ABSTRACT
The host cell restriction factor CD317/tetherin traps virions at the surface of producer cells to prevent their release. The HIV-1 accessory protein Vpu antagonizes this restriction. Vpu reduces the cell surface density of the restriction factor and targets it for degradation; however, these activities are dispensable for enhancing particle release. Instead, Vpu has been suggested to antagonize CD317/tetherin by preventing recycling of internalized CD317/tetherin to the cell surface, blocking anterograde transport of newly synthesized CD317/tetherin, and/or displacing the restriction factor from virus assembly sites at the plasma membrane. At the molecular level, antagonism relies on the physical interaction of Vpu with CD317/tetherin. Recent findings suggested that phosphorylation of a diserine motif enables Vpu to bind to adaptor protein 1 (AP-1) trafficking complexes via two independent interaction motifs and to couple CD317/tetherin to the endocytic machinery. Here, we used a panel of Vpu proteins with specific mutations in individual interaction motifs to define which interactions are required for antagonism of CD317/tetherin. Impairing recycling or anterograde transport of CD317/tetherin to the plasma membrane was insufficient for antagonism. In contrast, excluding CD317/tetherin from HIV-1 assembly sites depended on Vpu motifs for interaction with AP-1 and CD317/tetherin and correlated with antagonism of the particle release restriction. Consistently, interference with AP-1 function or its expression blocked these Vpu activities. Our results define displacement from HIV-1 assembly sites as active principle of CD317/tetherin antagonism by Vpu and support a role of tripartite complexes between Vpu, AP-1, and CD317/tetherin in this process.
IMPORTANCE CD317/tetherin poses an intrinsic barrier to human immunodeficiency virus type 1 (HIV-1) replication in human cells by trapping virus particles at the surface of producer cells and thereby preventing their release. The viral protein Vpu antagonizes this restriction, and molecular interactions with the restriction factor and adaptor protein complex 1 (AP-1) were suggested to mediate this activity. Vpu modulates intracellular trafficking of CD317/tetherin and excludes the restriction factor from HIV-1 assembly sites at the plasma membrane, but the relative contribution of these effects to antagonism remain elusive. Using a panel of Vpu mutants, as well as interference with AP-1 function and expression, we show here that Vpu antagonizes CD317/tetherin by blocking its recruitment to viral assembly sites in an AP-1-dependent manner. These results refine our understanding of the molecular mechanisms of CD317/tetherin antagonism and suggest complexes of Vpu with the restriction factor and AP-1 as targets for potential therapeutic intervention.
INTRODUCTION
As a complex retrovirus, human immunodeficiency virus (HIV) encodes the classical structural retroviral proteins Gag, Pol, and Env, the regulatory proteins Tat and Rev and so-called accessory proteins (Vif, Vpr, Vpu, and Nef in the case of HIV-1). Although structural and regulatory proteins are essential for HIV replication irrespective of the cellular context, accessory genes encode proteins that, among other functions, mediate the interaction of infected cells with the host immune system and can be dispensable for HIV spread in ex vivo cell line cultures. As an example for such an activity, Nef and Vpu promote the evasion of HIV-infected cells from recognition and thus lysis by cytotoxic T cells and natural killer cells (1–3). In addition, antagonism of cell-intrinsic immunity by counteraction of host cell restriction factors emerged over the past decade as a general theme of HIV accessory protein function (4). In HIV-1, this paradigm was first established for APOBEC3G, a cytidine deaminase that limits HIV replication by elevating the mutation rate during reverse transcription of incoming RNA genomes into DNA (5). This restriction factor is efficiently antagonized by HIV-1 Vif by targeting it for degradation and thereby preventing its incorporation into HIV particles (4). More recently, activities related to restrictions of HIV-1 replication were also identified for Vpr, which reduces production of antiviral cytokines by innate immune sensing through the premature activation of the SLX4 endonuclease complex (6). Likewise, Nef antagonizes the particle infectivity restriction imposed by SERINC3 and SERINC5 (7, 8).
Vpu is a 16-kDa multifunctional accessory protein encoded by HIV-1 and related primate lentiviruses. Initial studies of Vpu function revealed that the viral protein reduces the density of the HIV-1 entry receptor CD4 on the surface of an infected cell by targeting it for degradation (9). Reducing cell surface exposure of host cell receptors emerges as a general theme of Vpu function and an increasing array of target molecules, including natural killer cell ligands, amino acid transporters, and tetraspanins has been identified (3, 10–15). In addition, Vpu was recognized as a potent antagonist of the host cell restriction factor CD317/tetherin (also known as BST-2 or HM1.24), which prevents release of infectious virions by tethering virus particles to the surface of virus-producing cells (16–18). CD317/tetherin also elicits proinflammatory signaling upon virion binding by triggering activation of the transcription factor NF-κB (19–22) and sensitizes infected cells to antibody-dependent cellular cytotoxicity (23–26). Both effects are also antagonized by Vpu. CD317/tetherin is a glycosylated transmembrane protein with an unusual topology consisting of a short cytoplasmic tail (CT), a transmembrane domain (TMD), and a large extracellular domain attached to membranes via its glycosylphosphatidylinositol anchor (27). CD317/tetherin forms dimers that segregate into specialized membrane microdomains that serve as platforms for virus assembly and budding. Simultaneous insertion of CD317/tetherin molecules into the host cell plasma membrane (PM) and the envelope of budding virions leads to formation of a physical bridge between producer cell and virion and traps these particles and prevents their release (28–30).
Although the molecular mechanism by which Vpu antagonizes this function of CD317/tetherin remains a matter of debate, it appears to rely on the physical association of both proteins mediated by their TMDs (31, 32). The initial observation that Vpu moderately reduces the cell surface density of CD317/tetherin and triggers its degradation suggested that antagonism of the restriction factor may be mediated by these processes (17, 18, 33). Subsequent studies, however, uncoupled these activities and revealed that Vpu can antagonize CD317/tetherin without altering its steady-state surface exposure and/or stability (34–38). Rather, the ability of Vpu to exclude CD317/tetherin from HIV-1 assembly sites and thus from incorporation into virus particles emerged as a critical feature of antagonism (31, 39). Several, non mutually exclusive, mechanisms by which Vpu may exert this activity have been proposed: (i) active lateral displacement of CD317/tetherin from HIV assembly sites at the PM (31, 39), (ii) block of anterograde transport of newly synthesized CD317/tetherin molecules to the PM (40–42), and (iii) inhibition of CD317/tetherin recycling after internalization from the cell surface back to the PM (40).
On the molecular level, a diserine motif (S52 and S56 in most HIV-1 subtype B Vpu variants) in Vpu's cytoplasmic tail is involved in antagonism of the CD317/tetherin particle release restriction but the extent of its contribution remains controversial (33, 43–46). This motif allows Vpu to target cellular proteins to degradation by binding to the β-TrCP subunits 1 and 2 of the Skp1/Cullin1/F-box E3 ubiquitin ligase (47). This interaction is critical for Vpu-mediated degradation of the HIV entry receptor CD4 (47, 48). β-TrCP also facilitates the degradation of CD317/tetherin (36, 43, 45, 46), which involves the ESCRT-O complex (49). This process leads to elimination of complexes of the restriction factor with trapped virions after internalization from the PM and might thereby contribute to the antagonism of antiviral innate immune signaling that is elicited upon binding of HIV virion to cell surface CD317/tetherin (19, 21, 22). However, Kueck et al. recently reported that the role of the diserine motif in alleviating particle entrapment depends on the phosphorylation of the two serine residues by casein kinase II (44, 50), which promotes the physical interaction of Vpu with the clathrin machinery of the host cell (51). Coupling of Vpu to vesicular trafficking seems to rely particularly on the direct interaction with the adaptor protein complex 1 (AP-1) (52). In this scenario, phosphorylated diserine motifs enable Vpu to bind AP-1 complexes via two independent contacts made by a noncanonical, leucine-based binding motif in Vpu helix 1 and an acidic dileucine motif (ExxxLV) in Vpu helix 2 (51).
With these essential molecular interactions for antagonism of CD317/tetherin identified, the goal of the present study was to dissect at which step of CD317/tetherin trafficking they act, and which of the three proposed mechanisms contribute to Vpu antagonism. To this end, we combined the functional characterization of a panel of Vpu mutants with studying the effect of interference with AP-1 function or expression.
MATERIALS AND METHODS
Cell culture and transfections.
HT1080 and Tzm-bl cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) plus 20 mM HEPES (pH 7.5) and antibiotics. Tzm-bl cells were transfected using Lipofectamine2000 transfection reagent (1 μl/μg of DNA; catalog no. 11668-019; Life Technologies).
Expression plasmids.
The expression plasmids for CD317 with internal HA tag (CD317.HA) and Vpu with its TMD replaced by that of the vesicular stomatitis virus G protein (VSV-G) were kindly provided by Paul Bieniasz and Juan Bonifacino (28, 53). pN1-eGFP vector-based expression plasmids for wild-type (wt) and mutant Vpu from HIV-1 NL4.3 as green fluorescent protein (GFP) fusion proteins were described previously (13, 54). Additional mutations of recently identified Vpu protein interaction sites (51, 52, 55) were introduced by PCR amplification with primers that harbored the mutations of interest. In order to measure the influence of the different Vpu alleles on the colocalization of CD317 with the virus assembly site, each mutant was subcloned in the pcDNA3.1 vector with a 3′-end primer introducing a stop codon. The mouse Kir2.1 cDNA was acquired via Addgene (Addgene, catalog no. 32669), PCR amplified, and subcloned into the pN1-mCherry vector. The pCHIVΔVpu and pCHIVΔVpu Gag-eGFP constructs were generated from pCHIV or pCHIV (both described previously [56]), respectively, by exchange of an AgeI/XhoI fragment to the corresponding fragment from pNCLΔVpu, carrying a deletion from positions 6051 to 6180 of the pNL4-3 sequence (comprising codons 1 to 40 of vpu). The lack of Vpu expression was confirmed by immunoblot analysis of lysates from transfected cells. AP180 cDNA was obtained from OriGene (MC220758), and the sequence corresponding to the last 340 amino acids was PCR amplified and cloned into the pcDNA3.1 vector to obtain the pcDNA AP180-c expression construct.
