Abstract
The development of inhibitory control—the ability to suppress inappropriate actions in order to make goal-directed responses—is often impaired in autism spectrum disorders (ASD). In the present study, we examined whether the impairments in inhibitory control evident in ASD reflect—in part—differences in the development of the neural substrates of inhibitory control from adolescence into adulthood. We conducted a functional magnetic resonance imaging (fMRI) study on the anti-saccade task, a probe of inhibitory control, in high-functioning adolescents and adults with ASD compared to a matched group of typically developing (TD) individuals. The ASD group did not show the age-related improvements in behavioral performance from adolescence to adulthood evident in the typical group, consistent with previous behavioral work. The fMRI results indicated that much of the circuitry recruited by the ASD group was similar to the TD group. However, the ASD group demonstrated some unique patterns, including: (a) a failure to recruit the frontal eye field during response preparation in adolescence but comparable recruitment in adulthood; (b) greater recruitment of putamen in adolescence and precuneus in adolescence and adulthood than the TD group; and (c) decreased recruitment in the inferior parietal lobule relative to TD groups. Taken together, these results suggest that brain circuitry underlying inhibitory control develops differently from adolescence to adulthood in ASD. Specifically, there may be relative underdevelopment of brain processes underlying inhibitory control in ASD, which may lead to engagement of subcortical compensatory processes.
Keywords: autism, fMRI, inhibitory control, antisaccade, development, adolescence
Introduction
Autism spectrum disorders (ASD) are a class of neurodevelopmental disorders characterized in part by atypical brain development, which may lead to abnormalities in cognitive behaviors. In typical development, substantial improvements in inhibitory control of behavior and the brain systems that underlie it continue from adolescence into early adulthood [Luna, Garver, Urban, Lazar, & Sweeney, 2004; Velanova, Wheeler, & Luna, 2008, 2009]. Inhibitory control, defined as the ability to suppress automatic but inappropriate responses in favor of a goal-directed behavior [Bjorklund & Harnishfeger, 1995; Dempster, 1992] may be a likely candidate for cognitive differences in ASD, though the results are mixed, suggesting that deficits may be paradigm specific [Goldberg et al., 2005; Ozonoff & Strayer, 1997; Ozonoff, Strayer, McMahon, & Filloux, 1994; Schmitz et al., 2006] [Agam, Joseph, Barton, & Manoach, 2010; Luna, Doll, Hegedus, Minshew, & Sweeney, 2007; Minshew, Luna, & Sweeney, 1999; Ozonoff & Strayer, 1997; Russell, 1997] or highlight the need to study the development of these processes [Luna et al., 2007; Solomon et al., 2014]. As adolescence is a time of significant change in brain and behavior [Spear, 2000], the transition from adolescence to adulthood has become focus of investigation and represents a potential window of plasticity in the brain, which may be impacted in ASD.
On the inhibitory tasks shown to differ in ASD, studies have demonstrated that both children and adults with ASD have poorer performance than matched typical controls, suggesting that abnormalities may persist throughout development [Agam et al., 2010; Goldberg et al., 2002; Luna et al., 2007; Minshew et al., 1999; Mosconi et al., 2009; Solomon et al., 2014]. One index of inhibitory control, the anti-saccade(AS) task, is a well-established oculomotor paradigm that has previously been used to model the developmental trajectory of inhibitory control through adolescence into adulthood [Fischer, Biscaldi, & Gezeck, 1997; Fukushima, Hatta, & Fukushima, 2000; Klein & Foerster, 2001; Luna et al., 2001, 2004; Munoz, Broughton, Goldring, & Armstrong, 1998; Velanova et al., 2008, 2009]. During the AS task, participants must suppress an automatic reaction to a peripheral stimulus and generate a planned oculomotor response in the opposite direction. Prior evidence has suggested that TD adults and adolescents engage a widely distributed set of brain regions during performance of the AS task, including cortical and subcortical regions such as the frontal, supplementary, and parietal eye fields (FEF, SEF, and PEF, respectively), basal ganglia, dorsolateral prefrontal cortex (DLPFC), and cerebellum [for review, see Munoz & Everling, 2004]. Our previous fMRI studies using the anti-saccade task to examine normative development indicate significant developmental changes in brain function supporting anti-saccade performance and improved error processing. [Luna et al., 2001; Velanova et al., 2008, 2009].
Robust inhibitory control deficits on the AS task have been documented in adults with ASD [Agam et al., 2010; Luna et al., 2007; Minshew et al., 1999; Mosconi et al., 2009; Thakkar et al., 2008]. Adults with ASD demonstrate worse performance, decreased recruitment of the anterior cingulate cortex (ACC) and the FEF following correct trials, and reduced functional connectivity between the ACC and premotor regions compared to typical adults [Agam et al., 2010; Thakkar et al., 2008]. This is consistent with other inhibitory control tasks that result in reduced activation and functional connectivity in ACC, PFC, and posterior parietal regions, in adults with ASD relative to typically developed individuals [Kana, Keller, Minshew, & Just, 2007; Schmitz et al., 2006; Solomon et al., 2009]. We previously examined the development of inhibitory control behavior in ASD using the AS task in a cross-sectional sample from childhood to adulthood. The results indicated that despite performing worse than typically developing individuals in both adolescence and adulthood, the group with ASD showed significant age-related improvements similar to typically developing individuals [Luna et al., 2007]. That is, individuals with ASD show parallel improvements in inhibitory control through adolescence but do not catch up with typically developing peers. These behavioral findings suggest that similar brain maturation processes may be occurring in ASD from adolescence to adulthood, but to date, little is known about the development of the neural substrates of inhibitory control in ASD.
Identifying the neural substrates of the developmental delays, arrests, and abnormalities in ASD will provide insight into limitations in inhibitory control, and help to identify time points during which intervention will be particularly successful. Furthermore, assessing developmental changes into adulthood will identify cognitive deficits that may be late appearing and highlights how the final stage of development may deviate from typical development.
In the present study, we examined the neural correlates of the AS task in adolescents and adults diagnosed with ASD relative to age and IQ-matched typically developing adolescent and adult participants using functional magnetic resonance imaging (fMRI). Similar to our previous behavioral results [Luna et al., 2007], we predicted that the ASD group would show age-related improvements in anti-saccade performance from adolescence to adulthood similar to the typically developing group. However, we had no directional hypotheses for the interaction between age group and diagnosis group, given the lack of previous studies in this area.
