Abstract
Background:
Proliferation and differentiation of cancer cells are regulated by various cell cycle promoting and inhibiting factors. Our knowledge about these proteins and mechanisms regulating cell cycle progression has increased dramatically in recent years.
Aim:
The present study was undertaken to examine the expression profile of cell cycle regulatory proteins in normal proliferative endometrium, hyperplasias (simple, complex and atypical) and endometrial carcinoma in a quantitative approach as also to assess correlations of Cyclin D1 expression with Ki-67 a proliferation marker.
Settings and Design:
A retrospective case control study in a tertiary referral centre.
Materials and Methods:
We evaluated and compared the expression profile of Cyclin D1 and Ki-67 expressions in 61 endometrial samples submitted as either endometrial curetting or hysterectomy specimens, which were diagnosed as simple hyperplasia (n =11), complex hyperplasia (n = 13), atypical hyperplasia (n = 7), and endometrial carcinoma (n = 20).
Results:
There was increased expression of Cyclin D1 and Ki-67 in patients with endometrial carcinoma relative to proliferative endometrium and simple hyperplasia, but there was no such difference between cases of atypical hyperplasia and endometrial carcinoma. Cyclin D1 expression had a positive correlation with Ki-67 expression. Cyclin D1 together with Ki-67 may be a marker for endometrial carcinogenesis and tumor cell proliferation.
KEY WORDS: Atypical hyperplasia endometrium, complex hyperplasia, cyclin D1, endometrial carcinoma, endometrium, Ki-67
Introduction
Endometrial cancer (EC) is the fourth most common cancer occurring in women after breast, bowel and lung cancers and is derived from the endometrial epithelial lining of the uterine corpus. Clinicopathological, immunohistochemical and molecular genetic studies during the last two decades have generated substantial data to support the development of a dualistic model of endometrial carcinoma (EC)[1] and proposed two types of precursor lesions. Atypical hyperplasia (AH) is considered as a precursor of endometrioid type of EC and endometrial intraepithelial carcinoma (EIC) is considered the precursor of serous carcinoma.
Endometrial hyperplasia on the other hand refers to biologically, genetically, and clinically admixed groupings of hormonally altered and premalignant processes which are at times difficult to categorize. Poor reproducibility of endometrial WHO hyperplasia subgroup diagnoses has plagued pathologists, with studies reporting that half or fewer than half of diagnoses of atypical endometrial hyperplasia are confirmed by a second pathologist on review.[2,3]
Immunohistochemical methods have been useful for detecting several biomarkers of possible prognostic importance for a number of cancer types.[4] Cyclin D1 is a key protein in the regulation of the cell cycle at the G1 to S phase transition, and is essential for regulation of proliferation, differentiation and transcriptional control.[5,6] Overexpression of Cyclin D1, a cell cycle regulator that is essential for regulation of proliferation, differentiation and transcriptional control,[5,6] induces excessive cellular proliferation and is often overexpressed in human neoplasias e.g. in situ and infiltrating ductal breast carcinoma, colorectal carcinoma, bladder carcinoma, head and neck, lung and prostate cancers by rearrangement, amplification and mutation of the genes.[7,8,9,10] Several studies have reported increased cellular proliferation co-existing with progressive derailment of Cyclin D1 leading to progression of endometrial hyperplasia to EC (Type I). Nikaido et al.[11] reported that about 40% of ECs overexpress Cyclin D1 and proposed that Cyclin D1 deregulation may have a role in endometrial carcinogenesis. The present study aimed to study the expression pattern of Cyclin D1 in normal proliferative endometrium (PE), hyperplastic endometrium (simple, complex and atypical) and EC, to compare it with expression of Ki-67 which has a direct correlation with the degree of proliferation of cells[12] and to explore the possibility for Cyclin D1 as a potential diagnostic marker to distinguish these endometrial disorders.
Materials and Methods
Ethics: The Institute Ethics Committee of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, provided a consent waiver and approved the study. All samples were coded using unique identifiers.
Design: A retrospective study done in the Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi.
