Abstract
A lack of typical age-related improvement from adolescence to adulthood contributes to face recognition deficits in adults with autism on the Cambridge Face Memory Test (CFMT). The current studies examine if this atypical developmental trajectory generalizes to other tasks and objects, including parts of the face. The CFMT tests recognition of whole faces, often with a substantial delay. The current studies used the immediate memory (IM) task and the parts-whole face task from the Let's Face It! battery, which examines whole faces, face parts, and cars, without a delay between memorization and test trials. In the IM task, participants memorize a face or car. Immediately after the target disappears, participants identify the target from two similar distractors. In the part-whole task, participants memorize a whole face. Immediately after the face disappears, participants identify the target from a distractor with different eyes or mouth, either as a face part or a whole face.
Results indicate that recognition deficits in autism become more robust by adulthood, consistent with previous work, and also become more general, including cars. In the IM task, deficits in autism were specific to faces in childhood, but included cars by adulthood. In the part-whole task, deficits in autism became more robust by adulthood, including both eyes and mouths as parts and in whole faces. Across tasks, the deficit in autism increased between adolescence and adulthood, reflecting a lack of typical improvement, leading to deficits with non-face stimuli and on a task without a memory delay. These results suggest that brain maturation continues to be affected into adulthood in autism, and that the transition from adolescence to adulthood is a vulnerable stage for those with autism.
Keywords: ASD, Developmental disorders, Face, Holistic, Recognition, Eyes
1. Introduction
Individuals with autism exhibit impaired face recognition but the reasons for this deficit are unknown (Sasson, 2006; Weigelt, Koldewyn, & Kanwisher, 2012). It is unclear if this deficit is associated with the social impairment that is diagnostic of autism (e.g., a lack of social motivation leads to less expertise with faces; Dawson, Webb, & McPartland, 2005; Schultz, 2005), with general differences in visual processing (e.g., a ‘local bias’ undermines holistic processing important for face recognition; Behrmann, Thomas, & Humphreys, 2006b; Happe, 1999; Mottron, Dawson, Soulières, Hubert, & Burack, 2006), or with both. The lack of clarity on this issue reflects the evidence; deficits in recognition in autism are sometimes specific to faces (Bradshaw, Shic, & Chawarska, 2011; Wolf et al., 2008) and sometimes apply to a range of objects (e.g., motorcycles; Blair, Frith, Smith, Abell, & Cipolotti, 2002). In this paper, we examine how the deficit changes with age, with the hope that the progression of the visual differences in autism will clarify the etiology of the deficit and its impact on visual function.
Adolescent development has proven important for understanding visual differences in adults with autism (Kuschner, Bodner, & Minshew, 2009; O'Hearn, Lakusta, Schroer, Minshew, & Luna, 2011; O'Hearn, Schroer, Minshew, & Luna, 2010; Rump, Giovannelli, Minshew, & Strauss, 2009; Scherf, Luna, Kimchi, Minshew, & Behrmann, 2008), although most of the studies on age-related changes in autism focus on the initial development of this early-emerging disorder (e.g., Chawarska & Shic, 2009). We previously examined changes during adolescence in face recognition using a well-established face memory task (the Cambridge Face Memory Test, CFMT, described below). Performance on the CFMT improved from adolescence to adulthood typically, but did not improve during this transition in individuals with autism (O'Hearn et al., 2010). These results were surprising for two reasons. One, typical development of face recognition continued into adulthood, a finding later replicated in a larger sample of typically developing (TD) individuals (Germine, Duchaine, & Nakayama, 2011; while face recognition has long been considered late developing for vision, “late” was considered around age 12, Carey & Diamond, 1977; Mondloch, Geldart, Maurer, & Le, 2003). Two, face recognition deficits in autism became more robust in adulthood, despite the early emergence of autism and the potential for individuals with autism to learn compensatory strategies by adulthood. The lack of typical adolescent development in autism has far-reaching implications, because evidence suggests that it is quite general; some studies show a lack of development on visual tasks without face stimuli or a memory component (rapid enumeration of a few elements, O'Hearn, Franconeri, Wright, Minshew, & Luna, 2013; global shape recognition, Scherf et al., 2008), and in analyses that controlled for memory deficits (i.e., change detection with and without people, controlling for memory span; O'Hearn, Lakusta, et al., 2011). These differences in the face recognition deficits between the adolescents and the adults with autism may reflect cohort effects, an important possibility to examine with longitudinal data. However, one indication that cohort effects are not the entire explanation is that the increasing deficits in autism, at least on the CFMT, are driven by improvements in typical developing controls. Therefore, the increasing deficits with age probably do not reflect the substantial changes in treatment, education, etc. for individuals with autism in the last few years. Longitudinal data will also be crucial for understanding the increased variability in those with autism, and whether some individuals with autism do undergo improvement during this developmental transition. Pragmatically, this lack of development suggests that those with autism may be falling further behind during the crucial transition to adulthood (Taylor & Seltzer, 2010).
One goal of the current study was to examine whether the deficit in autism is specific to whole faces, which are a unique set of stimuli in many ways, in order to provide insight into what is ‘not developing’ in autism. Faces are the stimuli most likely to be rapidly and universally processed at an individual level. Increasing expertise for faces over age may be driven by the unique amount and the quality of experience with faces, embedded in learning mechanisms specific to the developmental stage. These experiences may be disrupted in autism (de Haan, Humphreys, & Johnson, 2002; Schultz, 2005). Though contentious, the “specialness” of face processing is apparent in evidence of an innate bias (Morton & Johnson, 1991; Pascalis & de Schonen, 1994), dedicated neural tissue (Kanwisher, McDermott, & Chun, 1997)/circuitry (Haxby, Hoffman, & Gobbini, 2002), and the importance of holistic visual processing (Tanaka & Farah, 1993). Holistic processing is operationalized as a decline in performance when the upright face (or other stimuli) is distorted, most commonly by rotation (inversion tasks), combining faces (composite faces), or showing only a face part (part-whole task). While these disruptions may impair performance with other stimuli, the impact is greater with faces, indicating that holistic processing is particularly important for face recognition in TD adults (Yin, 1969). In addition, face recognition has been proposed to rely on specific types of configural information (e.g., 2nd order configural information, which is spacing between features; Behrmann et al., 2006a) that may be particularly important for identifying individual faces.
Research in autism has long tried to pinpoint if the recognition deficit in autism is specific to faces, or perhaps one of the unique characteristics of faces. For instance, several studies have suggested that individuals with autism rely less on configural information, which may be uniquely important for face recognition, than do TD adults (Behrmann et al., 2006b; Dawson et al., 2005). This could potentially result from the general ‘local bias’ in visual processing or a lack of experience with faces that disrupts the maturation of configural processing (Webb et al., 2011). However, a recent review by Weigelt et al., 2012 concluded that individuals with autism use holistic and/or configural processes that are qualitatively similar to TD individuals when recognizing faces, a conclusion that parallels recent work on normative development (McKone et al., 2012). The review stresses that, instead of configural processes, memory demands are an important factor that contributes substantially to the face recognition deficits in autism. Weigelt and colleagues also suggest that the deficits may be specific to faces, and even more specifically, to recognition of the eyes, although they acknowledge that the evidence for this conclusion is more ambiguous than their conclusion of the importance of memory demands. The possibility of eye-specific deficits are supported by the limited evidence of decreased fixations to the eyes in autism, or increased fixations on the mouth (less reliably), compared to TD groups. These differences in fixations are important for performance. They are correlated with face recognition performance (Kirchner, Hatri, Heekeren, & Dziobek, 2011; Weigelt et al., 2012), as well as activation in the fusiform gyri both typically (Morris, Pelphrey, & McCarthy, 2007) and in autism (Dalton et al., 2005; Perlman, Hudac, Pegors, Minshew, & Pelphrey, 2011).
