ABSTRACT
Human immunodeficiency virus (HIV) replication is strongly dependent upon a programmed ribosomal frameshift. Here we investigate the relationships between the thermodynamic stability of the HIV type 1 (HIV-1) RNA frameshift site stem-loop, frameshift efficiency, and infectivity, using pseudotyped HIV-1 and HEK293T cells. The data reveal a strong correlation between frameshift efficiency and local, but not overall, RNA thermodynamic stability. Mutations that modestly increase the local stability of the frameshift site RNA stem-loop structure increase frameshift efficiency 2-fold to 3-fold in cells. Thus, frameshift efficiency is determined by the strength of the thermodynamic barrier encountered by the ribosome. These data agree with previous in vitro measurements, suggesting that there are no virus- or host-specific factors that modulate frameshifting. The data also indicate that there are no sequence-specific requirements for the frameshift site stem-loop. A linear correlation between Gag-polymerase (Gag-Pol) levels in cells and levels in virions supports the idea of a stochastic virion assembly mechanism. We further demonstrate that the surrounding genomic RNA secondary structure influences frameshift efficiency and that a mutation that commonly arises in response to protease inhibitor therapy creates a functional but inefficient secondary slippery site. Finally, HIV-1 mutants with enhanced frameshift efficiencies are significantly less infectious, suggesting that compounds that increase frameshift efficiency by as little as 2-fold may be effective at suppressing HIV-1 replication.
IMPORTANCE HIV, like many retroviruses, utilizes a −1 programmed ribosomal frameshift to generate viral enzymes in the form of a Gag-Pol polyprotein precursor. Thus, frameshifting is essential for viral replication. Here, we utilized a panel of mutant HIV strains to demonstrate that in cells, frameshifting efficiency is correlated with the stability of the local thermodynamic barrier to ribosomal translocation. Increasing the stability of the frameshift site RNA increases the frameshift efficiency 2-fold to 3-fold. Mutant viruses with increased frameshift efficiencies have significantly reduced infectivity. These data suggest that this effect might be exploited in the development of novel antiviral strategies.
INTRODUCTION
The genome of human immunodeficiency virus type 1 (HIV-1), like that of other retroviruses, has three genes that encode the structural proteins Gag, polymerase (Pol), and Env. The expression of the gag gene results in the synthesis of the Gag precursor protein, p55, which is subsequently processed by the viral protease to release the mature Gag proteins p17 (matrix protein), p24 (capsid), p15 (nucleocapsid), and p6 (late domain) and two so-called spacer peptides (SP) that flank p15, namely, p2 (SP1) and p1 (SP2), respectively (1). The synthesis of Gag precursor protein alone is sufficient for the assembly and release of noninfectious virus-like particles (VLPs) (2). The pol gene codes for the p160 polyprotein, which is subsequently processed into the mature enzymes reverse transcriptase (RT), integrase (IN), and protease (PR) (3). The pol gene is in the −1 reading frame relative to the gag gene. The synthesis of the Gag-Pol precursor results from a −1 programmed ribosomal frameshift (−1 PRF) that is stimulated by a conserved RNA structure and occurs at a frequency of ∼5% during translation, resulting in a Gag/Gag-Pol ratio of approximately 19:1 (4). Thus, frameshifting is essential for the expression of the viral enzymes and for maintaining the appropriate levels of Gag and Gag-Pol gene products required for viral assembly. Incorporation of Gag-Pol or its mature products into virions is required for infectivity, as the viral enzymes are necessary for the synthesis and integration of viral cDNA in infected cells (5).
The HIV-1 −1 PRF site is composed of a heptanucleotide slippery sequence (SS) (U UUU UUA [the spaces denote the 0 frame]) followed by a highly stable 11-bp RNA stem-loop that is 8 nucleotides downstream of the slippery sequence (6, 7) (Fig. 1). The slippery site allows near-cognate repairing of the A and P site tRNA anticodons in the −1 reading frame. The 8-nucleotide (nt) spacing between the slippery site and the stem-loop corresponds to the length of mRNA required to span the distance from the ribosomal A site to the mRNA entrance tunnel on the surface of the ribosome (6, 8). As the mRNA entrance tunnel is only wide enough to accommodate single-stranded mRNA, the first few base pairs of the stem-loop are expected to be engaged with ribosomal surface helicase-like proteins S3 and S9 (9, 10) at the time of frameshifting. Consistent with this, our previous experiments used a dual-luciferase assay in rabbit reticulocyte lysate to show that the local thermodynamic stability of the first 3 bp in the frameshift site stem-loop is highly correlated to frameshift efficiency in vitro (6). However, it is not known if a similar thermodynamic correlation exists within cells during viral replication.
FIG 1.
Representation of the wild-type HIV-1 frameshift site and the RNA constructs designed to discern the relative contributions of local stability (ΔGLocal), defined as the predicted free energy of the first 3 bp (including the adjacent stacking interaction with the nearest neighboring pair), and overall RNA stem-loop stability (ΔG) to HIV-1 frameshift efficiency.
The −1 PRF site of HIV-1 group M is contained within a larger 3-helix-junction secondary structure, which may serve to stall approaching ribosomes (11). Stalled ribosomes can lead to ribosome stacking (12), which may decrease frameshift efficiency if the leading ribosome unwinds the frameshift site RNA structure for the trailing ribosome to pass through unimpeded. Conversely, if every ribosome encounters the folded frameshift site stem-loop, decreased ribosome density may increase the relative frameshift efficiency. Consistent with this idea, Brakier-Gingras and coworkers found that decreased translation initiation of HIV-1 mRNA resulted in increased frameshifting (13). Additionally, we found that disruption of the genomic secondary structure by mutagenesis led to increased frameshifting in vitro (6, 14). SHAPE (selective 2-hydroxyl acylation analyzed by primer extension) data indicate that the genomic secondary structure is remodeled during translation, as regions of the RNA are unfolded and sequestered by the ribosome (14). The role of the genomic secondary structure surrounding the HIV frameshift site has not been investigated in living cells.
