ABSTRACT
An integrin-associated protein CD47, which is a ligand for the inhibitory receptor signal regulatory protein α, is expressed on B and T cells, as well as on most innate immune cells. However, the roles of CD47 in the immune responses to viral infection or vaccination remain unknown. We investigated the role of CD47 in inducing humoral immune responses after intranasal infection with virus or immunization with influenza virus-like particles (VLPs). Virus infection or vaccination with VLPs containing hemagglutinin from A/PR8/34 influenza virus induced higher levels of antigen-specific IgG2c isotype dominant antibodies in CD47-deficient (CD47KO) mice than in wild-type (WT) mice. CD47KO mice with vaccination showed greater protective efficacy against lethal challenge, as evidenced by no loss in body weight and reduced lung viral titers compared to WT mice. In addition, inflammatory responses which include cytokine production, leukocyte infiltrates, and gamma interferon-producing CD4+ T cells, as well as an anti-inflammatory cytokine (interleukin-10), were reduced in the lungs of vaccinated CD47KO mice after challenge with influenza virus. Analysis of lymphocytes indicated that GL7+ germinal center B cells were induced at higher levels in the draining lymph nodes of CD47KO mice compared to those in WT mice. Notably, CD47KO mice exhibited significant increases in the numbers of antigen-specific memory B cells in spleens and plasma cells in bone marrow despite their lower levels of background IgG antibodies. These results suggest that CD47 plays a role as a negative regulator in inducing protective immune responses to influenza vaccination.
IMPORTANCE Molecular mechanisms that control B cell activation to produce protective antibodies upon viral vaccination remain poorly understood. The CD47 molecule is known to be a ligand for the inhibitory receptor signal regulatory protein α and expressed on the surfaces of most immune cell types. CD47 was previously demonstrated to play an important role in modulating the migration of monocytes, neutrophils, polymorphonuclear neutrophils, and dendritic cells into the inflamed tissues. The results of this study demonstrate new roles of CD47 in negatively regulating the induction of protective IgG antibodies, germinal center B cells, and plasma cells secreting antigen-specific antibodies, as well as macrophages, upon influenza vaccination and challenge. As a consequence, vaccinated CD47-deficient mice demonstrated better control of influenza viral infection and enhanced protection. This study provides insights into understanding the regulatory functions of CD47 in inducing adaptive immunity to vaccination.
INTRODUCTION
Influenza viruses are common pathogens in the respiratory tract that are highly contagious and can cause pulmonary diseases. Seasonal influenza virus variants annually cause significant levels of morbidity and mortality, mostly in infants, the elderly, and sick people (1, 2). Vaccination is the most effective measure to prevent infections with a variety of pathogens, including influenza virus. Virus-like particles (VLPs) are able to effectively stimulate antigen-presenting cells (APCs), which in turn activate T and B cells (3–6). It has been demonstrated that immunization with influenza VLPs can induce protective humoral responses against seasonal and pandemic influenza virus infections (7–9). However, the mechanisms for evoking long-lasting immune responses are largely unknown.
CD47 is a transmembrane protein, which is first identified as integrin αvβ3. CD47 that is expressed on hematopoietic and nonhematopoietic cells can interact with an inhibitory receptor signal regulatory protein α (SIRPα) (10). SIRPα is also expressed on dendritic cells (DCs) and macrophages, whereas SIRPα is barely expressed on B and T cells (11, 12). It has been demonstrated that CD47/CD47 and CD47/SIRPα interactions are important for DC and neutrophil migration (13, 14). In addition, CD11b+ DCs in the lungs express both CD47 and SIRPα, but CD103+ DCs express only CD47. It was also demonstrated that CD47 helps CD11b+ DCs homing to draining lymph nodes during steady and inflammatory conditions (15). The populations of B220+ B cells and CD8+ T cells have been reported to remain unchanged in the spleens of SIRPα and CD47KO mice (16). However, a study reported that CD47-deficient (CD47KO) mice showed a defect in producing IgG antibodies to intravenous antigens (17). Another study using an allergic airway disease model demonstrated that antigen-specific antibody responses were lower in mucosal tissues from CD47KO mice (15). However, the role of CD47 in inducing specific antibodies in response to vaccination and protective immune responses against infectious viral disease remains largely unknown.
Influenza VLP vaccines have been suggested as promising alternative vaccine candidates (18, 19) and have also been tested in clinical trials (20, 21). Antibody responses to hemagglutinin (HA) after vaccination are the major immune correlates conferring protection against influenza virus infections. Thus, we investigated the possible roles of CD47 in inducing protective humoral immunity to influenza viral infection or vaccination using influenza VLP containing HA as a vaccine target antigen. We found that vaccination of CD47-deficient mice with influenza VLP yielded robust humoral responses and provided enhanced protection, resulting in reducing inflammatory responses that include reduced production of proinflammatory cytokines and reduced immune cell infiltration into the lungs after challenge with a lethal dose of influenza virus.
MATERIALS AND METHODS
Mice.
Wild-type (WT) C57BL/6 mice and CD47−/− (CD47KO) mice on a C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were bred at the Department of Animal Resources at Georgia State University.
Influenza VLPs and immunization.
Influenza VLPs were prepared as described previously (8). Briefly, to obtain influenza VLPs, Sf9 cells were coinfected with recombinant baculoviruses expressing M1 and influenza virus hemagglutinin (HA) from A/PR8/34 virus and cultured in SF900-II serum-free medium at 27°C for 3 days. Culture supernatants were collected by centrifugation at 2,000 × g for 20 min to remove cells. Collected supernatants containing influenza VLPs were further spun by ultracentrifugation at 100,000 × g for 60 min. The VLP pellets were resuspended in phosphate-buffered saline (PBS) at 4°C for overnight. Discontinuous sucrose gradient (20 to 30 to 60%) was used to purify VLPs by ultracentrifugation at 100,000 × g for 1 h at 4°C. The VLP bands were harvested, and the amount of incorporated HA level was analyzed by Western blot and hemagglutination activity assays.
Viral infection.
WT and CD47KO mice (6 to 8 weeks old) were intranasally (i.n.) immunized with influenza VLPs (3 μg) at weeks 0 and 4. Immunized mice were anesthetized by isoflurane (Baxter, Deerfield, IL) and i.n. infected with A/PR8/34 virus in 50 μl of PBS per mouse 6 to 7 weeks after secondary immunization. Challenge doses were 8× or 33× the 50% mouse lethal dose (LD50), where 1× LD50 dose contains ∼100 PFU. Mice were daily monitored for 14 days to record body weight changes and survival rates or else sacrificed at 6 days postinfection (dpi). Cases of >25% body weight loss were recorded as dead mice. To compare the efficacy of vaccination, unimmunized mice were used as a negative-control group. All animal studies were approved and conducted under the guidelines by Georgia State University's IACUC (permit A14025).
Serum preparation and humoral immune responses.
Antibody titers specific for influenza virus were determined by enzyme-linked immunosorbent assay (ELISA) using inactivated influenza A/PR8/34 virus (iPR8) as a coating antigen (8). Briefly, iPR8 (4 μg/ml) was coated onto 96-well microtiter plates (Nunc, Rochester, NY), followed by incubation overnight at 4°C. The 96-well plates were washed with PBS containing 0.05% Tween 20 (PBST), and 3% bovine serum albumin in PBST was used for blocking reagents for 90 min at 37°C. The collected sera were serially diluted and incubated for 90 min at 37°C. Horseradish peroxidase-conjugated goat anti-mouse IgG, IgG1, and IgG2c (Southern Biotech, Birmingham, AL) were used as secondary antibodies, and then tetramethylbenzidine (TMB) peroxidase substrate (Sigma-Aldrich, St. Louis, MO) was used as a substrate. The optical density at 450 nm (OD450) was measured using an optical spectrophotometer reader. The total antibody concentration was determined with respect to the quantitative standard antibody concentration by using purified IgG, IgG1, IgG2c, and IgM.
Cytokine assays.