Our initial work showing a lack of development in autism from adolescence to adulthood used the Cambridge Face Memory Test (CFMT; O'Hearn et al., 2010), developed to identify adults with prosopagnosia (Duchaine & Nakayama, 2006). This task has three conditions, with each condition increasing in difficulty. In the first condition, participants are told to memorize six target faces. Each face is memorized consecutively, across three memorization trials and three test trials. During the memorization trials, participants see the target face from three angles (3 s each). After the memorization trials, there are three test trials where participants identify the same images of the target face from two distractors. The second and third conditions are similar except that: (1) there is only one memorization trial, albeit longer (20 s), with the 6 target faces presented simultaneously. This means that the memory delay is increased when recognizing faces, especially for later test trials, and (2) the test stimuli images are not the same as the memorization images, but instead are displayed with novel angles/lighting and, in the third condition, blur. Performance in all three conditions displayed the same pattern of age-related improvement during adolescence typically but not in autism, despite the differences across conditions in the length of the delay and, in condition 3, the blurred images (thought to require more configural processing). Further work has replicated our findings of deficits on the CFMT in adults with autism (Kirchner et al., 2011), including individuals who do not display early communication deficits (Aspergers; Hedley, Brewer, & Young, 2011) and unaffected relatives of individuals with autism (Wilson, Freeman, Brock, Burton, & Palermo, 2010).
The current studies further characterize these age-related changes in recognition, including the typical improvements during adolescence, and how it differs in autism. We examine whether the deficit in autism is specific to face stimuli (by comparing faces vs. cars) or to holistic processing (by comparing whole vs. part faces). We also examined whether these distinct trajectories, typically and in autism, were evident on a task with no delay between memorization and test.1 To address these questions, we chose two tasks from the Let's Face It! (LFI) battery, one that tested immediate memory (IM task) for faces and cars, and one that tested holistic processing of faces (part-whole task). The LFI battery was developed for children and adolescents with autism (Tanaka et al., 2010; Wolf et al., 2008). Both tasks had no delay between initial presentation and test and, therefore, the memory demands are decreased compared to the CFMT. We examined specificity because of the inconsistent evidence of a face-specific deficit. We examined holistic processing because we thought the typical improvements during adolescence might be specific to whole objects. This seemed possible because the studies showing late typical development of visual processing, while diverse, all required the encoding of multiple elements, and this is a component of holistic processing (i.e., subitizing, O'Hearn et al., 2013; change detection in a dynamic scene, O'Hearn , Lakusta, et al., 2011, global shape integration, Scherf, Behrmann, Kimchi, & Luna, 2009, Kovács, Kozma, Fehér, & Benedek, 1999). However, the results of Wolf and colleagues using the part-whole task in a younger sample suggested that holistic processing in autism was similar to TD controls. This study did find that the relative skill for eyes vs. mouths was reduced in autism. Thus, in addition to holistic processing, the part-whole task also allowed us to test the relative focus on eyes and mouths across development.
In the present study, we predict that there will be age-related improvement during adolescence typically but that this development will be reduced in autism, replicating the basic pattern of results from the CFMT but with the decreased memory delay/task demands of the LFI. Another potential scenario is that, if the decreased memory demands of the LFI optimize performance in autism (Weigelt et al., 2012), adolescent improvements might be evident in the group with autism, which would provide important insight into development in autism. We also hypothesized that this pattern would not be specific to faces in adulthood, on the basis of the lack of typical adolescent improvements on visual tasks without face stimuli in those with autism (O'Hearn et al., 2013; O'Hearn, Lakusta, et al., 2011; Scherf et al., 2008). This is in contrast with the initial results with the LFI (Wolf et al., 2008) that revealed, across age, high functioning children/adolescents with autism (5–20 years of age) exhibited face-specific deficits that were not evident with car recognition (or house recognition in a different task). This suggests that the deficits might change, with age or other sample characteristics, from face-specific to more general. The current paper expands on these interesting results with a novel focus on age-related change, and the transition into adulthood.
2. Method
2.1. Participants
Twenty-five children (7–12), 25 adolescents (13–17), and 21 adults (18–35) with autism and 29 typically developing (TD) children, 25 adolescents, and 33 adults participated. The sample was mostly males, due to the prevalence of ASD. See Table 1 for demographic information. The age groups represent important developmental stages, and these divisions have proven useful in previous work (O'Hearn et al., 2010; Rump et al., 2009). Seven participants (4 TD, 3 autism) were not included in the analysis or in the demographic information: two with FSIQ scores > 135 and one aged > 35 years, dropped to improve matching across groups; two individuals who did not attend during testing; and two individuals with incomplete diagnoses. Age and IQ did not differ across groups, and IQ did not differ across the age groups (all p's > .47). ADOS and ADI scores were similar across the age groups in those with autism, though there was a trend (p < .10) in ADI-social score, with adults with autism tending to score higher than children with autism. The group of controls was larger than the group with autism. We did this to add power, particularly for understanding typical age-related improvements. However, for the adult group, we also report a second analysis that includes only individually matched TD and autism groups, to confirm the results with the larger group of controls did not reflect the disproportionate number of females or other differences in the TD sample. These smaller groups were matched for gender, IQ (within 15 points, but generally closer) and age (within 1.5 years for children, 3 years for adults), with all p's > .51 for t-tests comparing the TD and the autism group.
Table 1.
Participant demographics.
| Measure | Children |
Adolescents |
Adults |
|||
|---|---|---|---|---|---|---|
| Autism | TD | Autism | TD | Autism | TD | |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| N | 24 | 25 | 25 | 26 | 19 | 31 |
| Gender | 3 F, 21 M | 5 F, 20 M | 4 F, 21 M | 4 F, 22 M | 1 F, 18 M | 5 F, 26 M |
| Age | 11.4 (1.55) | 11.3 (1.39) | 15.3 (1.47) | 15.1 (1.44) | 24.4 (4.72) | 24.0 (5.10) |
| Full scale IQ | 111.5 (10.8) | 109.2 (11.7) | 106.8 (13.3) | 107.7 (9.5) | 110.6 (14.8) | 111.5 (10.4) |
| ADOS | ||||||
| Communication | 3.54 (1.41) | 3.96 (1.14) | 4.42 (1.39) | |||
| Social | 7.67 (1.83) | 7.84 (2.27) | 8.47 (2.78) | |||
| Total | 11.21 (3.02) | 11.80 (3.07) | 12.89 (3.59) | |||
| ADI | ||||||
| Social | 18.67 (5.76) | 19.63 (5.24) | 22.42 (3.52) | |||
| Communication | 16.13 (3.94) | 15.17 (3.89) | 16.74 (4.13) | |||
| Behavior | 6.42 (2.06) | 6.13 (2.35) | 6.32 (2.58) | |||
| Abnormality | 3.29 (1.33) | 2.71 (1.68) | 3.00 (1.05) | |||
Participants with autism were recruited through the University of Pittsburgh Autism Center of Excellence (ACE) subject core (HD#055748). Autism diagnoses were determined using the Autism Diagnostic Interview (ADI; Lord, Rutter, & Couteur, 1994) and the Autism Diagnostic Observation Schedule G (ADOS; Lord, Rutter, & Goode, 1989) and confirmed by expert clinical assessment. IQ was measured using the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Individuals with full-scale IQ scores < 80 or a known etiology for autism were excluded. TD participants were recruited through the ACE subject core and other studies, with recruitment methods including web postings and flyers. Controls had no personal history of psychiatric or neurological disorder, developmental delay or learning disability; no known family history of autism; and no first-degree relatives with a neuropsychiatric disorder considered to have a genetic component. No participant had a history of head injury, birth injury or seizure disorder. Far acuity of all participants was normal or corrected to at least 20/40.
Approval for the present study was obtained from the Internal Review Board at the University of Pittsburgh. Written informed consent was given by participants, or parents of minor participants, and all children assented to the study.