The HIV frameshift site genomic secondary structure contains a UUUUCUU sequence (nucleotides 1676 to 1682). In viruses resistant to protease inhibitors, a C1680-to-U mutation is frequently found in this region, which creates a UUUUUUU sequence that resembles the conserved UUUUUUA slippery sequence (15). This site could act as a secondary slippery sequence that increases the overall amount of frameshifting, thereby increasing the relative amount of protease in order to compensate for the reduction in protease activity in the presence of inhibitor (16). At the protein level, this causes a Leu-to-Phe mutation at the P1-p6 cleavage site that may affect cleavage efficiency and viral maturation. Previous studies investigating the effect of the C1680U mutation on frameshifting utilized a truncated RNA construct that did not contain the full frameshift site secondary structure (16, 17). In vitro, the full secondary structure is required in order to stimulate frameshifting at the C1680U secondary slippery site (14). Using a dual-luciferase assay in rabbit reticulocyte lysate, we found that the C1680U mutation creates a functional slippery sequence that can be detected when the primary slippery site is knocked out by mutagenesis (14).
Alteration of the Gag/Gag-Pol ratio from 20:1 to 20:21 has been achieved by cotransfection of an in-frame Gag-Pol expression vector, and the resulting increase in the level of Gag-Pol reduces the infectivity of progeny viruses and hinders the formation of stable virion RNA dimers through an unknown mechanism (18). Thus, the maintenance of normal Gag/Gag-Pol ratios is important for HIV-1 replication. Although both the proteolytic processing of HIV-1 proteins and a normal intracellular Gag/Gag-Pol ratio are required for RNA dimerization, the formation of stable RNA dimers is not essential for the packaging of HIV-1 genomic RNA (18). The importance of maintaining Gag/Gag-Pol levels for viral infectivity has encouraged the exploration of various approaches attempting to discover small molecules that could modulate HIV-1 frameshift efficiency (19–25).
MATERIALS AND METHODS
Plasmid construction.
Gene block DNAs (Integrated DNA Technologies [IDT], Inc.) of the different mutants were amplified by PCR and subcloned into pGEM-T Easy vector (Promega). The clones were grown using a dam-negative/dcm-negative (dam−/dcm−) competent Escherichia coli strain to obtain unmethylated DNA and were then cloned into the pHIV NL4-3 ΔEnv, ΔVPR, ΔNef, green fluorescent protein-positive (GFP+) provirus plasmid (26) between the ApaI and BclI restrictions sites. The resulting plasmid DNA (JB474 wild type [WT], M1 to M11, -SS, and -SS2) was purified from cell cultures (Qiagen), and the sequences of all constructs were verified (University of Wisconsin Biotechnology Center).
Cell culture, transfections, and Western blot analyses.
HEK293T cells were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% l-glutamine. Subconfluent monolayers were plated in six-well tissue culture-treated dishes prior to transfection with 2 μg of plasmids encoding each mutant version of HIV NL4-3 GFP reporter using polyethylenimine (PEI). At 24 h after transfection, the medium was replaced with fresh medium. When the goal of the assay was to measure frameshifting efficiency in the absence of proteolytic processing, the medium was supplemented with a 10 μM concentration of the protease inhibitor saquinavir. At 48 h after the transfection, the cells and supernatants were harvested as previously described (27). Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate), and filtered virions were pelleted through a 20% (wt/vol) sucrose cushion and lysed. Total protein concentrations from the cell lysates and purified virions were measured by the use of a bicinchoninic acid (BCA) protein assay kit (Pierce), and the same amounts of proteins were used for SDS-PAGE. SDS-PAGE was performed according to the method of Laemmli (28) on a 4% to 15% polyacrylamide gel, and the proteins were transferred into a nitrocellulose membrane. The membranes were incubated with relevant antisera as follows: a 1:1,000 dilution of the monoclonal anti-HIV-1 p24 antibody (derived from hybridoma 183-H12-5C) (29–31) obtained from the NIH AIDS Research and Reference Reagent Program (Bethesda, MD), a 1:500 dilution of rabbit polyclonal anti-HIV1 p15 antibody (Ab66951; Abcam), a 1:500 dilution of polyclonal anti-RT antibody (Ab63911; Abcam), a 1:2,500 dilution of rabbit anti-GFP antibody (AB10145; Millipore), and a 1:3,000 dilution of anti-heat shock protein 90 (HSP90) antibody (H-114 [catalog no. sc-7947]; Santa Cruz Biotechnology). The gene encoding HSP90 is a housekeeping gene and was detected to normalize band density values to total cell protein values. Secondary IRdye680- or IRdye800-conjugated antibodies (Li-Cor Biosciences) were used for quantitative immunoblotting with an Odyssey infrared scanner (Li-Cor Biosciences). All blot experiments were performed in triplicate.
Immunofluorescence.
Subconfluent monolayers of HEK293T cells cultured in 24-well glass-bottom dishes (Mattek Corporation, Ashland, MA, USA) were transfected with 1 μg of the different mutants cloned into pHIV NL4-3 plasmid using PEI. At 48 h posttransfection, cells were fixed using 4% paraformaldehyde, stained with DAPI (4′,6-diamidino-2-phenylindole), permeabilized with 0.2% Triton X-100 solution, and blocked in NGB buffer (50 mM NH4Cl, 1% goat serum, 1% bovine serum albumin) for 1 h. Gag was detected using mouse monoclonal anti-capsid (p24 CA) antiserum (hybridoma 183-H12-5C) (diluted 1:500 in NGB buffer), rabbit polyclonal anti-matrix (p17 MA) serum (UP595) (diluted 1:500 in NGB buffer) (32), secondary goat anti-mouse antibody conjugated to Alexa Fluor 546, and anti-rabbit antibody conjugated to Alexa Fluor 647 (Invitrogen). Imaging was performed on a Nikon Ti-Eclipse inverted wide-field microscope (Nikon Corporation, Melville, NY, USA) using a 100× Plan Apo objective lens (numerical aperture [NA], 1.45). Images were acquired using an Orca-Flash4.0 complementary-metal-oxide-semiconductor (CMOS) camera (Hamamatsu Photonics, Skokie, IL, USA) and Nikon NIS Elements software. Images were acquired using the following excitation/emission filter sets (nanometer ranges): 405/450 (DAPI), 490/525 (GFP), 555/605 (Alexa Fluor 546), and 645/705 (Alexa Fluor 647). Images were processed using FIJI/ImageJ2. For quantification, cells assessed in three independent experiments using ≥100 cells per condition (WT and M1 to M6) were scored for the presence of Gag (p24) immunofluorescence localized at the plasma membrane.
Single-cycle infectivity assays.