Anterograde transport assay for newly synthesized proteins.
To monitor the transport of newly synthesized protein pools, plasmid DNA was microinjected into cell nuclei, and the subcellular localization of proteins was determined at various time points postinjection. At 24 h prior to microinjection, 2 × 105 HT1080 cells were seeded on glass coverslips in six-well plates. The next day, the cells were microinjected into their nuclei with an AIS-2 microinjection apparatus using pulled borosilicate glass capillaries in principle, as reported previously (40). Constructs encoding a hemagglutinin (HA)-tagged tetherin (10 ng/μl) and plasmids encoding eGFP or NL4-3 Vpu.eGFP (30 ng/μl) (wt or mutant Vpu) were mixed in water and coinjected. Note that the results obtained with Vpu.eGFP fusion proteins in this assay do not differ from those obtained with untagged Vpu variants (40, 57). After microinjection, the cells were cultured for 0.5, 1, 2, or 6 h at 37°C to allow protein expression and trafficking. At the indicated time points the cells were fixed with 4% paraformaldehyde/phosphate-buffered saline (PBS) and permeabilized, and tetherin molecules were detected using a chicken anti-HA antibody (Abcam), followed by a goat anti-chicken Alexa-568 secondary antibody (Invitrogen). For colocalization analysis, the cells were fixed and permeabilized at 2 h postmicroinjection (p.m.) and stained for HA-tagged CD317/tetherin and one of the following antibodies against subcellular markers (Golgi, mouse anti-GM130 [BD]; trans-Golgi network [TGN], sheep anti-TGN46 [AbD Serotec]; early endosomes, goat anti-EEA1 [Santa Cruz]; endoplasmic reticulum [ER], rabbit anti-calreticulin [Stressmarq Biosciences, Inc.]), followed by staining with the appropriate secondary antibodies. Stained cells were imaged with a PerkinElmer UltraVIEW VoX spinning disc microscope equipped with a Hamamatsu ORCA Flash4.0 V2 camera and a ×60 Apo TIRF 1.49 oil objective lens. For quantification of the anterograde transport, the percentage of cells with clearly detectable PM localization of the cargo protein analyzed was determined. For each p.m. time point, at least 250 cells from three biological replicates were counted.
Recycling assay.
Recycling assays for CD317 were performed in principle as described previously (40). Tzm-bl cells were transfected with the constructs encoding the different Vpu alleles, or GFP. Note that results obtained with Vpu.eGFP fusion proteins in this assay do not differ from that of untagged Vpu variants (40). At 24 h posttransfection, the cells were first incubated with saturating concentrations of the unconjugated anti-CD317 Antibody (eBioscience) in ice-cold binding medium (DMEM supplemented with 20 mM HEPES [pH 7.5]) at 4°C. The cells were then washed in binding medium before being shifted to 37°C for 0 to 10 min. Subsequently, the cells were placed on ice, followed by incubation with either allophycocyanin (APC)-conjugated anti-CD317 antibody (eBioscience). For coupling of anti-CD317, an APC fluorophore lightning link kit (Innova Biosciences) was used according to the manufacturer's protocol. Recycling of receptors was quantified by flow cytometry analyzing the APC mean fluorescence intensity (MFI), which reflects receptor molecules newly exposed on the surface of viable cells with identical GFP intensities. The APC MFIs at different time points are presented as factors of enhancement relative to the MFI at t = 0, which was set to 1.
HIV particle release analysis.
Tzm-bl cells were seeded (105 cells per well in a 12 well-plate) and cotransfected with 0.65 μg of pcHIV-1 ΔVpu and pcHIV-1 ΔVpu Gag-eGFP and 0.4 μg for vectors encoding the different Vpu alleles. When the effects of AP180-c were analyzed, 1 μg of the AP180-c encoding plasmid or an empty vector control was added to the transfection mix. T20 (0.225 mg/ml) was added to the media at 4 h posttransfection to prevent the formation of syncytia. At 2 days posttransfection, the cells were harvested, lysed in sample buffer (Tris-HCl, sodium dodecyl sulfate, β-mercaptoethanol, glycerin; pH 6.8) and analyzed by Western blotting for cell-associated levels of Gag (sheep anti-capsid antibody), Vpu (rabbit anti-Vpu [FebGennix]), and transferrin receptor (mouse anti-transferrin receptor [Invitrogen]). Cell culture supernatants were analyzed for the amounts of reverse transcriptase activity (58) as a measure of virion production. Since the expression of AP180-c blocks virion packaging of Pol (59), the amounts of p24CA in the cell culture supernatant were determined by p24CA capture enzyme-linked immunosorbent assay (60).
Immunofluorescence staining and confocal microscopy.
Cells were fixed with 4% cold paraformaldehyde for 7 min and then permeabilized using 0.1% Triton X-100/PBS. The slides were blocked for 20 min using 5% FCS-PBS; incubated with the relevant primary and secondary antibodies for 40 min at 1:300 and 1:500 dilutions in 5% FCS-PBS, respectively; and mounted using Mowiol. Stained cells were imaged with a Leica SP5 confocal microscope with a ×63 HCX PL APO lambda blue 1.40 oil or a ×100 HCX PL APO CS 1.40 oil objective lens. Images for subcellular colocalization analysis were acquired using a Perkin-Elmer UltraVIEW VoX spinning disc microscope equipped with a Hamamatsu ORCA Flash4.0 V2 camera and a ×60 Apo TIRF 1.49 oil objective lens.
qPCR.
Total mRNA was extracted from cells using a NucleoSpin-RNA extraction kit (Macherey-Nagel) according to the manufacturer's instructions. Quantitative PCR was performed using GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a control and the following primer pairs for AP-1 μ1 (F_AP1M1, 5′-GAGTACATCACTCAGGAAGG; r_AP1M1, 5′-ACACCTCATTCTTCCGATAC) or AP-1 μ2 (F_AP1M2, 5′-TTTAAGAAACAGTCAGTGGC; r_AP1M2, 5′-CCGGGAAAGACTTAATACTC). The reaction was performed on an ABi7500 (Bio-Rad).
Quantification of CD317/tetherin at PM virus assembly sites.
Tzm-bl cells were cotransfected with 0.65 μg of pcHIV-1 ΔVpu and pcHIV-1 ΔVpu Gag-eGFP and 0.4 μg for the pcDNA vectors encoding the different Vpu variants. T20 (0.225 mg/ml) was added to the media at 4 h posttransfection to prevent the formation of syncytia. At 2 days posttransfection, the cells were fixed and stained with mouse anti-human CD317 (eBioscience) and a secondary antibody coupled to Alexa 568. Images were acquired on a Leica SP5 confocal microscope with a ×100 HCX PL APO CS 1.40 oil objective lens using a zoom factor of ×2 and an aperture of 0.8 AU. Images present confocal sections close to the cell-coverglass interface. All image analysis was performed with the latest version of ImageJ Fiji software (http://fiji.sc/wiki/index.php/Fiji). For each image, the cells were manually selected as the region of interest, and the colocalization value between CD317/tetherin and the eGFP-labeled assembly site was calculated. The Mander's split coefficient was used with a PSF value of 1 and a Costes randomization of 10.
Silencing of AP-1 expression.
Knockdown of AP-1 was achieved using a novel tricistronic AAV expression construct encoding three different shRNAs, the first two of which target AP-1 μ1: 5′-GGA CGT CAT CGA GTC TGT CAA TCA AGA GTT GAC AGA CTC GAT GAC GTC C-3′ and 5′-GCT CAT GTC CTA CCG TCT CAA TCA AGA GTT GAG ACG GTA GGA CAT GAG C-3′. The third shRNA is directed against AP-1 μ2: 5′-GGA TCC AGG TCC GAT ACA TGA TCA AGA GTC ATG TAT CGG ACC TGG ATC C-3′. The shRNAs were designed to encode, in 5′-3′ orientation, the sense strand followed by a loop sequence (underlined, TCAAGAG) and the antisense strand. In the order listed, these three shRNAs are expressed from the RNA polymerase III promoters U6, H1, or 7SK, respectively, each being terminated by a stretch of six thymidines. A detailed description of the underlying AAV-based triple shRNA cloning and expression system will be reported elsewhere (unpublished data). AAV vector-containing cell supernatants were produced as described previously (61), by providing the rep and cap genes of AAV serotype 2, as well as adenoviral helper functions in trans.
To analyze CD317/tetherin anterograde transport in the context of the knockdown of the μ subunits of AP-1, HT1080 cells were cultured for 48 h in media with the addition of 15 μl of AAVs encoding AP-1 μ1A- and μ1B-specific shRNAs (sh AP-1) or YFP (control). A total of 2 × 105 cells were seeded on glass coverslips in six-well plates, cultured overnight, and subjected to plasmid microinjection and anterograde transport assay. To investigate the influence of the AP-1 complex on the virus production and recruitment of CD317/tetherin to virus assembly sites, Tzm-bl cells were seeded (105 cells per well in a 12 well-plate) and 5 μl of AAVs encoding AP-1 μ1A- and μ1B-specific shRNAs (sh AP-1) or YFP (control) were added. After 24 h, the cells were transfected with 0.65 μg each of pcHIV-1 ΔVpu and pcHIV-1 ΔVpu Gag-eGFP, as well as 0.4 μg of the Vpu encoding plasmid. AAVs were added again during transfection, and 5 h later T20 (0.225 mg/ml) was added to prevent syncytium formation. At 2 days posttransfection, the cells were subjected to RNA extraction, confocal microscopy analysis, and quantification of particle production.