2.2. Procedure
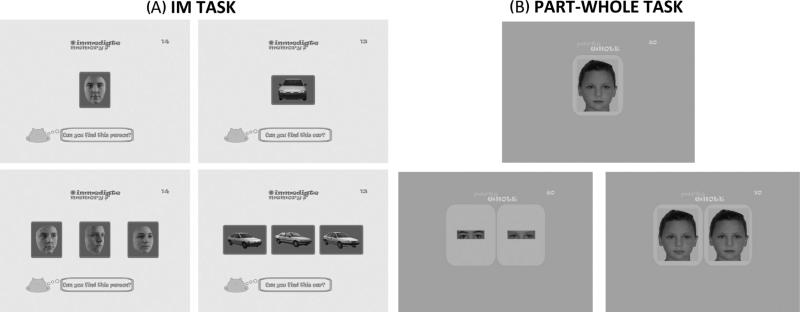
Three tasks from the Let's Face It! (LFI) battery (Wolf et al., 2008) were used. The order of the face and car IM tasks (Fig. 1A) was counterbalanced.
Fig. 1.
(A) Immediate memory (IM) task for faces and cars. Participants see the target face/car (top) for 1 s followed by the test stimuli with the target and two distractors. The participant clicks on the target face or car. (B) Parts-whole task for eyes and mouth. Participants see the target face (top) for 4 s followed by test stimuli with one target and one distractor, either the part alone (eyes, mouth) or the whole face. The participant clicks on the target or the target eyes or mouth.
2.2.1. Immediate memory (IM), faces (Fig. 1A)
This task assesses participants’ immediate memory for faces. A target face, from the front, was shown for 1 s. This face disappeared and was replaced with three probe faces, displayed at a 3/4 orientation (turned 1/4 of the way, slightly toward the right, with eyes not looking directly at the participant). The participant selected the image that corresponded with the target face. Face stimuli included 7 male and 7 female faces (N = 14 trials), presented in black and white with neutral expressions and the hair/hairline obscured.
2.2.2. Immediate memory (IM), cars (Fig. 1 A)
This task had the same format as face IM, with a target car displayed from the front for 1 s, followed by three probe cars displayed at right-ward 3/4 orientation (turned slightly toward the right). The participant selected the probe that corresponded with the target (N = 14 trials). The cars were sedans and presented in black and white with no identifying words/logos. Table 2 indicates that, in the current sample, performance was similar for faces and cars in TD children and adults. The results from Wolf and colleagues, using a different sample, also suggest that performance was relatively similar across faces and cars (67% faces, 70% cars in the TD group).
Table 2.
Comparison of accuracy for faces and cars within group, by age (columns) and groups (rows).
| Children | Adolescents | Adults | |
|---|---|---|---|
| TD | F = C, t(21) = .54, p = .60 | F > C*, t(23) = −1.94, p = .06 | F = C, t(26) = 1.14, p = .26 |
| ASD | F < C, t(21) = 6.86, p <.001 | F = C, t(22) = −.14, p = .89 | F < C, t(18) = −2.34, p = .03 |
Significance at the trend level.
2.2.3. Part-whole identity (Fig. 1 B)
In the part-whole identity task, participants were first presented with a whole face (the target) for 4 s. Immediately after the target disappeared, two whole faces (whole condition), or two pairs of eyes or two mouths (part condition) were presented. One of these was the target and one was a distractor, which had a different mouth or eyes, sometimes alone and sometimes embedded in the target face. The participant selected the target, either the whole or a part. The faces were 22 children's faces, males and females, with neutral expressions (N = 80 trials) in 2 blocks administered consecutively.
2.2.4. Outliers and analysis
Preliminary analyses identified and dropped data points 2 SD from the mean for their age/group. This was done separately for each condition in each task. Analyses included an initial omnibus analysis of variance (ANOVA), with follow-up ANOVAs and t-tests to clarify the results.
3. Results
3.1. IM (Fig. 2)
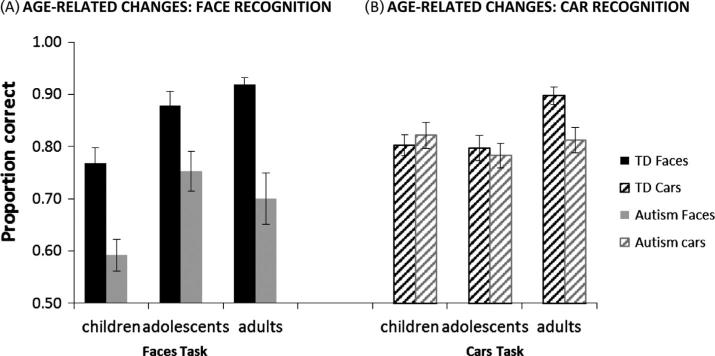
Fig. 2.
Mean proportion correct (y axis) on the Face (A, black bars) and Car (B, gray bars) IM tasks. Solid bars are TD, striped bars are the autism group. Age is on the x axis, divided into children, adolescents and adults. Error bars are the standard error of the mean.
The data was first examined with an omnibus repeated measures ANOVA, with condition (faces, cars) as a within-group, repeated factor, and group (autism, control) and age (children, adolescents, adults) as between-group factors. This ANOVA revealed main effects of condition, F(1,131) = 8.20, p = .005, η2p = .06, group, F(1,131) = 38.29, p < .001, η2p = .23, and age, F(2,131) = 7.59, p = .001, η2p = .10. These main effects were mitigated by significant interactions, including a condition × group interaction, F(1,131) = 21.05, p < .001, η2p = .14, condition × age interaction, F(2,131) = 8.72, p < .001, η2p = .12, and a borderline group × age interaction F(2,131) = 2.40, p < .10, η2p = .04. These interactions reflected that face recognition performance was more impaired than car recognition in autism; face recognition improved more with age than car recognition in both groups; and there was less age-related improvement in the group with autism than in the TD group. The difficulty of car and face IM was well-matched in the TD adult group. (Table 2 provides further insight into the relative difficulty of face and car recognition across group and age.) Due to the significant interactions, as well as our a priori interest in the pattern of development typically and in autism, we analyzed age-related changes in each group separately, using an ANOVA with post hoc analyses (Tamhane's t, which does not assume equal variance) to test whether performance changed significantly from one age to the next. Finally, to better understand the group × age interaction, we examined each age group separately to see if there were significant differences between those with and without autism.
3.1.1. Typical development
The omnibus ANOVA indicated that face recognition developed more than car recognition in both groups. However, in the ANOVA of the TD group only, there was a main effect of age, F(2,70) = 13.82, p < .001, η2p = .24, but no effect of condition nor age × condition interaction (p's > .14), suggesting similar age-related improvements across both conditions (significant age-related changes included from childhood to adulthood; and from adolescence to adulthood, p's < .01). While these post hoc analyses suggested no improvement from childhood to adolescence, visual inspection suggested improvement from childhood to adolescence on face but not car recognition. This possibility was supported by an analysis on face recognition alone, which exhibited a trend toward significant change from childhood to adolescence when analyzed separately (Tamhane's t, p < .08).
3.1.2. Development in autism
Although the omnibus ANOVA did not reveal a group × age × condition interaction, when analyzed separately, the age-related changes in the autism group suggested a different pattern from the TD individuals. Compared to the robust age-related improvement across conditions in TD individuals, individuals with autism exhibited less robust age-related improvements in performance. These improvements occurred in face recognition only, and they occurred from childhood to adolescence only. There was a main effect of condition, F(1,61) = 22.02, p < .001, η2p = .27, but no main effect of age, F(2,61) = 1.67, p = .20. Importantly there was a condition × age interaction, F(2,61) = 6.96, p = .002, η2p = .19. Follow-up ANOVAs revealed improvement with age on face recognition (F(2,61) = 4.98, p = .01) but not car recognition (F(2,61) = .99, p = .38). Post-hoc analyses (Tamhane's t) indicated that there was improvement on face recognition in autism from childhood to adolescence (p = .003) but no changes in performance from childhood to adulthood or adolescence to adulthood (p's > .27). The slight decline from adolescence to adulthood, though not significant, was enough to undermine the improvements from childhood to adolescence, leading to no significant improvements from childhood to adulthood.
