ABSTRACT
Nonsegmented negative-stranded RNA viruses, or members of the order Mononegavirales, share a conserved gene order and the use of elaborate transcription and replication machinery made up of at least four molecular partners. These partners have coevolved with the acquisition of the permanent encapsidation of the entire genome by the nucleoprotein (N) and the use of this N-RNA complex as a template for the viral polymerase composed of the phosphoprotein (P) and the large enzymatic protein (L). Not only is P required for polymerase function, but it also stabilizes the L protein through an unknown underlying molecular mechanism. By using NVP-AUY922 and/or 17-dimethylaminoethylamino-17-demethoxygeldanamycin as specific inhibitors of cellular heat shock protein 90 (HSP90), we found that efficient chaperoning of L by HSP90 requires P in the measles, Nipah, and vesicular stomatitis viruses. While the production of P remains unchanged in the presence of HSP90 inhibitors, the production of soluble and functional L requires both P and HSP90 activity. Measles virus P can bind the N terminus of L in the absence of HSP90 activity. Both HSP90 and P are required for the folding of L, as evidenced by a luciferase reporter insert fused within measles virus L. HSP90 acts as a true chaperon; its activity is transient and dispensable for the activity of measles and Nipah virus polymerases of virion origin. That the cellular chaperoning of a viral polymerase into a soluble functional enzyme requires the assistance of another viral protein constitutes a new paradigm that seems to be conserved within the Mononegavirales order.
IMPORTANCE Viruses are obligate intracellular parasites that require a cellular environment for their replication. Some viruses particularly depend on the cellular chaperoning apparatus. We report here that for measles virus, successful chaperoning of the viral L polymerase mediated by heat shock protein 90 (HSP90) requires the presence of the viral phosphoprotein (P). Indeed, while P protein binds to the N terminus of L independently of HSP90 activity, both HSP90 and P are required to produce stable, soluble, folded, and functional L proteins. Once formed, the mature P+L complex no longer requires HSP90 to exert its polymerase functions. Such a new paradigm for the maturation of a viral polymerase appears to be conserved in several members of the Mononegavirales order, including the Nipah and vesicular stomatitis viruses.
INTRODUCTION
Viruses with a nonsegmented negative-stranded RNA genome, or members of the order Mononegavirales, share a common and highly conserved genomic organization and replication machinery that are unique in the living world. Indeed, the L protein, or polymerase, which is endowed with all of the enzymatic activities required for the synthesis of RNA and the capping and polyadenylation of viral transcripts, cannot use naked viral genomic RNA in a processive way (1). Instead, L associates with the phosphoprotein (P) to dynamically anchor the polymerase complex to the nucleocapsid and/or to enable its processivity. This nucleocapsid is made up of a regular homopolymer of nucleoproteins, N, that entirely covers the viral genome (see references 2, to ,5 for reviews). The L polymerase processively synthesizes RNA transcripts only when associated with its P cofactor (1, 6–9). Virus replication also requires the association of N and P to form soluble N0P complexes (10) that are used as encapsidation substrates (7). N0P possibly associates with the P+L polymerase to form larger complexes (11, 12). Thus, the successful execution of viral transcription and replication relies on the coordinated interaction of the L, P, and N proteins.
The polymerase ensures both transcription from the negative-strand genome and replication by copying the negative-strand RNA genome into positive-strand mRNAs and an antigenome, respectively (13). According to the current model (14, 15), polymerases that are packaged within the viral particles start primary transcription upon delivery of the nucleocapsid into the cytoplasm. This results in the linear accumulation of viral mRNAs. As the resident nucleocapsid continues to recruit an increasing number of neopolymerases translated from these viral transcripts, the rate of viral transcript accumulation switches from linear to exponential. A few hours later, the polymerases start replicating the genome into a faithful antigenome copy that itself is used as a template for new genomes.
How P and L from members of the order Mononegavirales associate into a functional polymerase is not well understood. On the one hand, L is stabilized by P, as shown for vesicular stomatitis virus (VSV) (16, 17), Sendai virus (7), respiratory syncytial virus (RSV) (18), Ebola virus (EboV) (19), and measles virus (MeV) (20). In the case of VSV, P binding induces prominent conformational changes in L (21), the atomic structure of which, in complex with a P fragment, has been very recently solved by cryoelectron microscopy (22). On the other hand, the replication of VSV, parainfluenza virus 5 (PIV5), human PIV2 (HPIV2), HPIV3, simian virus 41 (23), EboV (24), and RSV (25) strictly requires active heat shock protein 90 (HSP90). Indeed, HSP90 seems to be necessary to ensure the stability of L, although its requirement for polymerase function in cells remains unknown. In this study, we deciphered the concerted action of P and HSP90 to give rise to a soluble, folded, and functional polymerase in the case of MeV and obtained similar findings on Nipah virus (NiV) and VSV.
MATERIALS AND METHODS
Plasmids, viruses, cells, and drugs.
L and P expression vectors were under the control of the T7 promoter. The L and P proteins of MeV (GenBank accession no. AF266287) were subcloned under the control of the T7 promoter and the encephalomyocarditis virus internal ribosome entry site (IRES) for protein expression with a pEMC plasmid backbone (26). We found that L expression was increased when L and P were both expressed from the same plasmid with a homemade single plasmid containing a duplicated expression cassette (each gene having its own T7 promoter and IRES). Plasmids pSC6-N and pSC6-M were used to express the MeV N and M proteins (26). Flag/L1-362 was cloned into the pEMC vector. All plasmids were made by PCR and InFusion-mediated recombination (Clontech). BSR-T7 (27) cells that stably express the T7 polymerase were used to express proteins from the T7 promoter. BSR-T7-Slam cells are BSR-T7 cells transduced by a LentiVector to stably express the human Slam receptor. HeLa cells (28), Vero/hSLAM cells (29), or BSR-T7-hSlam and brain slices from 9-day-old SLAM × IFNARko mice (30) were infected with the MeV Schwarz vaccine strain (31), recombinant MeV Mor-Flag/L expressing an L protein tagged with a Flag peptide at its N terminus and built according to reference 26, MeV-IC323-GFPm[1], MeV Mor-Flag/L[Gaussia], NiV-enhanced green fluorescent protein (EGFP) (32), or VSV-GFP (33). MeV-IC323-GFPm[1] was built to express GFP in an additional transcription unit located in the first position before the N gene. MeV Mor-Flag/L[Gaussia] was engineered to express L protein tagged with a Flag peptide at its N terminus and Gaussia coding sequence fused in frame between amino acids (aa) L1709 and L1710. Recombinant MeVs were rescued as previously described (26, 34). Viruses were titrated by the 50% tissue culture infective dose (TCID50) titration method (31). Cells were treated as indicated by MG132 (Peptide International, Inc.), 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), NVP-AUY922 (InvivoGen), cycloheximide, or 3-methyladenine (3-MA; Sigma). Stock solutions of MG132, cycloheximide, 17-DMAG, NVP-AUY922, and 3-MA were prepared in either dimethyl sulfoxide (the former two) or water (the latter three). Small interfering RNAs (siRNAs) targeting human HSP90α/β and a control siRNA (sc-35608) from Santa Cruz Biotech were transfected 1 day before MeV infection with Oligofectamine transfection reagent (Thermo Fisher Scientific).
Ex vivo organotypic brain explants.
Brain slices were prepared from SLAM × IFNARko mice (35) and maintained in culture as detailed previously (30). Briefly, hippocampi were isolated from the brains of 9-day-old mice and cut with a McIlwain tissue chopper (WPI-Europe) to obtain 350-μm-thick progressive slices. The brain slices were then dissociated in cold Hanks balanced salt solution buffer and laid out on Millipore cell culture insert membranes (Millicell cell culture insert, 30 mm, hydrophilic polytetrafluoroethylene). Slices were subsequently cultured in GlutaMAX minimal essential medium supplemented with 25% horse serum, 5 g/liter glucose, 1% HEPES, and 1 mg/ml human recombinant insulin at 37°C in 5% CO2 in a humidified atmosphere. The medium was changed 24 h later and then every 2 days. Infection of organotypic brain explants was performed after 2 days of culture by placing 104 PFU of MeV IC323-EGFP in the center of the hippocampal slices. Medium containing 17-DMAG was added at the concentrations indicated either 4 h before infection or 10 or 24 hpi. The progression of infection was monitored for 72 hpi, and pictures were obtained with an inverted fluorescence microscope (Zeiss) and an AxioCam camera (Zeiss).
RT-qPCR analysis.
For quantification of viral RNA accumulation, 105 Vero/hSLAM cells were infected with MeV IC323 or NiV at a multiplicity of infection (MOI) of 1 for 30 min at 4°C in fetal calf serum-free medium. The cells were then incubated at 37°C in 10% fetal calf serum-containing medium with or without 20 μg/ml cycloheximide or 2 μM 17-DMAG and harvested every 2 h. RNA was extracted with a column purification kit (NucleoSpin RNA; Macherey-Nagel) and measured by reverse transcription-quantitative PCR (RT-qPCR) as described previously for MeV (36) and NiV (37) with the following modifications to selected viral genomes. The negative-strand genome was reverse transcribed with sense 5′-tagged M primers 5′-GCAGGGCAATCTCACAATCAGGAAAACTGGTGTTCTACAACAA-3′ for MeV and 5′-GTGATCAAAGTGAGAATGAGCTAACGGCTGTTTGCTCAAATGGG-3′ for NiV. The cDNAs were diluted (1:10) and PCR quantified with sense tagged primer 5′-GCAGGGCAATCTCACAATCAGG-3′ and antisense M primer 5′-TGAAGGCCACTGCATT-3′ for MeV and tagged primer 5′-GTGATCAAAGTGAGAATGAGCT-3′ and antisense M primer 5′-GCTGCTACTCGGCTGATCTCAC-3′ for NiV.
Flow cytometry.
Cells infected with MeV IC323-EGFP were trypsinized, washed, and fixed in 1% paraformaldehyde for 10 min at room temperature. Fluorescence signals were quantified with Accuri C6 (BD Biosciences).
Western blotting.
Monolayers of Vero/hSLAM, BSR-T7, or BSR-T7-hSlam cells were either infected with MeV at the MOI indicated or transfected with plasmids coding for N, P, M, or Flag/L with JetPrime (Polyplus). Depending on the experiment, cells were treated with drugs or not treated and lysed in either urea-free buffer (50 mM Tris HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 0.1% NP-40, 5% glycerol, 1 mM dithiothreitol [DTT], cOmplete protease inhibitor [Roche]) or 8 M urea-containing buffer (50 mM Tris HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 0.1% NP-40, 5% glycerol, 1 mM DTT, 8 M urea, cOmplete protease inhibitor). The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto polyvinylidene difluoride membranes (Hybond-P; Amersham Biosciences). To optimize the efficiency of electroblotting transfer onto the membranes, SDS was omitted from both the running and stacking gels. The membranes were then incubated for 2 h with primary antibodies specific for N (cl25 antibody) (38), P (49.21 antibody) (39), M (anti-M antibody, MAB8910; Chemicon), Flag/L (anti-Flag antibody; Sigma), cellular glyceraldehyde 3-phosphate dehydrogenase (GAPDH; MAB374 antibody; Chemicon), or cellular HSP90 (H114; Santa Cruz); washed five times with Tris-buffered saline containing 0.05% Tween 20; and incubated with peroxidase-conjugated sheep anti-mouse or anti-rabbit IgG antibody (Promega) for 1 h at room temperature. The membranes were then washed five times with Tris-buffered saline–0.05% Tween 20 and incubated in Covalight reagent (Covalab), and chemiluminescent signals were measured with the VersaDoc Imaging System (Bio-Rad). To separate the soluble and insoluble fractions, cells were lysed in urea-free lysis buffer at 100 μl/well (for a six-well plate) for 20 min on ice and centrifuged for 15 min at 15,000 × g and 4°C, and the supernatants were collected (soluble fraction). To allow efficient electrophoretic migration of the L protein, urea was added to the supernatants to obtain 4 M urea. The pellets (insoluble fraction) were washed twice in urea-free lysis buffer before being dissolved in 100 μl of the urea-containing buffer for 20 min on ice and clarified by centrifugation for 15 min at 15,000 × g and 4°C. Proteins were detected by SDS-PAGE and Western blotting.