Cytokines were determined by ELISAs, performed as previously described by Quan et al. (8). Briefly, Ready-Set-Go interleukin-6 (IL-6), IL-10, and tumor necrosis factor alpha (TNF-α) kits (eBioscience, San Diego, CA) were used to determine the level of cytokines in the bronchoalveolar lavage fluid (BALF) and lung extracts according to the manufacturer's procedures.
Cell preparation and flow cytometry.
Nonadherent cells were collected from the BALF two times in PBS. The lung tissues were homogenized, and cells were then passed through a strainer and spun on 44/67% Percoll gradients at 2,800 rpm for 20 min. Cell band was harvested and washed with PBS. The frosted glass microscope slides were used to make cell suspensions from the mediastinal lymph nodes (MLN) and spleens. Red blood cells were lysed with ammonium chloride and lymphocytes were filtered through cell strainers. For flow cytometry analysis, all other markers were stained at 4°C using monoclonal antibodies specific for CD3, CD4, CD8, CD11b, CD11c, CD19, CD45, CD47, F4/80, and Ly6c (eBioscience or BD Pharmingen, San Diego, CA). The samples were analyzed on a LSR-II/Fortessa flow cytometer (Becton-Dickinson [BD]) and analyzed using the FlowJo software program (Tree Star, Inc.).
Intracellular cytokine staining analysis.
Lymphocytes were enriched from the BALF and lungs. Cells from the BALF of the same group were pooled to make enough cell numbers. Lymphocytes were in vitro stimulated in the presence of the synthetic peptides HA 211–225(YVQASGRVTVSTRRS) and HA441–455 (AELLVALENQHTIDL) (5 μg/ml) (32) with brefeldin A at 20 μg/ml for 5 h in an incubator at 37°C. Stimulated lymphocytes were stained with antibodies, including CD4 and CD8. The lymphocytes were then fixed and permeabilized using a BD Cytofix/Cytoperm Plus kit and stained with IFN-γ cytokine antibodies.
Lung viral titers.
Lung viral titers were determined using fertilized chicken eggs, which were incubated for 10 days at 37°C. Lung homogenates were serially diluted in PBS and injected into the allantoic sacs. Infected embryonated eggs were further incubated for 3 days and left in a cold room overnight to euthanize the embryos. The allantoic fluids were harvested and transferred into 96-well round-bottom plates containing citrated chicken blood. The plates were left at room temperature for 30 min, and the viral titers were calculated. Infected wells appeared as a uniform reddish color, but negative wells produced dots in the centers of round-bottom plates.
Statistics.
Unless otherwise stated, all results are presented as means ± the standard errors of the mean (SEM). The statistical significance for all experiments was determined by using an unpaired two-tailed Student t test or a one-way analysis of variance (ANOVA). Prism software (GraphPad Software, Inc., San Diego, CA) was used for all data analysis. Comparisons used to generate P values are indicated by horizontal lines (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
RESULTS
CD47KO mice induce increased virus-specific IgG antibodies after infection despite lower background IgG antibodies.
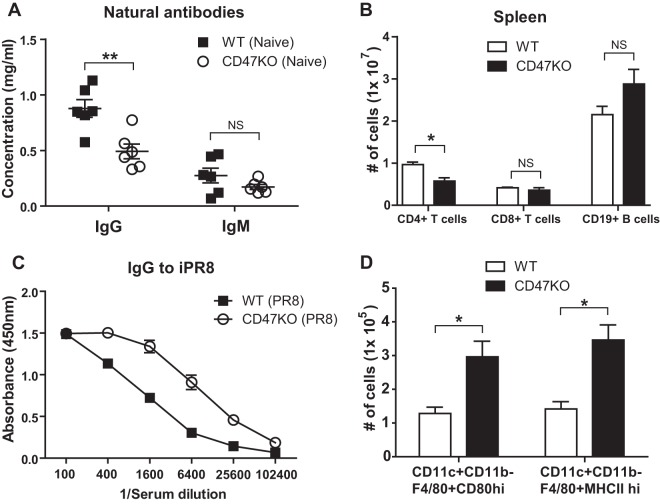
CD47 is expressed by hematopoietic cells, including T and B cells (23). To understand how CD47 deficiency intrinsically influences the production of natural immunoglobulin G (IgG) and IgM, blood samples were collected from naive C57BL/6 wild-type (WT) and CD47KO mice (n = 12), and the total amounts of antigen nonspecific background antibodies in sera were measured by ELISA. Both IgG and IgM antibodies were produced in CD47-deficient mice, but the serum IgG levels were significantly lower in CD47KO mice (0.5 mg/ml) than in WT mice (0.9 mg/ml) (Fig. 1A). In contrast, similar levels of IgM were observed in the sera of WT (0.3 mg/ml) and CD47KO (0.2 mg/ml) mice. To determine whether CD47 molecule influenced lymphocyte homeostasis, the numbers of T and B cells were compared in the spleens of naive CD47KO and WT mice. Similar numbers of CD8+ T and B cells were detected in the spleens of CD47KO and WT mice, but reduced CD4+ T cells in numbers were observed in the spleens of CD47-deficient mice (Fig. 1B).
FIG 1.
CD47-deficient mice show increased IgG antibody responses to viral infection despite low natural IgG antibody levels and macrophage activation to vaccination. (A) Natural antibodies in naive mice. Preimmune sera were collected from WT C57BL/6 (n = 6) and CD47KO (n = 6) mice. The total immunoglobulin (Ig) G and M levels were determined in the blood by ELISA and measured as the OD450. (B) T and B cells in naive mice. T and B cell numbers were analyzed specific markers such as CD3, CD4, CD8, and CD19 in the spleens of naive WT and CD47KO mice. (C) Virus-specific IgG antibodies after infection of naive mice. Naive WT and CD47KO mice were i.n. infected with a low dose of A/PR8 virus (0.5 × LD50). Antisera were serially diluted, and antigen-specific IgG levels were analyzed by ELISA against inactivated A/PR8/34 virus at 3 weeks after infection and measured as the OD450. (D) Macrophage responses to vaccination. The cells were collected from the lungs of WT and CD47KO mice 6 days after vaccination (3 μg of HA-VLPs). Activation markers, including CD80 and MHC-II, were used to analyze the status of macrophage activation. The statistical significance was determined by using an unpaired two-tailed Student t test. Error bars indicate means ± the SEM of the concentrations from individual animals. *, P < 0.05; **, P < 0.01; NS, no significant difference.
Next, we determined whether CD47KO mice would induce lower levels of virus antigen specific IgG antibodies after influenza virus infection. Naive WT and CD47KO mice (n = 8) were i.n. infected with a sublethal low dose of A/PR8/34 virus (0.5 × LD50), and then the levels of PR8 virus-specific IgG antibodies in sera were determined (Fig. 1C). Unexpectedly, CD47KO mice were found to induce a higher antibody response to influenza virus infection at a sublethal dose did WT mice (Fig. 1C). To understand the possible correlation of CD47 between antigen-presenting cells and T cells, naive WT and CD47KO mice were administered HA-VLPs, and the activation of antigen-presenting cells was monitored. Intranasal administration of VLP antigens induced an increase in the numbers of F4/80+ CD80high and F4/80+ MHC-IIhigh activated macrophages in the lungs of CD47KO mice than in WT mice (Fig. 1D). These results suggest that naive CD47KO mice maintain lower levels of background IgG antibodies than naive WT mice. However, it seems to be paradoxical that CD47KO mice can raise virus antigen-specific IgG antibodies at significantly higher levels after infection with influenza virus than in infected WT mice. Interestingly, CD47KO mice showed an increase in numbers of major histocompatibility complex class II (MHC-II)-positive activated antigen-presenting macrophages in response to i.n. VLP antigen.
Higher levels of antigen-specific IgG2c antibodies are induced in CD47KO mice after vaccination.
