Abstract
Hyperimmunoglobulin M (HIGM) type 3 due to CD40 deficiency is a very rare syndrome. Only 16 cases have been reported thus far. The clinical presentation is very variable. We present the first case of this rare disorder from India. The case is of a two-and-a-half-year-old female, with a history of repeated episodes of skin infections and diarrhea since birth. Laboratory evaluation revealed elevated absolute lymphocyte count and an absolute neutrophil count (ANC) of 1026/mm3. The lymphocyte subset analysis showed normal absolute counts of Natural Killer (NK) cells and elevated absolute counts of T-cells (CD4 and CD8) and B-cells. The serum immunoglobulin estimation showed low levels of IgG, IgA, IgE and an elevated level of IgM. The CD154 analysis was normal and expression of CD40 was absent on the B-cells. Molecular analysis showed a novel mutation, with deletion of 3bp (AAG) [p.Glu107GlyfsX84] in the homozygous state, in the CD40 gene. Thus the patient was diagnosed as HIGM type 3. The parents were screened and counseled regarding prenatal diagnosis at the time of next pregnancy.
KEY WORDS: CD40 gene, CD40 ligand, CD154, hyper IgM, recurrent infection
Introduction
The hyperimmunoglobulin-M syndrome (HIGM) is a group of genetic disorders in which the B-cells cannot switch to other kind of antibodies, resulting in the overproduction of IgM and underproduction of IgA, IgG, and IgE. Five types of HIGM have been characterized.
A majority of the HIGM cases are X-linked type-1. During the signaling between T- and B-cells, activated CD4+ T-cells express the CD40 ligand (CD154), which binds to CD40 on the resting B-cells, and accounts for the immune and inflammatory responses regulating B-cell proliferation, immunoglobulin class switching, germinal center formation, growth suppression, inflammation, and cell death. CD40 is a cysteine-rich type-I transmembrane cell surface protein belonging to the tumor necrosis factor (TNF) receptor family, which is expressed by a variety of cells including B-cells, macrophages, dendritic cells, and other nonimmune cell types.[1] In HIGM type-3, mutations of the CD40 gene cause lack of surface expression of CD40 and loss of interaction between CD40 and CD40L, resulting in a lack of immunoglobulin isotype switching, impaired generation of memory B-cells, defective somatic hypermutation, monocyte activation, and T lymphocyte priming.[2] Also CD40 expression may not always suggest a normal function. There are cases in literature where B-cells do express CD40, but are dysfunctional.[3]
Only 16 cases with CD40 mutation, a rare autosomal recessive disorder, have been reported thus far.[2,4,5,6,7] The clinical manifestations include recurrent sinopulmonary infections, Pneumocystis carinii pneumonia, and Cryptosporidium parvum infection, with very low levels of IgG, IgA, and normal/high levels of IgM. Flow cytometry analysis shows peripheral blood B-lymphocytes that lack expression of surface CD40.[2] Here we present possibly the first case of CD40 deficiency from India, evaluated clinically, immunophenotypically, and molecularly.
Case Report
A two-and-a-half-year-old first female child, born of a non-consanguineous marriage, was referred with a history of repeated episodes of infections since birth. Recurrent diarrhea and skin boils (culture showed growth of Staphylococcus aureus) were the predominant clinical manifestations, with increased leukocyte count and eosinophilia.
After informed consent 2 ml of blood was collected in Ethylenediaminetetraacetic acid (EDTA). Laboratory evaluation revealed a white blood cell (WBC) count of 11400/mm3 with absolute lymphocyte count of 9690/mm3 and absolute neutrophil count of 1026/mm3. The hemoglobin was 11.7gm/dl and platelet count was 624 × 103/μl.