Coimmunoprecipitation from infected cells.
Vero/hSLAM cells (7 × 106) were seeded into a 150-mm dish and infected with MeV-Flag/L 1 day later. At 28 h postinfection (hpi), the cells were lysed in 1 ml of NaMo-containing buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 0.1% NP-40, 5% glycerol, 1 mM DTT, cOmplete protease inhibitor, 20 mM NaMo), incubated for 20 min on ice, and centrifuged for 15 min at 15,000 × g and 4°C. The supernatant was incubated with anti-Flag antibody-coated magnetic beads (Sigma) for 2 h. The beads were then washed four times in washing buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 0.05% NP-40, 5% glycerol, 1 mM DTT, 20 mM NaMo), and Flag/L proteins were eluted for 1 h with 100 μl of washing buffer containing 3×Flag peptide (Sigma) at 300 ng/μl. To 100 μl of each protein sample, 90 μl of 10 M urea and 40 μl of 6× loading buffer (0.35 M Tris-HCl [pH 6.8], 10.3% SDS, 36% glycerol, 0.6 M DTT, 0.012% bromophenol blue) were added prior to SDS-PAGE and Western blot analysis.
Coimmunoprecipitation from transfected cells.
BSR-T7 cells (1.2. × 106) were seeded into a 60-mm dish and transfected with P, Flag/L-362, or Flag/N 1 day later. At 7 h posttransfection, the cells were treated with 2 μM 17-DMAG and 5 μM MG132 or not treated. At 24 h posttransfection, the cells were lysed in 300 μl of NaMo-containing buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 0.1% NP-40, 5% glycerol, cOmplete protease inhibitor, 20 mM NaMo), incubated for 20 min on ice, and centrifuged for 15 min at 4°C at 15,000 × g. The supernatant was incubated with anti-Flag antibody-coated magnetic beads (Sigma) for 2 h. The beads were then washed four times in washing buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 0.05% NP-40, 5% glycerol, 20 mM NaMo), and Flag-tagged proteins were eluted for 1 h with 50 μl of washing buffer containing 3×Flag peptide (Sigma) at 300 ng/μl. To 100 μl of each protein sample, 20 μl of 6× loading buffer (0.35 M Tris HCl [pH 6.8], 10.3% SDS, 36% glycerol, 0.6 M DTT, 0.012% bromophenol blue) was added, and the samples were boiled for 3 min at 100°C prior to SDS-PAGE and Western blot analysis. Anti-HSP90 antibody immunoprecipitation was performed with an anti-HSP90 monoclonal antibody (MAb; H114; Santa Cruz) and protein G coupled to Sepharose beads as detailed previously (39). For protein labeling, transfected BSR-T7 cells were starved for 30 min in Dulbecco's modified Eagle's medium minus methionine and cysteine (Gibco). This was then replaced with the same medium containing EasyTag EXPRESS 35S Protein Labeling Mix (PerkinElmer) at 100 μCi/ml for the times indicated (pulse). After one washing with phosphate-buffered saline (PBS), cells were chased with complete culture medium. 17-DMAG at 2 μM was added as indicated. Cells harvested at various times after the labeling pulse were lysed with NaMo lysis buffer. Supernatants obtained after centrifugation at 15,000 × g were subsequently immunoprecipitated with anti-Flag antibody-coated magnetic beads, and the resulting Flag-depleted supernatants were immunoprecipitated with anti-MeV P MAb 49.21 and protein G coupled to Sepharose beads (GE Healthcare) as described previously (39). Total 35S-labeled cellular proteins and immunoprecipitated viral proteins were resolved by SDS-PAGE and quantified with a Molecular Dynamics PhosphorImager. Protein half-life was estimated from the best exponential fit for r values of >0.97 whenever possible.
6×His-tagged protein precipitation.
BSR-T7 cells (2.5 × 106) were seeded into 100-mm culture dishes and cotransfected with 9 μg of an equal mixture of three plasmids coding for hemagglutinin-tagged ubiquitin (HA-Ub) (39), MeV P, and either a Flag-tagged or a Flag- and 6×His-tagged MeV L protein. Seven hours later, 2 μM 17-DMAG and 5 μM MG132 were added or not added. At 24 h posttransfection, the cells were lysed in 1 ml of DNPI-10 denaturing buffer (8 M urea, 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8.0), incubated for 20 min on ice, sonicated twice for 15 s each time, and centrifuged for 15 min at 15,000 × g and 4°C. The supernatant was incubated on Ni-nitrilotriacetic acid (NTA) agarose beads (Protino Ni-NTA Agarose; Macherey-Nagel) for 6 h. The beads were washed and eluted as recommended by the manufacturer. Loading buffer was added prior to SDS-PAGE and Western blot analysis.
Immunostaining.
Vero/hSLAM cells were seeded into Lab-Tek (0.5 × 105 cells/well; Iwaki) and infected 1 day later with MeV-Flag/L at an MOI of 0.1. At 24 hpi, the cells were fixed in 1% formaldehyde for 30 min, saturated, and permeabilized in PBS containing 4% fetal bovine serum (FBS) and 0.3% Triton X-100 (PBS-FBS-Triton buffer). Primary antibodies were diluted in PBS-FBS-Triton buffer and incubated on the cells overnight at 4°C. The Flag/L protein was stained with a murine anti-Flag MAb (1:200 dilution; Sigma), cellular HSP90 was stained with a rabbit anti-HSP90 polyclonal antibody (H114; 1:100; Santa Cruz), and P was stained with either a murine anti-P MAb 49.21 at 1:100 or a rabbit polyclonal antibody (39) at 1:500. The cells were then washed four times and incubated with 4′,6-diamidino-2-phenylindole (DAPI; 1:1,000) and either anti-mouse IgG Alexa Fluor 555 (1:750; Invitrogen) or anti-rabbit IgG Alexa Fluor 488 (1:750; Invitrogen). After four washes, slides were mounted with Fluoprep mounting medium (bioMérieux catalog no.75521).
Cell viability assays.
For each experiment with drug-treated cell lines, cell viability was assessed with the alamarBlue assay (Life Technologies) according to the manufacturer's protocol. Fluorescence emission was read with a Tecan Infinite 200 PRO series microplate reader.
Statistical analyses.
All statistical analyses were done with the Student t test.
Ethics statements and compliance with institutional rules and national laws.
All experiments were performed according to the French national charter on the ethics of animals and animal protocols approved by the CECCAPP animal ethical committee (CECCAPP_ENS_2014_033); class 2, 3, and 4 OGM authorization number 5854; and dual-use research MOT authorization no. ADE-021072012-7 and AMO-026352012-5 from the Agence Nationale de la Santé et du Médicament.
RESULTS
HSP90 activity is required for MeV growth at a postentry step.
HSP90 belongs to the essential cellular proteins, since several hundred client proteins, including major cellular kinases, rely on its chaperon activity (40–42). The involvement of HSP90 activity in an intracellular process is best studied with geldanamycin and its derivatives that compete with ATP for binding to HSP90's ATPase site (43). Inhibition of HSP90 ATPase activity with geldanamycin and its derivative 17-DMAG (44, 45) prevented the growth of MeV IC323-EGFP (Fig. 1A to D), as shown by the impairment of both the expression of a GFP reporter gene from MeV (Fig. 1A and C) and MeV production (Fig. 1B and D). NVP-AUY922, another known inhibitor of HSP90 belonging to another class of chemical compounds (46), also inhibited MeV infection in a dose-dependent manner (Fig. 1E). Neither 17-DMAG nor NVP-AUY922 significantly affected cellular reductase activities in the alamarBlue assay, a measurement that correlates with cell viability (47; see also reference 48 for a discussion of the use of the activity of a cellular enzyme to assess cell viability and/or growth) (Fig. 1F and G). The inhibition of HSP90 by 17-DMAG also prevented the growth of VSV and NiV (data not shown), in agreement with a previous report on VSV (23). Since HSP90 is very abundant and essential, its silencing with a specific siRNA is rather difficult. Yet, attenuation of the level of HSP90 expression with a specific siRNA in either Vero or HeLa cells resulted in less expression of MeV N protein (Fig. 1H, top) and infectious virus production (Fig. 1H, bottom) than in cells treated with a control siRNA. Considering the similar effects of the two different approaches (interfering RNA and drug treatment in different cells), we are confident that the apparent common antiviral effect is the result of the specific inhibition of cellular HSP90 activity. Indeed, the two chemically distinct HSP90 inhibitors we used decrease the chance of observing a biological effect related to any “off-target” activity, as reported in the case of 17-DMAG (49, 50). Furthermore, the mechanism of action of both drugs is well characterized, as shown by their perfect docking into the ATPase site of HSP90 (44, 51).
FIG 1.
HSP90 activity is required for efficient growth of MeV. (A to E) Inhibition of HSP90 activity with geldanamycin, 17-DMAG, or NVP-AUY922 abolishes MeV growth. Vero cells were infected with a recombinant MeV coding for GFP as a viral reporter at an MOI of 0.5 and treated with geldanamycin (A, B), 17-DMAG (C, D), or NVP-AUY922 (E) or not treated. Viral infection was assessed at 24 hpi by measuring GFP expression by flow cytometry (A, C, E) or by measuring MeV production (B, D). 17-DMAG or NVP-AUY922 treatment did not kill cells during 24 h of treatment, as shown by the limited reduction of cellular reductase activity observed at high concentrations with alamarBlue reagent (F, G). (H) Reduction of HSP90 expression by siRNA reduces MeV growth in simian Vero and human HeLa cells. Vero and HeLa cells were transfected with a siRNA targeting the HSP90 mRNA (siHSP90) or a control siRNA (siCtrl) and infected 1 day later with MeV (Schwarz strain) at an MOI of 1. The expression of HSP90 and MeV N protein was assessed by Western blotting (top) at 1 day postinfection, and the impact on MeV production was assessed by the TCID50 titration method (bottom).
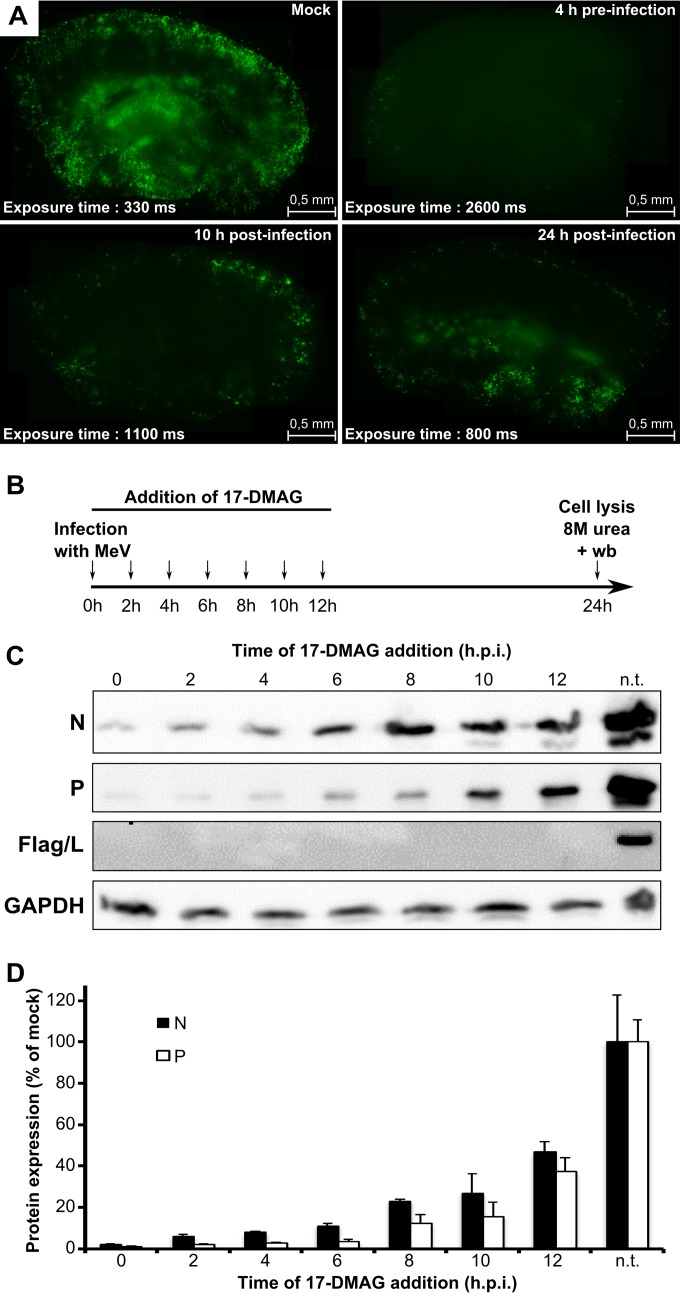
As HSP90 chaperoning mechanisms can be altered in cancer cells (40) and therefore in cell lines, the role of HSP90 in MeV replication was also assessed in a more relevant system based on MeV infection of primary slices of murine hippocampus conserving the three-dimensional architecture of the brain (30). HSP90 activity was also found to be critical in an ex vivo model of MeV infection of the mouse brain (Fig. 2A, compare the right and left top parts). Moreover, viral propagation throughout the brain slices was also blocked upon the late addition of 17-DMAG (Fig. 2A, bottom), while the brain tissues supported the drug with no visible side effect.