Subconfluent monolayers of HEK293T cells cultured in six-well plates were cotransfected with 2 μg of the different mutants cloned into pHIV NL4-3 plasmid and 0.2 μg vesicular stomatitis virus G (VSV-G) protein expression vector using PEI. At 48 h later, supernatants were harvested and the virus-like particles (VLPs) were purified through a 20% (wt/vol) sucrose cushion at 21,000 × g for 2 h at 4°C. Viral pellets were resuspended into 50 μl of phosphate-buffered saline solution. Subconfluent monolayers of HEK293T cells seeded in 35-mm cultures were infected using 10 μl of the virion resuspension. Infectivity assays were performed by two different methods: Western blotting and calculation of the relative percentages of infected HEK293T cells expressing GFP. For the Western blot analyses, the cells were harvested 48 h after the infection and Western blot analyses were carried out as described above. The membranes were incubated with a 1:2,500 dilution of rabbit anti-GFP antibody (AB10145; Millipore) and a 1:3,000 dilution of anti-heat shock protein 90 (HSP90) antibody (H-114 [catalog no. sc-7947]; Santa Cruz Biotechnology). HSP90 was detected to normalize band density values. To calculate the percentage of GFP-positive 293T cells, the cells were fixed at 48 h postinfection using 4% paraformaldehyde–phosphate-buffered saline (PBS) for 15 min at room temperature. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole), and coverslips were mounted using Mowiol (Sigma-Aldrich). Imaging experiments were performed using a Nikon Ti-Eclipse inverted wide-field microscope (Nikon Corp.) and a 10× objective lens (NA, 0.30), as previously described (33). Images were collected using a CoolSnap HQ camera (Photometrics) and Nikon NIS Elements software (v4.00.03). Images were acquired using differential interference contrast and the following fluorescence excitation/emission (nanometer ranges) filter sets: 325 to 375/435 to 485 (DAPI) and 490 to 520/520 to 550 (yellow fluorescent protein [YFP]). In each experiment, total cells and infected cells for 10 distinct fields of view were counted by two independent observers. Each mutant was assayed four times. GFP levels were also quantified by Western blot analysis.
Electron microscopy.
HEK293T cells were cultured in six-well dishes and transfected as described above. At 48 h posttransfection, culture supernatants were collected, filtered, and purified through a 20% sucrose cushion. Virion pellets were fixed in a solution of 2.5% glutaraldehyde–2.0% paraformaldehyde–0.1 M sodium phosphate buffer (PBS) (pH 7.4) for ∼2 h at room temperature. Samples were rinsed five times for 5 min each time in 0.1 M PBS. Rinsed pellets were then postfixed in 1% osmium tetroxide (OsO4)–1% potassium ferrocyanide–PBS for 1 h at room temperature. Following OsO4 postfixation, the samples were rinsed in PBS as described above followed by three 5-min rinses performed with distilled water to clear phosphates and then embedded using increasing concentrations (10 ml of components A and M, 10 ml of component B, 300 μl of component C, and 100 μl of component D) of Durcupan ACM resin (Fluka AG, Switzerland) at 60°C. Pellets were sectioned using a Leica electron microscopy (EM) UC6 ultramicrotome with 100-nm sections collected on 300-mesh Cu thin-bar grids and were contrasted using Reynolds lead citrate–8% uranyl acetate–50% ethyl alcohol (EtOH). The sections were observed with a Philips CM120 transmission electron microscope, and images were documented with a MegaView III (Olympus-SIS, Lakewood, CO) side-mounted digital camera.
Statistical analysis.
Data are presented as means ± standard errors of the means. Statistical significance was determined using one-way analysis of variance (ANOVA) followed by a Newman-Keuls test for multiple comparisons (GraphPad Prism Program v5.0). Differences were classified as significant for P values of <0.05 (#) and <0.001 (*).
RESULTS
Correlation between local thermodynamic stability of the stem-loop and frameshift efficiency in cells.
The sequence of the frameshift site stem-loop was modified in order to dissect the relative contributions of local and overall RNA thermodynamic stability to HIV-1 frameshift efficiency in HEK293T cells (Fig. 1). It is not possible to generate the range of thermodynamic stabilities needed to systematically test the correlation between RNA stability and frameshift efficiency using silent mutations. Therefore, the mutations shown in Fig. 1 also result in amino acid substitutions in the p1 spacer region (see Fig. S1 in the supplemental material). In order to measure frameshift efficiency, the highly effective protease inhibitor saquinavir (34) was used to inhibit proteolytic processing and allow the accurate quantification of Gag-Pol/Gag ratios. We observed that increased local stability of the first few base pairs results in significant increases in the frameshift efficiency (Fig. 2A and B; M1 to M3). No significant changes were observed for the mutants with lower local stability of the first 3 bp (Fig. 2A and B; M6, M7, and M8) or for the mutants with decreased overall stability of the frameshift site stem-loop (Fig. 2A and B; M5). Frameshift efficiency was plotted as a function of overall stability and local (3-bp) stability using previously described free energy values (6). The data from the mutants showed a modest correlation as a function of overall RNA stability (R2 = 0.67); however, we observed a strong correlation (R2 = 0.95) between frameshifting and the thermodynamic stability of the first 3 bp (Fig. 2E and F). These results are highly similar to previously described in vitro data (6). As expected, no frameshifting was detected when the slippery site was mutated to a nonslippery sequence (Fig. 2A and B).
FIG 2.
Effects of frameshift site mutations on Gag-Pol and Gag levels in cells and in virus-like particles (VLPs). (A and C) HEK293T cells were transfected with plasmids encoding the indicated mutations and cultured in the presence of saquinavir. Cell lysates (A) and virus-like particles (C) were harvested and measured using Western blots probed with a monoclonal anti-p24 antibody as described in Materials and Methods. (B and D) The ratio of Gag-Pol to Gag was used to calculate frameshift efficiencies in cells (B) and to quantify the Gag-Pol/Gag ratio in VLPs (D). *, P < 0.001; #, P < 0.05 (compared with WT values). Horizontal dashed lines represent the median value for the WT. (E) Overall thermodynamic stability versus frameshift efficiency. Data were fitted to a one-phase exponential decay function, Y = 0.045 × e−0.2X + 6.7. (F) Local stability of the first 3 bp versus the frameshift. Data were fitted to a one-phase exponential decay function, Y = 0.004 × e−0.8X + 7.8. (G) Correlation between Gag-Pol and Gag levels observed in cell lysates and purified VLPs.