Statistical analysis.
Statistical analysis of data sets was carried out using Microsoft Excel and GraphPad Prism. Statistical significance of parametrically or not normally distributed data sets was analyzed by using a Student t test or a Mann-Whitney U test, respectively. Unless specified otherwise, asterisks in the figures indicate the P value (*, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant).
RESULTS
Vpu determinants for interaction with AP-1 are required to block anterograde transport of CD317/tetherin.
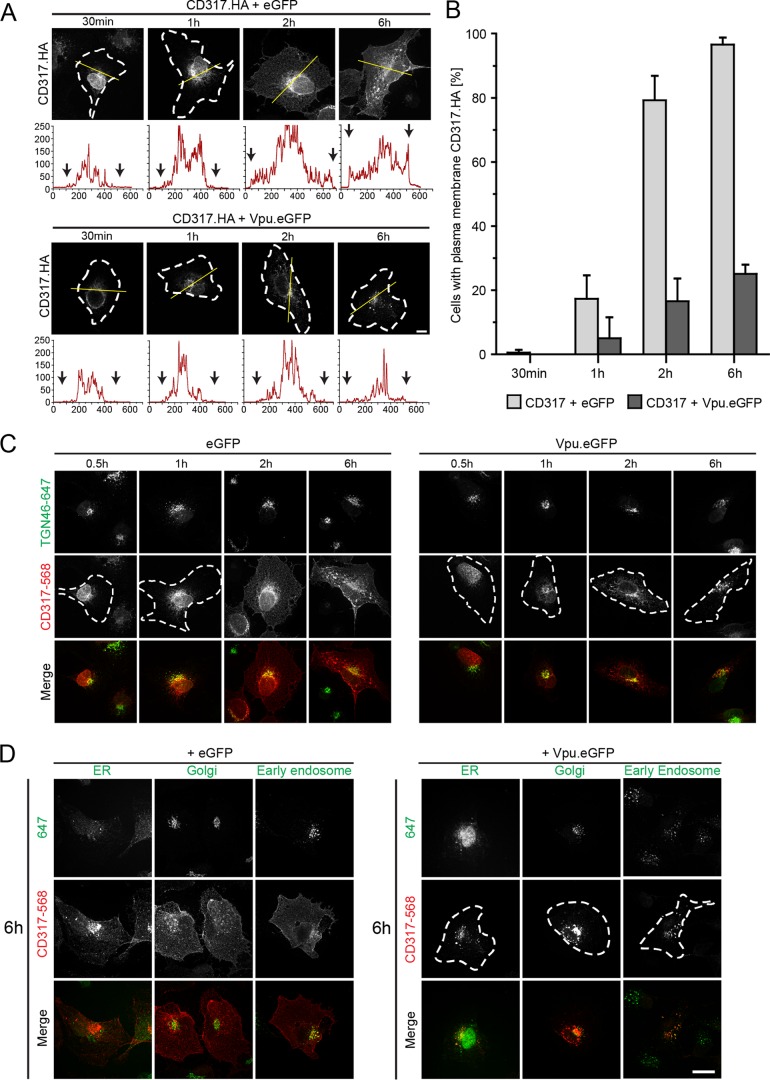
The goal of this study was to address which of the three proposed mechanisms contribute to the antagonism of HIV-1 Vpu of the particle release restriction imposed by CD317/tetherin. To this end, we characterized a panel of defined Vpu mutants in functional assays assessing effects of Vpu on CD317/tetherin recycling, anterograde transport, and localization to HIV-1 assembly sites. We first made use of our previously reported anterograde transport assay, in which the transport of newly synthesized protein to the PM is monitored over time after microinjection of an expression plasmid for CD317 with an internal HA tag (CD317.HA) into the nuclei of HT1080 cells (Fig. 1A) (40, 57). In eGFP-expressing control cells (Fig. 1A, upper panel), CD317.HA was first detected 30 min postmicroinjection (p.m.) in a perinuclear compartment. Increased protein expression and biosynthetic transport resulted in the appearance of CD317.HA at the PM at 2 h p.m. in 79.4% ± 7.6% of cells. At 6 h p.m., CD317.HA was detectable at the PM in virtually all cells analyzed (Fig. 1A and B). In contrast, coexpression of HIV-1 Vpu fused to eGFP (Vpu.eGFP) markedly reduced anterograde transport of CD317.HA to the PM (PM localization of CD317.HA detectable in 25.1% ± 2.9% of the cells at 6 h p.m.). Instead, CD317.HA accumulated in the perinuclear area where it colocalized with a marker of the trans-Golgi network (TGN) (40, 57) (Fig. 1C). Consistent with classical biosynthetic transport of transmembrane proteins, CD317/tetherin colocalized with markers of the endoplasmic reticulum and the Golgi apparatus before reaching the PM in GFP-expressing control cells but not in cells expressing Vpu.eGFP (Fig. 1D). Furthermore, disruption of the secretory pathway by brefeldin A resulted in a transport block similar to that induced by Vpu (data not shown).
FIG 1.
Vpu blocks anterograde transport of newly synthesized CD317/tetherin. (A) HT1080 cells were microinjected with expression plasmids for CD317.HA and eGFP or NL4-3 Vpu.eGFP, fixed at the indicated time points, and the subcellular localization of CD317.HA was analyzed. Shown are representative confocal micrographs. Scale bar, 10 μm. Where not visible by PM CD317.HA, cell boundaries are indicated by white dashed lines. The yellow line denotes the section represented below each image in the intensity plot profile (x axis, gray intensity × 1,000; y axis, distance in pixels). Black arrows indicate the cell limit. (B) Quantification of the experiment shown in panel A. Depicted is the mean of the percentage ± the standard deviations (SD) of cells with PM localization of CD317.HA from three independent experiments with over 70 cells analyzed per condition and experiment. (C and D) Time course of colocalization of newly synthesized CD317/tetherin. HT1080 cells were microinjected with expression plasmids for CD317.HA and eGFP or NL4-3 Vpu.eGFP, fixed at the indicated time points, and the subcellular localization of CD317.HA were analyzed using ER, Golgi, TGN, and early endosome subcellular markers. Shown are representative confocal micrographs. Scale bar, 10 μm. Where not visible by PM CD317.HA, cell boundaries are indicated by white dashed lines. (C) Time course of colocalization of newly synthesized CD317 with TGN46 in HT1080 cells expressing eGFP or NL4.3 Vpu.eGFP. Shown are representative confocal micrographs of cells stained for TGN46 and CD317 at 0.5, 1, 2, and 6 h p.m. (D) Representative confocal micrographs of newly synthesized CD317 with ER, Golgi, and early endosome markers in HT1080 cells expressing eGFP or NL4.3 Vpu.eGFP at 6 h p.m.
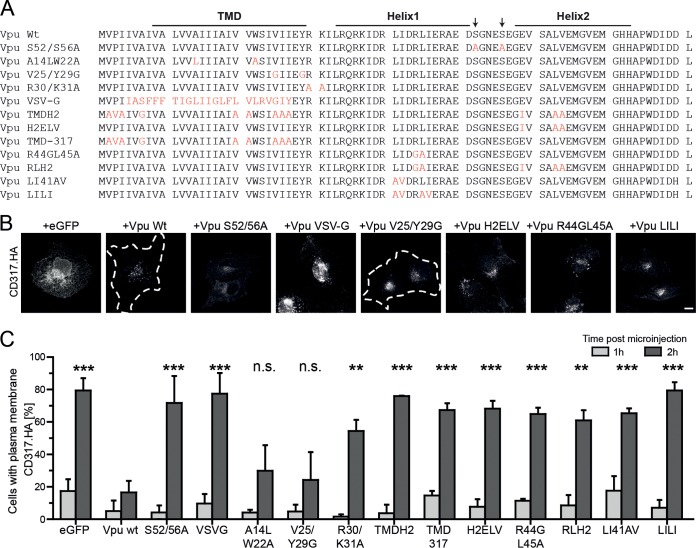
Next, we analyzed a series of Vpu mutants (Fig. 2A) for their ability to affect PM delivery of CD317.HA at 1 and 2 h p.m. (Fig. 2A to C). A Vpu mutant with pleiotropic defects in interactions with cellular ubiquitination and clathrin machineries due to disruption of the diserine motif (Vpu S52/56A) was used as a negative control. As expected (40, 57), Vpu S52/56A failed to block anterograde transport of CD317.HA. We next analyzed a set of Vpu mutants with defects in interaction with CD317/tetherin. Replacing the CD317/tetherin interacting TMD of Vpu with that of VSV-G (Vpu VSV-G) or mutating a series of residues that make physical contact to CD317/tetherin (31) (Vpu TMD-317) prevented Vpu from blocking CD317/tetherin PM delivery. In contrast, the A14LW22A mutation, which reduces but does not fully abrogate binding to CD317/tetherin (32, 62) was not sufficient to disrupt functional interactions, and the anterograde transport block was maintained. An association of Vpu with membrane microdomains (54) (impaired by mutant V25/Y29G) was dispensable for blocking CD317/tetherin PM delivery, and partial retargeting of Vpu away from the TGN (mutant R30/K31A) (63) resulted in intermediate blocking activity (Fig. 2C). A further series of mutants focused on determinants in Vpu recently described to mediate its interaction with AP-1 (51, 52). Mutations included residues required for binding AP-1 in Vpu helix 1 (Vpu R44GL45A, Vpu LI41AV, and Vpu LILI), the acidic dileucine motif in Vpu helix 2 (Vpu H2ELV), or combinations thereof (Vpu RLH2). All of these mutants were defective in blocking anterograde transport of newly synthesized CD317/tetherin, suggesting that all individual contacts of Vpu with AP-1 are critical for this activity. Together, these results suggest that interactions of Vpu with AP-1 at the level of the TGN are required for trapping the restriction factor en route to the PM.