Methods
Participants
Forty-two participants were recruited for the study, 14 typical adults (ages 18–31, M = 23.4 (+ /− 4.0); two females), and nine typical adolescents (ages 12–17, M = 13.9 (+ /− 1.4); two females) (CON groups) and eight adults with ASD (ages 19–33, M = 24.9 (+ /− 6.9), and eleven adolescents with ASD (ages 13–17, M = 15.1 (+ /− 1.2); two females) (ASD groups). The Autism Diagnostic Interview-Revised [Lord, Rutter, & Couteur, 1994] and the Autism Diagnostic Observation Schedule-General [Lord et al., 2000; Lord, Rutter, & Goode, 1989] were used to diagnose ASD. Diagnosis was confirmed by expert clinical opinion (NJM). Potential subjects were excluded if known to have an associated disorder such as tuberous sclerosis or fragile-X syndrome. All participants were required to have full scale and verbal IQ scores of 80 or above (Table 1). Medication status of the ASD participants is reported in Supporting Information Table S1. All participants were free of medications known to affect eye movements and had no history of seizure disorder.
Table 1.
Demographic Information
| ASD
|
CON
|
||||
|---|---|---|---|---|---|
| Adolescent n = 11 |
Adult n = 8 |
Adolescent n = 9 |
Adult n = 14 |
||
| Males | 9 | 8 | 7 | 12 | |
| Right handed, n (%) | 36 (88) | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ANOVA P | |
| Age (years) | 15.10 (1.19) | 24.88 (6.94) | 13.89 (1.36) | 23.36 (3.99) | 0.797 |
| Full scale IQ | 108.10 (10.94) | 98.88 (13.93) | 109.71 (10.13) | 109.53 (6.63) | 0.925 |
| ADOS | |||||
| Communication | 4.50 (1.58) | 4.38 (1.41) | – | – | |
| Social | 9.50 (2.27) | 10.5 (1.69) | – | – | |
| Total | 14.00 (4.43) | 14.88 (2.53) | – | – | |
| ADI | |||||
| Social | 23.90 (3.28) | 19.38 (4.87) | – | – | |
| Communication | 18.20 (3.42) | 15.25 (4.33) | – | – | |
| Restricted, repetitive behaviors | 6.30 (2.45) | 6.25 (1.98) | – | – | |
| Abnormal | 3.4 (1.43) | 2.62 (1.60) | – | – | |
dashes mean not relevant (CON individuals were not administered the ADOS or ADI).
There were no significant differences between groups in IQ (WASI) (Adult CON: 109.5 + /− 13.9, Adult ASD: 98.9 + /− 13.9, Adolescent CON: 109.7 + /− 10.1, Adolescent ASD: 106.2 + /− 12.1, Ps > .05). All but one participant were right handed. CON individuals had no personal or first-degree relative history of neurological disease or neuropsychiatric illness as determined by interview. Vision was normal (as stated by the participant or guardian for minor participants) or corrected to normal using MRI compatible glasses or contact lenses. Experimental procedures for this study complied with the Code of Ethics of the World Medical Association (1964 Declaration of Helsinki) and the Institutional Review Board at the University of Pittsburgh. Subjects were paid for their participation in the study. All participants and guardians provided informed consent prior to participation.
Paradigm
Participants performed an event-related task, which included AS and visually guided saccade (VGS) trials. Immediately prior to scanning, participants were trained on the AS and VGS tasks in a training room outside the scanner. During the AS trials, a red central fixation cross appeared on the screen for 2.5 sec signaling participants to prepare for presentation of the target stimulus. When the red cross disappeared, a peripheral target appeared at an unpredictable location on the horizontal meridian at 3, 6, or 9 degrees of visual angle to the left and right of the center fixation cross for 2.5 sec. Participants were instructed not to look at the stimulus, but instead make an eye movement to the mirror location. Peripheral target location was randomized within each run. During the VGS trials, participants were presented with a green fixation cross and instructed to look toward the peripheral stimulus when it appeared. These VGS trials were randomly interspersed between the AS trials (Fig. 1).
Figure 1.
Anti-saccade (AS) task schematic. A red fixation cross appeared for 2.5 sec. A peripheral light appeared for 2.5 sec during which participants were instructed to generate a saccade to its mirror location.
To uniquely estimate the hemodynamic response for the preparatory and response phases of the AS trials, our experimental design included additional AS partial “catch” trials, randomly inserted along with jittered intertrial intervals [Ollinger, Corbetta, & Shuldman, 2001; Ollinger, Shulman, & Corbetta, 2001]. This approach ensured that there were a sufficient number of independent linear equations to separately estimate the Blood-Oxygen-Level Dependent (BOLD) response associated with the preparatory and saccade response phases of each AS trial. The catch trials consisted of the presentation of a red central fixation cross, with no following target. The intertrial fixation period was uniformly distributed and jittered between intervals of 2.5, 5, or 7.5 sec and consisted of a white cross on a black background that subjects were instructed to fixate on in between trials. This allowed us to isolate activation specific to the fixation period when the inhibitory processes begin prior to making a correct inhibitory response [Everling, Dorris, & Munoz, 1998; Everling, Spantekow, Krappmann, & Flohr, 1998]. The three trial types (prep only, whole AS, whole VGS) were presented in a random order, separated by the intertrial intervals. Participants performed two functional runs of the task (duration = 6 min 50 sec each) for a total of 34 AS trials, 16 VGS trials, and 18 catch AS trials.
Eyetracking
Eye movement measurements during the scan using a long-range optics eye-tracking system (Model R-LRO6, Applied Science Laboratories, Bedford, MA). Nine-point calibrations were performed at the beginning of the session. Stimuli were presented using E-Prime (Psychology Software Tools, Inc., Pittsburgh, PA) projected onto a flat screen positioned behind the magnet. Participants viewed the screen using a mirror mounted on the head coil. Eye-movement data were analyzed and scored offline using ILAB [Gitelman, 2002] in conjunction with an in-house scoring program. The behavioral variables of interest were error rates for AS and VGS trials (the number of accurate eye movements/ total number of scorable trials). A correct response in the VGS task was one in which the first eye movement during the saccade response epoch was made toward the peripheral cue. A correct response in the AS task was one in which the first eye movement during the saccade response epoch was made toward the mirror location of the peripheral cue and extended beyond a 2.5 degrees/visual angle central fixation zone. Inhibitory errors occurred when the first saccade during the saccade response epoch was directed toward the suddenly appearing peripheral stimulus that they were to ignore and exceeded the 2.5 degrees/visual angle central fixation zone.
