/kg challenge tests. After retraining, all groups were injected with HAL 0.05 mg/kg (sc) 11 days after the last HAL treatment. Avoidance tests were conducted 60 min later. All data are expressed as mean+standard error of the mean (SEM). **p<0.01 for comparison to the VEH group; ##p<0.01 for comparison to the HAL-0.05 INT group. Adapted from Gao J and Li M (2014) Differential effects of intermittent versus continuous haloperidol treatment throughout adolescence on haloperidol sensitization and social behavior in adulthood. Prog Neuropsychopharmacol Biol Psychiatry 54: 67–75 with permission from Elsevier.
CONT: continuous; INT: intermittent.
Recently, we showed that persistent aripiprazole sensitization from adolescence to adulthood is sex-dependent (unpublished observation). In both the induction phase and the expression phase, male rats always had significantly lower avoidance than the females under aripiprazole, indicating that male rats might be more sensitive to aripiprazole treatment. This result suggests that antipsychotic sensitization might vary between sexes and clearly has a significant clinical implication if replicated.
As discussed at the beginning of this section, adolescent antipsychotic treatment is known to exert long-term impacts of basic behavioral and brain functions. However, no studies have examined whether adolescent antipsychotic treatment would affect social functioning in adulthood, one of seven primary cognitive domains that are affected in schizophrenia (Floresco et al., 2005; Green et al., 2004,). Gao and Li (2014) examined how intermittent and continuous haloperidol treatment may potentially impact social interaction and social memory using a paradigm that we validated in amphetamine and phencyclidine-based animal models of schizophrenia (Li et al., 2012). The social memory of rats was evidenced by the findings that a subject rat decreased its time investigating the same testing partner after a waiting period (~10 min) and increased its time on investigation if a novel partner was introduced (Akers et al., 2006; Holloway and Thor, 1988; Prediger et al., 2004,). Our results show that adolescent haloperidol treatment (continuous and intermittent) did not affect social behavior and social memory, as rats from the two haloperidol groups and the vehicle group exhibited a similar level of social interaction and showed a similar level of sensitivity to the change of social stimuli. This finding suggests that adolescent haloperidol treatment under both regimens did not fundamentally damage social functioning. Thus, the clinical significance of haloperidol sensitization needs to be further examined.
Collectively, these important findings firmly establish that antipsychotic treatment in adolescence can induce a long-term change in drug responsiveness that persists into adulthood. This altered sensitivity appears to be sex- and regimen-specific. Because antipsychotic drugs are being increasingly used in children and adolescents in the past two decades, findings from this study are important for understanding the impacts of adolescent antipsychotic treatment on the brain and behavioral developments. Furthermore, although we have demonstrated that environmental stimuli and behavioral response associated with drug treatment have a profound impact on the induction and expression of antipsychotic sensitization and tolerance in adult animals (Feng et al., 2013; Sun et al., 2014; Zhang and Li, 2012), there is no study that has examined how such factors could affect adolescent antipsychotic sensitization and tolerance. Given their potential effects on brain development, if we can identify the clinical and experimental conditions that modulate adolescent antipsychotic sensitization and tolerance, we could then better use them to our advantages. This work also has implications for clinical practice involving adolescent antipsychotic treatments in terms of drug choice, drug dose, and schedule, and treatment setting.
Neurobiological mechanisms
Although behavioral sensitization and tolerance induced by antipsychotic drugs are well established, the molecular mechanisms (e.g. receptor, intracellular signaling molecules) and neural basis of these effects are less clear. Given the fact that all antipsychotic drugs have immediate actions on dopamine D2 and 5-HT2A receptors (Meltzer et al., 1989; Meltzer et al., 2003; Miyamoto et al., 2005), and repeated antipsychotic treatment induces long-term changes in these receptors (Tarazi et al., 2001), one naturally suspects that changes in these receptors may account for the behavioral sensitization and tolerance induced by antipsychotic drugs. As the following results may show, antipsychotic-induced changes in D2 and 5-HT2A receptors in the various limbic areas are indeed in part involved in the mediation of the induction and/or expression of antipsychotic sensitization or tolerance. It should be noted that because many antipsychotics also have various degrees of affinity for a number of other neuronal receptors, including α-adrenergic, histamine H1, serotonin 5-HT1A, 5-HT6 and 5-HT7 receptors, and muscarinic receptors, and this multi-receptor action is likely to affect their efficacy and side effect profile (Lieberman et al., 2008), the magnitude and persistence of antipsychotic sensitization and tolerance might also be, to some extent, modulated by these receptor-binding affinities. Unfortunately, there is little research on the involvement of receptors other than D2 and 5-HT2A in antipsychotic sensitization and tolerance. Therefore, on the receptor mechanisms, we will have to limit ourselves to these two receptors.
Pharmacological studies on dopamine D2 and 5-HT2A receptor mechanisms
Li et al. (2012) took a pharmacological approach and compared the neuroreceptor mechanisms underlying acute and repeated treatment effects of haloperidol, clozapine, or olanzapine treatment, respectively. Specifically, they gave rats three days of repeated drug treatment and tested them in the conditioned avoidance response model. For some drug-treated rats, they were also concurrently administrated with either saline, quinpirole (a selective dopamine D2/3 agonist,), or 2,5-dimethoxy-4-iodo-amphetamine (DOI, a selective 5-HT2A/2C agonist). After two days drug-free retraining, a drug challenge test was conducted to examine the magnitude of haloperidol/olanzapine sensitization and clozapine tolerance. A previous study already shows that acute pretreatment of quinpirole, but not DOI, can dose-dependently reverse the haloperidol-induced disruption of active maternal responses, whereas acute pretreatment of DOI, but not quinpirole can reverse the disruption induced by clozapine (Zhao and Li, 2009a). Based on these findings and the receptor binding profiles of each antipsychotic (Miyamoto et al., 2005), it was hypothesized that acute and repeated effects of haloperidol may be mediated by its action on D2/3 receptor system, whereas those of olanzapine and clozapine may be mediated by their action on 5-HT2A/2C receptors. If this hypothesis were correct, quinpirole, but not DOI, should be able to attenuate acute haloperidol-induced disruption of avoidance response, and may also be effective in reducing haloperidol sensitization. In contrast, DOI, but not quinpirole, is expected to attenuate acute clozapine-induced disruption of avoidance, and may also be effective in reducing clozapine tolerance. For olanzapine, both quinpirole and DOI might have a reversal effect on its acute and repeated effects. This hypothesis was only partially confirmed. Specifically, pretreatment of quinpirole, but not DOI, did attenuate the acute haloperidol-induced disruption of avoidance responding and to a lesser extent, olanzapine-induced disruption. In contrast, pretreatment of DOI, but not quinpirole, attenuated the acute effect of clozapine. However, on the sensitization or tolerance effect, two unexpected findings were obtained. First, pretreatment of DOI, but not quinpirole, attenuated the haloperidol sensitization. Second, pretreatment of quinpirole enhanced the tolerance-like effect of clozapine and attenuated olanzapine sensitization. These results indicate that haloperidol sensitization may be mediated by its action on 5-HT2A/2C receptor system, whereas long-term effects of olanzapine and clozapine may be mediated by their action on the D2/3 receptor system. Although haloperidol is typically viewed as a strong D2 antagonist, it is also a 5-HT2A receptor inverse agonist (Weiner et al., 2001), and repeated haloperidol treatment causes a reduction in 5-HT2A receptor mRNA expression in various limbic regions (Buckland et al., 1997). Therefore, it is possible that haloperidol causes a sensitization effect by down-regulating 5-HT2A receptor. DOI may decrease this long-term impact of haloperidol by counteracting its effect on 5-HT2A receptor. This idea is also consistent with the well-known augmentation effect of 5-HT2A antagonism on the effects of haloperidol, as 5-HT2A-selective antagonist M100907 is shown to potentiate haloperidol-induced dopamine release in the medial prefrontal cortex (Bonaccorso et al., 2002), to reduce the reward-attenuating effect of haloperidol (Benaliouad et al., 2007), and to potentiate the avoidance disruptive effect of haloperidol (Wadenberg et al., 2001). The clozapine tolerance and olanzapine sensitization via D2/3 receptor systems could be understood in the context of their known long-term effect on D2/3 receptors (Atkins et al., 1999; Kapur et al., 2003; Moran-Gates et al., 2006). But why activation of D2/3 receptors reduces olanzapine sensitization but potentiates clozapine tolerance is not clear. One important lesson from this study is that the neuroreceptor mechanisms underlying the acute effect of an antipsychotic drug could be dissociable from those underlying its long-term effect. Thus, for the long-term effects such as sensitization and tolerance, the drug-initiated neural plasticity plays a more important role than the immediate targets of a drug.