FIG 2.
Molecular determinants in Vpu for the block of anterograde transport of newly synthesized CD317/tetherin. (A) Amino acid alignment of HIV-1 NL4.3 Vpu and the mutants used in this study. Mutated residues are shown in red. (B) Microphotographs of CD317.HA localization at 2 h p.m. with the indicated CD317.HA and Vpu.eGFP expression plasmids. (C) Quantification of the experiment from panel A. Depicted are the mean percentages ± the SD of cells with PM localization of CD317.HA from three independent experiments with over 70 cells analyzed per condition and experiment. Asterisks indicate the statistical significance of differences to Vpu wt for values at 2 h p.m.
Molecular determinants governing the block to CD317/tetherin recycling by Vpu.
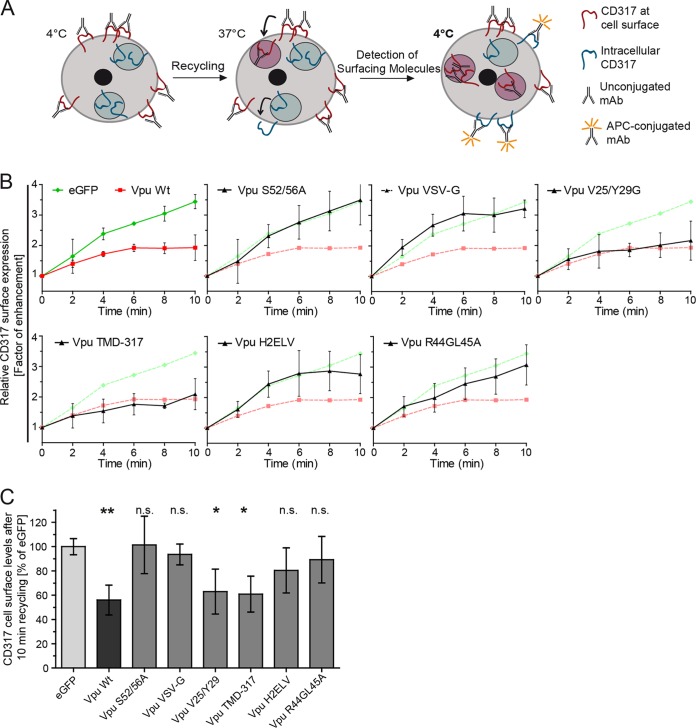
We next analyzed mutant Vpu proteins for their ability to affect the recycling of CD317/tetherin to the cell surface following internalization from the PM. To this end, we used our flow cytometry-based recycling assay in which the CD317/tetherin cell surface pool is saturated with unconjugated antibody in the cold, and the appearance of CD317/tetherin molecules not associated with antibodies at the cell surface was monitored over time after temperature shift (40) (Fig. 3A). Over the short observation period of this assay, inhibition of protein synthesis or anterograde transport does not reduce the resurfacing of CD317/tetherin molecules (40), indicating that exclusively recycling of previously internalized molecules is measured. Although CD317/tetherin efficiently recycled back to the cell surface in eGFP-expressing control cells, wt Vpu potently blocked this effect in a manner dependent on the diserine motif (as shown by mutant Vpu S52A/56A) (Fig. 3B and C). Similar to the results in the anterograde transport assay (Fig. 1), AP-1 interaction motifs were required for Vpu to block CD317/tetherin recycling, although the H2ELV and R44GL45A Vpu mutants retained some residual but not statistically significant blocking activity (Fig. 3B and C). Membrane microdomain association was dispensable for this effect (Vpu V25/Y29A). Differential results were obtained for alterations of the TMD of Vpu: (i) Vpu VSV-G, in which the TMD is replaced with that of VSV-G failed to inhibit CD317/tetherin recycling and, in contrast, (ii) Vpu TMD-317, in which contacts to the TMD of CD317/tetherin are mutated, retained significant ability to block recycling of CD317/tetherin. These findings suggest that similar to the anterograde transport block, AP-1 interaction motifs in Vpu are required for blocking CD317/tetherin recycling. However, based on differential requirements in the TMD in Vpu, mechanistic differences seem to exist between the Vpu-mediated blocks to CD317/tetherin recycling and anterograde transport.
FIG 3.
Molecular determinants in Vpu for blocking recycling of CD317/tetherin. (A) Schematic representation of the recycling assay (taken from Schmidt et al. [40]). See Materials and Methods for details. (B) Tzm-bl cells were transfected with the indicated eGFP or Vpu.eGFP expression plasmids. For clarity, results for each Vpu mutants are plotted (black) in a separate graph in comparison to the eGFP (green) and Vpu.eGFP (red) controls. Shown are relative CD317 surface levels over time as a factor of enhancement. Each point represents the mean ± the SD of three independent biological replicates. (C) Quantification of relative CD317 surface expression after 10 min of recycling (mean ± the SD of three independent experiments). Asterisks indicate the statistical significance of differences to the eGFP control.
AP-1 interaction sites in Vpu are required for antagonism and assembly site exclusion of CD317/tetherin.
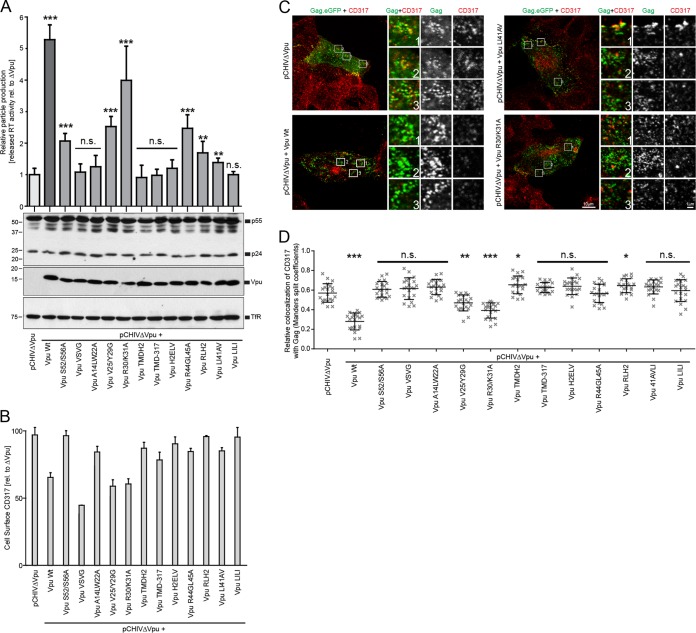
In order to assess the ability of our Vpu mutants to exclude CD317/tetherin from HIV assembly sites at the PM, Tzm-bl cells expressing endogenous CD317/tetherin were cotransfected with an equimolar mixture of pCHIVΔvpu and pCHIV.eGFPΔvpu, together with Vpu expression plasmids. Transfection with the pCHIV derivatives results in the assembly of HIV-1 budding sites detectable through Gag labeled with eGFP (64). In these experiments, coexpression of wt Vpu did not affect cell-associated levels of HIV-1 Gag but significantly increased particle release as judged by the quantification of reverse transcriptase (RT) activity in the cell culture supernatant. Vpu thus efficiently antagonized the CD317/tetherin mediated particle release restriction (Fig. 4A). The CD317/tetherin steady-state surface levels on these producer cells, as assessed by flow cytometry, were moderately reduced (Fig. 4B). Parallel confocal microscopy of particle-producing cells from the same experiment revealed a large number of Gag.eGFP punctae at the PM. In line with previous reports (29, 39), Gag punctae often colocalized with CD317/tetherin in the absence of Vpu, and this colocalization was markedly reduced when Vpu was expressed during particle production (Fig. 4C, left panels; see also Fig. 4D for quantification of the colocalization). This reduction of Gag-CD317/tetherin colocalization in the presence of Vpu can, in principle, result from the reduction of the overall density of the restriction factor at the PM and/or from specific exclusion of CD317/tetherin from viral assembly sites. Some Vpu mutants that do not reduce CD317/tetherin cell surface levels were shown to maintain full ability to displace the restriction factor from viral assembly sites, and this effect has thus been suggested as a correlate for antagonism of the particle release restriction (29, 39). The reduction of Gag-CD317/tetherin colocalization is therefore interpreted as a measure of the ability of Vpu to displace the restriction factor from HIV-1 assembly sites (29, 39).
FIG 4.
Molecular determinants in Vpu for antagonism of the CD317/tetherin release restriction and for exclusion of the restriction factor from PM sites of virus assembly. Tzm-bl cells were transfected with pcHIV-1 ΔVpu and pcHIV-1 ΔVpu Gag-eGFP, together with an empty control vector or the indicated Vpu expression plasmid. At 48 h posttransfection, the cells were analyzed for protein expression and colocalization of CD317/tetherin with Gag.eGFP, and the amounts of RT activity in the cell culture supernatant were quantified. (A) The top panel shows the quantification of RT activity in the cell culture supernatant relative to the pCHIVΔVpu control that was arbitrarily set to 1. Bars represent the means ± the SD from three independent biological replicates. Asterisks indicate the statistical significance of differences to the pCHIVΔVpu control. The lower panel shows Western blots of cell lysates using antibodies against p24CA, Vpu, and transferrin receptor (TfR). (B) Steady-state cell surface levels of CD317/tetherin on the virus-producing cells analyzed in panel A. Bars represent means ± the SD from three independent biological replicates and express cell surface levels relative to the pCHIVΔVpu control that was set to 100%. (C) Representative confocal micrographs of the PM area of virus producing cells. Scale bar, 10 μm. Shown are merged channels of Gag.eGFP and CD317.568 (left panels), as well as merged and individual channels of the zoomed areas (indicated by boxes; scale bar, 1 μm). (D) Relative colocalization values of CD317 with Gag expressed as the Mander's split coefficient. Values obtained from 20 cells per condition are displayed as individual data points, together with the median ± the SD. Asterisks indicate statistical differences to the pCHIVΔVpu control (*, P < 0.01; **, P < 0.001; ***, P < 0.0001).