3.1.3. Group differences at each age
In children (8–12 yo: 22 autism, 22 TD), there were significant main effects of condition F(1,42) = 22.89, p < .001, η2p = .35, group F(1,42) = 8.69, p = .005, η2p = .17, and a condition × group interaction, F(1,42) = 15.67, p < .001, η2p = .27, indicating that group differences varied across conditions. T-tests confirmed that the impairment in autism was evident during face recognition, t(45.98) = 4.16, p < .001, Cohen's d = 1.23, but not during car recognition, t(40.53) = −.57, p = .57.
In adolescents (13–17 yo: 23 autism, 24 TD), there was a main effect of group, F(1,45) = 6.11, p = .017, η2p = .12, indicating that individuals with autism performed more poorly than TD individuals. There was no main effect of condition nor a condition × group interaction (all p's > .17). While the condition × group interaction did not reach significance, follow-up t-tests on each condition separately suggested the deficit in autism was evident during face recognition but not car recognition (faces, t(41.92) = 2.66, p = .01, Cohen's d = .86; cars, t(47.98) = .43, p = .67).
In adults (18–35 yo: 19 autism, 27 TD), there was a main effect of group F(1,44) = 27.73, p < .001, η2p = .38, a trend-level main effect of condition, F(1,44) = 3.43, p = .07, η2p = .07, and a significant condition × group interaction F(1,44) = 8.28, p = .006, η2p = .16. T-tests indicated that the impairment in autism was evident on both conditions but was significantly greater for faces than cars (faces, t(20.39) = 4.30, p < .001, Cohen's d = 1.55; cars, t(34.13) = 2.94, p = .006, Cohen's d = .92). Since these groups were not as well matched as our other ages, we also did these analyses with smaller, individually matched groups (N = 19/group). These analyses revealed the same pattern of performance, with significant differences between adults with and without autism on both conditions (face IM, t(21.94) = 3.97, p = .001, Cohen's d = 1.70; car IM, t(33.17) = 3.39, p = .002, Cohen's d = 1.18).
3.1.4. Summary
In general, individuals with autism are more impaired at face recognition than at car recognition, and individuals in both groups exhibit more robust age-related improvements with faces than with cars. Although the three-way interaction of age, group and condition did not reach significance, this may reflect a lack of power (both condition × group and condition × age interactions were significant). We suggest this because the analyses of age in each group separately indicate distinct developmental trajectories for faces and cars in the two groups. In childhood, face recognition was impaired in the autism group but car recognition was not. By adulthood, after age-related improvements on car recognition occurred typically but not in autism, the impairment was evident in both face and car recognition. Longitudinal data is clearly warranted, to confirm the developmental trajectory in each condition and group, and to better understand if the increased variability in the autism group leads to more heterogeneity in the trajectories. Regardless of the need for further studies, the pattern of performance in this study indicates that age contributes to the inconsistent evidence on whether recognition deficits are face-specific in autism. When we analyze each age group separately, T-tests indicate that the impairment is: (1) face-specific in children with autism, (2) face-specific, but improving at a typical rate, in the adolescents with autism, and (3) robust and general across objects in adults with autism, but more notable for faces.
3.2. Part-whole task (Fig. 3)
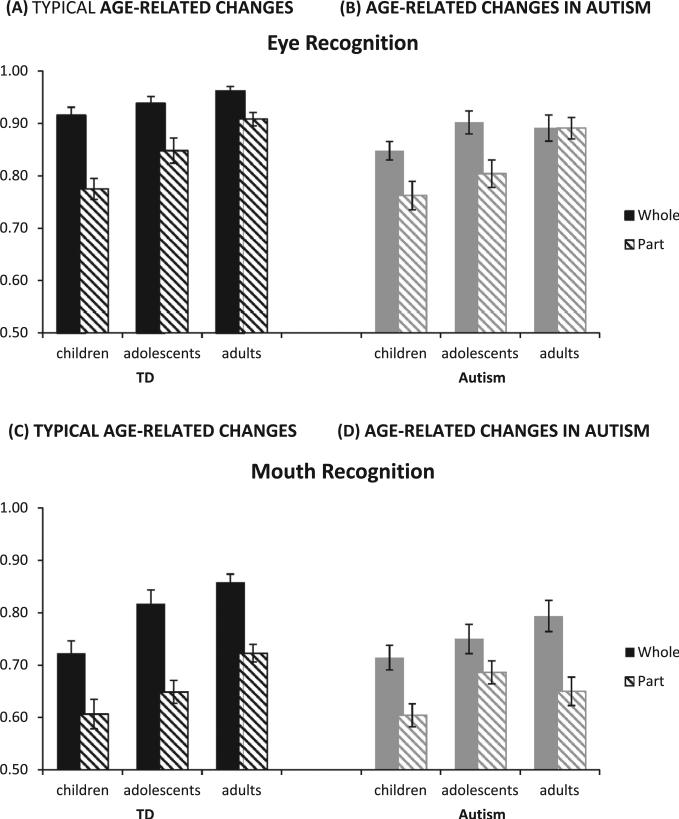
Fig. 3.
Eye recognition. (A) Typical development was evident for both parts and wholes. (B) In autism, there was development from adolescence to adulthood in the part eyes (only significant development in this stage in autism), and a trend from childhood to adolescence in the whole eyes condition. Mouth recognition. (C) Typical development was evident for both parts and wholes. (D) In autism, there was development from childhood to adolescence in the part mouth, but no significant developmental improvements in the whole mouth condition.
Proposals have suggested that impaired face recognition is related to less holistic or configural processing in autism, or relatively less attention to eyes than mouths. We examined both of these factors in the part-whole task, using an omnibus repeated measures ANOVA, with feature (eyes, mouth) and condition (part, whole) as within-group factors and group (autism, control) and age (children, adolescents, adults) as between-group factors. This analysis revealed main effects of condition, F(1,126) = 141.76, p < .001, η2p = .53, feature, F(1,126) = 467.97, p < .001, η2p = .79, group, F(1,126) = 6.06, p = .015, η2p = .05, and age, F(2,126) = 16.70, p < .001, η2p = .21, and significant condition × feature, F(1,126) = 18.86, p < .001, η2p = .13, condition × group, F(1,26) = 5.25, p = .02, η2p = .04, condition × feature × age F(2,126) = 6.48, p = .002, η2p = .09, and condition × feature group × age interactions, F(2,126) = 3.76, p = .03, η2p = .06. To understand this complex set of interactions, we examined age-related changes in each group separately, followed by group differences at each age.
3.2.1. Typical development
There were the expected main effects of condition, performance on whole > part, F(1,69) = 140.78, p < .001, η2p = .671; feature, eye > mouth, F(1,69) = 343.20, p < .001, η2p = .83, and age, older > younger, F(2,69) = 14.005, p < .01, η2p = .29. There was a 2-way interaction, condition × feature, F(1,69) = 10.79, p = .002, η2p = .14, but no condition × age interaction. However, a 3-way interaction, condition × feature × age F(2,69) = 3.79, p = .03, η2p = .10, indicated age-related changes in holistic processing were specific to the feature used. Each condition-feature combination showed significant age-related improvements (part eyes, F(1,70) = 22.20, p < .001, η2p = .24, Tamhane's t post hoc analyses indicate significant from childhood to adulthood, p < .05: part mouth F(1,70) = 11.07, p = .001, η2p = .14, significant from childhood to adulthood, p < .05: whole eyes, F(1,70) = 12.10, p = .001, η2p = .06, significant from childhood to adulthood, p < .05: whole mouth, F(1,70) = 21.34, p < .001, η2p = .23, significant from childhood to adulthood, and from childhood to adolescence, p < .05).