Behavioral studies on dopamine D2 receptor mechanism
Although we failed to show the involvement of D2 receptor in haloperidol sensitization, given its well characterized antagonism of D2 receptors, it seems premature to discount the role of this receptor system in the mediation of antipsychotic sensitization in general. Gao and Li (2013) used the quinpirole-induced hyperlocomotion test and further investigated the involvement of D2 receptor in antipsychotic sensitization. This test is a widely used method assessing drug or non-drug induced changes in D2 function (Tenk et al., 2007; Vorhees et al., 2009). Because quinpirole is a preferential D2/3 receptor agonist, and its psychomotor stimulating effect (i.e. increasing locomotor activity) is generally attributed to its selective agonism on D2, if a drug-treated rat shows a higher level of motor activity under quinpirole challenge than a vehicle-treated one, it would suggest that the drug causes an upregulation of D2 receptor (Luque-Rojas et al., 2013; Moreno et al., 2005). Indeed, this quinpirole-induced hyperlocomotion has been thought to be mediated through an increase in the efficacy of the post-synaptic D2 transduction (Szumlinski et al., 1997, 2000). Gao and Li (2013) observed that prior risperidone-treated adult rats showed a sensitization effect in the conditioned avoidance response test. Also, they exhibited a significantly higher level of motor activity than the vehicle-pretreated ones when they were all challenged with quinpirole, suggesting that risperidone sensitization is likely mediated by D2 receptor supersensitivity (Seeman, 2011). A more recent study from our laboratory showed that aripiprazole-induced sensitization in adult rats is also mediated by drug-induced upregulation of D2 receptor (Gao et al., 2015). However, antipsychotic sensitization induced during adolescence seems less dependent on D2 receptor upregulation, as adult rats that had been treated with risperidone or haloperidol in adolescence failed to show an increased motor activity under the quinpirole challenge, despite the fact that they exhibited a robust sensitization effect (Gao and Li, 2014; Qiao et al., 2014a). This finding highlights that antipsychotic treatment during the adolescent period may alter D2 receptors and others (e.g., 5-HT2A, 5-HT2B and 5-HT1A) in unique ways not seen in adult animals. Thus, adolescent antipsychotic sensitization (or tolerance) may rely on different receptor mechanisms than adulthood sensitization. This is because various neurotransmitter systems — especially the dopamine and serotonin systems in the prefrontal cortex, striatum, and hippocampus — are still undergoing maturational changes during adolescence (Benes et al., 2000; Teicher et al., 1995). At the present time, available evidence indicates that antipsychotic sensitization induced by olanzapine, risperidone and aripiprazole is likely mediated by D2 receptor upregulation, at least in adult rats. The 5-HT2A receptors may also play an important role in this effect as seen in haloperidol sensitization. Clearly, more work is needed to delineate the neuroreceptor mechanisms of antipsychotic sensitization and tolerance.
c-Fos immunocytochemistry study of the neural basis of antipsychotic sensitization
c-Fos, a protein product of immediate-early gene c-fos has been used as a molecular biomarker for identifying the neural basis of acute antipsychotic treatment (Robertson and Fibiger, 1992; Robertson et al., 1994). Acute administration of typical antipsychotic haloperidol and atypical drug clozapine produces a different induction pattern of c-Fos expression in the forebrain, with acute haloperidol increasing c-Fos-positive neurons in the dorsolateral striatum (DLSt), nucleus accumbens shell (NAs) and core (NAc), and lateral septal nucleus (LS) and acute clozapine producing such effects in the NAs, medial prefrontal cortex (mPFC) (Robertson and Fibiger, 1992; Robertson et al., 1994). Based on these observations, we postulated that by examining how repeated antipsychotic treatment alters c-Fos expression, we may be able to identify the neuroanatomical bases of antipsychotic sensitization or tolerance. In one study (Zhao et al., 2012), the c-Fos expression in the PCP-induced hyperlocomotion model was examined. Once daily for five days, adult male rats were injected with haloperidol, clozapine or saline, followed by an injection of PCP or saline 30 min later, and motor activity was measured for 90 min after PCP injection. c-Fos immunoreactivity was assessed either after acute (day 1) or repeated (day 5) haloperidol or clozapine tests. Based on the changes of c-Fos expression, a brain region had to meet the following three criteria in order to be considered as part of the neural circuit(s) by which haloperidol and clozapine act to achieve their sensitization or tolerance effect, respectively. First, it should show altered c-Fos expression in response to both acute and repeated treatment of PCP. Second, it should show altered PCP-induced c-Fos expression in response to acute and repeated treatment with haloperidol or clozapine. Third, it should show a change in c-Fos expression from day 1 to day 5. Based on these criteria, three regions including NAs, central amygdala (CeA) and VTA could be classified as part of the haloperidol neural circuit (likely mediating haloperidol sensitization), and three regions including mPFC, ventral part of lateral septal nucleus (LSv) and VTA as part of the clozapine neural circuit (likely mediating clozapine tolerance). It should be pointed out that while c-Fos is an important step in illuminating the differences in neuronal actions between haloperidol and clozapine in this task, these data should be regarded as one piece of evidence toward delineating the neural basis of these drug effects. Thus, other indices such as neurotransmitter release, receptor density changes should be used to validate the current findings in future work.