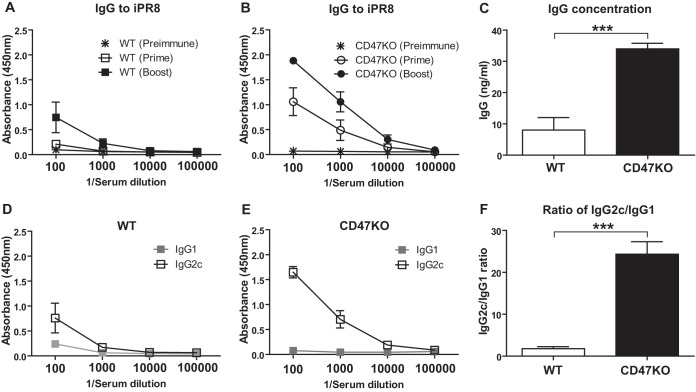
To determine the role of CD47 in inducing humoral responses to vaccination, WT and CD47KO mice (n = 12) were i.n. immunized with 3 μg of influenza virus HA-VLPs (A/PR8/34) twice at 0 and 4 weeks. Immune sera were collected 3 weeks after prime and boost immunization. After prime immunization, antibodies specific for inactivated A/PR8/34 (iPR8) virus were induced at significantly higher levels in CD47KO mice than those in WT mice (Fig. 2A and B). Importantly, antibodies specific for iPR8 virus were induced at consistently higher levels in CD47KO mice than those in WT mice, although the levels of vaccine antigen-specific antibodies were increased in WT mice after boost immunization. The concentration of antibodies showed 4.2-fold increases in CD47KO mice (34 ng/ml) compared to those in WT mice (8 ng/ml) (Fig. 2C).
FIG 2.
HA-VLP immunization induces enhanced antigen-specific antibody responses in CD47KO mice. WT and CD47KO mice were i.n. immunized with HA-VLPs (3 μg/mouse) at week 0 and boosted at week 4. Immune sera were collected 3 weeks after prime and boost immunization from animals. (A and B) Antisera were serially diluted, and antigen-specific IgG levels were analyzed by ELISA against inactivated A/PR8/34 virus and measured as the OD450. (C) Total concentration of IgG (ng/ml) against to virus was also calculated based on the standard mouse IgG concentration. (D and E) Isotypes of A/PR8/34-specific antibodies were determined using anti-mouse specific isotype antibodies. (F) Ratios of IgG2c relative to IgG1 measured in WT and CD47KO mice. The statistical significance was determined by using an unpaired two-tailed Student t test. Error bars indicate the means ± the SEM of the concentrations or ratios from individual animals. ***, P < 0.001.
A pattern of antigen-specific IgG1 and IgG2c isotype antibodies is known to be dictated by T helper type 2 (Th2)- and Th1-derived cytokines, respectively (24, 25). We compared a pattern of vaccine-specific antibody isotype levels in CD47KO and WT mice. CD47KO mice induced IgG2c isotype antibody predominantly, which was higher than the IgG2c level in WT mice (Fig. 2D and E). As a result, CD47KO mice showed a 14-fold increase in the IgG2c/IgG1 ratio compared to WT mice, indicating that CD47 may play a negative regulator in inducing IgG2c antibody, a Th1 type immune response (Fig. 2F). These results suggest that antigen-specific IgG2c humoral responses are induced more effectively than IgG1 antibody in CD47KO mice after immunization with influenza VLP vaccine or infection with influenza virus.
CD47KO mice are better protected against influenza after vaccination.
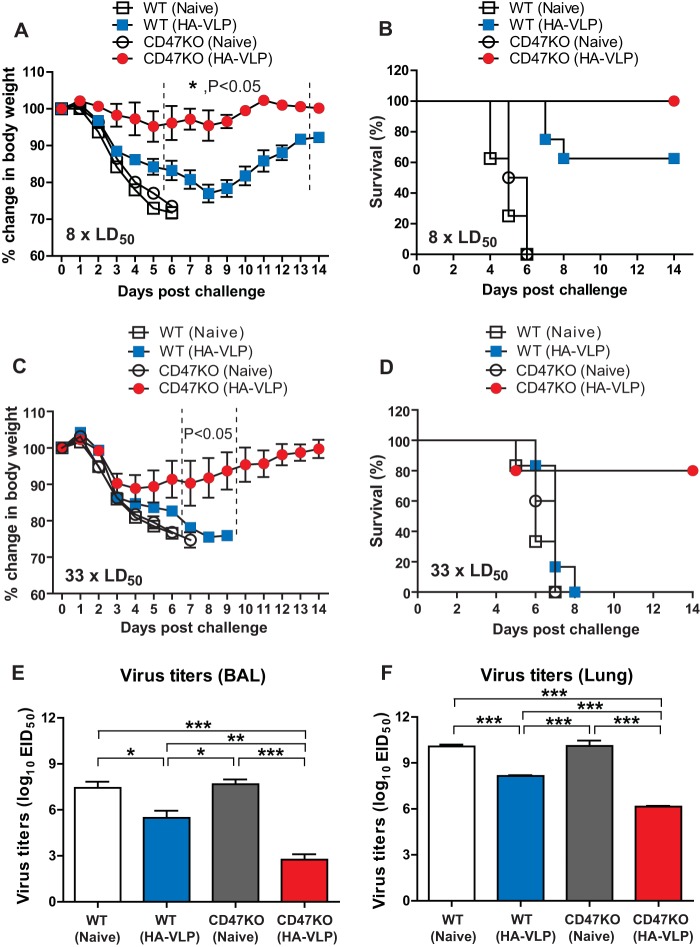
We determined whether CD47KO mice with antibody responses at higher levels would be better protected against influenza virus infection. CD47KO or WT mice (n = 12) after immunization with influenza VLPs were infected with a lethal dose of A/PR8/34 influenza virus (8 × LD50) (Fig. 3A). Naive WT and CD47KO mice displayed severe body weight loss of >25% (endpoints) and died or had to be euthanized by day 6 (Fig. 3B). Vaccinated WT mice lost their weight up to 22 to 25% resulting in 60% survival until day 8 and gradually recovered from A/PR8 infection. In contrast, immunized CD47KO mice with influenza VLPs did not show obvious weight loss after lethal challenge infection, indicating higher efficacy of protection (Fig. 3A and B).
FIG 3.
Immunization of CD47KO induces enhanced protection against influenza virus infection. Immunized WT and CD47KO mice were challenged with a lethal dose of A/PR8 influenza virus (8 × LD50 [A and B] or 33 × LD50 [C and D]) at 6 weeks after boost immunization. Body weight changes (A and C) and survival rates (B and D) were monitored daily for 14 days. (E and F) Viral titers in BALF (E) and lungs (F). Mice were infected with A/PR8/34 (8 × LD50), and the lung tissues were harvested at 6 days postchallenge. The viral titers were determined by inoculating chicken eggs with diluted lung samples. EID50, 50% egg infectious dose. “Naive” indicates unimmunized WT mice. The statistical significance was determined by using an unpaired two-tailed Student t test or one-way ANOVA. Error bars indicate the means ± the SEM of the concentrations or ratios from individual animals. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Furthermore, we tested whether HA-VLP vaccination was able to confer protection of CD47KO mice against a high dose of A/PR8/34 virus challenge. Vaccinated WT and CD47KO mice (n = 10) were infected with A/PR8/34 influenza virus (33 × LD50). As expected, all unimmunized mice and immunized WT mice displayed severe weight loss of >25% or died 5 to 8 days after infection (Fig. 3C and D). In contrast, VLP-immunized CD47KO mice showed around 10% body weight loss by 3 days after infection and gradually recovered body weight until 14 dpi, suggesting that vaccination with HA-VLPs was able to confer protection in CD47-deficient mice even against a high dose of influenza virus infection.
Lung viral titers were analyzed to determine the replication of challenge virus. Mice (n = 12) were infected with A/PR8/34 (8 × LD50), BALF and the lung tissues were harvested 6 days postchallenge (Fig. 3E and F). To determine egg infectious titers, the BALF and lung lysates were serially diluted, injected into embryonated chicken eggs, and incubated for 3 days. Thus, these results suggest that CD47KO mice induce stronger protective immunity, inhibiting viral replication by >10,000-fold after influenza VLP vaccination and infection, a finding that is consistent with higher levels of vaccine-specific antibody responses in CD47KO mice. WT mice that received influenza VLP vaccination also controlled lung viral replication by ∼100-fold but with less efficacy than did CD47KO mice (Fig. 3E and F).
Vaccinated CD47KO mice reduce inflammatory responses after infection.