In purified VLPs from our panel of mutants, we observed a pattern of Gag-Pol/Gag ratios that mirrored the data obtained from cell lysates (Fig. 2A to D). We noted that the Gag-Pol levels in purified HIV-1 VLPs were higher than in cells (∼20% versus 7% for the WT), suggesting that molecular interactions assist in recruiting Gag-Pol into virions. However, the strong linear correlation (R2 = 0.93) between Gag-Pol/Gag ratios in cell lysates and VLPs (Fig. 2G) demonstrates that intracellular Gag-Pol concentrations determine the relative levels of Gag-Pol in virions. This observation is consistent with a stochastic mechanism regulating the relative rate of Gag-Pol incorporation, similar to that recently reported for the gammaretrovirus Moloney murine leukemia virus (35). Similar results were obtained when no protease inhibitor was added to the media (data not shown).
Influence of the frameshift site on genomic secondary structure.
We also studied the influence of the HIV-1 genomic secondary structure surrounding the frameshift site. A secondary structure model derived from SHAPE data was derived previously (11) (Fig. 3); more recently, an alternative secondary structure was proposed for this region of the RNA (36). The two secondary structures contained the highly stable stem-loop (Fig. 1) and “anchoring helix” (Fig. 3) but mainly differed in whether or not the slippery sequence was base paired. The weak thermodynamic stability of the slippery sequence helix is consistent with formamide titration data (14). Previously, we showed that the anchoring helix modulates frameshifting in vitro, with disruption of this helix causing an increase in frameshifting (6, 14). Analysis of cell lysate shows that disruption of the anchoring helix (mutant M9) significantly increased the frameshift efficiency in cells (Fig. 3A to C). In purified VLPs, the M9 mutant on average also incorporated more Gag-Pol, although the effect was not statistically significant at a P value of <0.05 (Fig. 3D and E).
FIG 3.
Effects of mutations in the anchoring helix on HIV-1 frameshifting and Gag/Gag-Pol levels in VLPs. (A) The wild-type HIV-1 secondary structure and mutations in M9 (bold) that disrupt the anchoring helix. (B to E) The ratio of Gag-Pol/Gag was determined as described in the Fig. 2 legend. #, P < 0.05 (compared with WT values).
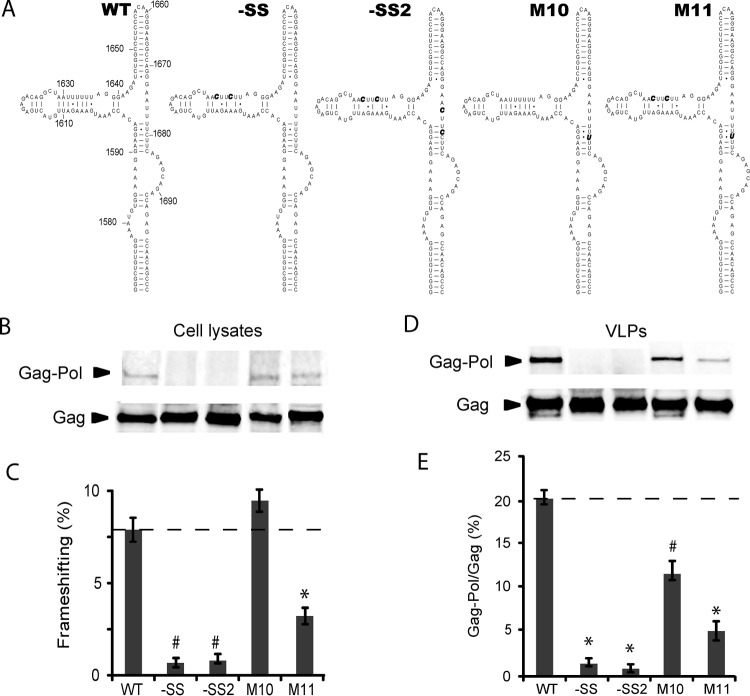
We next explored whether the C1680U mutation associated with patients on protease inhibitor therapy (15, 16) could function as a secondary slippery site in cells. When the primary slippery sequence was inactivated by double mutation (-SS), the frameshift efficiency decreased to near-background levels (Fig. 4). The same was true when both the primary and potential secondary slippery sites (-SS2) were knocked out (Fig. 4). The C1680U mutant (M10) produced slightly higher levels of frameshifting in cells, although the effect was not statistically significant. The slight increase in frameshifting seen with C1680U suggests that the secondary slippery site is not as efficient as the primary slippery site. Additionally, the M10 mutant had lower overall Gag-Pol levels in VLPs (see Discussion). Most notably, when the primary slippery site was knocked out, the C1680U mutant (M11) displayed frameshifting levels that were clearly enhanced relative to those seen with the -SS slippery site control mutant but not as high as those seen with the WT (Fig. 4). We conclude that the C1680U mutation does indeed induce frameshifting at the secondary site, albeit less efficiently than the primary frameshift site.
FIG 4.
Analysis of a putative secondary slippery site. (A) Secondary structures of RNAs and mutations (bold). -SS contains a double U-to-C mutation to knock out the primary slippery site. -SS2 also includes additional double U-to-C mutations to disrupt the putative secondary slippery site. M10 is a single C1680U mutation, while M11 has a knocked-out primary slippery site and a C1680U mutation. (B to E) Determination of frameshift efficiencies for these mutants performed as described for Fig. 2A. *, P < 0.001; #, P < 0.05 (compared with WT values).
Effects of altered Gag-Pol/Gag ratios on cell lysate and VLP protein processing.
The expression of the gag gene resulted in the synthesis of a precursor Gag protein, p55, which was subsequently processed by the viral protease into the mature Gag proteins p17 (matrix), p24 (capsid), p7 (nucleocapsid), and p6 (late domain) (Fig. 5). Since the expression of protease is dependent upon frameshifting, we wished to determine if the altered ratios of Gag-Pol/Gag induced by our various mutations influenced the proteolytic processing of viral proteins. We used Western blotting with antibodies against p24 or p15 nucleocapsid-p6 precursor to monitor protein processing 48 h after the transfection of HEK293T cells, in the absence of saquinavir, for both cell lysates and purified VLPs. The p15 precursor protein was detectable in purified VLPs but not in cell lysates. Protein levels were normalized to p55, p41, and p24 combined in order to detect possible differences in p24 levels.