FIG 2.
HSP90 activity is required at a postentry step and for viral propagation. (A) Inhibition of HSP90 activity with 17-DMAG blocks ongoing MeV propagation in ex vivo mouse brain explants. Brain slices in organotypic cultures were infected with a recombinant MeV IC323-GFP strain (104 PFU) and treated at different time points with 1 μM 17-DMAG. Observations were made at 3 days postinfection, and viral infection was assessed by measuring GFP expression by fluorescence microscopy (A) (note the increased exposure times for earlier treatment with 17-DMAG so as to visualize various levels of fluorescence intensity). (B to D) Addition of 17-DMAG at different times postinfection blocks the ongoing viral replication cycle. As schematized in panel B, 2 μM 17-DMAG was added at various times after the infection of Vero/hSLAM cells with Mor-Flag/L at an MOI of 1 and the production of MeV N, P, and Flag/L and cellular GAPDH from 8 M urea-treated whole-cell extracts was monitored by Western blotting (C) with quantification after normalization for GAPDH content (D). Data obtained in the presence of the solvent are indicated as nontreated (n.t.).
In cell culture, the addition of 17-DMAG at 4 hpi, i.e., after the virus entry step (14), still prevented the expression of the viral N, P, and L proteins (Fig. 2B to D). The expression of viral proteins was inhibited upon the addition of the drug at 6, 8, 10, or even 12 hpi. Interestingly L could be detected in significant amounts only in nontreated infected cells. Since the virus mostly undergoes transcription and replication at these time points (14, 34, 52), this suggests an inhibitory effect at the level of viral RNA synthesis.
L protein accumulation in the presence of P requires HSP90.
Since HSP90 is involved in the folding and/or stabilization of numerous client proteins (see reference 53 for a review), we evaluated the requirement of HSP90 activity for the production of the individual components involved in the synthesis of the viral RNA or its regulation (Fig. 3A shows the experimental setup). Surprisingly, whole-cell extracts solubilized in 8 M urea contained similar levels of the MeV N, P, M, and Flag/L proteins synthesized in the presence or absence of 17-DMAG (Fig. 3B). Accordingly, in in vitro translation assays with rabbit reticulocyte lysate, the addition of 17-DMAG did not affect translation from mRNAs with either a cap or an IRES as the recruiting signal for ribosomes (data not shown). This indicates that HSP90 and 17-DMAG are not involved in protein synthesis per se, in agreement with the HSP90-independent in vitro translation of p53 (54).
FIG 3.
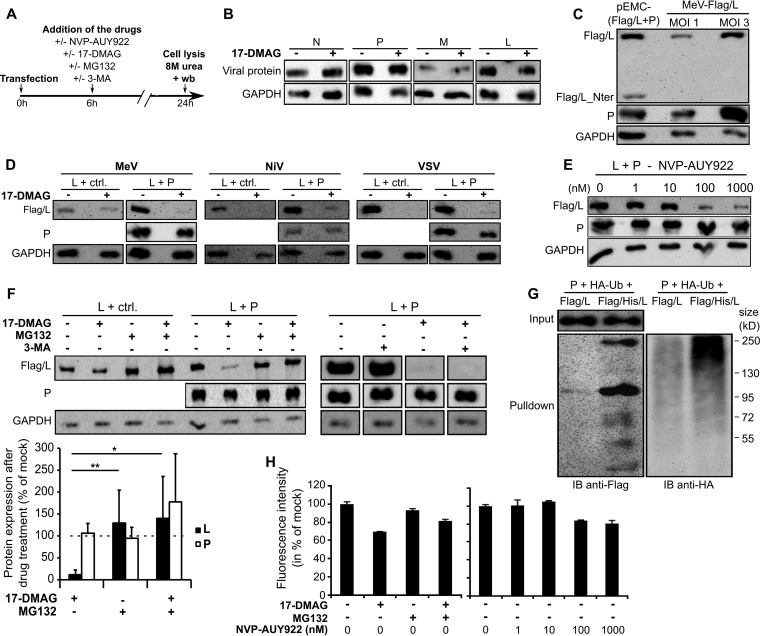
Requirement of HSP90 activity for the production of stable P+L polymerase complexes. (A) Schematic view of the experiment. BSR-T7 cells were transfected with plasmids and 6 h later treated with 2 μM 17-DMAG and/or 5 μM MG132, 5 mM 3-MA, or 1 to 1,000 nM NVP-AUY922 or not treated. The total protein content of cells was analyzed after cell lysis in an 8 M urea-containing lysis buffer at 28 h posttransfection by SDS-PAGE and Western blotting. (B) HSP90 activity is not required for individual production and stabilization of MeV N, P, M, and L proteins in BSR-T7 cells, as shown by the total protein levels in the absence or presence of 2 μM 17-DMAG added at 6 h posttransfection. (C) Similar levels of MeV Flag/L protein expressed from a pEMC-(Flag/L+P) expression vector and MeV-Flag/L in BSR-T7-hSlam cells as measured at 24 h posttransfection or postinfection. (D) Inhibition of HSP90 activity by 17-DMAG hampers the stability of MeV L protein when it is coexpressed with P and not with the control Renilla luciferase (ctrl., left side). Quantification of Western blotting signals from multiple analyses, expressed as percent inhibition after normalization to GAPDH protein levels, showed significant P-dependent L degradation upon 17-DMAG treatment (n = 5; P = 0.0054 [Student t test]). Inhibition of HSP90 also hampers the stability of both the NiV and VSV L proteins independently of the coexpression of their homologous P partner (D, middle and right parts). (E) Inhibition of HSP90 activity by NVP-AUY922 induces L loss. (F) The loss of L coexpressed with P in the absence of HSP90 activity is alleviated by 5 μM MG132 (F, L+P) but not by the autophagy inhibitor 3-MA, as shown by statistical analysis (F, histogram) by Student t test (17-DMAG versus MG132, P = 0.0083 [**]; 17-DMAG versus 17-DMAG + MG132, P = 0.0175 [*] [n = 4]), despite relatively large standard deviations because of intrinsic limitations of quantification by Western blotting and/or experiment-to-experiment variations. (G) MeV L protein is ubiquitinated upon HSP90 inhibition. Shown are the results of the pulling down on Ni beads of MeV Flag/L and Flag/6×His/L covalently bound to HA-Ub from BSR-T7 cells coexpressing Flag/L or Flag/6×His/L, P, and HA-Ub in the presence of HSP90 and proteasome inhibitors (2 μM 17-DMAG and 5 μM MG132, respectively). Eluates were analyzed by SDS-PAGE and Western immunoblotting (IB) with anti-Flag (left) and anti-HA (right) antibodies. (H) Cell viability was not strongly affected by drug treatment under the experimental conditions used. BSR-T7 cells were treated with drugs under the same conditions (time and concentrations) as in panels D to F. For each condition, 1 h before lysis time, 1× alamarBlue solution was added and 2 h later, fluorescence levels were measured.
When MeV Flag/L was coexpressed with P, the amount of detectable proteins reached levels similar to those observed after infection with recombinant MeV Flag/L (Fig. 3C) and higher than the level of Flag/L protein that accumulated in the absence of P (Fig. 3D, compare the L+ctrl and L+P parts on the left side). Note that an N-terminal ∼110-kDa fragment of Flag/L was detected only after transfection, suggesting artifactual generation linked to this particular mode of expression. Upon 17-DMAG treatment, MeV L expressed alone was poorly affected, while in the presence of P only a residual amount of L protein could be detected (Fig. 3D, compare the L+ctrl and L+P parts; see also panels B and F). The expression of P remained mostly unchanged (Fig. 3D, L+P parts) as observed when P was expressed alone (Fig. 3B). Similar phenotypes were observed with L proteins of VSV and NiV, which were found to disappear upon 17-DMAG treatment whether they were coexpressed with their homologous P partner or not (Fig. 3D). Moreover, the disappearance of MeV L when it was coexpressed with P was also observed upon treatment with 100 or 1,000 nM NVP-AUY922 (Fig. 3E), i.e., a dose that readily inhibits MeV growth (Fig. 1E). The 17-DMAG-mediated loss of MeV L protein detection was alleviated in the presence of the proteasome inhibitor MG132 (Fig. 3F, L+P parts and quantification in the histogram at the bottom), but not in the presence of 3-MA, an inhibitor of the autophagy pathway (Fig. 3F). In addition, in the presence of P, MeV L protein was found to be ubiquitinated after HSP90 inhibition in the presence of the proteasome inhibitor MG132, as shown by the anti-HA immunolabeling of Flag/6×His/L produced in the presence of HA-Ub and pulled down with Ni beads (Fig. 3G). Importantly, the variations in L protein levels observed upon drug treatment could not be explained by variations in cell viability assessed by measuring cellular reductase activities (Fig. 3H). All of these results suggest that when it is coexpressed with P in the absence of HSP90 activity, L is ubiquitinated and efficiently targeted to the proteasomal protein degradation pathway. This feature is not so prominent in the absence of P, where the L produced seems rather stable, an observed feature of MeV L (see the statistical analysis in the legend to Fig. 3) but neither NiV L nor VSV L.
Accumulation of a soluble form of L protein requires both P and HSP90.
In the previous experiments, an 8 M urea-containing lysis buffer was used to detect all of the MeV L proteins present in cells. Since misfolded proteins can form insoluble aggregates, cells were alternatively lysed in urea-free lysis buffer and both soluble and insoluble fractions were separated by centrifugation at 15,000 × g before treatment with urea and analysis by Western blotting (Fig. 4A).
FIG 4.