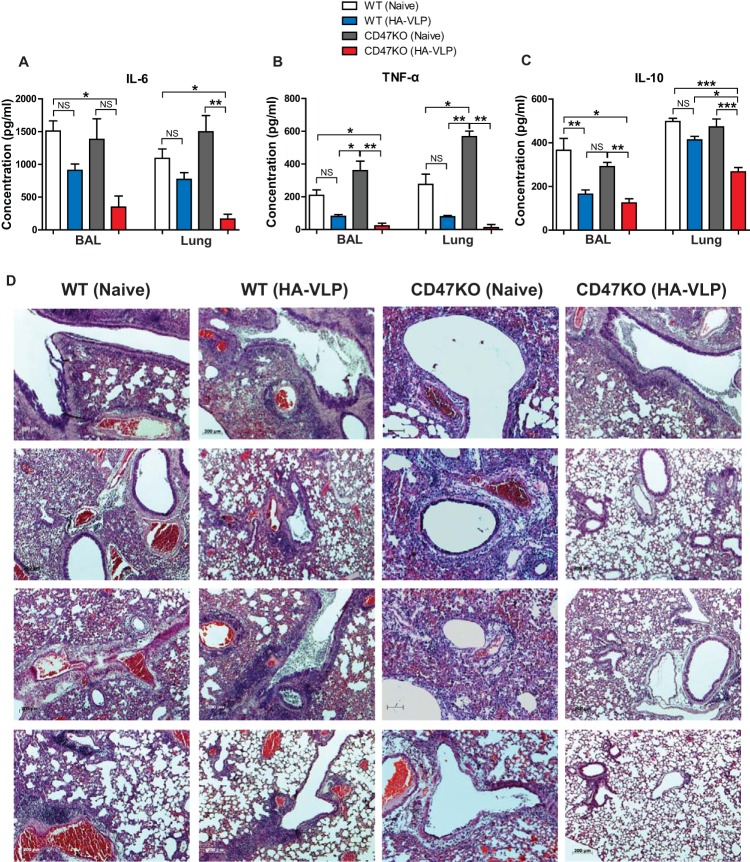
Overinduction of proinflammatory cytokines contributes to tissue damages during highly pathogenic influenza virus infections, including H5N1 and H1N1 strains (26, 27). To compare inflammatory responses, the levels of cytokines in BALF and lung extracts of animals (n = 6) immunized with influenza VLPs were determined by cytokine ELISA at 6 days postinfection with A/PR8/34 virus. High levels of IL-6 were detected in the BALF and lungs from naive WT and CD47KO mice, as well as from immunized WT mice, compared to immunized CD47KO mice (Fig. 4A). Interestingly, the level of TNF-α was lower in both immunized groups than those in unimmunized WT and CD47KO mice (Fig. 4B). Similar to the IL-6 levels, unimmunized naive WT and CD47KO mice showed the highest levels of IL-10 compared to immunized groups. Importantly, immunized WT mice had larger amounts of IL-10 than did immunized CD47KO mice (Fig. 4C). To better understand lung inflammation, histological analysis was performed 6 days after challenge with A/PR8/34 virus (Fig. 4D). Lung histology results showed severe inflammation in lung tissues from unimmunized WT and CD47KO mice and also substantial inflammation in immunized WT mice compared to immunized CD47KO mice (Fig. 4D). Immunized CD47KO mice exhibited the lowest levels of lung inflammation and inflammatory cytokines, as expected, due to better control of early influenza virus replication (Fig. 2). Therefore, inflammation of lung histopathology appeared to be correlated with high lung viral titers and high levels of proinflammatory cytokines.
FIG 4.
Vaccination of CD47KO mice results in reduction of inflammatory responses. BALF and lung extracts were used to determine inflammatory cytokine levels at 6 days after challenge with A/PR8 virus. IL-6 (A), TNF-α (B), and IL-10 (C) levels were determined in the BALF and lungs of unimmunized and immunized groups. (D) Lung histology. The lungs were harvested from vaccinated WT and CD47KO mice 6 days after challenge with a lethal dose of A/PR8 virus (8 × LD50). The tissues were fixed with 10% neutral buffered formalin solution. The lungs were embedded in paraffin, and 5-μm sections were stained with hematoxylin and eosin. Photographs were taken at a magnification of ×50. The statistical significance was determined by using one-way ANOVA. Error bars indicate the means ± the SEM of the concentrations or ratios from individual animals. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, no significant difference.
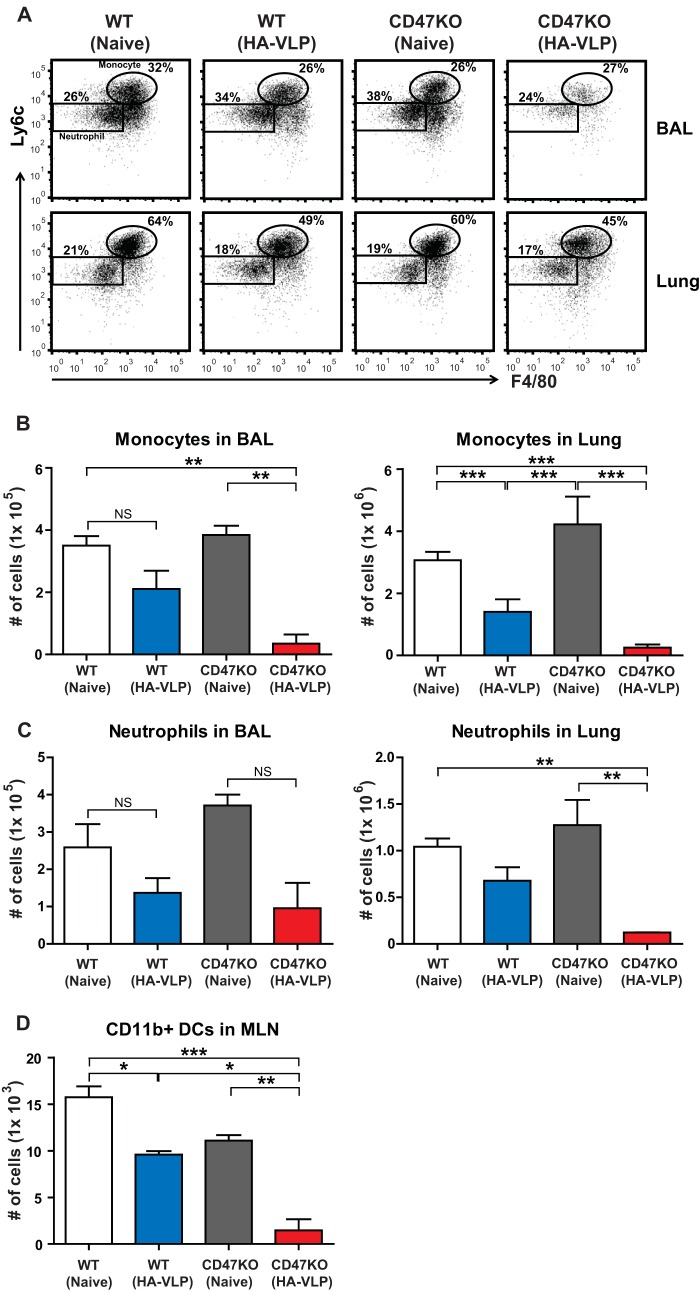
Vaccinated CD47KO mice show less innate immune cell recruitment upon virus infection.
Increased inflammatory responses may induce a large number of cell infiltrates into the lungs in response to viral infection. The cell numbers were analyzed in the BALF and lungs at 6 dpi with a lethal dose of A/PR8/34 virus. The markers for flow cytometry were used to separate populations, such as neutrophils and monocytes (28, 29). Importantly, we observed that the numbers of monocytes (CD11b+ Ly6chi F4/80+) in the BALF and lungs of immune WT mice were lower than those in nonimmune WT and CD47KO mice, but they were higher than those in immune CD47KO mice (Fig. 5A and B). Similar to monocyte infiltration, lower numbers of neutrophils (CD11b+ Ly6c+ F4/80−) were found in the BALF and lungs of immune WT mice than in nonimmune WT mice, but there was no significance (Fig. 5A and C). We observed a significant difference between nonimmune CD47KO and immune CD47KO mice because immune CD47KO mice showed the lowest levels of neutrophil recruitment. Thus, the results suggest that a high viral load and high levels of inflammatory cytokines are correlated with an increase in innate immune cell recruitment.