FIG 5.
(A) Effects of frameshifting mutants on Gag/Gag-Pol processing. (B and C) Gag processing was evaluated by Western blot analysis for cell lysates (B) and purified VLPs (C). Normalization was carried out using the total amount of p55, p41, and p24 for each mutant relative to the WT. Data shown represent averages of results from 4 individual experiments. *, P < 0.001; #, P < 0.05 (compared with WT values).
The mutants M4, M5, M6, and M7 all exhibited WT levels of frameshifting, and no significant differences in proteolytic processing patterns were observed for these mutants in cell lysates and purified VLPs (Fig. 5A and B). The mutants with increased frameshift efficiency (M1, M2, M3, and M9) also did not show significant differences in Gag processing in cells, with the exception of a significantly increased amount of p24 for M1, perhaps because this mutant had the highest Gag-Pol levels (and therefore the highest level of protease expression) (Fig. 5A). In the VLPs, the M1, M2, and M3 mutants had decreased levels of p55 precursor and corresponding increases in p24 abundance. These data are consistent with higher levels of proteolytic processing for mutants M1 to M3 with increased frameshifting.
As expected, the mutants that cannot express Gag-Pol due to a knocked-out primary slippery site sequence (-SS) and alternate slippery sequence (-SS2) showed an increase in relative amounts of p55 precursor in both cell lysates and VLPs, while p24 levels were nearly undetectable (Fig. 5). Interesting expression and processing differences were observed in VLPs for the M10 and M11 mutants with the alternate slippery site mutation (Fig. 5C). M10 exhibited decreased p55 and increased p24 levels, consistent with an increase in proteolytic processing. In contrast, M11 showed the inverse pattern—increased p55 and decreased p24 levels (Fig. 5C). These data are consistent with the significantly lower levels of Gag-Pol expression for M11 due to knockout of the primary slippery site (Fig. 4).
Assessment of the effect of increased frameshift efficiency on viral infectivity.
A single-cycle infectivity assay was used to assess the effect of the frameshift site mutations on viral infectivity. The viral Nef gene was replaced by GFP to enable quantification of viral gene expression in infected cells using fluorescence microscopy. Representative images of the assay are shown in Fig. 6A. For each mutant, the top panel shows the nucleus stained with DAPI (4′,6-diamidino-2-phenylindole) to indicate cell density and the bottom panel shows GFP fluorescence at 48 h postinfection. Mutants M1 to M3, which had significantly higher frameshift efficiencies than the WT, showed large decreases in relative infectivity (Fig. 6B). We note that these RNA mutations alter the amino acid sequence in the p7-p1-p6 region (predominately p1) (see Fig. S1 in the supplemental material). The p1 peptide sequence is thought to be nonessential as long as it is proteolytically cleaved, because the majority of it can be deleted without significantly impacting infectivity (37). The mutations also alter the sequence of p6* in Gag-Pol, which overlaps p1-p6 and is also widely dispensable for infectivity (38). The M4 and M5 mutants have altered p1 peptide sequences and were nearly as infectious as the WT (Fig. 6; see also Fig. S1 in the supplemental material). In contrast, the M6 and M7 mutants exhibited normal levels of frameshifting (Fig. 2) but were poorly infectious (Fig. 6), perhaps because these mutants have a glycine-to-tryptophan mutation directly adjacent to the p1-p6 cleavage site that may interfere with cleavage (see Fig. S1) (37). We were unable to address this hypothesis because the p1-p6 cleavage event could not be resolved in our Western blot analyses.
FIG 6.
Viral infectivity as measured by a single-cycle infectivity assay. (A) Representative images of the infectivity assay for the WT and each mutant. (B) Viral GFP expression was quantified and normalized relative to the WT virus. Two different negative controls were used: WT-VSV (cells transfected only with the WT vector and without VSV-G protein expression vector) and neg (no transfected cells). Four single-cycle infectivity assays were carried out. *, P < 0.001; #, P < 0.05 (compared with WT values).
The M8 mutant was 10-fold less infectious than the WT (Fig. 6). In this mutant, the RNA mutations conservatively altered a single amino acid in the middle of p1 (Trp to Phe; see Fig. S1 in the supplemental material), whereas much of the frameshift site stem-loop was disrupted. For this mutant, Gag-Pol levels were slightly lower in cell lysates and significantly lower in VLPs (Fig. 2). In contrast, M9 showed an increase in frameshifting due to disruption of the anchoring helix (Fig. 3) and also exhibited lowered infectivity (Fig. 6). The M11, -SS, and -SS2 mutants all exhibited low levels of frameshifting due to mutation of the primary slippery site (Fig. 4). Correspondingly, these mutants had low infectivity, as expected (Fig. 6).
Frameshift site mutations do not affect Gag expression and localization in cells.
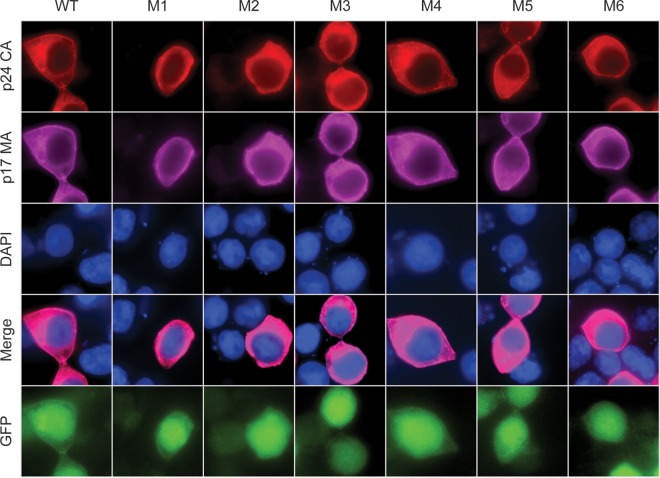
We tested a subset of mutants (M1 to M6) using immunofluorescence to investigate whether the frameshift site mutations affect Gag stability or localization in cells (Fig. 7). Antibodies against capsid and matrix showed no detectable differences in protein expression or cellular localization. Transfected cells were scored for Gag localization to the plasma membrane, which revealed that >90% of cells showed plasma membrane localization of Gag for the WT and M1 to M6 (Fig. 7 and data not shown). Therefore, the decreased infectivity associated with the M1-to-M3 frameshift mutants is not due to changes in Gag stability or localization.