Central microinjection studies on dopamine D2 and 5-HT2A mechanisms
Previous studies indicate that down-regulation of 5-HT2A receptors is one of the mechanisms underlying the therapeutic effects of chronic treatment with antipsychotic drugs (Moreno et al., 2013). Furthermore, our own c-Fos study suggested that the mPFC is part of the neural circuit that mediates the repeated effect of clozapine, e.g. clozapine tolerance. Therefore, it is possible that 5-HT2A receptors in the mPFC might be one of the central receptor mechanisms of clozapine tolerance. We are aware of only one study that tested this hypothesis in the CAR model (Feng et al., 2015). In this microinjection study, adult male rats were first trained in the avoidance test and then repeatedly injected with vehicle or clozapine for five days; their avoidance response was tested daily. Fifteen minutes before each daily test, they were also centrally infused with selective 5-HT2A/2C agonist DOI at 0.0, 5.0, or 25.0 µg/0.5 µL/side into the mPFC. It was shown that intra-mPFC infusions of DOI had no effect on the acute avoidance-disruptive effect of clozapine throughout the five test days. One day after the 5th clozapine test, all rats were retrained drug-free to bring their avoidance back to the pre-drug level before the final challenge test to assess the expression of clozapine tolerance. In the challenge test, we found that rats centrally infused with DOI 25.0 µg/0.5 µL/side during the repeated clozapine treatment days did not show higher avoidance than their corresponding vehicle controls, indicating an absence of clozapine tolerance. In other words, activation of 5–HT2A/2C serotonergic receptors in the mPFC by DOI did not affect the acute effect of clozapine, but only abolished clozapine tolerance, suggesting clozapine tolerance is mediated by 5–HT2A/2C receptors in the mPFC. This notion is supported by the subsequent experiment in which we centrally injected DOI 25.0 µg/0.5 µL/side immediately prior to the challenge test. We found that the intra-mPFC infusion of DOI at 25.0 µg/0.5 µL/side prior to the challenge test blocked the expression of clozapine tolerance. Thus, findings from this study confirmed that the mPFC is one critical brain region where clozapine acts to achieve its behavioral effects. It also suggests that the expression of clozapine tolerance, but not the tolerance induction is dependent on 5-HT2A/2C receptors in the mPFC.
Possible intracellular mechanisms
Different classes of clinically effective antipsychotics all share a common molecular mechanism involving inhibition of D2/β-arrestin-mediated signaling (Li et al., 2007; Masri et al., 2008). GSK3β is a key substrate of the dopamine-mediated β-arrestin/Akt signaling pathway and plays a critical role in neuronal development and function, including neurogenesis, axon/dendrite differentiation, neuronal positioning, synaptic transmission and plasticity, and neural apoptosis (Kaidanovich-Beilin et al., 2012; Kim and Snider, 2011). Dysregulation of this enzyme activity (e.g. reduced GSK3β protein levels in the prefrontal cortex) has been reported in patients with schizophrenia and mood disorders and in animal models of these mental disorders (Kozlovsky et al., 2005; Nadri et al., 2003). Antipsychotic drugs are demonstrated to cause an increase in the phosphorylation of GSK3β and concomitant inhibition of GSK3β activity via antagonizing D2 and 5-HT2A, and this GSK3β action is thought to mediate the therapeutic effects of antipsychotic treatment (Beaulieu et al., 2007; Freyberg et al., 2010; Karam et al., 2010; Li et al., 2007). More importantly, such regulation of GSK3β activity has been reported after chronic treatment with antipsychotic drugs, leading us to speculate that inhibition of GSK3β activity by antipsychotic treatment might be one of the mechanisms leading to persistent antipsychotic sensitization from adolescence to adulthood. If this hypothesis were correct, we would expect that (a) antipsychotic treatment would cause a persistent decrease in GSK3β activity (increased phospho-GSK3β); and (b) increasing GSK3β activity would attenuate the antipsychotic sensitization effect induced in both adolescence and adulthood. We recently obtained promising preliminary data consistent with the first expected result. Rats that showed a persistent olanzapine sensitization from adolescence to adulthood had higher levels of p-Akt and p-GSK3β, suggesting that the elevated p-Akt and p-GSK3β may be responsible for this long-lasting effect. Future systemic work needs to further test this hypothesis and determines whether elevated p-Akt and p-GSK3β is responsible for the long-lasting antipsychotic sensitization and tolerance in general.
Summary and future research
It is well documented now that antipsychotic drugs are exogenous stimuli that impact the brain and cause long-term behavioral changes and associated neuroadaptations. Behaviorally, antipsychotic-induced changes reflect an increase (sensitization) or decrease (tolerance) in drug sensitivity and environmental cues and behavioral response associated with drug treatment have a profound impact on the induction and expression of antipsychotic sensitization and tolerance. Neurochemically, dopamine D2 and serotonin 5-HT2A receptors play a role in these two behavioral effects of antipsychotic treatment. Neuroanatomically, the mPFC-related neural circuitry is critically involved in the clozapine tolerance, while other regions (e.g. NAs, VTA, and CeA) may be involved in the mediation of antipsychotic sensitization.
The present paper reviews some of the important evidence in the literature, focusing on the drug-induced changes in antipsychotic response. It is fair to say that although the research community of antipsychotic drugs is relatively large and highly active, this particular field (i.e. research on antipsychotic sensitization and tolerance) is rather small, and much of the work comes from a limited number of laboratories and uses a limited number of animal models (e.g. conditioned avoidance, PCP-induced hyperlocomotion, etc.). Therefore, it is imperative for future research to raise the profile of this area by focusing several areas, as outlined below.
One major area of research is to determine the clinical relevance of antipsychotic sensitization and tolerance. Are they clinically relevant for the explanation of the clinical effects (both therapeutic and side effects) of antipsychotic treatment? What clinical effect could be explained by sensitization and what could be explained by tolerance? What pharmacological features account for the differences between sensitization and tolerance? Why does clozapine primarily cause a tolerance in several behavioral tests of antipsychotic activity, while others induce a sensitization? Could this difference explain the superior treatment effect of clozapine? etc. What clinical phenomena are associated with clozapine tolerance? Clinician scientists could help answer these questions by looking into these two mechanisms as explanatory tools. At this time, it is fair to say that preclinical findings on antipsychotic sensitization and tolerance have not made a good connection with clinical research. Thus basic psychopharmacologists also need to understand clinical phenomena better.
As mentioned, antipsychotic sensitization and tolerance have not been systematically studied in a preclinical disease model of schizophrenia. All the published work so far has been done in otherwise healthy male rats. Therefore, the face validity is low as only humans with severe mental disorders such as schizophrenia receive antipsychotic therapy. Animal work also suggests that antipsychotic treatment has differential effects on “diseased” rodents and normal controls (Meyer et al., 2010). Thus, one important future focus for basic scientists is to examine antipsychotic sensitization and tolerance in animal models of schizophrenia. Resolving this issue could also help determine the clinical significance of antipsychotic sensitization and tolerance.
To date, limited research is available on the effects of adolescent antipsychotic exposure on basic psychological functions such as attention, novelty-seeking, emotion, and learning and memory (Milstein et al., 2013; Vinish et al., 2013). How antipsychotic sensitization and tolerance contribute to these effects has never been explored. Furthermore, whether functional changes in D2 and 5-HT2A receptor expressions induced by adolescent antipsychotic treatment are related to drug-induced behavioral effects has not been examined. Future research should fill these knowledge gaps by connecting what we know about the basic behavioral effects of adolescent antipsychotic treatment with the treatment’s intrinsic property of altering drug sensitivity. This research will significantly enhance our understanding of the positive and negative impacts of adolescent antipsychotic treatment on drug response, behavioral functions, and brain functions.