Study material: Endometrial samples submitted either as endometrial curetting or hysterectomy specimens of the patients diagnosed as endometrial hyperplasia and EC over 45 months. Endometrial biopsies diagnosed as PE were selected for the control group. The hematoxylin and eosin (H&E)-stained sections and representative blocks were retrieved from the archives of the Department of Pathology.
Study procedure: Immunohistochemistry for Cyclin D1 and Ki-67 was done using biotin-free antibody (supersensitive polymer-HRP detection method) manufactured by Biogenex Inc., USA. The quantitative evaluation of immunostaining for Cyclin D1 and Ki-67 was done by assessing extent of nuclear positivity. Due to the heterogeneity of the reaction, the area with the highest level of nuclear staining was selected and the number of positively stained nuclei in 1000 glandular cells was counted under 40 × magnification and results were expressed as percentage staining.
Statistical analysis: Pairwise comparisons were performed by the Mann-Whitney U test. Comparisons across multiple groups were performed by Kruskal-Wallis nonparametric one-way analysis using the SPSS statistical software version 16. The results were considered significant with a P value of less than 0.05.
Results
Demographics: Over 45 months, 38 cases of EC, 26 cases of complex hyperplasia (CH) and 78 cases of simple hyperplasia (SH) were diagnosed. The age of 38 patients of EC ranged from 35 to 79 years with a median age of 46 years. Sixty-three percent of patients were between 40 and 59 years with the peak incidence (17 cases, 44.7%) observed at the age group of 40-49 years. Twenty-six cases of CH aged between 20 and 69 years and the median age was 45 years. The maximum number of patients (15 cases, 57.7%) was in the age group of 40-49 years. Seventy-eight patients of SH aged between 20 and 69 years with the median age of 43.5 years.
Immunohistochemistry analysis: Of the above-mentioned 142 cases (EC = 38, CH = 26, SH = 78) immunohistochemical analysis was performed in 61 cases which included 20 cases of EC (well-differentiated endometrioid carcinoma [WDEC], moderately differentiated endometrioid carcinoma [MDEC], poorly differentiated endometrioid carcinoma [PDEC], serous papillary endometrioid carcinoma [SPEC]), 7 cases of AH, 13 cases of CH without atypia, 11 cases of SH without atypia and 10 cases of normal PE as control. The median age of these cases on which immunohistochemistry has been done was 45 years in the category of EC (n = 20, age range 30-79 years) as well as in the category of CH (n = 17, age range 30-69 years). The median age of the patients in the group of SH was 40 years (n = 14, age range 20-69 years).
Cyclin D1 and Ki-67 expression: For all cases, these are shown in Table 1. The median expression of Cyclin D1 was 40% in the cases of EC (n = 20) [Figure 1b]. Minimum expression of 2% was recorded in mucinous adenocarcinoma, whereas highest expression of 80% staining was recorded in four cases (two cases of WDEC, one case each of MDEC and clear cell carcinoma). The median expression of Ki-67 in the cases of EC was 57.5%, the minimum expression of 2% and maximum expression value was 80% [Figure 1c]. In the seven cases of AH, Cyclin D1 expression varied in a range of 23% to 51% [Figure 1e] with a median value of 30%, whereas Ki-67 expression varied from 25% to 55% [Figure 1f] with the median value of 30%. The highest value of expression for Cyclin D1 and Ki-67 was 51% and 55%, respectively, in this category. In cases of CH, immunoreactivity for Cyclin D1 varied from 7% to 40% [Figure 1h], whereas the expression of Ki-67 varied from 10% to 40% [Figure 1i]. The median value for Cyclin D1 expression and Ki-67 expression was 20% and 27%, respectively, among the cases of CH. The expression of Cyclin D1 in 11 cases of SH varied from 5% to 20% [Figure 1k]. For Ki-67, the values ranged from 7-50%. [Figure 1l]. The median values for Cyclin D1 and Ki-67 were 10% and 20%, respectively. The expression profile of Cyclin D1 and Ki-67 is also shown in a box plot [Figures 2 and 3]. For Cyclin D1 it was found that that 55% of ECs, 57.14% of AH and 7.6% of CH displayed >30% nuclear staining, whereas <30% nuclear staining was observed in 45% of ECs 42.8% of AH93% of CH and 100% of SH and PEs [Table 2]. Less than 10% nuclear staining was found in 70% of PE, 54.5% of SH, 23.1% of CH and 30% of ECs. More than 30% nuclear staining for Ki-67 was observed in 70% of ECs, 28.5% of AH, 23% of CH and in 9% of SH whereas <30% nuclear staining for Ki-67 was observed in 30% of ECs, 71.4% of AH, 78% of CH and 90% of SH and 100% of PEs.