Image Acquisition
Imaging data were acquired using a 3.0 Tesla whole-body MR scanner (SIGNA; General Electric Medical Systems, Milwaukee, WI) with echo-planar imaging capability and a commercial head radiofrequency coil. The following gradient echo echo-planar acquisition parameters were used: echo time = 25 ms; repetition time = 2,500 ms; single shot; full k space; 64 × 64 acquisition matrix with field of view = 20 × 20 cm3, voxel dimension = 3.125 × 3.125 × 6 mm. Twenty-three 5-mm axial slices were prescribed with a 1-mm gap in order to image the whole brain. The first four volumes in each run were discarded to allow stabilization of longitudinal magnetization. A three-dimensional spoiled gradient recalled (SPGR) pulse sequence with 124 slices (6 mm each) was used to acquire structural images in the axial plane. These anatomical images were used to register to functional images and to localize regions of activation.
Image Preprocessing
Imaging data were preprocessed using FSL (Functional MRI of the Brain (FMRIB) Software Library; [Smith et al., 2004]. Our preprocessing procedures included the following: SPGR images were warped into Talairach space [Talairach & Tournoux, 1988] using a 12-parameter affine transformation in FSL [Jenkinson & Smith, 2001]. Functional images were first slice-time corrected, adjusting for interleaved slice acquisition. Images were then rigid-body motion corrected by aligning all volumes with the volume acquired in the middle of the fMRI session. Rotational and translational head movement estimates were calculated. Following brain extraction, functional images were affine registered and warped to structural SPGR images in Talairach space [Talairach & Tournoux, 1988]. Images were resampled to voxel sizes of 3 × 3 × 3 mm. No subjects were excluded due to motion: instead the temporal derivative of the relative displacement from the middle volume for each run was calculated for each volume in the x, y, and z directions. Magnitude of the velocity was then calculated by taking the square root of the sum of squares of the x, y, and z components for each volume. Volumes with a velocity of over 1.2 mm/TR were removed from subsequent analyses. Participant groups did not differ in number of volumes removed due to excessive motion (P > 0.05). Total number of volumes removed for each group were the following: Adult ASD: 2, Adult CON: 1, Teen ASD: 15, Teen CON: 8. Images were then spatially smoothed with a 5 mm full-width at half maximum Gaussian smoothing kernel, and high-pass filtered (sigma = 30 sec) to remove low frequency drift. Finally, data from each run were scaled to a mean of one hundred.
Image Analyses
Analysis and Visualization of Functional Neuroimages (AFNI, Bethesda, MD, USA) software [Cox, 1996] was used for individual subject deconvolution, followed by group analyses (see Supporting Information Fig. S1). Deconvolution methods followed steps delineated by Ward [2002]. Our model consisted of three orthogonal regressors of interest; the preparatory period of the AS (catch and correct trials only), the correct AS saccade response, and the correct VGS trials, as well as regressors for incorrect AS and VGS trials. Linear and nonlinear trends and six motion parameters were also included as nuisance regressors. A unique estimated impulse response function for each regressor of interest was determined by a weighted linear sum of sine basis functions, multiplied by data determined least squares estimated beta weights. The estimated impulse response function reflects the estimated BOLD response to a type of trial after controlling for variations in the BOLD signal due to other regressors. We specified the duration of the estimated response (AS prep and response: 20 sec; VGS: 24 sec post stimulus onset), a sufficient time window for the hemodynamic response to peak and return to baseline [Boynton, Engel, Glover, & Heeger, 1996; Buckner, 1998; Dale & Buckner, 1997]. This procedure produced one-time course estimate per voxel per condition of interest. No assumptions about the specific shape of the BOLD response were made beyond using zero as the start point. The finite impulse response method and deconvolution allowed for comparison of the shapes of the estimated time courses at several different time points in order to choose the peak time point. This approach allowed us to characterize potential differences (if any) in the shape of the response related to development or ASD that an assumed response shape via the classical hemodynamic response modeling might miss. Goodness-of-fit statistics were calculated including partial F-statistics for each regressor and t-scores comparing each of the 5 estimated beta weights (from each basis function) with zero.
For group analyses, impulse response function values associated with AS prep and response epochs from each subject were entered into voxel-wise linear mixed effects models (one for prep and one for saccade), with “subjects” as a random factor and time course values (prep and saccade: 6 time points) as a within-group factor, and “age-group” (adolescents, adults) and “diagnosis group” (patients, controls) as between-group fixed factors. The resulting statistical map produced “main effect of time” maps that were used as base images from which functional regions of interest (ROIs) were defined (Figures 3 and 5). The “main effect of time” map reveals all the regions that demonstrate a significant modulation of signal from baseline across groups and conditions within each epoch, making this approach unbiased with respect to the effects of interest across groups. Prior research suggests that this method is reliable in delineating the basic circuitry recruited for the AS task Velanova et al., 2008, Geier et al., 2010. We also ran a second linear mixed effects model including estimated time points associated with correct VGS trials only in order to delineate the circuitry involved in eye movement control.