Another important research area is to identify the neural mechanisms of antipsychotic sensitization and tolerance. Because antipsychotic drugs have a profound impact on a variety of brain signaling molecules involved in a variety of important yet different brain functions (e.g. synaptic neurotransmission, neuroendocrine regulation, oxidative stress, adult neurogenesis etc.) (Chou et al., 2015; Seeman, 2002; Stojkovic et al., 2012), and even though we are certain that the sensitization and tolerance induced by these drugs must reflect the consequences of drug-induced neuroplastic changes, we do not fully understand the exact and critical mechanisms underlying the long-term sensitization and tolerance effects. There is not much research that has made an effort to connect drug-induced brain changes at the molecular/system level to the sensitization and tolerance at the behavioral level. This line of inquiry should be one of the focuses of future antipsychotic research. One related area of research is to investigate the neurobiological mechanism underlying the environmental control of the induction and expression of antipsychotic sensitization and tolerance. This work will help us gain better understanding of the neurobiological mechanisms underlying sensitization and tolerance effects.
Also, clinical evidence suggests that women react more favorably to antipsychotic therapy than men (Szymanski et al., 1995), and preclinical evidence also suggests that sex of animals is an important factor in the modulation of antipsychotic response, with females tending to have increased sensitivity to antipsychotic treatment due to lower D2 affinity compared to males (Pohjalainen et al., 1998). Our recent study also indicates that the magnitude of aripiprazole sensitization differs between male and female rats. With NIH’s increased emphasis on sex as a biological variable in the design and analysis of NIH-funded research involving animals, determining antipsychotic sensitization and its impact on basic behavioral and brain functions in both male and female animals becomes especially urgent. Unfortunately, all published antipsychotic sensitization studies so far used only male animals. On the dosing regimens, much of this work only used a daily intermittent drug injection schedule for a short period of time (e.g. five days) and most only tested male normal rats, while both clinical and preclinical evidence suggests that females tend to have delayed onset of psychopathology of schizophrenia (Han et al., 2012; Piontkewitz et al., 2012) and increased sensitivity to antipsychotic treatment due to lower D2 affinity compared to males (Pohjalainen et al., 1998). It is thus important to examine sex differences in antipsychotic sensitization and tolerance and use a variety of different dosing regiments, such as continuous treatment via osmatic minipumps or drinking water or prolonged treatment for months.
Acknowledgments
The author is grateful to all the people who has contributed to this line of research over the years. The author also wishes to thank the three anonymous reviewers for their insightful comments, which greatly improved the quality of this version.
Footnotes
Declaration of Conflicting Interest: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Institute of Mental Health (NIMH) grants (R01MH085635 and 1R03HD079870-01A1) to Ming Li.
References
- Agid O, Kapur S, Arenovich T, et al. (2003) Delayed-onset hypothesis of antipsychotic action: A hypothesis tested and rejected. Arch Gen Psychiatry 60: 1228–1235. [DOI] [PubMed] [Google Scholar]
- Agid O, Seeman P, Kapur S. (2006) The “delayed onset” of antipsychotic action–an idea whose time has come and gone. J Psychiatry Neurosci 31: 93–100. [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Nakazawa M, Romeo RD, et al. (2006) Early life modulators and predictors of adult synaptic plasticity. Eur J Neurosci 24: 547–554. [DOI] [PubMed] [Google Scholar]
- Alfaro CL. (2001) Emerging role of drug interaction studies in drug development: The good, the bad, and the unknown. Psychopharmacol Bull 35: 80–93. [PubMed] [Google Scholar]
- Allen JA, Yost JM, Setola V, et al. (2011) Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A 108: 18488–18493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtage J, Schmidt WJ. (2003) Context-dependent catalepsy intensification is due to classical conditioning and sensitization. Behav Pharmacol 14: 563–567. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. (1996) Sensitization to the psychomotor stimulant effects of amphetamine: Modulation by associative learning. Behav Neurosci 110: 1397–1414. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Schallert T, Robinson TE. (2002) Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology 26: 703–715. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Kocan D, Edwards DJ, et al. (1986) Behavioral effects of a single neuroleptic treatment grow with the passage of time. Brain Res 385: 58–67. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Levine J, Gershon S. (2000) Time-dependent sensitization: the odyssey of a scientific heresy from the laboratory to the door of the clinic. Mol Psychiatry 5: 350–356. [DOI] [PubMed] [Google Scholar]
- Aquino CC, Lang AE. (2014) Tardive dyskinesia syndromes: Current concepts. Parkinsonism Relat Disord 20: S113–S117. [DOI] [PubMed] [Google Scholar]
- Arnt J. (1982) Pharmacological specificity of conditioned avoidance response inhibition in rats: Inhibition by neuroleptics and correlation to dopamine receptor blockade. Acta Pharmacol Toxicol (Copenh) 51: 321–329. [DOI] [PubMed] [Google Scholar]
- Atkins JB, Chlan-Fourney J, Nye HE, et al. (1999) Region-specific induction of deltafosb by repeated administration of typical versus atypical antipsychotic drugs. Synapse 33: 118–128. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Robinson B, Csernansky JG, et al. (1990) Sensitization versus tolerance to haloperidol-induced catalepsy: Multiple determinants. Pharmacol Biochem Behav 36: 883–887. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. (2007) The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci 28: 166–172. [DOI] [PubMed] [Google Scholar]
- Benaliouad F, Kapur S, Rompre PP. (2007) Blockade of 5-Ht2a receptors reduces haloperidol-induced attenuation of reward. Neuropsychopharmacology 32: 551–561. [DOI] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. (2000) Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: Implications for the development of psychopathology. Cereb Cortex 10: 1014–1027. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Phillips AG, Fibiger HC. (1983) Prior training and intermittent retraining attenuate pimozide-induced avoidance deficits. Pharmacol Biochem Behav 18: 619–624. [DOI] [PubMed] [Google Scholar]
- Bignami G. (1978) Effects of neuroleptics, ethanol, hypnotic-sedatives, tranquilizers, narcotics, and minor stimulants in aversive paradigms. In: Anisman H, Bignami G. (eds) Psychopharmacology of Aversively Motivated Behavior. New York: Plenum Press, pp.385–453. [Google Scholar]
- Bonaccorso S, Meltzer HY, Li Z, et al. (2002) Sr46349-B, a 5-Ht(2a/2c) receptor antagonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Neuropsychopharmacology 27: 430–441. [DOI] [PubMed] [Google Scholar]
- Boyd EM. (1960) Chlorpromazine tolerance and physical dependence. J Pharmacol Exp Ther 128: 75–78. [PubMed] [Google Scholar]
- Browman KE, Badiani A, Robinson TE. (1998) Modulatory effect of environmental stimuli on the susceptibility to amphetamine sensitization: A dose-effect study in rats. J Pharmacol Exp Ther 287: 1007–1014. [PubMed] [Google Scholar]
- Buckland PR, D’souza U, Maher NA, et al. (1997) The effects of antipsychotic drugs on the mrna levels of serotonin 5ht2a and 5ht2c receptors. Brain Res Mol Brain Res 48: 45–52. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Deveaugh-Geiss J. (1984) Treatment schedule as a determinant of the development of tolerance to haloperidol. Psychopharmacology (Berl) 82: 164–167. [DOI] [PubMed] [Google Scholar]