Table 1.
Percentage expression profile of cyclin D1 and Ki-67 in cases of proliferative endometrium, simple hyperplasia, complex hyperplasia, atypical hyperplasia and endometrial carcinoma
| Proliferative endometrium | Simple hyperplasia | Complex hyperplasia | Atypical hyperplasia | Endometrial carcinoma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S.No | Cyclin D | Ki-67 | S.No | Cyclin D | Ki-67 | S.No | Cyclin-D | Ki-67 | S.No | Cyclin-D | Ki-67 | S.No | Ki-67 | Cyclin-D |
| PE 1 | 20 | 30 | SH1 | 5 | 30 | CH1 | 40 | 10 | AH1 | 23 | 25 | WDEC | 20 | 52 |
| PE 2 | 0 | 10 | SH2 | 15 | 20 | CH2 | 30 | 10 | AH2 | 51 | 55 | WDEC | 20 | 55 |
| PE 3 | 10 | 10 | SH3 | 10 | 7 | CH3 | 25 | 25 | AH3 | 25 | 30 | MAEC | 2 | 2 |
| PE 4 | 15 | 25 | SH4 | 10 | 12 | CH4 | 15 | 20 | AH4 | 30 | 30 | WDEC | 40 | 70 |
| PE 5 | 7 | 15 | SH5 | 7 | 10 | CH5 | 20 | 10 | AH5 | 35 | 25 | CCEC | 18 | 60 |
| PE 6 | 5 | 20 | SH6 | 10 | 25 | CH6 | 25 | 20 | AH6 | 25 | 25 | MDEC | 25 | 60 |
| PE 7 | 10 | 30 | SH7 | 10 | 30 | CH7 | 20 | 15 | AH7 | 30 | 35 | WDEC | 80 | 60 |
| PE 8 | 10 | 10 | SH8 | 20 | 25 | CH8 | 10 | 35 | WDEC | 7 | 10 | |||
| PE 9 | 15 | 30 | SH9 | 10 | 50 | CH9 | 30 | 27 | PDEC | 60 | 12 | |||
| PE 10 | 10 | 30 | SH10 | 10 | 15 | CH10 | 7 | 10 | CCEC | 51 | 80 | |||
| SH11 | 12 | 15 | CH11 | 20 | 40 | MDEC | 40 | 80 | ||||||
| CH12 | 15 | 20 | WDEC | 51 | 55 | |||||||||
| CH13 | 20 | 40 | WDEC | 80 | 60 | |||||||||
| CCEC | 51 | 60 | ||||||||||||
| CCEC | 80 | 70 | ||||||||||||
| WDEC | 40 | 51 | ||||||||||||
| SPEC | 7 | 30 | ||||||||||||
| MDEC | 25 | 35 | ||||||||||||
| MDEC | 80 | 80 | ||||||||||||
| WDEC | 7 | 20 | ||||||||||||
| Median | 10 | 22.5 | 10 | 20 | 20 | 27 | 30 | 30 | 40 | 57.5 | ||||
PE – Proliferative endometrium; AH – Atypical hyperplasia; CH – Complex hyperplasia; SH – Simple hyperplasia; WDEC – Well-differentiated endometrioid carcinoma; MDEC – Moderately differentiated endometrioid carcinoma; PDEC – Poorly differentiated endometrioid carcinoma; CCEC – Clear cell endometrial carcinoma; SPEC – Serous papillary endometrial carcinoma
Figure 1.