Analyzing Vpu mutants revealed that Vpu S52/56A retained a moderate but statistically significantly ability to enhance particle release and failed to reduce colocalization of CD317/tetherin with assembly sites. Vpu mutants V25/Y29G and R30/K31A displayed moderate and more robust antagonistic activity, respectively, which was paralleled by intermediate and potent efficacies in eliminating CD317/tetherin from assembly sites. Importantly, mutations that affected Vpu's interaction with CD317/tetherin or with AP-1 failed to efficiently enhance particle release or to reduce Gag-CD317/tetherin colocalization (note that the differences of Vpu TMDH2 and R44GL45A in CD317-Gag colocalization are statistically significant compared to pCHIVΔvpu because even less colocalization was observed). Linear regression analysis between the ability of Vpu variants to enhance particle release and to exclude CD317/tetherin from viral assembly sites revealed a strong correlation (r2 = 0.7642; P < 0.0001). In contrast, the ability of Vpu to downregulate steady-state cell surface levels of CD317/tetherin did not correlate with Vpu antagonism of the particle release restriction (r2 = 0.1706; P = 0.1422; see, for example, Vpu VSV-G that downregulates CD317/tetherin levels efficiently but does not enhance particle release). Thus, Vpu determinants for interaction with CD317/tetherin as well as with AP-1 are critical for enhancing particle release and for displacement of the restriction factor from HIV budding sites, suggesting a mechanistic link between both activities.
Interference with AP-1 function and expression blocks Vpu activity.
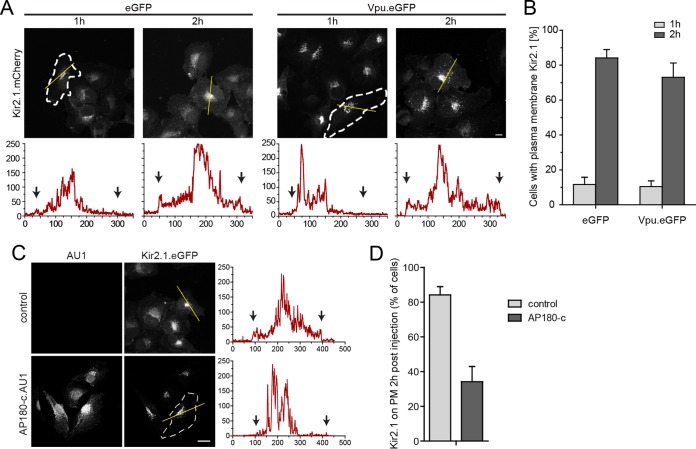
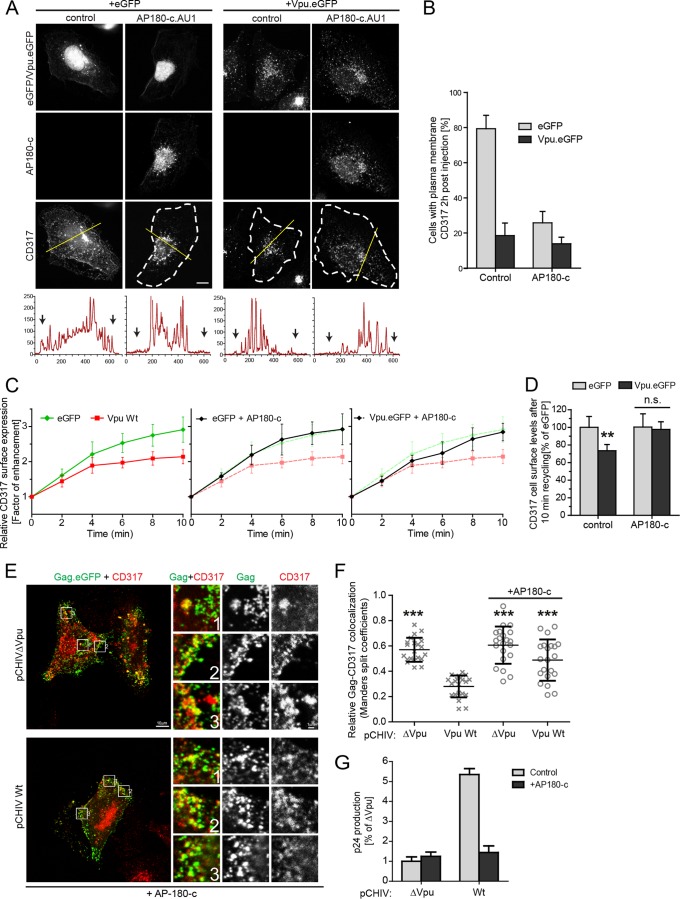
We next sought to determine directly which aspects of Vpu function AP-1 are involved. To this end, we first studied the anterograde transport of KIR2.1, a K+ channel known to be delivered to the PM in an AP-1-dependent manner (65) (Fig. 5). Newly synthesized KIR2.1 was rapidly transported to the PM within 2 h p.m., and coexpression of Vpu.eGFP had no significant impact on this anterograde transport process (Fig. 5A and B). Next, we analyzed the effect of AP180-c, a dominant-negative fragment of the endocytic adaptor AP180 that interferes with clathrin-membrane interactions (66) and thus affects both endocytosis and AP-1-dependent anterograde transport processes. In the context of Vpu-CD317/tetherin, AP180-c has been shown to prevent the reduction of steady-state CD317/tetherin cell surface levels (67) and counteract Vpu antagonism (55). However, it is still unknown which aspects of Vpu-mediated manipulation of intracellular CD317/tetherin transport are affected. In the anterograde transport assay, the expression of AU1-tagged AP180-c (AP180-c.AU1) significantly reduced PM delivery of KIR2.1 (Fig. 5C and D). These results demonstrate that Vpu affects PM delivery of specific cargo molecules and does not exert nonspecific effects on all AP-1 anterograde transport processes. Furthermore, they validate AP180-c as a tool for disruption of AP-1-dependent transport in our experimental system. Consistently, AP180-c.AU1 blocked anterograde transport of newly synthesized CD317.HA in GFP-expressing control cells in a manner indistinguishable from Vpu (Fig. 6A and B). The Vpu expression levels were unaffected by coexpression of AP180-c upon plasmid microinjection (compare the Vpu.eGFP signals in the two top right panels of Fig. 6A). No significant synergy of AP180-c and Vpu in blocking anterograde transport of CD317.HA was observed (Fig. 6A and B), suggesting that PM delivery of the restriction factor was already impaired with maximal efficacy by either AP180-c or Vpu alone. In contrast, AP180-c did not affect CD317/tetherin recycling in the absence of Vpu, suggesting that the restriction factor is recycled in an AP-1/clathrin-independent fashion (Fig. 6C and D). Of note, coexpression of AP180-c with Vpu almost completely blocked the ability of the viral protein to reduce CD317/tetherin recycling (Fig. 6C and D), indicating that the clathrin machinery is required for this Vpu activity. AP180-c also disrupted Vpu's ability to enhance HIV particle release and to displace CD317/tetherin from viral assembly sites (Fig. 6E to G) but did not affect these parameters in the absence of Vpu.
FIG 5.
Vpu does not exert unspecific effects on AP-1 mediated anterograde transport of transmembrane proteins. (A) HT1080 cells were microinjected with expression plasmids for Kir2.1.mCherry and eGFP or NL4-3 Vpu.eGFP, fixed at the indicated time points, and the subcellular localization of Kir2.1.mCherry was analyzed. Shown are representative confocal micrographs. Scale bar, 10 μm. Where not visible by PM Kir2.1.mCherry, cell boundaries are indicated by white dashed lines. The yellow line denotes the section represented below each image in the intensity plot profile (x axis, gray intensity × 1,000; y axis, distance in pixels). Black arrows indicate the cell limit. (B) Quantification of the experiment shown in panel A. Depicted are the mean percentages ± the SD of cells with PM localization of Kir2.1.mCherry from three independent experiments with >70 cells analyzed per condition and experiment. (C) HT1080 cells were microinjected with expression plasmids for Kir2.1.mCherry and pcDNA control or pcDNA AP180-c.AU1, fixed at 2 h p.m., and the subcellular localization of Kir2.1.mCherry was analyzed. Shown are representative confocal micrographs. Scale bar, 10 μm. Where not visible by PM Kir2.1.mCherry, the cell boundaries are indicated by white dashed lines. The yellow line denotes the section represented on the right of each image in the intensity plot profile (x axis, gray intensity × 1,000; y axis, distance in pixels). Black arrows indicate the cell limit. (D) Quantification of the experiment shown in panel C. Depicted are the mean percentages ± the SD of cells with PM localization of Kir2.1.mCherry from three independent experiments with >70 cells analyzed per condition and experiment.
FIG 6.