FIG 5.
Vaccinated CD47KO mice decrease immune cell recruitments upon influenza virus infection. Immune cells were harvested from the BALF, lungs, and MLN at 6 dpi with A/PR8/34 influenza virus. The frequencies and numbers of monocytes (A and B) and neutrophils (A and C) were analyzed at 6 dpi in the BALF and lungs in WT and CD47KO mice. (D) Respiratory DC subsets were analyzed in the MLN using specific markers, such as CD11c, CD11b, and MHC-II. The statistical significance was determined by using one-way ANOVA. Error bars indicate the means ± the SEM of the concentrations or ratios from individual animals. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, no significant difference.
A previous study has demonstrated that influenza virus infection induces an influx of respiratory DCs into the draining lymph nodes (30). We compared the numbers of CD11b+ DCs in the mediastinal lymph nodes (MLN) at 6 days after challenge with A/PR8/34 virus. Consistent with low lung viral loads from vaccinated CD47KO mice, reduced numbers of respiratory CD11b+ DCs were detected in the MLN of vaccinated CD47KO mice compared to those in vaccinated WT mice (Fig. 5D). Although similar levels of lung viral loads after challenge with influenza virus were found in unvaccinated naive WT and CD47KO mice, reduced numbers of CD11b+ DCs were detected in the MLN of CD47-deficient mice. These results indicate that enhanced protection against influenza virus infection in CD47KO mice reduces respiratory DC migration into the draining lymph nodes, as well as lowers the infiltration of monocytes and neutrophils into the lungs of CD47KO mice.
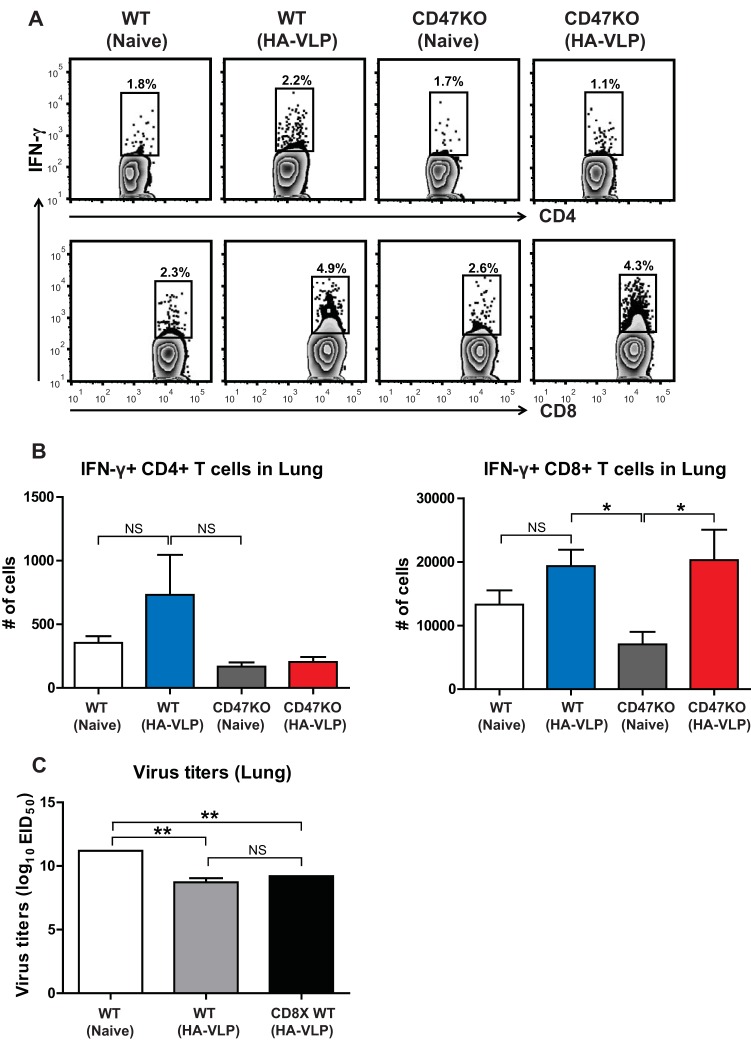
Vaccinated mice increase recall T cell recruitment in the lungs.
Memory T cells rapidly respond to antigen stimulation (31). To determine recall T cell recruitment in the site of infection in vaccinated mice after infection, T cells from the BALF and lungs were stimulated with the synthetic HA211–225 (YVQASGRVTVSTRRS) and HA441–455 (AELLVALENQHTIDL) peptides specific for CD4+ and CD8+ T cells, respectively (32). Intracellular IFN-γ expression was analyzed by flow cytometry. After influenza virus infection, similar numbers of IFN-γ-producing CD4+ T cells were observed in the lungs of nonimmune WT and CD47KO mice, as well as in immune WT and CD47KO mice (Fig. 6A and B). Interestingly, immunized WT and CD47KO mice showed increases in the numbers of memory-specific CD8+ T cells compared to naive mice by day 6 after challenge (Fig. 6A and B). To better understand the role of memory CD8+ T cells in protection, the CD8 population was depleted in immunized WT mice before influenza virus challenge. We observed no difference in lung viral clearance between immune and CD8-depleted immune WT mice (Fig. 6C). These results indicate that vaccination with HA-VLPs induces an increase in the numbers of recall T cell recruitment in infected tissues. Importantly, virus-specific antibodies play a major role in controlling influenza virus infection rather than IFN-γ-producing CD8+ T cells.
FIG 6.
Enhanced protective immunity in CD47KO appears to reduce recall T cell responses. Immune cells were harvested from the BALF and lungs of vaccinated mice 6 dpi with A/PR8 influenza virus. (A and B) The cells producing IFN-γ were determined by culture for 5 h in the presence of HA peptides. Antigen-specific T cells were determined by intracellular cytokine staining with anti-IFN-γ antibodies. (C) Lung viral titers in the lungs. HA-VLP immune WT mice received either anti-CD8 depletion antibodies or PBS prior to A/PR8/34 (8 × LD50) infection. The lung tissues were harvested at 6 dpi. Viral titers were determined by inoculation of chicken eggs with diluted lung samples. EID50, 50% egg infectious dose. The statistical significance was determined by using one-way ANOVA. Error bars indicate the means ± the SEM of the concentrations or ratios from individual animals. *, P < 0.05; **, P < 0.01; NS, no significant difference.
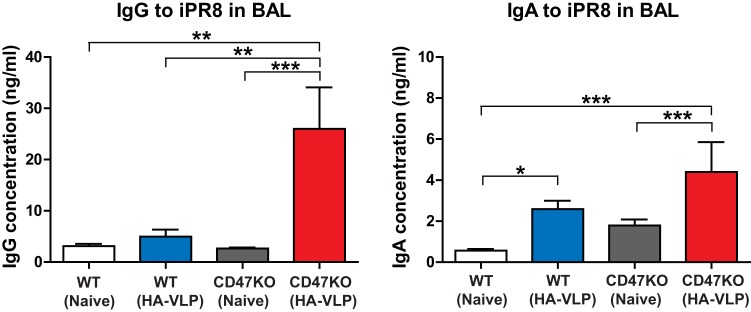
Intranasal vaccination induces higher levels of mucosal antibodies in CD47KO mice.
Respiratory tracts and lungs are the major sites of entry and replication for influenza virus. To determine whether humoral responses in mucosal tissues would have a correlation with enhanced protection in CD47KO mice, vaccine-specific antibody levels were determined in BALF by 6 days postchallenge (Fig. 7). Consistent with serum antibody responses, IgG antibodies were induced at significantly higher levels in the lungs of vaccinated CD47KO mice than those of naive WT, naive CD47KO, and vaccinated WT mice. In addition to IgG antibodies specific for iPR8 virus, WT and CD47KO mice with vaccination showed increased levels of IgA in the BALF compared to naive WT and CD47KO mice, respectively (Fig. 7). Interestingly, the level of specific IgA was ∼2-fold higher in vaccinated CD47KO mice than that in vaccinated WT. These results support that influenza VLP immunization efficiently induces specific IgG and IgA antibodies in mucosal tissues more effectively in CD47KO mice than in WT mice.