FIG 7.
Immunofluorescence of capsid (p24) and matrix (p17) expression in HEK 293T cells transfected with the WT and frameshift mutants M1 to M6. Cells were fixed 48 h posttransfection. Differences between WT and the frameshift mutants in capsid (p24) expression do not appear to have affected Gag localization within the cell. Viral GFP expression data indicate transfected cells.
We next investigated how frameshift site mutations affect the measurable levels of reverse transcriptase (RT). The M1, M2, and M3 mutants with increased frameshifting efficiencies all showed increased expression of RT in cell lysates (Fig. 8A and B). We observed an approximately 2-fold increase in RT levels, which agrees well with the 2-fold to 3-fold increase in frameshifting observed for these mutants. The M4-to-M7 mutants have wild-type frameshift efficiencies (Fig. 2) and also wild-type levels of RT (Fig. 8A and B). M8 showed relatively low RT expression consistent with its low infectivity (Fig. 6). Interestingly, the RT levels were much less variable in the VLPs than in the cell lysates (Fig. 8C and D). Similar per-particle RT normalization results were previously described by Mak and colleagues (39), and the data may imply that a set threshold level of RT is retained in association with the nucleocapsid postmaturation.
FIG 8.
Quantification of the expression of reverse transcriptase (RT). (A and C) Representative blots probed with the polyclonal anti-RT antibody and the monoclonal anti-p24 antibody using cell lysates (A) or purified VLPs (C). (B and D) Relative expression levels of RT in cell lysates (B) and in purified virus-like particles (D) were calculated using the total amount of p55, p41, and p24 for each construct and were normalized to the WT. *, P < 0.001; #, P < 0.05 (compared with WT values).
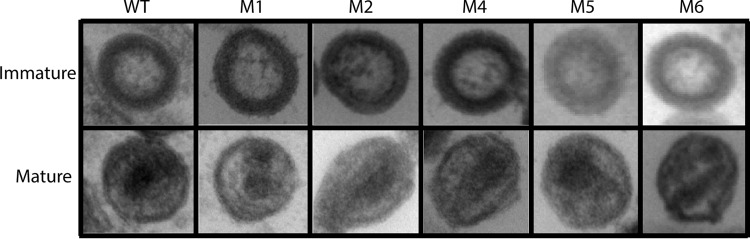
Virion morphology was analyzed by negative-stain electron microscopy for a subset of our mutants (Fig. 9). The purified particles were categorized as immature, i.e., as particles in which Gag molecules are radially arranged and in contact with the plasma membrane, or as mature, i.e., as particles containing electron density associated with conical or round cores. No significant morphology differences were observed between the WT and the mutants assayed, even for the mutants that exhibited strongly reduced infectivity. At least 40 purified virions were imaged for each sample. We found that for the WT and all mutants tested, >70% of the virions imaged contained density consistent with the presence of mature viral cores. The percentages of mature viral cores differed by less than 10% for all samples imaged. Thus, the observed decreased infectivity associated with the M1 and M2 mutants at this level of resolution is not due to large-scale defects in Gag assembly or virion morphogenesis. These results agree with the relatively normal proteolytic processing patterns detected for the VLPs (Fig. 5C), as well as with the normal RT levels associated with the mutant VLPs (Fig. 8C).
FIG 9.
Electron micrographs of immature and mature HIV-1 particles from WT cells and M1, M2, M4, M5, and M6 mutant cells.
DISCUSSION
Here we demonstrated that HIV-1 frameshift efficiency in cells is directly correlated (R2 = 0.95) with the local thermodynamic stability of the frameshift site stem-loop. In contrast, the correlation with overall thermodynamic stability is weaker (R2 = 0.67). A previous study concluded that overall thermodynamic stability correlates with frameshift efficiency but did not systematically (or quantitatively) parse relative thermodynamic contributions (39). Local stability determines frameshift efficiency because the ribosome unwinds mRNA helical structures 3 bp at a time, and this is the thermodynamic barrier that is encountered by the ribosome when it is engaged at the slippery site. The excellent agreement between these data and previous in vitro (rabbit reticulocyte lysate) measurements (6) indicates there are no additional viral or host factors that modulate frameshift efficiency. Our data also provide strong evidence that the functional frameshift site structure in cells is a stem-loop, as originally proposed by Jacks et al. (4), with no additional sequence-specific requirements. This is consistent with the observation that a stem-loop with an entirely different sequence but similar stability is fully functional in frameshifting (38). An alternative structure for the frameshift site involving tandem pseudoknots was recently proposed (40). However, our data are inconsistent with that model, as the mutations employed in the present study that either stimulate or have no effect on frameshifting would disrupt base pairs in the proposed pseudoknots.
The 3-helix-junction secondary structure surrounding the HIV frameshift site has a high pairing probability, as determined by evolutionary conservation, low SHAPE reactivity, and high predicted thermodynamic stability (11, 14). Here we show that this secondary structure modulates frameshift efficiency in cells. Disruption of the anchoring helix (M9) led to significantly increased frameshifting (Fig. 3) and lower infectivity (Fig. 6). The lower infectivity associated with this mutant may have been due to the W-to-Y mutation in the p7 (zinc knuckle 2 region) of nucleocapsid which is involved in genomic RNA recognition (41) or to a change in RNA structure and frameshifting efficiency or to a combination of these effects. M9 also has two mutations in the Pol p6* region (see Fig. S1 in the supplemental material), although this region has been reported to tolerate multiple amino acid substitutions without loss of infectivity (42).
The C1680U mutation induced a functional secondary slippery site (Fig. 4) that was less efficient than the primary slippery site. This was likely due the fact that there is only a weak (3-bp) helix downstream of the secondary slippery site, which is likely inefficient at inducing ribosomal pausing. The C1680U mutant frameshifted at levels only slightly higher than those seen with the WT (Fig. 4; M10) and yet had low infectivity (Fig. 6; M10). We hypothesize that the low infectivity associated with M10 was due to mutation of the p1-p6 cleavage site (NFL to NFF) (see Fig. S1 in the supplemental material). Interestingly, mutations at proteolytic processing sites associated with drug resistance have been previously observed to be profoundly codependent on the drug for processing and infectivity (43). Thus, it would be interesting to investigate whether the C1680U mutation operates through a similar mechanism. It is possible that the C1680 mutation is optimized to simultaneously allow enhanced frameshifting and increased proteolytic processing in the presence of inhibitor. Such coordination at both the RNA and protein levels may allow the C1680U mutant to achieve a new optimal Gag-Pol/Gag ratio in the presence of protease inhibitor.