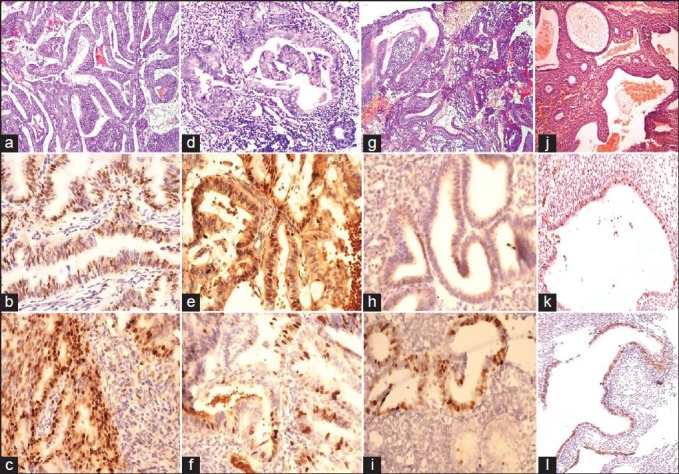
(a) Irregular endometrial glands with back to back arrangement, nuclear pleomorphism and hyperchromasia in a case of endometroid carcinoma (EC) (H&E 400×). (b) Cyclin D1 nuclear expression in papillary adenocarcinoma (400×). (c) Intense nuclear expression of Ki-67 in the case of EC (400×). (d) Atypical hyperplasia (AH) shows crowded, irregular and distorted glands lined by stratified columnar epithelial lining displaying nuclear atypia, loss of polarity, nuclear rounding and hyperchromatic nuclei. Stroma is present between the glands (H&E 400×). (e) Cyclin D1 expression in AH. Diffuse cytoplasmic staining also noted (400×). (f) Ki-67 expression in AH (400×). (g) Irregularly arranged crowded glands with intraluminal folding, stratifications in a case of complex hyperplasia (CH). No nuclear atypia present. (H&E 400×). (h) Cyclin D1 expression in CH without atypia (400×). (i) Ki-67 expression in CH without atypia (400×). (j) Cystically dilated endometrial glands filled with secretions in Simple cystic hyperplasia (SH), few tubular glands are also seen (H&E, 100×). (k) Cyclin D1 expression in SH without atypia (40×). (l) Ki-67 expression in SH without atypia (40×)
Figure 2.
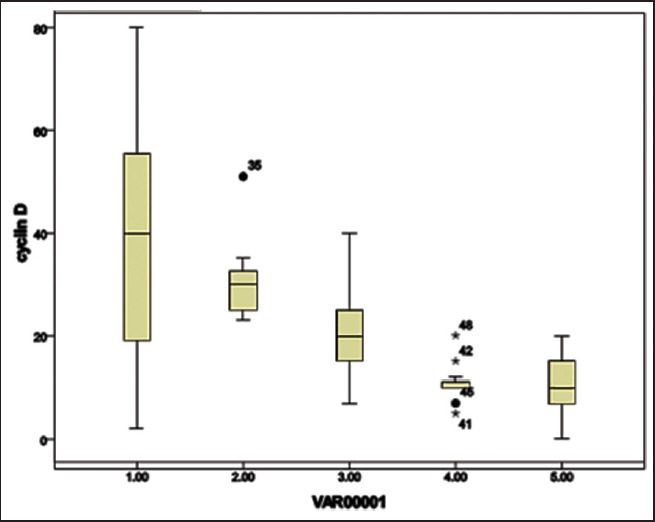
Box plot showing expression of Cyclin D1 over the spectrum of lesions. Cyclin D1 expression in percent is shown on the y-axis. Various groups on the x-axis:- 1 = Endometrial carcinoma [median 40, interquartile range 19.5-53.25]; 2 = Atypical hyperplasia [median 30; interquartile range 25-32.5). •35 represents extreme outlier (case no. 2 of Table 15); 3= Complex hyperplasia (median 20; interquartile range 15-25) 4 = Simple hyperplasia (median 10; interquartile range 10-11 with 4 extreme outliers *41.•45,*42,*48 representing case no. 1, 5, 2 and 8 of Table 17, respectively) and proliferative endometrium (median 10; interquartile range 7.75-13.5)
Figure 3.