The lymphocyte subset analysis, using a six color TBNK BD antibody (BD Biosciences, San Diego, CA, USA) showed normal counts of NK-cells and elevated counts of T-cells (CD4, CD8) and B-cells [Table 1]. The Nitroblue-tetrazolium and Dihydrorhodamine tests were normal. Serum immunoglobulin showed low levels of IgG, IgA, and IgE, and an elevated level of IgM [Table 2].
Table 1.
Analysis of different lymphocyte subsets with normal count
| Lymphocyte subpopulation | Result % lymphocyte | Absolute lymphocyte count/mm3 | Normal absolute count/mm3[8] |
|---|---|---|---|
| Lymphocytes | 85 | 9690 | 2300-5400 |
| CD19+ B-lymphocytes | 30 | 2907 | 390-1400 |
| CD3+ T-lymphocytes | 65 | 6298 | 1400-3700 |
| CD3+/CD4+ Th-lymphocytes | 44 | 4264 | 700-2200 |
| CD3+/CD8+ Tc-lymphocytes | 16 | 1550 | 490-1300 |
| CD3-/CD16+56+ NK-Cells | 5 | 484 | 130-720 |
Table 2.
Levels of serum immunoglobulin with normal control
| Immunoglobulin | Values | Normal range (Western)[9] |
|---|---|---|
| IgG | <1.37g/L | 4.0-15.9g/L |
| IgA | <0.255g/L | 0.17-2.90g/L |
| IgM | 6.16g/L | 0.34-3.48g/L |
| IgE | <17.5IU/ml | 3-423IU/ml |
CD154 analysis was normal. Expression of CD40 was absent on the B-cells [Figure 1]. After DNA extraction, polymerase chain reaction (PCR) amplification and the direct DNA sequencing technique were used for detection of mutations in the CD40 gene, using the Big Dye terminator v3.1 cycle sequencing kit (Applied Biosystems, USA), on an automated DNA sequencer, Applied Biosystems 3130xl Genetic Analyzer. This showed a novel deletion of 3bp (AAG) [g.4407_4409delAAG p.Glu107GlyfsX84] in a homozygous state in exon 4, leading to a frame-shift mutation and generation of a stop codon at p.192 in exon 7 [Figure 2]. This mutation was also found in a heterozygous condition in both the parents. The patient was managed using regular intravenous immunoglobulin supplements and prophylactic antibiotics. The parents were counseled regarding prenatal diagnosis for the next pregnancy.
Figure 1.
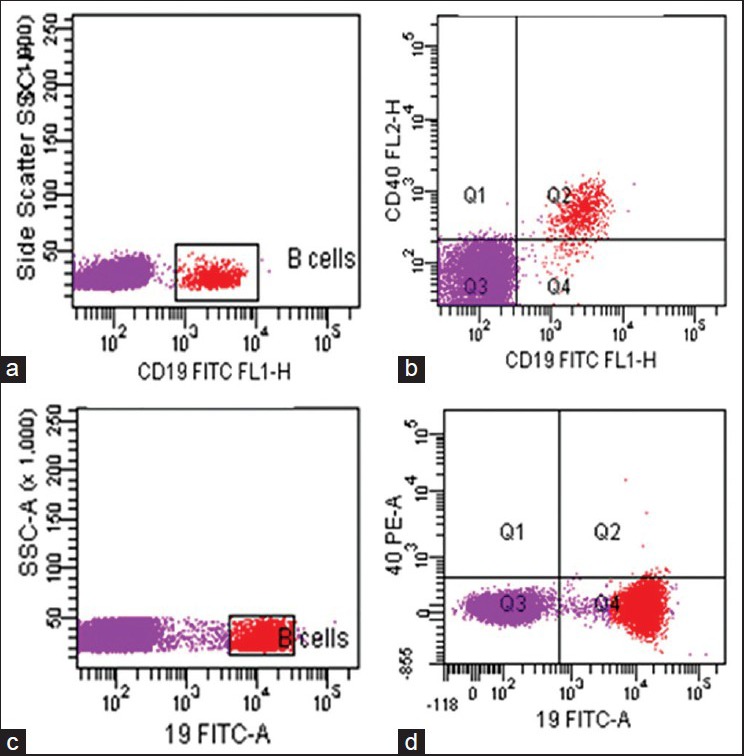
Expression of CD40 on B cells. Panel a and c: B-cell gating on side scatter and CD19 in normal and patient, respectively. Panel b: Normal expression of CD40 on B-cells. Panel d: B-cells with absence of CD40 expression in patient
Figure 2.