- Choi YK, Gardner MP, Tarazi FI. (2009) Effects of risperidone on glutamate receptor subtypes in developing rat brain. Eur Neuropsychopharmacol 19: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YK, Moran-Gates T, Gardner MP, et al. (2010) Effects of repeated risperidone exposure on serotonin receptor subtypes in developing rats. Eur Neuropsychopharmacol 20: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Jones S, Li M. (2015) Adolescent olanzapine sensitization is correlated with hippocampal stem cell proliferation in a maternal immune activation rat model of schizophrenia. Brain Res 1618: 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard G, Jones BD. (1980) Neuroleptic-induced supersensitivity psychosis: clinical and pharmacologic characteristics. Am J Psychiatry 137: 16–21. [DOI] [PubMed] [Google Scholar]
- Diamond BI, Borison RL. (1986) Basic and clinical studies of neuroleptic-induced supersensitivity psychosis and dyskinesia. Psychopharmacol Bull 22: 900–905. [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Goudie AJ. (1989) Psychoactive Drugs: Tolerance and Sensitization. Clifton, NJ: Humana Press. [Google Scholar]
- Ezrin-Waters C, Seeman P. (1977) Tolerance of haloperidol catalepsy. Eur J Pharmacol 41: 321–327. [DOI] [PubMed] [Google Scholar]
- Fallon P, Dursun SM. (2011) A naturalistic controlled study of relapsing schizophrenic patients with tardive dyskinesia and supersensitivity psychosis. J Psychopharmacol 25: 755–762. [DOI] [PubMed] [Google Scholar]
- Feng M, Gao J, Sui N, et al. (2015) Effects of central activation of serotonin 5-Ht2a/2c or dopamine D 2/3 receptors on the acute and repeated effects of clozapine in the conditioned avoidance response test. Psychopharmacology (Berl) 232: 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Sui N, Li M. (2013) Environmental and behavioral controls of the expression of clozapine tolerance: Evidence from a novel across-model transfer paradigm. Behav Brain Res 238: 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Geyer MA, Gold LH, et al. (2005) Developing predictive animal models and establishing a preclinical trials network for assessing treatment effects on cognition in schizophrenia. Schizophr Bull 31: 888–894. [DOI] [PubMed] [Google Scholar]
- Fregnan GB, Chieli T. (1980) Classical neuroleptics and deconditioning activity after single or repeated treatment. role of different cerebral neurotransmitters. Arzneimittelforschung 30: 1865–1870. [PubMed] [Google Scholar]
- Freyberg Z, Ferrando SJ, Javitch JA. (2010) Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry 167: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Li M. (2013) Time-dependence of risperidone and asenapine sensitization and associated D2 receptor mechanism. Behav Brain Res 257: 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Li M. (2014) Differential effects of intermittent versus continuous haloperidol treatment throughout adolescence on haloperidol sensitization and social behavior in adulthood. Prog Neuropsychopharmacol Biol Psychiatry 54: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Qin R, Li M. (2015) Repeated administration of aripiprazole produces a sensitization effect in the suppression of avoidance responding and phencyclidine-induced hyperlocomotion and increases D2 receptor-mediated behavioral function. J Psychopharmacol 29: 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelder MG, López Ibor JJ, Andreasen NC. (2000) New Oxford Textbook of Psychiatry. Oxford: Oxford University Press. [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, et al. (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology (Berl) 156: 117–154. [DOI] [PubMed] [Google Scholar]
- Gill KM, Cook JM, Poe MM, et al. (2014) Prior antipsychotic drug treatment prevents response to novel antipsychotic agent in the methylazoxymethanol acetate model of schizophrenia. Schizophr Bull 40: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason SD, Shannon HE. (1997) Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl) 129: 79–84. [DOI] [PubMed] [Google Scholar]
- Goudie AJ. (1993) Some future research directions: Commentary on Stewart and Badiani ‘Tolerance and sensitization to the behavioral effects of drugs’. Behav Pharmacol 4: 313–314. [PubMed] [Google Scholar]
- Goudie AJ, Cole JC. (2008) Switching antipsychotics. antipsychotic tolerance, withdrawal and relapse: Unresolved issues and research implications. J Psychopharmacol 22: 815–817. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Cole JC, Sumnall HR. (2007) Olanzapine and Jl13 induce cross-tolerance to the clozapine discriminative stimulus in rats. Behav Pharmacol 18: 9–17. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, et al. (2004) Approaching a consensus cognitive battery for clinical trials in schizophrenia: The Nimh-Matrics conference to select cognitive domains and test criteria. Biol Psychiatry 56: 301–307. [DOI] [PubMed] [Google Scholar]
- Grinevich VP, Papke RL, Lippiello PM, et al. (2009) Atypical antipsychotics as noncompetitive inhibitors of alpha4beta2 and alpha7 neuronal nicotinic receptors. Neuropharmacology 57: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Huang XF, Chen Da C, et al. (2012) Gender differences in cognitive function of patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 39: 358–363. [DOI] [PubMed] [Google Scholar]
- Holland PC. (1989) Transfer of negative occasion setting and conditioned inhibition across conditioned and unconditioned stimuli. J Exp Psychol Anim Behav Process 15: 311–328. [PubMed] [Google Scholar]
- Holloway WR, Jr, Thor DH. (1988) Social memory deficits in adult male rats exposed to cadmium in infancy. Neurotoxicol Teratol 10: 193–197. [DOI] [PubMed] [Google Scholar]
- Kaempf GL, Porter JH. (1987) Differential effects of pimozide and clozapine on schedule-controlled and scheduled-induced behaviors after acute and chronic administration. J Pharmacol Exp Ther 243: 437–445. [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Beaulieu JM, Jope RS, et al. (2012) Neurological functions of the masterswitch protein kinase - GSK-3. Front Mol Neurosci 5: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalverdijk LJ, Tobi H, Van Den Berg PB, et al. (2008) Use of antipsychotic drugs among Dutch youths between 1997 and 2005. Psychiatr Serv 59: 554–560. [DOI] [PubMed] [Google Scholar]
- Kapur S. (1998) A new framework for investigating antipsychotic action in humans: Lessons from pet imaging. Mol Psychiatry 3: 135–140. [DOI] [PubMed] [Google Scholar]
- Kapur S. (2003) Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 160: 13–23. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D. (2003) Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 27: 1081–1090. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. (2001) Atypical antipsychotics: New directions and new challenges in the treatment of schizophrenia. Annu Rev Med 52: 503–517. [DOI] [PubMed] [Google Scholar]
- Kapur S, Agid O, Mizrahi R, et al. (2006) How antipsychotics work–from receptors to reality. NeuroRx 3: 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Arenovich T, Agid O, et al. (2005) Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry 162: 939–946. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mizrahi R, Li M. (2005) From dopamine to salience to psychosis–linking biology, pharmacology and phenomenology of psychosis. Schizophr Res 79: 59–68. [DOI] [PubMed] [Google Scholar]
- Kapur S, Vanderspek SC, Brownlee BA, et al. (2003) Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: A suggested solution based on in vivo occupancy. J Pharmacol Exp Ther 305: 625–631. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, et al. (2000) Relationship between dopamine D(2) occupancy, clinical response, and side effects: A double blind pet study of first-episode schizophrenia. Am J Psychiatry 157: 514–520. [DOI] [PubMed] [Google Scholar]