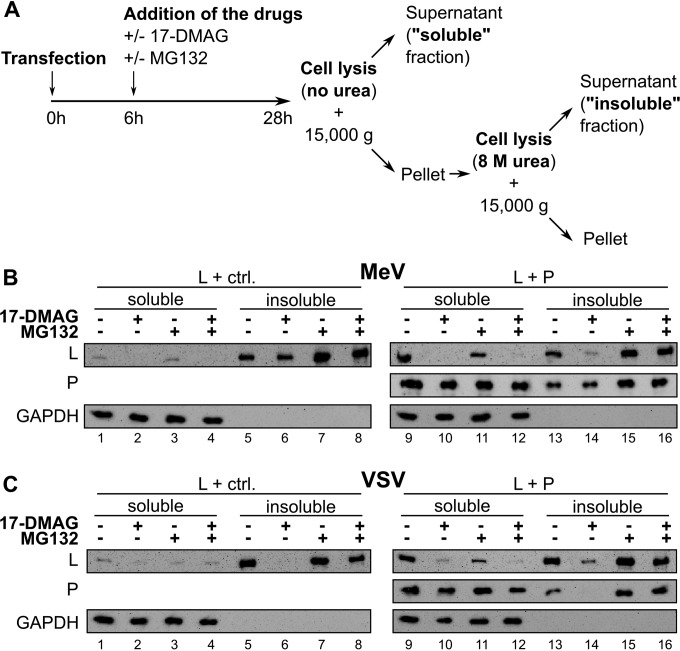
L and P proteins associate into soluble complexes in the presence of HSP90 activity. (A) Schematic view of the experiment. BSR-T7 cells were transfected with plasmids, and 6 h later, cells were treated with 2 μM 17-DMAG and/or 5 μM MG132 or not treated. Cells were lysed in urea-free lysis buffer at 28 h posttransfection, and soluble proteins were separated by centrifugation at 15,000 × g for 15 min (soluble fraction). The pellet was then washed twice in urea-free lysis buffer and incubated in 8 M urea-containing buffer. Denatured protein aggregates were finally separated after 15 min of centrifugation at 15,000 × g (insoluble fraction). (B, C) Western blot analysis of soluble and insoluble fractions from BSR-T7 cells cotransfected with L and a control (ctrl.) plasmid (left) or a P plasmid (right). Data for MeV are in panel B, and those for VSV are in panel C.
In the absence of P, most of the L protein was found in the insoluble fraction, with only trace amounts of soluble L (Fig. 4B, compare lanes 1 and 5). In the presence of MG132, the amount of insoluble L increased, whereas that of its soluble counterpart was stable (Fig. 4B, compare lanes 1 and 5 with lanes 3 and 7, respectively). Very similar data were obtained upon the addition of 17-DMAG in the absence or presence of MG132 (Fig. 4B, compare lanes 5 and 7 with lanes 6 and 8, respectively). The loss of only a small amount of soluble L was observed (Fig. 4B, compare lanes 1 and 3 with lanes 2 and 4, respectively). As a control, GAPDH was only recovered in the soluble fractions under all of the conditions tested (Fig. 4B, lanes 1 to 8, two bottom parts, and lanes 9 to 16).
When L was coexpressed with P, its distribution in both the supernatant and pellet of the cell lysate changed radically, with approximately equal proportions of soluble and insoluble L (Fig. 4B, compare lanes 9 and 13). P was also found in both the soluble and insoluble fractions. While treatment with the proteasome inhibitor did not strongly affect the soluble and insoluble L fractions (Fig. 4B, lanes 11 and 15), treatment with 17-DMAG resulted in the disappearance of both soluble and insoluble L protein (Fig. 4B, compare lanes 9 and 13 with lanes 10 and 14, respectively). Combined treatment with both 17-DMAG and MG132 resulted in enhanced recovery of L almost exclusively in the insoluble fraction (Fig. 4B, lanes 12 and 16). Treatment with MG132 and/or 17-DMAG hardly changed the soluble/insoluble P ratio. Similar results were obtained with VSV L (Fig. 4C), with the major difference that VSV L made in the absence of P accumulated mostly as insoluble material, as observed for MeV L, and was readily degraded upon treatment with 17-DMAG (Fig. 4, compare lane 6 in panel C for VSV and lane 6 in panel B for MeV), as detected upon coexpression with VSV P (Fig. 4C, compare lanes 6 and 14). Therefore, both P and HSP90 activity are required for accumulation of the L protein in a soluble form.
Association of HSP90 with L and P complexes.
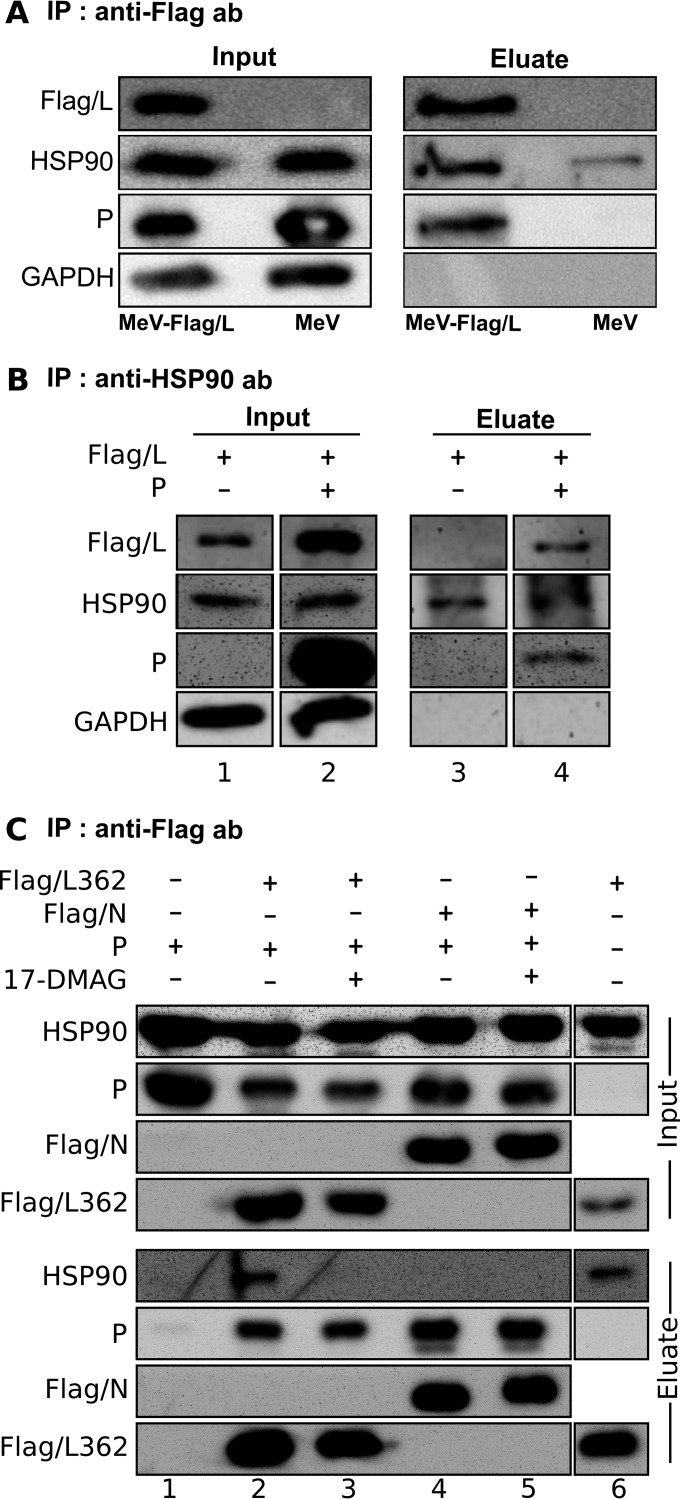
In order to perform its chaperon activity, HSP90 must, at least transiently, form a complex with its client protein. Therefore, we searched for evidence of any colocalization and/or complex formation between HSP90 and the P and L proteins. MeV infection is characterized by the accumulation of growing cytoplasmic inclusion bodies made up of all of the components of the viral transcription and replication machinery, the N, P, and L proteins (55–59). HSP90 was found to be diffused within the cytoplasm and enriched in the viral inclusions, as observed by colocalization with individually labeled P and L proteins in cells infected with recombinant MeV-Flag/L and imaged by confocal microscopy (Fig. 5), while in noninfected cells, HSP90 only displayed a diffused cytoplasmic distribution (Fig. 5, mock). In addition, both HSP90 and P could be coimmunoisolated with Flag/L protein upon gentle elution from anti-Flag antibody-coated beads with a Flag peptide (Fig. 6A), with HSP90 being recovered in greater amounts than those found in control eluates. In a converse experiment, both Flag/L and P were recovered in the eluate from anti-HSP90 beads from cells coexpressing Flag/L and P in the absence of 17-DMAG (Fig. 6B, lane 4). When produced in the absence of P, Flag/L was not found in the eluate from anti-HSP90 beads, most likely because of the low solubility of L without P (Fig. 6B, lane 3). Thus, both colocalization imaging and coimmunoprecipitation experiments indicate that L, P, and HSP90 can associate in protein complexes within infected cells.
FIG 5.
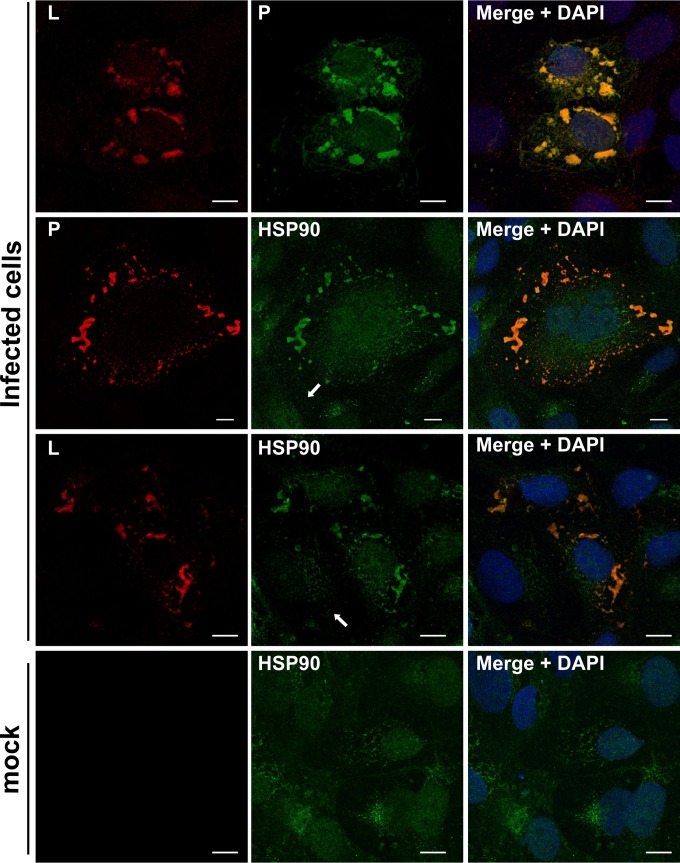
MeV L, P, and HSP90 colocalize within virus-induced inclusion bodies. (A) Vero/hSLAM cells were infected at an MOI of 0.1 with a recombinant MeV expressing a Flag-tagged L protein or not infected. At 24 hpi, cells were fixed, the L or P (left side) and P or HSP90 (middle) proteins were immunolabeled, and images were overlaid for colocalization (right side). White arrows indicate noninfected cells. White bars represent 10 μm.
FIG 6.
HSP90 is coimmunoprecipitated with P and L from infected and transfected cells but is dispensable for the stability of P-L362 complexes. (A) Coimmunoprecipitation of HSP90 and P with L from infected cells. Vero/hSLAM cells were infected at an MOI of 1 with MeV strain Moraten expressing L protein tagged with the Flag peptide or not tagged (MeV-Flag/L and MeV, respectively). At 28 hpi, immunoprecipitation (IP) from the supernatant of infected cells lysed with urea-free buffer was performed with anti-Flag antibody (ab)-coated magnetic beads. Flag/L, HSP90, P, and GAPDH were analyzed by SDS-PAGE and Western blotting as detailed in Materials and Methods. Note the faint nonspecific immunoprecipitation of HSP90 from extracts of cells infected with MeV expressing L protein without the Flag tag. (B) Both P and Flag/L are coimmunoprecipitated with HSP90 on immobilized anti-HSP90 antibodies. BSRT-7 cells were cotransfected with protein constructs at 24 h posttransfection, and proteins were immunoprecipitated by anti-HSP90 antibodies and protein G beads. Proteins in the cell extracts (input) and in the eluate from the Flag-tagged beads were detected by Western blotting. (C) The coimmunoprecipitation of P and L362 is not affected by inhibition of HSP90 activity. BSR-T7 cells were transfected to express P, Flag/N, and Flag/L362 with or without P. At 17 h posttransfection, cells were treated with 2 μM 17-DMAG or not treated. At 24 h posttransfection, Flag-tagged proteins were immunoprecipitated with anti-Flag antibody-coated beads, and the cell extracts (input) and the eluate from the Flag-tagged beads were subjected to Western blotting.