3.2.2. Development in autism
The pattern was similar in autism. There were significant main effects of condition, F(1,57) = 33.69, p < .001, η2p = .37, feature, F(1,57) = 159.03, p < .001, η2p = .74, and age, (F(2,57) = 5.49, p = .007, η2p = .16, as well as significant condition × feature, F(1,57) = 8.27, p = .006, η2p = .13, and condition × feature × age interactions F(2,57) = 5.51, p = .007, η2p = .16. Both part conditions and the whole mouth condition showed at least trend-level age-related improvements. The improvements were most striking with part eyes, F(1,58) = 11.64, p = .001, η2p = .17, which post hoc analyses indicated improved from childhood to adulthood, p < .01. With the whole mouth, there was trend-level age-related improvement, F(1,58) = 3.96, p = .05, η2p = .06, but post hoc analyses indicated that these changes did not reach significance at either developmental transition (childhood to adolescence, adolescence to adulthood). With the part mouth, there was a trend for significant age-related changes, F(1,58) = 3.34, p = .07, η2p = .05. However post hoc analyses indicated that, while performance improved significantly from childhood to adolescence in autism, p < .05, there was not significant improvement from childhood to adulthood, hinting that there may be some decline in performance from adolescence to adulthood.
3.2.3. Group differences at each age
In children (22 autism, 21 TD), there were few differences between those with and without autism. There were main effects of condition, F(1,41) = 48.26, p < .001, η2p = .54, and feature, F(1,41) = 165.47, p < .001, η2p = .80, but no main effect of group or significant interactions. Although there were no significant effects of group in the omnibus ANOVA, planned comparisons using t-tests on each feature suggested that eye recognition in the whole face was poorer in children with autism than in TD children t(43.51) = 2.84, p = .007, Cohen's d = .89.
In adolescents (22 autism, 24 TD), there were main effects of condition, F(1,44) = 53.67, p < .001, η2p = .55, and feature, F(1,44) = 131.43, p < .001, η2p = .75, but no main effect of group. However, the 3-way interaction of condition × feature × group was significant F(1,44) = 4.98, p = .03, η2p = .10. Planned compari sons on each condition/feature examined this interaction, but found only a trend for a group difference when recognizing mouths in the whole face condition t(48.67) = 1.76, p = .08, Cohen's d = .53.
In adults (16 autism, 27 TD), there were main effects of group, F(1,41) = 9.41, p = .004, η2p = .18, condition, F(1,41) = 42.24, p < .001, η2p = .51, and feature, F(1,41) = 180.57, p < .001, η2p = .82, but no interactions. The pattern was the same when we used the better-matched, smaller groups of adults, with a main effect of group, F(1,31) = 10.26, p = .003, η2p = .25, but no significant interactions. The group differences across conditions/features suggest general recognition deficits.
3.2.4. Summary
Holistic processing for faces (whole > part) is evident in all ages and groups, except with eyes in adults with autism. With age, both groups appear to rely less on holistic processing with eyes, but this likely reflects ceiling effects. Only a few group differences are evident in childhood and adolescence. Post-hoc analyses indicate that children with autism perform more poorly than TD children with eyes in a whole face (consistent with Wolf and colleagues, although their results were in both whole and part faces), and that adolescents with autism perform more poorly with mouths in a whole face than TD adolescents. The impairment in children and adolescents with autism is evident with whole objects only; this suggests that there may be differences in holistic processing in autism. However, any holistic differences are not robust, nor are they stable across age and feature. By adulthood, individuals with autism perform more poorly than TD individuals with eyes and mouths, in whole or part face conditions.
3.3. Correlations (Table 3)
Table 3.
Correlations between performance and holistic processing.
| Partial correlations | CFMT | Holistic processing with eyes | Holistic processing with mouth |
|---|---|---|---|
| Face IM | Autism: r(54) = .514, p < .001 | Autism: r(54) = .289, p = .031 | Autism: r(54) = .383, p = .004 |
| TD: r(62) = .345, p = .005 | TD: r(62) = .267, p = .033 | TD: r(62) = .378, p = .002 | |
| Car IM | Autism: r(54) = .154, p = .256 | Autism: r(54) = −.207, p = .125 | Autism: r(54) = −.019, p = .888 |
| TD: r(62) = −.021, p = .867 | TD: r(62) = −.196, p = .121 | TD: r(62) = −.013, p = .919 | |
| Whole eyes | Autism: r(54) = .439, p = .001 | Autism: r(54) = .438, p = .001 | |
| TD: r(62) = .380, p = .002 | TD: r(62) = .411, p = .001 | ||
| Whole mouth | Autism: r(54) = .516, p<.001 | Autism: r(54) = .449, p = .001 | |
| TD: r(62) = .408, p = .001 | TD: r(62) = .149, p = .240 | ||
| Part eyes | Autism: r(54) = .215, p = .112 | Autism: r(54) = .166, p = .222 | |
| TD: r(62) = .289, p = .02 | TD: r(62) = .394, p = .001 | ||
| Part mouth | Autism: r(54) = .165, p = .223 | Autism: r(54) = .136, p = .318 | |
| TD: r(62) = .202, p = .109 | TD: r(62) = −.089, p = .486 |
Recent work indicates an association between whole face recognition and holistic processing in typical adults (DeGutis, Wilmer, Mercado, & Cohan, 2013). Since the current studies examine both face recognition and holistic processing, both of which may differ in autism, we were able to explore the relation between these constructs. If the association between face recognition and holistic processing differs in those with autism, this provides novel insight into distinct strategies or processes used to recognize faces in autism. To explore this, we formed two ‘holistic’ composite scores, separately for the eye and mouth conditions, from performance in the part-whole task, using standardized residuals extracted from regression analyses. The method, from DeGutis et al., 2013, uses the residuals that remained when performance on the part condition was regressed from performance on the whole condition, much like a difference score but potentially more sensitive. We then used partial correlations (2-tailed, with age partialled out) in each group separately, to measure the association between performance on the current tasks (IM and part-whole) with the holistic composite scores for the eyes or mouth (from the part-whole task) and CFMT performance in this sample (a new study replicating the group × age interaction, F(2,144) = 4.54, p = .01, previously reported in O'Hearn et al., 2010). We did not correlate recognition of eyes (or alternately mouth) in the part-whole task with the holistic composite score for eyes (or mouth) because they are derived from the same scores.
The pattern (Table 3) was very similar across groups, with face recognition (but not car or mouth recognition) associated with holistic processing. In both groups, with age controlled, performance on the current whole-face recognition tasks (face IM/whole faces in part-whole) was significantly correlated with the CFMT, despite the longer delay, and face IM was correlated with holistic composite scores for both eyes and mouths extracted from the part-whole task. In contrast, car IM and the part mouth condition did not correlate with the CFMT, face IM, or holistic composite scores in either group.
There was one difference in the correlations across groups. In the TD group, but not in those with autism, recognition of eyes when they were presented as a part was related to CFMT and holistic processing for mouths. This hints that eyes by themselves, but not mouths, may be treated like a whole entity in the TD group but not in those with autism. This also makes the holistic composite score comparing whole and part conditions less robust with eyes in TD groups (potentially leading to the lack of correlation between the eye composite score and mouths in a whole face in the TD group).
4. Discussion and conclusion
Differences in face recognition in adults with autism reflect decreased age-related improvements in recognition generally. For face recognition, early deficits in autism are compounded by a lack of typical age-related improvements. For car recognition, deficits emerge from adolescence to adulthood, again due to a lack of improvement in the group with autism. This indicates that the trajectory of development in autism continues to be atypical into adulthood. Atypical trajectories are often described at earlier ages in autism (Elsabbagh et al., 2011), and this view of developmental disorders has a strong theoretical background in constructivist proposals (López, 2013; Thomas et al., 2009). However, little is known about the development of visual recognition during adolescence, or how it might differ in autism. The current study indicates that a lack of late improvement leaves adults with autism at a serious disadvantage on recognition tasks, compared to TD adults, even when memory demands are low and the stimuli are not people.