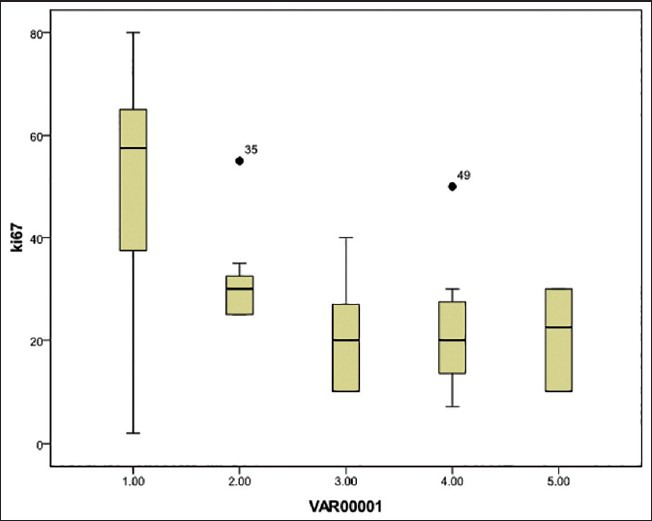
Box plot showing expression of Ki-67 over the spectrum of lesions representing endometrial carcinogenesis. Ki-67 expression in percent is shown on the y-axis. Various groups are plotted on the x-axis. 1= Endometrial carcinoma group [median 57.5; interquartile range 38.75-62.5]; 2 = Atypical hyperplasia group [median 30; interquartile range 25-32.5] • 35 = Extreme outlier case no. 2 of Table 15; 3= Complex hyperplasia group [median 20; interquartile range 10-27] 4= Simple hyperplasia group [median 20; interquartile range 13.5-27.5, • 49= Extreme outlier case no. 9 of Table 17]; and 5 = Proliferative endometrium group [median 22. 5; interquartile range 11.25-30]
Table 2.
Expression of cyclin D1 and Ki-67 in endometrial carcinoma, atypical hyperplasia, complex hyperplasia, simple hyperplasia, proliferative endometrium
| Expression (%) | Endometrial carcinoma n = 20 (%) | Atypical hyperplasia n = 7 (%) | Complex hyperplasia n = 13 (%) | Simple hyperplasia n = 11 (%) | Proliferative endometriumn = 10 (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin D1 | Ki-67 | Cyclin D1 | Ki-67 | Cyclin D1 | Ki-67 | Cyclin D1 | Ki-67 | Cyclin D1 | Ki-67 | |
| <10 | 2(10) | 6(30) | Nil | Nil | 4(30.7) | 3(23.1) | 7(63.63) | 6(54.5) | 3(30) | 7(70) |
| 11-30 | 4(20) | 3(15) | 5(71.4) | 3(42.8) | 6(46.15) | 9(69.23) | 3(27.27) | 5(45.45) | 7(70) | 3(30) |
| 31-60 | 9(45) | 7(35) | 2(28.57) | 4(57.14) | 3(23.07) | 1(7.69) | 1(9.09) | Nil | Nil | Nil |
| >60 | 5(25) | 4(20) | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
Between group analysis: Comparing the Cyclin D1 expression in all the five study groups (EC, AH, CH, SH, PE), Table 3, a statistically significant difference (P = 0.00) was observed between the groups. Also the expression profile of Cyclin D1 was compared in paired groups and a statistically significant difference was observed between the results of EC and SH (P = 0.04), SH and CH (P = 0.03), CH and AH (P = 0.02), SH and AH (P = 0.00). No statistical significant difference was seen between the results of EC and CH (P = 0.08), and EC and AH (P = 0.717). The Ki-67 expression pattern was compared between all the five study groups EC, AH, CH, SH, PE, and a statistically significant difference (P = 0.00) was observed between the groups. Further, comparing the expression profile of Ki-67 among the various (paired) groups, a statistically significant difference was observed among the results of EC and CH (P = 0.01), EC and SH (P = 0.001), EC and AH (P = 0.02), CH and AH (P = 0.01), SH and AH (P = 0.048), whereas there was no statistical difference seen between CH and SH (P > 0.05).