Effect of AP180-c on the ability of Vpu to alter CD317/tetherin trafficking and particle release restriction. (A) HT1080 cells were microinjected with expression plasmids for CD317.HA and eGFP or NL4-3 Vpu.eGFP, and with a control plasmid or an expression plasmid for AP180-c.AU1, fixed at 2 h p.m., and the subcellular localization of CD317.HA was analyzed. Shown are representative confocal micrographs. Scale bar, 10 μm. Where not visible by PM CD317.HA, the cell boundaries are indicated by white dashed lines. The yellow line denotes the section represented below each image in the intensity plot profile (x axis, gray intensity × 1,000; y axis, distance in pixels) where the black arrows indicate the cell limit. (B) Quantification of the cells shown in panel A with a detectable presence of CD317.HA on the PM. Depicted are the mean percentages ± the SD of cells with PM localization of CD317.HA from three independent experiments with >70 cells analyzed per condition and experiment. (C) Tzm-bl cells were transfected with plasmids expressing eGFP or Vpu.eGFP, together with an empty vector control or the AP180-c.AU1 expression plasmid. Shown are the relative CD317 surface levels over time as a factor of enhancement. Each point represents the mean ± the SD of three independent biological replicates. (D) Quantification of the relative CD317 surface expression after 10 min of recycling (mean ± the SD of three independent experiments). Asterisks indicate the statistical significance of differences compared to the corresponding eGFP control. (E to G) Tzm-bl cells were transfected with pcHIV ΔVpu and pcHIV ΔVpu Gag-eGFP, together with an empty control vector or the indicated Vpu expression plasmid, as well as the expression plasmid for AP180-c or the corresponding empty vector control. At 48 h posttransfection, the cells were analyzed for localization of CD317/tetherin to assembly sites, and the amounts of p24CA activity in the cell culture supernatant were quantified. (E) Representative confocal micrographs of the PM area of cells expressing AP180-c. Scale bar, 10 μm. Shown are merged channels of Gag.eGFP and CD317.568 (left panels), as well as merged and individual channels of the zoomed areas (indicated by boxes; scale bar, 1 μm). (F) Relative colocalization values of CD317 with Gag expressed as the Mander's split coefficient. Values obtained from 20 cells per condition are displayed as individual data points, together with the median ± the SD. Asterisks indicate the statistical significance of differences to wt Vpu. Values for controls are the same as in Fig. 3C. (G) Quantification of p24CA in the cell culture supernatant. Bars represent the mean p24CA amounts ± the SD from three independent biological replicates relative to the ΔVpu control without AP180-c, which was arbitrarily set to 1.
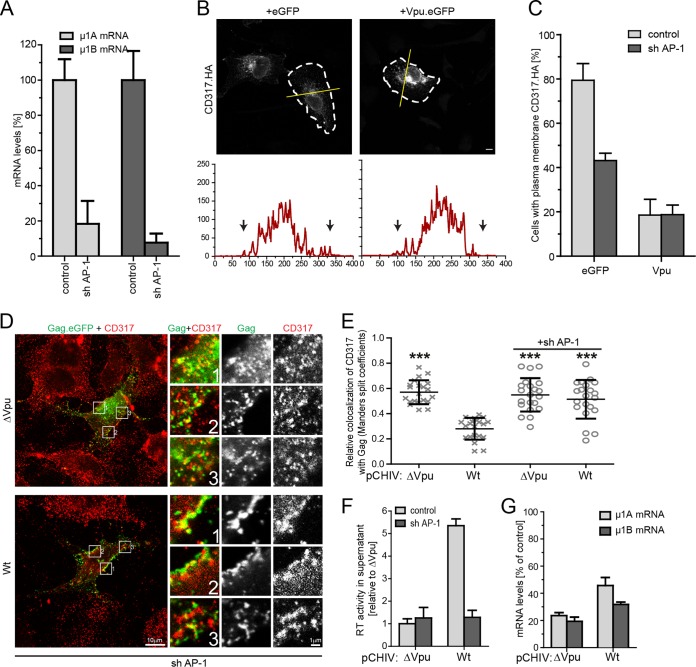
To address the role of endogenous AP-1 complexes in Vpu function, we wanted to silence their expression (Fig. 7). APs are heterotetrameric complexes comprising two large subunits that recruit accessory factors assisting in cargo sorting, vesicle scission, and uncoating (α or γ and β), a medium subunit required for binding of cargo (μ), and a small subunit (σ) (68). Functional knockdown of AP-1 complexes by targeting expression of individual subunits is complicated in some cell types by the expression of two μ subunit isoforms that assemble into AP-1 complexes with at least partially overlapping functions (69). We therefore aimed at the simultaneous silencing of both AP-1 μ isoforms and engineered a tricistronic RNA interference expression cassette encoding two shRNAs targeting AP-1 μ1A and one shRNA against AP-1 μ1B. Since the HT1080 cells used in our transport studies are difficult to transfect, the shRNA expression construct was delivered via an adeno-associated virus (AAV) vector (sh AP-1). Transduction with sh AP-1 AAV vector particles resulted in a robust reduction of AP-1 μ1A and μ1B mRNA levels (Fig. 7A). In line with the results obtained with AP180-c overexpression, anterograde transport of newly synthesized CD317/tetherin was reduced in GFP-expressing control cells, and the ability of Vpu to block this process was not affected (Fig. 7B and C). Analysis of CD317/tetherin upon sh AP-1 treatment introduced marked variations in basal rates of recycling and could therefore not be analyzed (data not shown). In the context of HIV-1 particle release, interference with expression of AP1 μ1A and μ1B prevented Vpu from excluding CD317/tetherin from viral budding sites (Fig. 7D and E) and disrupted its ability to enhance particle release (Fig. 7F and G). Together, these results demonstrate that AP-1 expression and function are critical for the ability of HIV-1 Vpu to alter CD317/tetherin trafficking and to antagonize the barrier to particle release imposed by the restriction factor.
FIG 7.
Effect of silencing AP-1 μ expression on the ability of Vpu to alter CD317/tetherin trafficking and particle release restriction. (A to C) HT1080 cells were exposed to the indicated AAVs for 3 days and then subjected to plasmid microinjection with expression plasmids for CD317.HA and eGFP or NL4-3 Vpu.eGFP, followed by anterograde transport analysis. (A) μ1A and μ1B mRNA levels at the time of microinjection. Depicted are mRNA levels relative to the AAV control (mean ± the SD from three independent measurements). (B) Subcellular localization of CD317.HA at 2 h p.m. Shown are representative confocal micrographs. Scale bar, 10 μm. Cell boundaries are indicated by white dashed lines; the yellow line denotes the section represented below each image in the intensity plot profile (x axis, gray intensity × 1,000; y axis, distance in pixel), where the black arrows indicate the cell limit. (C) Quantification of the experiment shown in panel B. Depicted are the mean percentages ± the SD of cells with PM localization of CD317.HA from three independent experiments with >70 cells analyzed per condition and experiment. (D to G) Tzm-bl cells were subjected to the indicated AAVs for 24 h and then transfected with pcHIV ΔVpu and pcHIV ΔVpu Gag-eGFP, together with an empty control or a Vpu expression plasmid. At 48 h posttransfection, cells were analyzed for subcellular localization of Gag and CD317/tetherin, AP-1 μ1A and μ1B mRNA levels and the amount of RT activity in the cell culture supernatant was quantified. (D) Representative confocal micrographs of the PM area of cells exposed to AAVsh1/2. Scale bar, 10 μm. Shown are merged channels of Gag.eGFP and CD317.568, as well as merged and individual channels of the zoomed areas (indicated by boxes; scale bar, 1 μm). (E) Relative colocalization values of CD317 with Gag expressed as the Mander's split coefficient. Values obtained from 20 cells per condition are displayed as individual data points, together with the median ± the SD. Asterisks indicate the statistical significance of differences to wt Vpu. Values for controls are the same as in Fig. 3C. (F) Quantification of RT activity in the cell culture supernatant. Bars represent the mean RT activity levels ± the SD from three independent biological replicates relative to the ΔVpu control in the presence of the AAV control that was arbitrarily set to 1. (G) μ1A and μ1B mRNA levels (mean ± the SD from three independent experiments relative to the AAV control that was arbitrarily set to 100%).
DISCUSSION
The aim of this study was to define the molecular determinants that mediate manipulations of CD317/tetherin trafficking by HIV-1 Vpu and to unravel their contribution to antagonism of the particle release restriction. To this end, we functionally characterized a panel of Vpu mutants with specific defects in interacting with CD317/tetherin or AP-1 for their ability to interfere with CD317/tetherin recycling, anterograde transport, and recruitment to viral assembly sites. These studies were complemented with interference with AP-1 function and expression in these experimental systems. The results reveal that Vpu antagonism of the CD317/tetherin release restriction is correlated to the ability to block recruitment of the restriction factor to viral assembly sites. Furthermore, our data suggest that interactions with AP-1 are essential for these activities.