P binding to the N terminus of L does not require HSP90 activity.
We then asked whether P binding to L can occur without HSP90 activity. Within the MeV L protein, the P binding site has been reported to map to the L1-408 fragment (20, 60). In fact, the L protein consists of six conserved regions (CRI to CRVI) throughout the Mononegavirales order, except for the Bornaviridae family, which lacks CRVI (61). According to the alignment of L from Mononegavirales with the recently solved VSV-L structures (22), the L-362 fragment (which covers most of CRI) can be designed as a possible independent folding unit. Indeed, the structure of the corresponding crystalized VSV L N-terminal domain (Protein Data Bank [PDB] code 5CHS) (62) superimposes well with that solved within the entire L protein (PDB code 5a22) (22). In contrast to full-length L, this fragment accumulated well in soluble form in cells, and its accumulation level remained unchanged upon the inhibition of HSP90 by 17-DMAG even when it was coexpressed with P (Fig. 6C, input, compare lanes 2 and 3). P was efficiently coimmunoprecipitated with Flag/L-362 even when they were coexpressed in the presence of 17-DMAG, as it was with the Flag/N protein, another P-binding protein (Fig. 6C, eluate, compare lanes 2 and 3). Small but detectable amounts of HSP90 were also isolated with Flag/L-362 produced with or without P but not with Flag/N (Fig. 6C, eluate, compare lanes 2 and 6 with lane 4). Upon HSP90 inhibition, no HSP90 could be detected after Flag/L-362 precipitation (Fig. 6C, eluate, compare lanes 2 and 3). Thus, P and HSP90 may bind to the N terminus of L independently of each other and the interaction between L and HSP90 requires HSP90 activity.
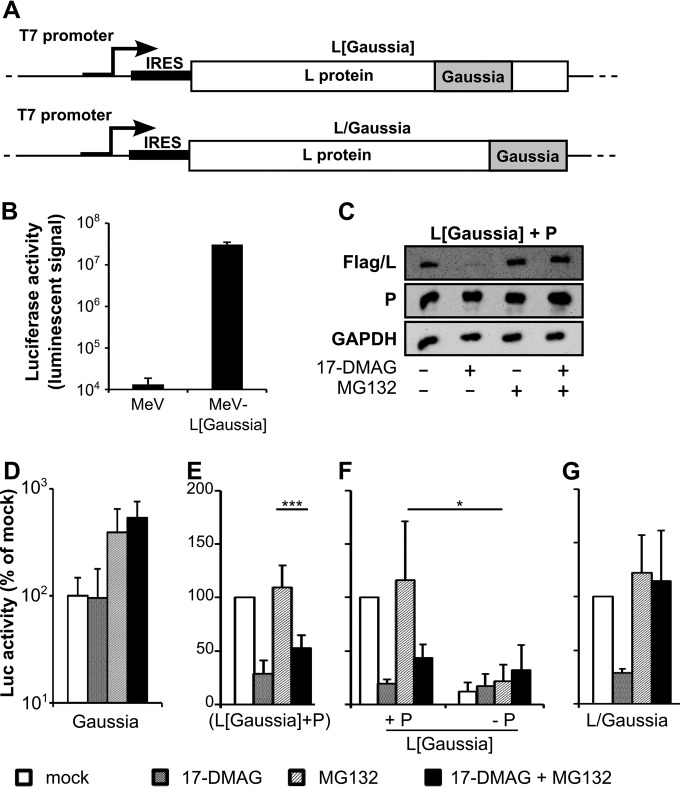
Chimeric L/luciferase proteins allow intracellular evaluation of L protein folding upon HSP90 assistance in the presence of P.
Taken together, the above data suggest that HSP90 can act as a chaperon of L to allow proper folding. To monitor the folding state of accumulated MeV L, Gaussia luciferase was fused between residues 1709 and 1710 of L (L[Gaussia]), i.e., within the flexible linker preceding the methyltransferase domain that encompasses CRVI (22) (Fig. 7A, top). This position tolerates a foreign protein graft, as shown by the rescue and growth of recombinant MeV L[Gaussia] (Fig. 7B), in agreement with similar observations made with other members of the order Mononegavirales (55, 63–65). We hypothesize that misfolding of the nascent L protein will induce the misfolding of the downstream fused luciferase and consequently loss of luciferase activity. According to Western blot analysis, chimeric MeV L[Gaussia] coexpressed with P exhibited the same biochemical phenotype as that observed with the L protein (compared Fig. 7C and 3F). While the activity of Gaussia luciferase per se was insensitive to 17-DMAG in the absence or presence of MG132 (Fig. 7D), that of L[Gaussia] was strongly inhibited by the addition of 17-DMAG in the presence of P (Fig. 7E). However, enzymatic activity could not be recovered upon simultaneous inhibition of the proteasome, suggesting that, in this case, L[Gaussia] remains misfolded when its degradation by the proteasome is prevented (compare Fig. 7E and C). P seems also to be required for the proper folding of L, since L[Gaussia] expressed in the absence of P displayed only marginal luciferase activity that was not affected by any treatment (Fig. 7F).
FIG 7.
Enzymatic activity from MeV L[Gaussia] and L/Gaussia hybrid molecules confirms that stable L folding is dependent on HSP90 activity. (A) Schematic view of MeV L[Gaussia] chimeric protein and L/Gaussia with luciferase coding sequence inserted between residues 1709 and 1710 (top) and appended to the C terminus of L (bottom), respectively. (B) Luciferase activity in cells infected with recombinant MeV-L[Gaussia] virus (MOI of 1) at 24 hpi illustrating tolerance of the internal graft of the luciferase domain. (C) L[Gaussia] chimeric protein recapitulates the detection phenotype of wild-type L protein observed in the presence of P with inhibition by 2 μM 17-DMAG treatment that is relieved by treatment with 5 μM MG132 (see the legend to Fig. 3 for experimental details). (D) Gaussia luciferase activity is insensitive to 17-DMAG treatment. Cells were cotransfected with glu1-P and glu2-P constructs that reconstitute the Gaussia luciferase thanks to the P tetramerization domain (34). Note the similar slight enhancement of the luciferase signal upon the inhibition of the proteasome by MG132 in the absence or presence of 17-DMAG. (E, F) Luciferase activity from the L[Gaussia] chimeric protein in the presence of P is inhibited by 2 μM 17-DMAG but not restored upon simultaneous treatment with MG132. MG132 versus 17-DMAG+MG132, P = 0.0002 (***; Student t test; n = 6) (E). (F) In the absence of P, luciferase activity from L[Gaussia] is marginal (L+P versus L in the presence of MG132, P = 0.0168 (*; Student t test; n = 4). (G) Gaussia luciferase appended to the C terminus of L remains active upon simultaneous HSP90 and proteasome inhibition, while it is lost upon the sole inhibition of HSP90.
When the Gaussia protein was appended at the C terminus of L via a short flexible linker (L/Gaussia) (Fig. 7A, bottom), the loss of luciferase activity induced by 17-DMAG (likely because of the P-dependent induced degradation of L/Gaussia) was fully prevented upon the addition of MG132 (Fig. 7G). This suggests that when appended to the C terminus of L instead of being embedded within the L structure, the Gaussia protein retains a functional fold independently of the upstream L fold status. Alternatively, the two last methyltransferase domain and C-terminal domain (CTD) of L may fold somehow independently of the upstream domains. Indeed, the enzymatically active methyltransferase domain of the human metapneumovirus L protein (aa 1599 to 2005) has been produced and its crystal structure has been solved (66) and active MeV polymerase has been reconstituted by the reassembly via an unrelated dimerization domain of two L split parts that encompassed the CRI-to-CRV and methyltransferase-CTD regions, respectively (67).
In the presence of P, HSP90 acts as a bona fide chaperon of the viral polymerase.
HSP90 client proteins require its activity either for the folding step that occurs during their synthesis and/or maturation (68) or to support the active conformation of their enzymatic activity, as is the case for many kinases and ubiquitin E3 ligases (69). In the first case, once the clients have achieved their mature folding, their stability and/or function usually no longer rely on HSP90 (68). The type of HSP90 client the L polymerase is was evaluated by two complementary approaches.
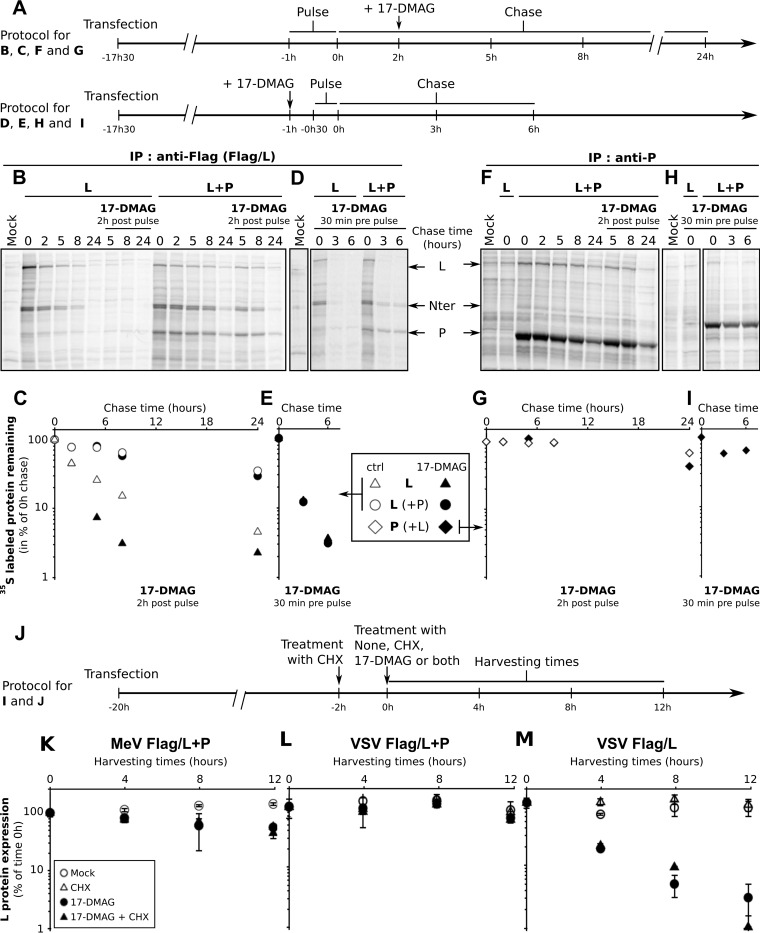
In a first set of experiments, the impact of 17-DMAG treatment on the fate of neosynthesized Flag/L protein was investigated after metabolic labeling with 35S-labeled cysteine and methionine, followed by immunoprecipitation of soluble Flag/L by anti-Flag antibody-coated beads. As expected, the coexpression of P and L slows down the decay of 35S-labeled Flag/L (Fig. 8B and C, compare white circles and triangles), as shown by a roughly 2.5-fold increase in the estimated half-life (∼14.8 h with P versus ∼5.9 h without P). Furthermore, the addition of 2 μM 17-DMAG on cells after a 2-h chase delay (Fig. 8A shows the experimental scheme) resulted in an ∼4-fold decrease in the rate of 35S-labeled Flag/L produced without P (Fig. 8B and C, black triangles, half-life of ∼1.4 h). In contrast, the decay of 35S-labeled Flag/L produced in the presence of P was mostly unaffected by this treatment (Fig. 8B and C, black circles, half-life of ∼12.8 h). When 17-DMAG was applied before the metabolic labeling and maintained thereafter (Fig. 8A shows the experimental scheme), 35S-labeled Flag/L quickly disappeared after the end of the pulse, independently of whether or not it was coexpressed with P (Fig. 8D and E, half-life of ∼1.2 h in both cases), and in agreement with Fig. 3D and F. The decay of P protein was also determined after immunoprecipitation with a P-specific antibody and found not to be sensitive to treatment with 17-DMAG (Fig. 8F to I), exhibiting ∼14.4-h and ∼13.9-h half-lives, respectively. In both pulse-chase experiments done in the presence of P, amounts of 35S-labeled P coimmunoprecipitated with anti-Flag/L beads (Fig. 8B and D) and those of 35S-labeled Flag/L coimmunoprecipitated with anti-P beads (Fig. 8F and H) nicely parallel the fate of 35S-labeled Flag/L and that of 35S-labeled P, respectively. As mentioned earlier, an ∼110-kDa 35S-labeled Flag/L N-terminal fragment that could correspond to the RNA-dependent RNA polymerase domain (22) was clearly identified in anti-Flag eluates (Fig. 8B and D). Since this fragment was detected only in the transfection system and not in the anti-P eluate (Fig. 8F and H), it likely represents an intermediate degradation product of Flag/L not bound to P. Of note, the level of 35S labeling of all cell proteins did not differ between samples by more than 13% over the first 8 h, as determined by SDS-PAGE of labeled cell extracts, thus excluding any bias during metabolic labeling of the cells (data not shown). These results confirm that L is very sensitive to the lack of HSP90 activity during its biosynthesis and show that L proteins having matured for 2 h in the presence of P and HSP90 activity become stable without any further chaperoning by HSP90.