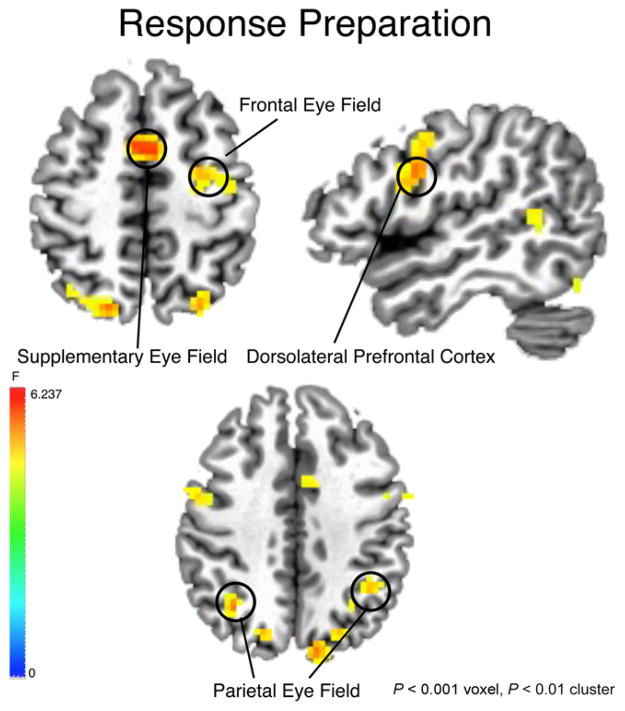
Figure 3.
Activation map for main effect of time during response preparation epoch collapsed across age group and diagnosis group. Threshold set at P < .001(corrected). Right side of image = right brain.
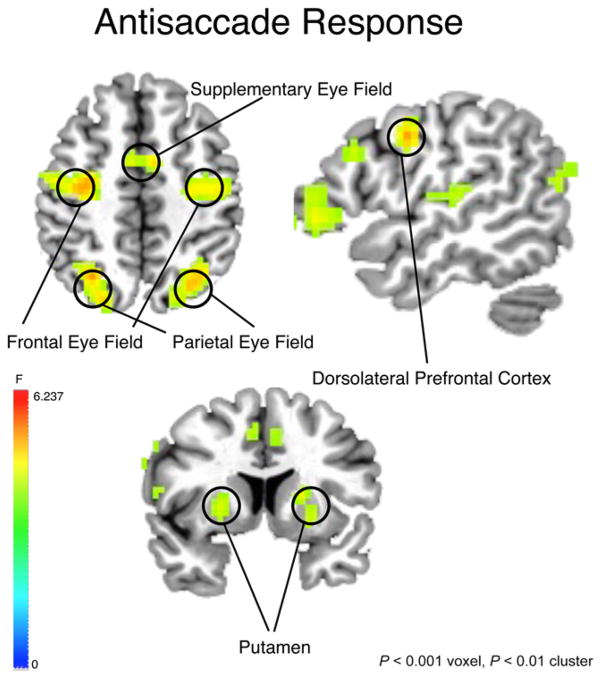
Figure 5.
Activation map for main effect of time during anti-saccade (AS) response epoch collapsed across age group and diagnosis group. Threshold set at P < .001(corrected). Right side of image = right brain.
The main effect of time map was corrected for multiple comparisons using a combination of cluster size and individual voxel probabilities, with the parameters determined by a Monte Carlo simulation using AFNI’s AlphaSim program. This analysis specified that 23 contiguous voxels (27 mm3 voxels) along with a single-voxel threshold of P < 0.001 was required to achieve a corrected, cluster-level alpha value of 0.05, and these regions were designated as clusters. Next, peak voxels within these significant clusters that were more than 12 mm apart in the corrected main effect of time map were identified using an automatic search algorithm. Twelve-millimeter diameter spheres were then centered on these peak voxels, resulting in a “sphere map.” Finally, a conjunction of the “sphere map” and the corrected main effect of time map yielded a functional ROI map, with each ROI consisting of at least 23 contiguous voxels. The resulting ROI map was used as a mask for subsequently extracting time course values for each participant.
We focused our analyses on functionally defined clusters that fell within the boundaries of a priori anatomical regions of interest that are known to be involved in oculomotor control. These included the SEF adjacent to the paracentral lobule, the FEF on the superior aspect of the precentral sulcus, the PEF on the inferior parietal sulcus, the putamen, the ACC, and the DLPFC [Curtis & Connolly, 2008; Luna et al., 1998] (Table 2).
Table 2.
Region Locations and Characteristics for Significant Regions of Interest During Preparatory and Outcome Anti-saccade (AS) Epochs
| Talairach coordinates
|
||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Epoch | Max F |
| L. FEF | 6 | 22 | 5 | 55 | Prep | 57.1 |
| SEF | 6 | 1 | −1 | 49 | Prep | 49.8 |
| L. FEF | 6 | 22 | 11 | 55 | Outcome | 51.2 |
| SEF | 6 | 4 | −1 | 49 | Outcome | 49.9 |
| R. IPL | 39, 40 | −32 | 59 | 40 | Outcome | 50.1 |
| L.Precuneus | 17, 18 | −1 | 62 | 28 | Outcome | 27.6 |
| L.Putamen | N/A | 19 | −4 | 7 | Outcome | 29.3 |
FEF, frontal eye field; SEF, supplementary eye field; IPL, inferior parietal lobule; IPL, inferior parietal lobule; L, left; R, right; BA, Brodmann’s Area.
Mean estimated time courses from each participant were extracted from each ROI of interest. The values from these mean estimated time courses at each time point for each subject response were entered into a repeated measures analysis of variance (ANOVA) using age group and diagnosis group as between-subjects factors and time as a within-subject factor. Below, we report regions that demonstrated significantly different modulations across time by age group, diagnosis group, and/or an age group by diagnosis group interaction. We also ran regression analyses with peak time course values for each subject response using age as a continuous variable, and diagnosis group as a categorical variable and report any significant effects as a linear function of age below. In Figures 4 and 6, we show graphs of average peak time course values for each group.
Figure 4.
Peak values from time courses during response preparation. Error bars represent + /− 1 standard error of the mean. For visualization purposes, filled black circles indicate location of the masks above slices of the Analysis and Visualization of Functional Neuroimages (AFNI) Talairach atlas, drawn using AFNI. The circles do not reflect the actual shape of the mask.
Figure 6.
Peak values from time courses showing regions that demonstrated an effect during anti-saccade (AS) response. See materials and methods for how data were extracted. Error bars represent + /− 1 standard error of the mean. For visualization purposes only, filled black circles indication location of the masks are schematically shown above slices of the Analysis and Visualization of Functional Neuroimages (AFNI) Talairach atlas, drawn using AFNI. The circles do not reflect the actual shape of the mask.