With the block of recycling, anterograde transport, and virus assembly site recruitment, several, non mutually exclusive mechanisms have been suggested to mediate Vpu's antagonism of the CD317/tetherin particle release restriction. Our results reveal a strong correlation between the ability of Vpu to antagonize the CD317/tetherin restriction and to exclude the restriction factor form viral assembly sites (r2 = 0.7642; P < 0.0001) and therefore strongly support the notion that displacement of CD317/tetherin is an essential active principle of antagonism. Similar correlations with enhancement of particle release were not observed for inhibition of anterograde transport of CD317/tetherin (r2 = 0.0915; P < 0.2548) or CD317/tetherin recycling (r2 = 0.3046; P < 0.1561), indicating that these activities are not sufficient for antagonism. However, while we were able to assess colocalization with assembly sites and particle release within the same experiment, anterograde and recycling assays had to be carried out under different experimental conditions. We can therefore not exclude that, e.g., the requirements for Vpu activity differ in the various cell systems used herein or are affected by variations in per cell expression levels. Within these limitations, modifications of its TMD allowed us to identify Vpu variants that retained the ability to interfere with intracellular trafficking of CD317/tetherin but failed to antagonize the particle release restriction. Heterologous replacement of the Vpu TMD (Vpu VSV-G) disrupted Vpu's ability to block anterograde CD317/tetherin transport, as well as its recycling. In contrast, replacing interaction contacts in the TMD of CD317/tetherin resulted in Vpu mutants that retained the ability to block anterograde CD317/tetherin transport (Vpu A14LW22A) or recycling (Vpu TMD-317). This indicates that Vpu may indirectly affect these transport pathways rather than trapping CD317/tetherin molecules by physical interaction. In this scenario, the Vpu TMD would harbor determinants for manipulations of host cell trafficking that differ from the residues required for interactions with CD317/tetherin. However, Vpu TMD-317 did not block anterograde transport but interfered with recycling of CD317/tetherin. All results together could thus also reflect that interactions of Vpu with CD317/tetherin of different stringency are required to block CD317/tetherin anterograde transport or recycling, respectively. Conceivably, the residual interaction of Vpu A14LW22A with CD317/tetherin (62) is further reduced by mutating a series of interacting residues (Vpu TMD-317) and entirely suppressed in Vpu variants with heterologous TMDs (Vpu VSV-G). Precisely assessing the requirements of Vpu-CD317/tetherin interactions for the ability of Vpu to interfere with intracellular trafficking of the restriction factor will require the determination of binding affinities between both proteins at the subcellular sites at which Vpu acts.
Another important aspect of this study is the emerging role of the interaction with AP-1 for the biological activities of Vpu. Mutating residues known to mediate AP-1 interactions, as well as interfering with AP-1 function or expression, blocked the ability of Vpu to enhance HIV particle release and to exclude CD317/tetherin from viral assembly sites. These results confirm and extend the recently suggested role of AP-1 in CD317/tetherin antagonism by Vpu (51, 52) and imply that AP-1 interactions enable Vpu to exclude the restriction factor from virus assembly sites. How AP-1 governs the exclusion of the restriction factor from HIV assembly sites at the PM is currently unclear. Since alterations in transport of the restriction factor to and from the PM are dispensable for antagonism, assembly site exclusion of CD317/tetherin may result from lateral displacement at the PM as originally proposed (31). Considering that AP-1 interactions are required for a broad range of Vpu activities and recruitment of CD317/tetherin to virus assemble sites in the absence of Vpu occurs independently of AP-1, AP-1 likely acts on Vpu directly, e.g., by controlling its subcellular localization. This implies that CD317/tetherin antagonism by virus assembly site exclusion is mediated a PM-resident pool Vpu, an aspect that warrants further investigation. Alternatively, Vpu may act in concert with AP-1 at the level of TGN to alter the transport route and specific destination of CD317/tetherin. This may result in the use of a noncanonical transport pathway depositing the restriction factor in a PM environment with little connectivity to the microdomains that serve as platforms for virus assembly and budding. Finally, an as-yet-unidentified additional mechanism may contribute to CD317/tetherin antagonism since Vpu S52/56A retained residual activity in enhancing particle release despite its complete inability to exclude the restriction factor from viral assembly sites. Irrespective of the precise mechanisms at work, the results presented herein emphasize the compartmentalized localization of CD317/tetherin at the PM as key principle of its antiviral activity, as well as the central role of interactions with AP-1 for antagonism by Vpu.
ACKNOWLEDGMENTS
This study was supported by the Deutsche Forschungsgemeinschaft (grants FA 378/11-2 [O.T.F.], KE 742/4-2 [O.T.K.], and SFB1129 projects 2 [D.G.], 6 [B.M.], 8 [O.T.F.], and 14 [S.B.]). O.T.F., S.B., and D.G. are members of the Cluster of Excellence CellNetworks (EXC81).
REFERENCES
- 1.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med 2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 3.Shah AH, Sowrirajan B, Davis ZB, Ward JP, Campbell EM, Planelles V, Barker E. 2010. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe 8:397–409. doi: 10.1016/j.chom.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon V, Bloch N, Landau NR. 2015. Intrinsic host restrictions to HIV-1 and mechanisms of viral escape. Nat Immunol 16:546–553. doi: 10.1038/ni.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop KN, Holmes RK, Sheehy AM, Malim MH. 2004. APOBEC-mediated editing of viral RNA. Science 305:645. doi: 10.1126/science.1100658. [DOI] [PubMed] [Google Scholar]
- 6.Laguette N, Bregnard C, Hue P, Basbous J, Yatim A, Larroque M, Kirchhoff F, Constantinou A, Sobhian B, Benkirane M. 2014. Premature activation of the SLX4 complex by Vpr promotes G2/M arrest and escape from innate immune sensing. Cell 156:134–145. doi: 10.1016/j.cell.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Rosa A, Chande A, Ziglio S, De Sanctis V, Bertorelli R, Goh SL, McCauley SM, Nowosielska A, Antonarakis SE, Luban J, Santoni FA, Pizzato M. 2015. HIV-1 Nef promotes infection by excluding SERINC5 from virion incorporation. Nature 526:212–217. doi: 10.1038/nature15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usami Y, Wu Y, Gottlinger HG. 2015. SERINC3 and SERINC5 restrict HIV-1 infectivity and are counteracted by Nef. Nature 526:218–223. doi: 10.1038/nature15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willey RL, Maldarelli F, Martin MA, Strebel K. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol 66:7193–7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haller C, Muller B, Fritz JV, Lamas-Murua M, Stolp B, Pujol FM, Keppler OT, Fackler OT. 2014. HIV-1 Nef and Vpu are functionally redundant broad-spectrum modulators of cell surface receptors, including tetraspanins. J Virol 88:14241–14257. doi: 10.1128/JVI.02333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matheson NJ, Sumner J, Wals K, Rapiteanu R, Weekes MP, Vigan R, Weinelt J, Schindler M, Antrobus R, Costa AS, Frezza C, Clish CB, Neil SJ, Lehner PJ. 2015. Cell surface proteomic map of HIV infection reveals antagonism of amino acid metabolism by Vpu and Nef. Cell Host Microbe 18:409–423. doi: 10.1016/j.chom.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolduan S, Hubel P, Reif T, Lodermeyer V, Hohne K, Fritz JV, Sauter D, Kirchhoff F, Fackler OT, Schindler M, Schubert U. 2013. HIV-1 Vpu affects the anterograde transport and the glycosylation pattern of NTB-A. Virology 440:190–203. doi: 10.1016/j.virol.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Tibroni N, Sauter D, Galaski J, Miura T, Alter G, Mueller B, Haller C, Walker BD, Kirchhoff F, Brumme ZL, Ueno T, Fackler OT. 2015. Modest attenuation of HIV-1 Vpu alleles derived from elite controller plasma. PLoS One 10:e0120434. doi: 10.1371/journal.pone.0120434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galaski J, Ahmad F, Tibroni N, Pujol FM, Muller B, Schmidt RE, Fackler OT. 2016. Cell surface downregulation of NK cell ligands by patient-derived HIV-1 Vpu and Nef alleles. J Acquir Immune Defic Syndr 72:1–10. doi: 10.1097/QAI.0000000000000917. [DOI] [PubMed] [Google Scholar]
- 15.Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, Santoni A, Doria M. 2007. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol 88:242–250. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 16.Neil SJ, Eastman SW, Jouvenet N, Bieniasz PD. 2006. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog 2:e39. doi: 10.1371/journal.ppat.0020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 18.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galao RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. 2012. Innate sensing of HIV-1 assembly by tetherin induces NF-κB-dependent proinflammatory responses. Cell Host Microbe 12:633–644. doi: 10.1016/j.chom.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galao RP, Pickering S, Curnock R, Neil SJ. 2014. Retroviral retention activates a Syk-dependent HemITAM in human tetherin. Cell Host Microbe 16:291–303. doi: 10.1016/j.chom.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cocka LJ, Bates P. 2012. Identification of alternatively translated tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog 8:e1002931. doi: 10.1371/journal.ppat.1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. 2013. Stimulation of NF-κB activity by the HIV restriction factor BST2. J Virol 87:2046–2057. doi: 10.1128/JVI.02272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, Fierer DS, Simon V, Chen BK. 2014. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J Virol 88:6031–6046. doi: 10.1128/JVI.00449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pham TN, Lukhele S, Hajjar F, Routy JP, Cohen EA. 2014. HIV Nef and Vpu protect HIV-infected CD4+ T cells from antibody-mediated cell lysis through down-modulation of CD4 and BST2. Retrovirology 11:15. doi: 10.1186/1742-4690-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veillette M, Desormeaux A, Medjahed H, Gharsallah NE, Coutu M, Baalwa J, Guan Y, Lewis G, Ferrari G, Hahn BH, Haynes BF, Robinson JE, Kaufmann DE, Bonsignori M, Sodroski J, Finzi A. 2014. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arias JF, Heyer LN, von Bredow B, Weisgrau KL, Moldt B, Burton DR, Rakasz EG, Evans DT. 2014. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A 111:6425–6430. doi: 10.1073/pnas.1321507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G. 2003. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Caballero D, Zang T, Ebrahimi A, McNatt MW, Gregory DA, Johnson MC, Bieniasz PD. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatesh S, Bieniasz PD. 2013. Mechanism of HIV-1 virion entrapment by tetherin. PLoS Pathog 9:e1003483. doi: 10.1371/journal.ppat.1003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauter D. 2014. Counteraction of the multifunctional restriction factor tetherin. Front Microbiol 5:163. doi: 10.3389/fmicb.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNatt MW, Zang T, Bieniasz PD. 2013. Vpu binds directly to tetherin and displaces it from nascent virions. PLoS Pathog 9:e1003299. doi: 10.1371/journal.ppat.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigan R, Neil SJ. 2010. Determinants of tetherin antagonism in the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein. J Virol 84:12958–12970. doi: 10.1128/JVI.01699-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goffinet C, Allespach I, Homann S, Tervo HM, Habermann A, Rupp D, Oberbremer L, Kern C, Tibroni N, Welsch S, Krijnse-Locker J, Banting G, Krausslich HG, Fackler OT, Keppler OT. 2009. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 5:285–297. doi: 10.1016/j.chom.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Goffinet C, Homann S, Ambiel I, Tibroni N, Rupp D, Keppler OT, Fackler OT. 2010. Antagonism of CD317 restriction of human immunodeficiency virus type 1 (HIV-1) particle release and depletion of CD317 are separable activities of HIV-1 Vpu. J Virol 84:4089–4094. doi: 10.1128/JVI.01549-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyagi E, Andrew AJ, Kao S, Strebel K. 2009. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc Natl Acad Sci U S A 106:2868–2873. doi: 10.1073/pnas.0813223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tervo HM, Homann S, Ambiel I, Fritz JV, Fackler OT, Keppler OT. 2011. beta-TrCP is dispensable for Vpu's ability to overcome the CD317/Tetherin-imposed restriction to HIV-1 release. Retrovirology 8:9. doi: 10.1186/1742-4690-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy N, Pacini G, Berlioz-Torrent C, Janvier K. 2014. Mechanisms underlying HIV-1 Vpu-mediated viral egress. Front Microbiol 5:177. doi: 10.3389/fmicb.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jafari M, Guatelli J, Lewinski MK. 2014. Activities of transmitted/founder and chronic clade B HIV-1 Vpu and a C-terminal polymorphism specifically affecting virion release. J Virol 88:5062–5078. doi: 10.1128/JVI.03472-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewinski MK, Jafari M, Zhang H, Opella SJ, Guatelli J. 2015. Membrane anchoring by a C-terminal tryptophan enables HIV-1 Vpu to displace bone marrow stromal antigen 2 (BST2) from sites of viral assembly. J Biol Chem 290:10919–10933. doi: 10.1074/jbc.M114.630095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt S, Fritz JV, Bitzegeio J, Fackler OT, Keppler OT. 2011. HIV-1 Vpu blocks recycling and biosynthetic transport of the intrinsic immunity factor CD317/tetherin to overcome the virion release restriction. mBio 2:e00036-11. doi: 10.1128/mBio.00036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dube M, Paquay C, Roy BB, Bego MG, Mercier J, Cohen EA. 2011. HIV-1 Vpu antagonizes BST-2 by interfering mainly with the trafficking of newly synthesized BST-2 to the cell surface. Traffic 12:1714–1729. doi: 10.1111/j.1600-0854.2011.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauser H, Lopez LA, Yang SJ, Oldenburg JE, Exline CM, Guatelli JC, Cannon PM. 2010. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology 7:51. doi: 10.1186/1742-4690-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V. 2009. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its β-TrCP2-dependent degradation. PLoS Pathog 5:e1000574. doi: 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindler M, Rajan D, Banning C, Wimmer P, Koppensteiner H, Iwanski A, Specht A, Sauter D, Dobner T, Kirchhoff F. 2010. Vpu serine 52 dependent counteraction of tetherin is required for HIV-1 replication in macrophages, but not in ex vivo human lymphoid tissue. Retrovirology 7:1. doi: 10.1186/1742-4690-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douglas JL, Viswanathan K, McCarroll MN, Gustin JK, Fruh K, Moses AV. 2009. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a βTrCP-dependent mechanism. J Virol 83:7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell RS, Katsura C, Skasko MA, Fitzpatrick K, Lau D, Ruiz A, Stephens EB, Margottin-Goguet F, Benarous R, Guatelli JC. 2009. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog 5:e1000450. doi: 10.1371/journal.ppat.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. 1998. A novel human WD protein, h-β TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell 1:565–574. doi: 10.1016/S1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 48.Binette J, Dube M, Mercier J, Halawani D, Latterich M, Cohen EA. 2007. Requirements for the selective degradation of CD4 receptor molecules by the human immunodeficiency virus type 1 Vpu protein in the endoplasmic reticulum. Retrovirology 4:75. doi: 10.1186/1742-4690-4-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janvier K, Roy N, Berlioz-Torrent C. 2012. Role of the endosomal ESCRT machinery in HIV-1 Vpu-induced down-regulation of BST2/tetherin. Curr HIV Res 10:315–320. doi: 10.2174/157016212800792414. [DOI] [PubMed] [Google Scholar]
- 50.Schubert U, Schneider T, Henklein P, Hoffmann K, Berthold E, Hauser H, Pauli G, Porstmann T. 1992. Human immunodeficiency virus type 1-encoded Vpu protein is phosphorylated by casein kinase II. Eur J Biochem 204:875–883. doi: 10.1111/j.1432-1033.1992.tb16707.x. [DOI] [PubMed] [Google Scholar]
- 51.Kueck T, Foster TL, Weinelt J, Sumner JC, Pickering S, Neil SJ. 2015. Serine phosphorylation of HIV-1 Vpu and its binding to tetherin regulates interaction with clathrin adaptors. PLoS Pathog 11:e1005141. doi: 10.1371/journal.ppat.1005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia X, Weber E, Tokarev A, Lewinski M, Rizk M, Suarez M, Guatelli J, Xiong Y. 2014. Structural basis of HIV-1 Vpu-mediated BST2 antagonism via hijacking of the clathrin adaptor protein complex 1. Elife 3:e02362. doi: 10.7554/eLife.02362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magadan JG, Perez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS. 2010. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog 6:e1000869. doi: 10.1371/journal.ppat.1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fritz JV, Tibroni N, Keppler OT, Fackler OT. 2012. HIV-1 Vpu's lipid raft association is dispensable for counteraction of the particle release restriction imposed by CD317/Tetherin. Virology 424:33–44. doi: 10.1016/j.virol.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Kueck T, Neil SJ. 2012. A cytoplasmic tail determinant in HIV-1 Vpu mediates targeting of tetherin for endosomal degradation and counteracts interferon-induced restriction. PLoS Pathog 8:e1002609. doi: 10.1371/journal.ppat.1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lampe M, Briggs JA, Endress T, Glass B, Riegelsberger S, Krausslich HG, Lamb DC, Brauchle C, Muller B. 2007. Double-labeled HIV-1 particles for study of virus-cell interaction. Virology 360:92–104. doi: 10.1016/j.virol.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Kluge SF, Mack K, Iyer SS, Pujol FM, Heigele A, Learn GH, Usmani SM, Sauter D, Joas S, Hotter D, Bibollet-Ruche F, Plenderleith LJ, Peeters M, Geyer M, Sharp PM, Fackler OT, Hahn BH, Kirchhoff F. 2014. Nef proteins of epidemic HIV-1 group O strains antagonize human tetherin. Cell Host Microbe 16:639–650. doi: 10.1016/j.chom.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trotard M, Tsopoulidis N, Tibroni N, Willemsen J, Binder M, Ruggieri A, Fackler OT. 2015. Sensing of HIV-1 infection in Tzm-bl cells with reconstituted expression of STING. J Virol 90:2064–2076. doi: 10.1128/JVI.02966-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang F, Zang T, Wilson SJ, Johnson MC, Bieniasz PD. 2011. Clathrin facilitates the morphogenesis of retrovirus particles. PLoS Pathog 7:e1002119. doi: 10.1371/journal.ppat.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fackler OT, Moris A, Tibroni N, Giese SI, Glass B, Schwartz O, Krausslich HG. 2006. Functional characterization of HIV-1 Nef mutants in the context of viral infection. Virology 351:322–339. doi: 10.1016/j.virol.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 61.Senis E, Fatouros C, Grosse S, Wiedtke E, Niopek D, Mueller AK, Borner K, Grimm D. 2014. CRISPR/Cas9-mediated genome engineering: an adeno-associated viral (AAV) vector toolbox. Biotechnol J 9:1402–1412. doi: 10.1002/biot.201400046. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida T, Koyanagi Y, Strebel K. 2013. Functional antagonism of rhesus macaque and chimpanzee BST-2 by HIV-1 Vpu is mediated by cytoplasmic domain interactions. J Virol 87:13825–13836. doi: 10.1128/JVI.02567-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dube M, Roy BB, Guiot-Guillain P, Mercier J, Binette J, Leung G, Cohen EA. 2009. Suppression of tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J Virol 83:4574–4590. doi: 10.1128/JVI.01800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivanchenko S, Godinez WJ, Lampe M, Krausslich HG, Eils R, Rohr K, Brauchle C, Muller B, Lamb DC. 2009. Dynamics of HIV-1 assembly and release. PLoS Pathog 5:e1000652. doi: 10.1371/journal.ppat.1000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma D, Taneja TK, Hagen BM, Kim BY, Ortega B, Lederer WJ, Welling PA. 2011. Golgi export of the Kir2.1 channel is driven by a trafficking signal located within its tertiary structure. Cell 145:1102–1115. doi: 10.1016/j.cell.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- 67.Lau D, Kwan W, Guatelli J. 2011. Role of the endocytic pathway in the counteraction of BST-2 by human lentiviral pathogens. J Virol 85:9834–9846. doi: 10.1128/JVI.02633-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park SY, Guo X. 2014. Adaptor protein complexes and intracellular transport. Biosci Rep 34:e00123. doi: 10.1042/BSR20140069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo X, Mattera R, Ren X, Chen Y, Retamal C, Gonzalez A, Bonifacino JS. 2013. The adaptor protein-1 μ1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells. Dev Cell 27:353–366. doi: 10.1016/j.devcel.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]