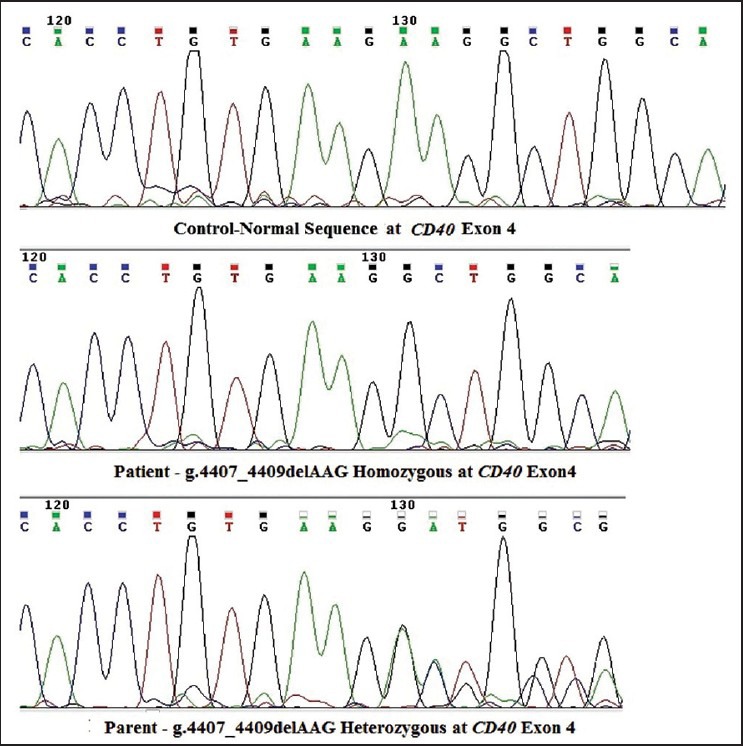
Sequence of novel mutation in the CD40 gene along with a wild-type normal sequence
Discussion
Here we discuss the clinical presentation, immunophenotypic analysis, and characterization of a novel mutation leading to CD40 deficiency. The largest reported study of HIGM type- 3 was in 11 patients from Saudi Arabia collected retrospectively over a eight year period. The age at presentation was 1-18 months and showed variable clinical severity. They presented with respiratory infection of viral origin followed by bacterial infections. Chronic diarrhea was reported in 63% of the patients.[7] It was also reported to be the most common clinical presentation in two cohorts of HIGM from Europe and USA.[10] The opportunistic infection in these patients could be due to improper maturation of dendritic cells leading to a defect in T-cell priming and interferon-γ secretion, besides CD40 deficiency.[4] Our patient presented with recurrent infection and chronic diarrhea since birth. However, opportunistic infection was not documented. Only S. aureus was isolated from the skin infection.
Most reports suggest low levels of IgA, IgG, and IgE and normal or high levels of IgM, which was in agreement with our patient.[2,4,5,6,7] Al-Saud et al. reported reduced NK-cells in few of the patients on more than one occasion, whereas, our patient showed normal NK-cells.[7]
The molecular analysis reported by Al-Saud et al. showed missense and splice site mutations in exon 3 in all 11 patients,[7] whereas, we got a novel mutation in exon 4 of the CD40 gene, with a 3bp deletion, leading to the absence of CD40 expression. Lanzi et al. demonstrated three CD40 mutations and showed that mutated proteins that could not fold were either retained in the endoplasmic reticulum causing stress and cell apoptosis or were degraded by the endoplasmic reticulum degradation pathway. However, different CD40 mutants showed different cellular behavior and elicited different cellular responses.[1]
A total of 3/11 patients reported by Al Saud et al. died due to severe sepsis suggesting the condition was fatal even after regular supplements of intravenous immunoglobulin and Granulocyte colony-stimulating factor (G-CSF), whereas, the others were doing fairly well.[7] Similarly, our patient showed reduction in the infection rate after regular intravenous immunoglobulin supplements and prophylactic antibiotics. She now occasionally has infection, which is managed with antibiotics.