- Karam CS, Ballon JS, Bivens NM, et al. (2010) Signaling pathways in schizophrenia: Emerging targets and therapeutic strategies. Trends Pharmacol Sci 31: 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Snider WD. (2011) Functions of GSK-3 signaling in development of the nervous system. Front Mol Neurosci 4: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Alphs L, Buchanan RW. (1992) The concept of supersensitivity psychosis. J Nerv Ment Dis 180: 265–270. [DOI] [PubMed] [Google Scholar]
- Klein A, Schmidt WJ. (2003) Catalepsy intensifies context-dependently irrespective of whether it is induced by intermittent or chronic dopamine deficiency. Behav Pharmacol 14: 49–53. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S. (2001) Antipsychotic drugs and neuroplasticity: Insights into the treatment and neurobiology of schizophrenia. Biol Psychiatry 50: 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky N, Nadri C, Agam G. (2005) Low GSK-3beta in schizophrenia as a consequence of neurodevelopmental insult. Eur Neuropsychopharmacol 15: 1–11. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Tadokoro S. (1981) Correlation between antiavoidance activities of antipsychotic drugs in rats and daily clinical doses. Pharmacol Biochem Behav 14: 181–192. [DOI] [PubMed] [Google Scholar]
- Leucht S, Busch R, Hamann J, et al. (2005) Early-onset hypothesis of antipsychotic drug action: A hypothesis tested, confirmed and extended. Biol Psychiatry 57: 1543–1549. [DOI] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, et al. (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 382: 951–962. [DOI] [PubMed] [Google Scholar]
- Li M, Fletcher PJ, Kapur S. (2007) Time Course of the antipsychotic effect and the underlying behavioral mechanisms. Neuropsychopharmacology 32: 263–272. [DOI] [PubMed] [Google Scholar]
- Li M, He W, Mead A. (2009a) An investigation of the behavioral mechanisms of antipsychotic action using a drug-drug conditioning paradigm. Behav Pharmacol 20: 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, He W, Mead A. (2009b) Olanzapine and risperidone disrupt conditioned avoidance responding in phencyclidine-pretreated or amphetamine-pretreated rats by selectively weakening motivational salience of conditioned stimulus. Behav Pharmacol 20: 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, He W, Munro R. (2012) Differential effects of acute amphetamine and phencyclidine treatment and withdrawal from repeated amphetamine or phencyclidine treatment on social interaction and social memory in rats. PsyCh Journal 1: 56–68. [DOI] [PubMed] [Google Scholar]
- Li M, He E, Volf N. (2011) Time course of the attenuation effect of repeated antipsychotic treatment on prepulse inhibition disruption induced by repeated phencyclidine treatment. Pharmacol Biochem Behav 98: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Parkes J, Fletcher PJ, et al. (2004) Evaluation of the motor initiation hypothesis of apd-induced conditioned avoidance decreases. Pharmacol Biochem Behav 78: 811–819. [DOI] [PubMed] [Google Scholar]
- Li X, Rosborough KM, Friedman AB, et al. (2007) Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol 10: 7–19. [DOI] [PubMed] [Google Scholar]
- Li M, Sun T, Zhang C, et al. (2010) Distinct neural mechanisms underlying acute and repeated administration of antipsychotic drugs in rat avoidance conditioning. Psychopharmacology (Berl) 212: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, et al. (2008) Antipsychotic drugs: Comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev 60: 358–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J, Jody D, Geisler S, et al. (1993) Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry 50: 369–376. [DOI] [PubMed] [Google Scholar]
- Loonen AJ, Ivanova SA. (2013) New insights into the mechanism of drug-induced dyskinesia. CNS Spectr 18: 15–20. [DOI] [PubMed] [Google Scholar]
- Luque-Rojas MJ, Galeano P, Suarez J, et al. (2013) Hyperactivity induced by the dopamine D2/D3 receptor agonist quinpirole is attenuated by inhibitors of endocannabinoid degradation in mice. Int J Neuropsychopharmacol 16: 661–676. [DOI] [PubMed] [Google Scholar]
- Masri B, Salahpour A, Didriksen M, et al. (2008) Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A 105: 13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead A, Li M. (2010) Avoidance-suppressing effect of antipsychotic drugs is progressively potentiated after repeated administration: An interoceptive drug state mechanism. J Psychopharmacol 24: 1045–1053. [DOI] [PubMed] [Google Scholar]
- Mead A, Li M, Kapur S. (2008) Clozapine and olanzapine exhibit an intrinsic anxiolytic property in two conditioned fear paradigms: Contrast with haloperidol and chlordiazepoxide. Pharmacol Biochem Behav 90: 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Li Z, Kaneda Y, et al. (2003) Serotonin receptors: Their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 27: 1159–1172. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. (1989) The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull 25: 390–392. [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, et al. (2010) Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull 36: 607–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein JA, Elnabawi A, Vinish M, et al. (2013) Olanzapine treatment of adolescent rats causes enduring specific memory impairments and alters cortical development and function. PLoS One 8: e57308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, et al. (2005) Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10: 79–104. [DOI] [PubMed] [Google Scholar]
- Moran-Gates T, Gan L, Park YS, et al. (2006) Repeated antipsychotic drug exposure in developing rats: Dopamine receptor effects. Synapse 59: 92–100. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Gonzalez-Maeso J. (2013) Preclinical models of antipsychotic drug action. Int J Neuropsychopharmacol 16: 2131–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Lopez-Moreno JA, Rodriguez De, Fonseca F, et al. (2005) Behavioural effects of quinpirole following withdrawal of chronic treatment with the Cb1 agonist, Hu-210, in rats. Behav Pharmacol 16: 441–446. [DOI] [PubMed] [Google Scholar]
- Nadri C, Lipska BK, Kozlovsky N, et al. (2003) Glycogen synthase kinase (GSK)-3beta levels and activity in a neurodevelopmental rat model of schizophrenia. Brain Res Dev Brain Res 141: 33–37. [DOI] [PubMed] [Google Scholar]
- Nordstrom AL, Farde L, Halldin C. (1992) Time course of D2-dopamine receptor occupancy examined by pet after single oral doses of haloperidol. Psychopharmacology (Berl) 106: 433–438. [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu L, et al. (2006) National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry 63: 679–685. [DOI] [PubMed] [Google Scholar]
- Patel NC, Crismon ML, Hoagwood K, et al. (2005) Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry 44: 548–556. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25: 192–216. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. (2011) Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr Bull 37: 1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. (2012) Tracing the development of psychosis and its prevention: What can be learned from animal models. Neuropharmacology 62: 1273–1289. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. (2009) Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry 66: 1038–1046. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Bernstein HG, Dobrowolny H, et al. (2012) Effects of risperidone treatment in adolescence on hippocampal neurogenesis, parvalbumin expression, and vascularization following prenatal immune activation in rats. Brain Behav Immun 26: 353–363. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, et al. (1998) Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 155: 768–773. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Hinson R. (1982) Pavlovian conditional tolerance to haloperidol catalepsy: Evidence of dynamic adaptation in the dopaminergic system. Science 218: 491–492. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Hinson RE, Siegel S. (1981) The role of Pavlovian processes in drug tolerance and dependence: Implications for treatment. Addict Behav 6: 205–211. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Miyoshi E, et al. (2004) Facilitation of short-term social memory by ethanol in rats is mediated by dopaminergic receptors. Behav Brain Res 153: 149–157. [DOI] [PubMed] [Google Scholar]