Table 3.
Comparison of immunoreactivity of Cyclin D1 and Ki-67 between two groups
| Comparing groups | Cyclin D1 (P value) | Ki-67 (P value) |
|---|---|---|
| Endometrial carcinoma and atypical hyperplasia | 0.717 | 0.02* |
| Endometrial carcinoma and complex hyperplasia | 0.08 | 0.001* |
| Endometrial carcinoma and simple hyperplasia | 0.04* | 0.001* |
| Complex hyperplasia and atypical hyperplasia | 0.02* | 0.01* |
| Simple hyperplasia and atypical hyperplasia | 0.00* | 0.048* |
| Simple hyperplasia and complex hyperplasia | 0.03* | 1.00 |
| Proliferative /SH/CH/Carcinoma | 0.001* | 0.00* |
| Carcinoma/AH/CH/SH/PE | 0.00* | 0.00* |
*Significant (P < 0.05); SH – Simple hyperplasia; CH – Complex hyperplasia; AH – Atypical hyperplasia; PE – Proliferative endometrium (Using Mann-Whitney test)
Discussion
Endometrial (endometrioid) carcinoma is often preceded by characteristic histopathologic lesions designated as endometrial hyperplasia[13] and currently it is accepted that there is a continuum of changes that evolve to endometrioid carcinoma. Hyperplasia is usually associated with exogenous estrogen stimulation therefore estrogen is considered as an endometrial carcinogen. Other mechanisms of endometrial carcinogenesis include mutations in p53 and PTEN tumor suppressor genes. Several authors[11,14,15] had documented overexpression of Cyclin D1 in EC and in a recent study Cyclin D1 has been shown to be a promising marker to predict lymph nodal metastasis in the patients of EC.[16] Very few studies have compared the expression of Cyclin D1 in ECs and spectrum of hyperplastic lesions.
A gradual and progressive increase in expression of both Cyclin D1 and Ki-67 levels was observed when the results were compared between normal PE, hyperplastic endometrium, atypical hyperplastic proliferations and EC. Our study revealed that expression of Cyclin D1 progressively increases from a median 10% in normal PE and SH to a median of 20% CH to 30% median expression in AH to highest median (40%) expression in EC which was statistically significant. A simultaneous and corresponding increase in the levels of Ki-67 was also observed in these groups (median 22.5%, 20%, 27%, 30% and 57.5%, respectively). A positive correlation found between median value of median Ki-67 levels and median Cyclin D1 levels in the present study is expected with cell cycle accelerating effects of high Cyclin D1 levels. A relatively higher mean Ki-67 expression in PE may be explained due to physiological influence of estrogenic stimuli. More than 50% cases of ECs showed >30% nuclear staining for both Ki-67 and Cyclin D1 whereas 57.14% and 28.5% cases of AH showed > 30% expression of Cyclin D1 and Ki-67 respectively. None of the cases of AH showed <10% staining of either Cyclin D1 for Ki-67. Statistically significant difference was observed in the expression profile of Cyclin D1 when compared between EC and SH (P = 0.04), CH and AH (P = 0.02), SH and CH (P = 0.03), SH and AH (P = 0.00). Non-significant difference was observed among the categories EC and AH (P = 0.717), EC and CH (P = 0.08) for Cyclin D1 expression results. Analyzing the results of Ki-67 expression, statistically significant difference was seen in the category of EC and AH (P = 0.01), EC and CH (P = 0.001), EC and SH (P = 0.001), SH and AH (P = 0.048) and non-significant difference was observed between the results of SH and CH.