FIG 7.
Mucosal vaccination induces enhanced antigen-specific IgG and IgA in CD47KO mice. BALF was used to determine mucosal antibody responses at 6 dpi. Antigen-specific IgG and IgA levels were analyzed by ELISA against inactivated A/PR8/34 virus and measured as the OD450. The statistical significance was determined by using one-way ANOVA. Error bars indicate the means ± the SEM of the concentrations or ratios from individual animals. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
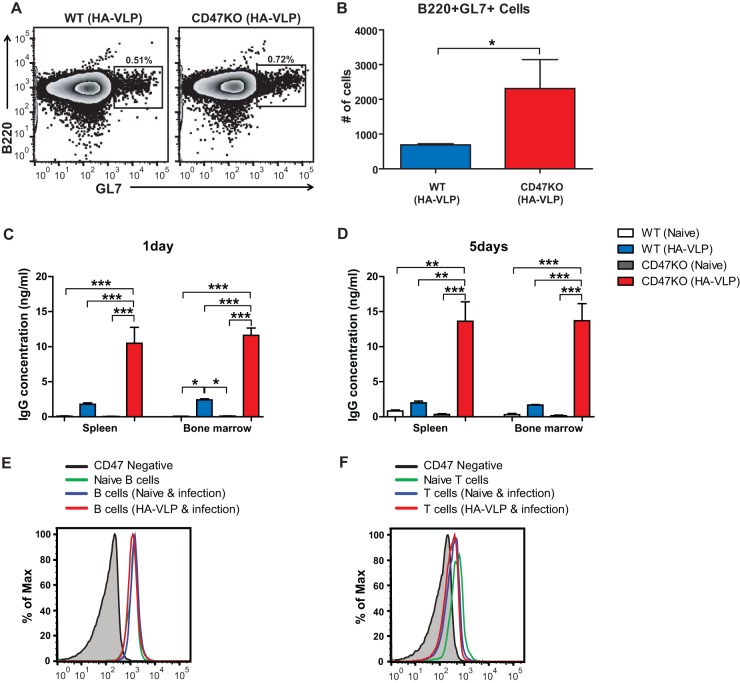
VLP immunization enhances memory B and plasma cell responses in CD47KO mice.
GL7 is used as a marker for germinal center B cells (33). To determine whether CD47 deficiency would influence B cell activation and the formation of the germinal center, GL7+ germinal center B cells (CD19+ B220+) were analyzed by flow cytometry in MLN of immunized mice (n = 6) at 6 dpi. The frequency of GL7+ B cells in CD47KO mice was higher than in WT mice (Fig. 8A). In addition, the total number of GL7+ MLN B cells in CD47KO mice was 3-fold higher than in WT mice (Fig. 8B). We analyzed in vitro antibody production to determine whether CD47 deficiency would contribute to generating antigen-specific memory B cells, and plasma cells, splenocytes, and bone marrow cells were harvested and cultured in 96-well plates coated with iPR8 virus. Larger amounts of specific IgG antibodies were detected in the spleens and bone marrow of vaccinated CD47KO mice than in those of vaccinated WT mice at 1 or 5 days after in vitro incubation (Fig. 8C and D). As expected, very low levels of specific antibodies were observed in naive WT and CD47KO mice compared to vaccinated WT and CD47KO mice. More importantly, vaccinated CD47KO mice showed significantly higher levels of virus-specific antibody-secreting cell responses. We next determined CD47 expression levels on B and T cells under naive and infection conditions. The levels of CD47 expression on B cells were similarly observed regardless of infection or immunization of mice (Fig. 8E). B cells are likely to express higher levels of CD47 compared to those in T cells (Fig. 8F). Interestingly, CD47 expression on T cells may be downregulated slightly after viral infection compared to that on T cells in naive mice, although these differences were minor due to low expression levels (Fig. 8F). Therefore, these results suggest that CD47 would have a more significant role in B cell immunity than in T cells and that CD47 may act as a negative regulator in B cell activation and differentiation.
FIG 8.
CD47 deficiency enhances memory humoral immune responses. B cell activation and memory antibody responses were determined in the MLN, spleen, and bone marrow 6 days after challenge with influenza virus. The frequency (A) and number (B) of GL7+ germinal center B cells were analyzed in the MLN of immunized mice. The cells from the spleens and bone marrows were collected and cultured for 1 day (C) or 5 days (D) to detect IgG specific to inactivated A/PR8 virus. The CD47 expression levels on B cells (E) and T cells (F) were determined under naive and infection conditions using flow cytometry. The statistical significance was determined by using an unpaired two-tailed Student t test or one-way ANOVA. Error bars indicate the means ± the SEM of the concentrations or ratios from individual animals. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, no significant difference.
DISCUSSION
In this study, we have shown that CD47 deficiency in mice resulted in generating enhanced levels of antigen-specific IgG antibodies and a higher efficacy of protection after vaccination despite low levels of antigen-nonspecific natural IgG serum antibodies. We provide evidence here that CD47 plays a role as a negative regulator on B cells in inducing antigen-specific antibodies. In addition, CD47 appeared to have a regulatory role in generating germinal center B cells and antibody-secreting plasma cells after VLP vaccination and virus challenge.
Reduced CD4+ T cells in numbers were observed in the spleen of either CD47 or SIRPα deficient mice, suggesting that interaction between CD47 and its ligand SIRPα plays an important role in T cell homeostasis (16). In line with a previous study, we found that the numbers of CD4+ T cells, but not of CD8+ T and B cells, were reduced in the spleens of CD47-deficient mice (Fig. 1B). Also, naive CD47KO mice maintain low levels of IgG antibodies. Therefore, it was expected that CD47KO mice would have defects in inducing protective antibody immune responses after influenza VLP vaccination. Unexpectedly, higher levels of antigen-specific IgG and IgG2c antibodies were observed in VLP-vaccinated CD47KO mice. It has been shown that DCs and the cytokine milieu influence a Th1 immune response. Consistent with the Th1 type IgG isotype responses in this study, a previous study demonstrated that CD47 deficiency enhanced a Th1 dominant cell response, producing IFN-γ and a Th1-biased antibody response (22), implying that CD47 is a negative regulator of the Th1 immune response. Thus, we expected that vaccination with VLPs might promote an enhanced Th1 dominant response and in turn induce a high ratio of IgG2c isotype antibody, although similar numbers of antigen-specific CD4+ and CD8+ T cells were observed in the lungs of nonimmune WT and CD47KO mice, as well as in immune WT and CD47KO mice. One possible explanation for the low Th1 cell responses in CD47KO mice is that high viral loads in immune WT mice might be a driving factor in boosting higher levels of IFN-γ+ CD4+ T cells primed in immune WT mice compared to those in immune CD47KO mice. The results in this study show that CD47KO mice vaccinated with influenza VLP are more effective at generating protective immunity against a lethal dose of A/PR8/34 virus, as evidenced by the lower viral titers at day 6 and no loss in body weight after challenge. Consistent with this active immunization and protection, naive CD47KO mice were found to induce higher levels of virus-specific IgG antibodies and to be more effectively protected after viral infection compared to WT mice. Thus, these results suggest that CD47 negatively modulates protective immune responses, which is consistent with previous findings (22).
In contrast to our findings, others have demonstrated that CD47KO mice have a defect in producing antigen-specific antibodies in response to intravenous particulate antigens due to the fact that the levels of CD11c+ 33D1+ marginal-zone DCs were low in the spleen, which is important for taking up blood-borne antigens (34). However, the B cell responses to soluble antigens were normal, implying that the T and B cells are not defective (17). Oral immunization induced a significantly lower level of intestinal ovalbumin (OVA)-IgA from CD47KO mice compared to WT mice, but similar levels of serum OVA-IgG were detected in CD47KO and WT mice (35). We found that viral infection of CD47KO mice at a sublethal dose induced higher levels of virus specific IgG antibodies compared to WT mice, which is consistent with the results from VLP vaccination. Thus, although it is possible that CD47 is a key regulator in inducing specific humoral immunity to viral and VLP vaccine antigens, further studies are required to better understand the immunological roles of CD47 in terms of the different routes of vaccination.