Results
Behavior
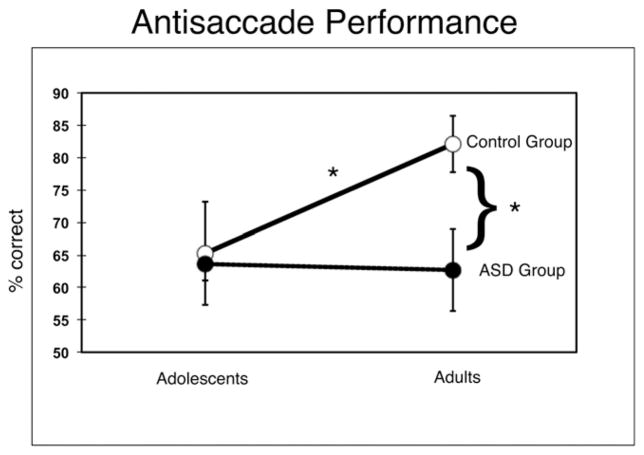
We ran an ANOVA with two factors (age group and diagnosis) on performance (percent correct values). There was a significant main effect of age group, and an age group by diagnosis interaction. The CON group performed significantly better than the ASD group (t40 = −2.325, P = 0.025). Collapsed across diagnosis groups, there was no effect of age group (t40 = 1.999, P = 0.052). Planned comparison two tailed t-tests revealed that AS performance improved significantly between CON adolescents and CON adults (t21 = 2.384, P = .027) but not between ASD adolescents and ASD adults (t17 = −.122, P = .904). Adolescents in the ASD group performed similarly to the CON adolescent group (t18 = −.192, P = .85). In contrast, adults with ASD performed significantly worse than adults in the typical group (t20 = −3.356, P = .003), consistent with prior findings [Agam et al., 2010; Luna et al., 2007; Mosconi et al., 2009; Thakkar et al., 2008] (Fig. 2). When considering age as a continuous variable, there was no significant effect of age or diagnosis group, or age by diagnosis group interactions. No differences were found between any of the groups in VGS error rates.
Figure 2.
Behavioral results. Anti-saccade (AS) performance (% correct) for adolescents (left) and adults (right) in both CON (solid line, open circles) and ASD groups (dashed line, closed circles). *P < .05. Error bars denote standard error.
Imaging Results
VGS
All groups similarly recruited a widely distributed set or regions during the VGS trials including the cortical eye fields (FEF, SEF, and PEF) and subcortical regions (caudate, putamen, thalamus). There were no diagnosis or age group effects.
AS response preparation
During the preparatory period of correct whole trials and all catch trials, all four participant groups similarly recruited a distributed set of brain regions known to support AS response preparation, including the FEF, SEF, DLPFC and IPL (Fig. 3). Across diagnosis groups, in the SEF, adults demonstrated greater activation than adolescents (F6,240 = 2.219, P = .042) (Fig. 4). In the left FEF, a significant age group by diagnosis group interaction (F6,228 = 2.468, P = .024) revealed that the ASD adolescents demonstrated reduced activation relative to the ASD adults (F6,102 = 2.687, P = .018) and CON adolescent (F6,108 = 2.925, P = .011) groups (Fig. 4). Secondary regression analyses confirmed the age effect in SEF (β = 0.015, t = 2.433, P = 0.020), showing linear increases in activation with age. In the left FEF, there were no significant effects when considering age as a continuous variable.
AS response
All groups recruited a set of regions known to be involved in making a correct AS response including the FEF, SEF, IPL, DLPFC, and striatum (Fig. 5). A significant age group effect was observed in SEF (F8,304 = 3.305, P = .001), with adults demonstrating increased activation relative to adolescents in both ASD and typical groups (Fig. 6I). A significant age group effect was also observed in left FEF (F8,304 = 2.080, P = .038), again with CON and ASD adults demonstrating increased activation relative to CON and ASD adolescents (Fig. 6I). In left precuneus, there was a significant main effect of diagnosis (F8,304 = 2.532, P = .011), revealing that individuals with ASD displayed increased activation relative to the typical groups (Fig. 6II). In the left putamen, a diagnosis group by age group interaction (F8,304 = 2.452, P = .014) indicated that adolescents with ASD demonstrated increased activation relative to typical adolescents and adults with ASD (Fig. 6III). In right IPL, there was a significant age group by diagnosis group interaction (F8,304 = 11.819, P = .0000), revealing an age-related increase in activation in the CON group but not in the ASD group (Fig. 6III). There were no significant effects when considering age as a continuous variable in SEF, FEF, IPL, precuneus, or putamen.
Secondary analyses
We ran secondary analyses using equal groups (eight participants in each group) that were matched for age and IQ to ensure that our findings for AS performance and in the right IPL were not due differences in group sizes. These results revealed a significant effect of group on performance (F3,28 = 3.504, P = 0.028). Post-hoc tests confirmed that adult ASD participants performed significantly worse than adult CON (t14 = −2.867, P = 0.012). Adolescent ASD participants performed similarly to Adolescent CON (t14 = 0.096, P = 0.925) and Adult ASD participants (t14 = .118, P = 0.908). Adolescent CON participants performed significantly worse than Adult CON participants (t14 = 2.689, P = 0.018). In right IPL, we also found a significant age-group by diagnosis interaction (F8,224 = 7.33, P = 0.01).
Discussion
We aimed to characterize age-related differences in inhibitory control in ASD during adolescence and adulthood. We used the AS task, which reliably shows a protracted maturation through adolescence and a behavioral deficit in ASD [Goldberg et al., 2002; Luna et al., 2001, 2007]. Overall, our findings suggest that brain development continues between adolescence and adulthood in individuals both with and without ASD, as expected on the basis of behavior.
While the ASD adolescent group performed at equivalent levels to the CON adolescent group, there were no changes between adolescence and adulthood in ASD, which was unlike our previous behavioral findings [Luna et al., 2007]. The CON groups showed characteristic age-related improvements in AS performance (e.g. [Geier, et al., 2010; Luna et al., 2001; Padmanabhan, 2011; Velanova et al., 2008]). The lack of age-related improvement in behavior in the ASD group suggests that development is delayed or arrested. The current study employed an interleaved paradigm, mixing VGS trials with AS trials, adding a task-switching component to the task. Prior research has suggested that mixing VGS and AS trials results in worse AS performance relative to pure blocks of AS trials [Cherkasova, Manoach, Intriligator, & Barton, 2002; Manoach et al., 2002; Reuter, Herzog, & Kathmann, 2006]. This added complexity may have particularly affected age-related improvements in individuals with ASD and suggests particular limitations in higher order cognitive processes may be delayed or arrested.