These results expand on our previous results which used whole faces on the CFMT (O'Hearn et al., 2010), by showing the same lack of late improvement in a task without a memory delay, and with cars and face parts as stimuli. This lack of adolescent improvement is consistent with results from other visual tasks (O'Hearn, Lakusta, et al., 2011; O'Hearn et al., 2013; Rump et al., 2009; Scherf et al., 2008), suggesting a developmental plateau in autism that encompasses a variety of visual functions. This also provides some insight into the inconsistencies in the literature on face processing in autism. If we had tested each age group alone, we would have come to different conclusions. In our sample, high-functioning children with autism display deficits specific to whole faces (face IM, eye recognition in whole face) compared to TD children. Adolescents with autism display an impairment similar to children with autism, but potentially less severe, with post hoc analyses indicating deficits in face IM but not the part-whole task. Adults with autism display a generalized recognition deficit, including cars and face parts, compared to TD adults.
Individuals with autism show improvements from childhood into adolescence, resulting in performance quite similar to TD individuals by adolescence. Considering the early deficits, this improvement in face recognition skills is an encouraging pattern, suggesting plasticity in the system. This similarity in performance between TD adolescents and adolescents with autism is consistent with some previous evidence (O'Hearn et al., 2013), and reflects improvements in performance from childhood to adolescence in autism, as well as relatively less improvement in the TD group. This limited improvement in TD individuals does not necessarily indicate a lack of development: instead, it may reveal a reorganization of visual processes that is needed for adult-level performance (Carey, Diamond, & Woods, 1980; Scherf & Scott, 2012). Meanwhile, the relatively skilled performance in adolescents with autism may reflect compensatory strategies that are helpful earlier in development but constrain performance by adulthood. From adolescence to adulthood, the only condition that improves in autism is recognition of eyes as a part. This potentially reflects the use of alternate, feature-based processes, a distinct style, or perhaps a compensatory strategy, often associated with autism. There appears to be almost a decline in performance from adolescence to adulthood in autism.
This work reveals that more developmental research is needed to elucidate exactly how visual processing in autism changes with age and experience. Limitations to the current study include the use of cross-sectional data and the sample size (although it is comparable to other studies of face recognition). The use of cross-sectional data does not allow us to analyze the shape of the trajectories or individual patterns of development, or to discern whether the trajectories differ across condition. These limitations also restrict our ability to examine the increased variability that is often evident in autism, and that may provide insight into potential subtypes in the group with autism. The use of cross-sectional data also leads to the concern that the results reflect cohort effects. This is an important possibility that we are currently examining, as it would indicate that experience (different in each cohort) has a substantial impact on visual processing in individuals with autism. However, this possibility seems less likely as the group differences reflect primarily the improvements in the TD group, which may be less liable to vary across cohort than the autism group, in which cohort effects could reflect different treatments emerging at different times. A final limitation of this work is that it tests only high functioning individuals with autism, mostly males. This ‘developmental plateau’ may not be evident in individuals with lower IQs, or in females with autism (Rhodes, Jeffery, Taylor, & Ewing, 2013). Such patterns would provide unique insight into adolescent development in autism, making it an important direction for further study.
These results, combined with previous evidence, indicate that visual differences in autism are modulated by sample characteristics (e.g., age) and task demands (e.g., delay), and that these factors may interact. Age is very likely to be an important factor, though certainly not the only factor, for explaining the mixed results on visual recognition in autism. Our results showing a face-specific deficit in children and adolescents (but not adults) with autism is consistent with Wolf et al. (2008) – however, their study showed face-specific deficits in a sample that included individuals with autism from 5–20 years old. Why the difference in results in these two samples? This discrepancy may reflect the composition of the TD groups used in each study. The control group in Wolf and colleagues were 5–18 years old, leading to not only a younger average age, but also probably excluding the stage of typical development from adolescence to adulthood, and potentially underestimating the deficit in adults with autism. Another potential reason for the discrepancy is that the group with autism in Wolf et al. (2008) included more individuals with IQs in the lower range. Of the three studies that have examined only adults with autism (high functioning), two have also reported general recognition deficits (Behrmann et al., 2006a; Blair et al., 2002). One study in adults only indicated a face-specific deficit in autism but also reports substantial heterogeneity in the sample (Wallace, Coleman, & Bailey, 2008).
Task demands also appear crucially important to whether differences in visual recognition are evident in autism. Even though the CFMT and LFI were both collected in our laboratory and were highly correlated, these two tasks showed different patterns of deficits in children with autism. The CFMT showed significant improvement in the TD group into adulthood (O'Hearn et al., 2010; see also Germine et al., 2011), with little impairment in face recognition in autism until adulthood. In contrast, the LFI showed less improvement from adolescence to adulthood in TD individuals, as well as face recognition deficits in autism in each age group, though the effect size of the face recognition deficit increased significantly by adulthood. This inconsistency across face recognition tasks may reflect that the CFMT was more ‘difficult’ than the LFI, with longer delays. This difference in difficulty may have led to less variation and decreased power at younger ages on the CFMT, and less variation and decreased power at older ages on the LFI (though performance was not at a true floor or ceiling). Another reason for such discrepancies are the task and the stimuli themselves. Both our results and those of Wolf et al. (2008) suggest that the part-whole task is less impacted by autism than is the face IM task, even though both tasks use whole face stimuli. This may reflect that the part-whole task uses children's faces as stimuli, or that the stimuli are less naturalistic (i.e., features from different faces are combined). Our results on the part-whole task also differ from previous studies (Joseph & Tanaka, 2003; Wolf et al., 2008) in that participants with autism did not perform relatively more poorly on eyes than mouths compared to the TD group. The eye deficit was evident in the current sample, but only in childhood and only with whole faces. By adulthood, our sample with autism exhibited holistic processing for mouths but not eyes, a pattern similar to that of adults with developmental prosopagnosia (DeGutis, Cohan, Mercado, Wilmer, & Nakayama, 2012) and to performance with face expression recognition in autism (Tanaka et al., 2012). Thus, although our work focuses on the importance of age, task demands that influence attention and eye movements are clearly important to face recognition performance.
Additional analyses indicate that individuals with autism show qualitatively typical processing for faces, with holistic processing related to recognition of faces but not cars or mouths alone. This is consistent with the analysis of Weigelt et al. (2012) showing qualitatively similar holistic processing in autism, though the decreased memory requirements did not improve development, as might have been predicted. One exception to the similarity between the TD group and the group with ASD is that TD participants appear to treat eyes alone as a ‘whole’ while those with autism do not. This interesting difference hints that TD individuals are more likely to use holistic processes with important parts, elevating them to “whole” status.
What underlies the recognition deficits in autism? Our evidence suggests at least two factors: (1) an early face-specific deficit which may be related to social impairments in autism, perhaps due to a lack of an early bias for faces or, over development, decreased attention to faces (Schultz, 2005), and (2) impaired maturation of domain-general visual processing, again potentially due to innate differences in autism that emerge during adolescence (e.g., sleeper effects; Maurer, Mondloch, & Lewis, 2007) or, over development, different visual experiences in autism (Scerif, 2010). The possibility that multiple visual differences underlie the face recognition deficit in autism, and that they may contribute differently depending on the sample characteristics and task demands, provides a unique viewpoint that may help to accommodate inconsistencies in the literature. This work also elucidates the typical maturation of brain function, if we assume behavioral development has a neural correlate. The regions underlying face recognition have been shown to develop into adolescence, and this late development is often considered to be specific for faces (Golarai, Liberman, Yoon, & Grill-Spector, 2010; Golarai et al., 2007; Scherf, Behrmann, Humphreys, & Luna, 2007; Scherf, Luna, Minshew, & Behrmann, 2010). The current evidence suggests that these systems continue to change past adolescence, into adulthood, and these changes are not specific to faces. Indeed, considering the overlapping nature of the representations in the ventral stream (Haxby et al., 2001), development of one category might naturally affect the representation of other categories (O'Hearn, Roth, Courtney, Street, & Luna, 2011).