FIG 8.
Impact of HSP90 activity on the fate of MeV L polymerase. (A to H) Monitoring of soluble MeV L and P after metabolic 35S labeling performed in the absence or presence of 17-DMAG. (A) Schematic view of the experiment. BSR-T7 cells were transfected with P and/or L plasmid(s) and the day after were pulse-labeled with 35S-labeled methionine and cysteine for either 1 h (top) or 0.5 h (bottom). At the times indicated, cells were lysed and soluble supernatants were analyzed by immunoprecipitation (IP) with anti-Flag antibody-coated beads and anti-P antibody plus protein G beads. In some cases, 2 μM 17-DMAG was added after (top) or before (bottom) the pulse-labeling. Shown are autoradiograms (B, D) and quantification (C, E) of material eluted from anti-Flag antibody-coated beads incubated with the soluble cell extracts harvested at the times indicated after 1 h (B, C) or 0.5 h (D, E) of pulse-labeling according to top and bottom schemes in panel A, respectively. Panels F and G and panels H and I show data on the immunoprecipitation (with anti-P antibodies) of the same extracts as in panels B and C and panels D and E, respectively. Note the comparable stability of L and P in panels C and G when the two proteins were coexpressed. (J) Schematic view of experiments shown in panels K to M. Decay of premade MeV (K) and VSV (L, M) Flag/L proteins coexpressed (K, L) or not coexpressed (M) with P that have been allowed to be synthesized and stabilized for 18 h. After a protein synthesis block with 20 μg/ml cycloheximide for 2 h, cycloheximide was replaced with fresh medium supplemented with 2 μM 17-DMAG, 20 μg/ml cycloheximide (CHX), or both or not supplemented. Protein decay was measured from Western blotting signals (mean values and standard deviations from two independent samples) and expressed as a percentage of the Western blotting signal determined at the end of the cycloheximide block. Note that the stability of MeV L produced without P could not be technically studied because the level was too low for detection (see Fig. 4B).
In a second set of experiments, we studied the fate of steady-state L and P that have accumulated in the presence of active HSP90 for 18 h. To ensure that we studied the fate of only the preexisting pool of stabilized L proteins, the observation started after an initial 2-h window of blocking of cellular translation with cycloheximide (see the scheme in Fig. 8J). MeV Flag/L premade in the presence of P and HSP90 activity displayed a similar decay upon treatment with cycloheximide, 17-DMAG, or both (Fig. 8K), with an apparent half-life of ∼13 to 14 h, i.e., in a range similar to that observed in the pulse-chase study. We conclude that HSP90 is dispensable for the maintenance of stable MeV P+L polymerase complexes once they have been fully assembled and/or folded. Similar results were obtained by studying the fate of VSV Flag/L produced in the presence of P that remained stable for 12 h (Fig. 8L), as well as VSV P (data not shown). In contrast, VSV Flag/L produced without P decayed much more quickly in the presence of 17-DMAG (Fig. 8M, compare black and white symbols), with an apparent ∼2- to 3-h half-life, while premade Flag/L remained stable for almost 12 h in the absence of 17-DMAG.
Functions of MeV polymerase do not rely on HSP90 activity.
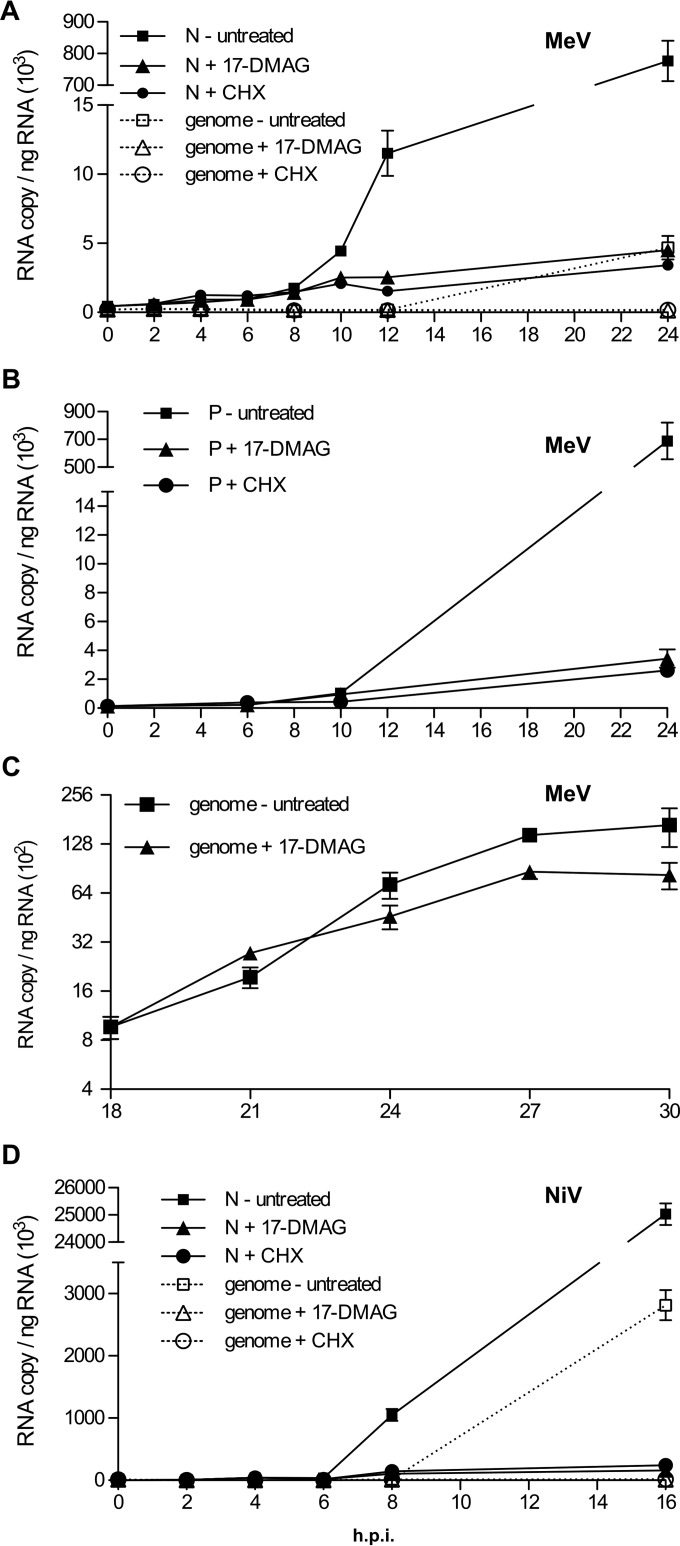
We then logically evaluated whether, once made, biochemically stable L (in complex with P) can be functional in the absence of any HSP90 activity. We took advantage of the active polymerases that are resident in virions and ready to start primary viral transcription upon nucleocapsid delivery into the cytoplasm of the host cell (14). Being mediated by the fixed number of polymerases found within the virion, this primary transcription is characterized by a linear mRNA accumulation phase for several hours until newly made polymerases are recruited to the incoming nucleocapsid, resulting in a shift from a linear to an exponential viral transcript accumulation rate, thus constituting a secondary transcription phase. During these two transcription phases that precede the onset of replication, every polymerase is very efficiently recycled and/or recruited on the incoming nucleocapsid template, as experimentally illustrated once more in Fig. 9. In the absence of any treatment, the N and P mRNAs quantified by RT-qPCR accumulated linearly for the first ∼8 h and then exponentially further on (Fig. 9A and B). When MeV-infected cells were treated with cycloheximide at 30 min postinfection to block the neosynthesis of any newly active viral polymerase, the linear phase of N mRNA accumulation proceeded without switching to the exponential phase, in agreement with our previous report (14). Interestingly, the addition of 17-DMAG mimicked the protein translation inhibition, with a similar rate of linear mRNA accumulation (Fig. 9C). This suggests that preformed polymerases brought by infecting virions do not depend on HSP90 activity to synthesize viral mRNA. Furthermore, the absence of a switch of the mRNA accumulation rate to the exponential phase indicates that HSP90 activity is required for the production of new polymerases, in agreement with our previous results showing the misfolding and degradation of L proteins under these conditions. The similar rate of N mRNA accumulation upon treatment with cycloheximide and 17-DMAG is in agreement with the biochemical stability of fully mature L protein in complex with P shown in Fig. 8. The reinitiation of viral transcription at the next gene start signal/sequence was also found to be unaffected upon treatment with either cycloheximide or 17-DMAG, since similar linear accumulation of P mRNA occurred (Fig. 9B). Finally, MeV genome replication did not require HSP90 activity since genomes were still accumulating after a late addition of 17-DMAG (Fig. 9C). Note, however, that upon the early addition of 17-DMAG, the onset of the replication phase was fully prevented (Fig. 9A, dotted lines), likely because of the inhibition of the exponential transcription phase. Similarly, secondary but not primary transcription of NiV was also blocked in the presence of 17-DMAG, as observed in the presence of cycloheximide (Fig. 9D). These data support the hypotheses that inhibition of HSP90 activity prevents the production of new functional polymerases and that stable and mature P+L polymerases are no longer sensitive to this inhibition.
FIG 9.
Once made, MeV and NiV polymerases remain functional in the absence of HSP90 activity. (A and B) MeV polymerases of virion origin do not require HSP90 activity for their RNA synthesis activity. Shown are rates of accumulation of MeV N transcripts and genome RNA (A) and MeV P transcripts (B). (C) MeV polymerases can function in the replicase mode in the absence of HSP90 activity. The same experiment as in panels A and B was performed with addition of the drug at 18 hpi, i.e., during the ongoing replication phase. (D) Virion-resident NiV polymerases do not require HSP90 activity for their RNA synthesis activity. Shown are rates of accumulation of N transcripts and genome RNA. Vero/hSLAM cells were infected with MeV IC323 or NiV at an MOI of 1 and treated 30 min later with either 2 μM 17-DMAG or 20 μg/ml cycloheximide (CHX) or not treated. RNA were extracted every 2 h, and viral RNAs were quantified by RT-qPCR. The mean values and standard deviations shown are from three independent infection samples.
DISCUSSION
Our overall data offer strong support for the idea that HSP90 acts as a transient chaperon for Mononegavirales L polymerase that needs to bind to its viral P partner in order to be stable and functional. Since we used three known inhibitors of HSP90, geldanamycin, 17-DMAG, and NVP-AUY922, and HSP90-targeted silencing, it is unlikely that our results are related to off-target activities (46, 49, 50). Moreover, the phenotype observed here perfectly fits what has been reported so far for hundreds of HSP90 clients (69).