Contrary to prior research showing brain activation differences during VGS trials in ASD relative to TD [Takarae, Minshew, Luna, & Sweeney, 2007], we found no VGS differences across diagnostic groups. This difference from previous work may be due to the fact that this task had a basic sensorimotor task (VGS) in the context of a more cognitively demanding inhibition task, which engages the same sensorimotor regions, but may require more effort in processing. Our findings of similar VGS performance and activation patterns between ASD and TD mirror recent findings demonstrating that, in the context of AS trials, VGS performance and brain activation is similar in ASD [Agam et al., 2010]. Prior research has also suggested that cortical eye movement control regions such as the FEF are recruited to a higher degree for inhibitory processes (AS) relative to sensorimotor processes (VGS), and disruptions in FEF and pre-supplementary motor area (SMA) have been found to impair inhibitory control while keeping simple saccadic processes intact [Muggleton, Chen, Tzeng, Hung, & Juan, 2010].
All participant groups recruited a largely similar network of relevant brain regions during both the preparation and response components of AS trials, including the FEF, SEF, IPL, ACC, and DLPFC, suggesting that the core circuitry required for making inhibitory responses is in place by adolescence and is functional in ASD. However, there were distinct age and diagnosis group differences in activation during both the preparatory and saccade response phases of the task that are discussed in detail below.
Three major patterns of differences were observed in brain activation during AS trials relative to baseline fixation. First, results revealed that the right IPL demonstrated an age group by diagnosis group interaction, mirroring the behavioral findings, providing evidence for distinct developmental trajectories between ASD and typically developing individuals. Second, the ASD groups demonstrated increased activation in precuneus relative to typical groups, suggesting increased reliance on attention-specific mechanisms to perform the task. Third, results showed that—in adulthood—both ASD and typical groups demonstrated increased recruitment relative to their adolescent counterparts in FEF and SEF, suggesting parallel age related change between diagnosis groups.
Although the typical groups demonstrated an age-related increase in lateral IPL activation, the ASD groups did not. This finding mirrored the behavioral findings of a lack of developmental improvement in AS performance in ASD relative to typically developing individuals. The lateral IPL has been previously associated with AS generation and in transforming sensory information to a motor plan [Barash & Zhang, 2006]. The ASD groups recruited this region to a lesser degree than the typical groups during correct anti-saccades, which may point to a general deficit in sensorimotor programming resulting in more errors overall. These results remained significant even with smaller group sizes, suggesting that they are unlikely to be due to unequal groups.
Furthermore, the groups with ASD demonstrated increased recruitment of left precuneus relative to the typical groups during the generation of a voluntary response. The precuneus has direct connections to regions of the IPL that are involved in visuo-spatial processing [Andersen, Asanuma, Essick, & Siegel, 1990; Leichnetz, 2001; Selemon & Goldman-Rakic, 1988], as well as the SMA and dorsal premotor areas [Cavada & Goldman-Rakic, 1989; Petrides & Pandya, 1984] including oculomotor regions such as the FEF [Cavanna & Trimble, 2006; Leichnetz, 2001; Leichnetz & Goldberg, 1988; Leichnetz & Gonzalo-Ruiz, 1996; Tian & Lynch, 1996a, 1996b]. Evidence suggests that the precuneus is engaged when attending to visuospatial tasks [Berman et al., 1999] and the response phase of the AS task [Brown, Goltz, Vilis, Ford, & Everling, 2006; Brown, Vilis, & Everling, 2007]. In the current study, increased reliance on the precuneus suggests the ASD group may rely on attention-related circuitry to generate a voluntary response as a compensatory mechanism for possible limitations in utilizing the cortical eye fields, specifically the IPL. The precuneus is also part of the putative “default-mode” network (a network of regions that are suppressed during goal-directed processes) [Raichle & Snyder, 2007]. Thus, it is also possible that a failure to attenuate default mode activation hampers inhibitory control performance.
Both ASD and typical groups showed age-related increased activation in SEF during response preparation, and SEF and FEF during the generation of the AS. Single-cell recordings demonstrate that neurons in the SEF and FEF are active prior to making correct AS responses, suggesting that these regions support planned responses and that response preparation is an essential component in the AS task [Schlag & Schlag-Rey, 1987]. Furthermore, the SEF and FEF have connections to oculomotor regions in the superior colliculus, with direct routes for planning eye movements [De Weijer et al., 2010; Everling & Munoz, 2000; Huerta & Kaas, 1990; Shook, Schlag-Rey, & Schlag, 1990]. Neurons in the FEF are also engaged during response preparation, supporting the ability to inhibit saccade motor neurons in the superior colliculus, thus stopping the prepotent motor response to the target [Munoz & Everling, 2004; Schall, Stuphorn, & Brown, 2002].
Our previous developmental studies have shown increased recruitment of SEF and FEF with age across blocks of AS trials, paralleled with developmental differences in performance [Luna et al., 2001]. Although prior event-related fMRI studies have demonstrated that adolescents do not differ from adults in recruitment of cortical eye fields during correct trials [Velanova et al., 2008], the added demand of task-switching in the current task may have tapped into a process that is not yet mature in typical adolescent populations. Furthermore, the parallels in age-related increases in the recruitment of FEF and SEF during correct AS responses from adolescence to adulthood in both typical and ASD groups suggest that some brain maturational processes underlying AS performance may be preserved in ASD.