CD47 helps in the homing of respiratory CD11b+ DCs to the draining lymph nodes during steady- and inflammatory-state conditions (15). However, no difference was found in the expression of costimulatory molecules such as CD40, CD80, or CD86 (17). Consistent with a previous finding, we also observed that reduced numbers of respiratory DCs were detected in the MLN of nonimmune CD47KO mice compared to those in nonimmune WT mice at day 6 after influenza virus infection. Delayed DC migration into the MLN may increase the duration of antigen stimulation to activate T cells in the infection site. In other words, antibodies can be rapidly induced at the site of T and B cell zones (extrafollicular sites) in the draining lymph nodes, but short-lived plasma cells can be generated (36). However, the slower antigen stimulation in the germinal centers (intra- and/or extrafollicular sites) can generate antigen-specific memory B cells and long-lived plasma cells (37, 38). Influenza virus infection induces antigen persistence, which can induce polyclonal memory CD4+ T cell responses 3 to 4 weeks after viral clearance (39), suggesting that sustained CD4+ T cells may contribute to increasing antigen-specific antibody production. In addition, prolonged antigens, which may have adjuvant-like depot effects, are able to continuously stimulate antibody-secreting cells, resulting in high levels of antibody production (40). This hypothesis is further supported by our findings that influenza VLP vaccination of CD47KO mice induced higher levels of germinal center phenotypic B cells, memory B cells, and antibody-secreting plasma cells upon antigen exposure. Importantly, we found that B cells expressed higher levels of CD47 compared to those in T cells and that antigen-specific antibody response was enhanced in the absence of CD47. These results provide evidence that CD47 is a negative regulator in humoral immune responses to viral infection or vaccination. This finding suggests that CD47 may have a more significant role in B cell immunity than in T cells.
Alternatively, other cell types may be involved in increased antigen-specific antibody production. The SIRPα signal pathway is not blocked in antigen-presenting cells in the absence of CD47. The interaction of SIRPα with CD47 on macrophages induces the phosphorylation of the cytoplasmic immunoreceptor tyrosine-based inhibition motif (ITIM) of SIRPα by Src homology region 2 domain-containing phosphatase 1 (SHP-1) and SHP-2, which can inhibit signaling pathways, including the mitogen-activated protein kinases and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (41). Furthermore, a previous study demonstrated that ligation of CD47 on human DCs with antibodies limited inflammatory cytokine responses but not their functions (42), which implied enhanced cytokine production during vaccination with influenza VLPs in CD47KO mice. It is possible that CD47 deficiency may influence antigen-presenting cells and CD4+ T cell activation pathway, as well as B cell activation. In line with previous findings, we detected that CD47KO mice induced more F4/80+ CD80high and F4/80+ MHC-IIhigh activated macrophages in the lungs than did WT mice after VLP vaccination. This suggests that F4/80+ CD80high and F4/80+ MHC-IIhigh activated macrophages further activate CD4+ T cells and/or induce cytokines, which can provide a biased help to the B cells to induce IgG2c isotype antibodies more effectively. In addition, it was reported that macrophages showed increased phagocytosis of cancer cells in the absence of CD47-mediated signaling (43). Interestingly, SIRPα-Fc treatment interfering with the CD47 pathway was able to reduce Th2-driven allergic airway inflammation (44), a finding which is in line with the results of IgG2c dominant antibody responses in CD47KO mice after i.n. VLP vaccination.
Our understanding of the mechanisms for triggering respiratory disease is largely unknown. However, there is evidence that inflammatory cytokines and the infiltration of leukocytes can cause severe respiratory illness during influenza virus infection. We have demonstrated that unimmunized WT and CD47KO mice, as well as immunized WT mice, showed high levels of TNF-α, IL-6, and IL-10, as well as the infiltration of leukocytes after challenge (Fig. 4 and 5), whereas immunized CD47KO mice with large amounts of specific antibodies yielded only small amounts of inflammatory and anti-inflammatory cytokines. In support for our findings, TNF-α and IL-1 are able to upregulate leukocyte adhesion molecules and IL-6 (45). High levels of TNF-α and IL-6 cytokines therefore indicate a severe inflammatory condition upon influenza virus challenge. In addition, the levels of an anti-inflammatory cytokine, IL-10, were high in an unimmunized WT group compared to immunized WT and CD47KO groups, suggesting that IL-10 may be required to downregulate excessive lung inflammation in a naive host after infection. In support of our observation, a study suggested that a high IL-10 level may be an indicator of disease progression and severity (46). Defective neutrophil migration was observed in the absence of CD47 expression (13). A deficiency or blockade of CD47 is protected from lipopolysaccharide (LPS)-induced acute inflammatory disease and Th2-driven airway inflammation by inhibiting the migration of leukocytes and neutrophils, respectively (44, 47). However, in the present study, naive CD47KO mice showed higher or similar cellularity of neutrophils in the BALF and lungs compared to those in naive WT mice upon influenza virus infection, suggesting no defect in the migration of CD47-deficient neutrophils into the lungs. Also, naive CD47KO mice were able to induce higher levels of virus specific antibodies, resulting in more resistance to a lower dose of viral infection than for WT mice. It is also possible that the roles of CD47 in modulating monocyte and neutrophil migration into the inflamed tissues might be different between LPS injection and influenza virus infection. Eventually, high levels of virus-specific IgG antibody responses by influenza VLP vaccination of CD47KO mice resulted in effective control of lung viral loads, leading to a reduction in inflammation and less leukocyte recruitment.
Taken together, our results demonstrate that influenza VLP vaccination can induce larger amounts of virus-specific antibodies and is able to confer enhanced protection against lethal doses of influenza virus infections in the absence of CD47. In addition, CD47KO mice generated high numbers of germinal center B cells and antibody-secreting memory cells. These studies provide insights into possible negative regulatory roles of CD47 on B cell activation in developing antigen-specific antibody responses. Further study is required to elucidate the underlying mechanisms by which CD47 negatively regulates B cell activation.