Our findings suggest that both these cellular proteins are deregulated and therefore may have a role in endometrial carcinogenesis. Since there was no statistically significant difference in Cyclin D1 expression between AH, CH and EC, it appears that the deregulation is maximal at the level of AH and CH and that Cyclin D1 overexpression may be an early event in endometrial carcinogenesis. Our findings support the significance of CH and AH as a precancerous lesion, and the assumption that SH is not a precancerous lesion. Ruhul Quddus et al. analyzed immunoreactivity in five groups (Proliferative, Secretary, Simple hyperplasia, Complex Hyperplasia with or without atypia and EC) and have shown that they are statistically different groups. Their observations were similar to ours and have documented no difference between CH and carcinoma and between proliferative, secretory and simple hyperplasia.[16] They did not segregate AH as a separate category. Instead they merged it with the CH category in their study. They suggested that maximal dysregulation occurs at the CH state and concluded that some alterations might be responsible for the different morphologic features and behavior of CH and carcinoma. Authors have also suggested that Cyclin D1 overexpression might be an early event in endometrial carcinogenesis. Ozuysal et al. (2005)[14] observed higher mean Ki-67 values in Cyclin D1 immunoreactive endometrial adenocarcinoma when compared with Cyclin D1 nonreactive cases of endometrial adenocarcinoma (P < 0.05). Mean Ki-67 values were higher in cases with PE compared to EC and AH (P < 0.01) and non-significant difference was found between the mean Ki-67 values of cases with SH and PE. They reported no difference in Cyclin D1 immunoreactivity among AH, SH, and PE groups in contrast to our findings. Cao et al. (2002)[15] reported Ki-67 positivity in all types of mitotically active endometria: Proliferative, hyperplastic, and all forms of carcinomas. The percentage of positive cells was greatest in poorly differentiated subset; however, there was no statistically significant difference observed between the benign proliferating groups and low-grade endometrioid carcinomas. Ozuysal et al. (2005) documented the Ki-67 expression value in the cases of PE, SH, AH, and EC groups as 529.0+29.0, 463.3+33.6, 353.5+54.0, and 354.6+34.3, respectively. Mean Ki-67 values were higher with those cases with EC and AH (P < 0.01). There was no statistically significant difference between the mean values of cases with SH and PE. Liang et al.[17] observed no statistical significant difference (P > 0.05) on comparing Cyclin D1 expression of SH, atypical CH, endometrioid carcinoma clear cell carcinoma and endometrial serous carcinoma (ESC) though there was a gradual increase in the expression, except ESC. They concluded that though Cyclin D1 exhibited a promising potential to predict the prognosis of patients with EC, Cyclin D1 exhibited a poor ability to differentiate neoplastic lesions from non-neoplastic lesions; thus, the application of Cyclin D1 only is not so credible for differentiation between benign and malignant lesions.
Our findings suggest the significance of CH and AH as a precancerous lesion, but not SH. Further, these findings also indicate that both Cyclin D1 and Ki-67 together may serve as informative biomarkers to recognize subsets of lesions that may be precancerous (AH and CH) and thus amenable for early surgical therapy and at the same time exclude lesions that may be responsive to hormonal therapy. Thus they have the potential to serve as an useful adjunct to categorize these lesions. We observed statistically significant difference in expression of Cyclin D1 (median 30; P = 0.02) as well as K-67 (median 30; P = 0.01) when the results were compared between the AH and CH. Though EC showed variable expression of Cyclin D1 (median 40%) and results were statistically non-significant when expression for the same was compared with that of AH, statistically significant difference was seen for the expression of Ki-67 (median 57%) between them (EC and AH). Our findings also suggest that Cyclin D1 expression of >30% together with Ki-67 expression of >30% may indicate AH while a Ki-67 expression of >57% in the same may indicate EC. Our findings are different from that of Lange et al.[17] who conclude that Cyclin D1 has a poor ability to differentiate neoplastic lesions from non-neoplastic lesions. Therefore, further studies with larger samples are needed to validate or refute these findings.