In the FEF, adolescents with ASD demonstrated decreased preparatory activation relative to the other three participant groups. This suggests a maturational delay in recruiting key inhibitory regions required to perform successful anti-saccades, as ASD adults showed the same magnitude of activation as CON adults. However, given that this activation difference in the FEF was found during correct AS trials and adolescents with ASD demonstrated the same level of performance as typical adolescents, compensatory mechanisms may have been recruited to perform at equivalent levels. Adolescents with ASD demonstrated increased engagement of putamen during correct AS trials relative to adults with ASD and typical adolescents. The putamen has direct projections to the FEF and is integral to oculomotor processing [O’Driscoll et al., 1995; Petit et al., 1993]. Increased activation in the putamen in the adolescent ASD group may reflect a compensatory process in order to overcome possible delayed development in cortical (i.e., FEF) control in order to generate a successful AS response. There is little fMRI evidence showing activation differences in putamen in ASD, with one study linking reduced activation in putamen with repetitive behavior symptoms and verbal fluency in ASD [Kenworthy et al., 2013]. However, converging lines of research from PET and structural MRI have suggested that the putamen may be a locus of dysfunction in ASD. Adults with ASD have also demonstrated decreased glucose metabolism in putamen [Haznedar et al., 2006] and decreased white matter connectivity strength between putamen and prefrontal cortex in ASD relative to typical individuals [Langen et al., 2011]. Individual differences in putamen shape have previously been correlated with deficits in motor skills in ASD [Qiu, Adler, Crocetti, Miller, & Mostofsky, 2010] and differences in putamen volume with repetitive and stereotyped behavior [Estes et al., 2011, Hollander et al., 2005]. There is however, a lack of developmental studies in this area, with one study showing differences in rates of change over age in putamen volume in individuals with ASD relative to typically developing individuals between childhood and early adulthood [Langen et al., 2009].
Conclusions
The present study was a cross-sectional study. Our results as such provide indirect evidence of developmental changes, which in the future may benefit from a longitudinal approach with a larger cohort especially given known variation in this spectrum. Furthermore, the number of trials per condition limited our ability to look at AS error versus correct trials. Given prior results suggesting limitations in AS circuitry in ASD, such as the dorsal ACC [Agam et al., 2010] that are involved in error processing over adolescence, an interesting future direction would be to explore this further in ASD. While the sample size is also a limitation, given the scarcity of developmental studies of inhibitory control in ASD that currently exist in the literature, the results from the present study can inform future work in this area.
We demonstrate that inhibitory control is affected in ASD and that age related change in both brain function and behavior from adolescence to adulthood may be distinct relative to typically developing individuals. These results suggest that there are underlying limitations in ASD in the ability to recruit the optimal circuitry to support inhibitory control. Furthermore, individuals with ASD may not show the optimal transitions in brain function through adolescence evident in typical maturation. Lastly, much of the circuitry supporting cognitive control was intact in ASD changes. Taken together, the results suggest that the well-delineated circuitry underlying the AS task is recruited in ASD, but specific refinements in the underlying circuitry evident in typically developing individuals may be compromised in ASD. Furthermore, age related change in AS performance and underlying brain function in key areas such as the FEF and IPL may be affected in ASD.
Acknowledgments
We thank Melanie Wilds for assistance with participant recruitment and data collection. We also thank all participants and families who volunteered for this study.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Figure S1. Schematic of single subject and group data analysis steps. Images for the single subject analysis were taken from a representative participant.
Table S1. Medication status of ASD participants.
References
- Agam Y, Joseph RM, Barton JJ, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. The Journal of Comparative Neurology. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- Barash S, Zhang M. Switching of sensorimotor transformations: Antisaccades and parietal cortex. Novartis Foundation Symposium. 2006;270:59–71. [PubMed] [Google Scholar]
- Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, et al. Cortical networks subserving pursuit and saccadic eye movements in humans: An fMRI study. Human Brain Mapping. 1999;8:209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund DF, Harnishfeger KK. The evolution of inhibition mechanisms and their role in human cognition and behavior. In: Dempster FN, Brainerd CJ, editors. Interference & inhibition in cognition. San Diego, CA: Academic Press, Inc; 1995. pp. 141–173. [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. The Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Goltz HC, Vilis T, Ford KA, Everling S. Inhibition and generation of saccades: Rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. Neuroimage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brown MR, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. Journal of Neurophysiology. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Event-related fMRI and the hemodynamic response. Human Brain Mapping. 1998;6:373–377. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<373::AID-HBM8>3.0.CO;2-P. [Clinical Trial Randomized Controlled Trial] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with frontal lobe. The Journal of Comparative Neurology. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain: A Journal of Neurology. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- Cherkasova MV, Manoach DS, Intriligator JM, Barton JJS. Antisaccades and task-switching: Interactions in controlled processing. Experimental Brain Research. 2002;144:528–537. doi: 10.1007/s00221-002-1075-z. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. Journal of Neurophysiology. 2008;99:133–145. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- De Weijer AD, Mandl RC, Sommer IE, Vink M, Kahn RS, Neggers SF. Human fronto-tectal and fronto-striatal-tectal pathways activate differently during anti-saccades. Frontiers in Human Neuroscience. 2010;4:41. doi: 10.3389/fnhum.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Developmental Review. 1992;12:45–75. [Google Scholar]
- Estes A, Shaw DW, Sparks BF, Friedman S, Giedd JN, Dawson G, … Dager SR. Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism research : official journal of the International Society for Autism Research. 2011;4:212–220. doi: 10.1002/aur.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Munoz DP. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. Journal of Neurophysiology. 1998;80:1584–1589. doi: 10.1152/jn.1998.80.3.1584. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Spantekow A, Krappmann P, Flohr H. Event-related potentials associated with correct and incorrect responses in a cued antisaccade task. Experimental Brain Research. 1998;118:27–34. doi: 10.1007/s002210050252. [DOI] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Research Brain Research Reviews. 1997;754:285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Hatta T, Fukushima K. Development of voluntary control of saccadic eye movements. I. Age-related changes in normal children. Brain & Development. 2000;22:173–180. doi: 10.1016/s0387-7604(00)00101-7. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR. ILAB: A program for postexperimental eye movement analysis. Behavior Research Methods, Instruments, & Computers: A Journal of the Psychonomic Society, Inc. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Landa RJ, Tien A. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40:2039–2049. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Mostofsky SH, Cutting LE, Mahone EM, Astor BC, et al. Subtle executive impairment in children with autism and children with ADHD. Journal of Autism & Developmental Disorders. 2005;35:279–293. doi: 10.1007/s10803-005-3291-4. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. American Journal of Psychiatry. 2006;163:1252–1263. doi: 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, … Buchsbaum M. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psychiatry. 2005;58:226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Kaas JH. Supplementary eye field as defined by intracortical microstimulation: Connections in macaques. The Journal of Comparative Neurology. 1990;293:299–330. doi: 10.1002/cne.902930211. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: Decreased activation and underconnectivity in inhibition networks. Biological Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Weinblatt R, Abrams DN, Wallace GL. Motor demands impact speed of information processing in autism spectrum disorders. Neuropsychology. 2013;27:529–536. doi: 10.1037/a0033599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38:179–189. [PubMed] [Google Scholar]
- Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, … Murphy DG. Fronto-striatal circuitry and inhibitory control in autism: Findings from diffusion tensor imaging tractography. Cortex; a journal devoted to the study of the nervous system and behavior. 2011 doi: 10.1016/j.cortex.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Langen M, Schnack HG, Nederveen H, Bos D, Lahuis BE, et al. Changes in the developmental trajectories of striatum in autism. Biological Psychiatry. 2009;66:327–333. doi: 10.1016/j.biopsych.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. Connections of the medial posterior parietal cortex (area 7 m) in the monkey. The Anatomical Record. 2001;263:215–236. doi: 10.1002/ar.1082. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Goldberg ME. Higher centers concerned with eye movement and visual attention: Cerebral cortex and thalamus. Reviews of Oculomotor Research. 1988;2:365–429. [Review] [PubMed] [Google Scholar]
- Leichnetz GR, Gonzalo-Ruiz A. Prearcuate cortex in the Cebus monkey has cortical and subcortical connections like the macaque frontal eye field and projects to fastigial-recipient oculomotor-related brainstem nuclei. Brain Research Bulletin. 1996;41:1–29. doi: 10.1016/0361-9230(96)00154-2. [Comparative Study Research Support, U.S. Gov’t, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur AL. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll S, Hegedus SJ, Minshew N, Sweeney J. Maturation of executive function in autism. Biological Psychiatry. 2007;61:474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, et al. Dorsal cortical regions subserving visually-guided saccades in humans: An fMRI study. Cerebral Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Lindgren KA, Cherkasova MV, Goff DC, Halpern EF, et al. Schizophrenic subjects show deficient inhibition but intact task switching on saccadic tasks. Biological Psychiatry. 2002;51:816–826. doi: 10.1016/s0006-3223(01)01356-7. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52:917–922. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D’Cruz AM, Seidenfeld A, Guter S, et al. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychological Medicine. 2009;39:1559–1566. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggleton NG, Chen CY, Tzeng OJ, Hung DL, Juan CH. Inhibitory control and the frontal eye fields. J Cogn Neurosci. 2010;22:2804–2812. doi: 10.1162/jocn.2010.21416. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: The anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, Holzman PS. Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:925–929. doi: 10.1073/pnas.92.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shuldman GL. Separating processes within a trial in event-related functional MRI: Part II. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI: Part I. Neuroimage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL. Inhibitory function in nonretarded children with autism. Journal of Autism and Developmental Disorders. 1997;27:59–77. doi: 10.1023/a:1025821222046. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL, McMahon WN, Filloux F. Executive function abilities in autism and Tourette syndrome: An information processing approach. Journal of Child Psychology and Psychiatry. 1994;35:1015–1032. doi: 10.1111/j.1469-7610.1994.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Geier CF, Ordaz SJ, Teslovich T, Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev Cogn Neurosci. 2011;1:517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Orssaud C, Tzourio N, Salamon G, Mazoyer B, Berthoz A. PET study of voluntary saccadic eye movement in humans: Basal ganglia-thalamocortical system and cingulate cortex involvement. Journal of Neurophysiology. 1993;69:1009–1017. doi: 10.1152/jn.1993.69.4.1009. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. The Journal of Comparative Neurology. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Qiu A, Adler M, Crocetti D, Miller MI, Mostofsky SH. Basal ganglia shapes predict social, communication, and motor dysfunctions in boys with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:539–551. doi: 10.1016/j.jaac.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Reuter B, Herzog E, Kathmann N. Antisaccade performance of schizophrenia patients: Evidence of reduced task-set activation and impaired error detection. Journal of Psychiatric Research. 2006;40:122–130. doi: 10.1016/j.jpsychires.2005.02.007. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Russell J. Autism as an executive disorder. New York: Oxford University Press; 1997. [Google Scholar]
- Schall JD, Stuphorn V, Brown JW. Monitoring and control of action by the frontal lobes. Neuron. 2002;36:309–322. doi: 10.1016/s0896-6273(02)00964-9. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. Journal of Neurophysiology. 1987;57:179–200. doi: 10.1152/jn.1987.57.1.179. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biological Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: Evidence for a distributed neural network subserving spatially guided behavior. Journal of Neuroscience. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field: I. Comparative aspects of mesencephalic and pontine connections. The Journal of Comparative Neurology. 1990;301:618–642. doi: 10.1002/cne.903010410. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, et al. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Yoon JH, Ragland JD, Niendam TA, Lesh TA, Fairbrother W, Carter CS. The development of the neural substrates of cognitive control in adolescents with autism spectrum disorders. Biol Psychiatry. 2014;76:412–421. doi: 10.1016/j.biopsych.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Neurobehavioral changes in adolescence. Current Directions in Psychological Science. 2000;9:111–114. [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Sweeney JA. Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Research. 2007;156:117–127. doi: 10.1016/j.pscychresns.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain: A Journal of Neurology. 2008;131(Pt 9):2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JR, Lynch JC. Corticocortical input to the smooth and saccadic eye movement subregions of the frontal eye field in cebus monkeys. Journal of Neurophysiology. 1996a;76:2754–2771. doi: 10.1152/jn.1996.76.4.2754. [DOI] [PubMed] [Google Scholar]
- Tian JR, Lynch JC. Functionally defined smooth and saccadic eye movement subregions in the frontal eye field of cebus monkeys. Journal of Neurophysiology. 1996b;76:2740–2753. doi: 10.1152/jn.1996.76.4.2740. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. Journal of Neuroscience. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Deconvolution analysis of fMRI time series data: documentation for the AFNI software package. 2002 Available at: http://afni.nimh.nih.gov/pub/dist/doc/manual/3dDeconvolve.pdf.