It is interesting that the M1 and M2 mutants had frameshifting levels that were 2-fold to 3-fold higher (Fig. 2) and low infectivity (Fig. 6). We detected no gross defects in processing (Fig. 5C), RT content (Fig. 8C), or viral morphology (Fig. 9) for these mutants. However, we did observe that the M1 and M2 mutants had protein patterns consistent with increased proteolytic processing relative to the WT, including lower levels of p55 Gag precursor and higher levels of p24 capsid (Fig. 5C). Therefore, the low infectivity of these mutants might be a consequence of protease overexpression. Other interesting mutants include M8 and M9, which have a disrupted stem-loop and anchoring helix, respectively. Surprisingly, M8 frameshifted only slightly less well than the WT (Fig. 2). This was likely due to the fact that the slippery sequence plays a dominant role in frameshifting (Fig. 4) and also suggests that the stem-loop can tolerate mismatches.
The ratios of Gag-Pol/Gag in VLPs correlate well with those observed in cell lysates (Fig. 2, 3, and 4), consistent with a stochastic mechanism for Gag-Pol incorporation into budding virions. However, we also observed that Gag-Pol/Gag ratios were enriched in virions relative to lysates, consistent with the selective recruitment of Gag-Pol into VLPs. The mechanism for preferential recruitment of Gag-Pol into assembling capsids is not understood but is likely to reflect one or more features of the pol gene relevant to the assembly pathway.
Finally, our observation that a very modest (2-to-3-fold) increase in frameshift efficiency can significantly decrease HIV-1 infectivity is encouraging in considering frameshifting as a potential therapeutic target. Indeed, compounds designed to bind to the frameshift site are capable of inhibiting viral replication (20). Therefore, further development and optimization of compounds that can target frameshifting seem warranted.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants R01 AI110221 to N.M.S. and R01 GM072447 to S.E.B. J.T.B. is supported by a NSF Graduate Research Fellowship. B.E.B. is supported by NIH training grant GM008349.
We gratefully acknowledge Randall Massey and the University of Wisconsin SMPH Electron Microscopy Facility for assistance with processing and imaging EM samples. We thank David S. Goodsell for inspiring illustrations and discussions and Allison L. Didychuk for assistance with data analysis.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00149-16.
REFERENCES
- 1.Wang WK, Chen MY, Chuang CY, Jeang KT, Huang LM. 2000. Molecular biology of human immunodeficiency virus type 1. J Microbiol Immunol Infect 33:131–140. [PubMed] [Google Scholar]
- 2.Wills JW, Craven RC. 1991. Form, function, and use of retroviral gag proteins. AIDS 5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Frankel AD, Young JA. 1998. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem 67:1–25. doi: 10.1146/annurev.biochem.67.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Jacks T, Power MD, Masiarz FR, Luciw PA, Barr PJ, Varmus HE. 1988. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature 331:280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Liu H, Xiao H, Conway JA, Hunter E, Kappes JC. 1997. Functional RT and IN incorporated into HIV-1 particles independently of the Gag/Pol precursor protein. EMBO J 16:5113–5122. doi: 10.1093/emboj/16.16.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouzakis KD, Lang AL, Vander Meulen KA, Easterday PD, Butcher SE. 2013. HIV-1 frameshift efficiency is primarily determined by the stability of base pairs positioned at the mRNA entrance channel of the ribosome. Nucleic Acids Res 41:1901–1913. doi: 10.1093/nar/gks1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staple DW, Butcher SE. 2005. Solution structure and thermodynamic investigation of the HIV-1 frameshift inducing element. J Mol Biol 349:1011–1023. doi: 10.1016/j.jmb.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Yusupova GZ, Yusupov MM, Cate JHD, Noller HF. 2001. The path of messenger RNA through the ribosome. Cell 106:233–241. doi: 10.1016/S0092-8674(01)00435-4. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M. 2011. The structure of the eukaryotic ribosome at 3.0 A resolution. Science 334:1524–1529. doi: 10.1126/science.1212642. [DOI] [PubMed] [Google Scholar]
- 10.Takyar S, Hickerson RP, Noller HF. 2005. mRNA helicase activity of the ribosome. Cell 120:49–58. doi: 10.1016/j.cell.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Watts JM, Dang KK, Gorelick RJ, Leonard CW, Bess JW Jr, Swanstrom R, Burch CL, Weeks KM. 2009. Architecture and secondary structure of an entire HIV-1 RNA genome. Nature 460:711–716. doi: 10.1038/nature08237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolin SL, Walter P. 1988. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J 7:3559–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charbonneau J, Gendron K, Ferbeyre G, Brakier-Gingras L. 2012. The 5′ UTR of HIV-1 full-length mRNA and the Tat viral protein modulate the programmed −1 ribosomal frameshift that generates HIV-1 enzymes. RNA 18:519–529. doi: 10.1261/rna.030346.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low JT, Garcia-Miranda P, Mouzakis KD, Gorelick RJ, Butcher SE, Weeks KM. 2014. Structure and dynamics of the HIV-1 frameshift element RNA. Biochemistry 53:4282–4291. doi: 10.1021/bi5004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyon L, Croteau G, Thibeault D, Poulin F, Pilote L, Lamarre D. 1996. Second locus involved in human immunodeficiency virus type 1 resistance to protease inhibitors. J Virol 70:3763–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doyon L, Payant C, Brakier-Gingras L, Lamarre D. 1998. Novel Gag-Pol frameshift site in human immunodeficiency virus type 1 variants resistant to protease inhibitors. J Virol 72:6146–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girnary R, King L, Robinson L, Elston R, Brierley I. 2007. Structure-function analysis of the ribosomal frameshifting signal of two human immunodeficiency virus type 1 isolates with increased resistance to viral protease inhibitors. J Gen Virol 88:226–235. doi: 10.1099/vir.0.82064-0. [DOI] [PubMed] [Google Scholar]