Our results reveal an intricate interplay between HSP90 and P for the production of a stable and soluble L polymerase. On the one hand, in the absence of P, MeV L is unstable and undergoes degradation by the proteasome for a small part and aggregation into insoluble forms for the majority. In the absence of P, HSP90 is not able to properly fold L according to the data obtained with our engineered luciferase-based folding assay. On the other hand, in the presence of P, blocking of HSP90 at an early stage after transfection of the expression vectors induces the degradation of nearly all L proteins by the proteasome. When the latter is inhibited, L accumulates in likely misfolded aggregates. Furthermore, the stable recruitment of P by binding to the N-terminal region of MeV L polymerase, possibly during protein synthesis (20, 60), occurs without the need for any HSP90 activity. As HSP90 is not able to fold L into a stable form without P, we propose that P binds and stabilizes L protein possibly during its biosynthesis, thanks to a P binding site residing in the N-terminal part of MeV L (L1-362). Indeed, in the absence of P, MeV L levels quickly fall off, mostly by aggregation into insoluble complexes. P thus appears to act as a prop that prevents the collapse of folded L until it reaches its final form as part of an active L-P polymerase. In support of this hypothesis, stable P+L complexes that are no longer sensitive to HSP90 inhibition were observed only when 17-DMAG treatment was delayed by 2 h after 35S metabolic labeling (Fig. 8B and D) and not when it was immediately applied just after the labeling pulse (data not shown). Whether, as expected from a prop, MeV L that has undergone proper folding can dissociate from and reassociate with P to form a functional enzyme, as experimentally documented for VSV (8, 70) and Newcastle disease virus (9), remains to be investigated.
Once folded and stabilized by P, the L polymerase appears to be long-lived, with an estimated half-life of 13 to 14 h, from the fate of both 35S pulse-labeled and steady-state accumulated proteins. This half-life was similar to that of MeV P, indicating that binding to P drives L toward a similar long-lived state. This nicely fits the observation of primary transcription at a constant rate by virion-derived polymerases observed over 24 hpi in Vero cells when either protein synthesis or L protein folding is prevented by cycloheximide or an HSP90 inhibitor, respectively.
We anticipate the conservation of these mechanisms for the polymerase of other members of the Mononegavirales order. However, the maturation of the polymerase complex from other members of the Mononegavirales order likely differs in at least some mechanical details. Indeed, without their P partner, the L proteins of RSV (71), VSV, and NiV are more efficiently targeted for protease degradation upon HSP90 inhibition than their MeV counterpart. Such a divergent behavior of HSP90 clients has also been reported for cellular kinases, some of them preferentially ending in intracellular aggregates and others being quickly degraded by the proteasome (6). Rapid aggregation of MeV L in the absence of P likely occurs and diverts most of it from degradation. That some properly folded L proteins can be produced without P binding is further supported by purified L proteins endowed with some enzymatic activities (RNA synthesis and capping) (1, 72, 73) and by the successful association into processive transcriptases of P and L proteins separately overproduced in insect cells in the case of VSV (16) and SeV (74) and also in mammalian cells in the case of VSV (16). However, P and L from PIV5 (75) and SeV (7) individually produced in mammalian cells cannot associate. Likewise, the RSV L protein cannot be detected in the absence of P even when overproduced in insect cells (18). Finally, even VSV L produced alone is rather unstable (16, 17) and largely stabilized by P with a similar transient requirement for HSP90 activity (Fig. 8L and M).
An analysis of the polymerase complex of the influenza virus, a segmented negative-stranded RNA virus, suggested that HSP90 would be required for its polymerase activity (76). In contrast, in the cases of MeV and NiV, HSP90 is clearly dispensable for the various enzymatic activities and function of the viral polymerase. The linear accumulation of MeV N and P transcripts upon infection in the presence of 17-DMAG over 24 h (i.e., well over the estimated 2 h needed to consecutively transcribe the six viral genes [14]) indicates that the polymerases recycle and are efficiently switched on upon recycling to the 3′ leader region of the genome containing the transcription promoter. Although the other polymerase activities (RNA capping and polyadenylation) were not directly investigated, the fact that N and P transcripts accumulate at similar rates after the inhibition of either HSP90 or protein translation suggests that the viral mRNAs have similar half-lives under these conditions and therefore that mRNA processing remains correctly fulfilled. Virus replication per se was also found not to rely on HSP90 activity.
In conclusion, HSP90 appears to be indispensable for MeV, NiV, and VSV replication by acting as a faithful chaperon of the L polymerase of Mononegavirales. We propose a prop-like activity for P behind the HSP90 chaperoning of L polymerase as a new paradigm. Future work will address how and when P may prop up the nascent L protein during its assistance with folding by the HSP90 chaperoning machinery and to what extent this mechanism is conserved throughout the Mononegavirales order. To this end, we will benefit from the new intracellular luciferase-based folding assay we have set up to probe the interactions between P and L polymerase components. Finally the strong antiviral activity of HSP90 inhibitors raises the possibility of their use as antiviral drugs from which the emergence of an escape mutant virus is unlikely to occur, as recently reported for RSV (25). Such a lack of an escape mutant is not unexpected, owing to the fact that anti-HSP90 inhibitors do not exert a direct antiviral activity by targeting a viral enzymatic activity. Instead, they prevent the indispensable chaperoning of any newly made viral L polymerase by the cellular HSP90 machinery.
ACKNOWLEDGMENTS
We thank M. Billeter, R. Cattaneo, P. Pothier, M. Takeda, J. Perrault, K. Conzelmann, J. K. Rose, Y. Jacob, Y. Yanagi, and L. Naldini for useful reagents, A. Bouchardon for technical help, J. Brunel for having derived the BSR-T7-Slam cell line, and P. Lawrence for English language revision. This work has benefited from the Imaging (CIQLE) and Cytometry and Genetic Analysis (ANIRA) facilities of SFR BioSciences Gerland—Lyon Sud (CNRS UMS3444 INSERM US8) and SFR Santé Lyon Est (CNRS UMS3453, INSERM US7).
The research reported here was supported by ANR CARDINNATE (ANR-12-BSV3-010-01) and NITRODEP (ANR-13-PDOC-0010-01) grants (http://www.agence-nationale-recherche.fr/), and B.G.'s salary was supported by a Sanofi-Pasteur grant to D.G. L.M.B.'s salary was supported by the Fondation pour la Recherche Médicale (FRM). The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
L.M.B., B.G., S.D.B., and D.G. designed the research. L.M.B., J.W., B.H., and C.M. designed and/or contributed new reagents. L.M.B., F.E., B.G., J.W., S.D.B., and C.M. performed the research. L.M.B., F.E., J.W., C.M., B.G., S.D.B., and D.G. analyzed the data. All of us drafted or revised the manuscript and approved the final version.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Morin B, Rahmeh AA, Whelan SP. 2012. Mechanism of RNA synthesis initiation by the vesicular stomatitis virus polymerase. EMBO J 31:1320–1329. doi: 10.1038/emboj.2011.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morin B, Kranzusch PJ, Rahmeh AA, Whelan SP. 2013. The polymerase of negative-stranded RNA viruses. Curr Opin Virol 3:103–110. doi: 10.1016/j.coviro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blocquel D, Bourhis J-M, Eléouët J-F, Gerlier D, Habchi J, Jamin M, Longhi S, Yabukarski F. 2012. The original mechanics of Mononegavirales transcription and replication. Virologie 16:225–257. [DOI] [PubMed] [Google Scholar]
- 4.Gerlier D, Lyles DS. 2011. Interplay between innate immunity and negative-strand RNA viruses: towards a rational model. Microbiol Mol Biol Rev 75:468–490. doi: 10.1128/MMBR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolakofsky D, Le Mercier P, Iseni F, Garcin D. 2004. Viral RNA polymerase scanning and the gymnastics of Sendai virus RNA synthesis. Virology 318:463–473. doi: 10.1016/j.virol.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 6.De BP, Banerjee AK. 1984. Specific interactions of vesicular stomatitis virus L and NS proteins with heterologous genome ribonucleoprotein template lead to mRNA synthesis in vitro. J Virol 51:628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horikami SM, Curran J, Kolakofsky D, Moyer SA. 1992. Complexes of Sendai virus NP-P and P-L proteins are required for defective interfering particle genome replication in vitro. J Virol 66:4901–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emerson SU, Yu Y. 1975. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol 15:1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamaguchi M, Yoshida T, Nishikawa K, Naruse H, Nagai Y. 1983. Transcriptive complex of Newcastle disease virus. I. Both L and P proteins are required to constitute an active complex Virology 128:105–117. [DOI] [PubMed] [Google Scholar]
- 10.Guryanov SG, Liljeroos L, Kasaragod P, Kajander T, Butcher SJ. 2015. Crystal structure of the measles virus nucleoprotein core in complex with an N-terminal region of phosphoprotein. J Virol 90:2849–2857. doi: 10.1128/JVI.02865-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qanungo KR, Shaji D, Mathur M, Banerjee AK. 2004. Two RNA polymerase complexes from vesicular stomatitis virus-infected cells that carry out transcription and replication of genome RNA. Proc Natl Acad Sci U S A 101:5952–5957. doi: 10.1073/pnas.0401449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta AK, Shaji D, Banerjee AK. 2003. Identification of a novel tripartite complex involved in replication of vesicular stomatitis virus genome RNA. J Virol 77:732–738. doi: 10.1128/JVI.77.1.732-738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamb RA, Parks GD. 2007. Paramyxoviridae: the viruses and their replication, p 1449–1496. In Fields BN, Howley PM (ed), Fields virology, 3rd ed Lippincott-Raven, Philadelphia, PA. [Google Scholar]
- 14.Plumet S, Duprex WP, Gerlier D. 2005. Dynamics of viral RNA synthesis during measles virus infection. J Virol 79:6900–6908. doi: 10.1128/JVI.79.11.6900-6908.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rima BK, Duprex WP. 2009. The measles virus replication cycle. Curr Top Microbiol Immunol 329:77–102. [DOI] [PubMed] [Google Scholar]
- 16.Canter DM, Jackson RL, Perrault J. 1993. Faithful and efficient in vitro reconstitution of vesicular stomatitis virus transcription using plasmid-encoded L and P proteins. Virology 194:518–529. doi: 10.1006/viro.1993.1290. [DOI] [PubMed] [Google Scholar]
- 17.Canter DM, Perrault J. 1996. Stabilization of vesicular stomatitis virus L polymerase protein by P protein binding: a small deletion in the C-terminal domain of L abrogates binding. Virology 219:376–386. doi: 10.1006/viro.1996.0263. [DOI] [PubMed] [Google Scholar]
- 18.Sourimant J, Rameix-Welti MA, Gaillard AL, Chevret D, Galloux M, Gault E, Eleouet JF. 2015. Fine mapping and characterization of the L-polymerase-binding domain of the respiratory syncytial virus phosphoprotein. J Virol 89:4421–4433. doi: 10.1128/JVI.03619-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trunschke M, Conrad D, Enterlein S, Olejnik J, Brauburger K, Muhlberger E. 2013. The L-VP35 and L-L interaction domains reside in the amino terminus of the Ebola virus L protein and are potential targets for antivirals. Virology 441:135–145. doi: 10.1016/j.virol.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horikami SM, Smallwood S, Bankamp B, Moyer SA. 1994. An amino-proximal domain of the L protein binds to the P protein in the measles virus RNA polymerase complex. Virology 205:540–545. doi: 10.1006/viro.1994.1676. [DOI] [PubMed] [Google Scholar]