Acknowledgment
The authors sincerely thank Mr Vikas Aggarwal, Mr Kanahiya Lal for their untiring technical help and Dr Alok Kumar, Assistant Professor, Department of Community Medicine, Institute of Medical Sciences, Banaras Hindu University, for his help in stastical analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–7. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 2.Kendall BS, Ronnett BM, Isacson C, Cho KR, Hedrick L, Diener-West M, et al. Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well-differentiated carcinoma. Am J Surg Pathol. 1998;22:1012–9. doi: 10.1097/00000478-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Zaino RJ, Kauderer J, Trimble CL, Silverberg SG, Curtin JP, Lim PC, et al. Reproducibility of the diagnosis of atypical endometrial hyperplasia: Agynecologic oncology group study. Cancer. 2006;106:804–11. doi: 10.1002/cncr.21649. [DOI] [PubMed] [Google Scholar]
- 4.Ito K, Sasano H, Matsunaga G, Sato S, Yajima A, Nasim S, et al. Correlations between p21 expression and clinicopathological findings, p53 gene and protein alterations, and survival in patients with endometrial carcinoma. J Pathol. 1997;183:318–24. doi: 10.1002/(SICI)1096-9896(199711)183:3<318::AID-PATH925>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Sherr CJ, Roberts JM. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 6.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 7.Russell A, Thompson MA, Hendley J, Trute L, Armes J, Germain D. Cyclin D1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene. 1999;18:1983–91. doi: 10.1038/sj.onc.1202511. [DOI] [PubMed] [Google Scholar]
- 8.Simpson JF, Quan DE, O’Malley F, Odom-Maryon T, Clarke PE. Amplification of CCND1 and expression of its protein product, cyclin D1, in ductal carcinoma in situ of the breast. Am J Pathol. 1997;151:161–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Schraml P, Kononen J, Bubendorf L, Moch H, Bissig H, Nocito A, et al. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res. 1999;5:1966–75. [PubMed] [Google Scholar]
- 10.Kaltz-Wittmer C, Klenk U, Glaessgen A, Aust DE, Diebold J, Löhrs U, et al. FISH analysis of gene aberrations (MYC, CCND1, ERBB2, RB, and AR) in advanced prostatic carcinomas before and after androgen deprivation therapy. Lab Invest. 2000;80:1455–64. doi: 10.1038/labinvest.3780152. [DOI] [PubMed] [Google Scholar]
- 11.Nikaido T, Li SF, Shiozawa T, Fujii S. Coabnormal expression of cyclin D1 and p53 protein in human uterine endometrial carcinomas. Cancer. 1996;78:1248–53. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1248::AID-CNCR12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.McCormick D, Chong H, Hobbs C, Datta C, Hall PA. Detection of the Ki-67 antigen in fixed and wax-embedded sections with the monoclonal antibody MIB1. Histopathology. 1993;22:355–60. doi: 10.1111/j.1365-2559.1993.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 13.Silverberg SG, Kurman RJ. Atlas of Tumor Pathology, 3rd series, fascicle 3. Washington, DC: Armed Forces Institute of Pathology; 1991. Tumors of the uterine corpus and gestational trophoblastic diseases; pp. 191–218. [Google Scholar]
- 14.Ozuysal S, Oztürk H, Bilgin T, Filiz G. Expression of cyclin D1 in normal, hyperplastic and neoplastic endometrium and its correlation with Ki-67 and clinicopathological variables. Arch Gynecol Obstet. 2005;271:123–6. doi: 10.1007/s00404-003-0595-5. [DOI] [PubMed] [Google Scholar]
- 15.Cao QJ, Einstein MH, Anderson PS, Runowicz CD, Balan R, Jones JG. Expression of COX-2, Ki-67, cyclin D1, and P21 in endometrial endometrioid carcinomas. Int J Gynecol Pathol. 2002;21:147–54. doi: 10.1097/00004347-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Ruhul Quddus M, Latkovich P, Castellani WJ, James Sung C, Steinhoff MM, Briggs RC, et al. Expression of cyclin D1 in normal, metaplastic, hyperplastic endometrium and endometrioid carcinoma suggests a role in endometrial carcinogenesis. Arch Pathol Lab Med. 2002;126:459–63. doi: 10.5858/2002-126-0459-EOCDIN. [DOI] [PubMed] [Google Scholar]
- 17.Liang S, Mu K, Wang Y, Zhou Z, Zhang J, Sheng Y, et al. CyclinD1, a prominent prognostic marker for endometrial diseases. DiagnPathol. 2013;8:138. doi: 10.1186/1746-1596-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]