Although stem cell transplant is the only cure for the disease, early screening in all newborns within the affected kindred can be considered along with prenatal diagnosis in this disease subject to cost effectiveness.
Acknowledgment
We acknowledge the Indian Council of Medical Research for providing the support for this project.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Lanzi G, Ferrari S, Vihinen M, Caraffi S, Kutukculer N, Schiaffonati L, et al. Different molecular behavior of CD40 mutants causing hyper-IgM syndrome. Blood. 2010;116:5867–74. doi: 10.1182/blood-2010-03-274241. [DOI] [PubMed] [Google Scholar]
- 2.Karaca NE, Forveille M, Aksu G, Durandy A, Kutukculer N. Hyper-immunoglobulin M syndrome type 3 with normal CD40 cell surface expression. Scand J Immunol. 2012;76:21–5. doi: 10.1111/j.1365-3083.2012.02697.x. [DOI] [PubMed] [Google Scholar]
- 3.Blaeser A, McGlauchlen K, Vogel LA. Aged B lymphocytes retain their ability to express surface markers but are dysfunctional in their proliferative capability during early activation events. Immun Ageing. 2008;5:15. doi: 10.1186/1742-4933-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontana S, Moratto D, Mangal S, De Francesco M, Vermi W, Ferrari S, et al. Functional defects of dendritic cells in patients with CD40 deficiency. Blood. 2003;102:4099–106. doi: 10.1182/blood-2003-04-1244. [DOI] [PubMed] [Google Scholar]
- 5.Kutukculer N, Moratto D, Aydinok Y, Lougaris V, Aksoylar S, Plebani A, et al. Disseminated cryptosporidium infection in an infant with hyper-IgM syndrome caused by CD40 deficiency. J Pediatr. 2003;142:194–6. doi: 10.1067/mpd.2003.41. [DOI] [PubMed] [Google Scholar]
- 6.Mazzolari E, Lanzi G, Forino C, Lanfranchi A, Aksu G, Ozturk C, et al. First report of successful stem cell transplantation in a child with CD40 deficiency. Bone Marrow Transplant. 2007;40:279–81. doi: 10.1038/sj.bmt.1705713. [DOI] [PubMed] [Google Scholar]
- 7.Al-Saud BK, Al-Sum Z, Alassiri H, Al-Ghonaium A, Al-Muhsen S, Al-Dhekri H, et al. Clinical, immunological, and molecular characterization of hyper-IgM syndrome due to CD40 deficiency in eleven patients. J Clin Immunol. 2013;33:1325–35. doi: 10.1007/s10875-013-9951-9. [DOI] [PubMed] [Google Scholar]
- 8.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Pediatric AIDS Clinical Trials Group. Lymphocyte subsets in healthy children from birth through 18 years of age: The Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Haynes B, Fauci A. Disorders of the immune systems, connective tissue and joints. In: Fauci AS, Braunwald E, Isselbacher K, Wilson J, Martin J, Kasper D, et al., editors. Harrison's Principles of Internal Medicine. 14th ed. New York, NY: McGraw Hill Co; 1998. pp. 1753–76. [Google Scholar]
- 10.Winklestein J, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, et al. The X-linked hyper-IgM syndrome: Clinical and immunologic features of 79 patients. Medicine (Baltimore) 2003;82:373–84. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]