- Qiao J, Gao J, Shu Q, et al. (2014a) Long-lasting sensitization induced by repeated risperidone treatment in adolescent Sprague-Dawley rats: A possible D2 receptor mediated phenomenon? Psychopharmacology (Berl) 231: 1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Li H, Li M. (2013) Olanzapine sensitization and clozapine tolerance: From adolescence to adulthood in the conditioned avoidance response model. Neuropsychopharmacology 38: 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Zhang Q, Li M. (2014b) Long-term impacts of adolescent risperidone treatment on behavioral responsiveness to olanzapine and clozapine in adulthood. Prog Neuropsychopharmacol Biol Psychiatry 48: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin R, Chen Y, Li M. (2013) Repeated asenapine treatment produces a sensitization effect in two preclinical tests of antipsychotic activity. Neuropharmacology 75C: 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani F, Murray ML, Byrne PJ, et al. (2008) Epidemiologic features of antipsychotic prescribing to children and adolescents in primary care in the United Kingdom. Pediatrics 121: 1002–1009. [DOI] [PubMed] [Google Scholar]
- Remington G, Kapur S. (2010) Antipsychotic dosing: How much but also how often? Schizophr Bull 36: 900–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington G, Seeman P, Feingold A, et al. (2011) "Extended" antipsychotic dosing in the maintenance treatment of schizophrenia: A double-blind, placebo-controlled trial. J Clin Psychiatry 72: 1042–1048. [DOI] [PubMed] [Google Scholar]
- Remington G, Seeman P, Shammi C, et al. (2005) "Extended" antipsychotic dosing: Rationale and pilot data. J Clin Psychopharmacol 25: 611–613. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Welge JA, Logue AD, et al. (2007) Dopamine and serotonin receptor binding and antipsychotic efficacy. Neuropsychopharmacology 32: 1715–1726. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Fibiger HC. (1992) Neuroleptics increase c-fos expression in the forebrain: Contrasting effects of haloperidol and clozapine. Neuroscience 46: 315–328. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Matsumura H, Fibiger HC. (1994) Induction patterns of fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther 271: 1058–1066. [PubMed] [Google Scholar]
- Robinson TE, Becker JB. (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Res 396: 157–198. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, et al. (1998) Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev 22: 347–354. [DOI] [PubMed] [Google Scholar]
- Rosenheck RA, Davis S, Covell N, et al. (2009) Does switching to a new antipsychotic improve outcomes? Data from the Catie trial. Schizophr Res 107: 22–29. [DOI] [PubMed] [Google Scholar]
- Rupnow MF, Greenspan A, Gharabawi GM, et al. (2007) Incidence and costs of polypharmacy: data from a randomized, double-blind, placebo-controlled study of risperidone and quetiapine in patients with schizophrenia or schizoaffective disorder. Curr Med Res Opin: [DOI] [PubMed] [Google Scholar]
- Salomon C, Hamilton B, Elsom S. (2014) Experiencing antipsychotic discontinuation: Results from a survey of Australian consumers. J Psychiatr Ment Health Nurs 21: 917–923. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Reckless GE, Seeman P, et al. (2008) Less is more: Antipsychotic drug effects are greater with transient rather than continuous delivery. Biol Psychiatry 64: 145–152. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Seeman P, Stewart J, et al. (2007) "Breakthrough" dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci 27: 2979–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandson NB, Armstrong SC, Cozza KL. (2005) An overview of psychotropic drug-drug interactions. Psychosomatics 46: 464–494. [DOI] [PubMed] [Google Scholar]
- Sanger DJ. (1985) The effects of clozapine on shuttle-box avoidance responding in rats: Comparisons with haloperidol and chlordiazepoxide. Pharmacol Biochem Behav 23: 231–236. [DOI] [PubMed] [Google Scholar]
- Schmidt WJ, Tzschentke TM, Kretschmer BD. (1999) State-dependent blockade of haloperidol-induced sensitization of catalepsy by Mk-801. Eur J Neurosci 11: 3365–3368. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Weber S, Jatzko A, et al. (2004) Hippocampal volume and cell proliferation after acute and chronic clozapine or haloperidol treatment. J Neural Transm 111: 91–100. [DOI] [PubMed] [Google Scholar]
- See RE, Kalivas PW. (1996) Tolerance and sensitization to the effects of antipsychotic drugs on dopamine transmission. In: Csernansky JG. (ed.) Antipsychotics. Vol. 120 Berlin: Springer, pp.203–224. [Google Scholar]
- Seeman P. (2000) Antipsychotic drugs, dopamine D2 receptors and schizophrenia. In: Lidow MS. (ed.) Neurotransmitter Receptors in Actions of Antipsychotic Medications. Boca Raton, FL: CRC Press, pp.43–63. [Google Scholar]
- Seeman P. (2002) Atypical antipsychotics: Mechanism of action. Can J Psychiatry 47: 27–38. [PubMed] [Google Scholar]
- Seeman P. (2008) Dopamine D2(high) receptors moderately elevated by sertindole. Synapse 62: 389–393. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2011) All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci Ther 17: 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, et al. (2005) Dopamine supersensitivity correlates with D2high states, implying many paths to psychosis. Proc Natl Acad Sci U S A 102: 3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala N, Kovio C, Leinonen E. (2005) Effect of anticholinergics in preventing acute deterioration in patients undergoing abrupt clozapine withdrawal. CNS Drugs 19: 1049–1055. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Hart JC, Bymaster FP, et al. (1999) Muscarinic receptor agonists, like dopamine receptor antagonist antipsychotics, inhibit conditioned avoidance response in rats. J Pharmacol Exp Ther 290: 901–907. [PubMed] [Google Scholar]
- Shu Q, Hu G, Li M. (2014a) Adult response to olanzapine or clozapine treatment is altered by adolescent antipsychotic exposure: A preclinical test in the phencyclidine hyperlocomotion model. J Psychopharmacol 28: 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Q, Qin R, Chen Y, et al. (2014b) Asenapine sensitization from adolescence to adulthood and its potential molecular basis. Behav Brain Res 273: 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. (1978) Pavlovian conditioning analysis of morphine tolerance. NIDA Res Monogr 18: 27–53. [PubMed] [Google Scholar]
- Siegel S, Baptista MA, Kim JA, et al. (2000) Pavlovian psychopharmacology: The associative basis of tolerance. Exp Clin Psychopharmacol 8: 276–293. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Li M. (2012) Drug-drug conditioning between citalopram and haloperidol or olanzapine in a conditioned avoidance response model: Implications for polypharmacy in schizophrenia. Behav Pharmacol 23: 658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sramek JJ, Gaurano V, Herrera JM, et al. (1990) Patterns of neuroleptic usage in continuously hospitalized chronic schizophrenic patients: Evidence for development of drug tolerance. DICP 24: 7–10. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Fowler SC. (1997) Subchronic effects of clozapine and haloperidol on rats’ forelimb force and duration during a press-while-licking task. Psychopharmacology (Berl) 130: 249–253. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Denney D, Szot P. (1997) Sensitization with clozapine: Beyond the dopamine hypothesis. Biol Psychiatry 42: 771–780. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. (1993) Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol 4: 289–312. [PubMed] [Google Scholar]
- Stewart J, Vezina P. (1991) Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behav Pharmacol 2: 65–71. [PubMed] [Google Scholar]