Finally, further work is needed to characterize the visual processes that are still improving into adulthood typically, and why this differs in autism. Based on previous studies with very simple tasks (e.g., enumeration), these age-related changes are likely to reflect fundamental visual processes (O'Hearn et al., 2013) not higher-order executive functions such as task-switching or the fine-tuning of memory (Luna, Doll, Hegedus, Minshew, & Sweeney, 2007). Nonetheless, executive function and memory also develop into adulthood and may contribute to performance on some tasks and the variability in results. However, our results suggest that age-related changes are related to visual or attentional processes (Scerif, 2010), such as the rapid allocation of attention to important parts of a visual display. That these improvements do not occur in autism provides intriguing hints at what is developing typically. Decreased global or holistic visual processing in children with autism, advantageous in some tasks (O'Riordan & Plaisted, 2001), may ultimately limit visual function over development, as TD individuals develop a greater ‘global bias’ into adulthood (Navon, 1977). However this relation is not simple; the results from the part-whole task indicate the deficits in autism are not specific to ‘whole’ objects only. Instead, TD individuals may be more likely to elevate stimuli to ‘holistic processing’, as the TD group does with eyes as a part. Alternately, the improvement evident typically may reflect adolescent experiences, when more attention may be allocated to complex visual information, often dynamic social interactions. The social deficits or repetitive behaviors in autism may undermine these experiences, resulting in less expertise with a variety of visual information. This differential development during adolescence is likely to reflect continued divergence in both experience and brain maturation between those with and without autism. Compensatory strategies, often helpful, may ultimately limit the final level of performance. These important possibilities underscore the need for further study of development during adolescence in autism. This is a vulnerable and understudied developmental stage, when many high functioning individuals with autism are no longer receiving services, and are instead transitioning to adult-level duties, such as full-time work, which often prove challenging (Taylor & Seltzer, 2010).
Acknowledgments
This work was completed at the University of Pittsburgh and supported by Autism Speaks Grant 04593 (PI Luna), NIMH 5 R01 MH067924 (PI Luna), NIH HD055748 (PI Minshew) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, and NIMH K01 MH081191 (PI O'Hearn). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Recruitment was supported by NICHD ACE grant HD055648 and CPEA grant HD35469. We thank Catherine Wright, the participants and their families, and the staff at the Autism Center of Excellence for their generous help. Preliminary analysis was presented at The International Meeting for Autism Research in Toronto ON in May, 2012.
Abbreviations
- TD
typically developing
- IM
immediate memory
- LFI
Let's Face It
- CFMT
Cambridge Face Memory Test
Footnotes
We did not use a task with simultaneous presentation, because these tasks differ from typical face recognition and the stimuli have to be challenging in some way.
References
- Behrmann M, Avidan G, Leonard GL, Kimchi R, Luna B, Humphreys K, et al. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006a;44(1):110–129. doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: Visual processing in autism. Trends in Cognitive Sciences. 2006b;10(6):258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Frith U, Smith N, Abell F, Cipolotti L. Fractionation of visual memory: Agency detection and its impairment in autism. Neuropsychologia. 2002;40(1):108–118. doi: 10.1016/s0028-3932(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Bradshaw J, Shic F, Chawarska K. Brief report: Face-specific recognition deficits in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2011;41(10):1429–1435. doi: 10.1007/s10803-010-1150-4. http://dx.doi.org/10.1007/s10803-010-1150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey S, Diamond R. From piecemeal to configurational representation of faces. Science. 1977;195(4275):312–314. doi: 10.1126/science.831281. [DOI] [PubMed] [Google Scholar]
- Carey S, Diamond R, Woods B. Development of face recognition: A maturational component? Developmental Psychology. 1980;16:257–269. [Google Scholar]
- Chawarska K, Shic F. Looking but not seeing: Atypical visual scanning and recognition of faces in 2 and 4-year-old children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39(12):1663–1672. doi: 10.1007/s10803-009-0803-7. http://dx.doi.org/10.1007/s10803-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. http://dx.doi.org/10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. http://dx.doi.org/10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- DeGutis J, Cohan S, Mercado RJ, Wilmer J, Nakayama K. Holistic processing of the mouth but not the eyes in developmental prosopagnosia. Cognitive Neuropsychology. 2012;29(5–6):419–446. doi: 10.1080/02643294.2012.754745. http://dx.doi.org/10.1080/02643294.2012.754745. [DOI] [PubMed] [Google Scholar]
- DeGutis J, Wilmer J, Mercado RJ, Cohan S. Using regression to measure holistic face processing reveals a strong link with face recognition ability. Cognition. 2013;126(1):87–100. doi: 10.1016/j.cognition.2012.09.004. http://dx.doi.org/10.1016/j.cognition.2012.09.004. [DOI] [PubMed] [Google Scholar]
- De Haan M, Humphreys K, Johnson MH. Developing a brain specialized for face perception: A converging methods approach. Developmental Psychobiology. 2002;40(3):200–212. [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 2006;44(4):576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Holmboe K, Gliga T, Mercure E, Hudry K, Charman T, et al. Social and attention factors during infancy and the later emergence of autism characteristics. Progress in Brain Research. 2011;189:195–207. doi: 10.1016/B978-0-444-53884-0.00025-7. http://dx.doi.org/10.1016/B978-0-444-53884-0.00025-7. [DOI] [PubMed] [Google Scholar]
- Germine LT, Duchaine B, Nakayama K. Where cognitive development and aging meet: Face learning ability peaks after age 30. Cognition. 2011;118(2):201–210. doi: 10.1016/j.cognition.2010.11.002. http://dx.doi.org/10.1016/j.cognition.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Golarai G, Ghahremani D, Whitfield-Gabrieli S, Reiss A, Eberhardt J, Gabrieli J, et al. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neuroscience. 2007;10(4):512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Liberman A, Yoon JMD, Grill-Spector K. Differential development of the ventral visual cortex extends through adolescence. Frontiers in Human Neuroscience. 2010;3(80) doi: 10.3389/neuro.09.080.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F. Autism: Cognitive deficit or cognitive style? Trends in Cognitive Sciences. 1999;3(6):216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51(1):59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Hedley D, Brewer N, Young R. Face recognition performance of individuals with Asperger syndrome on the Cambridge Face Memory Test. Autism Research: Official Journal of the International Society for Autism Research. 2011;4(6):449–455. doi: 10.1002/aur.214. http://dx.doi.org/10.1002/aur.214. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tanaka J. Holistic and part-based face recognition in children with autism. Journal of Child Psychology and Psychiatry. 2003;44(4):529–542. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner JC, Hatri A, Heekeren HR, Dziobek I. Autistic symptomatology, face processing abilities, and eye fixation patterns. Journal of Autism and Developmental Disorders. 2011;41(2):158–167. doi: 10.1007/s10803-010-1032-9. http://dx.doi.org/10.1007/s10803-010-1032-9. [DOI] [PubMed] [Google Scholar]
- Kovács I, Kozma P, Fehér A, Benedek G. Late maturation of visual spatial integration in humans. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(21):12204–12209. doi: 10.1073/pnas.96.21.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschner ES, Bodner KE, Minshew NJ. Local vs. global approaches to reproducing the Rey Osterrieth Complex Figure by children, adolescents, and adults with high-functioning autism. Autism Research. 2009;2(6):348–358. doi: 10.1002/aur.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López B. Beyond modularisation: The need of a socio-neuro-constructionist model of autism. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1966-9. http://dx.doi.org/10.1007/s10803-013-1966-9. [DOI] [PubMed]