- 18.Shehu-Xhilaga M, Crowe SM, Mak J. 2001. Maintenance of the Gag/Gag-Pol ratio is important for human immunodeficiency virus type 1 RNA dimerization and viral infectivity. J Virol 75:1834–1841. doi: 10.1128/JVI.75.4.1834-1841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brakier-Gingras L, Charbonneau J, Butcher SE. 2012. Targeting frameshifting in the human immunodeficiency virus. Expert Opin Ther Targets 16:249–258. doi: 10.1517/14728222.2012.665879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilimire TA, Bennett RP, Stewart RA, Garcia-Miranda P, Blume A, Becker J, Sherer N, Helms ED, Butcher SE, Smith HC, Miller BL. 2015. N-Methylation as a strategy for enhancing the affinity and selectivity of RNA-binding peptides: application to the HIV-1 frameshift-stimulating RNA. ACS Chem Biol doi: 10.1021/acschembio.5b00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcheschi RJ, Mouzakis KD, Butcher SE. 2009. Selection and characterization of small molecules that bind the HIV-1 frameshift site RNA. ACS Chem Biol 4:844–854. doi: 10.1021/cb900167m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcheschi RJ, Tonelli M, Kumar A, Butcher SE. 2011. Structure of the HIV-1 frameshift site RNA bound to a small molecule inhibitor of viral replication. ACS Chem Biol 6:857–864. doi: 10.1021/cb200082d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ofori LO, Hilimire TA, Bennett RP, Brown NW Jr, Smith HC, Miller BL. 2014. High-affinity recognition of HIV-1 frameshift-stimulating RNA alters frameshifting in vitro and interferes with HIV-1 infectivity. J Med Chem 57:723–732. doi: 10.1021/jm401438g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palde PB, Ofori LO, Gareiss PC, Lerea J, Miller BL. 2010. Strategies for recognition of stem-loop RNA structures by synthetic ligands: application to the HIV-1 frameshift stimulatory sequence. J Med Chem 53:6018–6027. doi: 10.1021/jm100231t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staple DW, Venditti V, Niccolai N, Elson-Schwab L, Tor Y, Butcher SE. 2008. Guanidinoneomycin B recognition of an HIV-1 RNA helix. Chembiochem 9:93–102. doi: 10.1002/cbic.200700251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherer NM, Swanson CM, Papaioannou S, Malim MH. 2009. Matrix mediates the functional link between human immunodeficiency virus type 1 RNA nuclear export elements and the assembly competency of Gag in murine cells. J Virol 83:8525–8535. doi: 10.1128/JVI.00699-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Wehrly K, Chesebro B. 1997. p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods 12:288–293. doi: 10.1006/meth.1997.0481. [DOI] [PubMed] [Google Scholar]
- 30.Chesebro B, Wehrly K, Nishio J, Perryman S. 1992. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol 66:6547–6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toohey K, Wehrly K, Nishio J, Perryman S, Chesebro B. 1995. Human immunodeficiency virus envelope V1 and V2 regions influence replication efficiency in macrophages by affecting virus spread. Virology 213:70–79. doi: 10.1006/viro.1995.1547. [DOI] [PubMed] [Google Scholar]
- 32.Swanson CM, Puffer BA, Ahmad KM, Doms RW, Malim MH. 2004. Retroviral mRNA nuclear export elements regulate protein function and virion assembly. EMBO J 23:2632–2640. doi: 10.1038/sj.emboj.7600270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aligeti M, Behrens RT, Pocock GM, Schindelin J, Dietz C, Eliceiri KW, Swanson CM, Malim MH, Ahlquist P, Sherer NM. 2014. Cooperativity among Rev-associated nuclear export signals regulates HIV-1 gene expression and is a determinant of virus species tropism. J Virol 88:14207–14221. doi: 10.1128/JVI.01897-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vella S, Floridia M. 1998. Saquinavir. Clinical pharmacology and efficacy. Clin Pharmacokinet 34:189–201. [DOI] [PubMed] [Google Scholar]
- 35.Johnson SF, Collins JT, D'Souza VM, Telesnitsky A. 2014. Determinants of Moloney murine leukemia virus Gag-Pol and genomic RNA proportions. J Virol 88:7267–7275. doi: 10.1128/JVI.03513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sükösd Z, Andersen ES, Seemann SE, Jensen MK, Hansen M, Gorodkin J, Kjems J. 2015. Full-length RNA structure prediction of the HIV-1 genome reveals a conserved core domain. Nucleic Acids Res 43:10168–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Marco A, Heuser AM, Glass B, Krausslich HG, Muller B, Briggs JA. 2012. Role of the SP2 domain and its proteolytic cleavage in HIV-1 structural maturation and infectivity. J Virol 86:13708–13716. doi: 10.1128/JVI.01704-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leiherer A, Ludwig C, Wagner R. 2009. Uncoupling human immunodeficiency virus type 1 Gag and Pol reading frames: role of the transframe protein p6* in viral replication. J Virol 83:7210–7220. doi: 10.1128/JVI.02603-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill MK, Shehu-Xhilaga M, Crowe SM, Mak J. 2002. Proline residues within spacer peptide p1 are important for human immunodeficiency virus type 1 infectivity, protein processing, and genomic RNA dimer stability. J Virol 76:11245–11253. doi: 10.1128/JVI.76.22.11245-11253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang X, Yang Y, Wang G, Cheng Q, Du Z. 2014. Highly conserved RNA pseudoknots at the Gag-Pol junction of HIV-1 suggest a novel mechanism of −1 ribosomal frameshifting. RNA 20:587–593. doi: 10.1261/rna.042457.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Guzman RN, Wu ZR, Stalling CC, Pappalardo L, Borer PN, Summers MF. 1998. Structure of the HIV-1 nucleocapsid protein bound to the SL3 psi-RNA recognition element. Science 279:384–388. doi: 10.1126/science.279.5349.384. [DOI] [PubMed] [Google Scholar]
- 42.Paulus C, Ludwig C, Wagner R. 2004. Contribution of the Gag-Pol transframe domain p6* and its coding sequence to morphogenesis and replication of human immunodeficiency virus type 1. Virology 330:271–283. doi: 10.1016/j.virol.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Waki K, Durell SR, Soheilian F, Nagashima K, Butler SL, Freed EO. 2012. Structural and functional insights into the HIV-1 maturation inhibitor binding pocket. PLoS Pathog 8:e1002997. doi: 10.1371/journal.ppat.1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.