- 21.Rahmeh AA, Schenk AD, Danek EI, Kranzusch PJ, Liang B, Walz T, Whelan SP. 2010. Molecular architecture of the vesicular stomatitis virus RNA polymerase. Proc Natl Acad Sci U S A 107:20075–20080. doi: 10.1073/pnas.1013559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang B, Li Z, Jenni S, Rahmeh AA, Morin BM, Grant T, Grigorieff N, Harrison SC, Whelan SP. 2015. Structure of the L protein of vesicular stomatitis virus from electron cryomicroscopy. Cell 162:314–327. doi: 10.1016/j.cell.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connor JH, McKenzie MO, Parks GD, Lyles DS. 2007. Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology 362:109–119. doi: 10.1016/j.virol.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DR, McCarthy S, Chrovian A, Olinger G, Stossel A, Geisbert TW, Hensley LE, Connor JH. 2010. Inhibition of heat-shock protein 90 reduces Ebola virus replication. Antiviral Res 87:187–194. doi: 10.1016/j.antiviral.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geller R, Andino R, Frydman J. 2013. Hsp90 inhibitors exhibit resistance-free antiviral activity against respiratory syncytial virus. PLoS One 8:e56762. doi: 10.1371/journal.pone.0056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter MA. 1995. Rescue of measles viruses from cloned DNA. EMBO J 14:5773–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finke S, Conzelmann KK. 1999. Virus promoters determine interference by defective RNAs: selective amplification of mini-RNA vectors and rescue from cDNA by a 3′ copy-back ambisense rabies virus. J Virol 73:3818–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones HW Jr, McKusick VA, Harper PS, Wuu KD. 1971. George Otto Gey. (1899–1970). The HeLa cell and a reappraisal of its origin. Obstet Gynecol 38:945–949. [PubMed] [Google Scholar]
- 29.Tatsuo H, Ono N, Tanaka K, Yanagi Y. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 30.Welsch JC, Talekar A, Mathieu C, Pessi A, Moscona A, Horvat B, Porotto M. 2013. Fatal measles virus infection prevented by brain-penetrant fusion inhibitors. J Virol 87:13785–13794. doi: 10.1128/JVI.02436-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grigorov B, Rabilloud J, Lawrence P, Gerlier D. 2011. Rapid titration of measles and other viruses: optimization with determination of replication cycle length. PLoS One 6:e24135. doi: 10.1371/journal.pone.0024135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoneda M, Guillaume V, Ikeda F, Sakuma Y, Sato H, Wild TF, Kai C. 2006. Establishment of a Nipah virus rescue system. Proc Natl Acad Sci U S A 103:16508–16513. doi: 10.1073/pnas.0606972103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostertag D, Hoblitzell-Ostertag TM, Perrault J. 2007. Overproduction of double-stranded RNA in vesicular stomatitis virus-infected cells activates a constitutive cell-type-specific antiviral response. J Virol 81:503–513. doi: 10.1128/JVI.01218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunel J, Chopy D, Dosnon M, Bloyet LM, Devaux P, Urzua E, Cattaneo R, Longhi S, Gerlier D. 2014. Sequence of events in measles virus replication: role of phosphoprotein-nucleocapsid interactions. J Virol 88:10851–10863. doi: 10.1128/JVI.00664-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sellin CI, Jegou JF, Renneson J, Druelle J, Wild TF, Marie JC, Horvat B. 2009. Interplay between virus-specific effector response and Foxp3 regulatory T cells in measles virus immunopathogenesis. PLoS One 4:e4948. doi: 10.1371/journal.pone.0004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plumet S, Gerlier D. 2005. Optimized SYBR green real-time PCR assay to quantify the absolute copy number of measles virus RNAs using gene specific primers. J Virol Methods 128:79–87. doi: 10.1016/j.jviromet.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Mathieu C, Pohl C, Szecsi J, Trajkovic-Bodennec S, Devergnas S, Raoul H, Cosset FL, Gerlier D, Wild TF, Horvat B. 2011. Nipah virus uses leukocytes for efficient dissemination within a host. J Virol 85:7863–7871. doi: 10.1128/JVI.00549-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giraudon P, Jacquier MF, Wild TF. 1988. Antigenic analysis of African measles virus field isolates: identification and localisation of one conserved and two variable epitope sites on the NP protein. Virus Res 10:137–152. doi: 10.1016/0168-1702(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 39.Chen M, Cortay JC, Logan IR, Sapountzi V, Robson CN, Gerlier D. 2005. Inhibition of ubiquitination and stabilization of human ubiquitin E3 ligase PIRH2 by measles virus phosphoprotein. J Virol 79:11824–11836. doi: 10.1128/JVI.79.18.11824-11836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khurana N, Bhattacharyya S. 2015. Hsp90, the concertmaster: tuning transcription. Front Oncol 5:100. doi: 10.3389/fonc.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Röhl A, Rohrberg J, Buchner J. 2013. The chaperone Hsp90: changing partners for demanding clients. Trends Biochem Sci 38:253–262. doi: 10.1016/j.tibs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Boulon S, Bertrand E, Pradet-Balade B. 2012. HSP90 and the R2TP co-chaperone complex: building multi-protein machineries essential for cell growth and gene expression. RNA Biol 9:148–154. doi: 10.4161/rna.18494. [DOI] [PubMed] [Google Scholar]
- 43.Neckers L, Workman P. 2012. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jez JM, Chen JC, Rastelli G, Stroud RM, Santi DV. 2003. Crystal structure and molecular modeling of 17-DMAG in complex with human Hsp90. Chem Biol 10:361–368. doi: 10.1016/S1074-5521(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 45.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. 1994. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A 91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voruganti S, Lacroix JC, Rogers CN, Rogers J, Matts RL, Hartson SD. 2013. The anticancer drug AUY922 generates a proteomics fingerprint that is highly conserved among structurally diverse Hsp90 inhibitors. J Proteome Res 12:3697–3706. doi: 10.1021/pr400321x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Brien J, Wilson I, Orton T, Pognan F. 2000. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 48.Gerlier D, Thomasset N. 1986. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods 94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 49.Samuni A, Goldstein S. 2012. Redox properties and thiol reactivity of geldanamycin and its analogues in aqueous solutions. J Phys Chem B 116:6404–6410. doi: 10.1021/jp304206n. [DOI] [PubMed] [Google Scholar]
- 50.Guo W, Reigan P, Siegel D, Ross D. 2008. Enzymatic reduction and glutathione conjugation of benzoquinone ansamycin heat shock protein 90 inhibitors: relevance for toxicity and mechanism of action. Drug Metab Dispos 36:2050–2057. doi: 10.1124/dmd.108.022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharp SY, Roe SM, Kazlauskas E, Cikotiene I, Workman P, Matulis D, Prodromou C. 2012. Co-crystalization and in vitro biological characterization of 5-aryl-4-(5-substituted-2-4-dihydroxyphenyl)-1,2,3-thiadiazole Hsp90 inhibitors. PLoS One 7:e44642. doi: 10.1371/journal.pone.0044642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plumet S, Herschke F, Bourhis JM, Valentin H, Longhi S, Gerlier D. 2007. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS One 2:e279. doi: 10.1371/journal.pone.0000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Picard D. 2012. Preface to Hsp90. Biochim Biophys Acta 1823:605–606. doi: 10.1016/j.bbamcr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Blagosklonny MV, Toretsky J, Bohen S, Neckers L. 1996. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci U S A 93:8379–8383. doi: 10.1073/pnas.93.16.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duprex WP, Collins FM, Rima BK. 2002. Modulating the function of the measles virus RNA-dependent RNA polymerase by insertion of green fluorescent protein into the open reading frame. J Virol 76:7322–7328. doi: 10.1128/JVI.76.14.7322-7328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vincent S, Spehner D, Manie S, Delorme R, Drillien R, Gerlier D. 1999. Inefficient measles virus budding in murine L.CD46 fibroblasts. Virology 265:185–195. doi: 10.1006/viro.1999.0064. [DOI] [PubMed] [Google Scholar]
- 57.Devaux P, Cattaneo R. 2004. Measles virus phosphoprotein gene products: conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function. J Virol 78:11632–11640. doi: 10.1128/JVI.78.21.11632-11640.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwasaki M, Takeda M, Shirogane Y, Nakatsu Y, Nakamura T, Yanagi Y. 2009. The matrix protein of measles virus regulates viral RNA synthesis and assembly by interacting with the nucleocapsid protein. J Virol 83:10374–10383. doi: 10.1128/JVI.01056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, Pavlovic J, Aguzzi A, Billeter MA, Cattaneo R. 1998. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J 17:3899–3908. doi: 10.1093/emboj/17.14.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cevik B, Holmes DE, Vrotsos E, Feller JA, Smallwood S, Moyer SA. 2004. The phosphoprotein (P) and L binding sites reside in the N-terminus of the L subunit of the measles virus RNA polymerase. Virology 327:297–306. doi: 10.1016/j.virol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Ogino T, Banerjee AK. 2011. An unconventional pathway of mRNA cap formation by vesiculoviruses. Virus Res 162:100–109. doi: 10.1016/j.virusres.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu S, Ogino M, Luo M, Ogino T, Green TJ. 2016. Structure and function of the N-terminal domain of the vesicular stomatitis virus RNA polymerase. J Virol 90:715–724. doi: 10.1128/JVI.02317-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruedas JB, Perrault J. 2009. Insertion of enhanced green fluorescent protein in a hinge region of vesicular stomatitis virus L polymerase protein creates a temperature-sensitive virus that displays no virion-associated polymerase activity in vitro. J Virol 83:12241–12252. doi: 10.1128/JVI.01273-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown DD, Rima BK, Allen IV, Baron MD, Banyard AC, Barrett T, Duprex WP. 2005. Rational attenuation of a morbillivirus by modulating the activity of the RNA-dependent RNA polymerase. J Virol 79:14330–14338. doi: 10.1128/JVI.79.22.14330-14338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silin D, Lyubomska O, Ludlow M, Duprex WP, Rima BK. 2007. Development of a challenge-protective vaccine concept by modification of the viral RNA-dependent RNA polymerase of canine distemper virus. J Virol 81:13649–13658. doi: 10.1128/JVI.01385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paesen GC, Collet A, Sallamand C, Debart F, Vasseur JJ, Canard B, Decroly E, Grimes JM. 2015. X-ray structure and activities of an essential Mononegavirales L-protein domain. Nat Commun 6:8749. doi: 10.1038/ncomms9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dochow M, Krumm SA, Crowe JE Jr, Moore ML, Plemper RK. 2012. Independent structural domains in paramyxovirus polymerase protein. J Biol Chem 287:6878–6891. doi: 10.1074/jbc.M111.325258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Echtenkamp FJ, Freeman BC. 2012. Expanding the cellular molecular chaperone network through the ubiquitous cochaperones. Biochim Biophys Acta 1823:668–673. doi: 10.1016/j.bbamcr.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. 2012. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naito S, Ishihama A. 1976. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem 251:4307–4314. [PubMed] [Google Scholar]
- 71.Munday DC, Wu W, Smith N, Fix J, Noton SL, Galloux M, Touzelet O, Armstrong SD, Dawson JM, Aljabr W, Easton AJ, Rameix-Welti MA, de Oliveira AP, Simabuco FM, Ventura AM, Hughes DJ, Barr JN, Fearns R, Digard P, Eleouet JF, Hiscox JA. 2015. Interactome analysis of the human respiratory syncytial virus RNA polymerase complex identifies protein chaperones as important cofactors that promote L-protein stability and RNA synthesis. J Virol 89:917–930. doi: 10.1128/JVI.01783-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta AK, Mathur M, Banerjee AK. 2002. Unique capping activity of the recombinant RNA polymerase (L) of vesicular stomatitis virus: association of cellular capping enzyme with the L protein. Biochem Biophys Res Commun 293:264–268. doi: 10.1016/S0006-291X(02)00217-6. [DOI] [PubMed] [Google Scholar]
- 73.Mathur M, Das T, Banerjee AK. 1996. Expression of L protein of vesicular stomatitis virus Indiana serotype from recombinant baculovirus in insect cells: requirement of a host factor(s) for its biological activity in vitro. J Virol 70:2252–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogino T, Kobayashi M, Iwama M, Mizumoto K. 2005. Sendai virus RNA-dependent RNA polymerase L protein catalyzes cap methylation of virus-specific mRNA. J Biol Chem 280:4429–4435. doi: 10.1074/jbc.M411167200. [DOI] [PubMed] [Google Scholar]
- 75.Parks GD. 1994. Mapping of a region of the paramyxovirus L protein required for the formation of a stable complex with the viral phosphoprotein P. J Virol 68:4862–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Momose F, Naito T, Yano K, Sugimoto S, Morikawa Y, Nagata K. 2002. Identification of Hsp90 as a stimulatory host factor involved in influenza virus RNA synthesis. J Biol Chem 277:45306–45314. doi: 10.1074/jbc.M206822200. [DOI] [PubMed] [Google Scholar]