- Lord C, Rutter M, Couteur AL. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Maurer D, Mondloch CJ, Lewis TL. Sleeper effects. Developmental Science. 2007;10(1):40–47. doi: 10.1111/j.1467-7687.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- McKone E, Stokes S, Liu J, Cohan S, Fiorentini C, Pidcock M, et al. A robust method of measuring other-race and other-ethnicity effects: The Cambridge Face Memory Test format. PLoS ONE. 2012;7(10):e47956. doi: 10.1371/journal.pone.0047956. http://dx.doi.org/10.1371/journal.pone.0047956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondloch CJ, Geldart S, Maurer D, Le GR. Developmental changes in face processing skills. Journal of Experimental Child Psychology. 2003;86(1):67–84. doi: 10.1016/s0022-0965(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Controlled scanpath variation alters fusiform face activation. Social Cognitive and Affective Neuroscience. 2007;2(1):31–38. doi: 10.1093/scan/nsl023. http://dx.doi.org/10.1093/scan/nsl023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J, Johnson MH. CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychological Review. 1991;98(2):164–181. doi: 10.1037/0033-295x.98.2.164. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. http://dx.doi.org/10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Navon D. Forest before trees: The precedence of global features in visual perception. Cognitive Psychology. 1977;9:353–383. [Google Scholar]
- O'Hearn K, Franconeri S, Wright C, Minshew N, Luna B. The development of individuation in autism. Journal of Experimental Psychology Human Perception and Performance. 2013;39(2):494–509. doi: 10.1037/a0029400. http://dx.doi.org/10.1037/a0029400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hearn K, Lakusta L, Schroer E, Minshew N, Luna B. Deficits in adults with autism spectrum disorders when processing multiple objects in dynamic scenes. Autism Research. 2011;4(2):132–142. doi: 10.1002/aur.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hearn K, Roth J, Courtney S, Street W, Luna B, Terwilliger R, Landau B. Object recognition in Williams syndrome: Uneven ventral stream activation. Developmental Science. 2011;14(3):549–565. doi: 10.1111/j.1467-7687.2010.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hearn K, Schroer E, Minshew N, Luna B. Lack of developmental improvement on a face memory task during adolescence in autism. Neuropsychologia. 2010;48(13):3955–3960. doi: 10.1016/j.neuropsychologia.2010.08.024. http://dx.doi.org/10.1016/j.neuropsycho logia.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Riordan M, Plaisted K. Enhanced discrimination in autism. The Quarterly Journal of Experimental Psychology. 2001;54(4):961–979. doi: 10.1080/713756000. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Schonen S. Recognition memory in 3- to 4-day-old human neonates. NeuroReport. 1994;5(14):1721–1724. doi: 10.1097/00001756-199409080-00008. [DOI] [PubMed] [Google Scholar]
- Perlman SB, Hudac CM, Pegors T, Minshew NJ, Pelphrey KA. Experimental manipulation of face-evoked activity in the fusiform gyrus of individuals with autism. Social Neuroscience. 2011;6(1):22–30. doi: 10.1080/17470911003683185. http://dx.doi.org/10.1080/17470911003683185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G, Jeffery L, Taylor L, Ewing L. Autistic traits are linked to reduced adaptive coding of face identity and selectively poorer face recognition in men but not women. Neuropsychologia. 2013;51(13):2702–2708. doi: 10.1016/j.neuropsychologia.2013.08.016. http://dx.doi.org/10.1016/j.neuropsychologia.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Rump KM, Giovannelli JL, Minshew NJ, Strauss MS. The development of emotion recognition in individuals with autism. Child Development. 2009;80(5):1434–1447. doi: 10.1111/j.1467-8624.2009.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ. The development of face processing in autism. Journal of Autism and Developmental Disorders. 2006;36(3):381–394. doi: 10.1007/s10803-006-0076-3. http://dx.doi.org/10.1007/s10803-006-0076-3. [DOI] [PubMed] [Google Scholar]
- Scerif G. Attention trajectories, mechanisms and outcomes: At the interface between developing cognition and environment. Developmental Science. 2010;13(6):805–812. doi: 10.1111/j.1467-7687.2010.01013.x. http://dx.doi.org/10.1111/j.1467-7687.2010.01013.x. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, Humphreys K, Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Developmental Science. 2007;10(4):F15–F30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, Kimchi R, Luna B. Emergence of global shape processing continues through adolescence. Child Development. 2009;80(1):162–177. doi: 10.1111/j.1467-8624.2008.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Luna B, Kimchi R, Minshew N, Behrmann M. Missing the big picture: Impaired development of global shape processing in autism. Autism Research. 2008;1(2):114–129. doi: 10.1002/aur.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Luna B, Minshew N, Behrmann M. Location, location, location: Alterations in the functional topography of face- but not object- or place-related cortex in adolescents with autism. Frontiers in Human Neuroscience. 2010;4:26. doi: 10.3389/fnhum.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Scott LS. Connecting developmental trajectories: Biases in face processing from infancy to adulthood. Developmental Psychobiology. 2012;54(6):643–663. doi: 10.1002/dev.21013. http://dx.doi.org/10.1002/dev.21013. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. Parts and wholes in face recognition. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 1993;46(2):225–245. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Wolf JM, Klaiman C, Koenig K, Cockburn J, Herlihy L, et al. Using computerized games to teach face recognition skills to children with autism spectrum disorder: The Let's Face It! program. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2010;51(8):944–952. doi: 10.1111/j.1469-7610.2010.02258.x. http://dx.doi.org/10.1111/j.1469-7610.2010.02258.x. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Wolf JM, Klaiman C, Koenig K, Cockburn J, Herlihy L, et al. The perception and identification of facial emotions in individuals with autism spectrum disorders using the Let's Face It! Emotion Skills Battery. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2012;53(12):1259–1267. doi: 10.1111/j.1469-7610.2012.02571.x. http://dx.doi.org/10.1111/j.1469-7610.2012.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Seltzer MM. Changes in the autism behavioral phenotype during the transition to adulthood. Journal of Autism and Developmental Disorders. 2010;40(12):1431–1446. doi: 10.1007/s10803-010-1005-z. http://dx.doi.org/10.1007/s10803-010-1005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MSC, Annaz D, Ansari D, Scerif G, Jarrold C, Karmiloff-Smith A. Using developmental trajectories to understand developmental disorders. Journal of Speech, Language, and Hearing Research: JSLHR. 2009;52(2):336–358. doi: 10.1044/1092-4388(2009/07-0144). http://dx.doi.org/10.1044/1092-4388(2009/07-0144) [DOI] [PubMed] [Google Scholar]
- Wallace S, Coleman M, Bailey A. Face and object processing in autism spectrum disorders. Autism Research. 2008;1(1):43–51. doi: 10.1002/aur.7. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Venema K, Greenson J, Murias M, et al. Developmental change in the ERP responses to familiar faces in toddlers with autism spectrum disorders versus typical development. Child Development. 2011;82(6):1868–1886. doi: 10.1111/j.1467-8624.2011.01656.x. http://dx.doi.org/10.1111/j.1467-8624.2011.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neuroscience and Biobehavioral Reviews. 2012;36(3):1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. http://dx.doi.org/10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Wilson CE, Freeman P, Brock J, Burton AM, Palermo R. Facial identity recognition in the broader autism phenotype. PLoS ONE. 2010;5(9):e12876. doi: 10.1371/journal.pone.0012876. http://dx.doi.org/10.1371/journal.pone.0012876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JM, Tanaka JW, Klaiman C, Cockburn J, Herlihy L, Brown C, et al. Specific impairment of face-processing abilities in children with autism spectrum disorder using the Let's Face It! skills battery. Autism Research: Official Journal of the International Society for Autism Research. 2008;1(6):329–340. doi: 10.1002/aur.56. http://dx.doi.org/10.1002/aur.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin RK. Looking at upside-down faces. Journal of Experimental Psychology. 1969;81(1):141–145. http://dx.doi.org/10.1037/h0027474. [Google Scholar]