REFERENCES
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. 2007. The annual impact of seasonal influenza in the United States: measuring disease burden and costs. Vaccine 25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Buonaguro L, Tornesello ML, Tagliamonte M, Gallo RC, Wang LX, Kamin-Lewis R, Abdelwahab S, Lewis GK, Buonaguro FM. 2006. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J Virol 80:9134–9143. doi: 10.1128/JVI.00050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moron VG, Rueda P, Sedlik C, Leclerc C. 2003. In vivo, dendritic cells can cross-present virus-like particles using an endosome-to-cytosol pathway. J Immunol 171:2242–2250. doi: 10.4049/jimmunol.171.5.2242. [DOI] [PubMed] [Google Scholar]
- 5.Song H, Wittman V, Byers A, Tapia T, Zhou B, Warren W, Heaton P, Connolly K. 2010. In vitro stimulation of human influenza-specific CD8+ T cells by dendritic cells pulsed with an influenza virus-like particle (VLP) vaccine. Vaccine 28:5524–5532. doi: 10.1016/j.vaccine.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Sailaja G, Skountzou I, Quan FS, Compans RW, Kang SM. 2007. Human immunodeficiency virus-like particles activate multiple types of immune cells. Virology 362:331–341. doi: 10.1016/j.virol.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM. 2010. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 405:165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quan FS, Huang C, Compans RW, Kang SM. 2007. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol 81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan FS, Vunnava A, Compans RW, Kang SM. 2010. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS One 5:e9161. doi: 10.1371/journal.pone.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown EJ, Frazier WA. 2001. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 11:130–135. doi: 10.1016/S0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 11.Matozaki T, Murata Y, Okazawa H, Ohnishi H. 2009. Functions and molecular mechanisms of the CD47-SIRPα signaling pathway. Trends Cell Biol 19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Seiffert M, Brossart P, Cant C, Cella M, Colonna M, Brugger W, Kanz L, Ullrich A, Buhring HJ. 2001. Signal-regulatory protein alpha (SIRPα) but not SIRPβ is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34+ CD38− hematopoietic cells. Blood 97:2741–2749. doi: 10.1182/blood.V97.9.2741. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, O'Connor MB, Mandell KJ, Zen K, Ullrich A, Buhring HJ, Parkos CA. 2004. Peptide-mediated inhibition of neutrophil transmigration by blocking CD47 interactions with signal regulatory protein alpha. J Immunol 172:2578–2585. doi: 10.4049/jimmunol.172.4.2578. [DOI] [PubMed] [Google Scholar]
- 14.Van VQ, Lesage S, Bouguermouh S, Gautier P, Rubio M, Levesque M, Nguyen S, Galibert L, Sarfati M. 2006. Expression of the self-marker CD47 on dendritic cells governs their trafficking to secondary lymphoid organs. EMBO J 25:5560–5568. doi: 10.1038/sj.emboj.7601415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raymond M, Rubio M, Fortin G, Shalaby KH, Hammad H, Lambrecht BN, Sarfati M. 2009. Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of TH2-mediated allergic inflammation. J Allergy Clin Immunol 124:1333–1342 e1331. doi: 10.1016/j.jaci.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Sato-Hashimoto M, Saito Y, Ohnishi H, Iwamura H, Kanazawa Y, Kaneko T, Kusakari S, Kotani T, Mori M, Murata Y, Okazawa H, Ware CF, Oldenborg PA, Nojima Y, Matozaki T. 2011. Signal regulatory protein alpha regulates the homeostasis of T lymphocytes in the spleen. J Immunol 187:291–297. doi: 10.4049/jimmunol.1100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagnerud S, Manna PP, Cella M, Stenberg A, Frazier WA, Colonna M, Oldenborg PA. 2006. Deficit of CD47 results in a defect of marginal zone dendritic cells, blunted immune response to particulate antigen and impairment of skin dendritic cell migration. J Immunol 176:5772–5778. doi: 10.4049/jimmunol.176.10.5772. [DOI] [PubMed] [Google Scholar]
- 18.Kang SM, Kim MC, Compans RW. 2012. Virus-like particles as universal influenza vaccines. Expert Rev Vaccines 11:995–1007. doi: 10.1586/erv.12.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang SM, Pushko P, Bright RA, Smith G, Compans RW. 2009. Influenza virus-like particles as pandemic vaccines. Curr Top Microbiol Immunol 333:269–289. doi: 10.1007/978-3-540-92165-3_14. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Macias C, Ferat-Osorio E, Tenorio-Calvo A, Isibasi A, Talavera J, Arteaga-Ruiz O, Arriaga-Pizano L, Hickman SP, Allende M, Lenhard K, Pincus S, Connolly K, Raghunandan R, Smith G, Glenn G. 2011. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine 29:7826–7834. doi: 10.1016/j.vaccine.2011.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khurana S, Wu J, Verma N, Verma S, Raghunandan R, Manischewitz J, King LR, Kpamegan E, Pincus S, Smith G, Glenn G, Golding H. 2011. H5N1 virus-like particle vaccine elicits cross-reactive neutralizing antibodies that preferentially bind to the oligomeric form of influenza virus hemagglutinin in humans. J Virol 85:10945–10954. doi: 10.1128/JVI.05406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouguermouh S, Van VQ, Martel J, Gautier P, Rubio M, Sarfati M. 2008. CD47 expression on T cell is a self-control negative regulator of type 1 immune response. J Immunol 180:8073–8082. doi: 10.4049/jimmunol.180.12.8073. [DOI] [PubMed] [Google Scholar]
- 23.Gresham HD, Dale BM, Potter JW, Chang PW, Vines CM, Lowell CA, Lagenaur CF, Willman CL. 2000. Negative regulation of phagocytosis in murine macrophages by the Src kinase family member, Fgr. J Exp Med 191:515–528. doi: 10.1084/jem.191.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. 1996. Essential role of Stat6 in IL-4 signaling. Nature 380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 25.Snapper CM, Paul WE. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 26.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. 2007. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol 81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kash JC, Basler CF, Garcia-Sastre A, Carter V, Billharz R, Swayne DE, Przygodzki RM, Taubenberger JK, Katze MG, Tumpey TM. 2004. Global host immune response: pathogenesis and transcriptional profiling of type A influenza viruses expressing the hemagglutinin and neuraminidase genes from the 1918 pandemic virus. J Virol 78:9499–9511. doi: 10.1128/JVI.78.17.9499-9511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith KA, Harcus Y, Garbi N, Hammerling GJ, MacDonald AS, Maizels RM. 2012. Type 2 innate immunity in helminth infection is induced redundantly and acts autonomously following CD11c+ cell depletion. Infect Immun 80:3481–3489. doi: 10.1128/IAI.00436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. 2008. Gr1+ inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legge KL, Braciale TJ. 2003. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 18:265–277. doi: 10.1016/S1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 31.Masopust D, Vezys V, Marzo AL, Lefrancois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 32.Crowe SR, Miller SC, Brown DM, Adams PS, Dutton RW, Harmsen AG, Lund FE, Randall TD, Swain SL, Woodland DL. 2006. Uneven distribution of MHC class II epitopes within the influenza virus. Vaccine 24:457–467. doi: 10.1016/j.vaccine.2005.07.096. [DOI] [PubMed] [Google Scholar]
- 33.Pasare C, Medzhitov R. 2005. Control of B-cell responses by Toll-like receptors. Nature 438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 34.Martin F, Kearney JF. 2002. Marginal-zone B cells. Nat Rev Immunol 2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 35.Westlund J, Livingston M, Fahlen-Yrlid L, Oldenborg PA, Yrlid U. 2012. CD47-deficient mice have decreased production of intestinal IgA following oral immunization but a maintained capacity to induce oral tolerance. Immunology 135:236–244. doi: 10.1111/j.1365-2567.2011.03536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. 1996. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol 26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 37.Wolniak KL, Shinall SM, Waldschmidt TJ. 2004. The germinal center response. Crit Rev Immunol 24:39–65. doi: 10.1615/CritRevImmunol.v24.i1.20. [DOI] [PubMed] [Google Scholar]
- 38.Chappell CP, Jacob J. 2006. Identification of memory B cells using a novel transgenic mouse model. J Immunol 176:4706–4715. doi: 10.4049/jimmunol.176.8.4706. [DOI] [PubMed] [Google Scholar]
- 39.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. 2005. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med 202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stills HF., Jr 2005. Adjuvants and antibody production: dispelling the myths associated with Freund complete and other adjuvants. ILAR J 46:280–293. doi: 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- 41.Kong XN, Yan HX, Chen L, Dong LW, Yang W, Liu Q, Yu LX, Huang DD, Liu SQ, Liu H, Wu MC, Wang HY. 2007. LPS-induced down-regulation of signal regulatory protein α contributes to innate immune activation in macrophages. J Exp Med 204:2719–2731. doi: 10.1084/jem.20062611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. 2000. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol 164:2193–2199. doi: 10.4049/jimmunol.164.4.2193. [DOI] [PubMed] [Google Scholar]
- 43.Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, Weissman IL. 2013. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A 110:11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raymond M, Van VQ, Rubio M, Welzenbach K, Sarfati M. 2010. Targeting SIRP-alpha protects from type 2-driven allergic airway inflammation. Eur J Immunol 40:3510–3518. doi: 10.1002/eji.201040797. [DOI] [PubMed] [Google Scholar]
- 45.Van Reeth K. 2000. Cytokines in the pathogenesis of influenza. Vet Microbiol 74:109–116. doi: 10.1016/S0378-1135(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, Zhang X, Zhao B, Wang J, Zhu Z, Teng Z, Shao J, Shen J, Gao Y, Yuan Z, Wu F. 2011. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS One 6:e28680. doi: 10.1371/journal.pone.0028680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su X, Johansen M, Looney MR, Brown EJ, Matthay MA. 2008. CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J Immunol 180:6947–6953. doi: 10.4049/jimmunol.180.10.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]