- Stille G, Lauener H, Eichenberger E. (1971) The pharmacology of 8-chloro-11-(4-methyl-1-piperazinyl)-5h-dibenzo(B,E)(1,4)diazepine (clozapine). Farmaco Prat 26: 603–625. [PubMed] [Google Scholar]
- Stojkovic T, Radonjic NV, Velimirovic M, et al. (2012) Risperidone reverses phencyclidine induced decrease in glutathione levels and alterations of antioxidant defense in rat brain. Prog Neuropsychopharmacol Biol Psychiatry 39: 192–199. [DOI] [PubMed] [Google Scholar]
- Sun T, He W, Hu G, et al. (2010) Anxiolytic-like property of risperidone and olanzapine as examined in multiple measures of fear in rats. Pharmacol Biochem Behav 95: 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Hu G, Li M. (2009) Repeated antipsychotic treatment progressively potentiates inhibition on phencyclidine-induced hyperlocomotion, but attenuates inhibition on amphetamine-induced hyperlocomotion: elevance to animal models of antipsychotic drugs. Eur J Pharmacol 602: 334–342. [DOI] [PubMed] [Google Scholar]
- Sun T, Liu X, Li M. (2014) Effect of environmental cues on the behavioral efficacy of haloperidol, olanzapine, and clozapine in rats. Behav Pharmacol 25: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N, Li M. (2012) Parametric studies of antipsychotic-induced sensitization in the conditioned avoidance response model: Roles of number of drug exposure, drug dose, and test-retest interval. Behav Pharmacol 23: 380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Allan M, Talangbayan H, et al. (1997) Locomotor sensitization to quinpirole: Environment-modulated increase in efficacy and context-dependent increase in potency. Psychopharmacology (Berl) 134: 193–200. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Goodwill AM, Szechtman H. (2000) Locomotor sensitization to quinpirole in rats: Effects of drug abstinence and sex. Psychopharmacology (Berl) 152: 304–311. [DOI] [PubMed] [Google Scholar]
- Szymanski S, Lieberman JA, Alvir JM, et al. (1995) Gender differences in onset of illness, treatment response, course, and biologic indexes in first-episode schizophrenic patients. Am J Psychiatry 152: 698–703. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Zhang K, Baldessarini RJ. (2001) Long-term effects of olanzapine, risperidone, and quetiapine on dopamine receptor types in regions of rat brain: Implications for antipsychotic drug treatment. J Pharmacol Exp Ther 297: 711–717. [PubMed] [Google Scholar]
- Tarsy D, Baldessarini RJ. (1974) Behavioural supersensitivity to apomorphine following chronic treatment with drugs which interfere with the synaptic function of catecholamines. Neuropharmacology 13: 927–940. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Jones C, Remington G, et al. (2002) Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry 7: 317–321. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr (1995) Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res 89: 167–172. [DOI] [PubMed] [Google Scholar]
- Tenk CM, Foley KA, Kavaliers M, et al. (2007) Neonatal immune system activation with lipopolysaccharide enhances behavioural sensitization to the dopamine agonist, quinpirole, in adult female but not male rats. Brain Behav Immun 21: 935–945. [DOI] [PubMed] [Google Scholar]
- Touyz SW, Saayman GS, Zabow T. (1978) A psychophysiological investigation of the long-term effects of clozapine upon sleep patterns of normal young adults. Psychopharmacology (Berl) 56: 69–73. [DOI] [PubMed] [Google Scholar]
- Trevitt J, Atherton A, Aberman J, et al. (1998) Effects of subchronic administration of clozapine, thioridazine and haloperidol on tests related to extrapyramidal motor function in the rat. Psychopharmacology (Berl) 137: 61–66. [DOI] [PubMed] [Google Scholar]
- Turrone P, Remington G, Kapur S, et al. (2003) Differential effects of within-day continuous vs. transient dopamine D2 receptor occupancy in the development of vacuous chewing movements (Vcms) in vats. Neuropsychopharmacology 28: 1433–1439. [DOI] [PubMed] [Google Scholar]
- Turrone P, Remington G, Kapur S, et al. (2005) Continuous but not intermittent olanzapine infusion induces vacuous chewing movements in rats. Biol Psychiatry 57: 406–411. [DOI] [PubMed] [Google Scholar]
- Van Der Heyden JA, Bradford LD. (1988) A rapidly acquired one-way conditioned avoidance procedure in rats as a primary screening test for antipsychotics: Influence of shock intensity on avoidance performance. Behav Brain Res 31: 61–67. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Vann RE, Wise LE, et al. (2002) Effects of antipsychotic drugs on operant responding after acute and repeated administration. Psychopharmacology (Berl) 160: 182–191. [DOI] [PubMed] [Google Scholar]
- Vezina P, Giovino AA, Wise RA, et al. (1989) Environment-specific cross-sensitization between the locomotor activating effects of morphine and amphetamine. Pharmacol Biochem Behav 32: 581–584. [DOI] [PubMed] [Google Scholar]
- Villanueva HF, Porter JH. (1993) Differential tolerance to the behavioral effects of chronic pimozide and clozapine on multiple random interval responding in rats. Behav Pharmacol 4: 201–208. [PubMed] [Google Scholar]
- Vinish M, Elnabawi A, Milstein JA, et al. (2013) Olanzapine treatment of adolescent rats alters adult reward behaviour and nucleus accumbens function. Int J Neuropsychopharmacol 16: 1599–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Johnson HL, Burns LN, et al. (2009) Developmental treatment with the dopamine D2/3 agonist quinpirole selectively impairs spatial learning in the Morris water maze. Neurotoxicol Teratol 31: 1–10. [DOI] [PubMed] [Google Scholar]
- Wadenberg MG, Browning JL, Young KA, et al. (2001) Antagonism at 5-Ht(2a) receptors potentiates the effect of haloperidol in a conditioned avoidance response task in rats. Pharmacol Biochem Behav 68: 363–370. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Hicks PB. (1999) The conditioned avoidance response test re-evaluated: Is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci Biobehav Rev 23: 851–862. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Soliman A, Vanderspek SC, et al. (2001) Dopamine D(2) receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology 25: 633–641. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Burstein ES, Nash N, et al. (2001) 5-Hydroxytryptamine2a receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther 299: 268–276. [PubMed] [Google Scholar]
- Williams SA, Wesnes K, Oliver SD, et al. (1996) Absence of effect of sertraline on time-based sensitization of cognitive impairment with haloperidol. J Clin Psychiatry 57: S7–S11. [PubMed] [Google Scholar]
- Wolgin DL, Moore J. (1992) Sensitization to haloperidol-induced suppression of milk intake: Effect of interdose interval. Psychopharmacology (Berl) 107: 290–296. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li M. (2012) Contextual and behavioral control of antipsychotic sensitization induced by haloperidol and olanzapine. Behav Pharmacol 23: 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Fang Y, Li M. (2011) Olanzapine and risperidone disrupt conditioned avoidance responding by selectively weakening motivational salience of conditioned stimulus: Further evidence. Pharmacol Biochem Behav 98: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Li M. (2009a) The receptor mechanisms underlying the disruptive effects of haloperidol and clozapine on rat maternal behavior: A double dissociation between dopamine D(2) and 5-Ht(2a/2c) receptors. Pharmacol Biochem Behav 93: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Li M. (2009b) Sedation and disruption of maternal motivation underlie the disruptive effects of antipsychotic treatment on rat maternal behavior. Pharmacol Biochem Behav 92: 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Sun T, Li M. (2012) Neural basis of the potentiated inhibition of repeated haloperidol and clozapine treatment on the phencyclidine-induced hyperlocomotion. Prog Neuropsychopharmacol Biol Psychiatry 38: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrunnen TL, Jann MW. (1998) Drug interactions with antipsychotic agents: Incidence and therapeutic implications. CNS Drugs 9: 381–401. [Google Scholar]